Abstract
Background
Alcoholism is a heterogeneous disease, with subjects possibly differing both in the best measure that predicts their excess consumption and in their most effective pharmacotherapy. Two different measures, of high novelty-induced activity and high fat-induced triglycerides (TG), are known to identify subgroups of animals prone to consuming higher amounts of ethanol. The question investigated here is whether these subgroups are, in fact, similar in their neurochemical phenotype that may contribute to their overconsumption.
Methods
Ethanol-naïve, Sprague-Dawley rats were subgrouped based on the two predictor measures of activity or TG levels, and then quantitative real-time PCR and digoxigenin-labeled in situ hybridization were used to measure their expression of hypothalamic peptides that affect ethanol intake. In additional subgroups subsequently trained to drink 9% ethanol, the opioid antagonist and alcoholism medication, naltrexone, was tested at a low dose (0.02 mg/kg s.c.) to determine the rats’ sensitivity to its effects.
Results
The two measures, while both effective in predicting amount of ethanol intake, were found to identify distinctive subgroups. Rats with high compared to low activity exhibited significantly greater expression of galanin and enkephalin in the paraventricular nucleus (PVN) and of orexin in the perifornical lateral hypothalamus (PFLH), but no difference in melanin-concentrating hormone in PFLH or neuropeptide Y in arcuate nucleus. This contrasts with rats having high TG, which exhibited greater expression only of PVN galanin, along with reduced PFLH orexin. The high activity rats with elevated enkephalin, but not high TG rats, were also unusually sensitive to naltrexone, which significantly reduced their alcohol intake.
Conclusions
In addition to revealing differences in endogenous peptides and drug responsiveness in predicted high ethanol drinkers, this study demonstrates that these disturbances differ markedly between the two at-risk subgroups. This indicates that simple tests may be effective in identifying subjects most responsive to a specific pharmacotherapy.
Keywords: galanin, hypothalamus, naltrexone, novelty-seeking, triglycerides
Alcohol use disorders may appear similar phenotypically; however, they have considerable heterogeneity (Heilig et al., 2011). This may be due to genetic heterogeneity, with alcohol use disorders having a large degree of genetic susceptibility, suggesting that individuals require different interventions. This is supported by findings with the alcoholism medication and opioid antagonist, naltrexone, which is effective only in a subgroup of patients (Heilig et al., 2011), such as those with a specific allele of the mu-opioid receptor (Heilig et al., 2011; Oslin et al., 2003). While pharmacogenetic tests will be beneficial in implementing personalized medication, behavioral and physiological phenotypes that correspond to these genetic variations may also help to identify subjects who are not only more likely to be responsive to a drug treatment but also at greater risk for developing alcohol use disorders.
A number of measures can identify subjects at risk for drinking excess alcohol. In prospective clinical studies, the development of alcohol use disorders can be predicted by personality factors such as anxiety or novelty-seeking, and also metabolic factors such as low level of response to alcohol that reflects poor ethanol oxidation (Schmidt et al., 2007; Schuckit et al., 2011). Similarly, in outbred rats, novelty-seeking assessed by high locomotor activity in a novel environment and also circulating triglyceride (TG) levels after a high-fat meal can identify at-risk animals (Karatayev et al., 2010a; Nadal et al., 2002). This latter measure, reflecting less efficient hydrolysis of meal-induced TGs by lipoprotein lipase, may be related to the poor ethanol oxidation of certain at-risk patients (Schuckit et al., 2011) as well as the inhibition of lipoprotein lipase by alcohol (Liu et al., 2011). While both measures detect at-risk rats, they show little relationship to each other and thus may identify phenotypically different subgroups (Karatayev et al., 2010a). These subgroups, due to biological heterogeneity, may also differ in their responsiveness to pharmacological treatment. Indeed, rats with high compared to low novelty-induced activity are more responsive to naltrexone’s ability to reduce both activity levels (Radcliffe and Erwin, 1998) and ingestion of palatable food (White et al., 2004).
In determining biological mechanisms that drive subgroups to drink alcohol, it is preferable to study subjects in the absence of ethanol, as it alters neurochemicals (Vengeliene et al., 2008). Studies in ethanol-naïve rats bred to consume ethanol suggest that at-risk subjects already show dysregulation of specific systems. Selectively-bred P and HAD compared to NP and LAD rats exhibit altered levels of monoamines (Strother et al., 2005), and AA compared to ANA rats show elevated expression of the opioid enkephalin (ENK) in corticolimbic regions (Marinelli et al., 2000). While these reports provide information on neurochemicals that may contribute generally to ethanol intake, they do not distinguish between subgroups that may drink for different reasons. Humans with the novelty-seeking personality trait also have altered levels of monoamines (Heck et al., 2009). In addition, a recent study in our laboratory comparing high-risk subjects showed that novelty-seeking rats have elevated expression of ENK in the hypothalamic paraventricular nucleus (PVN) and neuropeptide Y (NPY) in the arcuate nucleus (ARC), whereas those with high fat-induced TGs exhibit elevated galanin (GAL) and ENK in the PVN but not NPY in the ARC (Karatayev et al., 2010a). Whereas this provides some evidence that high-risk subgroups differ in their hypothalamic neurochemical profile, it is unclear whether the peptides, assessed immediately after the predictor tests, were differentially affected by the tests themselves.
Neurochemicals may not only drive ethanol intake but may also contribute to the predictor measures themselves. The hypothalamic orexigenic peptides that may serve these functions include GAL and ENK in the PVN, orexin/hypocretin (OX) and melanin-concentrating hormone (MCH) in the perifornical lateral hypothalamus (PFLH), and NPY in the ARC. When injected, GAL, ENK, OX and MCH each promote ethanol intake (Barson et al., 2010; Morganstern et al., 2010b; Schneider et al., 2007), while NPY decreases drinking, at least in Myers' high ethanol-preferring rats (Karatayev et al., 2010b). Also, both GAL and ENK stimulate locomotor activity (Lyudyno et al., 2008; Vujic-Redzic et al., 2000), but only GAL decreases fat oxidation that determines TG levels (Gunion et al., 1991; Yun et al., 2005). With neurochemicals affecting both risk for drinking and the associated behavioral or metabolic profile, assessment of these latter measures could give insight into the neurochemical phenotypes of different high-risk subgroups.
With subjects prone to drinking ethanol for different reasons, the present study was designed to determine whether at-risk animals show peptide disturbances prior to exposure and whether this varies as a function of the specific predictor measure. In outbred rats with variability in their responses, we used novelty-induced locomotor activity and fat-induced TG levels to identify high-risk subjects, examining these measures first in the same set of rats to determine their relationship to each other. We then examined different subgroups for their expression of hypothalamic peptides and responsiveness to naltrexone. For peptide measurements, we kept subjects ethanol-naïve and gave them a one-week rest period following the predictor tests, to ensure that baseline expression was evaluated. We hypothesized that predicted high ethanol consumers would show disturbances in their peptide systems and responsiveness to medication, but that these disturbances would differ as a function of the predictor employed and thus subgroup identified.
MATERIALS AND METHODS
Subjects
Adult, male Sprague–Dawley rats (N = 120, 225–250 g, Charles River Laboratories International, Inc., Wilmington, MA) were housed individually, on a 12-hour reversed light/dark cycle. Animals were allowed one week to acclimate to housing conditions, during which they received ad libitum chow (LabDiet Rodent Chow 5001, St. Louis, MO) and water. The facility was fully accredited by AAALAC. Protocols were approved by the Rockefeller University Animal Care Committee and followed the NIH Guide for the Care and Use of Laboratory Animals.
Experimental Procedures
Experiment 1: Rats (N = 24) were tested for novelty-induced locomotor activity and then fat-induced TG levels. They were sorted by their activity counts or TG levels and separated by tertile split into “high”, “middle”, and “low” subgroups (n = 8/subgroup), then trained to consume 9% ethanol.
Experiments 2 and 3: Rats (N = 96; n = 24/experiment) were tested for novelty-induced locomotor activity (Exp. 2) or fat-induced TG levels (Exp. 3) and separated into “high”, “middle”, and “low” subgroups (n = 8/subgroup). One week later, with chow removed 1 h prior, they were sacrificed by rapid decapitation during the middle of the dark cycle for quantitative real time polymerase chain reaction (qRT-PCR) (Exps. 2A and 3A) or digoxigenin-labeled in situ hybridization (DIG) (Exps. 2B and 3B).
