Abstract
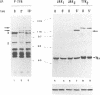
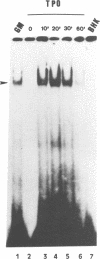
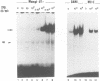
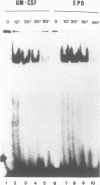
Thrombopoietin (TPO) is a newly cloned cytokine which is the major regulator of circulating platelet levels, acting on both proliferation and differentiation of megakaryocytes. We have investigated the ability of TPO to activate the JAK/STAT pathway in megakaryocytic cell lines. We used either the granulocyte-macrophage colony-stimulating factor (GM-CSF)- and/or erythropoietin (EPO)-dependent UT7 cell line in which the murine TPO receptor (mumpl) had been transfected (mumpl-UT7 transfectants) or the MO7E and DAMI cells which express endogenous human TPO receptors. We demonstrated that TPO activates the kinase JAK2 and a STAT5-like transcriptional factor but not STAT1, STAT2, STAT3 or STAT4, in a very rapid and transient manner. In order to better ascertain the specificity of the activation of STAT5-related factor by TPO, we investigated the effect of other cytokines/growth factors. Both GM-CSF and EPO activated the STAT5-like factor. In contrast, neither interferon (IFN)-gamma nor the mitogenic stem cell factor (SCF) activated STAT5, although IFN-gamma did activate STAT1 in those cells. The hematopoietic DNA binding activity related to STAT5 was identified as a p97 tyrosine-phosphorylated protein band which exhibited identical gel mobility to the mammary STAT5. Because v-mpl, a truncated form of the TPO receptor c-mpl, was shown to be oncogenic, we tested the activity of v-mpl on STAT5 and found STAT5 constitutively activated in two different v-mpl-expressing cells, the transiently transfected Cos7 cells and the stable v-mpl-UT7 transfectants. Overall, our data indicate that STAT5 is widely expressed in hematopoietic cells and activated by a number of cytokines, including TPO, GM-CSF and EPO, but not by IFN-gamma or SCF.
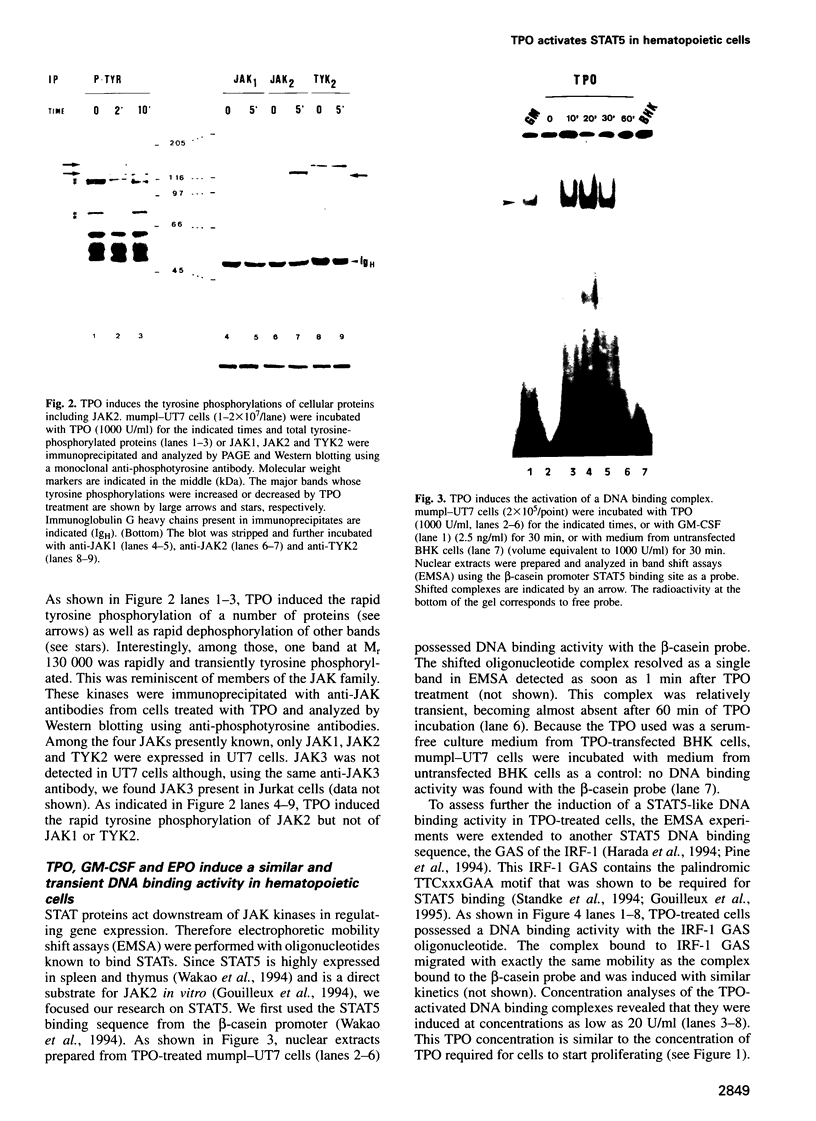
Full text
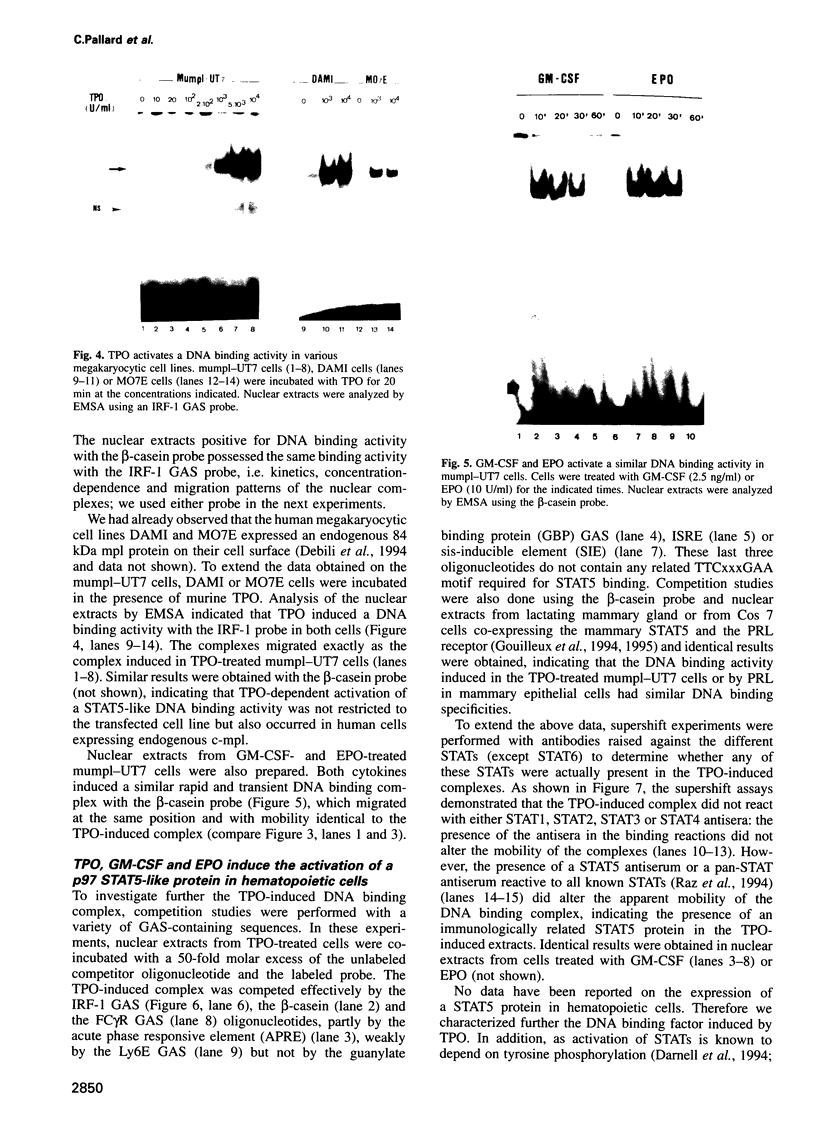
PDF
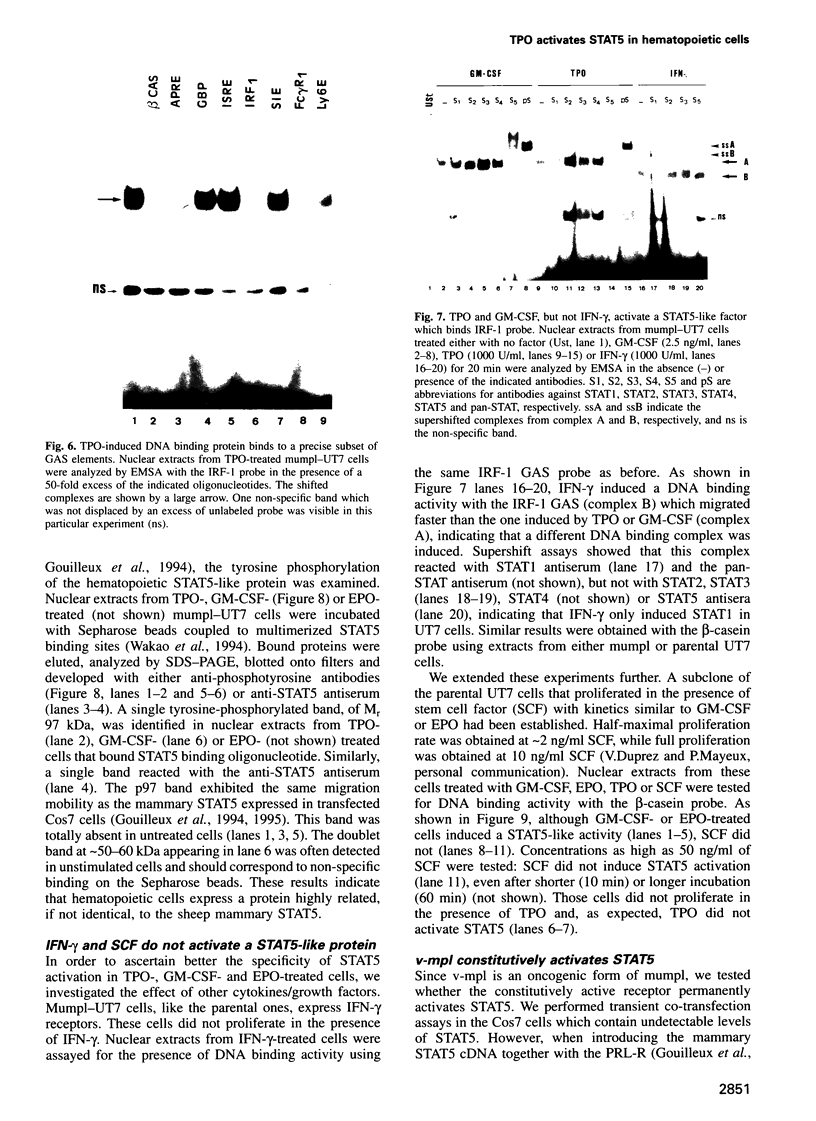
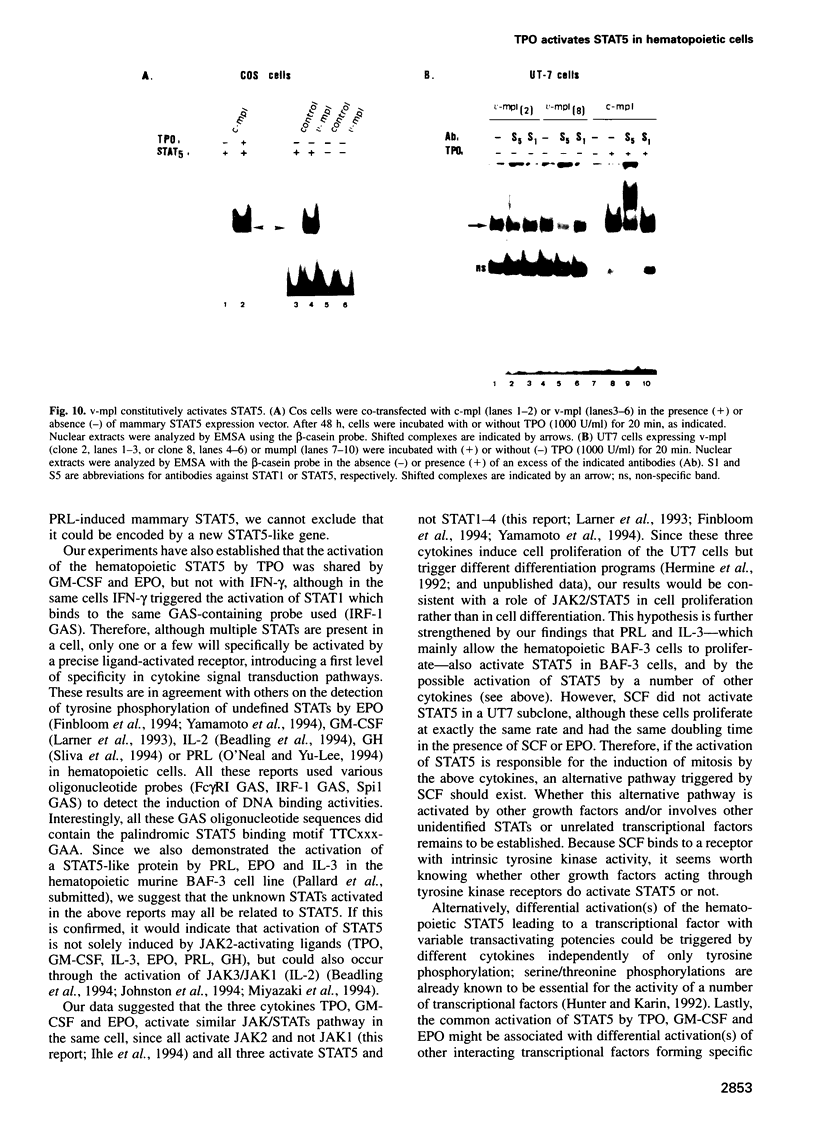



Images in this article
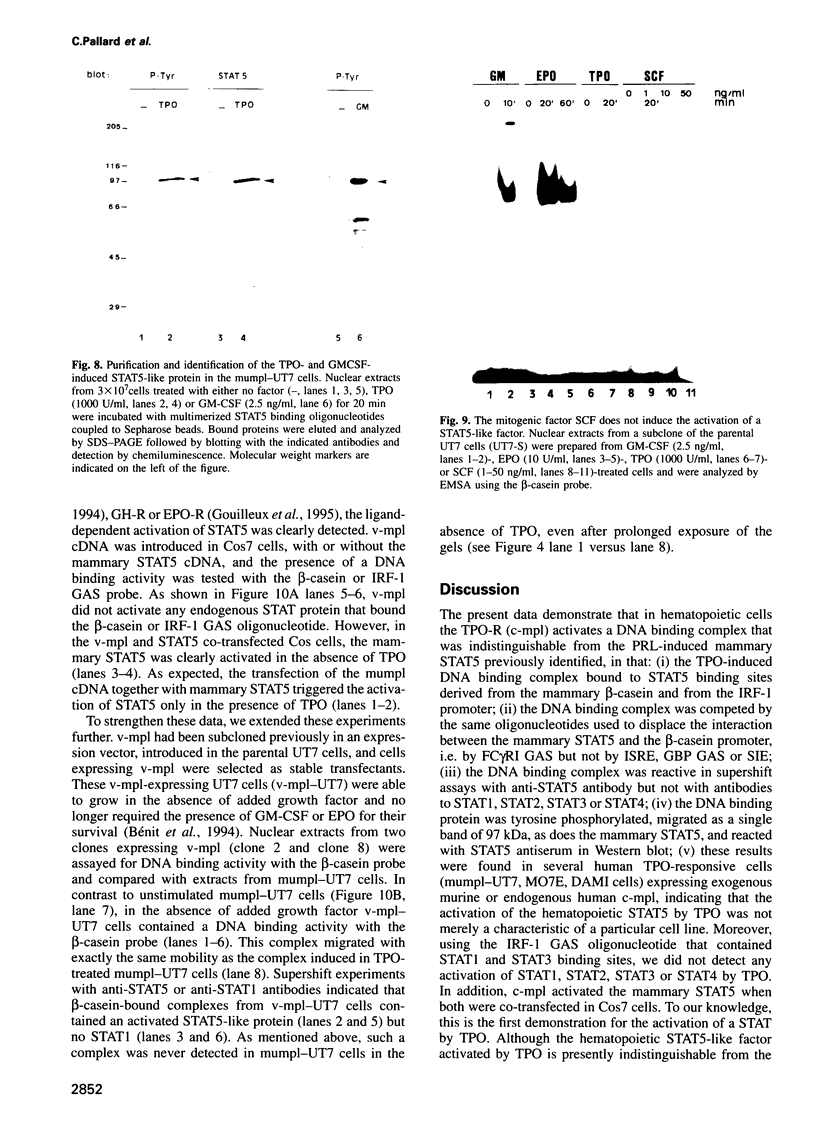
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdollahi A., Lord K. A., Hoffman-Liebermann B., Liebermann D. A. Interferon regulatory factor 1 is a myeloid differentiation primary response gene induced by interleukin 6 and leukemia inhibitory factor: role in growth inhibition. Cell Growth Differ. 1991 Aug;2(8):401–407. [PubMed] [Google Scholar]
- Akira S., Nishio Y., Inoue M., Wang X. J., Wei S., Matsusaka T., Yoshida K., Sudo T., Naruto M., Kishimoto T. Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell. 1994 Apr 8;77(1):63–71. doi: 10.1016/0092-8674(94)90235-6. [DOI] [PubMed] [Google Scholar]
