Abstract
Acute kidney injury is a major kidney disease associated with poor clinical outcomes. The pathogenesis of acute kidney injury is multifactorial and is characterized by tubular cell injury and death. Recent studies have demonstrated autophagy induction in proximal tubular cells during acute kidney injury. The regulatory mechanisms of tubular cell autophagy are poorly understood; however, some recent findings have set up a foundation for further investigation. Although autophagy may promote cell death under certain experimental conditions, pharmacological and autophagy-related gene knockout studies have established a renoprotective role for autophagy in acute kidney injury. The mechanisms by which autophagy protects cells from injury and how, possibly, its pro-survival role switches to pro-death under certain conditions are discussed. Further research is expected to help us understand the regulatory network of tubular cell autophagy, define its precise roles in specific context of acute kidney injury, and identify autophagy-targeting strategies for the prevention and treatment of acute kidney injury.
Keywords: acute kidney injury, autophagy, cell survival, cell death
Introduction
Acute kidney injury (AKI), mostly caused by renal ischemia-reperfusion, sepsis, and nephrotoxins, is characterized by a rapid loss of kidney function that leads to declined glomerular filtration, accumulation of nitrogenous wastes, and imbalance of water, electrolyte and acid-base. Despite advances in basic research and medical care during the past several decades, AKI is still a common and serious clinical problem in hospitalized patients and is associated with increasing incidence, high mortality, and few preventive and therapeutic options1. Moreover, AKI is an important risk factor for the development of chronic kidney disease and the eventual progression to end-stage renal disease, which further imposes enormous medical and economic burdens on the society2, 3.
The pathogenesis of AKI is very complex and multifactorial, with emerging tubular, microvascular and inflammatory mechanisms that interplay with and amplify each other. Tubular cell injury and death is the key pathological feature in AKI, which results in the generation of inflammatory and vasoactive mediators. These chemical mediators induce vasoconstriction and tissue inflammation, further exacerbating tubular damage and renal dysfunction4–6. In experimental models of AKI, the proximal tubules, especially the S3 segment located within the outer medulla of the kidney, suffer the most severe injury7, 8.
Autophagy is a “self-eating” response to stress and plays important roles in the pathogenesis of a variety of diseases9. The research in the last few years has demonstrated the induction of autophagy in proximal tubular cells and kidneys during AKI. This review summarizes our current insights on the role of autophagy and its regulation in experimental models of AKI. The therapeutic potential of targeting autophagy for AKI prevention and treatment is also discussed.
Autophagy induction in proximal tubular cells during AKI
In a rat model of renal ischemia-reperfusion, Lai and colleagues showed an increased expression of autophagy-related (ATG) proteins (BECLIN-1 and LC3) in renal tubules 10, 11. Suzuki et al. further showed increased numbers of LC3- and LAMP2-positive vacuoles in a human kidney proximal tubular cell line (HK-2) following hypoxia incubation, and in mouse kidneys during ischemia-reperfusion. Under these conditions, LC3-positive vacuoles colocalize with LAMP2-positive vacuoles, suggesting the fusion of autophagosomes with lysosomes for degradation12. The formation of autophagic vacuoles was further shown in tubular cells in transplanted human kidneys by electron microscopy (EM)12. Autophagy is also induced by hypoxia in a rat proximal tubular cell line (RPTC), as indicated by the formation of fluorescent puncta in GFP-LC3-transfected cells and the accumulation of LC3-II, a lipidated form of LC3 that localizes on autophagosomes. Importantly, induction of autophagy is an early response to hypoxic stress, prior to tubular cell apoptosis13. In anoxia-reoxygenation, an in vitro model of ischemia-reperfusion, anoxia alone induces LC3-II accumulation but not formation of GFP-LC3 puncta. Nonetheless, autophagy is induced undoubtedly during reoxygenation as indicated by both GFP-LC3 punctuated cells and LC3-II accumulation13. These observations are consistent with an autophagic response during in vitro ischemia-reperfusion of a cardiac cell line14. While the evidence of autophagy from these in vitro and in vivo studies is circumstantial, autophagy was recently demonstrated clearly during renal ischemia-reperfusion in C57BL/6 mice by biochemical and morphological analyses. Although not obvious during the ischemic period, LC3-II accumulates in renal tissues following reperfusion (Figure 1A). The appearance of autophagic vacuoles in proximal tubular cells is further revealed by EM (Figure 1B)13. Consistently, later studies using GFP-LC3 transgenic mice showed an increased formation of GFP-LC3 dots in proximal tubules during renal ischemia-reperfusion15, 16. It is noteworthy that autophagic flux was also determined by comparisons of LC3-II level and autophagosome number in the presence and absence of lysosomal inhibitors. Lysosomal inhibitors increase LC3-II accumulation in RPTC cells during hypoxia or anoxia incubation, and in kidney tissues during renal ischemia-reperfusion13. Similarly, Suzuki et al. showed that the number of LC3-positive vacuoles is significantly increased by lysosomal inhibitors during hypoxic treatment of HK-2 cells12. Together, these studies indicate that autophagy is indeed induced in tubular cells during renal ischemia-reperfusion.
Figure 1. Autophagy in acute kidney injury.
(A) Immunoblot analysis of LC3-II accumulation in kidney tissues after 30 minutes of bilateral renal ischemia, followed by 0–48 hours of reperfusion. The figure is adopted from Jiang et al.13, with permission from Elsevier. (B) Formation of autophagosomes and autolysosomes in tubular cells during renal ischemia-reperfusion. Kidney tissues were fixed for EM examination of autophagosomes (left panel, arrows: double or multiple membrane structures containing cytoplasm; middle panel, arrow: undigested organelles such as mitochondria) and autolysosomes (right panel, arrowheads: single membrane structures with remnants of cytoplasmic components). This figure is adopted from Jiang et al.13, with permission from Elsevier. (C) Cisplatin-induced autophagy in proximal tubular cells in kidney tissues. GFP-LC3 transgenic mice were treated with 20mg/kg cisplatin for 24 hours to harvest kidney tissues for immunostaining of aquaporin 1 (AQP1; marker of proximal tubules). Co-localization of GFP-LC3 dots with AQP1 suggests autophagy induction in proximal tubular cells. This figure is adopted from Inoue et al.19, with permission from Springer.
