Abstract
Peripheral blood cells from 28 patients with leukemic cutaneous T-cell lymphoma including 25 patients with Sézary syndrome were evaluated for expression of regulatory T-cell-associated markers (FoxP3, CD25, CTLA-4, neurophilin-1), T-cell activation markers (CD28 and its ligands B7.1 and B7.2) and NK cell-associated markers (NKG2D and its ligands MicA and Mic-B) using real-time quantitative polymerase chain reaction. T-plastin served as a positive genetic marker, and its expression correlated to blood tumor burden. More than 90% of samples had transcripts for CD28 and Mic-B, but less than 30% of samples expressed FoxP3, CTLA-4 and CD25. Expression of Mic-B by neoplastic cells could provide another mechanism to inhibit anti-tumor immune responses. FoxP3 expression correlated with a poor prognosis. Although the underlying mechanisms accounting for this correlation remain unclear, the expression of the Foxp3 and CTLA-4 regulatory elements indicates that a subset of leukemic cases displays a regulatory T-cell phenotype.
Keywords: Lymphoma, Sézary syndrome, T-plastin, FoxP3, Mic-B, CD28, regulatory T-cell
Introduction
Cutaneous T-cell lymphoma (CTCL) is a heterogeneous group of primary cutaneous non-Hodgkin lymphomas that is composed predominantly of mycosis fungoides (MF) and Sézary syndrome (SS) [1]. According to recommendations of the International Society for Cutaneous Lymphomas (ISCL) [2], SS is currently defined as an erythrodermic expression of CTCL with high blood tumor burden or “leukemic” involvement, but some patients with the clinical findings more typical of MF also may fulfil the hematologic criteria for leukemic disease. For this reason, we use the inclusive term “leukemic CTCL” to include patients with SS and MF who meet the B2 blood rating defined by the ISCL [2].
The prevailing concept is that MF/SS are clonal proliferations of skin-homing memory T-cells based on the frequent expression of cutaneous lymphocyte-associated antigen (CLA) [3–5], and chemokine receptors CCR4 and CCR10 that are involved in trafficking into the skin [6–8]. The typical immunophenotype of the neoplastic T-cells is CD4 + CD45RO + CD26− with variable expression of CD7 and CD25 (interleukin-2 receptor α chain/Tac) among cases [9–13]. Functionally, neoplastic T-cells of MF/SS have properties of immunoregulatory cells. Initial studies in vitro showed that the neoplastic T-cells of SS potentiate B cell transition into immunoglobulin-secreting plasma cells [14]. By contrast, malignant T-cells from patients with the adult T leukemia/lymphoma (ATL) frequently functioned as suppressor cells. In addition, the neoplastic T-cells observed in most cases of MF/SS appear to exhibit a TH2 cytokine secretion profile [15]. Most recently and relevant to this study, Berger showed that under certain conditions, neoplastic T-cells of CTCL can up-regulate expression of FoxP3, CD25, and cytotoxic lymphocyte-associated antigen-4 (CTLA-4/CD152) with enhanced capacity to secrete of IL-10 and transforming growth factor-β, i.e., the phenotype and functional properties of regulatory T (Treg) cells [16]. Wong extended these studies by showing that freshly isolated T-cells from patients with MF as well as neoplastic T-cells from SS markedly increase expression of CTLA-4 mRNA and surface protein when stimulated compared with normal cells [17]. If MF/SS is a tumor of Treg cells as proposed by Berger [16], then this might account for the profound immunosuppression that characterises SS [18].
FoxP3 (forkhead box P3) is the master regulatory gene for the development and function of both natural and induced Treg cells [19,20]. FoxP3 protein (scurfin) forms complexes with other coactivator or corepressor proteins, leading to Treg-related repression of interleukin-2 secretion and up-regulation of coding for CD25 and CTLA-4. Accordingly, FoxP3 gene expression and protein is used as a marker of Treg cells although it should be recognised that FoxP3 expression per se does not always identify cells with Treg functional properties [21].
CTLA-4 together with CD28 acts on T-cells as inhibitory and stimulatory receptors, respectively, when engaged to their ligands, B7-1/CD80 and B7-2/CD86 [22,23]. CD28 as well as B7-1 and B7-2 have been reported to be frequently expressed by neoplastic T-cells of MF/SS in the skin [4,24], suggesting the existence on an auto-stimulatory loop [25]. CTLA-4 is rapidly up-regulated following T-cell activation, inhibits T-cell responses, and regulates peripheral T-cell tolerance. Conversely, CD28, which is constitutively expressed on the surface of T-cells, acts in concert with TCR signalling to enhance T-cell activation and proliferation, and enhances T-cell survival by up-regulating anti-apoptotic proteins such as Bcl-XL. The interaction between CD28 and B7 is important for development and maintenance of natural Treg cells [26]. B7-2 is constitutively expressed on some resting T-cells whereas B7-1 is not present on resting T-cells, but both ligands can be up-regulated on activated T-cells.
Previous reports indicated a decline in the natural killer (NK) cell activity associated with CTCL progression [27–29]. However, it remained unclear whether this was due to a decrease of the NK cell numbers, impairment of their cytotoxic function, or both. Recent studies of SS have confirmed a quantitative decrease of NK cells, but also indicate that NK cells are capable of lyzing autologous neoplastic cells as well neoplastic cell lines derived from SS [30,31].
NK cells utilise a form of immune recognition known as “induced self recognition” that is exemplified by the NKG2D immunoreceptor-MicA/B ligand system [32–34]. Mic-A and its homologue Mic-B are MHC class I chain-related proteins that are not expressed abundantly by most normal cells, but are up-regulated on cells exposed to various cellular insults and neoplastic transformation [35]. These proteins are ligands for the activating immunoreceptor NKG2D on NK cells and cytotoxic T-cells, and provide a co-stimulatory signal that activates the cytolytic response (release of cytotoxic granules). In mice, NKG2D ligand expression by tumor cells results in tumor rejection by NK cells and/or CD8 T-cells, and its absence or down-regulation results in tumor escape from NK and T-cell attack. The anti-tumor response to naturally arising malignant cells appears to vary depend concentration of the expressed NKG2D ligands. Thus, NKG2D ligands appear to play a critical role in tumor immune surveillance, but there is no information about their role in MF/SS.
T-plastin, which is not normally expressed in lymphoid tissue, appears to be characteristically expressed by neoplastic T-cells of CTCL [36–40], and has been used as a molecular marker in studies that use real-time quantitative polymerase chain reaction (qPCR) [38]. Recent in vitro studies indicate that T-plastin is also expressed in cell lines derived from SS, but not T-cell lines derived from other T-cell lymphomas [39].
In this study we examined in leukemic cells from a cohort of CTCL patients, the mRNA gene expression profile of Treg-associated markers (FoxP3, CD25, CTLA-4, neurophilin-1), CD28 and its ligands B7.1 and B7.2 and NKG2D and its ligands Mic-A and Mic-B. T-plastin was used as a genetic marker of neoplastic T-cells in these experiments. We found that Mic-B is highly expressed by the neoplastic cells, and that FoxP3 is expressed in some cases that have a relatively poor prognosis.
Materials and methods
Real-time qPCR was performed on samples of peripheral blood lymphocytes from 28 patients with the leukemic phase (B2 blood rating) of CTCL as defined using hematologic criteria recommended by the ISCL [2]. The cells were thawed and washed in RPMI 1640 tissue culture medium. For qPCR analysis, mRNA was extracted and purified with Trizol from lysate of 5 – 10×105 thawed and washed cells with 500 μL Trizol (Invitrogen, Carlsbad, CA) reagent. Twenty-six of the samples with usable mRNA were collected by leukopheresis between November 1989 and September 1997 and stored in liquid nitrogen, and 2 samples (PT30 and PT31) were obtained from recently evaluated patients (Table I). Twenty-five cases had generalised erythroderma and therefore fulfilled the ISCL definition of SS, i.e., T4B2. The remaining 3 patients also had leukemic involvement, but had skin manifestations of extensive MF (PT20 and PT27) or primary cutaneous peripheral T-cell lymphoma with histologic features resembling Kimura disease (PT28). These studies were approved by the Institutional Review Board of Johns Hopkins University.
Table I.
