Abstract
Purpose of review
With progressive age, the immune system and the propensity for abnormal immunity change fundamentally. Individuals >50 years of age are more susceptible to infection and cancer, but also at higher risk for chronic inflammation and immune-mediated tissue damage. The process of immunosenescence is accelerated in rheumatoid arthritis.
Recent findings
Premature T cell senescence occurs not only in RA, it has also been involved in morbidity and mortality of chronic HIV infection. Senescent cells acquire the “senescence associated secretory phenotype (SASP)”, which promotes and sustains tissue inflammation. Molecular mechanisms underlying T cell aging are beginning to be understood. Besides the contraction of T cell diversity due to uneven clonal expansion, senescent T cells have defects in balancing cytoplasmic kinase and phosphatase activities, changing their activation thresholds. Also, leakiness in repairing DNA lesions and uncapped telomeres imposes genomic stress. Age-induced changes in the tissue microenvironment may alter T cell responses.
Summary
Gain- and loss-of-function in senescent T cells undermine protective immunity and create the conditions for chronic tissue inflammation, a combination typically encountered in RA. Genetic programs involved in T cell signaling and DNA repair are of high interest in the search for underlying molecular defects.
Keywords: immune aging, DNA damage, telomere, T cell signaling, SASP
Introduction
The progressive expansion of human lifespan, with billions of people in the global community reaching higher and higher ages, has given rise to one of the greatest social challenges. In essentially every society, an increasing proportion of individuals older than 65 years of age has led to a rising interest in the health status of the elderly, the diseases that eventually result in death and the cost burden imposed by the care for those with failing health [1–4]. In most societies, progressive age is associated with the development of cancers, cardiovascular disease, metabolic disorders, and neurodegenerative ailments. One of the common denominators of age-related morbidities is the process of immunosenescence. The aging of the immune system impairs protective immunity against malignant cells and pathogens, but paradoxically increases the risk for autoimmunity and goes hand-in-hand with a state of smoldering chronic inflammation [5,6]*. A causal relationship between deteriorating immunity and the risk to succumb to uncontrolled cellular malignancy or infection is relatively easy to envisage. More challenging is the conceptual and mechanistic understanding of how autoimmune disease, chronic inflammatory disease, tissue-degenerative disease and the immune aging process are related.
Like most biological processes, immune aging is a multifactorial cascade of events that affects the innate and adaptive arms of the immune system itself as well as the tissue environment in which immune responses occur. Different types of immune cells display differential susceptibility to aging. The long life span of adaptive immune cells makes them more susceptible to the impact of aging and T cells are particularly affected as the production of new T cells dwindles with the involution of the thymus that begins relatively early in life.
Over the last decade, evidence has accumulated that the immune aging process is accelerated in patients with rheumatoid arthritis (RA) [7–9]. RA patients share this feature with patients infected with the human immunodeficiency virus, although different mechanisms may underlie the faster progression of the immune aging process in the two conditions [10–12]*. Mechanistic insights as to how T cells age in healthy individuals as they progress through the second half of life have provided the opportunity to quantify immune aging and to begin to develop modes of interfering with what used to be considered an inevitable decline in cellular health and life span. This review will bring together recent information on how T cells age, how T cell aging is accelerated in patients with RA and which mechanistic pathways are beginning to be understood as potential targets in efforts to counteract the immune aging process.
T cell aging – mechanisms and consequences
T cells safeguard the host through their ability to recognize foreign antigen with utmost specificity, memorize this encounter and orchestrate a complex immune response that eliminates the offender while minimizing collateral damage. Three determinants make T cells explicitly susceptible to aging: (1) enormous proliferative stress, as they have to clonally expand massively with antigen exposure; (2) long life span as the carriers of immune memory; and (3) dependence on thymic intactness for repopulation. As somatic cells, T cells have only a limited number of cell cycles they can go through. In contrast to most other somatic cells, they have the ability to reactivate telomerase, elongate telomeres and thus lengthen their life span [13]. This mechanism of telomerase upregulation is defective in patients with RA, shortening the longevity of T cells [14].
T cells have a complex life cycle which begins in the thymus, and leads them into the periphery where they are mostly in a resting state. Stimulation initiates an activation and differentiation process that results in the loss of naivety, acquisition of memory features and differentiation into effector cells. As a general rule, the frequency of naïve T cells declines with progressive age and the frequency of memory populations increases (Table 1) [15]. CD4 T cells tend to age slower than their CD8 counterparts; the reason for this divergent behavior is not understood [16]. The following life events have been implicated in inducing T cell aging: (1) acute and chronic infections, especially with herpes viruses, e.g. cytomegalovirus (CMV); (2) cellular attrition and insufficient replacement of naïve cells; (3) the need to replicate peripheral T cells for replenishment, eventually imposing proliferative stress on naïve T cells and partially differentiating them; and (4) successive loss of tissue niches in which T cells are protected from stimulation. It is important to understand that the impact of these life experiences may be fundamentally different in humans and mice and that murine model systems have only partial applicability to human conditions. Comparative studies of inducible gene expression profiles in human and mouse cells have led to the recognition of noteworthy species differences, with most of the age-associated changes found in human CD4 T cells not reproduced in murine cells [17]. The human response pattern included a defect in the sustained inducibility of NF-κB target genes in the elderly, suggesting a defect in mounting inflammatory responses. However, this defect was combined with persistent upregulation of a subset of NF-κB target genes, including IL-1 and IL-6, even in the absence of NF-κB triggering. Fundamental differences between mice and men have also been described for pathogenic immune responses [18]. The emerging paradigm proposes that age-related immune dysregulation relies on multiple, but subtle, changes that in aggregate mediate major functional consequences.
