Abstract
Objective
There is vast evidence for brain aberrations in patients with fibromyalgia (FM) and it is possible that central plasticity is critical for the transition from acute to chronic pain. However, the relationship between brain structure and function is poorly investigated.
Methods
The present study, including 26 FM patients and 13 age- and gender-matched healthy controls, investigated the differences between patients and controls regarding functional connectivity during intermittent pressure pain and measures of brain structure. Magnetic resonance imaging (MRI) was used to obtain high-resolution anatomical images and functional MRI scans for measures of pain-evoked brain activity.
Results
FM patients displayed a distinct overlap between decreased cortical thickness, brain volumes and measures of functional regional coherence in the rostral anterior cingulate cortex. The morphometric changes were more pronounced with longer exposure to FM pain. In addition, we found associations between structural and functional changes in the mesolimbic areas of the brain and comorbid depressive symptoms in FM patients.
Conclusion
The combined integration of structural and functional measures allowed for a unique characterization of the impact of FM pain on the brain. Our data may lead to the identification of early structural and functional brain alterations in response to pain, which could be used to develop markers to predict the development of FM and other pain disorders.
Keywords: Magnetic Resonance Imaging, fibromyalgia, morphology, functional connectivity, ReHo
Chronic pain is a common health problem with limited treatment options. As a result, patients often suffer from persistent pain for decades, with inadequate access to effective pain relief and high rates of comorbid depression (1). In spite of the high prevalence of chronic pain, the impact of long-term exposure of on the human brain is still poorly investigated.
Fibromyalgia (FM) is a condition of widespread musculoskeletal pain, soft tissue tenderness, fatigue and sleep disturbances (2). Studies suggest that the presence of long-term localized pain is the most prevalent precursor of developing widespread pain (3) and FM (4). Moreover, long-term follow-ups demonstrate that FM remains chronic, with low probability of full recovery (5). There is evidence for central nervous system aberrations in FM (6, 7) for example, augmented responses to experimental pain stimuli (8, 9), changes in resting-state functional connectivity (10, 11) and altered function of brain neurotransmitters (12–15). In addition, studies found that FM is associated with brain morphological changes, such as decreased gray matter in the insula, rostral anterior cingulate cortex (rACC) and medial prefrontal cortex, compared to age-matched healthy controls (16–20).
Neuroimaging has significantly contributed to our understanding of the brain mechanisms associated with FM (21). Nevertheless, the linkage between brain structure, brain function and clinical symptoms, which could potentially elucidate the pathology and development of the disorder, is still missing. The combination of several independent brain measures, in the same group of patients, may represent a critical step to elucidate the central mechanisms of FM and negative impact of long-term exposure to pain. To that end, we designed a study where we first investigated the difference between FM patients and healthy controls regarding measures of brain morphology and functional regional homogeneity. Then, we investigated how the duration of FM symptoms and comorbid depressive symptoms had affected the morphology and regional homogeneity within the patient group. We hypothesized that brain regions previously implicated in FM pathology would display both functional and morphometric changes compared to healthy controls. We also hypothesized that the altered brain structure and functional changes observed in FM patients would be associated with the duration of FM symptoms and presence of comorbid depressive symptoms.
Materials and Methods
Participants
A total of 92 female FM patients aged 25 to 55 years, mean age 44 (SD = 8.2) were enrolled. Of the 92 patients 9 were excluded from fMRI analyses due to image artifacts or en passant findings of intracranial anomalies, leaving 83 patients for analysis with matched healthy controls. A total of 13 healthy female controls were available; aged 24 to 48 years, mean age 34 (SD = 8.6). Due to the relatively large number of patients, each healthy control could be age-matched with two FM patients (n=26, range 24–48 years, mean 38, SD = 6.8). All patients in this study were investigated as part of the baseline assessments of a pharmacological fMRI clinical trial including three sites: one in England, Sweden and Germany. The same brain imaging assessments were also performed in healthy controls at all three sites, consecutively recruited throughout the study. The study was approved by the local Ethical Committee at each of the three participating sites and all participants gave written informed consent. Parts of the present dataset were used in Jensen et al 2010, where all 92 available FM patients were analyzed (22) and Jensen et al 2009, where 11/26 FM patients and 13/13 controls from the present study were included (23). None of the previous publications included analyses of brain morphology or regional functional connectivity.
Screening
Before inclusion in the study, both FM patients and healthy controls were carefully screened for the inclusion and exclusion criteria. All FM patients were recruited from primary care and at the beginning of the screening visit an experienced pain physician confirmed the FM diagnosis, using the 1990 American College of Rheumatology criteria (24). The inclusion criteria required that FM patients had an average pain intensity of at least 40 out of 100 mm on a visual analogue scale (VAS) during the week prior to screening. Exclusion criteria included, for example, severe psychiatric disorder, suicide risk or history of substance, drug, or alcohol abuse. The data in the present study was collected as the baseline measure of a pharmacological Randomized Controlled Trial (RCT) and therefore patients’ medications or other treatments were strictly limited. The inclusion criteria required discontinuation of all treatments that could influence the patients’ pain perception, i.e., antidepressants and mood stabilisers, analgesics (tramadol, codeine, dextropropoxyphene), strong opioids including patches, anesthetic patches, anticonvulsants, centrally acting relaxants, joint injections, trigger/tender point injections, biofeedback and transcutaneous electrical nerve stimulation.
Pain stimulation
Experimental pain stimuli were applied to the thumbnail using a standardized method where a computer-controlled stimulator applies pressure via a 1 cm2 hard rubber probe (25). The thumb is inserted into a cylindrical opening and the probe applies pressure to the nail bed. Pressure pain is commonly used in FM studies because pressure sensitivity is a diagnostic criterion for FM and elicits a deep pain sensation that is clinically valid in this group of patients.
