Abstract
We synthesize several lines of evidence supporting the hypothesis that at least one function of Aβ is to serve as a part of the acute response to brain hemodynamic disturbances intended to seal vascular leakage. Given the resilient and adhesive physicochemical properties of amyloid, an abluminal hemostatic repair system might be highly advantageous if deployed on a limited and acute basis in young individuals. However, in the aged, inevitable cardiovascular dysfunction combined with brain microvascular lesions may yield global, chronic hypoperfusion that may lead to continuous amyloid deposition and consequential negative effects on neuronal viability. A large body of experimental evidence supports an Aβ rescue function gone astray. Preventing or inducing the removal of amyloid in Alzheimer’s disease (AD) has been simultaneously successful and disappointing. Amyloid deposits clearly play major roles in AD, but may not represent the preeminent factor in dementia pathogenesis. Successful application of AD preventative approaches may hinge on an accurate and comprehensive view of co-morbidities, including cardiovascular disease, diabetes and head trauma.
Introduction
A recent report from Alzheimer International projects that if existing trends continue, 115 million individuals worldwide will have Alzheimer’s disease (AD) by 2050 [1]. Despite a wealth of fundamental discoveries regarding AD pathogenesis, translation of potentially promising findings into clinically useful treatments has been repeatedly stymied.
Alzheimer’s disease is classically explained by a reductionist pathogenic mechanism positing amyloid deposition as the primary toxic entity in this dementia. The amyloid cascade hypothesis was reinforced by the discovery of familial cases of AD caused by mutations in the amyloid-beta precursor protein (APP) and in the presenilin (PS) genes which produce abundant amyloid deposition and early-onset dementia. Further support for the amyloid cascade hypothesis has also been provided by the engineering of transgenic (Tg) mouse models which mimic some aspects of AD amyloid pathology using mutant human APP and PS transgenes. Data revealing the significance of amyloid to AD pathology culminated in the therapeutic disruption of amyloid deposits in Tg mouse models and in AD patients. Despite the promising results in Tg mice, the successful disruption of amyloid plaques failed to yield commensurate effects on dementia in clinical trials [2-7]. Extrapolating Tg mouse model observations directly to humans while neglecting significant evolutionary and biological differences between the two species may have played an important role in the lack of success against dementia [8;9]. Despite the clear mitigation of amyloid plaque pathology in some patients, the striking lack of concurrent effect against dementia expression suggests the disappointing prospect that we have underestimated the complexity of the underlying pathology and thereby failed to address critical facets of the problem. Viewed as a whole, the results obtained with Tg mice and clinical trial experiences reveal that AD pathology involves more than amyloid accumulation and conquering dementia demands something more than eliminating amyloid plaque deposits once cognitive impairment becomes evident. Enticed by the clear abundance of amyloid deposits in AD brain tissue and vasculature, these neuropathological features have been the dominant target in therapeutic interventions. However, the fundamental question of why amyloid accumulates in the elderly brain has yet to be answered.
In this position paper, we explore the possibility that sustained cardiovascular disease or head trauma leads to amyloid deposition, a process intended to ensure vessel repair and integrity at specific sites of injury or leakage. However, this vital protective function becomes inadvertently deleterious when chronically activated in the elderly, creating excessive amyloid deposits around the brain vasculature and an anti-angiogenic environment which generates hypoxia/ischemia. In addition, during the aging process, evolving cardiovascular decline inevitably reduces brain perfusion. Coupled with escalating brain microvascular damage, these conditions may establish a vicious cycle of anomalous and excessive vascular amyloid deposition promoting capillary and arteriole wall strangulation and luminal occlusion, eventually producing blind capillary remnants and free extracellular amyloid cores which are manifested prior to the clinical onset of the disease [10]. The concept of amyloid serving as a hemostatic patch for leaky brain microvessels was first postulated in 1993 by Roher et al. [11] and further advanced by Atwood et al. [12] and Cullen et al. [13]. Our assumptions are compatible with recent observations postulating a series of events initiated by hypoxia/ischemia and followed by vascular injury, disruption of the blood-brain barrier (BBB) and vascular amyloid deposition which is complicated by a failure in Aβ clearance and potential increase of Aβ up-take from the circulation [14]. These series of biological events are conducive to neurovascular dysfunction, neuroinflammation and neurodegeneration [15].
The Role of Cardiovascular Disease, Diabetes and Head Trauma in AD
The cardiovascular system is pre-eminent in the development of the brain maintenance of its vital functions. The brain consumes a disproportionate share of total oxygen and metabolic resources. By age 80, the human heart and vessels have beaten, stretched and contracted about 3 billion times to propel approximately 200 million liters of blood through the vasculature. This situation may explain why AD rates expand almost exponentially with advancing age in parallel with an increased incidence of morbidity and mortality resulting from cardiovascular disease [16-21]. As time elapses, the cumulative harmful effects of wear and tear on cardiovascular function become more apparent. This is well illustrated by a significant decline in cardiac output and cardiac index with advancing age [22;23]. Numerous cross-sectional and longitudinal studies, using various imaging and ultrasound techniques, have shown that in AD there is an statistically significant reduction in total and regional cerebral blood flow when compared to age matched controls [24-32]. Echocardiographic investigations demonstrated that AD subjects exhibited a statistically significant diastolic dysfunction revealed by increased transmitral vortex formation time [33]. Likewise, duplex Doppler carotid ultrasound showed a consistent and significant decrease in diastolic flow along the path of the carotid artery in AD patients, suggesting a loss of arterial elastic capacity [34]. These parameters can be construed as risk factors for pathologic brain aging and, by extension, potential harbingers of AD. Aging imposes alterations in both the intracranial resistance of arterioles and capillaries [35-39] thus reducing cerebral blood flow and inducing cognitive dysfunction [40;41]. Recent hemodynamic studies using transcranial Doppler ultrasound confirmed decreased arterial mean flow velocity and increased pulsatility index in probable AD patients compared to non-demented controls [34], revealing that diffuse microvascular pathology, increased arterial rigidity and vascular resistance contribute to overall cognitive decline.
Brain hemodynamic alterations due to severe stenosis and hardening of the neck and intracranial arteries will impact brain perfusion while promoting lacunar infarcts and strokes. By age 80 atherosclerosis of the circle of Willis, carotid and vertebral arteries is widespread. These arteries exhibit a significantly increased degree of atherosclerosis in AD subjects compared to age-matched controls [42]. Chronic hypoxia/ischemia can lead to gross disruption of the BBB integrity [43-45], conditions that may be accentuated by hypertension and diabetes. These prevalent and progressive pathologies eventually exert negative effects on energy metabolism and neuronal transmission that are detrimental to memory and cognition.
Hypertension is an important risk factor for AD due to its microangiopathic effects on the brain [21;46] and its relationship to brain microhemorrhages [47]. This condition affects approximately 25% of the adult population in the USA [48], and increases to 60-65% in those older than age 65 [21;49]. As a highly perfused organ offering low resistance to blood flow, hypertension will ultimately elicit difficult-to-repair vascular injuries and irreversible structural and functional damage in the brain [50;51]. With aging, systolic and pulse pressure increases result in endothelial cell tearing, breaches in the BBB, smooth muscle cell disruption, small arterial dilations, vascular fragility, lipohyalinosis and fibrinoid necrosis [52]. As age advances, there is a direct relationship between stiffening of large elastic arteries and brain microvascular disease [50] as well as with increased pulse pressure and pulse velocity which are correlated to cognitive decline [53].