Table 1.
| Markers of T cell aging |
|---|
| Shrinking naïve and expanding memory compartment |
| Shortening of telomeres |
| Loss of CD28 expression |
| Excessive production of cytokines (Senescence-associated phenotype proteins) |
| Gain of cytotoxicity |
| Altered tissue trafficking |
| Expression of KLRG1, ILT-2 and KIR family members |
A hallmark of immune aging is the accumulation of end-differentiated effector T cells which typically lack expression of the costimulatory receptor CD28 (Table 1) [19,20]. CD8+CD28− T cells regularly accompany the aging process, whereas CD4+CD28− T cells are typically present at lower frequencies. These T cell populations are oligoclonal; CD4+CD28− T cells are relatively apoptosis resistant, whereas CD8+CD28− T cells are more likely to die, at least in vitro. CD28− T cells are high producers for interferon-γ (IFN-γ), display cytotoxic functions and accumulate in inflammatory lesions [21–26]. CD8+CD28− T cells, and less so CD4+CD28− T cells, are expanded in individuals chronically infected with CMV [27]. A remarkable finding is that the expansion of CMV-specific T cells leads to oligoclonal populations that comprise a large fraction of the T cell repertoire. How the human host retains sufficient T cell diversity to respond to immune challenges in light of this overwhelming representation of CMV-reactive T cells remains unresolved. Data connecting CMV carrier status with survival and susceptibility to comorbidities of aging have been controversial [28–31]*, ranging from the claim that CMV infection ages the immune system to the concept that age-dependent decline in immune competence encourages CMV reactivation/reinfection. The sheer size of the T cell compartment reactive against individual CMV-derived antigens, which can now be quantified by tetramer technology and often reaches 1–5% of peripheral T cells for single peptides, has supported the idea of “memory expansion”, meaning that the entire T cell memory compartment grows in size as humans age and carry chronic CMV infection. A recent study exploring the avidity of CMV-specific CD8+ T cells has demonstrated that CD8+ effector memory cells reexpressing CD45RA typically have low avidity and appear to expand mostly in response to antigen-nonspecific cytokine stimulation [32]. These data question memory expansion as the lead process skewing the T cell repertoire and refocus attention to the proinflammatory cytokine milieu in the aging host.
The senescence-associated secretory phenotype (SASP)
Aging inevitably is associated with the accumulation of DNA mutations and thus fosters malignancy. The risk for aging-associated outgrowth of cancerous cells is curbed through the cellular senescence program, which forces cells that have undergone repetitive replications into permanent cell cycle arrest [33]. While senescent cells slow down or totally arrest their replication, they remain functionally highly active. This process has been named senescence-associated secretory phenotype (SASP) and in essence describes a critical role of old and end-differentiated cells in imposing an inflammatory milieu [34,35]* (Table 1; Figure 1). Products secreted by senescent cells have been implicated in tissue repair, but are mostly active in damaging aging tissue sites through sustaining a persistent inflammatory reaction. It is tempting to implicate SASP in the tissue-injurious immune responses that characterize RA, especially as SASP may support loss and gain of tissue, such as the hyperplastic responses of the synovial pannus formation. A formal proof that SASP contributes to the tissue lesion in the RA joint is lacking, but SASP-sustained abnormalities need to be considered as pathogenic mechanisms in autoimmune disease. The dual-sided function of SASP has been emphasized by recent data showing that IFN-γ and TNF-α, two classical proinflammatory cytokines that are released by senescent T cells, drive cancerous cells into senescence and thus are ultimately host-protective [36]*. While long known for its anti-pathogen functions, this work broadens the critical role of IFN-γ, even when released in an antigen-nonspecific fashion. Therapeutic efforts aiming to eliminate aging T cells need to keep in mind that tissue inflammation is only one of their functions and that protection from cancer is critical in securing survival. The dual function of T cells in promoting host protection and tissue damage also holds for IL-17 producing TH17 cells, which have recently been characterized as terminally differentiated memory T cells, distinct from both central memory T cells and exhausted T cells. Human TH17 cells were shown to have a high potential for self-renewal, potent persistence, apoptotic resistance in vivo and plasticity to convert into other cell types. Their typifying gene signature included hypoxia-inducible factor1a and NOTCH which collaboratively regulate expression of survival genes [37]. TH1 and TH17 cells have been identified as key drivers of the pathogenic immunity in RA [38,39].