Experiment 4: With naltrexone being one of the few medications approved by the United States Food and Drug Administration for the treatment of alcoholism (Heilig et al., 2011), the rats from Experiment 1 (N = 24), after one week of 9% ethanol access, were injected immediately prior to daily 9% ethanol in a counterbalanced design on consecutive days with naltrexone hydrochloride (0.02 mg/kg s.c.) (Sigma-Aldrich, St. Louis, MO) in 0.9% sodium chloride (Hospira, Lake Forest, IL) or an equivalent volume (~0.3 ml) of sodium chloride (Williams and Broadbridge, 2009). Ethanol, food and water intake was then measured for 4 hours.
Behavioral Testing
Novelty-Induced Locomotor Activity
During the middle of the dark period, each rat was moved to a sound-attenuated room, placed for 15 minutes in a 17.0" × 17.0" chamber (Med Associates, Inc., St. Albans, VT) and activity counts (number of infrared beam breaks) were recorded.
Fat-Induced Triglycerides
Rats were acclimated to the 50% high-fat diet (Karatayev et al., 2010a) over 3 days with a daily 15-kcal meal. For the following 3 days, chow was removed at dark onset, rats 1 h later were given a 15-kcal high-fat meal for 30 minutes, and tail vein blood was sampled for serum TGs 30 min after that. TG values were averaged for each subject.
Ethanol Drinking
Ethanol was made available, in addition to the water, in a second 8 oz bottle with a non-drip sipper tube at the top of the cage (PETCO Animal Supplies, Inc, San Diego, CA), with the relative positions alternated each day to prevent side preference. Ethanol was provided for 8 hours each day, starting 3.5 hours after dark onset. The concentration was increased every 4 days, from 1% to 2%, 4%, 7%, and finally 9% v/v. Water and chow were available ad libitum. Ethanol, water and food were measured daily. Spillage of solution is less than 1 g per day even with extensive handling. Daily intake at 9% was averaged across the first 4 days of access. After the end of experiments, with intake consistent with initial 9% intake, tail vein blood was sampled 45 min after the start of daily access for blood ethanol concentration (BEC).
Brain Dissections
Immediately after sacrifice, the brain was placed in a matrix slicing guide with the ventral surface facing up. Four coronal cuts were made, starting with the anterior middle optic chiasm (Bregma −0.8 mm) (Paxinos and Watson, 2005). The second cut was 1.0 mm caudal to this, yielding a slice (Bregma −0.8 to −1.8 mm) for microdissection of the PVN. The third and fourth cuts were 1.0 mm caudal to the prior cuts, yielding a slice (Bregma −2.8 to −3.8 mm) for the PFLH and ARC.
Under a microscope, the PVN was rapidly dissected as a reversed isosceles triangle, 1.0 mm bilateral to the third ventricle and between the fornix structures. The PFLH was taken bilaterally, 0.2 mm medial and ventral to the fornix, 0.3 mm dorsal, and 0.4 mm lateral. The ARC was dissected as an isosceles triangle dorsal to the median eminence, 0.5 mm bilateral to the third ventricle. These dissections were stored in RNAlater (Sigma–Aldrich).
Quantitative Real-Time PCR
Total RNA from each sample was purified using the RNeasy Mini Kit (Qiagen Inc., Valenia, CA) and DNA removed using RNase-free DNase 1 (Qiagen Inc.). The yield was quantified with a Nanodrop ND-8000 spectrophotometer (NanoDrop, Wilmington, DE), with resulting A260/A280 ratios between 1.91 and 2.18. cDNA was reverse transcribed from 1 µg of RNA using high capacity RNA-to-cDNA master mix (Applied Biosystems, Foster City, CA). Minus RT controls were synthesized by replacing DNA polymerase with distilled water. The SYBR Green PCR core reagents kit (Applied Biosystems) was used for qRT-PCR. qRT-PCR was performed in MicroAmp Optic 96-well Reaction Plates (Applied Biosystems), using 12.5 ng of cDNA template in a 25 µl reaction volume, and run on an ABI PRISM 7900 Sequence Detection system (Applied Biosystems), under the condition of 2 min at 50 °C, 10 min at 95 °C, 40 cycles of 15 s at 95 °C and 1 min at 60 °C. Each sample was run in triplicate, and each run included a no-template and negative RT control. The specificities of PCR products were confirmed by a single band of corresponding molecular weight revealed by agarose gel electrophoresis. Target gene expression was quantified relative to the level of cyclophilin using the relative quantification method and average Ct values for the endogenous control versus the target genes differed by no more than 4 Ct. The primers were designed with ABI Primer Express V.1.5a software (Table 1). All reagents, unless indicated, were from Invitrogen (Carlsbad, CA).
Table 1. Primers used for quantitative real time polymerase chain reaction.
Primer sequences and concentrations used for quantitative real time polymerase chain reaction experiments (Exp. 2A and Exp. 3A).
| Primer | Sequence | Concentration |
|---|---|---|
| Cyclophilin | 5′-GTGTTCTTCGACATCACGGCT-3′ (forward) 5′-CTGTCTTTGGAACTTTGTCTGCA-3′ (reverse) |
200 nM |
| Enkephalin | 5′-GGACTGCGCTAAATGCAGCTA-3′(forward) 5′-GTGTGCATGCCAGGAAGTTG-3′ (reverse) |
100 nM |
| Galanin | 5'-TTCCCACCACTGCTCAAGATG-3' (forward) 5'-TGGCTGACAGGGTTGCAA-3' (reverse) |
100 nM |
| Melanin concentrating hormone | 5′-ATCGGTTGTTGCTCCTTCTCTG-3′ (forward) 5′-TCT GCT TGG AGC CTG TGT TCT T-3′ (reverse) |
100 nM |
| Neuropeptide Y | 5′-CACAGAAAATGCCCCCAGAA-3′ (forward) 5′-GTCAGGAGAGCAAGTTTCATTTCC-3′ (reverse) |
100 nM |
| Orexin | 5′-AGATACCATCTCTCCGGATTGC-3′ (forward) 5′-CCAGGGAACCTTTGTAGAAGGA-3′ (reverse) |
100 nM |
In Situ Hybridization
In situ hybridization with digoxigenin-labeled probes was used to measure the density of neurons expressing the peptide gene. With brains from Experiments 2B and 3B processed separately, frozen brains were cut into 30 µm coronal sections with every third section saved for analysis of a specific peptide, GAL or ENK through the PVN and OX or MCH through the PFLH. As NPY was not altered in either group using qRT-PCR, it was not tested. DIG-labeled cRNA probes were synthesized as previously described (Wortley et al., 2003). Free-floating sections were processed as described (Chang et al., 2010; Leibowitz et al., 2007). Gene expression level was measured by semiquantification with Image-Pro Plus software (Version 4.5, Media Cybernetics Inc., Silver Spring, MD) as described (Leibowitz et al., 2007) and expressed as “cells/mm2.” Sections through the entire nucleus were examined, resulting in 13–14 slices for the PVN and 15–17 for the PFLH.
Blood Assessment
Blood serum was assayed for TGs using a Triglyceride Assay kit (Sigma-Aldrich) and for BEC using an Analox GM8 Alcohol Analyzer (Lunenberg, MA).
Data Analysis
Correlations were made using the Pearson product-moment correlation coefficient. Multiple regression analysis was performed using the enter method. Differences between subgroups were tested with a one-way analysis of variance followed up by multiple comparisons with a Bonferroni correction when appropriate. Effects of drug injection (Experiment 4) were tested with a repeated-measures analysis of variance with the simple effects tested with paired, two-tailed t-tests. Differences between two groups were tested using unpaired, two-tailed t-tests. Significance was determined at p < 0.05. All data are reported as mean ± standard error of the mean (S.E.M.) except for data from Experiment 4, which are mean ± standard error of the difference (S.E.D.) (Keppel and Wickens, 2004).