Diabetes is an additional important risk factor for AD because of its vascular pathological repercussions and impact on energy metabolism [54-57]. By age 60 years and older, about 23% of Americans have diabetes [58]. A large body of research supports the contention that diabetes is more frequent in patients with AD [59-61]. Diabetics face a considerably higher risk of developing cardiovascular disease, hypertension, atherosclerosis and obesity [57;62] as well as brain microvascular changes leading to dysfunctional BBB associated with hypoperfusion and cognitive deficiencies [63-65]. It has been suggested that sporadic AD should be classified as type-3 diabetes due to insulin resistance and reduced expression of insulin and insulin-like growth factors in the AD brain [54-57;62].
Acute head trauma is a risk factor for AD development [66-68]. In comparison to the general population, AD and other memory loss-related diseases are 19- and 5-fold more frequent in National Football League players 30-49 and 50 plus years of age, respectively [69]. Both APP and amyloid-beta (Aβ) levels increase after acute brain injury [70-73], suggesting an acute phase protein response involved in brain salvage function. The capacity of Aβ to produce vasoconstriction [74;75], coupled with its potent anti-angiogenic activity [76;77] and the ability of the Aβ peptides to act as metal chelating agents [78-80] may reduce the generation of deleterious reactive species [81;82] from extravasated hemoglobin-bound iron [83;84] resulting from concussive microhemorrhages. Thus, while amyloid deposition may increase the probability of surviving acute brain injury, it also confers a threat for future dementia development. Despite evidence for clearance of trauma-associated Aβ [12], even minute remnants of vasculature-associated deposits could act as seeding templates for the future propagation of widespread amyloid pathology analogous to the chain reaction expansion characteristic of prion diseases [85].
In summary, cardiovascular dysfunction, common in middle age and elderly individuals, whether due to hypertension, intrinsic cardiac diastolic and systolic failure, lost of vascular compliance, atherosclerotic stenosis/thrombosis, brain diffuse microvascular disease and/or damaged BBB will eventually cause brain hypoxia/ischemia. These perturbations could be synergistically aggravated by respiratory disease, diabetes or by concussive head trauma. A disturbed microvasculature will need to be efficiently repaired to maintain the integrity of the BBB and an efficient blood flow to prevent energy metabolism failure and ultimately dementia.
Amyloid as a vascular repair mechanism
From a structural viewpoint, amyloid filaments exhibit high mechanical strength, are highly insoluble and resistant to degradation [86;87]. In addition, amyloid filaments are plastic, have cement-like bonding properties [86] and readily interact with the extracellular matrix [88] as well as with a reduced turnover suitable for vascular injury repair. Animal cements, based on amyloid polymerization aid in wound healing, maintenance of tissue integrity and exhibit biochemical processes analogous to blood clotting [89;90]. An abluminal amyloid molecular lattice would permit continued vascular blood flow while sealing breaches in the BBB [11;12]. This putative function would prevent the classical coagulation cascade from blocking the lumen of the capillaries and arterioles [12]. Moreover, Aβ may also act as an anti-microbial peptide capable of inhibiting and entrapping invading bacteria that otherwise could have harmful consequences for brain survival [91].
The above properties suggest that amyloid deposits may act as dynamic hydrophobic, insoluble sealants to halt vascular leakage due to vascular disease, trauma or intrinsic agingfailure of the brain vasculature [11;12] as well as serving as an acute phase protective function by sequestering excess heme, iron and other metal ions [92;93]. Blood components and their breakdown products free in the brain tissue have grave functional and pathological consequences as amply illustrated by the effects of hemorrhagic stroke [94] and brain edema [95]. Hypothesizing a brain-specific microvascular repair mechanism has important pathophysiological implications. A breached BBB will permit the infiltration of plasma proteins directly into the parenchyma or the creation of overt microhemorrhages. Small vascular lesions resulting in blood permeation into the neuropil are documented in AD by imaging techniques [96] and histological studies [97]. High levels of thrombin and matrix metalloproteinase-2 participate in the disruption of the BBB [98-101]. Hemin can generate oxidative stress through the production of superoxide and hydroxyl radicals by redox-active iron moieties resulting in membrane peroxidation attack, reduction of NADPH and depletion of glutathione levels [102]. Morphological studies have demonstrated a physical overlap between heme deposits and vascular-associated amyloid cores in the AD brain [13;103]. In brief, the grave consequences of extravasated plasma proteins and free-hemoglobin in the brain parenchyma, produced by a breached BBB and brain microhemorrhages, may be remediated by amyloid deposition.
The deleterious effects of excessive amyloid deposition
Vascular amyloid deposition ultimately evolves into a devastating condition resulting in progressive hypoxia/ischemia, failure in energy metabolism and permanent brain injury [97;104]. It is tempting to speculate that these consequences manifested in the aging brain are the ultimate tradeoff for a pathway selected through evolutionary processes to safeguard vascular continuity in younger individuals, but becomes progressively destructive under physiologic conditions in the elderly [105].
Microscopic examination of whole-mounted vascular specimens revealed that in AD, the cortical microvascular network harbors abundant fibrillar amyloid deposits at different degrees of condensation (Figure 1). At higher magnification, some microvessels appear constricted, particularly at sites surrounded by large cores of fibrillar amyloid [11;13]. Continuous Aβ accretion around the microvessel wall should generate increasing pressure on the expanding deposit, eventually occluding the vascular lumen. Conceivably, luminal occlusion is followed by degeneration and disappearance of the vessel wall, leaving insoluble amyloid cores apparently ‘floating’ free within the brain parenchyma entirely detached from the remaining vascular stumps (Figure 2). The chemical composition and post-translational modification similarities between microvasculature-attached and ‘free’ amyloid plaque Aβ peptides, rich in insoluble Aβ42 with abundant post-translational modifications [11;106-108] as well as the tenacious association of developing amyloid deposits with the brain vascular walls support this tenet. In advanced vascular amyloidosis, heavy amyloid deposition within the cortical arteries’ periarterial spaces compromises interstitial fluid removal from the white matter, dilating the periarterial spaces (etat criblé) [109-111]. Retention of interstitial fluid and poisonous metabolic waste may negatively compound the severe demyelination present in two-thirds of patients with AD [109]. Furthermore, it may also explain gross ventricle enlargement, a nearly universal signature of this type of dementia. From a hydrodynamic point of view, relentless enlargement of the ventricles will drastically compress the white matter, thereby promoting degeneration of this tissue.