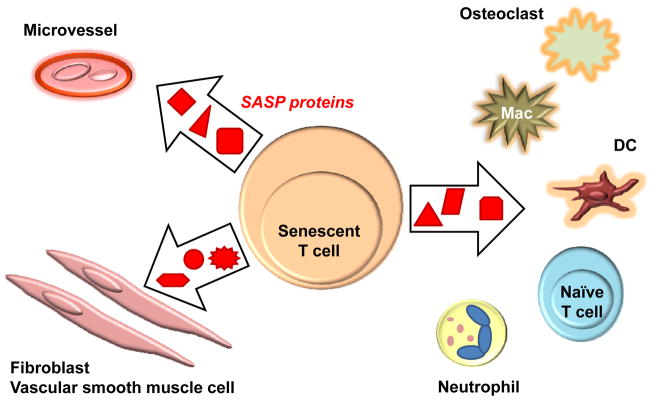
Figure 1. Senescence-associated secretory phenotype (SASP) and tissue damage.
Cells that enter the senescence program slow cell cycle progression and eventually stop proliferating. However, proliferative arrest is not associated with functional silence. Rather, senescent cells acquire the senescence-associated secretory phenotype (SASP) characterized by the secretion of proteins, such as cytokines, through which they regulate neighboring cells and tissues. SASP proteins typically elicit an inflammatory response. Cells and tissue structures potentially exposed to senescent T cells are shown.
Rewiring of the old T cell
In humans older than 50 years of age, both naïve and memory T cells undergo considerable changes in trafficking, response to stimulation, growth behavior, cytokine production and interaction with other cells in the immune system (Table 1) [6]*. Based on phenotype and function, senescent T cells must not be confused with exhausted T cells induced by prolonged antigen stimulation [40–42]. The phenotypic signature of exhausted T cells includes TIM-3, PD-1 and LAG-3, whereas human senescent cells express KLRG1, ILT2 and varying members of the KIR family. Thus, T cell aging is not simply a reflection of chronic antigenic activation.
Variably, the tumor suppressor gene p16(INK4a) has been implicated in mediating the senescence program. Deletion of p16(INK4a) in T cells has been effective in counteracting several aging phenotypes, such as thymic involution, decreased release of naïve T cells, and attenuation of antigen-specific immune responses. Conversely, somatic ablation of p16(INK4a) in B cells has cancer promoting effects, emphasizing the importance of cell specificity in the aging process [43]. Increased expression of p16(INK4a) in T cells of HIV-infected subjects and an inverse relationship between p16(INKp4a) expression and therapy-induced CD4 T cells recovery has suggested a possible role of this senescence-associated gene as a biomarker of T cell aging [44]. A search for additional markers of T cell aging in individuals with HIV infection has identified adenosine deaminase (ADA), glucose uptake receptor 1 (GLUT1) and leucine-rich repeat neuronal 3 (LRRN3) as the most robust predictors of CD8 T cell senescence [45]. This profile of senescence-related markers predicts that purinergic responses and metabolic activities are distinct in old T cells.
Gene expression studies have yielded valuable insights into functional abnormalities of aged T cells that may be helpful in delineating why aged T cells fail to promote protective immunity and are skewed towards SASP [46–48]. The balance between kinases and phosphatases is shifted towards activation-reducing phosphatase activity. Specifically, CD4 T cells from elderly donors suffer from signaling inhibition due to overexpression of the nuclear dual specific phosphatase 4 (DUSP4) [49]*, impairing T-cell dependent B cell responses. Similarly, overexpression of cytoplasmic DUSP6 has been implicated in the functional decline of aging CD4 T cells, making a connection between abnormalities in cytoplasmic signaling pathways and age-dependent competence of CD4 T cells [50**]. Specifically, dampened T cell receptor-induced ERK signaling could be attributed to overactivity of DUSP6, which, in turn, increased due to failing repression by miR-181a in aging T cells. CD4 T cells from RA patients are characterized by dysregulated signaling cascades, shifting their threshold to activating signals and possibly removing them from protective tolerance mechanisms [51**]. How abnormalities in signaling pathways, some of which appear to be a consequence of the cellular aging process, are connected to the breakdown of tolerance and the tissue-injurious immunity in rheumatoid arthritis needs further studies [52,53].
T cell aging in rheumatoid arthritis
Patients with RA have a signature of premature immune aging (Table 2). In essence, the T cell repertoire in RA is characterized by the accumulation of terminal-stage effector T cells, typically identified by CD28 loss, telomeric shortening, lack of telomerase activity, and restriction in clonal proliferation capacity [54–57]*. The biologic impact of such T cells remains controversial, but their presence in the elderly has been associated with a decline in functionality, reduced vaccine responses, and increased risk of infection, neurocognitive problems and cardiovascular disease. Interestingly, a similar signature of accelerated immune aging is now recognized in individuals with chronic HIV infection [58**]. There is an emerging consensus in the scientific community that HIV infection hastens the process of aging and that illnesses that appear typically in the elderly are highly frequent among HIV-infected individuals, even in those with therapy-induced suppression of the virus below detection limits [59,60]. The magnitude of the problem is substantial. It has been estimated that a 20 year-old adult initiating ART may already have lost one-third of the remaining life expectancy [61]. Based on loss in telomeric sequence reserve, RA patients age about 25–30 years faster than non-RA controls. A side-by-side comparison of HIV infection and RA demonstrates a surprising degree of similarities between both conditions (Table 3). Despite such similarities, important discrepancies remain; it is far from understood whether the accelerated aging process in RA and HIV is mechanistically identical to what happens with normal aging or whether the stressors that lead to premature decline in immunocompetence are fundamentally different. A common denominator of physiologic aging, RA and HIV infection is the co-occurrence of immune deficiency, smoldering inflammation and tissue degeneration. A substantial step forward has derived from the recognition that senescent cells are in essence pro-inflammatory cells through the release of SASP proteins, which function in an autocrine, paracrine and juxtacrine fashion (Figure 1). Understanding how immune aging and persistent inflammation are causally related could provide valuable insights for diverse areas of medicine.