Expression levels of T-plastin, FoxP3 and other gene transcripts in neoplastic cell samples from the leukemic phase of cutaneous T-cell lymphoma.*,‡
| PT | DX§ | %SC (mm3) | CD4/8 | %CD4 +7-or CD4 +26- | T-plastin | FoxP3 | CD25 | CTLA-4 | CD28 | Mic-A | Mic-B |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | SS | 30 (357) | 47/13 | 35 | + | 0 | 0 | 0 | + | 0 | 0 |
| 2 | SS | 61 (1909) | 83/9 | ND | ++ | 0 | 0 | 0 | ++ | 0 | +++ |
| 3 | SS | 34 (1071) | 50/8 | 56 | ++ | 0 | 0 | 0 | + | 0 | +++ |
| 5 | SS | 62 (3889) | 72/13 | 48 | ++ | 0 | 0 | 0 | + | 0 | 0 |
| 6 | SS | 21 (840) | 78/8 | 72 | ++ | 0 | 0 | 0 | ++ | 0 | +++ |
| 7 | SS | 24 (2359) | 94/1 | 92 | ++ | 0 | 0 | 0 | 0 | 0 | ++ |
| 8 | SS | 42 (1438) | 67/13 | ND | ++ | 0 | 0 | 0 | + | 0 | +++ |
| 9 | SS | 84 (5645) | 94/1 | 75 | ++++ | 0 | 0 | 0 | ++++ | 0 | ++++ |
| 10 | SS | 27 (525) | 75/5 | 1 | + | 0 | ++ | + | + | + | + |
| 11 | SS | 53 (1823) | 91/0.4 | 92 | ++ | + | 0 | 0 | + | 0 | ++ |
| 13 | SS | 22 (1391) | 75/1 | 61 | ++ | 0 | 0 | 0 | ++ | 0 | +++ |
| 14 | SS1 | 35 (660) | 80/5 | 39 | +++ | 0 | 0 | 0 | ++ | 0 | ++++ |
| 15 | SS | 71 (1189) | 76/6 | 64 | ++++ | 0 | 0 | 0 | 0 | 0 | ++++ |
| 16 | SS | 67 (22011) | 93/5 | 91 | +++ | 0 | ++++ | 0 | +++ | 0 | ++++ |
| 17 | SS1 | 53 (10330) | 91/6 | 91 | + | + | 0 | 0 | + | 0 | 0 |
| 18 | SS | 37 (492) | 65/2 | 37 | + | 0 | 0 | 0 | + | + | + |
| 20 | MFT | 32 (1156) | 86/8 | 86 | ++ | 0 | 0 | 0 | + | 0 | ++ |
| 21 | SS | 50 (1066) | 85/5 | 78 | + | 0 | +++ | + | + | 0 | + |
| 22 | SS | 50 (15314) | 96/0.3 | 95 | +++ | 0 | ++++ | 0 | ++ | 0 | +++ |
| 23 | SS | 50 (385) | 82/8 | ND | + | 0 | +++ | 0 | + | + | ++ |
| 24 | SS | 46 (789) | 84/3 | 81 | + | + | ++ | + | + | + | ++ |
| 25 | SS | 64 (37901) | 99/0.4 | 99 | ++ | 0 | 0 | 0 | + | 0 | ++++ |
| 26 | SS | 68 (3225) | 79/10 | ND | + | 0 | 0 | 0 | ++ | 0 | +++ |
| 27 | MFPQ | 28 (1614) | 84/7 | 81 | ++ | 0 | 0 | 0 | + | 0 | 0 |
| 28 | PTL¶ | 15 (188) | 70/4 | ND | ++ | 0 | 0 | 0 | + | + | +++ |
| 29 | SS | 91 (93020) | 98/0.4 | 99 | ++ | ++ | 0 | + | +++ | + | +++ |
| 30† | SS | 26 (816) | 86/3 | 75 | + | + | + | + | + | + | + |
| 31† | SS | 56 (2399) | 98/2 | 82 | ++++ | +++ | ++++ | + | +++ | ++ | +++ |
Abbreviations: SS, Sézary syndrome; SC, Sézary cell; MFPQ, mycosis fungoides, plaque phase; MFT, mycosis fungoides, tumor phase.
Gene/GAPDH transcripts: 0, not detected; +, gene expression less than 10-fold that of GAPDH expression; ++, gene expression 10 to <100 fold that of GAPDH; +++, gene expression 100 to <1000 fold that of GAPDH, ++++, gene expression 1000 or more that of GAPDH.
Recently collected samples.
Results for B7.1, B7.2, neurophilin-1 not shown (transcripts absent or rarely detected). Cases 4, 12 and 19 with SS excluded because GADPH failed to amplify.
Diagnosis of SS rendered if erythrodermic CTCL and at least one criterion for leukemic blood involvement per recommendations of the ISCL [2].
Cutaneous plaques and nodules with histologic features suggestive of Kimura disease; abnormal T-cells in blood with CD2+CD3-CD4+CD7-CD8-immunophenotype.
Sézary syndrome preceded by mycosis fungoides (secondary SS).
The immunophenotype of the neoplastic cells in all cases was CD3+CD4+CD8- with a CD4/CD8 ratio of 10 or more at the time of collection for 21 (75%) cases. Absolute Sézary cell counts exceeded 1.0 K/μL in 20 (71%) of the cases, and absolute lymphocyte counts exceeding 4.0 K/μL occurred in 13 patients of which 10 had evidence of a dominant T-cell clone by Southern blot (3 cases), PCR analysis of the T-cell receptor gamma chain (5 cases) or both methods (2 cases). Chromosome studies showed an abnormal clone in the blood for 23 (82%) cases. Taken together, 4 patients had one ISCL criterion for leukemic involvement, 5 patients had 2 criteria, 4 patients had 3 criteria, 8 patients had 4 criteria and 7 patients had all 5 criteria [2]. At the time of analysis, all but 2 patients (PT2, PT28) have died as a result of disease.
Target gene expression (T-plastin, FoxP3, CD25, CD28, CTLA-4, B7.1, B7.2, neurophilin-1, NKG2D, Mic-A and Mic-B) was assessed by qPCR using an ABI PRISM 7000 Sequence Detection System. Reagents and primer/probe constructs for target genes were purchased from Applied Biosystems, Inc. (Foster City, CA) [41,42]. Each sample was multiplexed with an endogenous control, glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Relative transcripts were determined by the formula: . Real-time PCR efficiencies of target genes and the reference gene (GAPDH) were approximately equal over a concentration of 0.1–200 ng total cDNA.
Multi-colour flow cytometry was utilised to evaluate the number of CD3 + CD4 + cells that express Foxp3 + and to correlate the relative number of Foxp3 + cells with Foxp3 + mRNA transcript levels assessed by qPCR. Briefly, to inhibit clumping of thawed cells, the initial diluent contained DNAase, 0.01%, RPMI 1640 and 20% normal human serum. The cells were then stained with directly conjugated anti-CD3 and anti-CD4 antibodies followed by intracellular staining for Foxp3 (clone PCH101, Human Regulatory T Cell Staining Kit; (e-Biosciences, San Diego, CA) according to the instructions supplied by the manufacturer. Flow cytometric analysis was assessed on a dual laser FACSCanto II (Becton-Dickinson, San Jose, CA) flow cytometer.
Statistical analysis
Because most continuous variables did not have a normal distribution, non-parametric tests (Mann–Whitney and Kruskal–Wallis tests) were used to test for differences among 2 and 3 or more groups, respectively. Fisher and Pearson chi-square exact tests were used to test categorical data in 2 by 2 and R by C tables, respectively. Spearman rank order test was used to test for correlation between two variables. The Cox proportional hazards model was used to examine the relationship between prognostic factors and survival. Survival probabilities were calculated using the method of Kaplan–Meier and survival curves were compared using the log-rank test of Mantel–Cox. Survival was determined from time of blood sampling to the death of death or last known date alive. Censoring was not required because the status of each patient was known at the time of the analysis. Death from any cause used to define overall survival. The statistical packages used for data analysis and graphs were SYSTAT10 and SPSS 13.0 for Windows, SPSS, Inc., Chicago, IL; StatXact 6 and EGRET for Windows, Cytel, Inc., Cambridge, MA; and SigmaPlot 9.0, Systat Software, Inc., Point Richman, CA.
Results
Gene mRNA expression was found in the majority of samples for T-plastin, CD28, and Mic-B (100%, 93% and 86% of samples, respectively; Table I. In contrast, mRNA transcripts were detected in a minority of the samples for CD25 (29% of samples), and FoxP3, CTLA-4 and Mic-A (21% of samples each). Transcripts for neurophilin-1, B7.1 and B7.2 were absent or rarely detected. Interestingly, when mRNA transcripts were detected for these genes (T-plastin, Foxp3, CTLA-4, CD25, CD28, Mic-A and Mic-B) in the patient samples, they greatly exceeded on average concentrations detected in normal peripheral mononuclear cells from control subjects (n = 6) (Figure 1).
Figure 1.
