Abstract
Background
It is well known that naltrexone, an FDA-approved medication for treatment of alcohol dependence, is effective for only a subset of individuals. Recent studies have examined the utility of a functional A118G single nucleotide polymorphism (SNP) of the mu-opioid receptor gene (OPRM1) as a predictor of naltrexone treatment response. Although the findings to date have generally been consistent with a moderating effect of the SNP, further evaluation of this hypothesis is warranted.
Objective
To evaluate whether problem drinkers with one or two copies of the 118G allele respond better to naltrexone treatment. The treatment goal in this cohort of high functioning men who have sex with men (MSM) was to reduce heavy drinking, rather than to promote abstinence.
Method
112 subjects of European ancestry from a randomized clinical trial of naltrexone and behavioral therapy for problem drinking MSM were included in the analysis. Subjects were treated for 12 weeks with 100 mg/day of oral naltrexone hydrochloride. All participants received medical management with a modified version of the Brief Behavioral Compliance Enhancement Treatment (BBCET), alone or in combination with Modified Behavioral Self-control Therapy (MBSCT).
Results
Naltrexone-treated subjects with one or two 118G alleles had a significantly greater percentage of non-hazardous drinking (NoH) (p < 0.01) than those treated with placebo or A118 homozygotes in either medication group.
Conclusions
These results are consistent with a modest moderating effect of the OPRM1 118G allele on the reduction of heavy drinking by naltrexone treatment.
Keywords: Alcohol, Moderation, Hazardous drinking, Pharmacogenetics
Introduction
Naltrexone is an opioid receptor antagonist approved by the US Food and Drug Administration (FDA) for the treatment of alcohol dependence. Naltrexone reduces heavy drinking rates, particularly among individuals who are compliant with the medication [1–4]. However, individual treatment responses to naltrexone vary, with only some problem drinkers benefiting from treatment with the medication.
A promising strategy to increase the effectiveness of naltrexone is through patient-treatment matching. Oslin et al. [5] reported that a functional single nucleotide polymorphism (the A118G SNP, rs17799971, which encodes an Asn40Asp amino acid substitution) in exon 1 of the mu-opioid receptor gene (OPRM1), predicted the efficacy of naltrexone in reducing the likelihood of heavy drinking in alcohol dependent patients. Similar results were subsequently reported in a large, well-controlled, multisite study of combined medications and behavioral interventions for alcohol dependence, the COMBINE study. In that study, carriers of the 118G allele had a significantly better outcome when treated with naltrexone than placebo [6].
However, not all studies have shown these effects. For example, a study in male Veterans reported no moderating effect of the A118G SNP [7], and recently a small study reported a positive effect, in terms of a longer time to relapse but not on abstinence, of the 118G allele in Asians [8]. Results by Ray and Oslin [9] did not support the efficacy of naltrexone on the percentage of days abstinent, time to first heavy drinking day, or global clinical outcome among African Americans. Of note, the estimated minor allele (118G) frequency is approximately 0.15 in individuals of European ancestry, 0.35 in Asians, and less than 0.05 in individuals of African descent (www.ncbi.nlm.nih.gov/projects/SNP).
The absence of conclusive findings [4,10] in relation to this pharmacogenetic effect may reflect interactions among genetic and environmental factors, as well as variability in the populations, subtypes of alcoholism, methods used, and treatment goals, e.g., abstinence vs. moderation [11]. Several lines of evidence converge to suggest that naltrexone may be more effective at reducing heavy drinking than establishing abstinence [4,12]. Naltrexone acts to blunt the rewarding properties of alcohol, probably through effects on the dopaminergic/ opioidergic reinforcement system. Naltrexone-treated individuals experience less arousal and more sedation as they continue to drink [13]. Because carriers of the minor 118G allele may experience greater alcohol-induced reward [14], there may also be an enhanced blunting of reward when naltrexone is administered [15]. In the present study, we tested the hypothesis that the Asn40Asp polymorphism moderated the treatment responses to naltrexone in a high functioning group of men who have sex with men (MSM), whose goal in treatment was to reduce their drinking, rather than becoming abstinent from alcohol.
Methods
Participants and procedures
Subjects were participants in Project SMART, a randomized controlled trial of combined medication and psychotherapy to reduce problem drinking in MSM [16].
