Abstract
The MRI technique has been used in diagnosis of manganism in humans and non-human primates. This cross-sectional study was designed to explore whether the pallidal signal intensity in T1-weighted MRI correlated with Mn levels in the blood compartment among Mn-exposed workers and to understand to what extent the MRI signal could reflect Mn exposure. A group of 18 randomly selected male Mn-exposed workers of which 13 were smelting workers with high exposure (mean of airborne Mn in work place: 1.26 mg/m3; range: 0.31–2.93 mg/m3), and 5 power distribution control workers with low exposure (0.66 mg/m3 and 0.23–0.77 mg/m3) from a ferroalloy factory, and another group of 9 male subjects as controls from a non-smelting factory who were office or cafeteria workers (0.01 mg/m3 and 0–0.03 mg/m3) were recruited for neurological tests, MRI examination, and analysis of Mn in whole blood (MnB), plasma (MnP) or red blood cells (MnRBC). No clinical symptoms and signs of manganism were observed among these workers. MRI data showed average increases of 7.4% (p < 0.05) and 16.1% (p < 0.01) in pallidal index (PI) among low- and high-exposed workers, respectively, as compared to controls. Fourteen out of 18 Mn-exposed workers (78%) had intensified PI values, while this proportion was even higher (85%) among the high Mn-exposed workers. Among exposed workers, the PI values were significantly associated with MnRBC (r = 0.55, p = 0.02). Our data suggest that the workers exposed to airborne Mn, but without clinical symptoms, display an exposure-related, intensified MRI signal. The MRI, as well as MnRBC, may be useful in early diagnosis of Mn exposure.
Keywords: Manganese, MRI, Red blood cells, Trace element, Smelting workers, Biomarker
1. Introduction
Severe manganese (Mn) intoxication (manganism) causes irreversible neurodegenerative parkinsonian syndromes which can be recognized and characterized by patient’s clinical manifestations (Aschner et al., 1999; Crossgrove and Zheng, 2004; Huang et al., 1998). Because there is scarcely any effective treatment for manganism, the early diagnosis of Mn neurotoxicity, particularly with low-level Mn exposure in occupational settings, has become critical in disease prevention and possibly clinical intervention. Recent clinical research efforts have been devoted to exploring the utility of magnetic resonance imaging (MRI) technique to identify brain regional Mn status among Mn-exposed workers (Kim, 2004; Kim et al., 1999c; Lucchini et al., 1999, 2000; Shinotoh et al., 1995) or to seek biochemical markers that are associated with Mn exposure (Husain et al., 2001; Lu et al., 2005).
MRI identification of brain regional Mn accumulation takes advantage of the paramagnetic properties of Mn, which allows the shortening of the T1 relaxation time and hence increases the signal intensity unique to Mn ions. An increased T1-weighted Mn signal in the globus pallidus area has been found among workers occupationally exposed to Mn (Dietz et al., 2001; Kim et al., 1999b; Lucchini et al., 2000; Nelson et al., 1993), in patients receiving long-term total parenteral nutrition (Alves et al., 1997; Ejima et al., 1992; Fell et al., 1996; Fredstrom et al., 1995; Iwase et al., 2000; Komaki et al., 1999; Nagatomo et al., 1999; Quaghebeur et al., 1996), and in clinical cases reported with hepatic failure (Butterworth et al., 1995; Chetri and Choudhuri, 2003; Hauser et al., 1994; Hazell and Butterworth, 1999; McKinney et al., 2004; Spahr et al., 2002). While in animal models the MRI intensity has been linked to external Mn exposure (Eriksson et al., 1992; Kim et al., 1999a; Misselwitz et al., 1995; Newland et al., 1989a,b; Shinotoh et al., 1995), the question as to how the change in MRI signals in humans is associated with external exposure conditions and whether this consequentially leads to alternations of the internal Mn exposure indices or the fluctuation in blood chemistry remains elusive.
Historically, biological monitoring of internal Mn exposure has relied on determination of Mn concentrations in the whole blood, serum or plasma. One of the perplexing problems in clinics is that blood levels of Mn usually poorly reflect the body burden of Mn and the ensuing disease status. There is a discrepancy between blood Mn levels and intracellularly distributed tissue Mn contents. For example, the terminal-phase elimination half life (t1/2) following i.v. injection of MnCl2 in rats is only about 2 h; such a short half life, in theory, would not result in the accumulation of Mn in the body (Zheng et al., 2000). However, tissue analyses suggest that the biological half life of Mn in brain tissues is between 51 and 74 days (Newland et al., 1987; Takeda et al., 1995). Thus, it is possible that the intracellular binding and sequestration of Mn ions may prevent the metal from migration to the extracellular space. As such, a simple measurement of extracellular Mn such as in plasma or serum may not accurately reflect Mn concentrations in the blood compartment, including blood cells. Additionally, Mn is known to be transported by the transferrin receptor (TfR) and/or divalent metal transporter (DMT1) (Crossgrove and Zheng, 2004). Both transporters have been identified in the RBC (Andrews, 1999; Poola et al., 1990; Weiss et al., 1997). Thus, it is reasonable to postulate that Mn in the blood compartment may tend to accumulate in the blood cells, leaving less in the extracellular fluid of plasma or serum. Thus, a direct analysis of Mn in the blood cells, e.g. in red blood cells (RBC), is deemed necessary.
This study was designed to investigate the relationship between MRI signal intensities in brains and Mn concentrations in the RBCs among active smelting workers. Most studies prior to this work on MRI and Mn exposure were conducted on exposed welders (Josephs et al., 2005; Kim et al., 1999c; Nelson et al., 1993; Ono et al., 2002). Since the welding fumes generated during the welding process possesses at least 13 metals (Li et al., 2004), the exposure to multiple metals, notably iron (Fe), may complicate the exposure scenario for accurate assessment of Mn exposure. In addition, the welders’ job assignments vary frequently between indoor and outdoor environments and amid open or closed compartments, bringing about the day-to-day variations in exposure conditions. Thus, we chose smelting workers whose job assignments are more stable and whose exposure scenario is relatively less complicated than those of welders. For example, our occupational air monitoring data indicate that air samples of smelting environment contain mainly MnO (20%) and SiO2 (22%), in addition to other trace metals including Fe2O3 (4%), CaO (4.5%), MgO (4%) and Al2O3 (5%).
Specifically, this study was aimed at evaluating (1) if there were changes in MRI signals in Mn-exposed smelting workers, as compared to non-exposed control subjects, (2) whether the prevalence of the increased MRI signal intensities correlated with external Mn exposure indices, and (3) if there was an association between MRI signal changes and Mn concentrations in the whole blood, plasma, serum or RBCs.
2. Subjects and methods
2.1. Factory and production processes
The study site chosen was a ferroalloy manufacture company located in the central region of Guangxi Province and not adjacent to any other metal industries. This Group Company has more than 10,000 employees. A subordinate smelting factory, where smelting workers were recruited and air Mn levels monitored, was selected for its day-to-day production of ferroalloy. The smelting workshop has more than 2000 workers working in front of smelting furnaces who have a direct exposure to airborne Mn and about 100 workers in a power distribution/control room, which is located within the smelting workshop but in a separate room.