- Avanzi G. C., Lista P., Giovinazzo B., Miniero R., Saglio G., Benetton G., Coda R., Cattoretti G., Pegoraro L. Selective growth response to IL-3 of a human leukaemic cell line with megakaryoblastic features. Br J Haematol. 1988 Jul;69(3):359–366. doi: 10.1111/j.1365-2141.1988.tb02374.x. [DOI] [PubMed] [Google Scholar]
- Barbieri G., Velazquez L., Scrobogna M., Fellous M., Pellegrini S. Activation of the protein tyrosine kinase tyk2 by interferon alpha/beta. Eur J Biochem. 1994 Jul 15;223(2):427–435. doi: 10.1111/j.1432-1033.1994.tb19010.x. [DOI] [PubMed] [Google Scholar]
- Bartley T. D., Bogenberger J., Hunt P., Li Y. S., Lu H. S., Martin F., Chang M. S., Samal B., Nichol J. L., Swift S. Identification and cloning of a megakaryocyte growth and development factor that is a ligand for the cytokine receptor Mpl. Cell. 1994 Jul 1;77(7):1117–1124. doi: 10.1016/0092-8674(94)90450-2. [DOI] [PubMed] [Google Scholar]
- Bazan J. F. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beadling C., Guschin D., Witthuhn B. A., Ziemiecki A., Ihle J. N., Kerr I. M., Cantrell D. A. Activation of JAK kinases and STAT proteins by interleukin-2 and interferon alpha, but not the T cell antigen receptor, in human T lymphocytes. EMBO J. 1994 Dec 1;13(23):5605–5615. doi: 10.1002/j.1460-2075.1994.tb06898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénit L., Courtois G., Charon M., Varlet P., Dusanter-Fourt I., Gisselbrecht S. Characterization of mpl cytoplasmic domain sequences required for myeloproliferative leukemia virus pathogenicity. J Virol. 1994 Aug;68(8):5270–5274. doi: 10.1128/jvi.68.8.5270-5274.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J. M., Johnson G. R. Gene transfer into hemopoietic stem cells using retroviral vectors. Int J Cell Cloning. 1989 Sep;7(5):264–280. doi: 10.1002/stem.5530070502. [DOI] [PubMed] [Google Scholar]
- Cosman D., Lyman S. D., Idzerda R. L., Beckmann M. P., Park L. S., Goodwin R. G., March C. J. A new cytokine receptor superfamily. Trends Biochem Sci. 1990 Jul;15(7):265–270. doi: 10.1016/0968-0004(90)90051-c. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr, Kerr I. M., Stark G. R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994 Jun 3;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- David M., Petricoin E. F., 3rd, Igarashi K., Feldman G. M., Finbloom D. S., Larner A. C. Prolactin activates the interferon-regulated p91 transcription factor and the Jak2 kinase by tyrosine phosphorylation. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):7174–7178. doi: 10.1073/pnas.91.15.7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusanter-Fourt I., Muller O., Ziemiecki A., Mayeux P., Drucker B., Djiane J., Wilks A., Harpur A. G., Fischer S., Gisselbrecht S. Identification of JAK protein tyrosine kinases as signaling molecules for prolactin. Functional analysis of prolactin receptor and prolactin-erythropoietin receptor chimera expressed in lymphoid cells. EMBO J. 1994 Jun 1;13(11):2583–2591. doi: 10.1002/j.1460-2075.1994.tb06548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finbloom D. S., Petricoin E. F., 3rd, Hackett R. H., David M., Feldman G. M., Igarashi K., Fibach E., Weber M. J., Thorner M. O., Silva C. M. Growth hormone and erythropoietin differentially activate DNA-binding proteins by tyrosine phosphorylation. Mol Cell Biol. 1994 Mar;14(3):2113–2118. doi: 10.1128/mcb.14.3.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X. Y. A transcription factor with SH2 and SH3 domains is directly activated by an interferon alpha-induced cytoplasmic protein tyrosine kinase(s). Cell. 1992 Jul 24;70(2):323–335. doi: 10.1016/0092-8674(92)90106-m. [DOI] [PubMed] [Google Scholar]
- Fujita T., Reis L. F., Watanabe N., Kimura Y., Taniguchi T., Vilcek J. Induction of the transcription factor IRF-1 and interferon-beta mRNAs by cytokines and activators of second-messenger pathways. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9936–9940. doi: 10.1073/pnas.86.24.9936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour K. C., Reich N. C. Receptor to nucleus signaling by prolactin and interleukin 2 via activation of latent DNA-binding factors. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):6850–6854. doi: 10.1073/pnas.91.15.6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouilleux F., Pallard C., Dusanter-Fourt I., Wakao H., Haldosen L. A., Norstedt G., Levy D., Groner B. Prolactin, growth hormone, erythropoietin and granulocyte-macrophage colony stimulating factor induce MGF-Stat5 DNA binding activity. EMBO J. 1995 May 1;14(9):2005–2013. doi: 10.1002/j.1460-2075.1995.tb07192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouilleux F., Wakao H., Mundt M., Groner B. Prolactin induces phosphorylation of Tyr694 of Stat5 (MGF), a prerequisite for DNA binding and induction of transcription. EMBO J. 1994 Sep 15;13(18):4361–4369. doi: 10.1002/j.1460-2075.1994.tb06756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg S. M., Rosenthal D. S., Greeley T. A., Tantravahi R., Handin R. I. Characterization of a new megakaryocytic cell line: the Dami cell. Blood. 1988 Dec;72(6):1968–1977. [PubMed] [Google Scholar]