Brain imaging
Brain images were collected using 1.5 Tesla scanners at each of the three study sites, respectively. In London, a General Electric HDx scanner was used, in Stockholm a General Electric Twinspeed Signa Horizon and in Cologne a PHILIPS scanner was used. Multiple T2*-weighted single-shot gradient echo EPI sequences were used to acquire blood oxygen level dependent (BOLD) contrast images. The following parameters were used: repetition time: 3000 ms (35 slices acquired), echo time: 40 ms, flip angle: 90 degrees, field of view: 24 × 24 cm, 64 × 64 matrix, 4 mm slice thickness with 0.4 mm gap and sequential image acquisition order. Visual distraction during scans was minimized by placing a blank screen in front of the patient’s field of view from inside the scanner. In addition to the functional scans, high-resolution T1-weighted structural images were acquired in coronal orientation for analysis of brain morphology. Parameters were: Spoiled Gradient Recalled 3D sequence, repetition time: 24 ms, echo time: 6 ms, flip angle 35 degrees with a voxel size of 0.9 × 1.5 × 0.9 mm3.
The scanning procedure was standardized between sites, by using written manuscripts for all oral instructions and practical training for the investigators. All FM patients and healthy controls were site-matched in order to make sure there was an equal number of patients and controls from every site. We also performed several statistical analyses to validate that the site-factor had no significant effect on brain data. Previous evidence (26) validate that the variance between individuals in neuroimaging studies is greater than the variance between scanners at different sites.
Procedure - Behavioral data
The day before fMRI scanning, each participant was calibrated for subjective pain ratings by receiving one ascending and one randomized series of pressure stimuli. Pressures (duration 2.5 seconds) were delivered at 30 seconds intervals. Participants were instructed to rate the intensity of the pain evoked by each stimulus by putting a mark on a 0–100 mm VAS ranging from “no pain” to “worst imaginable pain”. Each participant’s pain threshold (first VAS > 0 mm) and stimulation maximum (first rating > 60 mm VAS) was used to compute five different pressure intensities within the range of the threshold and maximum. In total, 15 stimuli, each lasting 2.5 seconds, were delivered in a randomized order and a polynomial regression was used to determine each individual’s pressure at VAS 50 mm, hereafter referred to as P50.
The Beck Depression Inventory (BDI) was used to quantitatively assess depressive symptoms. The BDI is a 21-item measure of the severity of depressive symptoms, and it has been extensively validated for use with both medical and mental health populations (27). A score of 0–9 indicates minimal depression, 10–18 indicates mild depression, 19–29 indicates moderate depression and 30–63 indicates severe depression.
Procedure - Functional imaging
Experimental pain during fMRI scanning was induced by means of painful pressures, individually calibrated to represent each participant’s 50 mm VAS (P50), as well as non-painful pressures, representing 0 mm VAS. Again, all stimulations were randomly distributed over the scanning time, preventing participants from anticipating the onset time and event type. The time interval between stimuli had a mean stimulus onset asynchronicity of 15 seconds (range 10–20 seconds). The study design included four different random sequences but the particular order was randomized for each participant. Each participant received at least two sequences. The total duration of two sequences was 16 minutes. Participants were instructed to focus on the pressures on the thumb and to not use any distraction or coping techniques.
Statistical analyses - Behavioral data
The correlations between duration of FM symptoms, pain ratings and sensitivity to pressure-pain were performed by means of partial correlations, controlling for physical age and depression scores, using the statistical software SPSS, version 18.0.
Statistical analyses – Neuroimaging data
Cortical Thickness
The cortical surface reconstruction was performed using FreeSurfer version 5.1 (http://surfer.nmr.mgh.harvard.edu). FreeSurfer uses a series of computationally intensive steps on the T1-weighted structural volumes to estimate the gray/white interface (28–30). These steps include motion correction and averaging, computing Talairach transforms, intensity normalization, skull stripping, tessellation of the gray matter/white matter boundary, automated topology correction, automatic volume labeling and white matter segmentation. Any inaccuracies in the reconstruction of white and pial surfaces of individual subjects were manually corrected before calculating cortical thickness. The cortical thickness measure was computed as the distance between the pial and white surfaces at each point across the cortical mantle.
Group analyses were performed by resampling each subject’s data to the FreeSurfer average atlas, distributed as a part of FreeSurfer. Cortical thickness maps were smoothed using a Gaussian kernel with a full width half maximum of 10 mm. General Linear Model was calculated at each vertex on the surface covarying for physical age. To test for significant differences in cortical thickness between the two groups, the slope of the lines for the two groups, representing the rate of change of thickness over time, was assumed to be constant and the offset/ intercept representing cortical thickness was varied to get the best fit for each group. In order to determine the relationship between cortical thickness and clinical scores in patients, thickness was regressed on a vertex-by vertex basis against scores of duration and depression while controlling for physical age. For this analysis an initial vertex wise threshold of p<.001 was used, uncorrected.
MRI Volumes
The automated procedure for labeling different brain structures, and getting their volumetric measures, is described in detail by Fischl et al (29). This procedure assigns a neuroanatomical label to each voxel in an MRI volume based on probabilistic information automatically estimated from a manually labeled training set, including both grey and white matter. This technique has been shown to be comparable in accuracy to manual labeling. The automatic segmentations were also visually inspected for accuracy. In the present paper, we report results of total brain volumes (supra-tentoral), volumes for cortical and sub-cortical gray matter, white matter, and the a priori regions of interest including rostral Anterior Cingulate Cortex (rACC), amygdala, lateral orbitofrontal cortex (lOFC).