For comparative purposes, the workers in another factory within the same ferroalloy industry group whose job functions were not relevant to smelting, such as office or cafeteria services, located 20 km upwind direction and in the same urban area, was chosen as the control.
2.2. Study population
This is a cross-sectional study. A group of 18 male workers in the ferroalloy factory were randomly selected as the exposed group. Among them, 13 workers engaged in furnace smelting who had direct exposure to a high level of airborne Mn were considered as the high Mn-exposed group and 5 workers working in the power distribution/control room with a low level of Mn exposure were considered as the low Mn-exposed group. The workers worked 7–8 h per day with the average employment history of 14 years (range: 5–33 years). A control group of nine workers were then recruited, frequency-matched to the Mn exposed group by age and years of employment, from a non-smelting factory. The mean ages were 35.8 years (range: 25–55) and 40.3 years (range: 27–54), respectively, for the Mn-exposed group and controls. Both groups were also matched for socioeconomic status (salary, education, etc.) and background environmental factors (place of residence, etc.). These and other demographic data of the study population are summarized in Table 1.
Table 1.
Summary of demographic data among Mn-exposed and control workers
| Parameters | Control | Mn exposed
|
||
|---|---|---|---|---|
| Low | High | Combine | ||
| Number of cases | 9 | 5 | 13 | 18 |
| Mean age (years) (95% CI) | 40.3 ± 9.2 (33.3–47.4) | 42.8 ± 9.6 (30.8–54.8) | 33.2 ± 8.8 (27.9–38.4) | 35.8 ± 9.8 |
| Years in employment (95% CI) | 17.6 ± 8.0 (12.0–27.5) | 21.6 ± 11.1 (7.9–35.4) | 11.3 ± 9.1 (5.8–16.8) | 14.1 ± 10.5* |
| Initial working age (years) (95% CI) | 21.0 ± 2.9 (18.9–23.2) | 21.4 ± 2.5 (18.3–24.5) | 22.2 ± 3.9 (19.9–24.6) | 22.0 ± 3.5 |
| Smoking (%) | 33.3 | 40.0 | 46.2 | 44.4 |
| Alcohol (%) | 33.3 | 40.0 | 30.1 | 33.3 |
| Airborne MnO2 (mg/m3) (95% CI) | 0.01 (0–0.03) | 0.66* (0.36–0.96) | 1.26**▲ (1.05–1.48) | 0.60** |
| MnB (μg/mL) (95% CI) | 0.04 ± 0.02 (0.02–0.05) | 0.05 ± 0.01 (0.04–0.06) | 0.05 ± 0.03 (0.03–0.07) | 0.05 ± 0.03 |
| MnP (μg/mL) (95% CI) | 0.05 ± 0.03 (0.04–0.08) | 0.04 ± 0.01* (0.03–0.05) | 0.05 ± 0.02 (0.04–0.07) | 0.05 ± 0.02 |
| MnRBC (μg/mL) (95% CI) | 0.14 ± 0.01 (0.14–0.16) | 0.15 ± 0.02 (0.12–0.18) | 0.16 ± 0.05 (0.12–0.18) | 0.15 ± 0.04 |
Data represent mean ± S.D.
p < 0.05,
p < 0.01 compared with the control workers;
p < 0.01 compared with low-exposed workers.
Note. The statistical significance among groups was further analyzed by ANCOVA (see Table 4).
Subjects in both groups at the time of interview had reported no exposure to other toxic substances, radiation therapy, or substance abuse. There were no statistically significant differences in smoking and alcohol consumption between the Mn-exposed workers and the controls (Table 1).
2.3. Collection of personal data and biological samples
The standardized interviews, clinical examinations, and MRI evaluations took place in the First Affiliated Hospital of Guangxi Medical University. The written informed consent forms were obtained from all subjects prior to interview and physical examination. A scheduled interview with a questionnaire lasting approximately 60 min was conducted by trained interviewers to obtain detailed information on occupational history, job description, socioeconomic status, lifestyle, and family and personal medical history. The participants were asked to fast overnight prior to the study. Blood samples were collected in the morning of the day of examination, followed by neurological examination on the same day. One millilitre of venous blood was drawn from a cubital vein into a heparinized tube as the whole blood fraction; 0.5 mL of the whole blood was used for testing routine blood parameters as well as measurement of trace elements. Four millilitres of venous blood was collected into a heparinized tube and maintained at room temperature for 30 min. The sample was then centrifuged at 600 × g/min for 5 min to separate the red blood cell (RBC) fraction. The supernatant was collected as the plasma fraction. All samples were stored at −20 °C until analyses. All test tubes used in the study were free of metal contamination, as pre-tested by atomic absorption spectrophotometry (AAS).
2.4. MRI examination
MRI examinations were performed using a 1.5 T Signa superconducting system (Signa Horizon LX; GE Medical Systems; Milwaukee, WI, USA) with a quadrature coil. The method and result interpretation have been described in detail by Kim et al. (1999c). In general, the spin echo (SE) technique was applied with two acquisitions in the T1-weighted sequence and one acquisition in the T2-weighted sequence. T1-weighted images were acquired in the axial and sagittal planes by using the following parameters: 466/14 [repetition time (TR)/echo time (TE) ms] two excitations, a 22 cm field-of-view, a 256 × 160 matrix, and slice thickness of 7 mm. Axial T2-weighted (TR/TE = 4000/100 ms) images were also obtained in the similar manner. The signal intensity of the globus pallidus relative to that recorded from frontal white matter was subjectively evaluated by a radiologist without prior knowledge of the disease status of the T1-weighted images. For quantitative evaluation of signal intensities, the region of interest (ROI) was placed in areas with the highest intensity by visual assessment in the globus pallidus and the subcortical frontal white matter. The pallidal index (PI), which is defined as the ratio of the signal intensity in the globus pallidus (SIGP) to that in the subcortical frontal white matter (SIFW) in axial T1-weighted MRI planes multiplied by 100, was then estimated (Krieger et al., 1995).
2.5. Air sample collection and analysis
For evaluation of the external exposure, the locations where workers usually worked in the smelting workshop (15 sites), the power distribution/control room (7 sites), or the offices or cafeteria (10 sites) were identified. Airborne manganese concentrations were determined in the breathing zones of workers by station air samplers. Air samples were collected using a Model BFC-35 pump equipped with a micro-porous filter, which has a diameter of 40 mm and the pore size of 0.8 μm. Air flow was pumped at a flow rate of 5 L/min for 4 min 1 h after smelting started. At each monitoring site, samples were collected in duplicates every other hour and two more times in the same day (total 5 h). The mean values of all four duplicated samples are presented in Table 1.
The filters were digested with 5 mL of HClO4–HNO3 mixture (1:9 v/v) at 200 °C. The dry residues were dissolved in 10 mL of 1% HCl. The solutions were diluted by 20–50-fold prior to AAS. Air Mn concentrations in working place were determined by a model HITACHI Z-5000 flame atomic absorption spectrophotometer (AAS) according to a China National Standard Operation Protocol (GB/T16018-1995) for occupational safety surveillance.