- Harada H., Kitagawa M., Tanaka N., Yamamoto H., Harada K., Ishihara M., Taniguchi T. Anti-oncogenic and oncogenic potentials of interferon regulatory factors-1 and -2. Science. 1993 Feb 12;259(5097):971–974. doi: 10.1126/science.8438157. [DOI] [PubMed] [Google Scholar]
- Harada H., Takahashi E., Itoh S., Harada K., Hori T. A., Taniguchi T. Structure and regulation of the human interferon regulatory factor 1 (IRF-1) and IRF-2 genes: implications for a gene network in the interferon system. Mol Cell Biol. 1994 Feb;14(2):1500–1509. doi: 10.1128/mcb.14.2.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermine O., Mayeux P., Titeux M., Mitjavila M. T., Casadevall N., Guichard J., Komatsu N., Suda T., Miura Y., Vainchenker W. Granulocyte-macrophage colony-stimulating factor and erythropoietin act competitively to induce two different programs of differentiation in the human pluripotent cell line UT-7. Blood. 1992 Dec 15;80(12):3060–3069. [PubMed] [Google Scholar]
- Hou J., Schindler U., Henzel W. J., Ho T. C., Brasseur M., McKnight S. L. An interleukin-4-induced transcription factor: IL-4 Stat. Science. 1994 Sep 16;265(5179):1701–1706. doi: 10.1126/science.8085155. [DOI] [PubMed] [Google Scholar]
- Hunter T., Karin M. The regulation of transcription by phosphorylation. Cell. 1992 Aug 7;70(3):375–387. doi: 10.1016/0092-8674(92)90162-6. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Witthuhn B. A., Quelle F. W., Yamamoto K., Thierfelder W. E., Kreider B., Silvennoinen O. Signaling by the cytokine receptor superfamily: JAKs and STATs. Trends Biochem Sci. 1994 May;19(5):222–227. doi: 10.1016/0968-0004(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Johnston J. A., Kawamura M., Kirken R. A., Chen Y. Q., Blake T. B., Shibuya K., Ortaldo J. R., McVicar D. W., O'Shea J. J. Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2. Nature. 1994 Jul 14;370(6485):151–153. doi: 10.1038/370151a0. [DOI] [PubMed] [Google Scholar]
- Kaushansky K., Lok S., Holly R. D., Broudy V. C., Lin N., Bailey M. C., Forstrom J. W., Buddle M. M., Oort P. J., Hagen F. S. Promotion of megakaryocyte progenitor expansion and differentiation by the c-Mpl ligand thrombopoietin. Nature. 1994 Jun 16;369(6481):568–571. doi: 10.1038/369568a0. [DOI] [PubMed] [Google Scholar]
- Kirchhoff S., Schaper F., Hauser H. Interferon regulatory factor 1 (IRF-1) mediates cell growth inhibition by transactivation of downstream target genes. Nucleic Acids Res. 1993 Jun 25;21(12):2881–2889. doi: 10.1093/nar/21.12.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T., Taga T., Akira S. Cytokine signal transduction. Cell. 1994 Jan 28;76(2):253–262. doi: 10.1016/0092-8674(94)90333-6. [DOI] [PubMed] [Google Scholar]