Participants
Potential participants expressed a desire to reduce their drinking but not quit altogether. To be eligible for the study, men: 1) were between the ages 18 to 65 years; 2) had an average consumption of at least 24 standard drinks per week over the last 90 days; 3) identified themselves as sexually active with other men; and 4) read English at an eighth grade level or higher. Participants were excluded if they: 1) had a lifetime diagnosis of bipolar disorder, schizophrenia, or other psychotic disorder; an untreated current major depressive disorder; or current dependence on drugs (with the exception of nicotine or cannabis); 2) started or changed psychotropic medication in the last 90 days; 3) were at risk for serious medication side effects from naltrexone (NTX); or 4) were enrolled in concurrent drug or alcohol treatment during the treatment phase of Project SMART.
Procedures
Procedures complied with and were approved by the New York State Psychiatric Institutional Review Board. Details of the procedures are provided in our recent report [16]. Briefly, the final sample consisted of 200 participants assigned via urn randomization to one of two medication conditions, NTX (100 mg/day orally) or placebo (PBO), and one of two counseling conditions, a modified version of Brief Behavioral Compliance Enhancement Treatment (BBCET, [17]) or BBCET in combination with Modified Behavioral Self-control Therapy (MBSCT). The treatment phase lasted 12 weeks and the medication was administered double-blind. One hundred eleven subjects of European ancestry who agreed to participate in the genetics study are included in the present report.
Substance use and other psychiatric disorders
The Composite International Diagnostic Instrument, Substance Abuse Module (CIDI-SAM, [18]) was used to evaluate substance dependence criteria. Participants were screened for psychosis and other thought disorders using the psychotic screening and bipolar disorder sections of the Structured Clinical Interview for DSM-IV, (SCID, [19]) and for cognitive impairment using the Mini-Mental State Examination (MMSE, [20]). Depressive symptoms were measured using the revised Beck Depression Inventory (BDI-II, [21]). All participants also a received psychiatric diagnostic interview by a psychiatrist for final determination of eligibility.
Alcohol and drug use patterns and problems
The Time-Line Follow-Back Interview (TLFB, [22]) was used to assess the frequency of alcohol and drug use during the previous 90 days at prescreen and at the end of treatment. Drinking and drug use were also assessed with the TLFB for the period between the prescreen and baseline interviews (typically 1–2 weeks). To assess the quantity and intensity of alcohol use, three variables were created from the TLFB data. Two primary outcomes--weekly sum of standard drinks (SSD), and weekly number of heavy drinking days (HDD)--were selected a priori because NIAAA safe drinking guidelines include measures of drinking quantity (no more than 14 drinks per week for men) and intensity (no heavy drinking days, i.e., no more than 4 drinks per day for men). In addition, the weekly proportion of subjects that achieved non-hazardous drinking (NoH) in the prior week (defined as drinking 14 or fewer standard drinks per week and having no heavy drinking days) was selected as a dichotomous secondary outcome measure.
Genotyping
DNA was extracted from whole blood using standard methods. The OPRM1 SNP, the OPRM1 SNP, rs1799971, was genotyped using the TaqMan fluorogenic 5′ nuclease assay [23] and the ABI PRISM 7900 Sequence Detection System (ABI, Foster City, CA, USA). 10 μL reactions were prepared containing 2.5 ng genomic DNA, 1 ng bovine serum albumin, 1X ABI TaqMan master mix, 6 pM of each primer, CCCAGCCCCGGTTCCT and TGATGGCCGTGATCATGGA, 0.3 pM of a Vic-AGATGGCGACCTGTCC-MGB, probe for the Asp G-allele and 0.6 pM of a Fam-AGATGGCAACCTGTCC-MGB probe for the Asn A-allele. Cycling parameters were 95°C for 10 min followed by 35 cycles of 95 and 58.5°C for 15 s and 60 s respectively. Genotyping for 17% of were repeated for quality control, with complete concordance.
Statistical analysis
All analyses were performed using SAS Statistical Software, version 9.1 (SAS Institute Inc., 2003). Generalized estimating equations (GEE; [24]) were used to analyze the non-normal, longitudinal data for the three dependent variables: weekly sum of standard drinks (SSD), weekly number of heavy drinking days (HDD) and weekly proportion of participants with non-hazardous drinking (NoH). GEE is a data analytic technique for longitudinal data that corrects for correlated observations. Of the 122 subjects of European ancestry who agreed to participate in the genetic sub-study, 112 provided at least some drinking data for the treatment period and are included in this report.
Within the GEE analyses, a normal distribution with identity function for SSD, a negative binomial distribution with log function for HDD, and a binomial distribution with logit link function for NoH were specified and provided good model ft. In addition, an exchangeable working correlation matrix was specified. Post hoc analyses were performed to explore the results of the primary analyses.