2.6. Determination of blood chemistry and metals in biological samples
Levels of white blood cells (WBC), red blood cells (RBC), hemoglobin (Hgb), haematocrit (Hct), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), platelets (PIT), lymphocyte percentage (LY), and lymphocyte count (LY#) among Mn exposed workers and control subjects were determined by using well established routine assay procedures in the hospital or an automated COULTER LH 750 Hematology Analyzer (Beckman, USA).
Concentrations of Mn, Zn, Cu, Fe, Ca and Mg in the whole blood, plasma, or RBC fractions were quantified by a model JY-70PII inductively coupled plasma-atomic emission spectrophotometer (ICP-AES, JY70P Type II, Jobin-Yvon Company, France). Aliquots (0.1 mL) of samples were diluted (5–20-fold) with an appropriate volume of 0.8% Triton X-100/0.5% EDTA in distilled, deionized water prior to ICP-AES analysis. The standard curves were established using freshly made metal standards on the day of analysis. The detection limit of this method was 0.3, 5.7, 3.9, 3.3 9.9, 0.6 ng/mL for Mn, Fe, Cu, Zn, Ca and Mg, respectively.
2.7. Statistical analyses
Records of interviews and other reports were reviewed and abstracted for demographic data. All data are expressed as the mean ± S.D. The data were initially analyzed using ANOVA linear contract analysis. If the ANOVA showed an overall significance at p < 0.05, Student’s t-test was used to identify the significant differences among subgroups. Analysis of covariance (ANCOVA) using Years in Employment as covariate was further employed to analyze statistical significance among groups for parameters such as air level of MnO2 (MnA), Mn in the whole blood (MnB), Mn in plasma (MnP), Mn in RBC (MnRBC), white blood cell counts (WBC), mean corpuscular hemoglobin concentration (MCHC), signal intensity in globus pallidus (SIGP), signal intensity in frontal white matter (SIFW) and PI. Associations between the PI and MnA, MnRBC, SIGP or SIFW in exposed workers were analyzed by stepwise multiple regression analysis by controlling variables including age, years in employment, initial working age, concentration of Mn, Zn, Cu, Fe, Ca and Mg in the whole blood, plasma and RBC, and all routine blood parameters. Statistical software SPSS/PC+ for Windows (V.10.0) was used in data analysis.
2.8. Materials
Chemicals were obtained from the following sources: AAS standards of all metals from Alfa Products (Danvers, MA, USA). All reagents were of analytical grade, HPLC grade or the highest available pharmaceutical grade.
3. Results
3.1. Airborne Mn levels in working zones
Table 1 summarizes the demographic characteristics of the study population. There were no significant differences between Mn-exposed workers and controls in their age, years in employment, initial age at employment, smoking, and alcohol consumption.
The maximum allowable concentration (MAC) and the threshold limit value (TLV) for airborne Mn in the work place is 0.2 mg/m3, according to the Chinese Ministry of Public Health (TJ36-79) and the American Conference of Governmental Industrial Hygienists. In this cross-sectional study, the geometric mean of airborne Mn concentrations in the furnace smelting workplace (i.e., the high exposure group) was 1.3 (range: 0.3–2.9) mg/m3. Among 15 worksites monitored, 81% had Mn exposure levels above the MAC value. The geometric mean of Mn concentrations in the air of the power distribution/ control room (i.e., the low exposure group) was 0.7 (range: 0.2–0.8) mg/m3; about 86% of the seven monitored worksites had Mn exposure levels above the MAC value. These two geometric means of the low and high exposure groups were 3.3-fold and 6.3-fold, respectively, above the MAC. It was noticed that the air samples in the current study were taken during a summer season when all windows in this semi-open designed smelting workshop were open, allowing outdoor air flow to cool down the workshop.
3.2. Blood chemistry and Mn concentrations in the whole blood (MnB), Plasma (MnP) and red blood cells (MnRBC) in Mn-exposed and control workers
Of 10 parameters monitored for blood chemistry of Mn-exposed workers, all were in the normal range as compared to control subjects (Table 2).
Table 2.
Routine blood parameters in Mn-exposed and control workers
| Parameters | Control | Mn exposed
|
||
|---|---|---|---|---|
| Low | High | Combine | ||
| Case number | 9 | 5 | 13 | 18 |
| WBC (×109 L−1) | 6.56 ± 0.75 | 7.40 ± 1.29 | 6.35 ± 0.97* | 6.64 ± 1.14 |
| RBC (×1012 L−1) | 5.03 ± 0.56 | 5.08 ± 0.18 | 5.40 ± 0.69 | 5.31 ± 0.61 |
| Hgb (g/L) | 145 ± 15.8 | 151 ± 8.08 | 145 ± 12.3 | 147 ± 11.4 |
| Hct (%) | 43.6 ± 3.85 | 45.1 ± 2.40 | 44.4 ± 3.35 | 44.6 ± 3.06 |
| MCV (fL) | 87.5 ± 10.1 | 88.8 ± 3.74 | 83.0 ± 8.25 | 84.6 ± 7.64 |
| MCH (pg) | 29.1 ± 3.81 | 29.8 ± 1.16 | 27.1 ± 3.20 | 27.9 ± 3.01 |
| MCHC (g/L) | 332 ± 9.86 | 335 ± 2.59 | 326 ± 7.61▲ | 329 ± 7.66 |
| PIT (×109 L−1) | 197 ± 73.2 | 225 ± 40.8 | 203 ± 36.9 | 209 ± 38.1 |
| LY (%) | 34.6 ± 5.85 | 32.4 ± 3.55 | 36.1 ± 3.80 | 35.0 ± 4.00 |
| LY# (×109 L−1) | 2.26 ± 0.34 | 2.40 ± 0.55 | 2.28 ± 0.25 | 2.31 ± 0.35 |
Data represent mean ± S.D.
p < 0.05 compared with low-exposed workers.
The statistical significance among groups for WBC and MCHC was further analyzed by ANCOVA (see Table 4). WBC, white blood cell counts; RBC, red blood cell counts; Hgb, hemoglobin; Hct, hematocrit; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; PIT, platelet; LY, lymphocyte percentage; LY#, lymphocyte counts.
Concentrations of MnB, MnP, and MnRBC in Mn-exposed workers were not statistically significantly different from those in controls (Table 1), although a slight increase was observed in MnRBC of high Mn-exposed workers. It is noteworthy that MnRBC levels in both exposed workers and controls were about three-fold higher than the values of MnB and MnP. Moreover, the variations of MnRBC values (S.D./ mean × 100%) were found to be between 1 and 27% of the means, whereas the variations of MnB or MnP were between 40 and 60% of the means, suggesting that the levels of MnRBC were less variable than the traditional MnB and MnP.
3.3. MRI analysis and correlations between the PI and MnB, MnP, or MnRBC
Among active smelting workers who were exposed to high levels of airborne Mn, the T1-weighted MRI showed a distinct, whitened signal in globus pallidus area (Fig. 1A). The enhanced signal was also evident among low Mn-exposed workers (Fig. 1B). The prevalence of enhanced T1-weighted MRI signals was 60 and 85% among low and high Mn-exposed workers, respectively, with 100% occurrence among smelting workers who had more than 5 years of working experience (Table 3).