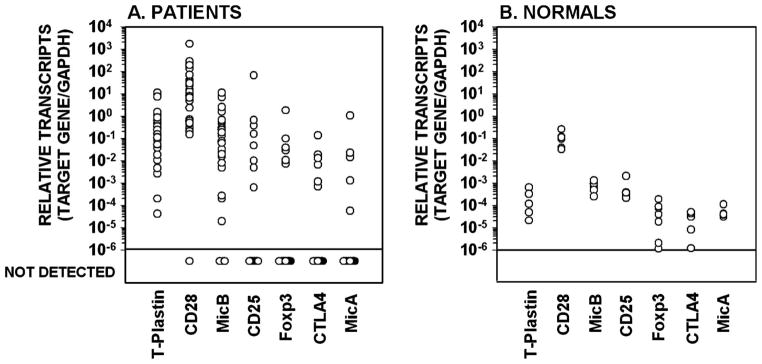
The gene expression profile of T-plastin, FoxP3 and other markers of T-cell activation in peripheral blood mononuclear cell samples from patients with Sézary syndrome compared with normal controls.
Expression of T-plastin mRNA transcripts
Transcripts for T-plastin were detected in all 28 samples with a wide range of expression levels among cases (Table I, Figure 1). Because message for T-plastin has been proposed as a molecular marker of SS [37,38,40] and because qPCR provides a quantitative measure of gene transcripts in the sample, T-plastin transcript levels were compared with other measures of blood tumor burden. One such measure, the number of Sézary cells/mm3 (absolute Sézary cell count), was available on all cases of SS (n = 25) and correlated significantly to the level of T-plastin transcripts (ρ = + 0.425, p = 0.034). On a smaller subset of patients, positive, but not statistically significant, correlations were also observed between T-plastin transcript levels and absolute counts of CD4 + CD26- cells (n = 21, ρ = + 0.286, p = 0.205), and Vβ + cells (n = 12, ρ = + 0.315, p = 0.306). The lack of correlation with CD4 + CD7- cells (n = 21, ρ = + 0.096, p = 0.674) reflects the fact that CD7 is variably expressed by neoplastic T-cells [43].
The level of T-plastin transcripts also correlated strongly with the transcript levels for CD28 (ρ = + 0.457, p = 0.022) and Mic-B (ρ = + 0.720, p <0.001) as well as the chemokine receptor CCR10 (ρ = + 0.761, p <0.001) that is highly expressed by neoplastic T-cells (data not shown) [8]. The transcript levels of CD28 and CCR10 also significantly correlated with the absolute Sézary cell count (ρ = + 0.423, p = 0.035 and ρ = + 0.512, p = 0.009, respectively) whereas the positive correlation with Mic-B failed to reach statistical significance (ρ = + 0.332, p = 0.104). Although serum LDH also correlated significantly with the absolute Sézary cell count (ρ = + 0.448, p = 0.025), no correlation was found between T-plastin expression and serum LDH levels (ρ = + 0.095, p = 0.646). In contrast to other measures of blood tumor burden (absolute Sézary cell count, CD4/CD8 ratio, and serum LHD level), the level of T-plastin transcripts in samples from the 25 patients with SS did not correlate significantly with survival in the Cox model (p = 0.381).
Expression of FoxP3, CD25 and CTLA-4 mRNA Transcripts
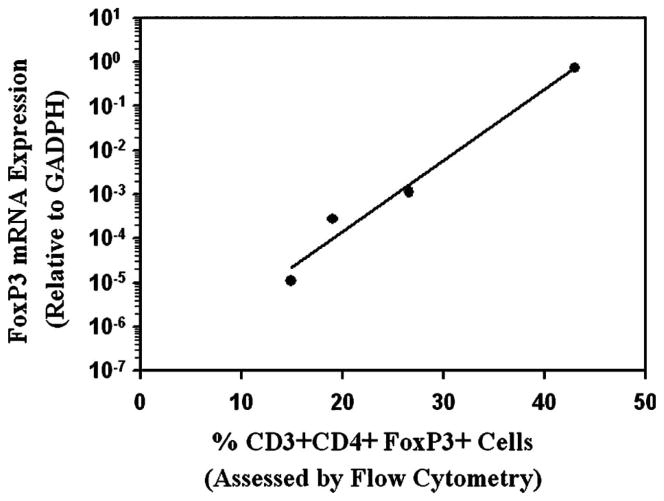
Transcripts for FoxP3 were detected in 6 (21%) samples from our patients with SS including PT31 whose neoplastic T-cells had extremely high expression levels for FoxP3 and several other genes including CD25, but not CTLA-4. Overall, transcripts for CD25 were found in only 8 (29%) and CTLA-4 message was found in 6 (21%) of our samples. Additional studies were undertaken to determine whether mRNA transcript levels for Foxp3 correlated with the levels of CD3 + CD4 + Foxp3 + cells detected by flow cytometry. As illustrated in Figure 2, there was significant correlation (p <0.001) of Foxp3 message levels with the number of cells expressing Foxp3 protein for a limited number of cases that could be studied.
Figure 2.
Correlation of Foxp3 mRNA transcript levels with the number of CD4 +Foxp3+ T-cells identified by multi-colour flow cytometry.
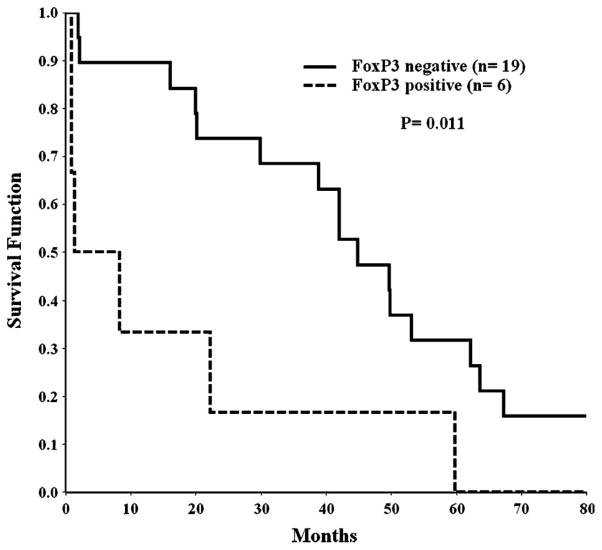
CD25 transcripts were found in 3 (50%) of the 6 FoxP3 expressing samples versus 5 of the 22 (23%) that did not express FoxP3, a difference that was not statistically significant (Fisher test, p = 0.311). Most of the FoxP3 + CD25 + cases exhibited low levels of CD25 transcripts except for PT31 who had relatively high FoxP3 expression plus very high expression of CD25, findings that are associated with a Treg phenotype. Of the remaining 22 FoxP3 negative samples, the level of CD25 transcripts was also often at high levels, which may be more indicative of cell activation. The correlation between FoxP3+ and CTLA-4+ cases and between CD25+ and CTLA-4+ cases also was significant (Fisher test, p = 0.01 and p = 0.003, respectively). This further suggests that some of the FoxP3 expressing cases may have acquired CTLA-4 expression as well as CD25. Although we recognise that statistical results based on such small numbers of cases must be interpreted with caution, these findings support the notion that a subset of leukemic CTCL patients might have acquired a phenotype of Treg cells. Of interest, the 6 patients with SS whose neoplastic cells expressed FoxP3 had a significantly worse prognosis than the other 19 patients whose cells lacked FoxP3 (Mantel test, p = 0.011; Figure 3). Cases with CTLA-4 mRNA expression also had a worse prognosis, but the difference in survival curves was not significantly different (Mantel test, p = 0.163), and there was no correlation with survival for cases with or without CD25 mRNA expression (Mantel test, p = 0.871).
Figure 3.
An adverse survival was observed for patients with Sézary syndrome whose circulating neoplastic T-cells expressed FoxP3 mRNA compared with patients without expression.
CD28, B7.1 and B7.2
In concert with reported flow cytometric studies [44,45], CD28 transcripts were detected in all but 2 samples. This is not surprising because CD28 is constitutively expressed by normal T-cells. Moreover, the level of CD28 transcripts in the cells varied widely among the cases which agrees with the study by Lima in which CD28 surface protein expression on Vβ+ neoplastic cells of 18 cases with SS was increased in 9 cases, normal in 2 cases, and decreased in 7 cases [45]. The level of CD28 transcripts was positively correlated with the level of T-plastin (ρ = + 0.426, p = 0.024), a finding that suggests that some of the variability of CD28 expression among our samples was related to the percentage of neoplastic cells in the sample.
With regard to circulating neoplastic T-cells, Schwab found very low surface expression of B7.1, but B7.2 was increased in all 5 cases of SS studies by flow cytometry [44]. However, contrary to this report, transcripts for B7.2 were detected in only 2 of our cases overall including the sample from one patient with SS (PT31) who also had relatively high expression levels of FoxP3, CD25 and CD28.
NKG2D, MicA and MicB
An unexpected finding in our study was that Mic-B mRNA was present in 86% of samples with median transcript concentrations in positive samples second only to T-plastin. In addition, the level of Mic-B transcript levels was strongly correlated to that of T-plastin (ρ = 0.637, p <0.001), suggesting a relationship to blood tumor burden. Mic-A transcripts were present in 8 (29%) samples at low expression levels and NKG2D mRNA was detected in one sample. Neither the level of Mic-B transcripts nor the presence or absence of Mic-A transcripts correlated with survival.