The independent variables (IVs) for the models were medication condition (NTX or PBO) and genotype (AG/GG or AA) and the interaction of these conditions. To allow for simultaneous testing of both main and interaction effects, the IVs were orthogonally contrast coded. Time (weeks 1–12) and the interactions of time × medication condition, time × genotype, and time × medication × genotype were included in the models to test effects over time. The time variable was centered. Two covariates included in the models were based on the respective dependent variables (i.e., SSD, HDD): the baseline value at prescreen and the value for the week prior to baseline assessment. Also included was treatment condition (MBSCT/BBCET).
Results
Subject characteristics
Baseline demographics and personal characteristics of the subjects included in the present report were similar to those reported for the full sample [16]. The typical participant was approximately 40 years of age, European-American, single, with a baseline weekly sum of standard drinks of 43.1 (SD = 25.5), and approximately 8 drinks per drinking day. Eighty percent of participants completed college, and two-thirds of them attended graduate or professional school.
Genotype distribution
Table 1 summarizes the AA vs. G-carrier genotype frequencies for the OPRM1 A118G SNP grouped by treatments in the present study sample. AA vs. G-carrier genotypes were distributed equally in subjects who were randomized to receive naltrexone vs. placebo in this cohort (Pearson Chi-Square = 0.001, p = 0.981). Twenty-two percent of subjects carried at least one copy of the minor G allele.
Table 1.
AA vs. G-carrier genotype frequency (A118G) of OPRM1 grouped by treatments (Naltrexone, NTX vs. Placebo, PBO).
| NTX/PBO |
OPRM1 Genotype |
Total | |
|---|---|---|---|
| AG/GG | AA | ||
| PBO | 13 (22.4%) | 45 (77.6%) | 58 (100.0%) |
| NTX | 12 (22.2%) | 42 (77.8%) | 54 (100.0%) |
| Total | 25 (22.3%) | 87 (77.7%) | 112 (100.0%) |
Pearson Chi-Square = 0.001, p = 0.981
Asn40Asp SNP of the OPRM1 moderates the effect of naltrexone treatment on the likelihood of non-hazardous drinking (NoH)
Similar to the findings in the Morgenstern et al. [16] main effects paper, there were no significant main effects for naltrexone in relation to SSD or HDD in the cohort included in the present study. However, in regards to NoH, GEE analyses yielded a significant main effect for time (β = 0.05, SE = 0.02, p < 0.05; odds ratio [OR] = 1.057, CI 95% = 1.01, 1.11) indicating that the odds of drinking ≤ 14 standard drinks and having no heavy drinking days in a week increased almost 6% with each additional week of treatment. The interaction of time × naltrexone was significant (β = −0.14, SE = 0.05, p < 0.01; OR = 0.87, CI 95% = 0.79, 0.96). Further, the 3-way interaction of time × naltrexone × genotype also was significant (β = −0.29, SE = 0.10, p < 0.01; OR = 0.75, CI 95% = 0.62, 0.91). To better understand these interactions, models were run within genotype which revealed that the effect of NTX over time was non-significant within the AA genotype (β = 0.01, SE = 0.04, p > 0.05). However, time × naltrexone was significant within the AG genotype (β = −0.29, SE = 0.09, p < 0.01. This effect within AG genotype appears to diminish slightly over time, but not significantly so (β = 0.04, SE = 0.06, p > 0.05). Figure 1 shows the respective weekly proportion of subjects that achieved non-hazardous drinking (NoH) at the end of the treatment (week 12) grouped by genotype and treatment. The estimate marginal means of NoH over the course of the treatment period were 0.27, 0.24, 0.13, 0.22 for NTX/AG, NTX/AA, PBO/AG, PBO/AA respectively.
Figure 1.