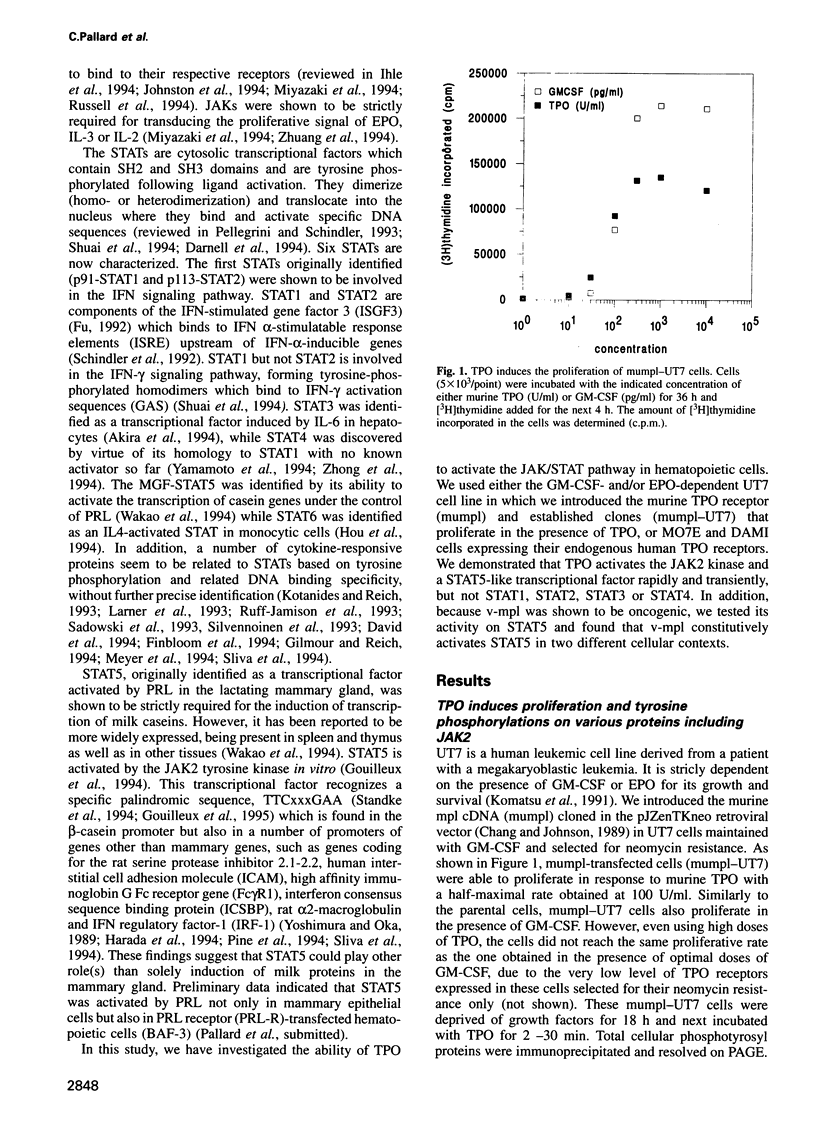
- Komatsu N., Nakauchi H., Miwa A., Ishihara T., Eguchi M., Moroi M., Okada M., Sato Y., Wada H., Yawata Y. Establishment and characterization of a human leukemic cell line with megakaryocytic features: dependency on granulocyte-macrophage colony-stimulating factor, interleukin 3, or erythropoietin for growth and survival. Cancer Res. 1991 Jan 1;51(1):341–348. [PubMed] [Google Scholar]
- Kotanides H., Reich N. C. Requirement of tyrosine phosphorylation for rapid activation of a DNA binding factor by IL-4. Science. 1993 Nov 19;262(5137):1265–1267. doi: 10.1126/science.7694370. [DOI] [PubMed] [Google Scholar]
- Larner A. C., David M., Feldman G. M., Igarashi K., Hackett R. H., Webb D. S., Sweitzer S. M., Petricoin E. F., 3rd, Finbloom D. S. Tyrosine phosphorylation of DNA binding proteins by multiple cytokines. Science. 1993 Sep 24;261(5129):1730–1733. doi: 10.1126/science.8378773. [DOI] [PubMed] [Google Scholar]
- Levy D. E., Kessler D. S., Pine R., Reich N., Darnell J. E., Jr Interferon-induced nuclear factors that bind a shared promoter element correlate with positive and negative transcriptional control. Genes Dev. 1988 Apr;2(4):383–393. doi: 10.1101/gad.2.4.383. [DOI] [PubMed] [Google Scholar]
- Lok S., Kaushansky K., Holly R. D., Kuijper J. L., Lofton-Day C. E., Oort P. J., Grant F. J., Heipel M. D., Burkhead S. K., Kramer J. M. Cloning and expression of murine thrombopoietin cDNA and stimulation of platelet production in vivo. Nature. 1994 Jun 16;369(6481):565–568. doi: 10.1038/369565a0. [DOI] [PubMed] [Google Scholar]
- Methia N., Louache F., Vainchenker W., Wendling F. Oligodeoxynucleotides antisense to the proto-oncogene c-mpl specifically inhibit in vitro megakaryocytopoiesis. Blood. 1993 Sep 1;82(5):1395–1401. [PubMed] [Google Scholar]
- Meyer D. J., Campbell G. S., Cochran B. H., Argetsinger L. S., Larner A. C., Finbloom D. S., Carter-Su C., Schwartz J. Growth hormone induces a DNA binding factor related to the interferon-stimulated 91-kDa transcription factor. J Biol Chem. 1994 Feb 18;269(7):4701–4704. [PubMed] [Google Scholar]
- Miyazaki T., Kawahara A., Fujii H., Nakagawa Y., Minami Y., Liu Z. J., Oishi I., Silvennoinen O., Witthuhn B. A., Ihle J. N. Functional activation of Jak1 and Jak3 by selective association with IL-2 receptor subunits. Science. 1994 Nov 11;266(5187):1045–1047. doi: 10.1126/science.7973659. [DOI] [PubMed] [Google Scholar]
- Morgenstern J. P., Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990 Jun 25;18(12):3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neal K. D., Yu-Lee L. Y. Differential signal transduction of the short, Nb2, and long prolactin receptors. Activation of interferon regulatory factor-1 and cell proliferation. J Biol Chem. 1994 Oct 21;269(42):26076–26082. [PubMed] [Google Scholar]