In nephrotoxic models of AKI, cisplatin was shown to induce autophagy in different renal proximal tubular cell lines including RPTC (rat)17, LLC-PK1 (porcine)18, and NRK-52E (rat)19. In all three cell lines, cisplatin induces autophagy in a treatment time-dependent manner, as shown by autophagosome formation and LC3-II accumulation. In addition, EM of cisplatin-treated RPTC cells identifies various characteristic structures that may represent the maturation of autophagic vesicle from phagophore through autolysosome17. Notably, autophagy is activated by cisplatin within hours, earlier than apoptosis17–19. Using GFP-LC3 transgenic mice, Inoue et al. further monitored autophagy in kidney tissues during cisplatin nephrotoxicity, unveiling autophagy mainly in proximal tubules (Figure 1C)19. These observations are consistent with an examination by EM, which demonstrated a time-dependent increase of autophagic vacuoles in proximal tubular cells following cisplatin treatment of C57BL/6 mice17. Autophagy is also induced in proximal tubular cells during cyclosporine nephrotoxicity and aristolochic acid nephropathy20, 21. In addition, some environmental toxins, such as cadmium and arsenic, were also shown to induce autophagy in proximal tubular cells and kidneys22, 23.
In addition to ischemic and nephrotoxic AKI, autophagy is induced in other settings of AKI as well. In a rat model that simulates human peritonitis by cecal ligation and puncture, autophagy increases transiently in kidneys at the early stage of sepsis, as indicated by LC3-positive aggregates primarily in proximal tubules and LC3-II accumulation. Interestingly, autophagy goes back to the basal level at the late stage, accompanied by renal dysfunction and tubular injury24. Autophagy induction during septic AKI is also shown in the kidneys of rats treated with lipopolysaccharide25. Moreover, LC3-II accumulates in kidney tissues following glycerol injection to rats, suggesting autophagy induction in the model of myoglobinuric AKI26.
Regulation of tubular cell autophagy during AKI
The regulation of autophagy has been extensively studied in the past few years, and tremendous progress has recently been made in understanding the molecular mechanism and signaling pathways of autophagy from yeast to mammals27–30. The ATG proteins constitute the core molecular machinery of autophagy and function at several successive steps of the autophagy cascade to orchestrate the process. Upstream of the core machinery, autophagy is regulated by a complex signaling network of multiple stimulatory and inhibitory inputs. Diverse signaling pathways regulate autophagy in response to various stresses. Moreover, these signaling pathways may interact with each other to determine the specificity and magnitude of autophagy. Despite these significant advances, regulation mechanisms that contribute to tubular cell autophagy in AKI remain unclear. Several recent studies have just started the journey of exploration.
Oxidative stress
Bolisetty et al. examined the regulation of autophagy by heme oxygenase 1 (HMOX1) during cisplatin-induced AKI. They found that cisplatin induces HMOX1, oxidative stress and autophagy in cultured renal tubular cells and mouse kidneys. In Hmox1-null models, oxidative stress is elevated, resulting in an increased sensitivity to autophagy and tubular cell death. Restoring HMOX1 in tubular cells reverses the autophagic response. Furthermore, overexpression of HMOX1 reduces oxidative stress, delays autophagy induction and protects against cisplatin-induced cell death31. These results suggest that oxidative stress may lead to autophagy in renal tubular cells. Lately, Parajuli et al. generated a mouse model with manganese superoxide dismutase (Mnsod) specifically deleted from distal nephrons. As a result of Mnsod knockout, mitochondrial oxidant production is increased, leading to autophagy activation32. Moreover, N-acetyl-L-cysteine, an antioxidant, inhibits both autophagosome formation and LC3-II accumulation in RPTC cells subjected to anoxia-reoxygenation, further suggesting the regulation of tubular cell autophagy by oxidative stress during AKI (Livingston M and Dong Z, unpublished data).
ER stress
In mammals, endoplasmic reticulum (ER) stress through unfolded protein response (UPR) and intracellular calcium has been implicated in autophagy regulation33. In cyclosporine-treated human renal tubular cells, ER stress is induced along with autophagy activation. Salubrinal, an ER stress inhibitor, suppresses cyclosporine-induced autophagy, suggesting the involvement of ER stress in tubular cell autophagy during cyclosporine nephrotoxicity20. A recent study examined ER stress response in cisplatin-treated NRK-52E cells and found that cisplatin at different doses elicits different UPR. 10μM cisplatin induces autophagy and upregulates two ER chaperones, glucose-regulated proteins 78 (GRP78) and 94 (GRP94). The expression of GRP78 and GRP94 is significantly reduced by 50μM cisplatin, leading to autophagy inhibition and apoptosis induction. Under this condition, pre-conditioning with taurine, an antioxidant, restores the expression level of both ER chaperones and switches the cellular response from apoptosis to autophagy34. The signaling pathways by which ER stress induces tubular cell autophagy were then studied in the models of tunicamycin-induced kidney injury. Gozuacik et al. showed that death-associated protein kinase (DAPK) is activated by ER stress through protein phosphatase 2A dephosphorylation. Importantly, ER stress-induced autophagy is inhibited in Dapk-null cells, suggesting that DAPK may be a positive mediator of autophagy35. Using inhibitors of the mitogen-activated protein kinases (MAPKs), Kawakami et al. demonstrated that activation of extracellular signal-regulated kinase (ERK), but not c-Jun N-terminal kinase (JNK) or p38, is necessary for the induction of tubular cell autophagy by ER stress36. Further investigations should test how DAPK and ERK regulate autophagy in tubular cells. Given that ER stress is activated by a variety of insults that cause AKI37, it would also be important to examine if ER stress is a common modulator of tubular cell autophagy.