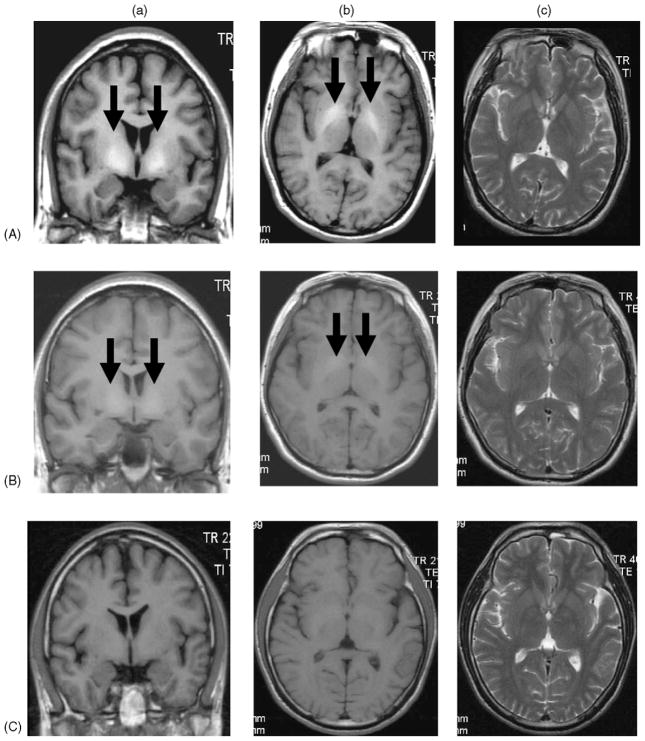
Fig. 1.
Representative MRI of Mn exposed workers and control workers. (A) Significantly increased T1-weighted MRI (PI = 121.5) in a smelting worker who was exposed to the high level of airborne Mn; (B) slightly increased T1-weighted MRI (PI = 112.2) in a power distribution/control worker exposed to low level of airborne Mn; and (C) normal T1-weighted MRI (PI = 102.7) in a worker without Mn exposure. (a) Coronal T1-weighted MRI, (b) axial T1-weighted MRI, and (c) axial T2-weighted MRI. Arrows indicate increased signal intensities at the globus pallidus.
Table 3.
Pallidal index (PI) of brain T1-weighted MRI among Mn-exposed and control workers
| Group | n | Workers with increased PI (%) | SIGP (mean ± S.D.) | SIFW (mean ± S.D.) | PI (mean ± S.D.) |
|---|---|---|---|---|---|
| Control | 9 | 0 (0.0) | 428.5 ± 15.0 | 419.4 ± 15.2 | 102.2 ± 1.5 |
| Mn-exposed | 18 | 14 (77.8)** | 529.1 ± 71.0** | 453.8 ± 33.6 | 116.2 ± 8.4** |
| Low-Mn | 5 | 3 (60.0) | 466.3 ± 27.5## | 425.0 ± 26.7# | 109.8 ± 4.5*# |
| High-Mn | 13 | 11 (84.6) | 533.3 ± 67.9** | 464.9 ± 29.8* | 118.7 ± 8.4** |
| <5 years | 5 | 3 (60.0) | 515.6 ± 72.0 | 450.6 ± 25.9 | 114.1 ± 9.9 |
| 5–10 years | 4 | 4 (100) | 572.2 ± 61.6 | 464.7 ± 30.6 | 123.0 ± 7.2 |
| >10 years | 4 | 4 (100) | 581.3 ± 61.7 | 483.0 ± 30.5 | 120.1 ± 5.8 |
Data represent mean ± S.D.
p < 0.05,
p < 0.01, compared with control group;
p < 0.05,
p < 0.01, compared with high-exposed group.
Note. The statistical significance among groups was further analyzed by ANCOVA (see Table 4). SIGP, signal intensities of globus pallidus; SIFW, signal intensities of frontal white matter.
The pallidal indices (PI), as calculated in reference to the signal intensity in the subcortical frontal white matter (SIFW), were increased by 7.4 and 16.1%, respectively, in the low and high Mn-exposed workers as compared to control workers ( p < 0.05). Among high Mn-exposed workers with 5–10 years of working experience, the PI values were increased by 20% as compared to that of non-exposed workers (Table 3). Scatter dot-plots of the distribution outcomes of the PI and SIGP values among control, low and high Mn-exposed workers are presented in Fig. 2.
Fig. 2.
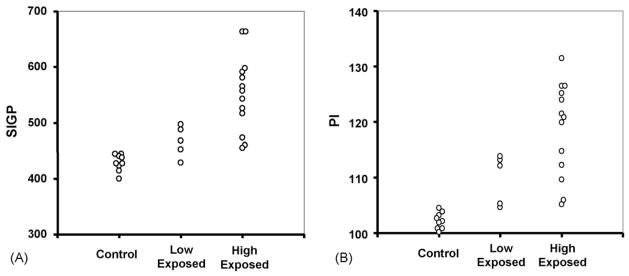
Scatter dot-plots of the distribution of individual MRI scores among control, low and high Mn-exposed workers. (A) Signal intensity in globus pallidus (SIGP); (B) pallidal index (PI), which is defined as the ratio of SIGP to that in the subcortical frontal white matter (SIFW) in axial T1-weighted MRI planes multiplied by 100.
It is possible that the year of employment, which is related to the duration of Mn exposure, may function as a confounder. Thus, an analysis of covariance (ANCOVA) was conducted for the following parameters: airborne MnO2, MnB, MnP and MnRBC (in Table 1), WBC and MCHC (in Table 2), and SIGP, SIFW and PI (in Table 3), by controlling years of employment as a covariate. ANCOVA revealed that for airborne MnO2, SIGP, SIFW, and PI, a statistically significant difference did indeed existed among control, low-exposed and high-exposed workers (Table 4).
Table 4.
Statistical significance among control, low-exposed, and high-exposed workers for critical parameters by ANCOVA
| Variables | F-value (d.f.) | p |
|---|---|---|
| MnO2 | 31.09 (2) | 0.000** |
| MnB | 0.361 (2) | 0.701 |
| MnRBC | 0.337 (2) | 0.717 |
| MnP | 3.110 (2) | 0.064 |
| WBC | 1.943 (2) | 0.166 |
| MCHC | 2.743 (2) | 0.085 |
| SIGP | 15.78 (2) | 0.000** |
| SIFW | 9.513 (2) | 0.001** |
| PI | 15.43 (2) | 0.000** |
Values were analyzed by ANCOVA by controlling years of employment as covariate. F-value, value of covariance analysis; d.f., degree of freedom; p, level of significance. For air MnO2, SIGP, SIFW, and PI, there existed statistically significant differences among groups.
Highly significant.