Statistical analyses for volumetric measures extracted from Freesurfer’s automated segmentation, were performed using SPSS 18.0. Group differences between FM patients and controls in cortical gray matter, sub-cortical gray matter, cortical white matter, sub-cortical white matter and segmentation volumes of the a priori ROIs were analyzed using independent samples t-tests. To get an estimate of the relationship between neuroanatomical volumes from the a priori ROIs and the behavioral measures depression scores and pain duration, partial correlation analyses were performed. The correlation between ROI volumes and FM duration was controlled for ‘age’ and ‘depression’ and, conversely, the correlation between ROI volumes and depression was controlled for ‘age’ and ‘duration’. For all comparisons, the resulting p-values were corrected using the Bonferroni method. Since there were six a priori ROIs (three bilateral structures), the resulting corrected threshold for all pairwise comparisons was p<.008.
Regional Homogeneity (ReHo) functional connectivity
All pre-processing and ReHo analyses were performed using DPARSFA (Data Processing Assistant for Resting State fMRI, Advanced) based on SPM8 (31–33). Pre-processing included the following steps: removal of the first 10 time points, slice timing correction using the middle slice as a reference, realignment (motion correction), normalization into MNI space using an EPI template, linear trend removal, and temporal filtering of results by 0.01–0.08 Hz. After this, the ReHo according to Kendall’s coefficient concordance (KCC) (34) was calculated using a cluster consisting of 27 voxels. At a given voxel, ReHo was defined as the KCC of the time series of this voxel with those of its 26 nearest neighbors. The resulting map of ReHo values at each voxel was then divided by the global mean value within the whole-brain mask. This result was then smoothed with a kernel of 4 mm. Ultimately, a two-sample t-test and two separate regression analyses were performed, using the second-level analysis function in the Statistical Parametric Mapping 8 (SPM8) software (SPM8, Wellcome Trust Centre for Neuroimaging, London, UK) and Matlab 7.4 (Mathworks). In line with previous studies, a threshold of voxel-wise p <.005 uncorrected with 20 contiguous voxels was used for predefined ROI’s. For non-ROI brain regions, the threshold was set at voxel-wise p <.001 (uncorrected) and p <.05 FWE-corrected at cluster level.
Results
Between group effects
Behavioral outcomes
A two sample t-test revealed a significant difference in pressure pain sensitivity (P50) between FM patients and healthy controls, t(37)=-4.0, p <.001, two-tailed; validating that patients needed significantly lower amounts of pressure than controls to experience comparable pain intensities (see Table 1).
Table 1. Subjects’ characteristics.
The mean value and Standard Deviation (SD) is given for each variable. ‘The pressure at VAS 50’ represents the pressure required to evoke pain that corresponds to a rating of 50 on a 0–100 mm visual analogue scale. The duration of FM symptoms is based on patients’ subjective report. ‘Weekly pain intensity’ refers to patients’ rating of the average clinical pain intensity over the last week, on a 0–100 mm visual analogue scale. The ‘BDI score’ refers to self-rated depressive symptoms where 0–9 indicates minimal depression, 10–18 indicates mild depression, 19–29 indicates moderate depression and 30–63 indicates severe depression. For the variables ‘Age’ and ‘Pressure at VAS 50’, the t-score and p-value from an independent samples t-test is also listed.
| Variable | FM patients (n=26) | Controls (n=13) | t-score | p-value |
|---|---|---|---|---|
| Age (years) | 38 (7) | 34 (9) | 1.8 | 0.08 |
| Pressure at VAS 50 (kPa) | 341 (133) | 572 (186) | 4.14 | 0.001* |
| Duration of FM symptoms (years) | 11(6) | Na | ||
| Weekly pain intensity (VAS mm) | 72 (14) | Na | ||
| BDI score | 21 (11) | Na |
significant
Neuroimaging outcomes
Cortical thickness
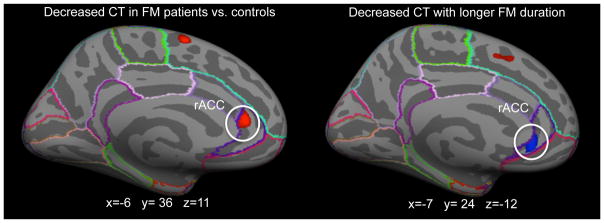
A vertex-wise whole brain analysis of cortical thickness generated significance maps for groups of vertices with significant differences between FM patients and controls. This analysis revealed several regions of significantly lower cortical thickness in FM patients compared to controls: the left rACC, which was part of our a priori hypothesis, as well as others beyond the territory of the hypothesis: left superior frontal gyrus, right superior temporal gyrus, right and left middle temporal gyrus and right fusiform gyrus. Only one group of vertices displayed higher cortical thickness in FM patients compared to controls, located in the right superior parietal gyrus (see Table 2 and Figure 1).
Table 2. Cortical thickness and functional differences between FM patients and healthy controls.
FreeSurfer analyses of cortical thickness and ReHo analyses of functional regional coherence. The anatomical locations of each cluster is given in Montreal Neurological Institute (MNI) coordinates (x, y, z). Regions in italics denote our a priori regions of interest.
| Cortical Thickness Controls > FM |
Laterality | Number of vertices | X | Y | Z | Max p-value |
|---|---|---|---|---|---|---|
| rACC | L | 136 | −6 | 36 | 11 | 0.00041 |
| Superior frontal | L | 85 | −7 | −3 | 62 | 0.00046 |
| Middle temporal | L | 48 | −56 | −14 | −20 | 0.00095 |
| Superior temporal | R | 250 | 55 | −22 | 4 | 0.00024 |
| Middle temporal | R | 106 | 63 | −16 | −16 | 0.00037 |
| Fusiform | R | 60 | 37 | −25 | −22 | 0.00082 |
| Cortical Thickness FM > Controls |
Laterality | Number of vertices | X | Y | Z | Max p-value |
|---|---|---|---|---|---|---|
| Superior parietal | R | 181 | 26 | −61 | 44 | 0.00021 |
| ReHo Controls > FM |
Laterality | Cluster size (voxels) | X | Y | Z | p-value cluster |
|---|---|---|---|---|---|---|
| rACC / MPFC | L | 756 | −4 | 49 | −11 | 0.05 |
Figure 1. Cortical Thickness measures in FM patients and healthy controls.