RESULTS
Experiment 1: “High Activity” and “High TG” Predict High Ethanol Intake but Identify Distinct Subgroups
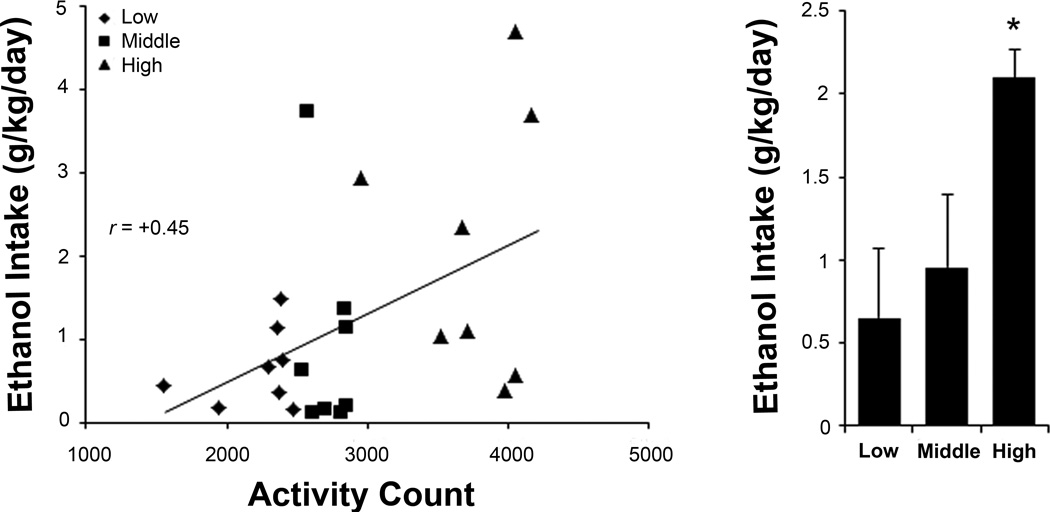
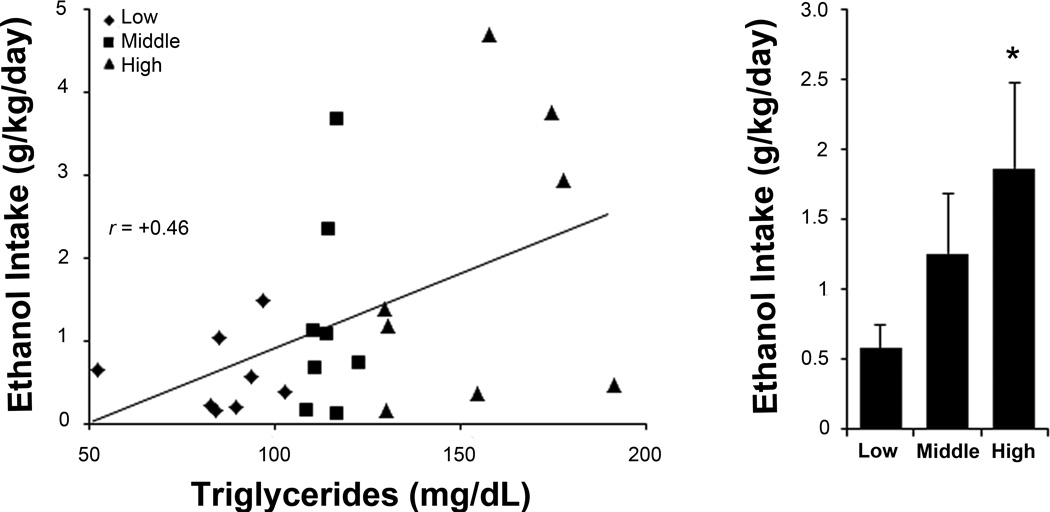
To reexamine the ability of novelty-induced locomotor activity and fat-induced TG levels to predict the level of ethanol intake, rats were separated into “high”, “middle”, and “low” subgroups for these measures and trained to drink ethanol. There was no difference in body weight between the subgroups (activity: F(2, 21) = 0.60, ns; TG: F(2, 21) = 0.31, ns). Ethanol intake ranged from 0.14 to 4.7 g/kg/day, and BEC ranging from 0 to 77 mg/dl was highly correlated both with daily intake (r = +0.79, p < 0.001) and 45-min intake prior to blood sampling (r = +0.96, p < 0.001). Multiple regression analysis of 9% ethanol intake revealed a significant model (F(2, 21) = 10.91, p < 0.01) that accounted for 51% of the variance, with both activity and TGs emerging as significant predictors (Table 2). Activity correlated significantly with intake of ethanol (r = +0.45, p < 0.05), but not water (r = −0.03, ns) or chow (r = −0.24, ns), and the high and low subgroups consumed significantly different levels of 9% ethanol (t(8) = 2.48, p < 0.05) (Fig. 1). Similarly, TGs correlated significantly with intake of ethanol (r = +0.46, p < 0.05), but not water (r = −0.21, ns) or chow (r = +0.11, ns), and the high and low subgroups also drank significantly different levels of 9% ethanol (t(8) = 2.38, p < 0.05) (Fig. 2). Further analyses showed that these activity and TG measures were not closely related. They were not correlated with each other (r = −0.22, ns), with only two animals high in both activity and TGs, and the activity subgroups showed no differences in their fat-induced TG levels (F(2, 21) = 2.31, ns). Neither measure correlated significantly with drinking at lower concentrations (Table 3). Thus, while both emerged as significant predictors of 9% ethanol intake, these two measures identify distinct subgroups of high-risk subjects.
Table 2. Predictor variables for 9% ethanol intake.
Both novelty-induced locomotor activity and fat-induced triglyceride (TG) levels emerge as significant predictor variables for 9% ethanol intake.
| Predictor Variable | Beta | p |
|---|---|---|
| Locomotor Activity | 0.56 | p < 0.01 |
| TG Levels | 0.58 | p < 0.01 |
Fig 1.
Sorting by activity counts in a novel open field predicts rats’ relative 9% ethanol intake. Left: activity counts positively correlate with later daily 9% ethanol intake. Right: rats sorted into “low”, “middle”, and “high” subgroups according to their different activity counts go on to drink different amounts of 9% ethanol. Values are mean ± S.E.M. *p < 0.05 vs. low subgroup.
Fig 2.
Sorting by triglyceride levels after a high-fat meal predicts rats’ relative 9% ethanol intake. Left: triglyceride levels positively correlate with later daily 9% ethanol intake. Right: rats sorted into “low”, “middle”, and “high” subgroups according to their different triglyceride levels go on to drink different amounts of 9% ethanol. Values are mean ± S.E.M. *p < 0.05 vs. low subgroup.
Table 3. Correlation of predictor measures and ethanol intake.
Novelty-induced locomotor activity and fat-induced triglyceride levels correlate significantly with ethanol intake at 9% but not at lower concentrations.
| Predictor Measure | 1% Ethanol | 2% Ethanol | 4% Ethanol | 7% Ethanol | 9% Ethanol |
|---|---|---|---|---|---|
| Locomotor Activity | r = −0.03 | r = 0.33 | r = 0.16 | r = 0.27 | r = 0.45* |
| Triglyceride Levels | r = −0.15 | r = −0.16 | r = 0.02 | r = 0.20 | r = 0.46* |
p < 0.05.