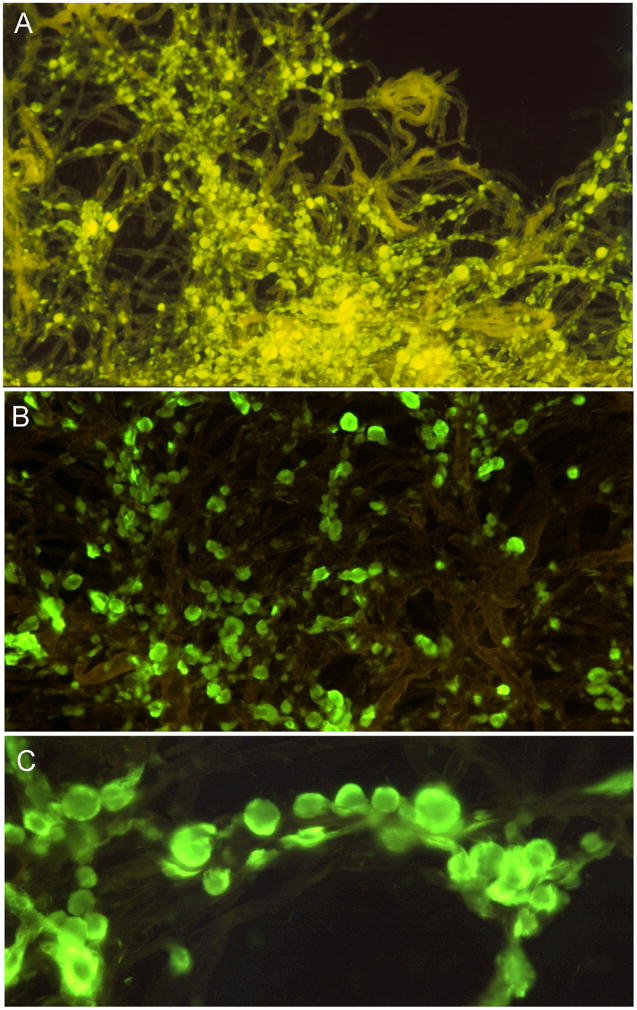
Figure 1. Alzheimer’s disease whole-mount preparations of isolated microvessel-associated amyloid deposits stained by thioflavine-S.
A) A tuft of cortical microvessels walls revealed as detergent (SDS) insoluble cross-linked extracellular matrix remnants demonstrating a wide range of amyloid core deposits intimately associated with the basal lamina. The early amyloid deposits are small, flat and ellipsoidal. In more advanced deposits, the amyloid deposits become spherical and completely surround the vascular wall. The continuous accretion of fibrillar Aβ onto the surface of the amyloid sphere by glial cells may eventually obliterate the microvessel, thereby compromising blood supply. B and C) Tufts of capillaries and arterioles with numerous amyloid cores attached to the vascular basal lamina. Note that in some instances the spherical amyloid cores are sparse while in other instances they are distributed in a rosary-like succession. For detailed technical information and interpretation see reference [11]. Figure A reproduced with permission from the Publisher: Proceeding of the National Academy of Sciences, USA. Magnification: A = 100X; B = 200X; C = 400X.
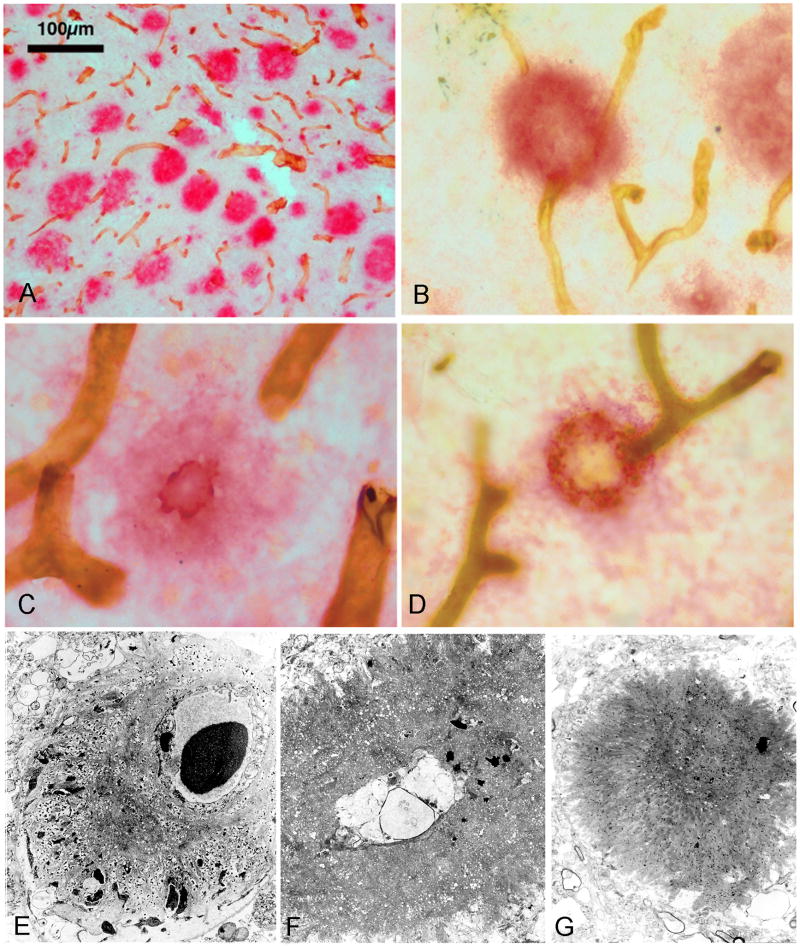
Figure 2. Spatial associations between amyloid plaque cores and microvessels in AD cortical areas.
A) Digital image of entorhinal cortex of amyloid deposits stained with anti-Aβ (red) and microvessels (brown) stained with anti-collagen IV monoclonal antibody demonstrating the association between the two structures. B, C and D) These images demonstrate the close relationship between amyloid plaques and the cerebral microvessels. In some instances, the vessel is surrounded by the amyloid plaque or the amyloid plaque appear to be ‘floating’ free in the neuropil surrounded by remnant vascular stumps. For complete technical description and interpretation of data for A, B, C and D see Cullen et al 2006 [13]. Figures A, B, C and D reproduced with permission from the Publisher: Elsevier Inc. E) Electron micrograph showing a core of amyloid attached to the surface of a cortical microvessel. The wisps of amyloid fibrils are inter-digitated with the extracellular matrix and cellular debris. F) As amyloid deposition advances, the swollen remnants of the blood vessel are entrapped at the center of an amyloid core. G) Once the blood vessel is totally obliterated and the vascular stumps retract, a dense core of radiating amyloid fibrils represent the ultimate permanent lesion. Magnification: E = 5500X; F = 5500X; G = 2500X.
Global therapeutic implications of amyloid removal
Immunotherapy has disrupted amyloid plaques in humans and Tg animal models. Although amyloid deposition creates noxious conditions and amyloid plaques have been correlated with AD dementia, this association is relatively weak [112;113]. The remarkable physical impact of immunotherapy coupled with the striking lack of corresponding effect on dementia suggests that amyloid plaques are not the sole or perhaps even primary pathogenic factor of AD.
The postmortem data from immunotherapy trial subjects reveal that although the disruption of amyloid plaques has been dramatic in some cases, the elimination was not total and persistent remnants may still harbour toxic Aβ peptides or other noxious molecular species [2;3;114]. In addition, plaque removal cannot reverse a legacy of destroyed vascular elements or neurons. The inability of Aβ immunization to totally eradicate amyloid plaques coupled with the failed or slow exit of amyloid from the brain [2;3;114] due to a congested vasculature means that the amyloid hypothesis per se has yet to be tested rigorously. Furthermore, additional findings suggest that amyloid plaques might reconstitute swiftly as soon as antibody levels decline [3]. Taken together, these observations strongly suggest that to avoid amyloid toxicity, applying interventions on a preventative rather than a therapeutic basis may be more efficacious.