HIF1α-BNIP3
Regulation of autophagy by hypoxia-inducible factor 1 (HIF1) and its transcriptional target BCL-2 nineteen-kilodalton interacting protein 3 (BNIP3) was first demonstrated in the Hif1α knockout MEF cells exposed to hypoxia38. The involvement of HIF1α-BNIP3 in tubular cell autophagy during AKI was then studied. Preliminary work showed that HIF1α is induced in RPTC cells within hours of hypoxia, paralleled with autophagy induction. BNIP3 is also induced in a HIF1α-dependent manner. Importantly, hypoxia-induced autophagy in RPTC cells is abolished by genetic knockdown of HIF1α (Livingston M and Dong Z, unpublished data). These observations were further extended by a recent study using a different proximal tubular cell line. BNIP3 is induced in rat proximal tubules after renal ischemia-reperfusion and in NRK-52E cells in response to hypoxia or cobalt chloride, a chemical inducer of HIF1. While overexpression of BNIP3 in NRK-52E cells induces formation of autophagosomes that mainly colocalize with mitochondria, knockdown of BNIP3 significantly suppresses hypoxia-induced autophagy and mitophagy39. These results suggest that HIF1α-BNIP3 may contribute to the regulation of autophagy in renal tubular cells.
p53
In cisplatin-treated renal tubular cells, autophagy is partially suppressed by chemical inhibition of p53, suggesting that p53 may positively regulate autophagy in this experimental condition17. The pro-autophagy role of p53 depends on its nuclear localization and is associated with an increasing number of transcriptional targets40. It has been shown that p53 transactivates 5′-AMP activated protein kinase (AMPK) to inhibit mammalian target of rapamycin complex 1(MTORC1), resulting in autophagy41. Alternatively, p53 can induce autophagy by activating damage-regulated autophagy modulator (DRAM), a lysosomal membrane protein that stimulates autophagosome-lysosome fusion42. In response to DNA damage, p53 upregulates ULK1 for sustained autophagy and subsequent cell death43. Novel p53 transcriptional targets that can activate autophagy are being discovered, including BNIP3 and Sestrin244, 45. A latest study has further identified a plethora of p53-bound genes that encode proteins involved in almost all steps of the autophagy pathway46. While promoting autophagy in these studies, p53 may induce TP53-induced glycolysis and apoptosis regulator (TIGAR) to suppress oxidative stress and inhibit autophagy via an mTOR-independent pathway47. Whether these mechanisms are responsible for autophagy regulation by p53 in tubular cells during AKI remains to be determined.
BCL-2 family proteins
Inhibitory effects of BCL-2 or BCL-2L1/Bcl-xl on tubular cell autophagy during AKI have been suggested by several studies. In rats, intrarenal arterial delivery of adenoviral Bcl-xl gene reduces autophagy in tubular cells following ischemia-reperfusion10. Suppression of autophagy by BCL-2 was also shown in Bcl-2/GFP-LC3 double transgenic mice under this condition48. Moreover, cisplatin-induced autophagy is almost completely blocked in a BCL-2 stable expression tubular cell line17. A well-recognized mechanism by which BCL-2 downregulates autophagy is that BCL-2 binds Beclin-1 through a BCL-2 homology 3 (BH3) domain to prevent Beclin-1 from assembling the class III PtdIns3K complex that is indispensable for autophagosome formation49, 50. Notably, only ER-targeted BCL-2 inhibits autophagy49. However, in BCL-2 overexpressing renal tubular cells BCL-2- Beclin-1 interaction is not detected by co-immunoprecipitation assay17. Recently, Chang et al. showed that nutrient-deprivation autophagy factor 1 (NAF1) binds BCL-2 at the ER and is required for the interaction of BCL-2 with Beclin-1, and BCL-2 inhibition of autophagy51. In addition to the association with Beclin-1, ER-localized BCL-2 may also suppress autophagy by diminishing stress-induced Ca2+ release and consequent activation of Ca2+/calmodulin-dependent protein kinase-AMPK signaling pathway52. These interesting possibilities need to be investigated in experimental models of AKI.
ULK1 and its regulation
ULK1 is a mammalian homolog of yeast Atg1, which functions as an initiating kinase in the autophagy core machinery53. ULK1 is activated in tubular cells during severe hypoxia or anoxia and following reoxygenation, and in kidney tissues during renal ischemia-reperfusion. Importantly, tubular cell autophagy induced under these conditions is significantly suppressed by ULK1 knockdown, suggesting a role for ULK1 on autophagy regulation in ischemic AKI. Mechanistically, ULK1 activation is coordinately mediated by multiple upstream signals including mTORC1, AMPK and JNK (Livingston M and Dong Z. unpublished data). Despite these findings, the mechanisms by which ULK1 regulates tubular cell autophagy during AKI remain unknown. Under starvation conditions, ULK1 initiates autophagy through the recruitment of other ATG proteins to the pre-autophagosomal structure (PAS) and phosphorylation of downstream substrates53. The search for ULK1 interacting proteins and substrates has gained impressive progress. ULK1 can phosphorylate both AMBRA1 and Beclin-1 to activate the class III PtdIns3K complex and thereby induce autophagy54, 55. Gammoh et al. further identified an interaction between FIP200 and ATG16L1, essential components of the ULK1 and ATG5 complexes, respectively56. ULK1 also participates in the feedback regulation of mTORC1 and AMPK to fine-tune autophagy57. These findings will certainly inspire research into the regulation mechanisms of ULK1 and its role in tubular cell autophagy during AKI.
Pathological role of tubular cell autophagy in AKI: pro-survival or pro-death?
Clearly, the observations that autophagy occurs prior to apoptosis in renal tubular cells during AKI suggest that autophagy is an early response of the cells to stress and not a result of apoptosis. However, what role autophagy plays under this condition is still controversial.