Table 2.
| T Cell Aging in Rheumatoid Arthritis |
|---|
| Premature loss of telomeres in naïve CD4 T cells |
| Contraction of the T cell repertoire |
| Telomeric shortening in CD34+ hematopoietic precursors |
| Accumulation of CD4+CD28- T cells |
| Telomerase deficiency in T cells |
| Restricted proliferative potential of naïve T cells |
| Defective repair of DNA damage due to ATM insufficiency |
| Genomic stress |
Table 3.
| Aging-related comorbidities | Chronic HIV infection | Rheumatoid arthritis |
|---|---|---|
| Cancer | ++ | ? |
| Cardiovascular disease | ++ | ++ |
| Metabolic syndrome | ++ | ++ |
| Infectious risk | ++ | ++ |
| Reduced vaccine response | ++ | ++ |
| Bone loss | ++ | ++ |
| Neurocognitive decline | ++ | ? |
| Frailty | ++ | ++ |
| Shortened life expectancy | ++ | ++ |
DNA damage-induced immunosenescence in rheumatoid arthritis
Molecular mechanisms causing immune aging in RA are not entirely clarified, but recent studies have shed some light on abnormal genetic and biochemical pathways relevant in T cell senescence (Figure 2) [62,63]. Chronic antigenic stimulation could be a simple explanation for the pre-aged phenotype of RA T cells. However, ongoing recognition of autoantigens should eventually lead to exhaustion and clonal deletion. This seems not to be the case. Also, the signifying abnormalities in RA T cells, including the premature loss of telomeres, have been extended to CD34+ hematopoietic progenitor cells, separating antigen recognition and cellular aging [64,65]. A similar dilemma exists in HIV infection, where complete viral suppression fails to protect the host from immunosenescence. The co-occurrence of deficient immunity and prematurity of age-related morbidities extends to the progeroid syndromes, in which affected individuals begin aging during the second decade of life [66–68]. Such progeroid syndromes have provided important clues as to the causative abnormalities underlying the immunosenescence process [69].
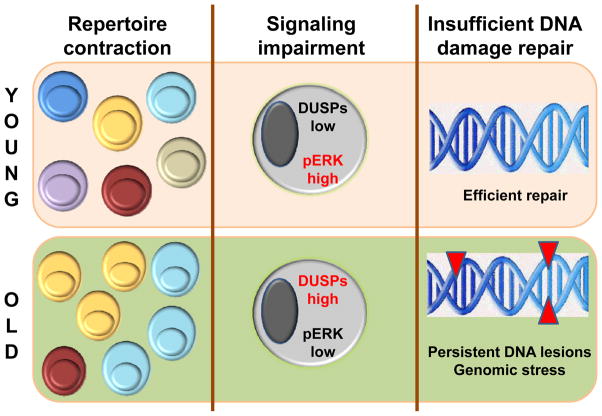
Figure 2. Mechanisms of T Cell Aging.
With progressive age, the T cell compartment undergoes profound changes induced by slowing T cell replenishment, replicative stress and chronic antigen-specific and nonspecific stimulation. Three major mechanisms have been implicated in the altered T cell functionality of the elderly and of individuals affected by premature immunosenescence. Repertoire contraction results from uneven clonal expansion and restricts T cell diversity. Recently, several molecular networks have been discovered which rewire the signaling apparatus of senescent T cells, leading to inappropriate responsiveness. Inefficiencies in DNA repair have a major impact on T cell survival, and possibly T cell differentiation, as they lower the apoptotic threshold and impose chronic genomic stress.
Replicative stress inevitably leads to loss of telomeric sequences as DNA replication is inefficient at the chromosomal ends. It is estimated that each cell cycle shortens telomeres by about 50 bp. Human T cells lose about 2000 bp between the age of 20 and 70 years, equivalent to about 40 division cycles. It is also estimated that naïve T cells expand by 30–40 population doublings during priming alone [14]. To extend their telomeric reserve, T cells are able to upregulate the activity of telomerase. This compensatory mechanism is defective in RA T cells [14,53]. Insufficient telomeric repair has also been reported for HIV T cells due to abnormalities in the telomeric protection complex of the shelterin proteins, thereby leaving chromosomal ends exposed [70]*.