If amyloid deposits perform a vascular rescue function or simply accumulate steadily with age and produce localized damage, specifically removing them may inadvertently promote BBB breaches. However, explaining this assumption as the consequence of the simple removal of an essential vascular patch [12] is not straightforward. Microhemorrhages are frequently observed in AD patients [97], implying that the unchecked accumulation of vascular amyloid can itself be intrinsically destructive. Postmortem examinations of AN-1792-immunized individuals revealed that vascular amyloid deposits were partially refractory to immune disruption [2;114;115]. In some AD patients, vascular amyloid may have increased as a consequence of immunotherapy [2;114;115]. Amyloid-β immunotherapy has induced deleterious side effects such as microhemorrhages [115-118], as well as vasogenic edema and aseptic meningoencephalitis (reviewed in: [119]) in some recipients. In these patients, it is possible that essential amyloid depositional processes in the vasculature were active when therapy commenced and the removal of functional amyloid “scabs” [12] breached the vessels directly or created failure-prone areas. Recognizing that amyloid accumulation may precede the onset of dementia by a substantial margin [10;120] and the empiric discovery that patient ApoE genotype exerts considerable influence over the type and emergence of lesions, suggest that careful patient selection, precise treatment timing and individual titration of immunotherapy may be essential for optimal efficacy.
An additional complication of Aβ immunotherapy is its potential interference with the coagulation cascade, a complex and highly conserved hemostatic mechanism that repairs injured blood vessels. Multiple, interdependent proteins participate in a chain of events terminating in the formation of an adhesive clot, mainly made of platelets and cross-linked fibrin. Although a well functioning coagulation cascade is essential for survival, the requirements for vascular integrity maintenance and repair in the brain may be more stringent than those of peripheral organs. The brain is an exceptional organ with finely tuned electrical activities generated by neurons assisted by glial cells that need to be maintained in a semi-secluded molecular compartment secured by the integrity of the BBB. While rapid coagulation mediates vascular recovery in many organs, severe microvascular damage within the brain may follow a fundamentally different response strategy to ensure adequate blood flow while minimizing the prospect of neuronal injury.
In AD, a breached BBB would release fibrinogen into the extracellular space of the brain microvasculature where it will contact Aβ peptides. In addition, damaged endothelial cells produce thrombin [121]. Recent in vivo and in vitro experiments demonstrate that the interaction between these two molecules results in altered thrombosis and fibrinolysis and generates lysis-resistant clots that may contribute to vascular constriction, brain hypoperfusion and neuroinflammation [122;123].
It is important to recognize that the production and distribution of Aβ is not restricted to the brain [124]. The long-term effects of chronic immunotherapy administration on vital cell signaling pathways and the coagulation system are not known. The available data suggest that potential interactions between protease nexin-2 (PN-2), an APP molecule carrying a Kunitz-type serine protease inhibitory domain, and anti-Aβ antibodies may have negative effects on the coagulation cascade resulting in thrombosis. Although antibodies against Aβ are directed against amino acid sequences within the Aβ peptide, the possibility of interactions with APP in patients undergoing immunotherapy remains open. Intriguingly, in recent clinical trials several AD patients treated with bapineuzumab, a monoclonal antibody against the N-terminal domain of Aβ, developed deep venous thrombosis (3.2%) or pulmonary embolism (0.8%), while none of these coagulation cascade-associated adverse events occurred in the placebo branch [125]. For comparison, acute venous thromboembolism has an annual incidence of about 1-2 cases for 1000 individuals in the general population [126;127]. The observation that Aβ immunotherapy in AD patients and APP Tg mice induces microhemorrhages [115;116;118;128] further suggests that the normal coagulation cascade may be perturbed. This may be a manifestation of PN-2 inhibition, steric hindrance effects or conformational changes induced by high titers of circulating anti-Aβ antibodies. Protease nexin-2 blocks the IXa, Xa and XIa factors and tissue factor:factor VIIa in the prothrombinase complex cascade, supporting the hypothesis that PN-2 functions in the focused regulation of the coagulation process at sites of vascular injury [129]. The net effects of such induced alterations may be more profound in the periphery, but unfortunately no data exist to settle the matter.
Summary statement and conclusions
The supreme challenge is to set the impressive knowledge regarding brain Aβ biochemistry into a larger physiological context that takes account of established AD systemic co-morbidities such as cardiovascular disease and diabetes. This is no small task since the normal function(s) of APP/Aβ, an evolutionarily-conserved molecule, remains nebulous. Several lines of evidence suggest that at least one function of Aβ might be as a part of the acute response to brain vascular trauma and degeneration intended to seal capillary and small vessel leakage. A brain vascular repair system mitigating ischemia/hypoperfusion might be highly advantageous deployed on an acute basis in young individuals. However, in the aged, inevitable cardiovascular dysfunction combined with microvascular lesions may yield chronic conditions that are misinterpreted as requiring repair which promotes a self-synergizing global cascade of insidious vascular occlusion and ultimately negative effects on cognition. Although amyloid deposits deployed in the acute response to brain vascular trauma are apparently reversed, especially in younger more functionally vigorous individuals, past head injuries are a recognized risk factor for AD development, suggesting that the salvaged regions of the brain harbour potential nucleation sites that may promote amyloid propagation in the future.
Confronting hypothesis with data, it seems likely that the long-prevailing view of AD pathogenesis may be too limited. The brain is heavily dependent on cardiac output and the functional integrity of the arterial and venous networks. The brain is unique among the organs in its extreme perfusion demands, energy requirements and strictly maintained biochemical separateness from the circulatory system. Considering the brain and its age-related AD in isolation, we may have overlooked the fact that all aspects of brain function ultimately depend on the cardiovascular system and adequate energy metabolism. The genesis of dementia is multifactorial and heterogeneous; its mitigation may be a complex undertaking as well.
Acknowledgments
This study was supported by the National Institute on Aging grants R01 AG019795 and R21 AG035078 and by the State of Arizona Alzheimer Disease Research Consortium. We would like to thank Ian D. Daugs for assistance with the figures Dr. Walter M. Kalback and Dr. Dean C. Luehrs for critical review of the manuscript.
List of Abbreviations
- Aβ
amyloid-beta
- AD
Alzheimer’s disease
- ApoE
apolipoprotein E
- APP
amyloid-beta precursor protein
- BBB
blood-brain barrier
- PN-2
protease nexin-2
- PS
presenilin
- RBC
red blood cell
- Tg
transgenic
- WBC
white blood cell
Footnotes
Declaration of Competing Interests
TAK, CLM and AER declare that they have no competing interests.