Experiment 2: High Activity Rats Have Elevated GAL, ENK and OX mRNA
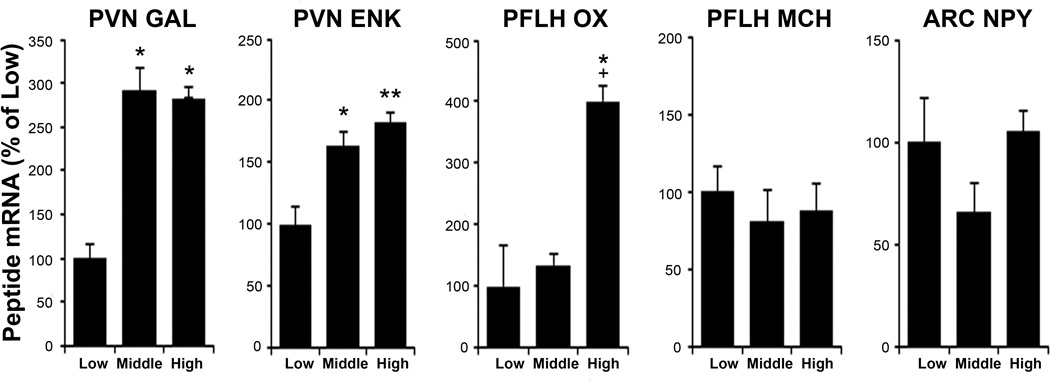
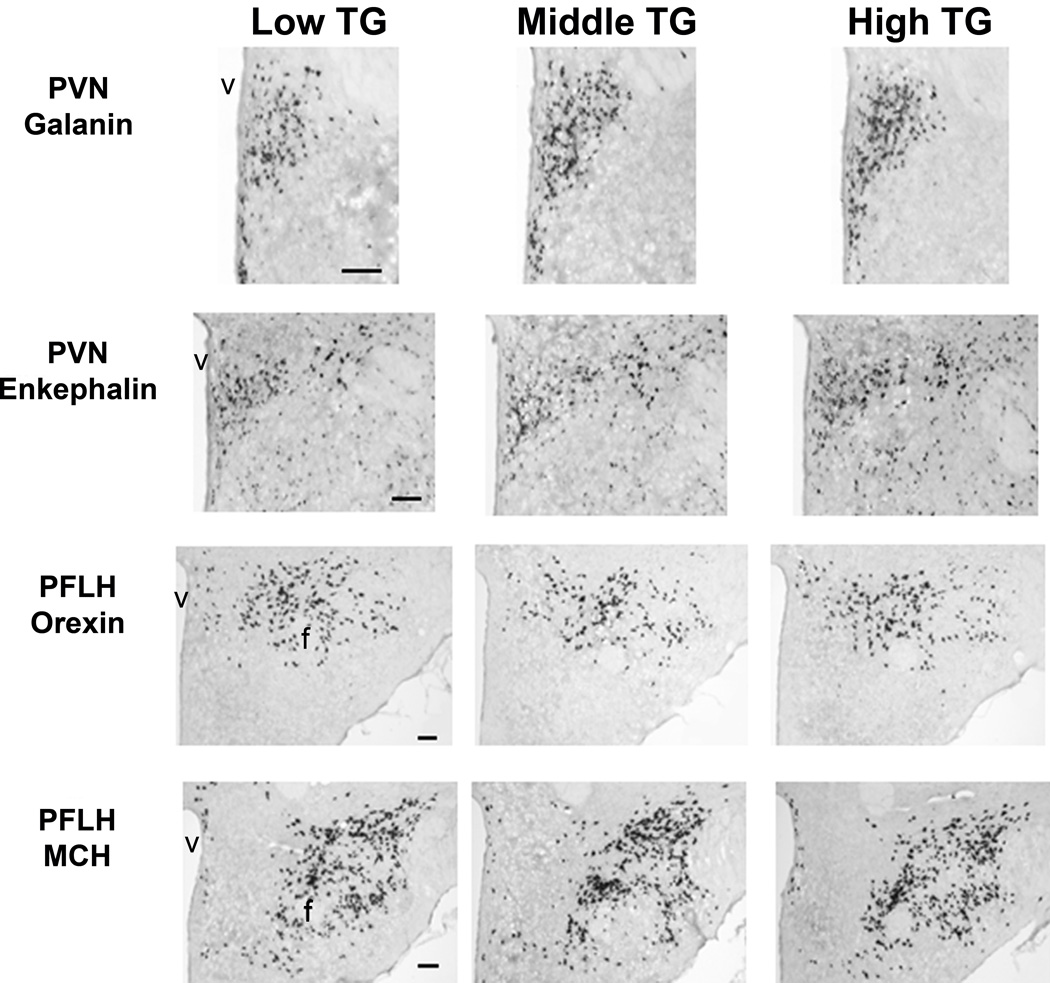
To understand the neurochemical mechanisms behind high novelty-induced locomotor activity, we next examined ethanol-naïve “high”, “middle”, and “low” activity subgroups (Table 4) using qRT-PCR and DIG. As assessed by qRT-PCR (Exp. 2A), these groups, while similar in PFLH MCH (F(2, 21) = 0.38, ns) and ARC NPY mRNA (F(2, 21) = 2.10, ns), showed significant differences in PVN GAL (F(2, 21) = 5.12, p < 0.05), PVN ENK (F(2, 21) = 8.19, p < 0.01) and PFLH OX (F(2, 21) = 5.69, p < 0.05) (Fig. 3). Specifically, the expression of both GAL and ENK was elevated in the high and middle groups compared to the low group (p < 0.05), and OX was elevated in the high compared to both the low and middle groups (p < 0.05). As assessed by DIG (Exp. 2B), the groups again exhibited significant differences in PVN GAL (F(2, 21) = 17.23, p < 0.001), PVN ENK (F(2, 21) = 9.50, p < 0.01), and PFLH OX (F(2, 21) = 5.51, p < 0.05) and no difference in PFLH MCH mRNA (F(2, 21) = 0.97, ns) (Table 5). The expression of GAL was elevated in the high and middle groups compared to low group (p < 0.01), and ENK and OX were increased in the high compared to both the low and middle groups (p < 0.05). Activity also correlated positively and generally significantly with both GAL, measured using qRT-PCR (r = +0.31, p = 0.15) and DIG (r = +0.74, p < 0.01), and ENK, using qRT-PCR (r = +0.49, p < 0.05) and DIG (r = +0.65, p < 0.05). It was weakly correlated with OX (r = +0.18, ns and r = +0.43, p = 0.16). As in Experiment 1, the groups showed no significant differences in their body weights (Exp. 2A: F(2, 21) = 1.54, ns; Exp. 2B: F(2, 21) = 0.17, ns) or basal TG levels (Exp. 2A: F(2, 21) = 0.61, ns; Exp. 2B: F(2, 21) = 0.53, ns). Thus, animals with high novelty-induced locomotor activity, which are more likely to drink higher levels of ethanol, have elevated mRNA expression of GAL and ENK in the PVN and OX in the PFLH.
Table 4. Activity counts and triglyceride levels in Experiments 2 and 3.
Separation by tertile split results in “low”, “middle”, and “high” subgroups with different activity counts or triglyceride levels. From left to right are novelty-induced locomotor activity in Experiments 2A and 2B and fat-induced triglyceride levels in Experiments 3A and 3B.
| Subgroup | Activity Count Exp. 2A |
Activity Count Exp. 2B |
Triglycerides (mg/dL) Exp. 3A |
Triglycerides (mg/dL) Exp. 3B |
|---|---|---|---|---|
| Low | 1851 ± 119 | 1599 ± 247 | 59 ± 4 | 65 ± 6 |
| Middle | 2631 ± 65 | 2436 ± 67 | 85 ± 6 | 96 ± 2 |
| High | 3663 ± 369 | 3282 ± 143 | 123 ± 4 | 122 ± 4 |
Values are mean ± S.E.M.
Fig 3.
High activity rats show increased hypothalamic galanin, enkephalin and orexin mRNA, as assessed by qRT-PCR (Experiment 2A). Values are mean ± S.E.M. **p < 0.01 vs. low subgroup; *p < 0.05 vs. low; +p < 0.05 vs. middle. Abbreviations: ARC, arcuate nucleus; ENK, enkephalin; GAL, galanin; MCH, melanin-concentrating hormone; NPY, neuropeptide Y; OX, orexin; PFLH, perifornical lateral hypothalamus; PVN, paraventricular nucleus of the hypothalamus.
Table 5. Digoxigenin-labeled in situ hybridization in activity subgroups.
High activity rats show increased hypothalamic galanin, enkephalin and orexin mRNA cell density, as assessed by digoxigenin-labeled in situ hybridization (Experiment 2B).
| Subgroup | PVN GAL (cells/µm × 10−5) |
PVN ENK (cells/µm × 10−5) |
PFLH OX (cells/µm × 10−5) |
PFLH MCH (cells/µm × 10−5) |
|---|---|---|---|---|
| Low | 2.11 ± 0.04 | 2.22 ± 0.26 | 3.42 ± 0.71 | 4.17 ± 0.13 |
| Middle | 2.82 ± 0.12** | 2.51 ± 0.12 | 3.91 ± 0.66 | 4.44 ± 0.27 |
| High | 3.15 ± 0.19** | 3.75 ± 0.36**+ | 8.55 ± 1.85*+ | 3.73 ± 0.55 |
Values are mean ± S.E.M.
p < 0.01 vs. low subgroup;
p < 0.05 vs. low;
p < 0.05 vs. middle.
See text or legend to Figure 3 for abbreviations.