When the PI values among 18 Mn exposed workers were correlated with MnB, MnP or MnRBC by a linear regression analysis, the PI values were significantly associated with MnRBC (r = 0.55, p = 0.02) (Fig. 3), less significantly with MnP (r = 0.42, p = 0.08), and not significantly with MnB (r = 0.09, p = 0.72) (Table 5A). When the values of the control subjects were also included in the linear regression analysis (n = 27), a significant, yet weaker, correlation continued to exist between PI and MnRBC (r = 0.41, p = 0.033) (data not shown); this observation is consistent with those reported by Kim et al. (2005a,b). No association was observed between the PI and Fe levels in the whole blood, plasma or RBC (data not shown). Interestingly, the MnB values were significantly associated with MnRBC (r = 0.56, p < 0.05), while they did not correlate with the PI, suggesting a large variation in MnB analysis.
Fig. 3.
Correlation between MnRBC and the PI values among 18 Mn-exposed workers. Data were analyzed by linear correlation, correlation coefficient (r) = 0.55, p < 0.05.
Table 5A.
Correlation on working year, Mn in blood, plasma, RBC and PI among Mn-exposed workers (n = 18)
| Index | Working years | MnB (μg/mL) | MnP (μg/mL) | MnRBC (μg/mL) | PI |
|---|---|---|---|---|---|
| Working years | – | 0.23 | 0.32 | 0.29 | −0.01 |
| MnB (μg/mL) | – | – | −0.01 | 0.56* | 0.09 |
| MnP (μg/mL) | – | – | – | 0.36 | 0.42 |
| MnRBC (μg/mL) | – | – | – | – | 0.55* |
| PI | – | – | – | – | – |
Values represent correlation coefficient (r).
p < 0.05.
A stepwise multiple regression analysis was used to determine associations between the PI and airborne MnO2, MnRBC, SIGP or SIFW in exposed workers by controlling independent variables including age, years in employment, initial working age, concentration of Mn, Zn, Cu, Fe, Ca and Mg in the whole blood, plasma and RBC, and all other routine blood parameters. The multiple regression analyses revealed the existence of statistically significant correlations between the PI and MnO2, MnRBC, SIGP, or SIFW (Table 5B).
Table 5B.
Correlations between PI and other parameters by stepwise multiple regression analysis
| Models | Variables | Unstandardized coefficients (B) | Standardized coefficients (β) | t | p | Adjusted R2 |
|---|---|---|---|---|---|---|
| Model I (n = 27) | MnO2 | 9.219 | 0.536 | 3.544 | 0.002** | 0.409 |
| MnRBC | 127.638 | 0.450 | 2.976 | 0.007** | ||
| Model II (n = 27) | SIGP | 0.119 | 0.935 | 13.140 | 0.000** | 0.868 |
| Model III (n = 27) | SIFW | 0.210 | 0.718 | 5.156 | 0.000** | 0.718 |
Model I: selected independent variables include age, years in employment, initial working age, air MnO2, concentrations of Mn, Zn, Cu, Fe, Ca and Mg in whole blood, red blood cell and plasma, and all routine blood parameters. Model II: SIGP was added to Model I with same selected independent variables but without MnO2 and MnRBC. Model III: SIFW was added to Model 1 with same selected independent variables but without blood Cu. Multiple regression analyses revealed the existence of statistically significant correlation between the PI and MnO2, MnRBC, SIGP, or SIFW.
Highly significant.
3.4. Concentrations of essential metals in the whole blood, plasma or RBC among Mn-exposed and control workers
Mn exposure did not cause any significant alterations in the levels of Fe, Zn, Ca and Mg in the whole blood, plasma or RBC, except for a significant increase in whole blood Cu concentrations in Mn-exposed groups as compared to controls (p < 0.05, Table 6). However, Cu levels in plasma and RBCs of Mn-exposed workers were normal when compared to those of the control subjects.
Table 6.
Concentrations of trace elements in the blood compartment of Mn-exposed and control workers
| Group | Fe | Cu | Zn | Ca | Mg |
|---|---|---|---|---|---|
| Blood (μg/mL) | |||||
| Control | 503 ± 64.2 | 0.64 ± 0.07 | 5.10 ± 1.78 | 48.6 ± 4.75 | 35.9 ± 4.24 |
| Exposed | 551 ± 66.6 | 0.73 ± 0.09* | 5.54 ± 0.70 | 48.2 ± 4.49 | 36.7 ± 3.02 |
| Plasma (μg/mL) | |||||
| Control | 1.26 ± 0.48 | 0.73 ± 0.34 | 1.86 ± 0.26 | 77.6 ± 28.7 | 17.9 ± 6.71 |
| Exposed | 1.58 ± 0.59 | 0.72 ± 0.25 | 1.86 ± 0.57 | 81.7 ± 26.6 | 19.6 ± 6.87 |
| RBC (μg/mL) | |||||
| Control | 935 ± 110 | 0.58 ± 0.13 | 11.7 ± 2.17 | 4.82 ± 2.07 | 42.3 ± 10.5 |
| Exposed | 850 ± 264 | 0.55 ± 0.15 | 10.2 ± 2.87 | 5.00 ± 2.94 | 40.8 ± 13.3 |
Data represent mean ± S.D.
p < 0.05, compared with control group.
3.5. Follow-up study of Mn-exposed workers with improved working environment
Upon being notified of the initial environmental monitoring results, the ferroalloy manufacturer has taken the steps to improve the working environment, including implement of additional high capacity ventilation fans and reinforcing the use of personal protective equipment. With the improved air quality, airborne Mn levels declined towithin the range of 0.2–0.3 mg/m3 6 month after the initial study. During a 1-year follow-up study, the geometric mean of airborne Mn level in smelting working place was 0.26 mg/m3, close to the MAC value. However, MnB and MnRBC among eight smelter workers, who were originally in the high Mn-exposed group, remained unchanged. The PI values among these workers were also not significantly different from the values obtained in the original examination (Table 7), however, no significant correlation was found between the PI values and the levels of MnRBC, MnP, or MnS.
Table 7.
Mn concentrations in the blood compartment and the PI of Mn-exposed workers in a 1-year follow-up study
| Original | Follow-up | |
|---|---|---|
| Number of cases | 8 | 8 |
| Airborne Mn (mg/m3) (range) | 0.72 (0.07–2.93) | 0.26* (0.01–2.40) |
| MnB (μg/mL) | 0.05 ± 0.03 | 0.09 ± 0.07 |
| MnP (μg/mL) | 0.05 ± 0.02 | – |
| MnS (μg/mL) | – | 0.10 ± 0.09 |
| MnRBC (μg/mL) | 0.17 ± 0.03 | 0.20 ± 0.08 |
| PI | 122 ± 6.20 | 123 ± 7.10 |
Data represent mean ± S.D.
p < 0.05, compared with the original study.
4. Discussion
The data presented in this human study support the view that T1-weighted MRI may serve as a sensitive non-invasive indicator in the evaluation of Mn exposure among asymptomatic Mn-exposed workers who are on active duty. Moreover, Mn concentrations in the RBC may be useful for biological monitoring Mn exposure.