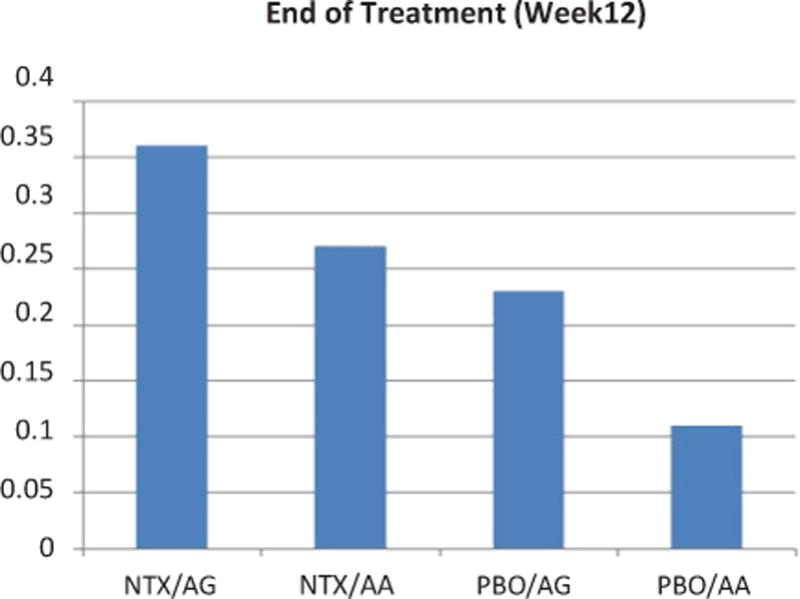
Proprotion of no-heavy drinking (NoH) subjects by genotype and treatment at the end of tretament (week 12). NTX: naltrexone, PBO: placebo. AG: AG or GG 118G-allele carriers. AA: A118 homozygotes.
Discussion
Although the efficacy of naltrexone treatment for alcoholism varies among studies, reviews indicate that naltrexone treatment appears to have larger effects when the outcome measure is a reduction in heavy drinking as opposed to the promotion of abstinence [12]. The analysis of data in the present study aimed to examine the underlying genetic factors that influence the response to naltrexone among high functioning MSM of European ancestry. Contrary to previous studies, for example the COMBINE Study [1], the present study did not test the efficacy of naltrexone on percent days abstinent, time to first heavy drinking day, or global clinical outcome. Rather, the goal of the present study was moderation of drinking, i.e., the achievement of non-hazardous drinking.
One limitation of the present study was that the sample size is relatively small for a genetic association study. However, this is not unusual for pharmacogenetic analyses. The small sample size in this study has also contributed to larger statistical variations of drinking outcomes across time. This has been addressed by sophisticated statistical models that we have applied to avoid spurious findings.
Despite no statistically significant association between rs1779971 and naltrexone treatment effects on the weekly sum of standard drinks (SSD) or the number of heavy drinking days, there was a significant moderator effect on the weekly proportion of subjects who achieved non-hazardous drinking (NoH). This dichotomous measure lends itself more readily to clinical interpretation than continuous measures. Similar types of dichotomous measures are now required by the U.S. Food and Drug Administration for the approval of new drug applications for medications to treat alcohol dependence [25]. This outcome may be of particular utility when the study treatment is designed to reduce heavy drinking, rather than promote abstinence.
Acknowledgments
Supported by the grants from the National Institutes of Health: R01 AA015553 (J.M.), K24 AA013736 (H.R.K), K23 AA018696 (A.C.H.C.) and M01 RR06192 (University of Connecticut GCRC).
References
- 1.Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 2.Chick J, Anton R, Checinski K, Croop R, Drummond DC, et al. A multicentre, randomized, double-blind, placebo-controlled trial of naltrexone in the treatment of alcohol dependence or abuse. Alcohol Alcohol. 2000;35:587–593. doi: 10.1093/alcalc/35.6.587. [DOI] [PubMed] [Google Scholar]
- 3.Morris PL, Hopwood M, Whelan G, Gardiner J, Drummond E. Naltrexone for alcohol dependence: a randomized controlled trial. Addiction. 2001;96:1565–1573. doi: 10.1046/j.1360-0443.2001.961115654.x. [DOI] [PubMed] [Google Scholar]
- 4.Rosner S, Hackl-Herrwerth A, Leucht S, Vecchi S, Srisurapanont M, et al. Opioid antagonists for alcohol dependence. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD001867.pub3. CD001867. [DOI] [PubMed] [Google Scholar]
- 5.Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, et al. A functional polymorphism of the mu-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003;28:1546–1552. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- 6.Anton RF, Oroszi G, O’Malley S, Couper D, Swift R, et al. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Arch Gen Psychiatry. 2008;65:135–144. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gelernter J, Gueorguieva R, Kranzler HR, Zhang H, Cramer J, et al. Opioid receptor gene (OPRM1, OPRK1, and OPRD1) variants and response to naltrexone treatment for alcohol dependence: results from the VA Cooperative Study. Alcohol Clin Exp Res. 2007;31:555–563. doi: 10.1111/j.1530-0277.2007.00339.x. [DOI] [PubMed] [Google Scholar]