Experiment 3: High TG Rats Have Increased GAL but Not ENK or OX mRNA
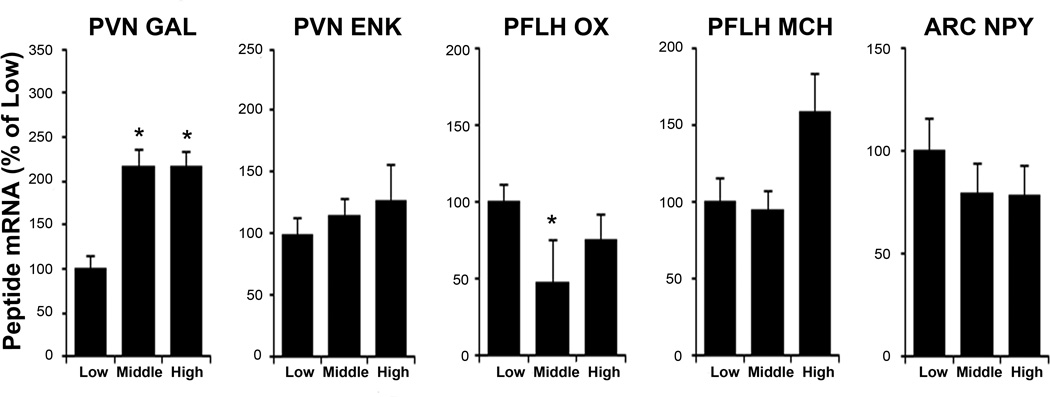
To understand the neurochemical mechanism underlying high fat-induced TGs, we examined ethanol-naïve “high”, “middle”, and “low” TG subgroups (Table 4), using qRT-PCR and DIG. The subgroups showed no significant differences in body weight (Exp. 3A: F(2, 21) = 0.95, ns; Exp. 3B: F(2, 21) = 2.50, ns). This experiment revealed a peptide expression pattern that differed from that of the activity groups. As assessed by qRT-PCR (Exp. 3A), the TG subgroups showed significant differences in PVN GAL (F(2, 21) = 4.60, p < 0.05) and PFLH OX (F(2, 21) = 5.63, p < 0.05) but not PFLH MCH (F(2, 21) = 2.08, ns), ARC NPY (F(2, 21) = 0.91, ns), or PVN ENK mRNA (F(2, 21) = 0.29, ns) (Fig. 4). Similar to the activity groups, GAL was elevated in the high and middle groups compared to the low group (p < 0.05). In contrast, OX was reduced in the middle compared to low group (p < 0.05) and slightly decreased in the high compared to low group (ns). Similar results were obtained with DIG (Exp. 3B), with the groups again exhibiting differences in PVN GAL (F(2, 21) = 21.32, p < 0.001) and PFLH OX (F(2, 21) = 162.85, p < 0.001), but not PFLH MCH (F(2, 21) = 0.00, ns) or PVN ENK mRNA (F(2, 21) = 1.35, ns) (Table 6 and Fig. 5). The expression of GAL was increased in the high and middle compared to low group (p < 0.01), and OX was reduced in the high and middle compared to low group (p < 0.01). Levels of fat-induced TG correlated positively and significantly with GAL measured using qRT-PCR (r = +0.45, p < 0.05) and DIG (r = +0.60, p < 0.05) but negatively with OX using qRT-PCR (r = −0.19, ns) and DIG (r = −0.64, p < 0.05). At sacrifice, basal TG levels were still different between the groups (Exp. 3A: F(2, 21) = 10.08, p < 0.01; Exp 3B: F(2, 21) = 3.63, p < 0.05), and while they correlated with fat-induced TGs (Exp. 3A: r = +0.67, p < 0.001; Exp 3B: r = +0.58, p < 0.01), they showed little relationship with peptide expression. Thus, animals with high fat-induced TGs, which are predicted to drink higher levels of ethanol, have elevated mRNA expression of GAL in the PVN but decreased OX in the PFLH.
Fig 4.
High triglyceride rats show increased hypothalamic galanin but slightly decreased orexin mRNA, as assessed by qRT-PCR (Experiment 2B). Values are mean ± S.E.M. *p < 0.05 vs. low subgroup. See text or legend to Figure 3 for abbreviations.
Table 6. Digoxigenin-labeled in situ hybridization in triglyceride subgroups.
High triglyceride rats show increased hypothalamic galanin but decreased orexin mRNA cell density, as assessed by digoxigenin-labeled in situ hybridization (Experiment 3B).
| Subgroup | PVN GAL (cells/µm × 10−5) |
PVN ENK (cells/µm × 10−5) |
PFLH OX (cells/µm × 10−5) |
PFLH MCH (cells/µm × 10−5) |
|---|---|---|---|---|
| Low | 2.09 ± 0.03 | 2.74 ± 0.14 | 7.42 ± 0.22 | 8.44 ± 0.22 |
| Middle | 2.72 ± 0.07** | 2.79 ± 0.05 | 6.31 ± 0.20** | 8.25 ± 0.25 |
| High | 3.02 ± 0.16** | 2.99 ± 0.13 | 6.54 ± 0.30** | 8.29 ± 0.19 |
Values are mean ± S.E.M.
p < 0.01 vs. low subgroup.
See text or legend to Figure 3 for abbreviations.
Fig 5.
Photomicrographs for the fat-induced triglyceride group showing digoxigenin-labeled in situ hybridization histochemistry analysis of cells expressing galanin and enkephalin mRNA in the paraventricular nucleus of the hypothalamus (PVN) and orexin and melanin-concentrating hormone in the perifornical lateral hypothalamus (PFLH). Abbreviations: f, fornix; v, third ventricle. 4X magnification. Scale bar = 125 µm.
Experiment 4: Naltrexone is More Effective in High Activity Rats
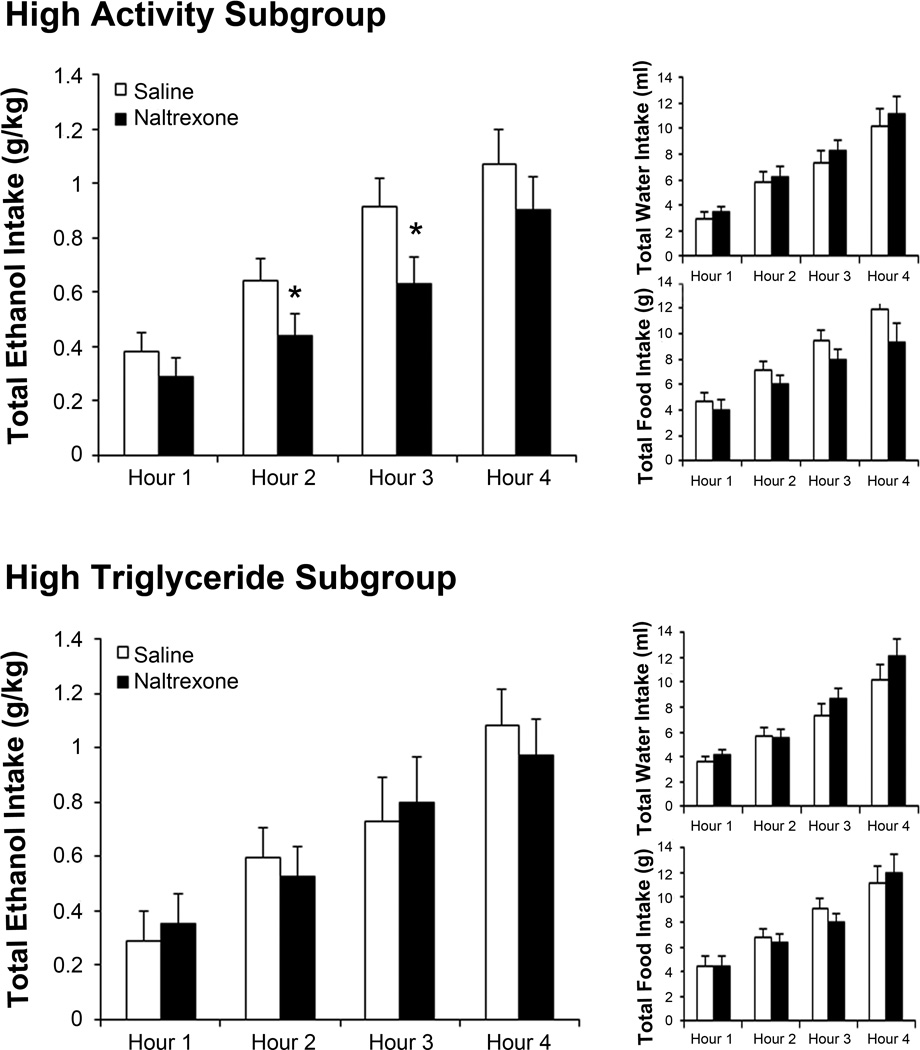
With high activity but not high TG rats having elevated expression of ENK, we examined whether they also exhibit greater sensitivity to the general opioid antagonist, naltrexone. Using the rats from Experiment 1, analysis of the whole group showed no effect of naltrexone on intake of ethanol (F(1, 23) = 2.45, ns) or water (F(1, 23) = 0.51, ns) and only a small decrease in food intake (F(1, 23) = 4.71, p < 0.05) during the third hour after injection (t(23) = 3.26, p < 0.01). Examination of the subgroups, however, revealed a significant effect only in the high activity rats. While naltrexone had no effect in the high TG and low activity subgroups on their intake of ethanol (TG: F(1, 7) = 0.01, ns; activity: F(1, 7) = 0.01, ns), water (TG: F(1, 7) = 0.56, ns; activity: F(1, 7) = 0.36, ns), or food (TG: F(1, 7) = 0.03, ns; activity: F(1, 7) = 0.99, ns), it had a significant effect in the high activity subgroup (Fig. 6), causing a decrease in ethanol intake (F(1, 7) = 5.73, p < 0.05) during the second (t(7) = 2.54, p < 0.05) and third (t(7) = 2.90, p < 0.05) hours. This change was specific to ethanol, as naltrexone did not significantly affect consumption of water (F(1, 7) = 1.00, ns) or food (F(1, 7) = 5.52, p = 0.05). Thus, the high activity rats, one subgroup of high ethanol drinkers, were uniquely responsive to naltrexone.
Fig 6.