In cisplatin-treated RPTC cells, inhibition of autophagy by pharmacological inhibitors (3-methyladenine or bafilomycin A1) or genetic knockdown of Beclin-1 or ATG5 increases apoptosis, suggesting a protective role for autophagy in cisplatin-induced tubular cell injury17. Similarly, Yang et al. demonstrated that cisplatin-induced autophagy in LLC-PK1 cells acts as a prosurvival mechanism against cell apoptosis18. Moreover, Rovetta et al. showed two “sensitivity-thresholds” to cisplatin in NRK-52E cells. Cisplatin at 10μM activates autophagy to preserve cell viability and prevent cell death. In contrast, autophagy is blocked in the cells treated with 50μM cisplatin and as a result, apoptosis is induced. These results also suggest autophagy plays a protective role34. Later in vivo work further confirmed and extended the in vitro findings. In a mouse model of cisplatin nephrotoxicity, pharmacological blockade of autophagic flux by chloroquine significantly enhances cisplatin-induced kidney injury whereas activation of autophagy by rapamycin protects proximal tubules from injury58. A cytoprotective role of autophagy was also shown in primary culture of human proximal tubular cells during cyclosporine nephrotoxicity20. Zeng et al. further suggested that autophagy induced in NRK-52E cells via ERK pathway is a protective mechanism for cell survival during aristolochic acid nephropathy21. Using both in vitro and in vivo experimental models, a recent study examined the role of autophagy in renal ischemia-reperfusion. In vitro, pharmacological or genetic suppression of autophagy sensitizes tubular cells to apoptosis induced by hypoxia incubation or anoxia-reoxygenation. Inhibition of autophagy in vivo by chloroquine or 3-methyladenine worsens ischemia-reperfusion renal injury, as indicated by renal function, histology, and tubular apoptosis. Together, these results suggest that autophagy is a renoprotective mechanism for cell survival in ischemic AKI13. A latest study demonstrated that autophagy may play a major role in mediating the renoprotective effects of caloric restriction preconditioning against renal ischemia-reperfusion injury in rats, suggesting a connection between these two renoprotective mechanisms59. The renoprotective role of autophagy was also shown in experimental models of septic AKI. In rats subjected to cecal ligation and puncture operation, the decline of an early increased autophagic response is associated with the development of kidney injury at the late stage of sepsis. Knockdown of ATG7 exaggerates, whereas preincubation of rapamycin diminishes tumor necrosis factor α-induced cell death in NRK-52E cells24.
In contrast, there are several studies suggesting that tubular cell autophagy may contribute to cell death during AKI. In a rat model of renal ischemia-reperfusion, Lai and colleagues showed increased Beclin-1 and LC3 expression as well as apoptosis in injured renal tubules. Both autophagy and apoptosis are suppressed by BCL-2 overexpression or ischemic preconditioning, accompanied by the amelioration of kidney injury10, 11. Similarly, Suzuki et al. found that autophagy occurs in renal tubules with disrupted morphology in GFP-LC3 transgenic mice after renal ischemia-reperfusion. While autophagy is reduced in BCL-2/GFP-LC3 double transgenic mice, tubular damage is also attenuated. Along with the in vitro observation that autophagy inhibitors protect HK2 cells from H2O2-induced cell death, it was concluded that autophagy might be detrimental during renal ischemia-reperfusion12. In tunicamycin-treated mice, Gozuacik et al. showed that ER stress induces apoptosis and autophagy concomitantly in the same damaged tubular cells. Interestingly, inhibition of autophagy by itself does not change cell death or survival; however, in combination with caspase blockade, autophagy inhibition increases cell viability. Furthermore, Dapk knockout mice are resistant to ER stress-induced kidney injury as tubular cell autophagy is suppressed in those mice. Based on these results, it was suggested that autophagy may serve as a second cell killing mechanism that acts in concert with apoptosis to trigger kidney injury during ER stress35. A cell death-promoting role of autophagy was also suggested by Inoue et al. showing that pharmacological or genetic inhibition of autophagy suppresses cisplatin-induced caspase activation and apoptosis in NRK-52E cells19.
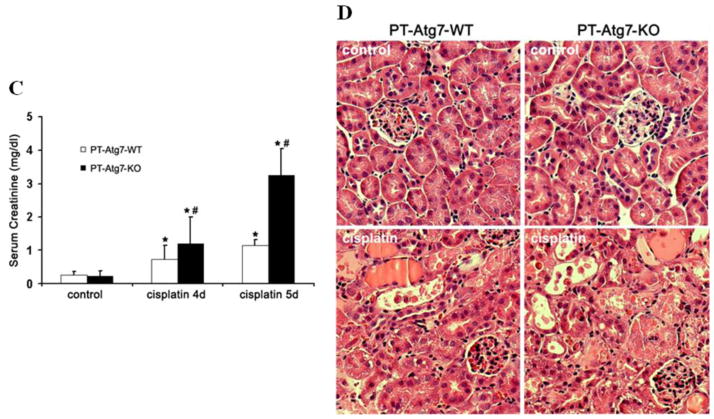
The cause of the obvious discrepancy among the aforementioned studies is unclear, although it is generally believed that, depending on experimental conditions, autophagy can be either protective or detrimental. It is important to note that the occurrence of autophagy and apoptosis in the same cells or cell population does not indicate that autophagy contributes to apoptosis in these cells; rather it suggests the presence of cellular stress leading to both apoptosis and autophagy. In the same line of thinking, simultaneous inhibition of apoptosis and autophagy (e.g. by BCL-2 or preconditioning10, 11) does not necessarily imply a role of autophagy in apoptosis. In addition, the non-specific effects of pharmacological inhibitors of autophagy have to be considered. In this regard, in vivo tests using kidney tissue-specific Atg gene knockout models have obvious advantages. To this end, Kimura et al. and Liu et al. established tubule-specific Atg5 knockout mouse models, which show the accumulation of deformed mitochondria, aberrant concentric membranous structures, and cytoplasmic inclusions including SQSTM1/P62- and ubiquitin-positive protein aggregates in renal tubules, leading to cellular degeneration. During renal ischemia-reperfusion, tubular cell autophagy is inhibited in the Atg5 conditional knockout mice and, importantly, more severe kidney injury is induced in these mice compared with wild type animals15, 16. Moreover, a mouse model of Atg7 knockout in proximal tubules (PT-Atg7 KO) was established recently. Knockout of Atg7 leads to impairment of the autophagy-conjugation systems, resulting in inhibition of autophagy and accumulation of autophagy-selective substrates such as SQSTM1/P62 during cisplatin treatment (Figure 2A, 2B). Compared with their wild-type littermates, PT-Atg7 KO mice show accelerated loss of renal function and aggravated kidney tissue damage and tubular apoptosis (Figure 2C, 2D). Primary proximal tubular cells isolated from PT-Atg7 KO mice are more sensitive to cisplatin-induced caspase activation and apoptosis than the cells from wild-type mice. PT-Atg7 KO mice are also more sensitive to renal ischemia-reperfusion injury than their wild-type littermates58. Further, Takahashi et al. demonstrated that Atg5 knockout in proximal tubules results in a more severe cisplatin-induced kidney injury, accompanied by accelerated DNA damage and P53 activation60. Together, these conditional knockout studies have demonstrated definitive evidence for a renoprotective role of tubular cell autophagy during AKI.