Measures of cortical thickness, using FreeSurfer, revealed lower cortical thickness measures in the left rACC (MNI x=−6, y=36, z=11) in FM patients, compared to age- and gender-matched healthy controls.
MRI volumes
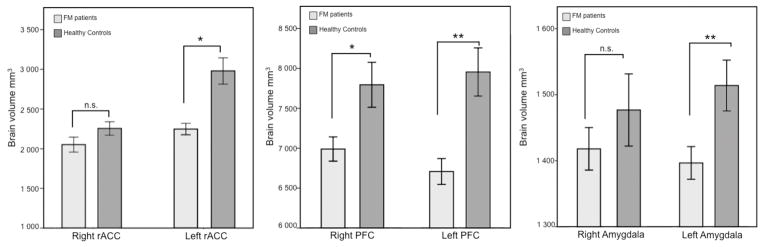
In an overall analysis of total brain volume (supratentorial), including both cortical and subcortical structures, patients displayed significantly lower total brain volume than controls, t(37)=−3.6, p=0.001. The automated segmentation of cerebral matter in FreeSurfer allowed for more specific comparisons of white and gray matter volumes. Analyses of gray matter volumes, both cortical and subcortical, revealed that patients had significantly lower volumes than controls in cortical gray matter t(37)=−3.2, p=.003, two-tailed, as well as subcortical gray matter t(37)=−2.7, p=.010, two-tailed. In addition to gray matter volumes, patients displayed significantly lower volume of cortical white matter compared to healthy controls t(37)=−2.9, p=.006, two-tailed indicating that the decreased brain volumes seen in FM were not only an effect of gray matter atrophy.
Comparisons of a priori ROI volumes (listed previously) between patients and controls revealed that patients had significantly lower volumes in the left rACC, t(37)=−4.7, p=.00003 and the left lOFC, t(37)=−3.79, p=.0005. The right lOFC and right amygdala approached significance but were not significant after Bonferroni correction: right lOFC, t(37)=−2.49, p=.017; and right amygdala, t(37)=−2.75, p=.009. The left amygdala and the right rACC were not significantly different between patients and controls.
ReHo functional connectivity
Analyses of functional regional homogeneity (ReHo) revealed significantly lower coherence in the rACC in FM patients, compared to controls. The cluster was partially located in the left cingulate cortex but also extended into the left medial prefrontal cortex. There were no regions that showed significantly higher regional coherence in patients than controls (see Table 2 and Figure 2).
Figure 2. Overlap of three different brain measures comparing FM patients and controls.
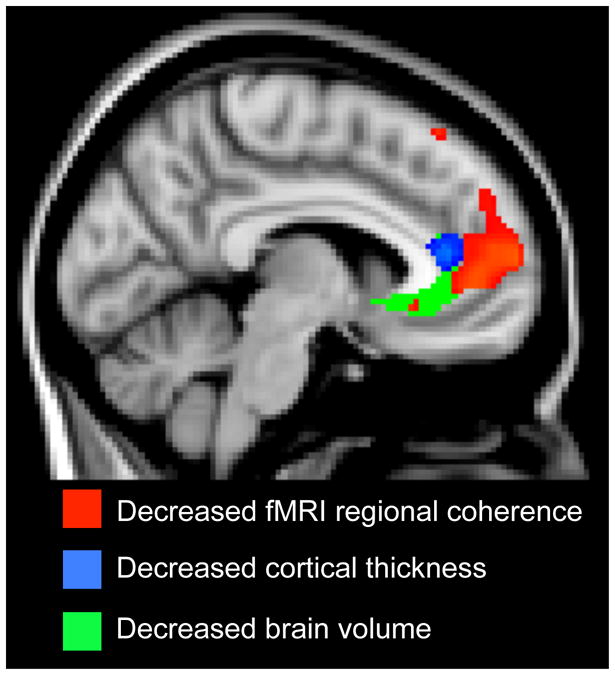
Spatial overlap between the results from volumetric, cortical thickness and regional functional connectivity analyses (ReHo). For all three analyses methods, the rostral ACC displayed decreased measures in FM patients, compared to controls, with regard to brain volume, cortical thickness and functional connectivity.
Within group effects - FM Duration
Behavioral outcomes
Within the patient group (n=26), a partial correlation displayed no significant association between the duration of FM and ratings of clinical pain intensity (VAS), r=.029, p=.892; but a moderate correlation between FM duration and sensitivity to P50 pressure pain, r=.405, p=.050; see Table 1.
Neuroimaging outcomes
Cortical thickness
Regression analyses were used to investigate the effects of FM duration on cortical thickness in patients (n=26). Significance maps indicating regions with significant associations between cortical thickness and FM duration revealed specific regions on the cortical surface (see Table 3). The middle temporal gyrus displayed a significant negative association between FM duration and cortical thickness, as well as the rACC, which was included in our a priori hypothesis, indicating lower cortical thickness with longer FM duration (see Figure 3). Several groups of vertices, specifically in the left middle frontal cortex, bilateral parietal lobes and bilateral temporal lobes, displayed higher cortical thickness with longer duration of FM pain (see Table 3).
Table 3.
Cortical thickness related to FM duration and depression.