- Pellegrini S., Schindler C. Early events in signalling by interferons. Trends Biochem Sci. 1993 Sep;18(9):338–342. doi: 10.1016/0968-0004(93)90070-4. [DOI] [PubMed] [Google Scholar]
- Pine R., Canova A., Schindler C. Tyrosine phosphorylated p91 binds to a single element in the ISGF2/IRF-1 promoter to mediate induction by IFN alpha and IFN gamma, and is likely to autoregulate the p91 gene. EMBO J. 1994 Jan 1;13(1):158–167. doi: 10.1002/j.1460-2075.1994.tb06245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz R., Durbin J. E., Levy D. E. Acute phase response factor and additional members of the interferon-stimulated gene factor 3 family integrate diverse signals from cytokines, interferons, and growth factors. J Biol Chem. 1994 Sep 30;269(39):24391–24395. [PubMed] [Google Scholar]
- Ruff-Jamison S., Chen K., Cohen S. Induction by EGF and interferon-gamma of tyrosine phosphorylated DNA binding proteins in mouse liver nuclei. Science. 1993 Sep 24;261(5129):1733–1736. doi: 10.1126/science.8378774. [DOI] [PubMed] [Google Scholar]
- Russell S. M., Johnston J. A., Noguchi M., Kawamura M., Bacon C. M., Friedmann M., Berg M., McVicar D. W., Witthuhn B. A., Silvennoinen O. Interaction of IL-2R beta and gamma c chains with Jak1 and Jak3: implications for XSCID and XCID. Science. 1994 Nov 11;266(5187):1042–1045. doi: 10.1126/science.7973658. [DOI] [PubMed] [Google Scholar]
- Sadowski H. B., Shuai K., Darnell J. E., Jr, Gilman M. Z. A common nuclear signal transduction pathway activated by growth factor and cytokine receptors. Science. 1993 Sep 24;261(5129):1739–1744. doi: 10.1126/science.8397445. [DOI] [PubMed] [Google Scholar]
- Schindler C., Shuai K., Prezioso V. R., Darnell J. E., Jr Interferon-dependent tyrosine phosphorylation of a latent cytoplasmic transcription factor. Science. 1992 Aug 7;257(5071):809–813. doi: 10.1126/science.1496401. [DOI] [PubMed] [Google Scholar]
- Schwarz L. A., Stevens A. M., Hrachovy J. A., Yu-Lee L. Y. Interferon regulatory factor-1 is inducible by prolactin, interleukin-2 and concanavalin A in T cells. Mol Cell Endocrinol. 1992 Jul;86(1-2):103–110. doi: 10.1016/0303-7207(92)90180-e. [DOI] [PubMed] [Google Scholar]
- Shuai K., Horvath C. M., Huang L. H., Qureshi S. A., Cowburn D., Darnell J. E., Jr Interferon activation of the transcription factor Stat91 involves dimerization through SH2-phosphotyrosyl peptide interactions. Cell. 1994 Mar 11;76(5):821–828. doi: 10.1016/0092-8674(94)90357-3. [DOI] [PubMed] [Google Scholar]