- 8.Kim SG, Kim CM, Choi SW, Jae YM, Lee HG, et al. A micro opioid receptor gene polymorphism (A118G) and naltrexone treatment response in adherent Korean alcohol-dependent patients. Psychopharmacology (Berl) 2009;201:611–618. doi: 10.1007/s00213-008-1330-5. [DOI] [PubMed] [Google Scholar]
- 9.Ray LA, Oslin DW. Naltrexone for the treatment of alcohol dependence among African Americans: results from the COMBINE Study. Drug Alcohol Depend. 2009;105:256–258. doi: 10.1016/j.drugalcdep.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chamorro AJ, Marcos M, Mirón-Canelo JA, Pastor I, González-Sarmiento R, et al. Association of micro-opioid receptor (OPRM1) gene polymorphism with response to naltrexone in alcohol dependence: a systematic review and meta-analysis. Addict Biol. 2012;17:505–512. doi: 10.1111/j.1369-1600.2012.00442.x. [DOI] [PubMed] [Google Scholar]
- 11.Kranzler HR, Edenberg HJ. Pharmacogenetics of alcohol and alcohol dependence treatment. Curr Pharm Des. 2010;16:2141–2148. doi: 10.2174/138161210791516387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pettinati HM, O’Brein CP, Rabibowitz AR, Wortman SP, Oslin CP, et al. The status of naltrexone in the treatment of alcohol dependence: specific effects on heavy drinking. J Clin Psychopharmacol. 2006;26:610–625. doi: 10.1097/01.jcp.0000245566.52401.20. [DOI] [PubMed] [Google Scholar]
- 13.O’Malley SS, Froehlich JC. Advances in the use of naltrexone: An integration of preclinical and clinical findings. Recent Dev Alcohol. 2003;16:217–245. [PubMed] [Google Scholar]
- 14.Ray LA, Hutchison KE. A polymorphism of the mu-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcohol Clin Exp Res. 2004;28:1789–1795. doi: 10.1097/01.alc.0000148114.34000.b9. [DOI] [PubMed] [Google Scholar]
- 15.Ray LA, Hutchison KE. Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: a double-blind placebo-controlled study. Arch Gen Psychiatry. 2007;64:1069–1077. doi: 10.1001/archpsyc.64.9.1069. [DOI] [PubMed] [Google Scholar]
- 16.Morgenstern J, Kuerbis AN, Chen AC, Kahler CW, Bux DA, et al. A Randomized Clinical Trial of Naltrexone and Behavioral Therapy for Problem Drinking Men Who Have Sex With Men. J Consult Clin Psychol. 2012;80:863–875. doi: 10.1037/a0028615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson BA, DiClemente CC, Ait-Daoud N, Stoks SM. Brief Behavioral Compliance Enhancement Treatment (BBCET) manual. In: Johnson BA, Ruiz P, Galanter M, editors. Handbook of Clinical Alcoholism Treatment. Lippincott Williams & Wilkins; Baltimore, MD: 2003. pp. 282–301. [Google Scholar]
- 18.Cottler LB, Robins LN, Helzer JE. The reliability of the CIDI-SAM: A comprehensive substance abuse interview. Br J Addict. 1989;84:801–814. doi: 10.1111/j.1360-0443.1989.tb03060.x. [DOI] [PubMed] [Google Scholar]
- 19.First MB, Spitzer RL, Gibbon M. Structured Clinical Interview for DSM-IV Biometric Department. New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- 20.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 21.Beck AT, Steer RA, Brown GK. Beck Depression Inventory, Second Edition Manual. Harcourt Brace; San Diego, CA: 1996. [Google Scholar]
- 22.Sobell MB. Developing a prototype for evaluating alcohol treatment effectiveness. In: Sobell LC, Ward E, editors. Evaluating alcohol and drug abuse treatment effectiveness: Recent advances. Pergamon; New York: 1980. pp. 129–150. [Google Scholar]
- 23.Livak KJ, Flood SJ, Marmaro J, Giusti W, Deetz K. Oligonucleotides with fuorescent dyes at opposite ends provide a quenched probe system useful for detecting PCR product and nucleic acid hybridization. PCR Methods Appl. 1995;4:357–362. doi: 10.1101/gr.4.6.357. [DOI] [PubMed] [Google Scholar]
- 24.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 25.Falk D, Wang XQ, Liu L, Fertig J, Mattson M, et al. Percentage of subjects with no heavy drinking days: Evaluation as an efficacy endpoint for alcohol clinical trials. Alcohol Clin Exp Res. 2010;34:1–13. doi: 10.1111/j.1530-0277.2010.01290.x. [DOI] [PubMed] [Google Scholar]