Discussion
T-plastin mRNA appears to be characteristically expressed by neoplastic T-cells of CTCL [36–38,40]. It is therefore not surprising that the T-plastin expression levels correlated to absolute Sézary cell counts, which is used to approximate blood tumor burden in SS [46,47]. T-plastin expression also strongly correlated with CCR10 expression (data not shown; ρ = + 0.686, p <0.001), which is characteristically expressed by neoplastic T-cells [8,48,49]. However, although the absolute Sézary cell count correlated significantly with prognosis in the Cox model (p = 0.011), neither T-plastin nor CCR10 transcript levels had prognostic importance at least for this cohort of CTCL patients with high blood tumor burden. Of interest, T-plastin was not identified as one of the genes associated with poor outcome in patients with high-count SS that were studied by a cDNA gene array even though it was highly expressed in the samples [37]. Therefore, T-plastin expression may be more useful as a molecular marker for diagnosis than for prognosis. Other significant correlations were observed between transcript levels of T-plastin and CD28 (ρ = + 0.426, p = 0.024) and Mic-B (ρ = + 0.637, p <0.001) that may be associated with blood tumor burden.
T-plastin, encoded by PLS3 located at chromosome segment Xq23, is an actin-bundling protein that is highly expressed in actively dividing cells of solid tissues and up-regulated in several carcinomas, but unlike its isoform L-plastin, is not expressed by lymphoid tissue [50]. T-plastin is involved in regulation of the actin cytoskeleton by cross-linking actin filaments into tight bundles, and has been implicated in DNA repair [50]. It has been proposed that the cortical cytoskeleton acts as a scaffold for various signal transduction pathways [51]. Considering that a translocation that partnered the L-plastin gene (LCP1), located at chromosome segment 13q14.3, with the BCL6 gene has been implicated in the pathogenesis of B-cell lymphoma [52], we wondered if a similar molecular alteration that involves the T-plastin gene might be important in CTCL. However, no translocations that involve this segment have to our knowledge been reported in CTCL [53].
The present study agrees with previous reports that CD28 is constitutively expressed by neoplastic T-cells similar to normal T-cells [4,24,44,45], but perhaps with more variability with regard to its density. No difference in CD28 protein expression by infiltrating T-cells in the skin was observed between MF and SS [4]. CD28 co-stimulation of normal CD4+ T-cells via its major ligands, B7.1/CD80 or B7.2/CD86, is essential for proliferation and survival via the Bcl-xL pathway, and promotes differentiation into TH2 cells with production of IL-4 and IL-5 [22,54]. However, in contrast to other studies [4,24,44], we did not find transcripts for B7.1 or B7.2 in neoplastic cells. In addition, if neoplastic T-cells were being co-stimulated through the T-cell receptor and CD28, one might expect up-regulation and more frequent expression of CTLA-4 that normally acts to antagonise the main effects of CD28. Thus, the results of the present study do not support the hypothesis that CD28-B7 interactions provide autostimulation of neoplastic T-cells in advanced stages of CTCL.
The present study also shows that Mic-B gene transcript levels are highly expressed by neoplastic cells of CTCL and that further studies are warranted to determine the significance of this finding in CTCL. However, it should be noted that high Mic-B expression was not found in two gene expression array studies that compared SS with controls, raising question about the specificity of this finding [40,55]. The Mic-B gene and its homologue Mic-A gene encode proteins that are ligands for the activating immunoreceptor NKG2D on NK cells and cytotoxic T-cells that triggers release of cytotoxic granules [32–35]. Because Mic-B protein expression by neoplastic T-cells was not studied nor was soluble Mic-B measured in the sera of our patients, we can only speculate as to the clinical significance of high Mic-B gene expression by neoplastic T-cells. However, assuming that high gene transcript levels indicate that Mic-B protein is being expressed as occurs with a variety of other malignancies including hematopoietic tumors [56–58], then the question arises how are these cells able to evade killing by NKG2D bearing NK or CD8+ T-cells considering the fact that NK cells harvested from patients with SS are capable of killing autologous neoplastic cells [30–31]. One potential mechanism is that the prolonged encounter with tumor cell-bound, but not soluble, NKG2D ligand alters NKG2D signalling in NK cells [59]. Another potential mechanism is that tumor-derived soluble Mic-B protein can down-regulate expression of NKG2D on CD8+ T-cells and suppress T-cell activation [60,61].
Lastly, our studies indicate that FoxP3 mRNA is not detectable in the majority (22/28 samples) of unstimulated leukemic cases, but when expressed it is associated with a poor prognosis. This finding agrees with other published studies that indicate that FoxP3 protein is not expressed to any significant degree by neoplastic cells in skin lesions or peripheral blood of most patients with MF/SS whereas the cutaneous infiltrates do have variable proportions of tumor-infiltrating FoxP3+ cells [62–65]. In recent study, Gjerdrum evaluated FoxP3 protein expression in 69 patients with MF further classified into early patch/plaque (n = 10), infiltrated plaque (n = 42), non-transformed tumor (n = 10), and transformed tumor (n = 7) cases [65]. Tumor-infiltrating FoxP3+ cells were present in varying numbers in the cutaneous infiltrates of MF, but a relative decrease was observed for tumor lesions compared with patch/plaque lesions. Moreover, the loss of FoxP3+ cells correlated with an adverse prognosis, and significantly both FoxP3 values and disease type were found to be independent risk factors for death when analysed as covariates in the Cox Proportional Hazards Model. This study also found that FoxP3 protein was not expressed by neoplastic cells in the skin infiltrate in any of these cases although it should be noted that negative FoxP3 staining was defined as absent or less than 10% staining by neoplastic cells, suggesting that occasional neoplastic cells could have been positive. Interestingly, the lack of FoxP3 protein in transformed MF in this study differs from the experience of Hallermann in which 4 of 5 cases of CD30+ transformed MF cases expressed FoxP3 [64]. The reason for this discrepancy is unclear but could be due to technical reasons (immunohistochemistry with or without antigen retrieval; FoxP3 antibody clones 236A/E7 versus hFoxy) [66].
Our observation that a small number of patients with SS have detectable transcripts for FoxP3 and that this is associated with a poor prognosis may signify that neoplastic cells in some cases have acquired the functional properties associated with Treg cells. FoxP3 expression by neoplastic cells in ATL also appears to adversely affect prognosis [62]. Although we were able to show a significant correlation between Foxp3 mRNA transcript levels with the number of CD4 + Foxp3+ T-cells identified by multi-colour flow cytometry in a few of our samples (Figure 2), attempts to correlate Foxp3 mRNA transcript levels with FoxP3 protein levels by flow cytometry or Western blotting was compromised due to the poor state of the stored cells. The possibility that the source of the FoxP3 message was from normal Treg cells in the sample cannot be excluded, but is considered unlikely because of the selection of cases with high blood tumor burdens. Nevertheless, Kwon observed that in 8 patients with SS, a CD4 + CD25 + FoxP3+ Treg phenotype was expressed by non-malignant T-cells in 7 instances, while the Treg phenotype was expressed by 60% of the Vβ+ neoplastic T-cells in one case [67]. Discordance between FoxP3 mRNA and protein expression has been observed for various tumor cell lines including the Sézary-derived HUT78 cells, suggesting regulation at the posttranslational level [68]. However, an alternative explanation is that FoxP3 mRNA may be easier to detect by the more sensitive qPCR technique than FoxP3 protein by standard methods which would account for the apparent lack of FoxP3 protein by neoplastic cells in most tissue samples and CTCL-derived cells lines. If so, an increased amount of FoxP3 protein detected by immunohistochemistry occurs in only certain unusual circumstances such as large cell transformation [64].
Thus, neoplastic T-cells appear capable of expressing FoxP3 mRNA and possibly protein in a small subset of aggressive MF/SS, but whether this is the result of cell activation or an intrinsic defect is unclear. In humans, expression of FoxP3 resides exclusively in CD4 + CD25+ T-cells, but in contrast to the mouse studies, activation of human CD4 + CD25− T-cells leads to expression of FoxP3 and generation of CD4 + CD25+ Treg cells with expression of FoxP3 correlated with regulatory activity although not always [21,69,70]. Because we found no correlation between FoxP3, CD25 or CTLA-4 expression in most cases, which one might have expected if neoplastic T-cells had been derived from Treg cells, the possibility that FoxP3 expression in our cases is related more to cell activation than a change in neoplastic phenotype remains plausible.
