Abstract
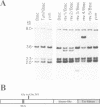
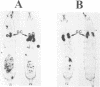
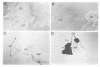
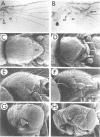
In mammals, many cytokines and growth factors stimulate members of the Janus kinase (JAK) family to transduce signals for the proliferation and differentiation of various cell types, particularly in hematopoietic lineages. Mutations in the Drosophila hopscotch (hop) gene, which encodes a JAK, also cause proliferative defects. Loss-of-function alleles result in lethality and underproliferation of diploid tissues of the larva. A dominant gain-of-function allele, Tumorous-lethal (hopTum-l), leads to formation of melanotic tumors and hypertrophy of the larval lymph glands, the hematopoietic organs. We show that a single amino acid change in Hop is associated with the hopTum-l mutation. Overexpression of either wild-type hop or hopTum-l in the larval lymph glands causes melanotic tumors and lymph gland hypertrophy indistinguishable from the original hopTum-l mutation. In addition, overexpression of Hop in other tissues of the larva leads to pattern defects in the adult or to lethality. Finally, overexpression of either hop or hopTum-l in Drosophila cell culture results in tyrosine phosphorylation of Hop protein. However, overexpression of hopTum-l results in greater phosphorylation than overexpression of the wild-type. We conclude that hopTum-l encodes a hyperactive Hop kinase and that overactivity of Hop in lymph glands causes malignant neoplasia of Drosophila blood cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argetsinger L. S., Campbell G. S., Yang X., Witthuhn B. A., Silvennoinen O., Ihle J. N., Carter-Su C. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell. 1993 Jul 30;74(2):237–244. doi: 10.1016/0092-8674(93)90415-m. [DOI] [PubMed] [Google Scholar]
- Baserga R. Oncogenes and the strategy of growth factors. Cell. 1994 Dec 16;79(6):927–930. doi: 10.1016/0092-8674(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Binari R., Perrimon N. Stripe-specific regulation of pair-rule genes by hopscotch, a putative Jak family tyrosine kinase in Drosophila. Genes Dev. 1994 Feb 1;8(3):300–312. doi: 10.1101/gad.8.3.300. [DOI] [PubMed] [Google Scholar]
- Brand A. H., Manoukian A. S., Perrimon N. Ectopic expression in Drosophila. Methods Cell Biol. 1994;44:635–654. doi: 10.1016/s0091-679x(08)60936-x. [DOI] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N. Raf acts downstream of the EGF receptor to determine dorsoventral polarity during Drosophila oogenesis. Genes Dev. 1994 Mar 1;8(5):629–639. doi: 10.1101/gad.8.5.629. [DOI] [PubMed] [Google Scholar]
- Brand A. H., Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993 Jun;118(2):401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Briscoe J., Guschin D., Müller M. Signal transduction. Just another signalling pathway. Curr Biol. 1994 Nov 1;4(11):1033–1035. doi: 10.1016/s0960-9822(00)00236-0. [DOI] [PubMed] [Google Scholar]
- Bryant P. J., Schmidt O. The genetic control of cell proliferation in Drosophila imaginal discs. J Cell Sci Suppl. 1990;13:169–189. doi: 10.1242/jcs.1990.supplement_13.16. [DOI] [PubMed] [Google Scholar]
- Bryant P. J., Watson K. L., Justice R. W., Woods D. F. Tumor suppressor genes encoding proteins required for cell interactions and signal transduction in Drosophila. Dev Suppl. 1993:239–249. [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Clifford R. J., Schüpbach T. Coordinately and differentially mutable activities of torpedo, the Drosophila melanogaster homolog of the vertebrate EGF receptor gene. Genetics. 1989 Dec;123(4):771–787. doi: 10.1093/genetics/123.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Jr, Kerr I. M., Stark G. R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994 Jun 3;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Diaz-Benjumea F. J., Hafen E. The sevenless signalling cassette mediates Drosophila EGF receptor function during epidermal development. Development. 1994 Mar;120(3):569–578. doi: 10.1242/dev.120.3.569. [DOI] [PubMed] [Google Scholar]
- Firmbach-Kraft I., Byers M., Shows T., Dalla-Favera R., Krolewski J. J. tyk2, prototype of a novel class of non-receptor tyrosine kinase genes. Oncogene. 1990 Sep;5(9):1329–1336. [PubMed] [Google Scholar]
- Fischer J. A., Giniger E., Maniatis T., Ptashne M. GAL4 activates transcription in Drosophila. Nature. 1988 Apr 28;332(6167):853–856. doi: 10.1038/332853a0. [DOI] [PubMed] [Google Scholar]
- Hanratty W. P., Dearolf C. R. The Drosophila Tumorous-lethal hematopoietic oncogene is a dominant mutation in the hopscotch locus. Mol Gen Genet. 1993 Apr;238(1-2):33–37. doi: 10.1007/BF00279527. [DOI] [PubMed] [Google Scholar]