Figure 2. Autophagy inhibition in PT-Atg7 KO mice worsens cisplatin-induced acute kidney injury.
Wild-type and PT-Atg7 KO mice were injected with 25mg/kg cisplatin or saline as control. (A) Kidneys were harvested to collect cortical tissues for immunoblot analysis of Atg7, LC3, Atg5 (Atg12 conjugated), p62, and β-actin. (B) Kidneys of cisplatin-treated mice were collected for immunofluorescence staining of LC3, fluorescein isothiocyanate (FITC)-labeled phaseolus vulgaris agglutinin (PHA; marker of proximal tubules), and Hoechst33342. Arrows indicate formation of LC3 dots and inset shows LC3 dots at high magnification. (C) Blood samples were collected for measurement of serum creatinine. Data are expressed as mean ± SD. * P < 0.05, significantly different from the control groups; # P < 0.05, significantly different from the relevant wild-type group. (D) Histology of kidney cortex (hematoxylin-eosin staining). This figure is adopted from Jiang et al.58, with permission from Nature Publishing Group.
It is not yet understood how autophagy protects tubular cells from injury or apoptosis. Upon metabolic stress in which the availability of oxygen and nutrient is poor, this catabolic pathway can generate amino acids and lipids that can be reused for protein synthesis and ATP production, which are essential for the adaptation to bioenergetic catastrophe9. As a cellular housekeeping process, autophagy can clear misfolded proteins and damaged organelles to maintain cellular homeostasis and thereby set a higher threshold against apoptosis induction9, 61. Indeed, when autophagy is impaired in proximal tubules by Atg5 knockout, damaged mitochondria and abnormal protein aggregates accumulate in the cells. As a result, these autophagy-deficient mice are more sensitive to kidney injury than wild-type mice that have intact autophagy15, 16, 60. Similar findings are shown in a study when autophagy is inhibited by chloroquine or ATG7 deficiency58. In addition, it is plausible that the signaling activated during autophagy can interfere with or compromise cell death pathways. This possibility has been implicated in the studies showing that, on one hand, liberation of BCL-2 and FLIP from activated autophagy protein complexes may block the intrinsic and extrinsic pathways of apoptosis49, 62; on the other hand, autophagic degradation of active caspase 8 is responsible for the inhibition of apoptotic cell death63. Moreover, certain stress stimuli can activate other cytoprotective responses to cooperate with autophagy to achieve optimal cellular repair and adaptation30. Finally, data obtained from studying programmed cell death in embryo development indicate that autophagy is required for the maintenance of high ATP levels that may in turn facilitate the elimination of apoptotic cells. This function of autophagy could prevent a detrimental inflammatory response both during normal development and after exposure to pathological stimuli61. Further research is needed to gain insight into the mechanisms underlying the renoprotective effect of tubular cell autophagy in AKI.
It is generally acknowledged that autophagy is induced to serve primarily as an adaptive and defensive mechanism for cell survival, because Atg gene knockdown or knockout accelerates rather than delays cell death; however, in certain settings64, uncontrolled massive autophagy may lead to cell death. How this prosurvival attempt fails and then switches to an alternative cell death pathway is unclear. It possibly is a result of an irreversible collapse of cell viability due to non-specific destruction of large proportions of cytoplasmic contents or a result of selective degradation of cytoprotective elements61. Some molecules, such as Draper, JNK and DAPK, have been shown to direct autophagy from a survival to a death pathway, although the exact mechanisms are unknown65. Notably, many examples of Atg gene-dependent cell death occur in cells whose apoptotic machinery is compromised; however, a caveat should be considered since knockout or overexpression of a single Atg gene could have unknown indirect effects beyond autophagy66. In addition to triggering cell death on its own, autophagy may also join apoptosis to coordinately determine a cell’s fate. The functional relationship between autophagy and apoptosis is complex and generally presents as three scenarios61, 64, 67. First, the two pathways share common regulatory signals and each can regulate and modify the activity of the other. Second, autophagy acts either upstream of apoptosis to enable apoptotic signaling or during the final stage of apoptosis to participate in certain morphological changes by providing ATP. Third, autophagy and apoptosis develop in a mutually exclusive manner under certain conditions, probably as a result of distinct thresholds for each process or mutual inhibition between the two processes. The intricate interplay and the cross-regulation between autophagy and apoptosis pathways further complicate the conundrum of how autophagy contributes to the life and death decisions of a stressed cell.
Autophagy as a therapeutic target for AKI?
Pharmacological approaches to activate or inhibit autophagy are currently receiving considerable attentions for therapeutic purposes of some diseases including neurodegenerative disease, infectious disease and cancer. Various screens have identified drugs and compounds that modulate autophagy. Although many of the compounds are tool compounds and may not be suitable for clinical use, a few of them are in clinical trials for the treatment of certain cancers and Huntington’s disease68. Given the evidence that autophagy is induced in AKI and manipulation of autophagy can affect the development and severity of AKI in many experimental settings, targeting autophagy could be a novel and potential strategy for the treatment of this disorder. There are, however, challenges that must be addressed before this strategy can be considered tenable and feasible.
The first challenge is the question of what precise role autophagy plays in AKI. Based on current evidence, particularly the evidence from kidney tubule-specific Atg gene knockout animals, autophagy is most likely renoprotective during AKI. Nonetheless, whether or not upregulating autophagy is “good” is not simplistic and may depend on magnitude, timing, and probably, other factors. It would be very important to determine an optimal condition and therapeutic window in which induction of autophagy will yield protective effects. To accomplish this, monitoring autophagy, especially autophagic flux, in the correct tissue at the correct time will be a major challenge in the kidney.