High activity rats respond to a low dose of naltrexone. Top left panel: high activity rats decrease their 9% ethanol drinking in response to naltrexone (0.02 mg/kg s.c.) compared to saline. Top right panel: high activity rats do not significantly alter water or food intake in response to naltrexone. Bottom left panel: high triglyceride rats do not significantly alter their 9% ethanol drinking in response to naltrexone. Bottom right panel: high triglyceride rats do not significantly alter water or food intake in response to naltrexone. Values are mean ± S.E.D. *p < 0.05 vs. saline.
DISCUSSION
“High Activity” and “High TG” Predict High Ethanol Intake but Identify Distinct Subgroups
The results show that novelty-induced locomotor activity and fat-induced TG levels measured in the same group of outbred rats can each positively and significantly predict the relative level of ethanol drinking. This confirms our findings in separate groups of rats (Karatayev et al., 2010a) and is consistent with other reports (Nadal et al., 2002). While both measures predict intake, they identify different subgroups, confirming and extending our earlier results in rats never tested with ethanol (Karatayev et al., 2010a). While high-risk subjects differ from low-risk subjects in their peptide expression prior to ethanol exposure, different high-risk subgroups also have a dissimilar neurochemical phenotype that reacts differently to pharmacotherapy. High activity rats have elevated expression of PVN GAL, PVN ENK and PFLH OX mRNA and a unique sensitivity to naltrexone, while high TG rats have elevated expression only of PVN GAL along with reduced PFLH OX. It is notable that this is evident prior to exposure to ethanol, which can affect peptide expression (Chang et al., 2007a; Morganstern et al., 2010a; Morganstern et al., 2010b), and also one week following the predictor tests, when effects from the tests themselves (Chang et al., 2007b; Ferenczi et al., 2010; Gaysinskaya et al., 2007; Yukhananov and Handa, 1997) are likely to have dissipated. While further testing involving outbred strains with greater ethanol consumption than Sprague-Dawleys might strengthen these conclusions, the results support the idea of disease heterogeneity as well as peptide specificity and suggest that particular neurochemical differences may underlie this phenomenon.
Galanin May Drive High Ethanol Intake and Predictors
One major result is that PVN GAL mRNA is elevated in both subgroups of predicted high ethanol drinkers. This is consistent with our previous finding showing high TG rats to have elevated PVN GAL after the fatty meal (Karatayev et al., 2010a), although the high activity subgroup in that same report failed to exhibit elevated GAL immediately after the predictor test (Karatayev et al., 2010a), possibly due to their sensitivity to the stress from the novel environment that suppresses hypothalamic GAL expression (Sergeyev et al., 2005). A role for GAL in driving ethanol intake is substantiated in other studies, showing that GAL overexpressing mice drink more ethanol while GAL knockouts drink less (Karatayev et al., 2009b; Karatayev et al., 2010b), that PVN GAL injection in rats stimulates consumption of ethanol (Rada et al., 2004), and that a GAL haplotype in humans is associated with alcoholism (Belfer et al., 2006). That GAL may also influence the predictor measures themselves is suggested by the finding that locomotor activity is increased in GAL overexpressing mice (Kuteeva et al., 2005) and that serum TGs are elevated in GAL overexpressors (Poritsanos et al., 2009) and likely to be enhanced by PVN injection of GAL as it reduces fat oxidation in muscle (Yun et al., 2005). Together, this evidence supports the idea that GAL drives not only ethanol intake but also high novelty-induced locomotor activity and fat-induced TG levels.
Enkephalin is Involved in Ethanol Intake in High Activity Subjects
Another important result is that PVN ENK mRNA is elevated in rats predicted to be high ethanol drinkers by the activity but not the TG measure. This finding, obtained a week after open field testing, substantiates our earlier result in rats examined immediately after this test (Karatayev et al., 2010a) and agrees with the finding that PVN injections of ENK analogues stimulate ethanol drinking (Barson et al., 2010). While this suggests that ENK can drive ethanol intake, the lack of change in rats with high TGs indicates that it may contribute only in certain high-risk subjects. In our earlier study (Karatayev et al., 2010a), ENK mRNA immediately after the high-fat meal was also elevated in high TG subjects, but this was likely from the meal itself, which stimulates ENK expression (Chang et al., 2007b). The present results, with examination a week after the predictor test, support the idea of biological heterogeneity behind alcohol use disorders and suggest that high ENK may lead to one specific phenotype. Whereas central and peripheral injections of ENK analogues, like GAL, increase locomotor activity (Dauge et al., 1988; Vujic-Redzic et al., 2000), they differ in having no effect on fat oxidation (Gunion et al., 1991) that would affect TG levels. Therefore, while ENK may drive the higher novelty-induced locomotor activity that predicts one subgroup’s propensity to consume ethanol, it likely does not affect fat-induced TG levels and ethanol drinking of this other subgroup.
Naltrexone is Specifically Effective in High Activity Rats
Our results additionally show that high activity rats with elevated PVN ENK mRNA, likely accompanied by higher peptide levels (Chang et al., 2007a), are particularly sensitive to naltrexone. Whereas the significant effect was on ethanol drinking, this subgroup also showed reduced chow intake, with an effect size nearly as large as for ethanol (ηp2 = 0.44 vs. ηp2 = 0.45), suggesting that they may be more sensitive in general to naltrexone’s effects. This substantiates findings with other behaviors in high activity rats and also studies of alcoholics showing only certain patients to benefit from this opioid antagonist (see Introduction). The possibility that this high activity subgroup has more opioid receptors to which both naltrexone and ENK bind is suggested by evidence in alcohol-preferring AA rats, showing a greater density of opioid receptors in concert with higher ENK expression in the prefrontal cortex (Marinelli et al., 2000). Also, it is consistent with findings that subjects with the Asp40 allele of the mu-opioid receptor, which has greater binding affinity, are more responsive to naltrexone (Oslin et al., 2003). The results lend some support to the Opioid Surfeit Hypothesis of alcoholism, which posits that individuals are vulnerable because of excess opioid activity (Oswald and Wand, 2004), as opposed to the Opioid Deficit Hypothesis, which holds that low opioids motivate compensatory consumption. Since naltrexone also affects other neurochemicals, as indicated by its ability to block OX-induced feeding (Sweet et al., 2004), the greater sensitivity of the high activity subgroup may also be related to their elevated OX mRNA. Whether it is due to their higher ENK or OX, which itself can stimulate PVN ENK (Barson et al., 2011; Karatayev et al., 2009a), novelty-seeking rats represent one subgroup responsive to the drinking-reducing effects of naltrexone.
Orexin is Differentially Involved in Ethanol Intake and Predictor Measures
The results with PFLH OX represent a striking example of the biological heterogeneity of alcohol use. Whereas rats predicted to be high ethanol drinkers by increased activity have elevated OX mRNA, those predicted by high TGs show reduced expression. This may parallel theorized roles for opioids, with high or low levels making individuals vulnerable to alcoholism (Oswald and Wand, 2004). It is also notable that elevated OX was the major difference between the high and middle activity rats, underscoring its importance in ethanol drinking. This finding of high OX in the high activity subgroup agrees with evidence that locomotor activity is stimulated by ventricular injection of OX (Samson et al., 2010) and reduced in OX knockout mice (Mori et al., 2010). Whereas the result of low OX in the high TG subgroup was unexpected given that elevated TGs from a high-fat meal can increase OX expression (Karatayev et al., 2009c), OX itself can stimulate lipid substrate utilization (Stricker-Krongrad et al., 2002), which would reduce TG levels. Thus, low OX may enable TG levels to remain high after a fatty meal. As hypothalamic OX injection stimulates ethanol intake (Schneider et al., 2007), the results suggest that OX, like ENK, is involved in driving ethanol intake in the high activity but not the high TG subgroup. Whereas we did not test an OX antagonist because it is not yet approved for clinical use and the receptor subtype involved is unknown, we might also hypothesize that the high activity subgroup would be more responsive to an OX antagonist.
Lack of Major Role for Melanin-Concentrating Hormone or Neuropeptide Y in Ethanol Intake
In both predictor models, we observed no differences in PFLH MCH or ARC NPY mRNA, suggesting that they do not play a major role in driving the enhanced exploratory behavior, lower lipid oxidation, or excess ethanol intake of the high-risk subgroups. These results are not surprising given the inconsistent relationship of these peptides with ethanol. Although hypothalamic MCH injection stimulates ethanol intake (Morganstern et al., 2010b), MCH receptor knockouts drink more than wild-type mice (Duncan et al., 2007). Also, hypothalamic NPY injection does not affect ethanol drinking in outbred rats (Karatayev et al., 2010b), and alcohol-preferring AA compared to alcohol-avoiding ANA rats show no difference in ARC NPY mRNA (Caberlotto et al., 2001). We previously found NPY to be elevated in high activity rats immediately after testing (Karatayev et al., 2010a), but this may have been due to test-induced stress (Ferenczi et al., 2010). With regard to the predictor measures, locomotor activity is not affected by central injection of either MCH (Sanchez et al., 1997) or NPY (Heilig et al., 1989), and while MCH receptor knockouts show increased fatty acid oxidation (Chung et al., 2010) that should reduce TG, they exhibit other neurotransmitter changes that could explain this (Duncan et al., 2007). Thus, while they affect ethanol intake under certain circumstances, MCH and NPY appear less important in promoting the excess ethanol drinking of subjects with higher activity or TG levels.