A possible explanation for the poor prognosis of FoxP3-expressing Sézary patients could be related to presumed Treg-like inhibitory effects of such cells on normal anti-tumor immune mechanisms as shown for other cancers [71,72]. Treg cells function as professional suppressor T-cells that are capable of inhibiting the in vitro proliferation of CD4 + CD25-T-cells stimulated via T-cell receptors [73,74]. In healthy individuals, thymic-derived natural Treg cells represent about 10% of CD4+ T-cells in the peripheral blood, and are identified by high surface expression of CD25 and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4/CD152), which contributes to the cell-cell suppressive effect of Treg cells [75]. However, in the case of CTCL, where tumor-infiltrating Treg cells might actually be inhibiting the growth of neoplastic T-cells either directly or indirectly via dendritic cell function as well as paradoxically inhibiting cytotoxic T-cell responses against the tumor cells (a dichotomy in terms of the anti-tumor immune response), the accumulation of neoplastic cells with Treg-like properties might alter the immune balance and favour the expansion of neoplastic cells that typically lack Treg-like properties. According to this hypothesis, only in some cases would the numbers of Treg-like neoplastic cells be detectable within the neoplastic pool.
The presence of neoplastic cells with Treg-like immunoregulatory properties in CTCL has therapeutic implications. For example, such cells might interfere with anti-tumor responses mediated by normal tumor-infiltrating Treg cells induced by extracorporeal photopheresis [76,77] or by cytotoxic cells induced by dendritic cell vaccines [78,79]. To enhance the effectiveness of such immunotherapeutic approaches, concurrent treatments that reduce or eliminate Treg-like neoplastic cells should be considered. For instance, a drug that targets CD25 such as denileukin diftitox might prove effective although not all FoxP3+ neoplastic T-cells appear to express CD25 and an inhibitory effect on tumor-infiltrating Treg cells that express high CD25 levels might not be desirable in this clinical setting. Similarly the suppressive effects of low dose cyclophosphamide or fludarabine on Treg cells might not selectively target Treg-like neoplastic T-cells vis-á-vis normal Treg cells [80–82]. At the present time, a non-myeloblative allogeneic bone marrow transplantation with the intent of generating a graft-versus-lymphoma effect may provide the best therapeutic option to gain long-term survival in these patients [83–86].
The hypothesis that neoplastic T-cells can acquire Treg-like properties has also been proposed for HTLV-1+ ATL, a neoplasm that shares many clinical and pathologic features with MF/SS [87–90]. At the mRNA level, FoxP3 expression in quite variable in ATL cells, and could be even down-regulated compared with Tregs from normal subjects [87]. Chen recently proposed that CD25+ ATL cells may be derived from 2 distinct subsets: one originating from primary FoxP3-expressing Treg cells, the other from activated T-cells [91]. The former subset, representing about one-third of ATL cases, exhibits high FoxP3 and CTLA-4 expression with associated immunosuppression and presumably a worse prognosis [62]. The latter subset is characterised by absent to low level of FoxP3 and negligible regulatory activity similar to conventional activated T-cells.
However, in contrast to leukemic ATL cells which constitutionally express high levels of CD25, expression of CD25 by the circulating neoplastic cells of leukemic CTCL is typically low, but also quite variable [9,92]. Considering that increased expression of CD25 on neoplastic cells in the skin may identify patients at risk to develop large cell transformation [93], and that there is an association between transformation and FoxP3 and CD25 expression by neoplastic cells, we wonder if high CD25 expression might also a marker of neoplastic T-cells with an activated Treg phenotype in MF/SS.
Taken together, we conclude that unstimulated circulating neoplastic T-cells in the majority of cases of SS do not constitutively express the phenotype of Treg cells, but when activated in vivo may up-regulate molecules like FoxP3 and CTLA-4, thereby acquiring Treg-like functional properties. In this regard, this subset of SS may be analogous to the activated form of ATL proposed by Chen [91]. The poor prognosis of Sézary patients whose circulating neoplastic T-cells express FoxP3 transcripts is a new finding that needs to be validated on fresh samples from a larger series of patients. If confirmed, then FoxP3 expression by neoplastic cells can be added to the list of other prognostic indicators for SS such as absolute Sézary cell count [46,47], serum level of lactate dehydrogenase [94,95] or soluble interleukin-2 receptor [96,97], and gene expression profile [37].
Acknowledgments
This work was supported by the Leonard and Ruth Levene Skin Research Fund and grants CA15396 and R01-CA89194 from the National Cancer Institute, National Institutes of Health.
References
- 1.Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–3785. doi: 10.1182/blood-2004-09-3502. [DOI] [PubMed] [Google Scholar]
- 2.Vonderheid EC, Bernengo MG, Burg G, Duvic M, Heald P, Laroche L, et al. Update on erythrodermic cutaneous T-cell lymphoma: report of the International Society for Cutaneous Lymphomas. J Am Acad Dermatol. 2002;46:95–106. doi: 10.1067/mjd.2002.118538. [DOI] [PubMed] [Google Scholar]
- 3.Heald PW, Yan SL, Edelson RL, Tigelaar R, Picker LJ. Skin-selective lymphocyte homing mechanisms in the pathogenesis of leukemic cutaneous T-cell lymphoma. J Invest Dermatol. 1993;101:222–226. doi: 10.1111/1523-1747.ep12364814. [DOI] [PubMed] [Google Scholar]
- 4.Kamarashev J, Burg G, Kempf W, Hess Schmid M, Dummer R. Comparative analysis of histological and immunohistological features in mycosis fungoides and Sézary syndrome. J Cutan Pathol. 1998;25:407–412. doi: 10.1111/j.1600-0560.1998.tb01766.x. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi T, Ohshima K, Tsuchiya T, Suehuji H, Karube K, Nakayama J, et al. The comparison of expression of cutaneous lymphocyte-associated antigen (CLA), and Th1- and Th2-associated antigens in mycosis fungoides and cutaneous lesions of adult T-cell leukemia/lymphoma. Eur J Dermatol. 2003;13:553–559. [PubMed] [Google Scholar]
- 6.Sokolowska-Wojdylo M, Wenzel J, Gaffal E, Lenz J, Speuser P, Erdmann S, et al. Circulating clonal CLA(+) and CD4(+) T-cells in Sézary syndrome express the skin-homing chemokine receptors CCR4 and CCR10 as well as the lymph node-homing chemokine receptor CCR7. Br J Dermatol. 2005;152:258–264. doi: 10.1111/j.1365-2133.2004.06325.x. [DOI] [PubMed] [Google Scholar]
- 7.Notohamiprodjo M, Segerer S, Huss R, Hildebrandt B, Soler D, Djafarzadeh R, et al. CCR10 is expressed in cutaneous T-cell lymphoma. Int J Cancer. 2005;115:641–647. doi: 10.1002/ijc.20922. [DOI] [PubMed] [Google Scholar]
- 8.Capriotti E, Vonderheid EC, Thoburn CJ, Bright EC, Hess AD. Chemokine receptor expression by leukemic T-cells of cutaneous T-cell lymphoma: clinical and histopathological correlations. J Invest Dermatol. 2007;127:2882–2892. doi: 10.1038/sj.jid.5700916. [DOI] [PubMed] [Google Scholar]