Second, the drugs that activate autophagy via inhibiting mTOR include rapamycin and its analogues68. A study in rats has suggested that the mTOR pathway is involved in tubular repair after AKI and rapamycin compromises recovery from AKI by inducing apoptosis and inhibiting proliferation of tubular cells69. Therefore, specific and selective inhibitors of the autophagic machinery would be more attractive for clinical use. There have been thus far no reports of deleterious effects associated with specific autophagy upregulation in vivo. From a therapeutic view, however, constitutive autophagy induction may not be necessary; instead, intermittent upregulation of autophagy may be more effective with fewer side effects68.
Third, thus far all studies suggesting that autophagy is associated with renoprotection were conducted in healthy adult animals. Given the fact that AKI is often a serious problem in hospitalized patients with other diseases, particularly in ICU patients with critical conditions, it will be necessary to determine: (1) whether induction of autophagy can still be renoprotective in those patients; (2) whether upregulation of autophagy for AKI treatment has side effects on or interferes with the treatment of other diseases. Eventually, the question of whether autophagy represents a useful target in AKI treatment will need to be addressed by conducting clinical trials in patients.
Conclusions and Perspectives
Despite some controversies, pharmacological and genetic knockdown or knockout studies have suggested a renoprotective role of autophagy in renal tubular cells in AKI. The mechanism by which autophagy protects tubular cells is currently unclear. In addition, whether and how autophagy changes its role from a pro-survival mechanism to a pro-death factor are currently unknown. The key signaling pathways that induce and regulate autophagy in AKI are also poorly understood. Further research should focus on these areas to elucidate the mechanism of autophagy induction in tubular cells in AKI, delineate the underlying signaling pathways, and define the precise roles played by autophagy in tubular regulation in this disease. A comprehensive understanding of the regulatory network of tubular cell autophagy will facilitate the discovery of genetic and pharmacological modulators for the prevention and treatment of kidney diseases including AKI.
Acknowledgments
Financial Support: The study was supported in part by grants from the National Institutes of Health and Department of Veterans Administration of USA.
Footnotes
Conflict of Interest Statement: The authors have no conflicts to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bellomo R, Kellum JA, Ronco C. Acute kidney injury. Lancet. 2012;380:756–66. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 2.Heung M, Chawla LS. Predicting progression to chronic kidney disease after recovery from acute kidney injury. Curr Opin Nephrol Hypertens. 2012;21:628–34. doi: 10.1097/MNH.0b013e3283588f24. [DOI] [PubMed] [Google Scholar]
- 3.Leung KC, Tonelli M, James MT. Chronic kidney disease following acute kidney injury-risk and outcomes. Nat Rev Nephrol. 2013;9:77–85. doi: 10.1038/nrneph.2012.280. [DOI] [PubMed] [Google Scholar]
- 4.Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
- 5.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–21. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol. 2011;7:189–200. doi: 10.1038/nrneph.2011.16. [DOI] [PubMed] [Google Scholar]
- 7.Lieberthal W, Nigam SK. Acute renal failure. I. Relative importance of proximal vs. distal tubular injury. Am J Physiol. 1998;275:F623–31. doi: 10.1152/ajprenal.1998.275.5.F623. [DOI] [PubMed] [Google Scholar]
- 8.Lieberthal W, Nigam SK. Acute renal failure. II. Experimental models of acute renal failure: imperfect but indispensable. Am J Physiol Renal Physiol. 2000;278:F1–F12. doi: 10.1152/ajprenal.2000.278.1.F1. [DOI] [PubMed] [Google Scholar]
- 9.Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chien CT, Shyue SK, Lai MK. Bcl-xL augmentation potentially reduces ischemia/reperfusion induced proximal and distal tubular apoptosis and autophagy. Transplantation. 2007;84:1183–90. doi: 10.1097/01.tp.0000287334.38933.e3. [DOI] [PubMed] [Google Scholar]
- 11.Wu HH, Hsiao TY, Chien CT, Lai MK. Ischemic conditioning by short periods of reperfusion attenuates renal ischemia/reperfusion induced apoptosis and autophagy in the rat. J Biomed Sci. 2009;16:19. doi: 10.1186/1423-0127-16-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki C, Isaka Y, Takabatake Y, Tanaka H, Koike M, Shibata M, et al. Participation of autophagy in renal ischemia/reperfusion injury. Biochem Biophys Res Commun. 2008;368:100–6. doi: 10.1016/j.bbrc.2008.01.059. [DOI] [PubMed] [Google Scholar]
- 13.Jiang M, Liu K, Luo J, Dong Z. Autophagy is a renoprotective mechanism during in vitro hypoxia and in vivo ischemia-reperfusion injury. Am J Pathol. 2010;176:1181–92. doi: 10.2353/ajpath.2010.090594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamacher-Brady A, Brady NR, Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem. 2006;281:29776–87. doi: 10.1074/jbc.M603783200. [DOI] [PubMed] [Google Scholar]
- 15.Kimura T, Takabatake Y, Takahashi A, Kaimori JY, Matsui I, Namba T, et al. Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J Am Soc Nephrol. 2011;22:902–13. doi: 10.1681/ASN.2010070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu S, Hartleben B, Kretz O, Wiech T, Igarashi P, Mizushima N, et al. Autophagy plays a critical role in kidney tubule maintenance, aging and ischemia-reperfusion injury. Autophagy. 2012;8:826–37. doi: 10.4161/auto.19419. [DOI] [PubMed] [Google Scholar]
- 17.Periyasamy-Thandavan S, Jiang M, Wei Q, Smith R, Yin XM, Dong Z. Autophagy is cytoprotective during cisplatin injury of renal proximal tubular cells. Kidney Int. 2008;74:631–40. doi: 10.1038/ki.2008.214. [DOI] [PubMed] [Google Scholar]