Cortical thickness measures for the duration of FM pain, using age and depression as covariates of no interest. Comorbid depression was measured by Beck’s Depression Inventory (BDI). Negative results indicate decreased cortical thickness with longer FM duration or higher depression scores. Conversely, positive results indicate increased cortical thickness with longer FM duration or high depression. The anatomical locations of each cluster is given in Montreal Neurological Institute (MNI) coordinates (x, y, z). Regions in italics denote our a priori regions of interest.
| Cortical Thickness FM Duration - Negative results |
Laterality | Number of vertices | X | Y | Z | Max p-value |
|---|---|---|---|---|---|---|
| Middle temporal | R | 99 | 63 | −27 | −16 | 0.0003 |
| rACC | R | 82 | 5 | 31 | −7 | 0.00097 |
| Cortical Thickness FM Duration - Positive results |
Laterality | Number of vertices | X | Y | Z | Max p-value |
|---|---|---|---|---|---|---|
| Supramarginal | L | 118 | −50 | −52 | 45 | 0.00011 |
| Rostral middle frontal | L | 118 | −30 | 43 | 18 | 0.00015 |
| Inferior temporal | L | 122 | −51 | −63 | −5 | 0.00040 |
| Superior frontal | L | 40 | −20 | 8 | 61 | 0.00073 |
| Pars opercularis | L | 82 | −33 | 22 | 12 | 0.00074 |
| Inferior temporal | R | 120 | 46 | −34 | −20 | 0.000082 |
| Precentral | R | 25 | 52 | −4 | 8 | 0.00037 |
| Caudal middle frontal | R | 41 | 39 | 19 | 38 | 0.00062 |
| Cortical Thickness Depression – Negative results |
Laterality | Number of vertices | X | Y | Z | Max p-value |
|---|---|---|---|---|---|---|
| Pericalcarine | R | 896 | 18 | −72 | 5 | 0.0000036 |
| Fusiform | R | 161 | 38 | −62 | −18 | 0.00010 |
| Fusiform | R | 20 | 34 | −46 | −14 | 0.0007 |
| Lateral occipital | R | 55 | 24 | −86 | 17 | 0.00056 |
| Paracentral | R | 64 | 17 | −26 | 39 | 0.00062 |
| Inferior parietal | L | 133 | −39 | −70 | 44 | 0.000059 |
| Caudal middle frontal | L | 66 | −25 | −2 | 45 | 0.00018 |
| Precuneus | L | 60 | −18 | −77 | 30 | 0.00059 |
| Fusiform | L | 14 | −37 | −29 | −25 | 0.00084 |
| Cortical Thickness Depression – Positive results |
Laterality | Number of vertices | X | Y | Z | Max p-value |
|---|---|---|---|---|---|---|
| Supramarginal | L | 265 | −54 | −51 | 27 | 0.00032 |
Figure 3. Duration of FM pain and brain volumes.
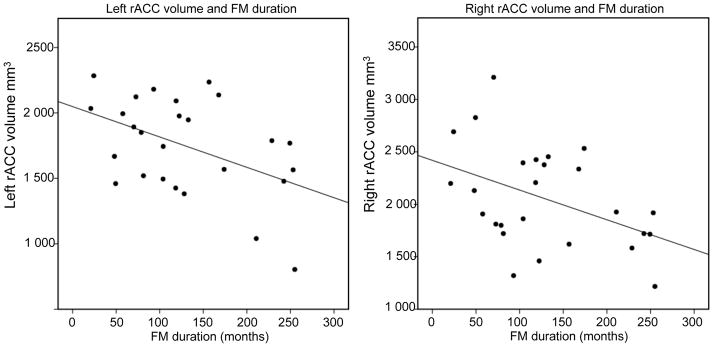
The duration of FM pain was negatively correlated to brain volumes in the right OFC and right rACC, controlling for physical age and depression (r=−.58, p=0.001, two-tailed) and (r=−.54, p=.007, two-tailed).
MRI volumes
A regression analysis revealed that the supratentorial brain volume, including both cortical and subcortical grey matter areas, was significantly decreased with longer FM duration, r=−.435, p=.030. In separate analyses of cortical and subcortical gray matter, there were significant decreases in gray matter volume with longer FM duration for subcortical gray matter, r=−.403, p=.046; but only a trend towards significance for the cortical gray matter, r=−.384, p=.058. A separate correlation between FM duration and white matter volumes displayed a significant decrease of white matter with longer FM duration, r=−.403, p=.046.
The volumes of the right and left rACC were negatively correlated to FM duration, suggesting decreased rACC volumes with longer FM duration; left rACC, r=−.44, p=.034; right rACC, r=−.54, p=.007. The left and right lOFC also displayed negative correlations with FM duration; left lOFC, r=−.42, p=.040; right lOFC r=−.58, p=.001. Amygdala volumes were not significantly correlated with FM duration; left amygdala, r=−.42, p=.052; right amygdala, r=−.29, p=.17. After Bonferroni correction for multiple comparisons, we conclude that the decreased volume observed in the right lOFC and right rACC were significantly correlated to FM duration (see Figure 3).
ReHo functional connectivity
The effect of FM duration on functional regional coherence was assessed by means of an fMRI regression analysis in FM patients (n=26), using FM duration as a covariate of interest. The regression of FM duration revealed no significant changes in functional regional coherence; negative or positive.
Within group effects – Depression
Behavioral outcomes
Within the patient group (n=26), a partial correlation displayed no significant association between BDI depression scores and FM duration, r=.23, p=.26; and no correlation between depression scores and ratings of clinical pain intensity, r=.10, p=.60.