- Hanratty W. P., Ryerse J. S. A genetic melanotic neoplasm of Drosophila melanogaster. Dev Biol. 1981 Apr 30;83(2):238–249. doi: 10.1016/0012-1606(81)90470-x. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Kerr I. M. Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet. 1995 Feb;11(2):69–74. doi: 10.1016/s0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Witthuhn B. A., Quelle F. W., Yamamoto K., Thierfelder W. E., Kreider B., Silvennoinen O. Signaling by the cytokine receptor superfamily: JAKs and STATs. Trends Biochem Sci. 1994 May;19(5):222–227. doi: 10.1016/0968-0004(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Taga T., Akira S. Cytokine signal transduction. Cell. 1994 Jan 28;76(2):253–262. doi: 10.1016/0092-8674(94)90333-6. [DOI] [PubMed] [Google Scholar]
- Klämbt C., Jacobs J. R., Goodman C. S. The midline of the Drosophila central nervous system: a model for the genetic analysis of cell fate, cell migration, and growth cone guidance. Cell. 1991 Feb 22;64(4):801–815. doi: 10.1016/0092-8674(91)90509-w. [DOI] [PubMed] [Google Scholar]
- Mahoney P. A., Weber U., Onofrechuk P., Biessmann H., Bryant P. J., Goodman C. S. The fat tumor suppressor gene in Drosophila encodes a novel member of the cadherin gene superfamily. Cell. 1991 Nov 29;67(5):853–868. doi: 10.1016/0092-8674(91)90359-7. [DOI] [PubMed] [Google Scholar]
- Müller M., Briscoe J., Laxton C., Guschin D., Ziemiecki A., Silvennoinen O., Harpur A. G., Barbieri G., Witthuhn B. A., Schindler C. The protein tyrosine kinase JAK1 complements defects in interferon-alpha/beta and -gamma signal transduction. Nature. 1993 Nov 11;366(6451):129–135. doi: 10.1038/366129a0. [DOI] [PubMed] [Google Scholar]
- Natesan S., Gilman M. Z. DNA bending and orientation-dependent function of YY1 in the c-fos promoter. Genes Dev. 1993 Dec;7(12B):2497–2509. doi: 10.1101/gad.7.12b.2497. [DOI] [PubMed] [Google Scholar]
- Perrimon N., Engstrom L., Mahowald A. P. A pupal lethal mutation with a paternally influenced maternal effect on embryonic development in Drosophila melanogaster. Dev Biol. 1985 Aug;110(2):480–491. doi: 10.1016/0012-1606(85)90105-8. [DOI] [PubMed] [Google Scholar]
- Perrimon N., Mahowald A. P. l(1)hopscotch, A larval-pupal zygotic lethal with a specific maternal effect on segmentation in Drosophila. Dev Biol. 1986 Nov;118(1):28–41. doi: 10.1016/0012-1606(86)90070-9. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuai K., Ziemiecki A., Wilks A. F., Harpur A. G., Sadowski H. B., Gilman M. Z., Darnell J. E. Polypeptide signalling to the nucleus through tyrosine phosphorylation of Jak and Stat proteins. Nature. 1993 Dec 9;366(6455):580–583. doi: 10.1038/366580a0. [DOI] [PubMed] [Google Scholar]
- Silvennoinen O., Ihle J. N., Schlessinger J., Levy D. E. Interferon-induced nuclear signalling by Jak protein tyrosine kinases. Nature. 1993 Dec 9;366(6455):583–585. doi: 10.1038/366583a0. [DOI] [PubMed] [Google Scholar]
- Silvers M., Hanratty W. P. Alterations in the production of hemocytes due to a neoplastic mutation of Drosophila melanogaster. J Invertebr Pathol. 1984 Nov;44(3):324–328. doi: 10.1016/0022-2011(84)90030-2. [DOI] [PubMed] [Google Scholar]
- Staehling-Hampton K., Jackson P. D., Clark M. J., Brand A. H., Hoffmann F. M. Specificity of bone morphogenetic protein-related factors: cell fate and gene expression changes in Drosophila embryos induced by decapentaplegic but not 60A. Cell Growth Differ. 1994 Jun;5(6):585–593. [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Sturgill T. W., Wu J. Recent progress in characterization of protein kinase cascades for phosphorylation of ribosomal protein S6. Biochim Biophys Acta. 1991 May 17;1092(3):350–357. doi: 10.1016/s0167-4889(97)90012-4. [DOI] [PubMed] [Google Scholar]
- Traugh J. A., Pendergast A. M. Regulation of protein synthesis by phosphorylation of ribosomal protein S6 and aminoacyl-tRNA synthetases. Prog Nucleic Acid Res Mol Biol. 1986;33:195–230. doi: 10.1016/s0079-6603(08)60024-0. [DOI] [PubMed] [Google Scholar]
- Watson K. L., Johnson T. K., Denell R. E. Lethal(1) aberrant immune response mutations leading to melanotic tumor formation in Drosophila melanogaster. Dev Genet. 1991;12(3):173–187. doi: 10.1002/dvg.1020120302. [DOI] [PubMed] [Google Scholar]
- Watson K. L., Konrad K. D., Woods D. F., Bryant P. J. Drosophila homolog of the human S6 ribosomal protein is required for tumor suppression in the hematopoietic system. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11302–11306. doi: 10.1073/pnas.89.23.11302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilks A. F., Harpur A. G. Cytokine signal transduction and the JAK family of protein tyrosine kinases. Bioessays. 1994 May;16(5):313–320. doi: 10.1002/bies.950160505. [DOI] [PubMed] [Google Scholar]
- Wilks A. F., Harpur A. G., Kurban R. R., Ralph S. J., Zürcher G., Ziemiecki A. Two novel protein-tyrosine kinases, each with a second phosphotransferase-related catalytic domain, define a new class of protein kinase. Mol Cell Biol. 1991 Apr;11(4):2057–2065. doi: 10.1128/mcb.11.4.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witthuhn B. A., Quelle F. W., Silvennoinen O., Yi T., Tang B., Miura O., Ihle J. N. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993 Jul 30;74(2):227–236. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- Woods D. F., Bryant P. J. Molecular cloning of the lethal(1)discs large-1 oncogene of Drosophila. Dev Biol. 1989 Jul;134(1):222–235. doi: 10.1016/0012-1606(89)90092-4. [DOI] [PubMed] [Google Scholar]