- Silvennoinen O., Schindler C., Schlessinger J., Levy D. E. Ras-independent growth factor signaling by transcription factor tyrosine phosphorylation. Science. 1993 Sep 24;261(5129):1736–1739. doi: 10.1126/science.8378775. [DOI] [PubMed] [Google Scholar]
- Skoda R. C., Seldin D. C., Chiang M. K., Peichel C. L., Vogt T. F., Leder P. Murine c-mpl: a member of the hematopoietic growth factor receptor superfamily that transduces a proliferative signal. EMBO J. 1993 Jul;12(7):2645–2653. doi: 10.1002/j.1460-2075.1993.tb05925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliva D., Wood T. J., Schindler C., Lobie P. E., Norstedt G. Growth hormone specifically regulates serine protease inhibitor gene transcription via gamma-activated sequence-like DNA elements. J Biol Chem. 1994 Oct 21;269(42):26208–26214. [PubMed] [Google Scholar]
- Souyri M., Vigon I., Penciolelli J. F., Heard J. M., Tambourin P., Wendling F. A putative truncated cytokine receptor gene transduced by the myeloproliferative leukemia virus immortalizes hematopoietic progenitors. Cell. 1990 Dec 21;63(6):1137–1147. doi: 10.1016/0092-8674(90)90410-g. [DOI] [PubMed] [Google Scholar]
- Standke G. J., Meier V. S., Groner B. Mammary gland factor activated by prolactin on mammary epithelial cells and acute-phase response factor activated by interleukin-6 in liver cells share DNA binding and transactivation potential. Mol Endocrinol. 1994 Apr;8(4):469–477. doi: 10.1210/mend.8.4.7519723. [DOI] [PubMed] [Google Scholar]
- Tanaka N., Ishihara M., Kitagawa M., Harada H., Kimura T., Matsuyama T., Lamphier M. S., Aizawa S., Mak T. W., Taniguchi T. Cellular commitment to oncogene-induced transformation or apoptosis is dependent on the transcription factor IRF-1. Cell. 1994 Jun 17;77(6):829–839. doi: 10.1016/0092-8674(94)90132-5. [DOI] [PubMed] [Google Scholar]
- Vigon I., Florindo C., Fichelson S., Guenet J. L., Mattei M. G., Souyri M., Cosman D., Gisselbrecht S. Characterization of the murine Mpl proto-oncogene, a member of the hematopoietic cytokine receptor family: molecular cloning, chromosomal location and evidence for a function in cell growth. Oncogene. 1993 Oct;8(10):2607–2615. [PubMed] [Google Scholar]
- Vigon I., Mornon J. P., Cocault L., Mitjavila M. T., Tambourin P., Gisselbrecht S., Souyri M. Molecular cloning and characterization of MPL, the human homolog of the v-mpl oncogene: identification of a member of the hematopoietic growth factor receptor superfamily. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5640–5644. doi: 10.1073/pnas.89.12.