Neuroimaging outcomes
Cortical thickness
The effect of BDI depression scores on cortical thickness was analyzed by means of regression analyses, creating a cortical thickness map revealing depression related thinning in FM patients. Several areas on the cortical surface displayed significant negative correlations between cortical thickness and depression scores. The significant regions were located primarily in the parietal and occipital cortex, indicating that cortical thinning in these regions were associated with higher BDI scores. Only one group of vertices, located in the left parietal lobe (supramarginal gyrus), displayed a positive correlation between depression and cortical thickness (see Table 3).
MRI volumes
Total brain volume
There were no significant correlations between BDI scores and the supratentorial grey matter brain volume, including both cortical and subcortical areas r=−.02, p=.92; cortical gray matter r=−.19; p=.37; subcortical gray matter r=.05, p=.82; or cortical white matter r=.14, p=.50.
ReHo functional connectivity
A regression analysis of functional regional coherence and BDI depression scores revealed significant changes in regional coherence in the ventral striatum/Nucleus Accumbens (NAc). With high levels of comorbid depressive symptoms, patients displayed relatively lower regional coherence in the NAc region of the striatum (MNI coordinates: x=21, y=15, z=0; cluster size: 57 voxels; peak T-value: 3.85; p<.005 cluster corrected). There were no significant results for the opposite depression contrast.
Discussion
In this study, we investigated structural and functional brain differences between FM patients and matched controls. Our results suggest that the rACC, a key region for modulation of pain, had significantly decreased cortical thickness, brain volume and regional functional connectivity in FM, as compared to healthy controls. The volumetric changes were more pronounced with longer exposure to FM pain. Also, there were structural and functional changes in the mesolimbic areas of the brain associated with comorbid depressive symptoms. The present study provides unique evidence for overlapping neuroanatomical and functional brain changes in patients with chronic pain.
Consistent with previous studies (35, 36) our study found that FM patients were significantly more sensitive to experimental pain than controls, reflecting a core symptom and diagnostic criterion in FM. In line with this behavioral difference, the analysis of regional functional connectivity in FM patients and controls revealed lower rACC coherence in FM patients, compared to controls. Impaired pain inhibitory responses in FM patients have previously been associated with lower activations of the rACC (23) and less functional connectivity between the rACC and other regions of the brain’s pain modulatory system (37). The functional connectivity method used in this study, ReHo, is a model-free estimation of the synchrony of spontaneous fMRI signal oscillations within spatially neighboring voxels and has recently proven to be sensitive to disease-related alterations, as demonstrated in several different disorders (38–40). Other functional connectivity methods such as seed based methods (which focus on connectivity between a particular seed and other brain regions) (41) and independent component analysis (ICA, which focuses on identifying different components of brain networks) (42) are used to identify key networks of the brain. ReHo, on the other hand, measures the local synchrony of different brain regions, facilitating the identification of key brain regions, rather than networks. This unique character of ReHo is important when investigating the relationship between brain structure and functional. Since the rACC is a key region for descending inhibition of pain (43, 44), the lower rACC coherence in FM patients provides support for the hypothesis that the FM pathology may be associated with dysfunction of descending pain modulation (36, 45, 46).
In consistency with previous morphometric studies in FM (16–20) we found decreased measures of cortical thickness and brain volumes in FM patients, compared to controls. As expected, the cortical thickness of the rACC was decreased with longer FM duration. In line with our hypothesis, there was a negative association between FM duration and rACC volumes, suggesting that rACC volumes are decreased with increased duration of FM pain. A study from 2007, found lower mu-opioid receptor function in the rACC (12), possibly reflecting significant atrophy in this region. Our results indicate that the rACC may an important element in the development and/or pathophysiology of FM. In line with previous morphometric reports of increased grey matter volumes in FM patients (20) we also found increased cortical thickness with increased FM duration in several temporal and frontal regions. It is possible that increased cortical thickness with longer FM duration reflects a compensatory mechanism, attempting to prevent the negative effects of a constant nociceptive input to the brain, however the anatomical specificity is not clear.
In a recent morphometric study (47) the authors found that the difference in gray matter volumes between FM and matched controls disappeared when controlling for comorbid affective measures, suggesting that brain atrophy might be more related to negative affect than pain. To address this concern, we used patients’ ratings of depressive symptoms (BDI) to assess the specific impact of comorbid depression on brain function and structure in FM. A regression analysis of fMRI data revealed a significant association between high depressive scores and decreased functional coherence in the ventral striatum. The ventral striatum is part of the mesolimbic reward circuitry and low activations have previously been associated with core symptoms of depression, such as anhedonia and reduced motivation (48). There were no brain regions with significantly greater regional coherence with higher depression. The analysis of cortical thickness revealed significant decreases in cortical thickness in 9 regions with higher depression scores, including the parietal cortex, which has previously been implicated in depression (48). There was no spatial overlap between the functional decrease in mesolimbic activation and decreases in brain volumes. Furthermore, we did not find any significant depression-related changes in any of the regions that were defined as regions of interest (functional or structural). Taken together, our results indicate that depressive symptoms are associated with cerebral changes, independent from pain. The results are in line with our findings from an earlier neuroimaging study in which we found independent neural mechanisms for FM pathology and depression (22), even if depression and pain are inherently related in the clinical context.
Recent neuroimaging studies have investigated the causality between chronic pain and measures of decreased brain volumes (49, 50). Data suggest that long-term exposure to pain causes reductions in specific brain regions, and not vice versa. Here, we found that FM duration was significantly correlated to brain changes; nevertheless, the sequence of these changes needs to be fully investigated in longitudinal studies. Such studies could potentially explore whether structural and functional alterations are early indicators of the development of widespread chronic pain and may help to develop predictive tools for chronic pain.