Cells recognize telomeres that become critically short or have structural defects through their DNA damage sensing machinery [71]. Breaks or chemical alterations of the DNA endanger cells as they may give rise to mutations and eventually malignant cell growth. Accordingly, cells possess highly effective machineries to recognize and repair DNA damage. DNA lesions resulting from genotoxic events recruit DNA damage sensing proteins that subsequently mobilize signaling pathways to repair breaks and exchange chemically altered bases. Of note, many of the progeroid syndromes are nuclear instability syndromes, caused by genetic mutations in DNA repair molecules. One of the aging syndromes, ataxia telangiectasia (AT), results from a genetic defect in the DNA damage sensing kinase ataxia telangiectasia mutated (ATM) [72,73]. Children with AT develop premature immune aging early in life, have frequent infections, are radiosensitive and have a high risk of lymphoma development. Patients with RA have ATM-deficient T cells which accumulate DNA breaks and fail to efficiently repair radiation-induced DNA lesions [74]. Forced overexpression of ATM rescues the abnormal phenotype of the RA T cells. Elevated tail length measurements in comet assays of RA cells have been reported in patients with active and inactive disease, suggesting a complicated relationship exists between the inflammatory milieu and the DNA repair defect [75]. Leakiness in DNA damage repair exposes RA T cells to genomic stress. Faced with an increased number of DNA breaks, RA T cells upregulate activity of the DNA repair enzyme DNA-PKcs [76**]. Chronic repair activity imposes cellular stress through the DNA-PKcs-JNK-axis, possibly skewing the functionality of T cells and biasing them towards excessive inflammatory activity.
CONCLUSIONS
As a system of long-lived cells that are under high proliferative demand, the adaptive immune system is prone to undergo age-related degeneration. The process of immunosenescence leads to declining protective immunity combined with excessive inflammatory activity. Prematurity of the immune aging process has been described in rheumatoid arthritis, in chronic HIV infection and in the progeroid syndromes, all of which are characterized by the early development of age-associated comorbidities, such as cardiovascular disease and metabolic syndrome. Mechanisms underlying T cell aging are beginning to be understood and encompass contraction of T cell diversity due to uneven clonal expansion, rewiring of the T cell signaling apparatus favoring phosphatase activity and defects in DNA damage and telomere repair. Molecules of interest include telomerase, DUSP4, DUSP6, mi-R181a, ATM and DNA-PKcs. Recent studies have identified a deficiency of the glycolytic enzyme phosphofructokinase-2 in RA T cells, causing metabolic rewiring and reduced intracellular energy generation [77**]. Understanding the connections between T cell aging, cellular metabolism, immunodeficiency, unopposed inflammation and tissue damage in RA promises to be a rich field of discovery.
Key points.
After the age of 50 years, the immune system undergoes dramatic changes, loses protective abilities and gains pro-inflammatory functions. Immunosenescence is accelerated in patients with rheumatoid arthritis.
Senescent T cells are functionally altered. Their signaling machinery is rewired. Senescent cells acquire the “senescence-associated secretory phenotype” (SASP). SASP proteins, often cytokines, induce tissue inflammation.
It remains unknown whether chronic inflammation can accelerate immune aging. Vice versa, immune aging is strongly associated with chronic smoldering inflammation and tissue damage.
Molecular mechanisms associated with T cell aging in rheumatoid arthritis include leakiness of DNA repair and inefficiency of telomeric maintenance/repair.
Acknowledgments
The authors were funded by grants from the National Institutes of Health, EY011916, AR042527, HL058000 (C.M.W.), the Govenar Discovery Fund (Z.Y.) and AI057266, AI090019, AG045779 (J.J.G.).
Footnotes
None of the authors has any conflicts of interest.
REFERENCES AND RECOMMENDED READING
- 1.Vigen R, Maddox TM, Allen LA. Aging of the United States population: impact on heart failure. Curr Heart Fail Rep. 2012;9:369–374. doi: 10.1007/s11897-012-0114-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McEniry M. Early-life conditions and older adult health in low- and middle-income countries: a review. J Dev Orig Health Dis. 2013;4:10–29. doi: 10.1017/S2040174412000499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catindig JA, Venketasubramanian N, Ikram MK, et al. Epidemiology of dementia in Asia: insights on prevalence, trends and novel risk factors. J Neurol Sci. 2012;321:11–16. doi: 10.1016/j.jns.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 4.Hurd MD, Martorell P, Delavande A, et al. Monetary costs of dementia in the United States. N Engl J Med. 2013;368:1326–1334. doi: 10.1056/NEJMsa1204629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goronzy JJ, Li G, Yang Z, et al. The janus head of T cell aging - autoimmunity and immunodeficiency. Front Immunol. 2013;4:131. doi: 10.3389/fimmu.2013.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6*.Goronzy JJ, Weyand CM. Understanding immunosenescence to improve responses to vaccines. Nat Immunol. 2013;14:428–436. doi: 10.1038/ni.2588. A review of the molecular mechanisms relevant in immune aging and a discussion of species similarities and disparities between humans and mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weyand CM, Fujii H, Shao L, et al. Rejuvenating the immune system in rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:583–588. doi: 10.1038/nrrheum.2009.180. [DOI] [PubMed] [Google Scholar]