Summary and Conclusions
These results provide the first evidence that measures which predict high ethanol consumption can identify different subgroups of high-risk subjects that have distinct hypothalamic peptide mRNA expression prior to drinking. This has major implications for the treatment for alcohol use disorders. The differences between the high activity and high TG subgroups in ENK and OX point to the likelihood that medications targeting these systems may be effective only in a subset of subjects. In contrast, as high-risk subjects from both models exhibit elevated GAL mRNA, this may be a major common factor in driving ethanol intake, and targeting this system could result in broadly effective pharmacotherapy. It is also possible that GAL plays a dominant role in promoting initial ethanol intake, while ENK and OX become more important with ethanol experience. Whereas the data are correlational, and additional studies are needed to establish causality, the expression alterations in the high-risk subgroups lead us to hypothesize that the peptides are responsible not only for the subsequent ethanol drinking but also for the predictor measures themselves. Thus, assessment of these phenotypes would allow insight into subjects’ neurochemical state and could ultimately allow for the implementation of personalized medicine for problematic alcohol use.
ACKNOWLEDGEMENTS
This research was supported by USPHS Grant AA12882. We extend gratitude to Sherry Liang, Olga Lukatskaya, Ambrose Carr and Jessica Baylan for technical assistance.
REFERENCES
- Barson JR, Carr AJ, Soun JE, Sobhani NC, Rada P, Leibowitz SF, Hoebel BG. Opioids in the hypothalamic paraventricular nucleus stimulate ethanol intake. Alcohol Clin Exp Res. 2010;34:214–222. doi: 10.1111/j.1530-0277.2009.01084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Chang GQ, Poon K, Morganstern I, Leibowitz SF. Galanin and the orexin 2 receptor as possible regulators of enkephalin in the paraventricular nucleus of the hypothalamus: relation to dietary fat. Neuroscience. 2011;193:10–20. doi: 10.1016/j.neuroscience.2011.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfer I, Hipp H, McKnight C, Evans C, Buzas B, Bollettino A, Albaugh B, Virkkunen M, Yuan Q, Max MB, Goldman D, Enoch MA. Association of galanin haplotypes with alcoholism and anxiety in two ethnically distinct populations. Mol Psychiatry. 2006;11:301–311. doi: 10.1038/sj.mp.4001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caberlotto L, Thorsell A, Rimondini R, Sommer W, Hyytia P, Heilig M. Differential expression of NPY and its receptors in alcohol-preferring AA and alcohol-avoiding ANA rats. Alcohol Clin Exp Res. 2001;25:1564–1569. [PubMed] [Google Scholar]
- Chang GQ, Barson JR, Karatayev O, Chang SY, Chen YW, Leibowitz SF. Effect of chronic ethanol on enkephalin in the hypothalamus and extra-hypothalamic areas. Alcohol Clin Exp Res. 2010;34:761–770. doi: 10.1111/j.1530-0277.2010.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Ahsan R, Avena NM, Lee C, Lewis MJ, Hoebel BG, Leibowitz SF. Effect of ethanol on hypothalamic opioid peptides, enkephalin, and dynorphin: relationship with circulating triglycerides. Alcohol Clin Exp Res. 2007a;31:249–259. doi: 10.1111/j.1530-0277.2006.00312.x. [DOI] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Ahsan R, Gaysinskaya V, Marwil Z, Leibowitz SF. Dietary fat stimulates endogenous enkephalin and dynorphin in the paraventricular nucleus: role of circulating triglycerides. Am J Physiol Endocrinol Metab. 2007b;292:E561–E570. doi: 10.1152/ajpendo.00087.2006. [DOI] [PubMed] [Google Scholar]
- Chung S, Wong T, Nagasaki H, Civelli O. Acute homeostatic responses to increased fat consumption in MCH1R knockout mice. J Mol Neurosci. 2010;42:459–463. doi: 10.1007/s12031-010-9358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauge V, Rossignol P, Roques BP. Comparison of the behavioural effects induced by administration in rat nucleus accumbens or nucleus caudatus of selective mu and delta opioid peptides or kelatorphan an inhibitor of enkephalin-degrading-enzymes. Psychopharmacology (Berl) 1988;96:343–352. doi: 10.1007/BF00216060. [DOI] [PubMed] [Google Scholar]
- Duncan EA, Sorrell JE, Adamantidis A, Rider T, Jandacek RJ, Seeley RJ, Lakaye B, Woods SC. Alcohol drinking in MCH receptor-1-deficient mice. Alcohol Clin Exp Res. 2007;31:1325–1337. doi: 10.1111/j.1530-0277.2007.00427.x. [DOI] [PubMed] [Google Scholar]
- Ferenczi S, Zelei E, Pinter B, Szoke Z, Kovacs KJ. Differential regulation of hypothalamic neuropeptide Y hnRNA and mRNA during psychological stress and insulin-induced hypoglycemia. Mol Cell Endocrinol. 2010;321:138–145. doi: 10.1016/j.mce.2010.02.036. [DOI] [PubMed] [Google Scholar]
- Gaysinskaya VA, Karatayev O, Chang GQ, Leibowitz SF. Increased caloric intake after a high-fat preload: relation to circulating triglycerides and orexigenic peptides. Physiol Behav. 2007;91:142–153. doi: 10.1016/j.physbeh.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Gunion MW, Rosenthal MJ, Morley JE, Miller S, Zib B, Butler B, Moore RD. mu-receptor mediates elevated glucose and corticosterone after third ventricle injection of opioid peptides. Am J Physiol. 1991;261:R70–R81. doi: 10.1152/ajpregu.1991.261.1.R70. [DOI] [PubMed] [Google Scholar]
- Heck A, Lieb R, Ellgas A, Pfister H, Lucae S, Roeske D, Putz B, Muller-Myhsok B, Uhr M, Holsboer F, Ising M. Investigation of 17 candidate genes for personality traits confirms effects of the HTR2A gene on novelty seeking. Genes Brain Behav. 2009;8:464–472. doi: 10.1111/j.1601-183X.2009.00494.x. [DOI] [PubMed] [Google Scholar]
- Heilig M, Goldman D, Berrettini W, O'Brien CP. Pharmacogenetic approaches to the treatment of alcohol addiction. Nat Rev Neurosci. 2011;12:670–684. doi: 10.1038/nrn3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Vecsei L, Widerlov E. Opposite effects of centrally administered neuropeptide Y (NPY) on locomotor activity of spontaneously hypertensive (SH) and normal rats. Acta Physiol Scand. 1989;137:243–248. doi: 10.1111/j.1748-1716.1989.tb08745.x. [DOI] [PubMed] [Google Scholar]
- Karatayev O, Barson JR, Carr AJ, Baylan J, Chen YW, Leibowitz SF. Predictors of ethanol consumption in adult Sprague-Dawley rats: relation to hypothalamic peptides that stimulate ethanol intake. Alcohol. 2010a;44:323–334. doi: 10.1016/j.alcohol.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatayev O, Barson JR, Chang GQ, Leibowitz SF. Hypothalamic injection of non-opioid peptides increases gene expression of the opioid enkephalin in hypothalamic and mesolimbic nuclei: Possible mechanism underlying their behavioral effects. Peptides. 2009a;30:2423–2431. doi: 10.1016/j.peptides.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatayev O, Baylan J, Leibowitz SF. Increased intake of ethanol and dietary fat in galanin overexpressing mice. Alcohol. 2009b;43:571–580. doi: 10.1016/j.alcohol.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatayev O, Baylan J, Weed V, Chang S, Wynick D, Leibowitz SF. Galanin knockout mice show disturbances in ethanol consumption and expression of hypothalamic peptides that stimulate ethanol intake. Alcohol Clin Exp Res. 2010b;34:72–80. doi: 10.1111/j.1530-0277.2009.01068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatayev O, Gaysinskaya V, Chang GQ, Leibowitz SF. Circulating triglycerides after a high-fat meal: predictor of increased caloric intake, orexigenic peptide expression, and dietary obesity. Brain Res. 2009c;1298:111–122. doi: 10.1016/j.brainres.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppel G, Wickens TD. Design and Analysis: A Researcher's Handbook. 4th Edition ed. Upper Saddle River, NJ: Prentice Hall; 2004. [Google Scholar]