- 18.Yang C, Kaushal V, Shah SV, Kaushal GP. Autophagy is associated with apoptosis in cisplatin injury to renal tubular epithelial cells. Am J Physiol Renal Physiol. 2008;294:F777–87. doi: 10.1152/ajprenal.00590.2007. [DOI] [PubMed] [Google Scholar]
- 19.Inoue K, Kuwana H, Shimamura Y, Ogata K, Taniguchi Y, Kagawa T, et al. Cisplatin-induced macroautophagy occurs prior to apoptosis in proximal tubules in vivo. Clin Exp Nephrol. 2010;14:112–22. doi: 10.1007/s10157-009-0254-7. [DOI] [PubMed] [Google Scholar]
- 20.Pallet N, Bouvier N, Legendre C, Gilleron J, Codogno P, Beaune P, et al. Autophagy protects renal tubular cells against cyclosporine toxicity. Autophagy. 2008;4:783–91. doi: 10.4161/auto.6477. [DOI] [PubMed] [Google Scholar]
- 21.Zeng Y, Yang X, Wang J, Fan J, Kong Q, Yu X. Aristolochic acid I induced autophagy extenuates cell apoptosis via ERK 1/2 pathway in renal tubular epithelial cells. PloS one. 2012;7:e30312. doi: 10.1371/journal.pone.0030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chargui A, Zekri S, Jacquillet G, Rubera I, Ilie M, Belaid A, et al. Cadmium-induced autophagy in rat kidney: an early biomarker of subtoxic exposure. Toxicol Sci. 2011;121:31–42. doi: 10.1093/toxsci/kfr031. [DOI] [PubMed] [Google Scholar]
- 23.Kimura A, Ishida Y, Wada T, Hisaoka T, Morikawa Y, Sugaya T, et al. The absence of interleukin-6 enhanced arsenite-induced renal injury by promoting autophagy of tubular epithelial cells with aberrant extracellular signal-regulated kinase activation. Am J Pathol. 2010;176:40–50. doi: 10.2353/ajpath.2010.090146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsiao HW, Tsai KL, Wang LF, Chen YH, Chiang PC, Chuang SM, et al. The decline of autophagy contributes to proximal tubular dysfunction during sepsis. Shock. 2012;37:289–96. doi: 10.1097/SHK.0b013e318240b52a. [DOI] [PubMed] [Google Scholar]
- 25.Wu MF, Li PC, Chen CC, Ye SS, Chien CT, Yu CC. Cordyceps sobolifera extract ameliorates lipopolysaccharide-induced renal dysfunction in the rat. Am J Chin Med. 2011;39:523–35. doi: 10.1142/S0192415X11009007. [DOI] [PubMed] [Google Scholar]
- 26.Funk JA, Schnellmann RG. Persistent disruption of mitochondrial homeostasis after acute kidney injury. Am J Physiol Renal Physiol. 2012;302:F853–64. doi: 10.1152/ajprenal.00035.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang Z, Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol. 2010;22:124–31. doi: 10.1016/j.ceb.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehrpour M, Esclatine A, Beau I, Codogno P. Overview of macroautophagy regulation in mammalian cells. Cell Res. 2010;20:748–62. doi: 10.1038/cr.2010.82. [DOI] [PubMed] [Google Scholar]
- 30.Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–93. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bolisetty S, Traylor AM, Kim J, Joseph R, Ricart K, Landar A, et al. Heme oxygenase-1 inhibits renal tubular macroautophagy in acute kidney injury. J Am Soc Nephrol. 2010;21:1702–12. doi: 10.1681/ASN.2010030238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parajuli N, MacMillan-Crow LA. Role of reduced manganese superoxide dismutase in ischemia-reperfusion injury: a possible trigger for autophagy and mitochondrial biogenesis? Am J Physiol Renal Physiol. 2013;304:F257–67. doi: 10.1152/ajprenal.00435.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoyer-Hansen M, Jaattela M. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ. 2007;14:1576–82. doi: 10.1038/sj.cdd.4402200. [DOI] [PubMed] [Google Scholar]
- 34.Rovetta F, Stacchiotti A, Consiglio A, Cadei M, Grigolato PG, Lavazza A, et al. ER signaling regulation drives the switch between autophagy and apoptosis in NRK-52E cells exposed to cisplatin. Exp Cell Res. 2012;318:238–50. doi: 10.1016/j.yexcr.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Gozuacik D, Bialik S, Raveh T, Mitou G, Shohat G, Sabanay H, et al. DAP-kinase is a mediator of endoplasmic reticulum stress-induced caspase activation and autophagic cell death. Cell Death Differ. 2008;15:1875–86. doi: 10.1038/cdd.2008.121. [DOI] [PubMed] [Google Scholar]
- 36.Kawakami T, Inagi R, Takano H, Sato S, Ingelfinger JR, Fujita T, et al. Endoplasmic reticulum stress induces autophagy in renal proximal tubular cells. Nephrol Dial Transplant. 2009;24:2665–72. doi: 10.1093/ndt/gfp215. [DOI] [PubMed] [Google Scholar]
- 37.Kitamura M. Endoplasmic reticulum stress and unfolded protein response in renal pathophysiology: Janus faces. Am J Physiol Renal Physiol. 2008;295:F323–34. doi: 10.1152/ajprenal.00050.2008. [DOI] [PubMed] [Google Scholar]
- 38.Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, et al. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem. 2008;283:10892–903. doi: 10.1074/jbc.M800102200. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Ishihara M, Urushido M, Hamada K, Matsumoto T, Shimamura Y, Ogata K, et al. Sestrin2 and BNIP3 (Bcl-2/adenovirus E1B 19kDa-interacting protein3) regulate autophagy and mitophagy in renal tubular cells in acute kidney injury. Am J Physiol Renal Physiol. doi: 10.1152/ajprenal.00642.2012. Epub 2013 May 22. Available from: www.ajprenal.physiology.org. [DOI] [PubMed]
- 40.Tasdemir E, Maiuri MC, Galluzzi L, Vitale I, Djavaheri-Mergny M, D’Amelio M, et al. Regulation of autophagy by cytoplasmic p53. Nat Cell Biol. 2008;10:676–87. doi: 10.1038/ncb1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng Z, Zhang H, Levine AJ, Jin S. The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci U S A. 2005;102:8204–9. doi: 10.1073/pnas.0502857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crighton D, Wilkinson S, O’Prey J, Syed N, Smith P, Harrison PR, et al. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell. 2006;126:121–34. doi: 10.1016/j.cell.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 43.Gao W, Shen Z, Shang L, Wang X. Upregulation of human autophagy-initiation kinase ULK1 by tumor suppressor p53 contributes to DNA-damage-induced cell death. Cell Death Differ. 2011;18:1598–607. doi: 10.1038/cdd.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang EY, Gang H, Aviv Y, Dhingra R, Margulets V, Kirshenbaum LA. p53 Mediates Autophagy and Cell Death by a Mechanism Contingent On Bnip3. Hypertension. 2013;62:70–7. doi: 10.1161/HYPERTENSIONAHA.113.01028. [DOI] [PubMed] [Google Scholar]