Reference List
- 1.Wimo A, Prince M. World Alzheimer Report 2010. Alzheimer’s Disease International. 2010 Sep 21; [Google Scholar]
- 2.Maarouf CL, Daugs ID, Kokjohn TA, Kalback WM, Patton RL, Luehrs DC, et al. The biochemical aftermath of anti-amyloid immunotherapy. Mol Neurodegener. 2010;5:39. doi: 10.1186/1750-1326-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roher AE, Maarouf CL, Daugs ID, Kokjohn TA, Hunter JM, Sabbagh MN, et al. Neuropathology and Aβ Spectrum in a Bapineuzumab Immunotherapy Recipient. J Alzheimers Dis. 2011;24:315–25. doi: 10.3233/JAD-2011-101809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, et al. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–23. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 5.Wong GT. FDA Deems U.S. Alzhemed Trial Results Inconclusive. Alzheimer Research Forum. 2007 Aug 28; [Google Scholar]
- 6.Strobel G. Chicago: Flurizan Postmortem. Alzheimer Research Forum. 2008 Aug 20; [Google Scholar]
- 7.Fagan T. Lilly Halts IDENTITY Trials as Patients Worsen on Secretase Inhibitor. Alzheimer Research Forum. 2010 Aug 18; [Google Scholar]
- 8.Kokjohn TA, Roher AE. Amyloid precursor protein transgenic mouse models and Alzheimer’s disease: understanding the paradigms, limitations, and contributions. Alzheimers Dement. 2009;5:340–347. doi: 10.1016/j.jalz.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roher AE, Kokjohn TA. Appraisal of AbetaPP Transgenic Mice as Models for Alzheimer’s Disease Amyloid Cascade. Curr Med Chem Immun Endo & Metab Agents. 2003;3:85–90. [Google Scholar]
- 10.Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–28. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roher AE, Lowenson JD, Clarke S, Woods AS, Cotter RJ, Gowing E, et al. beta-Amyloid-(1-42) is a major component of cerebrovascular amyloid deposits: implications for the pathology of Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:10836–40. doi: 10.1073/pnas.90.22.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atwood CS, Bowen RL, Smith MA, Perry G. Cerebrovascular requirement for sealant, anti-coagulant and remodeling molecules that allow for the maintenance of vascular integrity and blood supply. Brain Res Brain Res Rev. 2003;43:164–78. doi: 10.1016/s0165-0173(03)00206-6. [DOI] [PubMed] [Google Scholar]
- 13.Cullen KM, Kocsi Z, Stone J. Microvascular pathology in the aging human brain: evidence that senile plaques are sites of microhaemorrhages. Neurobiol Aging. 2006;27:1786–96. doi: 10.1016/j.neurobiolaging.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 14.Zlokovic BV, Deane R, Sagare AP, Bell RD, Winkler EA. Low-density lipoprotein receptor-related protein-1: a serial clearance homeostatic mechanism controlling Alzheimer’s amyloid beta-peptide elimination from the brain. J Neurochem. 2010;115:1077–89. doi: 10.1111/j.1471-4159.2010.07002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zlokovic BV. Neurodegeneration and the neurovascular unit. Nat Med. 2010;16:1370–1371. doi: 10.1038/nm1210-1370. [DOI] [PubMed] [Google Scholar]
- 16.Gaziano JM. Global Burden of Cardiovascular Disease. In: Zipes DP, Libby P, Bonow RO, Braunwald E, editors. Braunwald’s Heart Disease. Philadelphia: Elsevier Saunders; pp. 1–19. [Google Scholar]
- 17.Marin J. Age-related changes in vascular responses: a review. Mech Ageing Dev. 1995;79:71–114. doi: 10.1016/0047-6374(94)01551-v. [DOI] [PubMed] [Google Scholar]
- 18.de la Torre JC. Alzheimer’s disease is a vasocognopathy: a new term to describe its nature. Neurol Res. 2004;26:517–24. doi: 10.1179/016164104225016254. [DOI] [PubMed] [Google Scholar]
- 19.Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010;120:287–96. doi: 10.1007/s00401-010-0718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dickstein DL, Walsh J, Brautigam H, Stockton SD, Jr, Gandy S, Hof PR. Role of vascular risk factors and vascular dysfunction in Alzheimer’s disease. Mt Sinai J Med. 2010;77:82–102. doi: 10.1002/msj.20155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novak V, Hajjar I. The relationship between blood pressure and cognitive function. Nat Rev Cardiol. 2010;7:686–98. doi: 10.1038/nrcardio.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jefferson AL. Cardiac output as a potential risk factor for abnormal brain aging. J Alzheimers Dis. 2010;20:813–21. doi: 10.3233/JAD-2010-100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jefferson AL, Himali JJ, Beiser AS, Au R, Massaro JM, Seshadri S, et al. Cardiac index is associated with brain aging: the Framingham Heart Study. Circulation. 2010;122:690–697. doi: 10.1161/CIRCULATIONAHA.109.905091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Devanand DP, Van Heertum RL, Kegeles LS, Liu X, Jin ZH, Pradhaban G, et al. (99m)Tc hexamethyl-propylene-aminoxime single-photon emission computed tomography prediction of conversion from mild cognitive impairment to Alzheimer disease. Am J Geriatr Psychiatry. 2010;18:959–72. doi: 10.1097/JGP.0b013e3181ec8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Streitparth F, Wieners G, Kamena A, Schroder RJ, Stiepani H, Kokocinski T, et al. [Diagnostic value of multislice perfusion CT in dementia patients] Radiologe. 2008;48:175–83. doi: 10.1007/s00117-006-1443-y. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi S, Tateno M, Utsumi K, Takahashi A, Saitoh M, Morii H, et al. Quantitative analysis of brain perfusion SPECT in Alzheimer’s disease using a fully automated regional cerebral blood flow quantification software, 3DSRT. J Neurol Sci. 2008;264:27–33. doi: 10.1016/j.jns.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Nishimura T, Hashikawa K, Fukuyama H, Kubota T, Kitamura S, Matsuda H, et al. Decreased cerebral blood flow and prognosis of Alzheimer’s disease: a multicenter HMPAO-SPECT study. Ann Nucl Med. 2007;21:15–23. doi: 10.1007/BF03033995. [DOI] [PubMed] [Google Scholar]
- 28.Imabayashi E, Matsuda H, Asada T, Ohnishi T, Sakamoto S, Nakano S, et al. Superiority of 3-dimensional stereotactic surface projection analysis over visual inspection in discrimination of patients with very early Alzheimer’s disease from controls using brain perfusion SPECT. J Nucl Med. 2004;45:1450–1457. [PubMed] [Google Scholar]