- 9.Waldmann TA, Greene WC, Sarin PS, Saxinger C, Blayney DW, Blattner WA, et al. Functional and phenotypic comparison of human T-cell leukemia/lymphoma virus positive adult T-cell leukemia with human T-cell leukemia/lymphoma virus negative Sézary leukemia, and their distinction using anti-Tac. Monoclonal antibody identifying the human receptor for T-cell growth factor. J Clin Invest. 1984;73:1711–1718. doi: 10.1172/JCI111379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nasu K, Said J, Vonderheid E, Olerud J, Sako D, Kadin M. Immunopathology of cutaneous T-cell lymphomas. Am J Pathol. 1985;119:436–447. [PMC free article] [PubMed] [Google Scholar]
- 11.Harmon CB, Witzig TE, Katzmann JA, Pittelkow MR. Detection of circulating T-cells with CD4+ CD7− immunophenotype in patients with benign and malignant lymphoproliferative dermatoses. J Am Acad Dermatol. 1996;35(3 Part 1):404–410. doi: 10.1016/s0190-9622(96)90605-2. [DOI] [PubMed] [Google Scholar]
- 12.Bernengo MG, Novelli M, Quaglino P, Lisa F, De Matteis A, Savoia P, et al. The relevance of the CD4+ CD26− subset in the identification of circulating Sézary cells. Br J Dermatol. 2001;144:125–135. doi: 10.1046/j.1365-2133.2001.04014.x. [DOI] [PubMed] [Google Scholar]
- 13.Talpur R, Jones DM, Alencar AJ, Apisarnthanarax N, Herne KL, Yang Y, et al. CD25 expression is correlated with histological grade and response to denileukin diftitox in cutaneous T-cell lymphoma. J Invest Dermatol. 2006;126:575–583. doi: 10.1038/sj.jid.5700122. [DOI] [PubMed] [Google Scholar]
- 14.Broder S, Muul L, Marshall S, Waldmann TA. Neoplasms of immunoregulatory T-cells in clinical investigation. J Invest Dermatol. 1980;74:267–271. doi: 10.1111/1523-1747.ep12543356. [DOI] [PubMed] [Google Scholar]
- 15.Kim EJ, Hess S, Richardson SK, Newton S, Showe LC, Benoit BM, et al. Immunopathogenesis and therapy of cutaneous T-cell lymphoma. J Clin Invest. 2005;115:798–812. doi: 10.1172/JCI24826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berger CL, Tigelaar R, Cohen J, Mariwalla K, Trinh J, Wang N, et al. Cutaneous T-cell lymphoma: malignant proliferation of T-regulatory cells. Blood. 2005;105:1640–1647. doi: 10.1182/blood-2004-06-2181. [DOI] [PubMed] [Google Scholar]
- 17.Wong HK, Wilson AJ, Gibson HM, Hafner MS, Hedgcock CJ, Berger CL, et al. Increased expression of CTLA-4 in malignant T-cells from patients with mycosis fungoides – cutaneous T-cell lymphoma. J Invest Dermatol. 2006;126:212–219. doi: 10.1038/sj.jid.5700029. [DOI] [PubMed] [Google Scholar]
- 18.Heald P, Yan SL, Edelson R. Profound deficiency in normal circulating T-cells in erythrodermic cutaneous T-cell lymphoma. Arch Dermatol. 1994;130:198–203. [PubMed] [Google Scholar]
- 19.Fontenot JD, Gavin MA, Rudensky AY. FoxP3 programs the development and function of CD4 +CD25+ regulatory T-cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 20.Yagi H, Nomura T, Nakamura K, Yamazaki S, Kitawaki T, Hori S, et al. Crucial role of FOXP3 in the development and function of human CD25 +CD4+ regulatory T-cells. Int Immunol. 2004;16:1643–1656. doi: 10.1093/intimm/dxh165. [DOI] [PubMed] [Google Scholar]
- 21.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4 +FOXP3 T-cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S, Chen L. T lymphocyte co-signaling pathways of the B7-CD28 family. Cell Mol Immunol. 2004;1:37–42. [PubMed] [Google Scholar]
- 23.Sansom DM, Walker LS. The role of CD28 and cytotoxic T-lymphocyte antigen-4 (CTLA-4) in regulatory T-cell biology. Immunol Rev. 2006;212:131–148. doi: 10.1111/j.0105-2896.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- 24.Nickoloff BJ, Nestle FO, Zheng XG, Turka LA. T lymphocytes in skin lesions of psoriasis and mycosis fungoides express B7-1: a ligand for CD28. Blood. 1994;83:2580–2586. [PubMed] [Google Scholar]
- 25.McCusker ME, Garifallou M, Bogen SA. Sézary lineage cells can be induced to proliferate via CD28-mediated costimulation. J Immunol. 1997;158:4984–4991. [PubMed] [Google Scholar]
- 26.Tang Q, Henriksen KJ, Boden EK, Tooley AJ, Ye J, Subudhi SK, et al. Cutting edge: CD28 controls peripheral homeostasis of CD4 +CD25+ regulatory T-cells. J Immunol. 2003;171:3348–3352. doi: 10.4049/jimmunol.171.7.3348. [DOI] [PubMed] [Google Scholar]
- 27.Laroche L, Kaiserlian D. Decreased natural-killer-cell activity in cutaneous T-cell lymphomas. N Engl J Med. 1983;308:101–102. doi: 10.1056/NEJM198301133080213. [DOI] [PubMed] [Google Scholar]
- 28.Neilan BA, Vonderheid EC, O’Neill KJ. Natural cell-mediated cytotoxicity in cutaneous T-cell lymphomas. J Invest Dermatol. 1983;81:176–178. doi: 10.1111/1523-1747.ep12543616. [DOI] [PubMed] [Google Scholar]
- 29.Wood NL, Kitces EN, Blaylock WK. Depressed lymphokine activated killer cell activity in mycosis fungoides. A possible marker for aggressive disease. Arch Dermatol. 1990;126:907–913. [PubMed] [Google Scholar]
- 30.Wysocka M, Benoit BM, Newton S, Azzoni L, Montaner LJ, Rook AH. Enhancement of the host immune responses in cutaneous T-cell lymphoma by CpG oligodeoxynucleotides and IL-15. Blood. 2004;104:4142–4149. doi: 10.1182/blood-2004-03-1190. [DOI] [PubMed] [Google Scholar]
- 31.Bouaziz JD, Ortonne N, Giustiniani J, Schiavon V, Huet D, Bagot M, et al. Circulating natural killer lymphocytes are potential cytotoxic effectors against autologous malignanT-cells in Sézary syndrome patients. J Invest Dermatol. 2005;125:1273–1278. doi: 10.1111/j.0022-202X.2005.23914.x. [DOI] [PubMed] [Google Scholar]
- 32.Diefenbach A, Raulet DH. Innate immune recognition by stimulatory immunoreceptors. Curr Opin Immunol. 2003;15:37–44. doi: 10.1016/s0952-7915(02)00007-9. [DOI] [PubMed] [Google Scholar]
- 33.Raulet DH. Roles of the NKG2D immunoreceptor and its ligands. Nat Rev Immunol. 2003;3:781–790. doi: 10.1038/nri1199. [DOI] [PubMed] [Google Scholar]
- 34.Stastny P. Introduction: MICA/MICB in innate immunity, adaptive immunity, autoimmunity, cancer, and in the immune response to transplants. Hum Immunol. 2006;67:141–144. doi: 10.1016/j.humimm.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 35.Vivier E, Tomasello E, Paul P. Lymphocyte activation via NKG2D: towards a new paradigm in immune recognition? Curr Opin Immunol. 2002;14:306–311. doi: 10.1016/s0952-7915(02)00337-0. [DOI] [PubMed] [Google Scholar]
- 36.Su MW, Dorocicz I, Dragowska WH, Ho V, Li G, Voss N, et al. Aberrant expression of T-plastin in Sézary cells. Cancer Res. 2003;63:7122–7127. [PubMed] [Google Scholar]
- 37.Kari L, Loboda A, Nebozhyn M, Rook AH, Vonderheid EC, Nichols C, et al. Classification and prediction of survival in patients with the leukemic phase of cutaneous T-cell lymphoma. J Exp Med. 2003;197:1477–1488. doi: 10.1084/jem.20021726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tiemessen MM, Mitchell TJ, Hendry L, Whittaker SJ, Taams LS, John S. Lack of suppressive CD4 +CD25 + FOXP3+ T-cells in advanced stages of primary cutaneous T-cell lymphoma. J Invest Dermatol. 2006;126:2217–2223. doi: 10.1038/sj.jid.5700371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gibson HM, Wilson AJ, Wong HK. Analysis of T-plastin gene regulation, a gene highly expressed in Sézary cells. J Invest Dermatol. 2006;126:83. (abstr.) [Google Scholar]
- 40.Booken N, Gratchev A, Utikal J, Weiβ C, Yu X, Qadoumi M, et al. Sézary syndrome is a unique cutaneous T-cell lymphoma as identified by an expanded gene signature including diagnostic marker molecules CDO1 and DNM3. Leukemia. 2008;22:393–399. doi: 10.1038/sj.leu.2405044. [DOI] [PubMed] [Google Scholar]
- 41.Miura Y, Thoburn CJ, Bright EC, Chen W, Nakao S, Hess AD. Cytokine and chemokine profiles in autologous graft-versus-host disease (GVHD): interleukin-10 and interferon-gamma may be critical mediators in the development of autologous GVHD. Blood. 2002;100:2650–2658. doi: 10.1182/blood-2002-01-0176. [DOI] [PubMed] [Google Scholar]