In proton nuclear MRI, the paramagnetic property of Mn ions can shorten the T1-relaxation time and increase the signal intensity in targeted brain images, which results in a detectable increase of signal intensities in T1-weighted sequences (Eriksson et al., 1992; Kim et al., 2005a,b; Misselwitz et al., 1995; Newland et al., 1989a,b; Shinotoh et al., 1995). The usefulness of T1-weighted MRI in the diagnosis of Mn exposure has been demonstrated in animal and human studies. For example, non-human primates exposed to Mn display symmetrically enhanced signals in T1-weighted MRI (Eriksson et al., 1992; Newland et al., 1989a,b). In some cases, the enhanced signal can also be observed in the globus pallidus of Mn-exposed monkeys whose neurologic deficits are absent and whose behavioral changes are subtle (Newland and Weiss, 1992; Olanow et al., 1996; Shinotoh et al., 1995). Human studies among welders, who are exposed to Mn in welding fume, have also provided strong evidence of an increased MRI signal in welders’ globus pallidus (Dietz et al., 2001; Kim et al., 1999c; Nelson et al., 1993). Our data clearly showed an overall high incidence (78%) and significant increase in T1-weighted MRI among Mn-exposed workers in comparison to control workers. Furthermore, we found that the increased PI appeared to be associated with airborne Mn levels in exposure environment.
When the geometric mean of airborne Mn concentrations in the workers’ breathing zone of the power control room was 0.66 mg/m3 (three-fold above the MAC), three out of five power control workers (60%) displayed increased PI signals, although the PI values were not statistically significantly different from that of control. Under the high Mn-exposure condition, where the geometric mean of airborne Mn concentrations in the smelting environment was 1.26 mg/m3 (6.3-fold of the MAC), the increased signals in T1-weighted MRI were highly prevalent (84.6%); the workers with more than 5 year working experience showed nearly 100% occurrence of enhanced PI. A similar observation has been reported in a South Korean study where the enhanced signals in T1-weighted MRI were highly prevalent (73.5%) in Mn-exposed welders in comparison to unchanged PI values among control clerical workers (Kim et al., 1999c). Thus, the increase in MRI signal intensities in globus pallidus appears to be a sensitive marker for external Mn exposure.
The question as to whether the MRI can be used to reflect recent or long-term Mn exposure is still debatable. Our previous work on a manganism patient treated with para-aminosalicylic acid showed a normal T1-weighted MRI in the globus pallidus (Jiang et al., 2006). Kim and his colleagues have reported that a welder with more than 10-year Mn exposure whose clinical manifestations included masked face, asymmetric resting tremor, and bradykinesia had symmetrical high signal intensities in the globus pallidus on T1-weighted image. The intensity, however, nearly completely disappeared 6 months after he discontinued welding practice (Kim et al., 1999c). Similar observations among welders, either with or without chelating drug treatment, are also reported by other investigators (Arona et al., 1997; Discalzi et al., 2000; Nelson et al., 1993). These observations support the view that T1-weighted MRI may serve as a good indicator for recent exposure among active workers, but it may not be sensitive for patients who have been removed from the exposure scene. Our 1-year follow-up study revealed that the geometric mean of airborne Mn level in a smelting working environment had been significantly reduced nearly to the MAC level for about 6 months; however, the PI values among these exposed workers did not decline during the follow-up study. Notably, the workers in this study were not removed from their jobs and their blood and RBC concentrations of Mn were also unchanged. Thus, the possibility for sporadic exposure to low levels of Mn among these smelting workers cannot be excluded.
It should be noted also that none of the workers in the current study displayed any clinical signs and symptoms of Mn intoxication typically seen among manganism patients, although they did have the elevated PI values. The enhanced T1-weighted MRI signals have been observed in patients receiving total parenteral nutrition, presumably owing to excessive Mn intake (Mirowitz et al., 1991; Mirowitz and Westrich, 1992), and in patients with liver failure because of their inability to eliminate Mn (Krieger et al., 1995). Appreciably, these patients usually do not have permanent neurological damage. Taken together, these findings support the view that T1-weighted image among active workers is a reasonable indicator for Mn exposure before signs and symptoms appear in clinics, but not necessarily for severe cases of manganism.
Determination of Mn concentrations in the whole blood, plasma, or serum has been used in our previous clinical and experimental animal studies (Li et al., 2004; Lu et al., 2005; Zheng et al., 1999). Since Mn is primarily intracellularly distributed (Crossgrove and Zheng, 2004), it was reasonable to hypothesize that Mn in the blood compartment may accumulate in the blood cells, and a direct assay of Mn in the RBC fraction may serve as a better indicator for Mn concentrations in the blood circulation. Our current results revealed a slight increase in MnRBC in Mn exposed workers as compared to controls, although this difference did not reach any statistical sig-nificance due to the small sample size. However, when the brain MRI signal was plotted against MnRBC among smelting workers, the PI value was significantly associated with MnRBC, suggesting the existence of a relationship between Mn in brain tissue and Mn in RBC. Thus, further studies to explore the RBC as a useful matrix for monitoring Mn exposure are deemed necessary.
Upon entering the body, Mn may interfere with the metabolism of trace elements and alter their homeostasis, particularly those of Fe and Cu (Li et al., 2004; Nikolova, 1993; Zhang et al., 2001). The results from the current study did not reveal any substantial changes in four essential elements examined, i.e., Fe, Zn, Ca, and Mg. However, the Cu level in the whole blood of Mn-exposed group was significantly higher than that of the control group. It remains unclear whether this is secondary to Mn exposure or simply due to co-exposure to airborne Cu in the smelting environment. It is also unclear what is the clinical significance of elevated Cu level among smelting workers. Nonetheless, the subject on Cu homeostasis among smelting workers should be of interest for future investigation.
In summary, the present study demonstrates that the smelting workers with Mn exposure but without clinical symptoms of Mn intoxication display intensified MRI signals in the globus pallidus region. The increased PI values appear to be associated with the degree of environmental Mn exposure. Moreover, the PI values among Mn-exposed workers is correlates with Mn concentrations in red blood cells. Future research should be directed toward understanding the uses of MRI and MnRBC for early diagnosis of Mn exposure.
Acknowledgments
This study was partly supported by National Science Foundation of China Grant #30070663 (YMJ), Guangxi Science and Technology Commission Grant #0443004-42 (YMJ), U.S. NIH/National Institute of Environmental Health Sciences Grant #ES-08164 (WZ), and U.S. Department of Defense Contract #USAMRMC W81XWH-05-1-0239 (WZ).