- 8.Mayerl C, Prelog M. Immunosenescence and juvenile idiopathic arthritis. Autoimmun Rev. 2012;11:297–300. doi: 10.1016/j.autrev.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Tarjanyi O, Boldizsar F, Nemeth P, et al. Age-related changes in arthritis susceptibility and severity in a murine model of rheumatoid arthritis. Immun Ageing. 2009;6:8. doi: 10.1186/1742-4933-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erlandson KM, Allshouse AA, Jankowski CM, et al. Association of Functional Impairment with Inflammation and Immune Activation in HIV Type 1-Infected Adults Receiving Effective Antiretroviral Therapy. J Infect Dis. 2013;208:249–259. doi: 10.1093/infdis/jit147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith RL, de Boer R, Brul S, et al. Premature and accelerated aging: HIV or HAART? Front Genet. 2012;3:328. doi: 10.3389/fgene.2012.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12*.Deeks SG, Verdin E, McCune JM. Immunosenescence and HIV. Curr Opin Immunol. 2012;24:501–506. doi: 10.1016/j.coi.2012.05.004. An excellent summary of the current knowledge about prematurity of immune aging in HIV infected individuals and the proposed clinical consequences. [DOI] [PubMed] [Google Scholar]
- 13.Andrews NP, Fujii H, Goronzy JJ, et al. Telomeres and immunological diseases of aging. Gerontology. 2010;56:390–403. doi: 10.1159/000268620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fujii H, Shao L, Colmegna I, et al. Telomerase insufficiency in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2009;106:4360–4365. doi: 10.1073/pnas.0811332106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnold CR, Wolf J, Brunner S, et al. Gain and loss of T cell subsets in old age--age-related reshaping of the T cell repertoire. J Clin Immunol. 2011;31:137–146. doi: 10.1007/s10875-010-9499-x. [DOI] [PubMed] [Google Scholar]
- 16.Czesnikiewicz-Guzik M, Lee WW, Cui D, et al. T cell subset-specific susceptibility to aging. Clin Immunol. 2008;127:107–118. doi: 10.1016/j.clim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bektas A, Zhang Y, Wood WH, 3rd, et al. Age-associated alterations in inducible gene transcription in human CD4+ T lymphocytes. Aging (Albany NY) 2013;5:18–36. doi: 10.18632/aging.100522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seok J, Warren HS, Cuenca AG, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chou JP, Effros RB. T cell replicative senescence in human aging. Curr Pharm Des. 2013;19:1680–1698. doi: 10.2174/138161213805219711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Broux B, Markovic-Plese S, Stinissen P, et al. Pathogenic features of CD4+CD28- T cells in immune disorders. Trends Mol Med. 2012;18:446–453. doi: 10.1016/j.molmed.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Nakajima T, Schulte S, Warrington KJ, et al. T-cell-mediated lysis of endothelial cells in acute coronary syndromes. Circulation. 2002;105:570–575. doi: 10.1161/hc0502.103348. [DOI] [PubMed] [Google Scholar]
- 22.Warrington KJ, Takemura S, Goronzy JJ, et al. CD4+,CD28- T cells in rheumatoid arthritis patients combine features of the innate and adaptive immune systems. Arthritis Rheum. 2001;44:13–20. doi: 10.1002/1529-0131(200101)44:1<13::AID-ANR3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 23.Liuzzo G, Goronzy JJ, Yang H, et al. Monoclonal T-cell proliferation and plaque instability in acute coronary syndromes. Circulation. 2000;101:2883–2888. doi: 10.1161/01.cir.101.25.2883. [DOI] [PubMed] [Google Scholar]
- 24.Liuzzo G, Kopecky SL, Frye RL, et al. Perturbation of the T-cell repertoire in patients with unstable angina. Circulation. 1999;100:2135–2139. doi: 10.1161/01.cir.100.21.2135. [DOI] [PubMed] [Google Scholar]
- 25.Nakajima T, Goek O, Zhang X, et al. De novo expression of killer immunoglobulin-like receptors and signaling proteins regulates the cytotoxic function of CD4 T cells in acute coronary syndromes. Circ Res. 2003;93:106–113. doi: 10.1161/01.RES.0000082333.58263.58. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Nakajima T, Goronzy JJ, et al. Tissue trafficking patterns of effector memory CD4+ T cells in rheumatoid arthritis. Arthritis Rheum. 2005;52:3839–3849. doi: 10.1002/art.21482. [DOI] [PubMed] [Google Scholar]
- 27.Turner JE, Campbell JP, Edwards KM, et al. Rudimentary signs of immunosenescence in Cytomegalovirus-seropositive healthy young adults. Age (Dordr) 2013 doi: 10.1007/s11357-013-9557-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pawelec G, Derhovanessian E. Role of CMV in immune senescence. Virus Res. 2011;157:175–179. doi: 10.1016/j.virusres.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 29*.Savva GM, Pachnio A, Kaul B, et al. Cytomegalovirus infection is associated with increased mortality in the older population. Aging Cell. 2013;12:381–387. doi: 10.1111/acel.12059. A study analyzing whether cytomegalovirus infection, proposed to induce immune aging, affects survival. [DOI] [PubMed] [Google Scholar]