- 42.Miura Y, Thoburn CJ, Bright EC, Hess AD. Cytolytic effector mechanisms and gene expression in autologous graft-versus-host disease: distinct roles of perforin and Fas ligand. Biol Blood Marrow Transplant. 2004;10:156–170. doi: 10.1016/j.bbmt.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 43.Vonderheid EC, Bigler RD, Kotecha A, Boselli CM, Lessin SR, Bernengo MG, et al. Variable CD7 expression on T-cells in the leukemic phase of cutaneous T-cell lymphoma (Sézary syndrome) J Invest Dermatol. 2001;117:654–662. doi: 10.1046/j.1523-1747.2001.01456.x. [DOI] [PubMed] [Google Scholar]
- 44.Schwab C, Willers J, Niederer E, Ludwig E, Kundig T, Grob P, et al. The use of anti-T-cell receptor-Vbeta antibodies for the estimation of treatment success and phenotypic characterization of clonal T-cell populations in cutaneous T-cell lymphomas. Br J Haematol. 2002;118:1019–1026. doi: 10.1046/j.1365-2141.2002.03726.x. [DOI] [PubMed] [Google Scholar]
- 45.Lima M, Almeida J, dos Anjos Teixeira M, Queiros ML, Santos AH, Fonseca S, et al. Utility of flow cytometry immunophenotyping and DNA ploidy studies for diagnosis and characterization of blood involvement in CD4+ Sézary’s syndrome. Haematologica. 2003;88:874–887. [PubMed] [Google Scholar]
- 46.Scarisbrick JJ, Whittaker S, Evans AV, Fraser-Andrews EA, Child FJ, Dean A, et al. Prognostic significance of tumor burden in the blood of patients with erythrodermic primary cutaneous T-cell lymphoma. Blood. 2001;97:624–630. doi: 10.1182/blood.v97.3.624. [DOI] [PubMed] [Google Scholar]
- 47.Vonderheid EC, Pena J, Nowell P. Sézary cell counts in erythrodermic cutaneous T-cell lymphoma: implications for prognosis and staging. Leuk Lymphoma. 2006;47:1841–1856. doi: 10.1080/10428190600709655. [DOI] [PubMed] [Google Scholar]
- 48.Sokolowska-Wojdylo M, Wenzel J, Gaffal E, Lenz J, Speuser P, Erdmann S, et al. Circulating clonal CLA(+) and CD4(+) T-cells in Sézary syndrome express the skin-homing chemokine receptors CCR4 and CCR10 as well as the lymph node-homing chemokine receptor CCR7. Br J Dermatol. 2005;152:258–264. doi: 10.1111/j.1365-2133.2004.06325.x. [DOI] [PubMed] [Google Scholar]
- 49.Notohamiprodjo M, Segerer S, Huss R, Hildebrandt B, Soler D, Djafarzadeh R, et al. CCR10 is expressed in cutaneous T-cell lymphoma. Int J Cancer. 2005;115:641–647. doi: 10.1002/ijc.20922. [DOI] [PubMed] [Google Scholar]
- 50.Delanote V, Vandekerckhove J, Gettemans J. Plastins: versatile modulators of actin organization in (patho)physiological cellular processes. Acta Pharmacol Sin. 2005;26:769–779. doi: 10.1111/j.1745-7254.2005.00145.x. [DOI] [PubMed] [Google Scholar]
- 51.Juliano RL. Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu Rev Pharmacol Toxicol. 2002;42:283–323. doi: 10.1146/annurev.pharmtox.42.090401.151133. [DOI] [PubMed] [Google Scholar]
- 52.Galiegue-Zouitina S, Quief S, Hildebrand MP, Denis C, Detourmignies L, Lai JL, et al. Nonrandom fusion of L-plastin(LCP1) and LAZ3(BCL6) genes by t(3;13)(q27;q14) chromosome translocation in two cases of B-cell non-Hodgkin lymphoma. Genes Chromosomes Cancer. 1999;26:97–105. [PubMed] [Google Scholar]
- 53.Mitelman F, Johansson B, Mertens F, editors. Mitelman Database of Chromosome Aberrations in Cancer. 2006 http://cgap.nci.nih.gov/Chromosomes/Mitelman.
- 54.Bour-Jordan H, Blueston JA. CD28 function: a balance of costimulatory and regulatory signals. J Clin Immunol. 2002;22:1–7. doi: 10.1023/a:1014256417651. [DOI] [PubMed] [Google Scholar]
- 55.van Doorn R, Dijkman R, Vermeer MH, Out-Luiting JJ, van der Raaij-Helmer EM, et al. Aberrant expression of the tyrosine kinase receptor EphA4 and the transcription factor twist in Sézary syndrome identified by gene expression analysis. Cancer Res. 2004;64:5578–5586. doi: 10.1158/0008-5472.CAN-04-1253. [DOI] [PubMed] [Google Scholar]
- 56.Salih HR, Antropius H, Gieseke F, Lutz SZ, Kanz L, Rammensee HG, et al. Functional expression and release of ligands for the activating immunoreceptor NKG2D in leukemia. Blood. 2003;102:1389–1396. doi: 10.1182/blood-2003-01-0019. [DOI] [PubMed] [Google Scholar]
- 57.Girlanda S, Fortis C, Belloni D, Ferrero E, Ticozzi P, Sciorati C, et al. MICA expressed by multiple myeloma and monoclonal gammopathy of undetermined significance plasma cells costimulates pamidronate-activated γδ lymphocytes. Cancer Res. 2005;65:7502–7508. doi: 10.1158/0008-5472.CAN-05-0731. [DOI] [PubMed] [Google Scholar]
- 58.Carbone E, Neri P, Mesuraca M, Fulciniti MT, Otsuki T, Pende D, et al. HLA class I, NKG2D, and natural cytotoxicity receptors regulate multiple myeloma cell recognition by natural killer cells. Blood. 2005;105:251–258. doi: 10.1182/blood-2004-04-1422. [DOI] [PubMed] [Google Scholar]
- 59.Coudert JD, Zimmer J, Tomasello E, Cebecauer M, Colonna M, Vivier E, et al. Altered NKG2D function in NK cells induced by chronic exposure to NKG2D ligand-expressing tumor cells. Blood. 2005;106:1711–1717. doi: 10.1182/blood-2005-03-0918. [DOI] [PubMed] [Google Scholar]
- 60.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419:734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 61.Salih HR, Goehlsdorf D, Steinle A. Release of MICB molecules by tumor cells: mechanism and soluble MICB in sera of cancer patients. Hum Immunol. 2006;67:188–195. doi: 10.1016/j.humimm.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 62.Roncador G, Garcia JF, Garcia JF, Maestre L, Lucas E, Menarguez J, et al. FOXP3, a selective marker for a subset of adult T-cell leukaemia/lymphoma. Leukemia. 2005;19:2247–2253. doi: 10.1038/sj.leu.2403965. [DOI] [PubMed] [Google Scholar]
- 63.Klemke CD, Fritzsching B, Franz B, Kleinmann EV, Oberle N, Poenitz N, et al. Paucity of FOXP3+ cells in skin and peripheral blood distinguishes Sézary syndrome from other cutaneous T-cell lymphomas. Leukemia. 2006;20:1123–1129. doi: 10.1038/sj.leu.2404182. [DOI] [PubMed] [Google Scholar]
- 64.Hallermann C, Niermann C, Schulze HJ. Regulatory T-cell phenotype in association with large cell transformation of mycosis fungoides. Eur J Haematol. 2007;78:260–263. doi: 10.1111/j.1600-0609.2006.00809.x. [DOI] [PubMed] [Google Scholar]
- 65.Gjerdrum LM, Woetmann A, Odum N, Burton CM, Rossen K, Skovgaard GL, et al. FOXP3+ regulatory T-cells in cutaneous T-cell lymphomas: association with disease stage and survival. Leukemia. 2007;21:2512–2518. doi: 10.1038/sj.leu.2404913. [DOI] [PubMed] [Google Scholar]
- 66.Banham AH, Brown PJ, Lyne L, Schulze H-J, Hallerman C. Is FOXP3 expressed in cutaneous T-cell lymphomas. Eur J Haematol. 2008;80:90–91. doi: 10.1111/j.1600-0609.2007.00990.x. [DOI] [PubMed] [Google Scholar]
- 67.Kwon S, Geskin L, McCann S, Paley K, Falo LD., Jr CTCL patients exhibit elevated levels of regulatory T-cell that correspond with disease severity and response to therapy. J Invest Dermatol. 2006;126:110. (abstr.) [Google Scholar]
- 68.Yamamoto M, Tsuji-Takayama K, Suzuki M, Harashima A, Sugimoto A, Motoda R, et al. Comprehensive analysis of FOXP3 mRNA expression in leukemia and transformed cell lines. Leuk Res. 2008;32:651–658. doi: 10.1016/j.leukres.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 69.Walker MR, Kasprowicz DJ, Gersuk VH, Benard A, Van Landeghen M, Buckner JH, et al. Induction of FoxP3 and acquisition of T regulatory activity by stimulated human CD4 + CD25− T-cells. J Clin Invest. 2003;112:1437–1443. doi: 10.1172/JCI19441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morgan ME, van Bilsen JH, Bakker AM, Heemskerk B, Schilham MW, Hartgers FC, et al. Expression of FOXP3 mRNA is not confined to CD4 +CD25+ T regulatory cells in humans. Hum Immunol. 2005;66:13–20. doi: 10.1016/j.humimm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 71.Zou W. Regulatory T-cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 72.Yu P, Fu YX. Tumor-infiltrating T lymphocytes: friends or foes? Lab Invest. 2006;86:231–245. doi: 10.1038/labinvest.3700389. [DOI] [PubMed] [Google Scholar]