- 29.Kantarci K, Jack CR., Jr Neuroimaging in Alzheimer disease: an evidence-based review. Neuroimaging Clin N Am. 2003;13:197–209. doi: 10.1016/s1052-5149(03)00025-x. [DOI] [PubMed] [Google Scholar]
- 30.Hanyu H, Shimuzu T, Tanaka Y, Takasaki M, Koizumi K, Abe K. Effect of age on regional cerebral blood flow patterns in Alzheimer’s disease patients. J Neurol Sci. 2003;209:25–30. doi: 10.1016/s0022-510x(02)00456-2. [DOI] [PubMed] [Google Scholar]
- 31.Matsuda H, Kanetaka H, Ohnishi T, Asada T, Imabayashi E, Nakano S, et al. Brain SPET abnormalities in Alzheimer’s disease before and after atrophy correction. Eur J Nucl Med Mol Imaging. 2002;29:1502–5. doi: 10.1007/s00259-002-0930-2. [DOI] [PubMed] [Google Scholar]
- 32.Kogure D, Matsuda H, Ohnishi T, Asada T, Uno M, Kunihiro T, et al. Longitudinal evaluation of early Alzheimer’s disease using brain perfusion SPECT. J Nucl Med. 2000;41:1155–62. [PubMed] [Google Scholar]
- 33.Belohlavek M, Jiamsripong P, Calleja AM, McMahon EM, Maarouf CL, Kokjohn TA, et al. Patients with Alzheimer disease have altered transmitral flow: echocardiographic analysis of the vortex formation time. J Ultrasound Med. 2009;28:1493–500. doi: 10.7863/jum.2009.28.11.1493. [DOI] [PubMed] [Google Scholar]
- 34.Roher AE, Garami Z, Tyas SL, Maarouf CL, Kokjohn TA, Belohlavek M, et al. Transcranial Doppler ultrasound blood flow velocity and pulsatility index as systemic indicators for Alzheimer’s disease. Alzheimers Dement. 2011 doi: 10.1016/j.jalz.2010.09.002. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalaria RN. Cerebral vessels in ageing and Alzheimer’s disease. Pharmacol Ther. 1996;72:193–214. doi: 10.1016/s0163-7258(96)00116-7. [DOI] [PubMed] [Google Scholar]
- 36.Scheibel AB, Duong TH, Jacobs R. Alzheimer’s disease as a capillary dementia. Ann Med. 1989;21:103–7. doi: 10.3109/07853898909149194. [DOI] [PubMed] [Google Scholar]
- 37.Brown WR, Moody DM, Thore CR, Anstrom JA, Challa VR. Microvascular changes in the white mater in dementia. J Neurol Sci. 2009;283:28–31. doi: 10.1016/j.jns.2009.02.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown WR, Thore CR. Review: Cerebral microvascular pathology in aging and neurodegeneration. Neuropathol Appl Neurobiol. 2010 doi: 10.1111/j.1365-2990.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hardy JA, Mann DM, Wester P, Winblad B. An integrative hypothesis concerning the pathogenesis and progression of Alzheimer’s disease. Neurobiol Aging. 1986;7:489–502. doi: 10.1016/0197-4580(86)90086-2. [DOI] [PubMed] [Google Scholar]
- 40.de la Torre JC. Alzheimer disease as a vascular disorder: nosological evidence. Stroke. 2002;33:1152–62. doi: 10.1161/01.str.0000014421.15948.67. [DOI] [PubMed] [Google Scholar]
- 41.de la Torre JC. Hemodynamic consequences of deformed microvessels in the brain in Alzheimer’s disease. Ann N Y Acad Sci. 1997;826:75–91. doi: 10.1111/j.1749-6632.1997.tb48462.x. [DOI] [PubMed] [Google Scholar]
- 42.Roher AE, Tyas SL, Maarouf CL, Daugs ID, Kokjohn TA, Emmerling MR, et al. Intracranial atherosclerosis as a contributing factor to Alzheimer’s disease dementia. Alzheimers Dement. 2011 doi: 10.1016/j.jalz.2010.08.228. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vasilevko V, Passos GF, Quiring D, Head E, Kim RC, Fisher M, et al. Aging and cerebrovascular dysfunction: contribution of hypertension, cerebral amyloid angiopathy, and immunotherapy. Ann N Y Acad Sci. 2010;1207:58–70. doi: 10.1111/j.1749-6632.2010.05786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaur C, Ling EA. Blood brain barrier in hypoxic-ischemic conditions. Curr Neurovasc Res. 2008;5:71–81. doi: 10.2174/156720208783565645. [DOI] [PubMed] [Google Scholar]
- 45.Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 46.Cohuet G, Struijker-Boudier H. Mechanisms of target organ damage caused by hypertension: therapeutic potential. Pharmacol Ther. 2006;111:81–98. doi: 10.1016/j.pharmthera.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 47.Henskens LH, van Oostenbrugge RJ, Kroon AA, de Leeuw PW, Lodder J. Brain microbleeds are associated with ambulatory blood pressure levels in a hypertensive population. Hypertension. 2008;51:62–68. doi: 10.1161/HYPERTENSIONAHA.107.100610. [DOI] [PubMed] [Google Scholar]
- 48.Short Version of Long-term Plan for Research and Translation in Hypertension for Enhancing Public Health. National Heart, Lung, and Blood Institute; 2005. [Google Scholar]
- 49.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–23. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 50.O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005;46:200–204. doi: 10.1161/01.HYP.0000168052.00426.65. [DOI] [PubMed] [Google Scholar]
- 51.Inzitari M, Pozzi C, Rinaldi LA, Masotti G, Marchionni N, Di Bari M. Cognitive and functional impairment in hypertensive brain microangiopathy. J Neurol Sci. 2007;257:166–73. doi: 10.1016/j.jns.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 52.Feihl F, Liaudet L, Waeber B. The macrocirculation and microcirculation of hypertension. Curr Hypertens Rep. 2009;11:182–89. doi: 10.1007/s11906-009-0033-6. [DOI] [PubMed] [Google Scholar]
- 53.Waldstein SR, Rice SC, Thayer JF, Najjar SS, Scuteri A, Zonderman AB. Pulse pressure and pulse wave velocity are related to cognitive decline in the Baltimore Longitudinal Study of Aging. Hypertension. 2008;51:99–104. doi: 10.1161/HYPERTENSIONAHA.107.093674. [DOI] [PubMed] [Google Scholar]
- 54.Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease--is this type 3 diabetes? J Alzheimers Dis. 2005;7:63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- 55.de la Monte SM, Tong M, Lester-Coll N, Plater M, Jr, Wands JR. Therapeutic rescue of neurodegeneration in experimental type 3 diabetes: relevance to Alzheimer’s disease. J Alzheimers Dis. 2006;10:89–109. doi: 10.3233/jad-2006-10113. [DOI] [PubMed] [Google Scholar]
- 56.de la Monte SM. Insulin resistance and Alzheimer’s disease. BMB Rep. 2009;42:475–81. doi: 10.5483/bmbrep.2009.42.8.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Craft S. Insulin resistance and Alzheimer’s disease pathogenesis: potential mechanisms and implications for treatment. Curr Alzheimer Res. 2007;4:147–52. doi: 10.2174/156720507780362137. [DOI] [PubMed] [Google Scholar]