- 30.Derhovanessian E, Maier AB, Hahnel K, et al. Lower proportion of naive peripheral CD8+ T cells and an unopposed pro-inflammatory response to human Cytomegalovirus proteins in vitro are associated with longer survival in very elderly people. Age (Dordr) 2013;35:1387–1399. doi: 10.1007/s11357-012-9425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31*.Bartlett DB, Firth CM, Phillips AC, et al. The age-related increase in low-grade systemic inflammation (Inflammaging) is not driven by cytomegalovirus infection. Aging Cell. 2012;11:912–915. doi: 10.1111/j.1474-9726.2012.00849.x. An elegant prospective study analyzing whether chronic cytomegalovirus infection causes the smouldering inflammatory syndrome typical for the elderly. [DOI] [PubMed] [Google Scholar]
- 32.Griffiths SJ, Riddell NE, Masters J, et al. Age-associated increase of low-avidity cytomegalovirus-specific CD8+ T cells that re-express CD45RA. J Immunol. 2013;190:5363–5372. doi: 10.4049/jimmunol.1203267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodier F, Campisi J. Four faces of cellular senescence. J Cell Biol. 2011;192:547–556. doi: 10.1083/jcb.201009094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coppe JP, Desprez PY, Krtolica A, et al. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36*.Braumuller H, Wieder T, Brenner E, et al. T-helper-1-cell cytokines drive cancer into senescence. Nature. 2013;494:361–365. doi: 10.1038/nature11824. The first report implicating IFN-γ, a product of senescent T cells, in directly fighting cancer cell growth. [DOI] [PubMed] [Google Scholar]
- 37.Kryczek I, Zhao E, Liu Y, et al. Human TH17 cells are long-lived effector memory cells. Sci Transl Med. 2011;3:104ra100. doi: 10.1126/scitranslmed.3002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lubberts E. Th17 cytokines and arthritis. Semin Immunopathol. 2010;32:43–53. doi: 10.1007/s00281-009-0189-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Annunziato F, Cosmi L, Liotta F, et al. Type 17 T helper cells-origins, features and possible roles in rheumatic disease. Nat Rev Rheumatol. 2009;5:325–331. doi: 10.1038/nrrheum.2009.80. [DOI] [PubMed] [Google Scholar]
- 40.Youngblood B, Wherry EJ, Ahmed R. Acquired transcriptional programming in functional and exhausted virus-specific CD8 T cells. Curr Opin HIV AIDS. 2012;7:50–57. doi: 10.1097/COH.0b013e32834ddcf2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 42.Akbar AN, Henson SM. Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat Rev Immunol. 2011;11:289–295. doi: 10.1038/nri2959. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y, Johnson SM, Fedoriw Y, et al. Expression of p16(INK4a) prevents cancer and promotes aging in lymphocytes. Blood. 2011;117:3257–3267. doi: 10.1182/blood-2010-09-304402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nelson JA, Krishnamurthy J, Menezes P, et al. Expression of p16(INK4a) as a biomarker of T-cell aging in HIV-infected patients prior to and during antiretroviral therapy. Aging Cell. 2012;11:916–918. doi: 10.1111/j.1474-9726.2012.00856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chou JP, Ramirez CM, Wu JE, et al. Accelerated Aging in HIV/AIDS: Novel Biomarkers of Senescent Human CD8+ T Cells. PLoS One. 2013;8:e64702. doi: 10.1371/journal.pone.0064702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goronzy JJ, Li G, Yu M, et al. Signaling pathways in aged T cells - a reflection of T cell differentiation, cell senescence and host environment. Semin Immunol. 2012;24:365–372. doi: 10.1016/j.smim.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weng NP, Araki Y, Subedi K. The molecular basis of the memory T cell response: differential gene expression and its epigenetic regulation. Nat Rev Immunol. 2012;12:306–315. doi: 10.1038/nri3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen G, Lustig A, Weng NP. T cell aging: a review of the transcriptional changes determined from genome-wide analysis. Front Immunol. 2013;4:121. doi: 10.3389/fimmu.2013.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49*.Yu M, Li G, Lee WW, et al. Signal inhibition by the dual-specific phosphatase 4 impairs T cell-dependent B-cell responses with age. Proc Natl Acad Sci U S A. 2012;109:E879–888. doi: 10.1073/pnas.1109797109. Identification and characterization of the dual-specific phosphatase 4 as a molecular mediator of T cell aging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50**.Li G, Yu M, Lee WW, et al. Decline in miR-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nat Med. 2012;18:1518–1524. doi: 10.1038/nm.2963. The first study implicating miR-181a and dual specific phosphatases in signaling abnormalities of aged T cells and characterization of pharmacologic interventions to reverse T cell aging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51**.Singh K, Deshpande P, Li G, et al. K-RAS GTPase- and B-RAF kinase-mediated T-cell tolerance defects in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2012;109:E1629–1637. doi: 10.1073/pnas.1117640109. A study defining and characterizing signaling abnormalities in T cells from RA patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goronzy JJ, Weyand CM. Immune aging and autoimmunity. Cell Mol Life Sci. 2012;69:1615–1623. doi: 10.1007/s00018-012-0970-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hohensinner PJ, Goronzy JJ, Weyand CM. Telomere dysfunction, autoimmunity and aging. Aging Dis. 2011;2:524–537. [PMC free article] [PubMed] [Google Scholar]