- Kuteeva E, Hokfelt T, Ogren SO. Behavioural characterisation of transgenic mice overexpressing galanin under the PDGF-B promoter. Neuropeptides. 2005;39:299–304. doi: 10.1016/j.npep.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Leibowitz KL, Chang GQ, Pamy PS, Hill JO, Gayles EC, Leibowitz SF. Weight gain model in prepubertal rats: prediction and phenotyping of obesity-prone animals at normal body weight. Int J Obes (Lond) 2007;31:1210–1221. doi: 10.1038/sj.ijo.0803634. [DOI] [PubMed] [Google Scholar]
- Liu WY, Yin RX, Zhang L, Wu DF, Htet Aung LH, Hu XJ, Cao XL, Miao L. Interactions of the LIPG 584C>T polymorphism and alcohol consumption on serum lipid levels. Alcohol. 2011;45:681–687. doi: 10.1016/j.alcohol.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Lucas LA, McMillen BA. Effect of neuropeptide Y microinjected into the hypothalamus on ethanol consumption. Peptides. 2004;25:2139–2145. doi: 10.1016/j.peptides.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Lyudyno VI, Abdurasulova IN, Klimenko VM. The role of the neuropeptide galanin in forming type-specific behavioral characteristics. Neurosci Behav Physiol. 2008;38:93–98. doi: 10.1007/s11055-008-0013-3. [DOI] [PubMed] [Google Scholar]
- Marinelli PW, Kiianmaa K, Gianoulakis C. Opioid propeptide mRNA content and receptor density in the brains of AA and ANA rats. Life Sci. 2000;66:1915–1927. doi: 10.1016/s0024-3205(00)00517-8. [DOI] [PubMed] [Google Scholar]
- Morganstern I, Chang GQ, Barson JR, Ye Z, Karatayev O, Leibowitz SF. Differential effects of acute and chronic ethanol exposure on orexin expression in the perifornical lateral hypothalamus. Alcohol Clin Exp Res. 2010a;34:886–896. doi: 10.1111/j.1530-0277.2010.01161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morganstern I, Chang GQ, Chen YW, Barson JR, Zhiyu Y, Hoebel BG, Leibowitz SF. Role of melanin-concentrating hormone in the control of ethanol consumption: Region-specific effects revealed by expression and injection studies. Physiol Behav. 2010b;101:428–437. doi: 10.1016/j.physbeh.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T, Ito S, Kuwaki T, Yanagisawa M, Sakurai T, Sawaguchi T. Monoaminergic neuronal changes in orexin deficient mice. Neuropharmacology. 2010;58:826–832. doi: 10.1016/j.neuropharm.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Nadal R, Armario A, Janak PH. Positive relationship between activity in a novel environment and operant ethanol self-administration in rats. Psychopharmacology (Berl) 2002;162:333–338. doi: 10.1007/s00213-002-1091-5. [DOI] [PubMed] [Google Scholar]
- Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, Volpicelli JR, O'Brien CP. A functional polymorphism of the mu-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003;28:1546–1552. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- Oswald LM, Wand GS. Opioids and alcoholism. Physiol Behav. 2004;81:339–358. doi: 10.1016/j.physbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain, in Stereotaxic Coordinates. Fifth Edition ed. San Diego, CA: Academic Press, Inc; 2005. [Google Scholar]
- Poritsanos NJ, Mizuno TM, Lautatzis ME, Vrontakis M. Chronic increase of circulating galanin levels induces obesity and marked alterations in lipid metabolism similar to metabolic syndrome. Int J Obes (Lond) 2009;33:1381–1389. doi: 10.1038/ijo.2009.187. [DOI] [PubMed] [Google Scholar]
- Rada P, Avena NM, Leibowitz SF, Hoebel BG. Ethanol intake is increased by injection of galanin in the paraventricular nucleus and reduced by a galanin antagonist. Alcohol. 2004;33:91–97. doi: 10.1016/j.alcohol.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Radcliffe RA, Erwin VG. Genetic relationship between central beta-endorphin and novelty-induced locomotor activity. Pharmacol Biochem Behav. 1998;60:709–718. doi: 10.1016/s0091-3057(98)00045-8. [DOI] [PubMed] [Google Scholar]
- Samson WK, Bagley SL, Ferguson AV, White MM. Orexin receptor subtype activation and locomotor behaviour in the rat. Acta Physiol (Oxf) 2010;198:313–324. doi: 10.1111/j.1748-1716.2009.02056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez M, Baker BI, Celis M. Melanin-concentrating hormone (MCH) antagonizes the effects of alpha-MSH and neuropeptide E-I on grooming and locomotor activities in the rat. Peptides. 1997;18:393–396. doi: 10.1016/s0196-9781(96)00327-0. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Buckner JD, Keough ME. Anxiety sensitivity as a prospective predictor of alcohol use disorders. Behav Modif. 2007;31:202–219. doi: 10.1177/0145445506297019. [DOI] [PubMed] [Google Scholar]
- Schneider ER, Rada P, Darby RD, Leibowitz SF, Hoebel BG. Orexigenic peptides and alcohol intake: differential effects of orexin, galanin, and ghrelin. Alcohol Clin Exp Res. 2007;31:1858–1865. doi: 10.1111/j.1530-0277.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Trim RS, Allen RC, Fukukura T, Knight EE, Cesario EM, Kreikebaum SA. A prospective evaluation of how a low level of response to alcohol predicts later heavy drinking and alcohol problems. Am J Drug Alcohol Abuse. 2011;37:479–486. doi: 10.3109/00952990.2011.598590. [DOI] [PubMed] [Google Scholar]
- Sergeyev V, Fetissov S, Mathe AA, Jimenez PA, Bartfai T, Mortas P, Gaudet L, Moreau JL, Hokfelt T. Neuropeptide expression in rats exposed to chronic mild stresses. Psychopharmacology (Berl) 2005;178:115–124. doi: 10.1007/s00213-004-2015-3. [DOI] [PubMed] [Google Scholar]
- Stricker-Krongrad A, Richy S, Beck B. Orexins/hypocretins in the ob/ob mouse: hypothalamic gene expression, peptide content and metabolic effects. Regul Pept. 2002;104:11–20. doi: 10.1016/s0167-0115(01)00344-5. [DOI] [PubMed] [Google Scholar]
- Strother WN, Lumeng L, Li TK, McBride WJ. Dopamine and serotonin content in select brain regions of weanling and adult alcohol drinking rat lines. Pharmacol Biochem Behav. 2005;80:229–237. doi: 10.1016/j.pbb.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Sweet DC, Levine AS, Kotz CM. Functional opioid pathways are necessary for hypocretin-1 (orexin-A)-induced feeding. Peptides. 2004;25:307–314. doi: 10.1016/j.peptides.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujic-Redzic V, Dimitrijevic M, Stanojevic S, Kovacevic-Jovanovic V, Miletic T, Radulovi J. Peripheral effects of methionine-enkephalin on inflammatory reactions and behavior in the rat. Neuroimmunomodulation. 2000;8:70–77. doi: 10.1159/000026455. [DOI] [PubMed] [Google Scholar]
- White DA, Kalinichev M, Holtzman SG. Individual differences in locomotor reactivity to a novel environment and sensitivity to opioid drugs in the rat. II. Agonist-induced antinociception and antagonist-induced suppression of fluid consumption. Psychopharmacology (Berl) 2004;177:68–78. doi: 10.1007/s00213-004-1921-8. [DOI] [PubMed] [Google Scholar]
- Williams KL, Broadbridge CL. Potency of naltrexone to reduce ethanol self-administration in rats is greater for subcutaneous versus intraperitoneal injection. Alcohol. 2009;43:119–126. doi: 10.1016/j.alcohol.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wortley KE, Chang GQ, Davydova Z, Leibowitz SF. Peptides that regulate food intake: orexin gene expression is increased during states of hypertriglyceridemia. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1454–R1465. doi: 10.1152/ajpregu.00286.2002. [DOI] [PubMed] [Google Scholar]
- Yukhananov RY, Handa RJ. Estrogen alters proenkephalin RNAs in the paraventricular nucleus of the hypothalamus following stress. Brain Res. 1997;764:109–116. doi: 10.1016/s0006-8993(97)00432-0. [DOI] [PubMed] [Google Scholar]
- Yun R, Dourmashkin JT, Hill J, Gayles EC, Fried SK, Leibowitz SF. PVN galanin increases fat storage and promotes obesity by causing muscle to utilize carbohydrate more than fat. Peptides. 2005;26:2265–2273. doi: 10.1016/j.peptides.2005.04.005. [DOI] [PubMed] [Google Scholar]