References
- Alves G, Thiebot J, Tracqui A, Delangre T, Guedon C, Lerebours E. Neurologic disorders due to brain manganese deposition in a jaundiced patient receiving long-term parenteral nutrition. J Parenter Enteral Nutr. 1997;21:41–5. doi: 10.1177/014860719702100141. [DOI] [PubMed] [Google Scholar]
- Andrews NC. The iron transporter DMT1. Int J Biochem Cell Biol. 1999;31:991–4. doi: 10.1016/s1357-2725(99)00065-5. [DOI] [PubMed] [Google Scholar]
- Arona A, Mata M, Bonet M. Diagnosis of chronic manganese intoxication by magnetic resonance imaging. New Engl J Med. 1997;336:964–5. doi: 10.1056/nejm199703273361319. [DOI] [PubMed] [Google Scholar]
- Aschner M, Vrana KE, Zheng W. Manganese uptake and distribution in the central nervous system (CNS) Neurotoxicology. 1999;20:173–80. [PubMed] [Google Scholar]
- Butterworth RF, Spahr L, Fontaine S, Layrargues GP. Manganese toxicity, dopaminergic dysfunction and hepatic encephalopathy. Metab Brain Dis. 1995;10:259–67. doi: 10.1007/BF02109357. [DOI] [PubMed] [Google Scholar]
- Chetri K, Choudhuri G. Role of trace elements in hepatic encephalopathy: zinc and manganese. Indian J Gastroenterol. 2003;22(Suppl 2):S28–30. [PubMed] [Google Scholar]
- Crossgrove J, Zheng W. Manganese toxicity upon overexposure. NMR Biomed. 2004;17:544–53. doi: 10.1002/nbm.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz MC, Ihrig A, Wrazidlo W, Bader M, Jansen O, Triebig G. Results of magnetic resonance imaging in long-term manganese dioxide-exposed workers. Environ Res. 2001;85:37–40. doi: 10.1006/enrs.2000.4068. [DOI] [PubMed] [Google Scholar]
- Discalzi G, Pira E, Herrero Hernandez E, Valentini C, Turbiglio N, Meliga F. Occupational Mn parkinsonism: magnetic resonance imaging and clinical patterns following CaNa2EDTA chelation. Neurotoxicology. 2000;21:863–6. [PubMed] [Google Scholar]
- Ejima A, Imamura T, Nakamura S, Saito H, Matsumoto K, Momono S. Manganese intoxication during total parenteral nutrition. Lancet. 1992;339:426. doi: 10.1016/0140-6736(92)90109-g. [DOI] [PubMed] [Google Scholar]
- Eriksson H, Tedroff J, Thuomas KA, Aquilonius SM, Hartvig P, Fasth KJ, et al. Manganese induced brain lesions in Macaca fascicularis as revealed by positron emission tomography and magnetic resonance imaging. Arch Toxicol. 1992;66:403–7. doi: 10.1007/BF02035130. [DOI] [PubMed] [Google Scholar]
- Fell JM, Reynolds AP, Meadows N, Khan K, Long SG, Quaghebeur G, et al. Manganese toxicity in children receiving long-term parenteral nutrition. Lancet. 1996;347:1218–21. doi: 10.1016/s0140-6736(96)90735-7. [DOI] [PubMed] [Google Scholar]
- Fredstrom S, Rogosheske J, Gupta P, Burns LJ. Extrapyramidal symptoms in a BMT recipient with hyperintense basal ganglia and elevated manganese. Bone Marrow Transplant. 1995;15:989–92. [PubMed] [Google Scholar]
- Hazell AS, Butterworth RF. Hepatic encephalopathy: an update of pathophysiologic mechanisms. Proc Soc Exp Biol Med. 1999;222:99–112. doi: 10.1046/j.1525-1373.1999.d01-120.x. [DOI] [PubMed] [Google Scholar]
- Hauser RA, Zesiewicz TA, Rosemurgy AS, Martinez C, Olanow CW. Manganese intoxication and chronic liver failure. Ann Neurol. 1994;36:871–5. doi: 10.1002/ana.410360611. [DOI] [PubMed] [Google Scholar]
- Huang CC, Chu NS, Lu CS, Chen RS, Calne DB. Long-term progression in chronic manganism. Neurology. 1998;50:698–700. doi: 10.1212/wnl.50.3.698. [DOI] [PubMed] [Google Scholar]
- Husain M, Khanna VK, Roy A, Tandon R, Pradeep S, Set PK. Platelet dopamine receptors and oxidative stress parameters as markers of manganese toxicity. Hum Exp Toxicol. 2001;20:631–6. doi: 10.1191/096032701718890531. [DOI] [PubMed] [Google Scholar]
- Iwase K, Kondoh H, Higaki J, Tanaka Y, Yoshikawa M, Hori S, et al. Hyperintense basal ganglia on T1-weighted magnetic resonance images following postoperative parenteral nutrition in a pancreatoduodenectomized patient. Dig Surg. 2000;17:190–3. doi: 10.1159/000018830. [DOI] [PubMed] [Google Scholar]
- Jiang YM, Mo XA, Du FQ, Fu X, Zhu XY, Gao HY, et al. Effective treatment of manganese-induced occupational parkinsonism with PAS-Na: a case of 17-year follow-up study. J Occup Environ Med. 2006;48:644–9. doi: 10.1097/01.jom.0000204114.01893.3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Ahlskog JE, Klos KJ, Kumar N, Fealey RD, Trenerry MR, et al. Neurological manifestations in welders with pallidal MRI T1 hyperintensity. Neurology. 2005;64:2033–9. doi: 10.1212/01.WNL.0000167411.93483.A1. [DOI] [PubMed] [Google Scholar]
- Kim SH, Chang KH, Chi JG, Cheong HK, Kim JY, Kim YM, et al. Sequential change of MR signal intensity of the brain after manganese administration in rabbits. Correlation with manganese concentration and histopathologic findings. Invest Radiol. 1999a;34:383–93. doi: 10.1097/00004424-199906000-00001. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kim J, Ito K, Lim HS, Cheong HK, Kim JY, et al. Idiopathic parkinsonism with superimposed manganese exposure: utility of positron emission tomography. Neurotoxicology. 1999b;20:249–52. [PubMed] [Google Scholar]
- Kim Y, Kim KS, Yang JS, Shin YC, Park IJ, Kim E, et al. Increase in signal intensities on T1-weighted magnetic resonance images in asymptomatic manganese-exposed workers. Neurotoxicology. 1999c;20:901–7. [PubMed] [Google Scholar]
- Kim Y. High signal intensities on T1-weighted MRI as a biomarker of exposed to manganese. Ind Health. 2004;42:111–5. doi: 10.2486/indhealth.42.111. [DOI] [PubMed] [Google Scholar]
- Kim E, Kim Y, Cheong HK, Cho S, Shin YC, Sakong J, et al. Pallidal index on MRI as a target organ dose of manganese: structural equation model analysis. Neurotoxicology. 2005a;26:351–9. doi: 10.1016/j.neuro.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Kim Y, Park JK, Choi YH, Yoo CY, Lee CR, Lee H, et al. Blood manganese concentration is elevated in iron deficiency anemia patients, whereas globus pallidus signal intensity is minimally affected. Neurotoxicology. 2005b;26:107–11. doi: 10.1016/j.neuro.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Komaki H, Maisawa S, Sugai K, Kobayashi Y, Hashimoto T. Tremor and seizures associated with chronic manganese intoxication. Brain Dev. 1999;21:122–4. doi: 10.1016/s0387-7604(98)00074-6. [DOI] [PubMed] [Google Scholar]