- 45.Maiuri MC, Malik SA, Morselli E, Kepp O, Criollo A, Mouchel PL, et al. Stimulation of autophagy by the p53 target gene Sestrin2. Cell cycle. 2009;8:1571–6. doi: 10.4161/cc.8.10.8498. [DOI] [PubMed] [Google Scholar]
- 46.Kenzelmann Broz D, Spano Mello S, Bieging KT, Jiang D, Dusek RL, Brady CA, et al. Global genomic profiling reveals an extensive p53-regulated autophagy program contributing to key p53 responses. Genes Dev. 2013;27:1016–31. doi: 10.1101/gad.212282.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bensaad K, Cheung EC, Vousden KH. Modulation of intracellular ROS levels by TIGAR controls autophagy. EMBO J. 2009;28:3015–26. doi: 10.1038/emboj.2009.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Isaka Y, Suzuki C, Abe T, Okumi M, Ichimaru N, Imamura R, et al. Bcl-2 protects tubular epithelial cells from ischemia/reperfusion injury by dual mechanisms. Transplant Proc. 2009;41:52–4. doi: 10.1016/j.transproceed.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 49.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–39. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 50.Maiuri MC, Le Toumelin G, Criollo A, Rain JC, Gautier F, Juin P, et al. Functional and physical interaction between Bcl-X(L) and a BH3-like domain in Beclin-1. EMBO J. 2007;26:2527–39. doi: 10.1038/sj.emboj.7601689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang NC, Nguyen M, Germain M, Shore GC. Antagonism of Beclin 1-dependent autophagy by BCL-2 at the endoplasmic reticulum requires NAF-1. EMBO J. 2010;29:606–18. doi: 10.1038/emboj.2009.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and Bcl-2. Mol Cell. 2007;25:193–205. doi: 10.1016/j.molcel.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 53.Mizushima N. The role of the Atg1/ULK1 complex in autophagy regulation. Curr Opin Cell Biol. 2010;22:132–9. doi: 10.1016/j.ceb.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 54.Di Bartolomeo S, Corazzari M, Nazio F, Oliverio S, Lisi G, Antonioli M, et al. The dynamic interaction of AMBRA1 with the dynein motor complex regulates mammalian autophagy. J Cell Biol. 2010;191:155–68. doi: 10.1083/jcb.201002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Russell RC, Tian Y, Yuan H, Park HW, Chang YY, Kim J, et al. ULK1 induces autophagy by phosphorylating Beclin-1 and activating VPS34 lipid kinase. Nat Cell Biol. 2013;15:741–50. doi: 10.1038/ncb2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gammoh N, Florey O, Overholtzer M, Jiang X. Interaction between FIP200 and ATG16L1 distinguishes ULK1 complex-dependent and -independent autophagy. Nat Struc Mol Biol. 2013;20:144–9. doi: 10.1038/nsmb.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alers S, Loffler AS, Wesselborg S, Stork B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: cross talk, shortcuts, and feedbacks. Mol Cell Biol. 2012;32:2–11. doi: 10.1128/MCB.06159-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang M, Wei Q, Dong G, Komatsu M, Su Y, Dong Z. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int. 2012;82:1271–83. doi: 10.1038/ki.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lempiainen J, Finckenberg P, Mervaala EE, Sankari S, Levijoki J, Mervaala EM. Caloric restriction ameliorates kidney ischaemia/reperfusion injury through PGC-1alpha-eNOS pathway and enhanced autophagy. Acta physiol. 2013;208:410–21. doi: 10.1111/apha.12120. [DOI] [PubMed] [Google Scholar]
- 60.Takahashi A, Kimura T, Takabatake Y, Namba T, Kaimori J, Kitamura H, et al. Autophagy guards against cisplatin-induced acute kidney injury. Am J Pathol. 2012;180:517–25. doi: 10.1016/j.ajpath.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 61.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–52. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 62.Lee JS, Li Q, Lee JY, Lee SH, Jeong JH, Lee HR, et al. FLIP-mediated autophagy regulation in cell death control. Nat Cell Biol. 2009;11:1355–62. doi: 10.1038/ncb1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hou W, Han J, Lu C, Goldstein LA, Rabinowich H. Autophagic degradation of active caspase-8: a crosstalk mechanism between autophagy and apoptosis. Autophagy. 2010;6:891–900. doi: 10.4161/auto.6.7.13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scarlatti F, Granata R, Meijer AJ, Codogno P. Does autophagy have a license to kill mammalian cells? Cell Death Differ. 2009;16:12–20. doi: 10.1038/cdd.2008.101. [DOI] [PubMed] [Google Scholar]
- 65.Chen Y, Klionsky DJ. The regulation of autophagy - unanswered questions. J Cell Sci. 2011;124:161–70. doi: 10.1242/jcs.064576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat Rev Mol Cell Biol. 2008;9:1004–10. doi: 10.1038/nrm2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eisenberg-Lerner A, Bialik S, Simon HU, Kimchi A. Life and death partners: apoptosis, autophagy and the cross-talk between them. Cell Death Differ. 2009;16:966–75. doi: 10.1038/cdd.2009.33. [DOI] [PubMed] [Google Scholar]
- 68.Rubinsztein DC, Codogno P, Levine B. Autophagy modulation as a potential therapeutic target for diverse diseases. Nat Rev Drug Discov. 2012;11:709–30. doi: 10.1038/nrd3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lieberthal W, Fuhro R, Andry CC, Rennke H, Abernathy VE, Koh JS, et al. Rapamycin impairs recovery from acute renal failure: role of cell-cycle arrest and apoptosis of tubular cells. Am J Physiol Renal Physiol. 2001;281:F693–706. doi: 10.1152/ajprenal.2001.281.4.F693. [DOI] [PubMed] [Google Scholar]