Conclusion
The combination of functional and structural brain measures revealed that FM patients had overlapping decreases of cortical thickness, brain volumes and regional functional coherence in the rACC. The atrophy of the rACC, as measured by brain volumes, was associated with duration of FM pain. Given the crucial role of the rACC in descending pain modulation, our result implies that disrupted endogenous pain modulation is central in the development and pathophysiology of FM.
Acknowledgments
This study was performed in collaboration with the pharmaceutical company Pierre Fabre. The results are in part derived from a placebo controlled drug intervention study (EudraCT # 2004-004249-16) financed by Pierre Fabre. Dr. Jensen received support from the COFAS Marie Curie Postdoc Program. Other support for the authors include KO1AT003883 (NIH / NCCAM), R21AT004497 (NIH / NCCAM), R03AT218317 (NIH / NIDA), R01AT006364 (NIH / NCCAM) to Jian Kong, R01AT005280 (NIH / NCCAM) to Randy Gollub.
Footnotes
This study was performed in collaboration with the pharmaceutical company Pierre Fabre. The results are in part derived from a placebo controlled drug intervention study (EudraCT #2004-004249-16) financed by Pierre Fabre.
The authors have no conflicts of interest.
References
- 1.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and Pain Comorbidity: A Literature Review. Arch Intern Med. 2003;163(20):2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe F. New American College of Rheumatology criteria for fibromyalgia: a twenty-year journey. Arthritis care & research. 2010;62(5):583–4. doi: 10.1002/acr.20156. [DOI] [PubMed] [Google Scholar]
- 3.Bergman S, Herrström P, Jacobsson LT, Petersson IF. Chronic widespread pain: a three year followup of pain distribution and risk factors. The Journal of Rheumatology. 2002;29(4):818–825. [PubMed] [Google Scholar]
- 4.Forseth KO, Husby G, Gran JT, Forre O. Prognostic factors for the development of fibromyalgia in women with self-reported musculoskeletal pain. A prospective study. The Journal of rheumatology. 1999;26(11):2458–67. [PubMed] [Google Scholar]
- 5.Bengtsson A, Bäckman E, Lindblom B, Skogh T. Long term follow-up of Fibromyalgia patients: Clinical symptoms, muscular function, laboratory tests - en eight year comparison study. J Musculoskeletal pain. 1994;2:67–80. [Google Scholar]
- 6.Clauw DJ. Fibromyalgia: Update on Mechanisms and Management. Journal of Clinical Rheumatology. 2007;13(2):102–109. [PubMed] [Google Scholar]
- 7.Yunus MB. Fibromyalgia and Overlapping Disorders: The Unifying Concept of Central Sensitivity Syndromes. Seminars in Arthritis and Rheumatism. 2007;36(6):339–356. doi: 10.1016/j.semarthrit.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Cook DB, Lange G, Ciccione DS, Liu WC, Steffener J, Natelson BH. Functional Imaging of Pain in Patients with Primary Fibromyalgia. The Journal of Rheumatology. 2004;31(2):364–78. [PubMed] [Google Scholar]
- 9.Gracely RH, Petzke F, Wolf M, Clauw D. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis & Rheumatism. 2002;46(5):1333–1343. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 10.Napadow V, Kim J, Clauw DJ, Harris RE. Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis and rheumatism. 2012 doi: 10.1002/art.34412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Napadow V, LaCount L, Park K, AsSeine S, Clauw D, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthiritis and Rheumatism. 2010;62(8):2545–55. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK. Decreased Central μ-Opioid Receptor Availability in Fibromyalgia. J Neurosci. 2007;27(37):10000–10006. doi: 10.1523/JNEUROSCI.2849-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris RE, Sundgren PC, Craig AD, Kirshenbaum E, Sen A, Napadow V, et al. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis and rheumatism. 2009;60(10):3146–52. doi: 10.1002/art.24849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadetoff D, Lampa J, Westman M, Andersson M, Kosek E. Evidence of central inflammation in fibromyalgia-increased cerebrospinal fluid interleukin-8 levels. Journal of neuroimmunology. 2012;242(1–2):33–8. doi: 10.1016/j.jneuroim.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Sarchielli P, Mancini ML, Floridi A, Coppola F, Rossi C, Nardi K, et al. Increased Levels of Neurotrophins Are Not Specific for Chronic Migraine: Evidence From Primary Fibromyalgia Syndrome. The Journal of Pain. 2007;8(9):737–745. doi: 10.1016/j.jpain.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Burgmer M, Gaubitz M, Konrad C, Wrenger M, Hilgart S, Heuft G, et al. Decreased gray matter volumes in the cingulo-frontal cortex and the amygdala in patients with fibromyalgia. Psychosomatic medicine. 2009;71(5):566–73. doi: 10.1097/PSY.0b013e3181a32da0. [DOI] [PubMed] [Google Scholar]
- 17.Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated Brain Gray Matter Loss in Fibromyalgia Patients: Premature Aging of the Brain? J Neurosci. 2007;27(15):4004–4007. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lutz J, Jager L, de Quervain D, Krauseneck T, Padberg F, Wichnalek M, et al. White and gray matter abnormalities in the brain of patients with fibromyalgia: a diffusion-tensor and volumetric imaging study. Arthritis and rheumatism. 2008;58(12):3960–9. doi: 10.1002/art.24070. [DOI] [PubMed] [Google Scholar]
- 19.Robinson ME, Craggs JG, Price DD, Perlstein WM, Staud R. Gray matter volumes of pain-related brain areas are decreased in fibromyalgia syndrome. The journal of pain : official journal of the American Pain Society. 2011;12(4):436–43. doi: 10.1016/j.jpain.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt-Wilcke T, Luerding R, Weigand T, Jürgens T, Schuierer G, Leinisch E, et al. Striatal grey matter increase in patients suffering from fibromyalgia--a voxel-based morphometry study. Pain. 2007;132(1):109–16. doi: 10.1016/j.pain.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Schweinhardt P, Sauro KM, Bushnell MC. Fibromyalgia: A Disorder of the Brain? Neuroscientist. 2008 doi: 10.1177/1073858407312521. 1073858407312521. [DOI] [PubMed] [Google Scholar]