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakao H., Gouilleux F., Groner B. Mammary gland factor (MGF) is a novel member of the cytokine regulated transcription factor gene family and confers the prolactin response. EMBO J. 1994 May 1;13(9):2182–2191. doi: 10.1002/j.1460-2075.1994.tb06495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendling F., Maraskovsky E., Debili N., Florindo C., Teepe M., Titeux M., Methia N., Breton-Gorius J., Cosman D., Vainchenker W. cMpl ligand is a humoral regulator of megakaryocytopoiesis. Nature. 1994 Jun 16;369(6481):571–574. doi: 10.1038/369571a0. [DOI] [PubMed] [Google Scholar]
- Wendling F., Penciolelli J. F., Charon M., Tambourin P. Factor-independent erythropoietic progenitor cells in leukemia induced by the myeloproliferative leukemia virus. Blood. 1989 Apr;73(5):1161–1167. [PubMed] [Google Scholar]
- Willman C. L., Sever C. E., Pallavicini M. G., Harada H., Tanaka N., Slovak M. L., Yamamoto H., Harada K., Meeker T. C., List A. F. Deletion of IRF-1, mapping to chromosome 5q31.1, in human leukemia and preleukemic myelodysplasia. Science. 1993 Feb 12;259(5097):968–971. doi: 10.1126/science.8438156. [DOI] [PubMed] [Google Scholar]
- Witthuhn B. A., Silvennoinen O., Miura O., Lai K. S., Cwik C., Liu E. T., Ihle J. N. Involvement of the Jak-3 Janus kinase in signalling by interleukins 2 and 4 in lymphoid and myeloid cells. Nature. 1994 Jul 14;370(6485):153–157. doi: 10.1038/370153a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Quelle F. W., Thierfelder W. E., Kreider B. L., Gilbert D. J., Jenkins N. A., Copeland N. G., Silvennoinen O., Ihle J. N. Stat4, a novel gamma interferon activation site-binding protein expressed in early myeloid differentiation. Mol Cell Biol. 1994 Jul;14(7):4342–4349. doi: 10.1128/mcb.14.7.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M., Oka T. Isolation and structural analysis of the mouse beta-casein gene. Gene. 1989 May 30;78(2):267–275. doi: 10.1016/0378-1119(89)90229-1. [DOI] [PubMed] [Google Scholar]
- Zhong Z., Wen Z., Darnell J. E., Jr Stat3 and Stat4: members of the family of signal transducers and activators of transcription. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4806–4810. doi: 10.1073/pnas.91.11.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang H., Patel S. V., He T. C., Sonsteby S. K., Niu Z., Wojchowski D. M. Inhibition of erythropoietin-induced mitogenesis by a kinase-deficient form of Jak2. J Biol Chem. 1994 Aug 26;269(34):21411–21414. [PubMed] [Google Scholar]
- de Sauvage F. J., Hass P. E., Spencer S. D., Malloy B. E., Gurney A. L., Spencer S. A., Darbonne W. C., Henzel W. J., Wong S. C., Kuang W. J. Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature. 1994 Jun 16;369(6481):533–538. doi: 10.1038/369533a0. [DOI] [PubMed] [Google Scholar]