- Krieger D, Krieger S, Jansen O, Gass P, Theilmann L, Lichtnecker H. Manganese and chronic hepatic encephalopathy. Lancet. 1995;346(8970):270–4. doi: 10.1016/s0140-6736(95)92164-8. [DOI] [PubMed] [Google Scholar]
- Li GJ, Zhang L, Lu L, Wu P, Zheng W. Occupational exposure to welding fume among welders: alterations of manganese, iron, zinc, copper, and lead in body fluids and the oxidative stress status. J Occup Environ Med. 2004;46:241–8. doi: 10.1097/01.jom.0000116900.49159.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Zhang LL, Li GJ, Guo W, Liang W, Zheng W. Serum concentrations of manganese and iron as the potential biomarkers for manganese exposure in welders. Neurotoxicology. 2005;26:257–65. doi: 10.1016/j.neuro.2004.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchini R, Apostoli P, Perrone C, Placidi D, Albini E, Migliorati P, et al. Long-term exposure to “low levels” of manganese oxides and neurofunctional changes in ferroalloy workers. Neurotoxicology. 1999;20:287–97. [PubMed] [Google Scholar]
- Lucchini R, Albini E, Placidi D, Gasparotti R, Pigozzi MG, Montani G, et al. Brain magnetic resonance imaging and manganese exposure. Neurotoxicology. 2000;21:769–75. [PubMed] [Google Scholar]
- McKinney AM, Filice RW, Teksam M, Casey S, Truwit C, Clark HB, et al. Diffusion abnormalities of the globi pallidi in manganese neurotoxicity. Neuroradiology. 2004;46:291–5. doi: 10.1007/s00234-004-1179-1. [DOI] [PubMed] [Google Scholar]
- Mirowitz SA, Westrich TJ. Basal ganglial signal intensity alterations: reversal after discontinuation of parenteral manganese administration. Radiology. 1992;185:535–6. doi: 10.1148/radiology.185.2.1410368. [DOI] [PubMed] [Google Scholar]
- Mirowitz SA, Westrich TJ, Hirsch JD. Hyperintense basal ganglia on T1-weighted MR images in patients receiving parenteral nutrition. Radiology. 1991;181:117–20. doi: 10.1148/radiology.181.1.1909445. [DOI] [PubMed] [Google Scholar]
- Misselwitz B, Mühler A, Weinmann HJ. A toxicologic risk for using manganese complexes? A literature survey of existing data through several medical specialties. Invest Radiol. 1995;30:611–20. doi: 10.1097/00004424-199510000-00007. [DOI] [PubMed] [Google Scholar]
- Nagatomo S, Umehara F, Hanada K, Nobuhara Y, Takenaga S, Arimura K, et al. Manganese intoxication during total parenteral nutrition: report of two cases and review of the literature. J Neurol Sci. 1999;162:102–5. doi: 10.1016/s0022-510x(98)00289-5. [DOI] [PubMed] [Google Scholar]
- Nelson K, Golnick J, Korn T, Angle C. Manganese encephalopathy: utility of early magnetic resonance imaging. Br J Ind Med. 1993;50:510–3. doi: 10.1136/oem.50.6.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newland MC, Weiss B. Persistent effects of manganese on effortful responding and their relationship to manganese accumulation in the primate globus pallidus. Toxicol Appl Pharmacol. 1992;113:87–97. doi: 10.1016/0041-008x(92)90012-h. [DOI] [PubMed] [Google Scholar]
- Newland MC, Cox C, Hamada R, Oberdorster G, Weiss B. The clearance of manganese chloride in the primate. Fundam Appl Toxicol. 1987;9:314–28. doi: 10.1016/0272-0590(87)90054-6. [DOI] [PubMed] [Google Scholar]
- Newland MC, Ceckler TL, Kordower JH, Weiss B. Visualizing manganese in primate basal ganglia with magnetic resonance imaging. Exp Neurol. 1989a;106:251–8. doi: 10.1016/0014-4886(89)90157-x. [DOI] [PubMed] [Google Scholar]
- Newland MC, Ceckler TL, Kordower JH, Weiss B. Visualizing manganese in the primate basal ganglia with magnetic resonance imaging. Exp Neurol. 1989b;106:251–8. doi: 10.1016/0014-4886(89)90157-x. [DOI] [PubMed] [Google Scholar]
- Nikolova P. Effect of manganese on essential trace element metabolism tissue concentrations and excretion of manganese, iron, copper, cobalt and zinc. Trace Elements Med. 1993;10:141–7. [Google Scholar]
- Olanow CW, Good PF, Shinotoh H, Hewitt KA, Vingerhoets F, Snow BJ, et al. Manganese intoxication in the rhesus monkey: a clinical, imaging, pathologic, and biochemical study. Neurology. 1996;46:492–8. doi: 10.1212/wnl.46.2.492. [DOI] [PubMed] [Google Scholar]
- Ono K, Komai K, Yamada M. Myoclonic involuntary movement associated with chronic manganese poisoning. J Neurol Sci. 2002:93–6. doi: 10.1016/s0022-510x(02)00111-9. [DOI] [PubMed] [Google Scholar]
- Poola I, Mason AB, Lucas JJ. The chicken oviduct and embryonic red blood cell transferrin receptors are distinct molecules. Biochem Biophys Res Commun. 1990;171:26–32. doi: 10.1016/0006-291x(90)91351-r. [DOI] [PubMed] [Google Scholar]
- Quaghebeur G, Taylor WJ, Kingsley DP, Fell JM, Reynolds AP, Milla PJ. MRI in children receiving total parenteral nutrition. Neuroradiology. 1996;38:680–3. doi: 10.1007/s002340050333. [DOI] [PubMed] [Google Scholar]
- Shinotoh H, Snow BJ, Hewitt KA, Pate BD, Doudet D, Nugent R, et al. MRI and PET studies of manganese-intoxicated monkeys. Neurology. 1995;45:1199–204. doi: 10.1212/wnl.45.6.1199. [DOI] [PubMed] [Google Scholar]
- Spahr L, Burkhard PR, Grotzsch H, Hadengue A. Clinical significance of basal ganglia alterations at brain MRI and 1H MRS in cirrhosis and role in the pathogenesis of hepatic encephalopathy. Metab Brain Dis. 2002;17:399–413. doi: 10.1023/a:1021974321874. [DOI] [PubMed] [Google Scholar]
- Takeda A, Sawashita J, Okada S. Biological half-lives of zinc and manganese in rat brain. Brain Res. 1995;695:53–8. doi: 10.1016/0006-8993(95)00916-e. [DOI] [PubMed] [Google Scholar]
- Weiss G, Houston T, Kastner S, Johrer K, Grunewald K, Brock JH. Regulation of cellular iron metabolism by erythropoietin: activation of iron-regulatory protein and upregulation of transferrin receptor expression in erythroid cells. Blood. 1997;89:680–7. [PubMed] [Google Scholar]
- Zhang LL, Wu P, Lu L, Li GJ, Guo WR, Zheng W, et al. Alteration of 5 essential trace elements in serum of welder exposed to manganese. China Public Health. 2001;17:783–4. [Google Scholar]
- Zheng W, Zhao Q, Slavkovich V, Aschner M, Graziano H. Alteration of iron homeostasis following chronic exposure to manganese in rats. Brain Res. 1999;833:125–32. doi: 10.1016/s0006-8993(99)01558-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Kim H, Zhao Q. Comparative toxicokinetics of manganese chloride and methylcyclo-pentadienyl Mn tricarbonyl in male Sprague–Dawley rats. Toxicol Sci. 2000;54:295–301. doi: 10.1093/toxsci/54.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]