- 22.Jensen KB, Ingvar M, Petzke F, Carville S, Fransson P, Choy E, et al. Anxiety and depressive symptoms in Fibromyalgia are related to low health esteem but not to sensitivity or cerebral processing of pain. Arthiritis and Rheumatism. 2010;62(11):3488–3495. doi: 10.1002/art.27649. [DOI] [PubMed] [Google Scholar]
- 23.Jensen KB, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, et al. Evidence of dysfunctional pain inhibition in Fibromyalgia reflected in rACC during provoked pain. Pain. 2009;144(1–2):95–100. doi: 10.1016/j.pain.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 24.Wolfe F, Smythe H, Yunus M, Bennett R, Bombardier C, oldenberg D, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33(2):160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 25.Petzke F, Clauw DJ, Ambrose K, Khine A, Gracely RH. Increased pain sensitivity in fibromyalgia: effects of stimulus type and mode of presentation. Pain. 2003;105(3):403–413. doi: 10.1016/S0304-3959(03)00204-5. [DOI] [PubMed] [Google Scholar]
- 26.Yendiki A, Greve D, Wallace S, Vangel M, Bockholt J, Mueller B, et al. Multi-site characterization of an fMRI working memory paradigm: reliability of activation indices. Neuroimage. 2010;53(1) doi: 10.1016/j.neuroimage.2010.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 28.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 29.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 30.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 31.Chao-Gan Y, Yu-Feng Z. DPARSF: A MATLAB Toolbox for “Pipeline” Data Analysis of Resting-State fMRI. Frontiers in systems neuroscience. 2010;4:13. doi: 10.3389/fnsys.2010.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zang Y, Jiang T, Lu Y, He Y, Tian L. Regional homogeneity approach to fMRI data analysis. NeuroImage. 2004;22(1):394–400. doi: 10.1016/j.neuroimage.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 33.Zou Q, Wu CW, Stein EA, Zang Y, Yang Y. Static and dynamic characteristics of cerebral blood flow during the resting state. NeuroImage. 2009;48(3):515–24. doi: 10.1016/j.neuroimage.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kendall M, Gibbons J. Rank Correlation Methods. London: Edward Arnold; 1990. [Google Scholar]
- 35.Kosek E, Ekholm J, Hansson P. Sensory dysfunction in fibromyalgia patients with implications for pathogenic mechanisms. Pain. 1996;68(2–3):375–383. doi: 10.1016/s0304-3959(96)03188-0. [DOI] [PubMed] [Google Scholar]
- 36.Lautenbacher S, Rollman GB. Possible Deficiencies of Pain Modulation in Fibromyalgia. The Clinical journal of pain. 1997;13(3):189–96. doi: 10.1097/00002508-199709000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Jensen KB, Loitoile R, Kosek E, Petzke F, Carville S, Fransson P, et al. Patients With Fibromyalgia Display Less Functional Connectivity In The Brain’s Pain Inhibitory Network. Molecular pain. 2012;8(1):32. doi: 10.1186/1744-8069-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mankinen K, Long XY, Paakki JJ, Harila M, Rytky S, Tervonen O, et al. Alterations in regional homogeneity of baseline brain activity in pediatric temporal lobe epilepsy. Brain research. 2011;1373:221–9. doi: 10.1016/j.brainres.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Paakki JJ, Rahko J, Long X, Moilanen I, Tervonen O, Nikkinen J, et al. Alterations in regional homogeneity of resting-state brain activity in autism spectrum disorders. Brain research. 2010;1321:169–79. doi: 10.1016/j.brainres.2009.12.081. [DOI] [PubMed] [Google Scholar]
- 40.Qiu C, Liao W, Ding J, Feng Y, Zhu C, Nie X, et al. Regional homogeneity changes in social anxiety disorder: A resting-state fMRI study. Psychiatry research. 2011;194(1):47–53. doi: 10.1016/j.pscychresns.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 41.Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. Journal of neurophysiology. 2010;103(1):297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, et al. Toward discovery science of human brain function. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(10):4734–9. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bingel U, Lorenz J, Schoell E, Weiller C, Büchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120(1):8–15. doi: 10.1016/j.pain.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 44.Fields HL. State-dependent opioid control of pain. Nat Rev Neurosci. 2004;5:565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- 45.Kosek E, Hansson P. Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. Pain. 1997;70(1):41–51. doi: 10.1016/s0304-3959(96)03295-2. [DOI] [PubMed] [Google Scholar]
- 46.Julien N, Goffaux P, Arsenault P, Marchand S. Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain. 2005;114(1–2):295–302. doi: 10.1016/j.pain.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 47.Hsu MC, Harris RE, Sundgren PC, Welsh RC, Fernandes CR, Clauw DJ, et al. No consistent difference in gray matter volume between individuals with fibromyalgia and age-matched healthy subjects when controlling for affective disorder. Pain. 2009;143(3):262–7. doi: 10.1016/j.pain.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain structure & function. 2008;213(1–2):93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. The Journal of neuroscience. 2009;29(44):13746–50. doi: 10.1523/JNEUROSCI.3687-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seminowicz DA, Wideman TH, Naso L, Hatami-Khoroushahi Z, Fallatah S, Ware MA, et al. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. The Journal of neuroscience. 2011;31(20):7540–50. doi: 10.1523/JNEUROSCI.5280-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