- 54.Schmidt D, Goronzy JJ, Weyand CM. CD4+ CD7- CD28- T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J Clin Invest. 1996;97:2027–2037. doi: 10.1172/JCI118638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wagner UG, Koetz K, Weyand CM, et al. Perturbation of the T cell repertoire in rheumatoid arthritis. Proc Natl Acad Sci U S A. 1998;95:14447–14452. doi: 10.1073/pnas.95.24.14447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koetz K, Bryl E, Spickschen K, et al. T cell homeostasis in patients with rheumatoid arthritis. Proc Natl Acad Sci U S A. 2000;97:9203–9208. doi: 10.1073/pnas.97.16.9203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schonland SO, Lopez C, Widmann T, et al. Premature telomeric loss in rheumatoid arthritis is genetically determined and involves both myeloid and lymphoid cell lineages. Proc Natl Acad Sci U S A. 2003;100:13471–13476. doi: 10.1073/pnas.2233561100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58**.High KP, Brennan-Ing M, Clifford DB, et al. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr. 2012;60 (Suppl 1):S1–18. doi: 10.1097/QAI.0b013e31825a3668. An excellent summary of what is known about premature immune aging in HIV and its implication for morbidity and mortality. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lohse N, Hansen AB, Pedersen G, et al. Survival of persons with and without HIV infection in Denmark, 1995–2005. Ann Intern Med. 2007;146:87–95. doi: 10.7326/0003-4819-146-2-200701160-00003. [DOI] [PubMed] [Google Scholar]
- 60.Losina E, Schackman BR, Sadownik SN, et al. Racial and sex disparities in life expectancy losses among HIV-infected persons in the united states: impact of risk behavior, late initiation, and early discontinuation of antiretroviral therapy. Clin Infect Dis. 2009;49:1570–1578. doi: 10.1086/644772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cavanagh MM, Weyand CM, Goronzy JJ. Chronic inflammation and aging: DNA damage tips the balance. Curr Opin Immunol. 2012;24:488–493. doi: 10.1016/j.coi.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Georgin-Lavialle S, Aouba A, Mouthon L, et al. The telomere/telomerase system in autoimmune and systemic immune-mediated diseases. Autoimmun Rev. 2010;9:646–651. doi: 10.1016/j.autrev.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 64.Colmegna I, Diaz-Borjon A, Fujii H, et al. Defective proliferative capacity and accelerated telomeric loss of hematopoietic progenitor cells in rheumatoid arthritis. Arthritis Rheum. 2008;58:990–1000. doi: 10.1002/art.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Colmegna I, Weyand CM. Haematopoietic stem and progenitor cells in rheumatoid arthritis. Rheumatology (Oxford) 2011;50:252–260. doi: 10.1093/rheumatology/keq298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Goto M. Hierarchical deterioration of body systems in Werner's syndrome: implications for normal ageing. Mech Ageing Dev. 1997;98:239–254. doi: 10.1016/s0047-6374(97)00111-5. [DOI] [PubMed] [Google Scholar]
- 67.Carney EF, Srinivasan V, Moss PA, et al. Classical ataxia telangiectasia patients have a congenitally aged immune system with high expression of CD95. J Immunol. 2012;189:261–268. doi: 10.4049/jimmunol.1101909. [DOI] [PubMed] [Google Scholar]
- 68.Exley AR, Buckenham S, Hodges E, et al. Premature ageing of the immune system underlies immunodeficiency in ataxia telangiectasia. Clin Immunol. 2011;140:26–36. doi: 10.1016/j.clim.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 69.Reinhardt HC, Schumacher B. The p53 network: cellular and systemic DNA damage responses in aging and cancer. Trends Genet. 2012;28:128–136. doi: 10.1016/j.tig.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70*.Lichterfeld M, Cung T, Seiss K, et al. Shelterin dysfunction and p16(INK4a)-mediated growth inhibition in HIV-1-specific CD8 T cells. J Virol. 2012;86:5533–5540. doi: 10.1128/JVI.00196-12. First study investigating telomere protection proteins, the shelterins, in T cells from individuals chronically infected with HIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gardano L, Pucci F, Christian L, et al. Telomeres, a busy platform for cell signaling. Front Oncol. 2013;3:146. doi: 10.3389/fonc.2013.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reynolds JJ, Stewart GS. A nervous predisposition to unrepaired DNA double strand breaks. DNA Repair (Amst) 2013;12:588–599. doi: 10.1016/j.dnarep.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 73.Anheim M, Tranchant C, Koenig M. The autosomal recessive cerebellar ataxias. N Engl J Med. 2012;366:636–646. doi: 10.1056/NEJMra1006610. [DOI] [PubMed] [Google Scholar]
- 74.Shao L, Fujii H, Colmegna I, et al. Deficiency of the DNA repair enzyme ATM in rheumatoid arthritis. J Exp Med. 2009;206:1435–1449. doi: 10.1084/jem.20082251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karaman A, Binici DN, Melikoglu MA. Comet assay and analysis of micronucleus formation in patients with rheumatoid arthritis. Mutat Res. 2011;721:1–5. doi: 10.1016/j.mrgentox.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 76.Shao L, Goronzy JJ, Weyand CM. DNA-dependent protein kinase catalytic subunit mediates T-cell loss in rheumatoid arthritis. EMBO Mol Med. 2010;2:415–427. doi: 10.1002/emmm.201000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77**.Yang Z, Fujii H, Mohan SV, Goronzy JJ, Weyand CM. Phosphofructokinase deficiency impairs ATP generation, autophagy, and redox balance in rheumatoid arthritis T cells. J Exp Med. 2013:2119–34. doi: 10.1084/jem.20130252. First study demonstrating metabolic abnormalities in T cells from patients with rheumatoid arthritis. [DOI] [PMC free article] [PubMed] [Google Scholar]