- 73.Piccirillo CA, Shevach EM. Naturally-occurring CD4 + CD25+ immunoregulatory T-cells: central players in the arena of peripheral tolerance. Semin Immunol. 2004;16:81–88. doi: 10.1016/j.smim.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 74.Sakaguchi S, Setoguchi R, Yagi H, Nomura T. Naturally arising Foxp3-expressing CD25 +CD4+ regulatory T-cells in self-tolerance and autoimmune disease. Curr Top Microbiol Immunol. 2006;305:51–66. doi: 10.1007/3-540-29714-6_3. [DOI] [PubMed] [Google Scholar]
- 75.Read S, Greenwald R, Izcue A, Robinson N, Mandelbrot D, Francisco L, et al. Blockade of CTLA-4 on CD4 + CD25+ regulatory T-cells abrogates their function in vivo. J Immunol. 2006;177:4376–4383. doi: 10.4049/jimmunol.177.7.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maeda A, Schwarz A, Kernebeck K, Gross N, Aragane Y, Peritt D, et al. Intravenous infusion of syngeneic apoptotic cells by photopheresis induces antigen-specific regulatory T-cells. J Immunol. 2005;174:5968–5976. doi: 10.4049/jimmunol.174.10.5968. [DOI] [PubMed] [Google Scholar]
- 77.Biagi E, Di Biaso I, Leoni V, Gaipa G, Rossi V, Bugarin C, et al. Extracorporeal photochemotherapy is accompanied by increasing levels of circulating CD4 +CD25 +GITR + Foxp3 +CD62L+ functional regulatory T-cells in patients with graft-versus-host disease. Transplantation. 2007;84:31–39. doi: 10.1097/01.tp.0000267785.52567.9c. [DOI] [PubMed] [Google Scholar]
- 78.Maier T, Tun-Kyi A, Tassis A, Jungius KP, Burg G, Dummer R, et al. Vaccination of patients with cutaneous T-cell lymphoma using intranodal injection of autologous tumor-lysate-pulsed dendritic cells. Blood. 2003;102:2338–2344. doi: 10.1182/blood-2002-08-2455. [DOI] [PubMed] [Google Scholar]
- 79.Salskov-Iversen M, Berger CL, Edelson RL. Rapid construction of a dendritic cell vaccine through physical perturbation and apoptotic malignant T-cell loading. J Immune Based Ther Vaccines. 2005;3:4–19. doi: 10.1186/1476-8518-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bass KK, Mastrangelo MJ. Immunopotentiation with low-dose cyclophosphamide in the active specific immunotherapy of cancer. Cancer Immunol Immunother. 1998;47:1–12. doi: 10.1007/s002620050498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Quaglino P, Fierro MT, Rossotto GL, Savoia P, Bernengo MG. Treatment of advanced mycosis fungoides/Sézary syndrome with fludarabine and potential adjunctive benefit to subsequent extracorporeal photochemotherapy. Br J Dermatol. 2004;150:327–336. doi: 10.1111/j.1365-2133.2004.05712.x. [DOI] [PubMed] [Google Scholar]
- 82.Beyer M, Schultze JL. Regulatory T-cells in cancer. Blood. 2006;108:804–811. doi: 10.1182/blood-2006-02-002774. [DOI] [PubMed] [Google Scholar]
- 83.Guitart J, Wickless SC, Oyama Y, Kuzel TM, Rosen ST, Traynor A, et al. Long-term remission after allogeneic hematopoietic stem cell transplantation for refractory cutaneous T-cell lymphoma. Arch Dermatol. 2002;138:1359–1365. doi: 10.1001/archderm.138.10.1359. [DOI] [PubMed] [Google Scholar]
- 84.Soligo D, Ibatici A, Berti E, Morandi P, Longhi E, Venegoni L, et al. Treatment of advanced mycosis fungoides by allogeneic stem-cell transplantation with a nonmyeloablative regimen. Bone Marrow Transplant. 2003;31:663–666. doi: 10.1038/sj.bmt.1703872. [DOI] [PubMed] [Google Scholar]
- 85.Herbert KE, Spencer A, Grigg A, Ryan G, McCormack C, Prince HM. Graft-versus-lymphoma effect in refractory cutaneous T-cell lymphoma after reduced-intensity HLA-matched sibling allogeneic stem cell transplantation. Bone Marrow Transplant. 2004;34:521–525. doi: 10.1038/sj.bmt.1704641. [DOI] [PubMed] [Google Scholar]
- 86.Molina A, Zain J, Arber DA, Angelopolou M, O’Donnell M, Murata-Collins J, et al. Durable clinical, cytogenetic, and molecular remissions after allogeneic hematopoietic cell transplantation for refractory Sézary syndrome and mycosis fungoides. J Clin Oncol. 2005;23:6163–6171. doi: 10.1200/JCO.2005.02.774. [DOI] [PubMed] [Google Scholar]
- 87.Karube K, Ohshima K, Tsuchiya T, Yamaguchi T, Kawano R, Suzumiya J, et al. Expression of FoxP3, a key molecule in CD4CD25 regulatory T-cells, in adult T-cell leukaemia/lymphoma cells. Br J Haematol. 2004;126:81–84. doi: 10.1111/j.1365-2141.2004.04999.x. [DOI] [PubMed] [Google Scholar]
- 88.Kohno T, Yamada Y, Akamatsu N, Kamihira S, Imaizumi Y, Tomonaga M, et al. Possible origin of adult T-cell leukemia/lymphoma cells from human T lymphotropic virus type-1-infected regulatory T-cells. Cancer Sci. 2005;96:527–533. doi: 10.1111/j.1349-7006.2005.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Matsubara Y, Hori T, Morita R, Sakaguchi S, Uchiyama T. Phenotypic and functional relationship between adult T-cell leukemia cells and regulatory T-cells. Leukemia. 2005;19:482–483. doi: 10.1038/sj.leu.2403628. [DOI] [PubMed] [Google Scholar]
- 90.Yamaguchi T, Ohshima K, Karube K, Tutiya T, Kawano R, Suefuji H, et al. Clinicopathological features of cutaneous lesions of adult T-cell leukaemia/lymphoma. Br J Dermatol. 2005;152:76–81. doi: 10.1111/j.1365-2133.2004.06274.x. [DOI] [PubMed] [Google Scholar]
- 91.Chen S, Ishii N, Ine S, Ikeda S, Fujimura T, Ndhlovu, et al. Regulatory T-cell-like activity of FoxP3+ adult T-cell leukemia cells. Int Immunol. 2006;18:269–277. doi: 10.1093/intimm/dxh366. [DOI] [PubMed] [Google Scholar]
- 92.Jones D, Ibrahim S, Patel K, Luthra R, Duvic M, Medeiros LJ. Degree of CD25 expression in T-cell lymphoma is dependent on tissue site: implications for targeted therapy. Clin Cancer Res. 2004;10:5587–5594. doi: 10.1158/1078-0432.CCR-0721-03. [DOI] [PubMed] [Google Scholar]
- 93.Stefanato CM, Tallini G, Crotty PL. Histologic and immunophenotypic features prior to transformation in patients with transformed cutaneous T-cell lymphoma: is CD25 expression in skin biopsy samples predictive of large cell transformation in cutaneous T-cell lymphoma? Am J Dermatopathol. 1998;20:1–6. doi: 10.1097/00000372-199802000-00001. [DOI] [PubMed] [Google Scholar]
- 94.Foulc P, N’Guyen JM, Dreno B. Prognostic factors in Sézary syndrome: a study of 28 patients. Br J Dermatol. 2003;149:1152–1158. doi: 10.1111/j.1365-2133.2003.05677.x. [DOI] [PubMed] [Google Scholar]
- 95.Marti RM, Pujol RM, Servitje O, Palou J, Romagosa V, Bordes R, et al. Sézary syndrome and related variants of classic cutaneous T-cell lymphoma. A descriptive and prognostic clinicopathologic study of 29 cases. Leuk Lymphoma. 2003;44:59–69. doi: 10.1080/1042819021000054652. [DOI] [PubMed] [Google Scholar]
- 96.Wasik MA, Vonderheid EC, Bigler RD, Marti R, Lessin SR, Polansky M, et al. Increased serum concentration of the soluble interleukin-2 receptor in cutaneous T-cell lymphoma. Clinical and prognostic implications. Arch Dermatol. 1996;132:42–47. [PubMed] [Google Scholar]
- 97.Bernengo MG, Quaglino P, Novelli M, Cappello N, Doveil GC, Lisa F, et al. Prognostic factors in Sézary syndrome: a multivariate analysis of clinical, haematological and immunological features. Ann Oncol. 1998;9:857–863. doi: 10.1023/a:1008397323199. [DOI] [PubMed] [Google Scholar]