- 58.Freeman JS. The increasing epidemiology of diabetes and review of current treatment algorithms. J Am Osteopath Assoc. 2010;110:eS2–eS6. [PubMed] [Google Scholar]
- 59.Carlsson CM. Type 2 diabetes mellitus, dyslipidemia, and Alzheimer’s disease. J Alzheimers Dis. 2010;20:711–22. doi: 10.3233/JAD-2010-100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Luchsinger JA. Diabetes, related conditions, and dementia. J Neurol Sci. 2010;299:35–38. doi: 10.1016/j.jns.2010.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kopf D, Frolich L. Risk of incident Alzheimer’s disease in diabetic patients: a systematic review of prospective trials. J Alzheimers Dis. 2009;16:677–85. doi: 10.3233/JAD-2009-1011. [DOI] [PubMed] [Google Scholar]
- 62.Craft S. The role of metabolic disorders in Alzheimer disease and vascular dementia: two roads converged. Arch Neurol. 2009;66:300–305. doi: 10.1001/archneurol.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Horani MH, Mooradian AD. Effect of diabetes on the blood brain barrier. Curr Pharm Des. 2003;9:833–40. doi: 10.2174/1381612033455314. [DOI] [PubMed] [Google Scholar]
- 64.Mooradian AD. Central nervous system complications of diabetes mellitus--a perspective from the blood-brain barrier. Brain Res Brain Res Rev. 1997;23:210–218. doi: 10.1016/s0165-0173(97)00003-9. [DOI] [PubMed] [Google Scholar]
- 65.Mooradian AD. Effect of aging on the blood-brain barrier. Neurobiol Aging. 1988;9:31–39. doi: 10.1016/s0197-4580(88)80013-7. [DOI] [PubMed] [Google Scholar]
- 66.Jellinger KA. Head injury and dementia. Curr Opin Neurol. 2004;17:719–23. doi: 10.1097/00019052-200412000-00012. [DOI] [PubMed] [Google Scholar]
- 67.Szczygielski J, Mautes A, Steudel WI, Falkai P, Bayer TA, Wirths O. Traumatic brain injury: cause or risk of Alzheimer’s disease? A review of experimental studies. J Neural Transm. 2005;112:1547–64. doi: 10.1007/s00702-005-0326-0. [DOI] [PubMed] [Google Scholar]
- 68.Van Den Heuvel C, Thornton E, Vink R. Traumatic brain injury and Alzheimer’s disease: a review. Prog Brain Res. 2007;161:303–16. doi: 10.1016/S0079-6123(06)61021-2. [DOI] [PubMed] [Google Scholar]
- 69.Weir DR, Jackson JS, Sonnega A National Football League Player Care Foundation. Study of Retired NFL Players. University of Michigan Institute for Social Research; Sep 10, 2009. [Google Scholar]
- 70.Smith DH, Chen XH, Iwata A, Graham DI. Amyloid beta accumulation in axons after traumatic brain injury in humans. J Neurosurg. 2003;98:1072–77. doi: 10.3171/jns.2003.98.5.1072. [DOI] [PubMed] [Google Scholar]
- 71.Olsson A, Csajbok L, Ost M, Hoglund K, Nylen K, Rosengren L, et al. Marked increase of beta-amyloid(1-42) and amyloid precursor protein in ventricular cerebrospinal fluid after severe traumatic brain injury. J Neurol. 2004;251:870–876. doi: 10.1007/s00415-004-0451-y. [DOI] [PubMed] [Google Scholar]
- 72.Ikonomovic MD, Uryu K, Abrahamson EE, Ciallella JR, Trojanowski JQ, Lee VM, et al. Alzheimer’s pathology in human temporal cortex surgically excised after severe brain injury. Exp Neurol. 2004;190:192–203. doi: 10.1016/j.expneurol.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 73.Raby CA, Morganti-Kossmann MC, Kossmann T, Stahel PF, Watson MD, Evans LM, et al. Traumatic brain injury increases beta-amyloid peptide 1-42 in cerebrospinal fluid. J Neurochem. 1998;71:2505–9. doi: 10.1046/j.1471-4159.1998.71062505.x. [DOI] [PubMed] [Google Scholar]
- 74.Paris D, Town T, Mori T, Parker TA, Humphrey J, Mullan M. Soluble beta-amyloid peptides mediate vasoactivity via activation of a pro-inflammatory pathway. Neurobiol Aging. 2000;21:183–97. doi: 10.1016/s0197-4580(99)00111-6. [DOI] [PubMed] [Google Scholar]
- 75.Townsend KP, Obregon D, Quadros A, Patel N, Volmar C, Paris D, et al. Proinflammatory and vasoactive effects of Abeta in the cerebrovasculature. Ann N Y Acad Sci. 2002;977:65–76. doi: 10.1111/j.1749-6632.2002.tb04799.x. [DOI] [PubMed] [Google Scholar]
- 76.Paris D, Townsend K, Quadros A, Humphrey J, Sun J, Brem S, et al. Inhibition of angiogenesis by Abeta peptides. Angiogenesis. 2004;7:75–85. doi: 10.1023/B:AGEN.0000037335.17717.bf. [DOI] [PubMed] [Google Scholar]
- 77.Paris D, Patel N, Ganey NJ, Laporte V, Quadros A, Mullan MJ. Anti-Tumoral Activity of a Short Decapeptide Fragment of the Alzheimer’s Abeta Peptide. Int J Pept Res Ther. 2010;16:23–30. doi: 10.1007/s10989-010-9198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chaney MO, Webster SD, Kuo YM, Roher AE. Molecular modeling of the Abeta1-42 peptide from Alzheimer’s disease. Protein Eng. 1998;11:761–67. doi: 10.1093/protein/11.9.761. [DOI] [PubMed] [Google Scholar]
- 79.Atwood CS, Moir RD, Huang X, Scarpa RC, Bacarra NM, Romano DM, et al. Dramatic aggregation of Alzheimer abeta by Cu(II) is induced by conditions representing physiological acidosis. J Biol Chem. 1998;273:12817–26. doi: 10.1074/jbc.273.21.12817. [DOI] [PubMed] [Google Scholar]
- 80.House E, Collingwood J, Khan A, Korchazkina O, Berthon G, Exley C. Aluminium, iron, zinc and copper influence the in vitro formation of amyloid fibrils of Abeta42 in a manner which may have consequences for metal chelation therapy in Alzheimer’s disease. J Alzheimers Dis. 2004;6:291–301. doi: 10.3233/jad-2004-6310. [DOI] [PubMed] [Google Scholar]
- 81.Bishop GM, Robinson SR, Liu Q, Perry G, Atwood CS, Smith MA. Iron: a pathological mediator of Alzheimer disease? Dev Neurosci. 2002;24:184–87. doi: 10.1159/000065696. [DOI] [PubMed] [Google Scholar]
- 82.Bishop GM, Robinson SR. The amyloid paradox: amyloid-beta-metal complexes can be neurotoxic and neuroprotective. Brain Pathol. 2004;14:448–52. doi: 10.1111/j.1750-3639.2004.tb00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sercombe R, Dinh YR, Gomis P. Cerebrovascular inflammation following subarachnoid hemorrhage. Jpn J Pharmacol. 2002;88:227–49. doi: 10.1254/jjp.88.227. [DOI] [PubMed] [Google Scholar]
- 84.Bishop GM, Robinson SR. Quantitative analysis of cell death and ferritin expression in response to cortical iron: implications for hypoxia-ischemia and stroke. Brain Res. 2001;907:175–87. doi: 10.1016/s0006-8993(01)02303-4. [DOI] [PubMed] [Google Scholar]
- 85.Miller G. Neurodegeneration. Could they all be prion diseases? Science. 2009;326:1337–39. doi: 10.1126/science.326.5958.1337. [DOI] [PubMed] [Google Scholar]
- 86.Barlow DE, Dickinson GH, Orihuela B, Kulp JL, III, Rittschof D, Wahl KJ. Characterization of the adhesive plaque of the barnacle Balanus amphitrite: amyloid-like nanofibrils are a major component. Langmuir. 2010;26:6549–56. doi: 10.1021/la9041309. [DOI] [PubMed] [Google Scholar]
- 87.Smith JF, Knowles TP, Dobson CM, Macphee CE, Welland ME. Characterization of the nanoscale properties of individual amyloid fibrils. Proc Natl Acad Sci U S A. 2006;103:15806–11. doi: 10.1073/pnas.0604035103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Inoue S. Basement membrane and beta amyloid fibrillogenesis in Alzheimer’s disease. Int Rev Cytol. 2001;210:121–61. doi: 10.1016/s0074-7696(01)10005-7. [DOI] [PubMed] [Google Scholar]
- 89.Fowler DM, Koulov AV, Balch WE, Kelly JW. Functional amyloid--from bacteria to humans. Trends Biochem Sci. 2007;32:217–24. doi: 10.1016/j.tibs.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 90.Dickinson GH, Vega IE, Wahl KJ, Orihuela B, Beyley V, Rodriguez EN, et al. Barnacle cement: a polymerization model based on evolutionary concepts. J Exp Biol. 2009;212:3499–510. doi: 10.1242/jeb.029884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Soscia SJ, Kirby JE, Washicosky KJ, Tucker SM, Ingelsson M, Hyman B, et al. The Alzheimer’s disease-associated amyloid beta-protein is an antimicrobial peptide. PLoS One. 2010;5:e9505. doi: 10.1371/journal.pone.0009505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Atamna H, Boyle K. Amyloid-beta peptide binds with heme to form a peroxidase: relationship to the cytopathologies of Alzheimer’s disease. Proc Natl Acad Sci U S A. 2006;103:3381–86. doi: 10.1073/pnas.0600134103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Atamna H. Heme binding to Amyloid-beta peptide: mechanistic role in Alzheimer’s disease. J Alzheimers Dis. 2006;10:255–66. doi: 10.3233/jad-2006-102-310. [DOI] [PubMed] [Google Scholar]
- 94.Janjua N, Mayer SA. Cerebral vasospasm after subarachnoid hemorrhage. Curr Opin Crit Care. 2003;9:113–19. doi: 10.1097/00075198-200304000-00006. [DOI] [PubMed] [Google Scholar]
- 95.Hua Y, Keep RF, Hoff JT, Xi G. Brain injury after intracerebral hemorrhage: the role of thrombin and iron. Stroke. 2007;38:759–62. doi: 10.1161/01.STR.0000247868.97078.10. [DOI] [PubMed] [Google Scholar]
- 96.Atri A, Locascio JJ, Lin JM, Yap L, Dickerson BC, Grodstein F, et al. Prevalence and effects of lobar microhemorrhages in early-stage dementia. Neurodegener Dis. 2005;2:305–12. doi: 10.1159/000092317. [DOI] [PubMed] [Google Scholar]
- 97.Jellinger KA. Alzheimer disease and cerebrovascular pathology: an update. J Neural Transm. 2002;109:813–36. doi: 10.1007/s007020200068. [DOI] [PubMed] [Google Scholar]
- 98.Turgeon VL, Houenou LJ. The role of thrombin-like (serine) proteases in the development, plasticity and pathology of the nervous system. Brain Res Brain Res Rev. 1997;25:85–95. doi: 10.1016/s0165-0173(97)00015-5. [DOI] [PubMed] [Google Scholar]
- 99.Suo Z, Citron BA, Festoff BW. Thrombin: a potential proinflammatory mediator in neurotrauma and neurodegenerative disorders. Curr Drug Targets Inflamm Allergy. 2004;3:105–14. doi: 10.2174/1568010043483953. [DOI] [PubMed] [Google Scholar]
- 100.Yin X, Wright J, Wall T, Grammas P. Brain endothelial cells synthesize neurotoxic thrombin in Alzheimer’s disease. Am J Pathol. 2010;176:1600–1606. doi: 10.2353/ajpath.2010.090406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang Y, Hill JW, Rosenberg GA. Multiple roles of metalloproteinases in neurological disorders. Prog Mol Biol Transl Sci. 2011;99:241–63. doi: 10.1016/B978-0-12-385504-6.00006-3. [DOI] [PubMed] [Google Scholar]
- 102.Robinson SR, Dang TN, Dringen R, Bishop GM. Hemin toxicity: a preventable source of brain damage following hemorrhagic stroke. Redox Rep. 2009;14:228–35. doi: 10.1179/135100009X12525712409931. [DOI] [PubMed] [Google Scholar]
- 103.Wu CW, Liao PC, Yu L, Wang ST, Chen ST, Wu CM, et al. Hemoglobin promotes Abeta oligomer formation and localizes in neurons and amyloid deposits. Neurobiol Dis. 2004;17:367–77. doi: 10.1016/j.nbd.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 104.Rhodin JA, Thomas T. A vascular connection to Alzheimer’s disease. Microcirculation. 2001;8:207–20. doi: 10.1038/sj/mn/7800086. [DOI] [PubMed] [Google Scholar]
- 105.Marchesi VT. Alzheimer’s dementia begins as a disease of small blood vessels, damaged by oxidative-induced inflammation and dysregulated amyloid metabolism: implications for early detection and therapy. FASEB J. 2011;25:5–13. doi: 10.1096/fj.11-0102ufm. [DOI] [PubMed] [Google Scholar]
- 106.Roher AE, Kuo YM. Isolation of amyloid deposits from brain. Methods Enzymol. 1999;309:58–67. doi: 10.1016/s0076-6879(99)09006-0. [DOI] [PubMed] [Google Scholar]
- 107.Lowenson JD, Clarke S, Roher AE. Chemical modifications of deposited amyloid-beta peptides. Methods Enzymol. 1999;309:89–105. doi: 10.1016/s0076-6879(99)09009-6. [DOI] [PubMed] [Google Scholar]
- 108.Roher AE, Lowenson JD, Clarke S, Wolkow C, Wang R, Cotter RJ, et al. Structural alterations in the peptide backbone of beta-amyloid core protein may account for its deposition and stability in Alzheimer’s disease. J Biol Chem. 1993;268:3072–83. [PubMed] [Google Scholar]
- 109.Roher AE, Kuo YM, Esh C, Knebel C, Weiss N, Kalback W, et al. Cortical and leptomeningeal cerebrovascular amyloid and white matter pathology in Alzheimer’s disease. Mol Med. 2003;9:112–22. [PMC free article] [PubMed] [Google Scholar]
- 110.Weller RO, Massey A, Newman TA, Hutchings M, Kuo YM, Roher AE. Cerebral amyloid angiopathy: amyloid beta accumulates in putative interstitial fluid drainage pathways in Alzheimer’s disease. Am J Pathol. 1998;153:725–33. doi: 10.1016/s0002-9440(10)65616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Weller RO, Massey A, Kuo YM, Roher AE. Cerebral amyloid angiopathy: accumulation of A beta in interstitial fluid drainage pathways in Alzheimer’s disease. Ann N Y Acad Sci. 2000;903:110–117. doi: 10.1111/j.1749-6632.2000.tb06356.x. [DOI] [PubMed] [Google Scholar]
- 112.Terry RD. The pathogenesis of Alzheimer disease: an alternative to the amyloid hypothesis. J Neuropathol Exp Neurol. 1996;55:1023–25. [PubMed] [Google Scholar]
- 113.Pimplikar SW, Nixon RA, Robakis NK, Shen J, Tsai LH. Amyloid-independent mechanisms in Alzheimer’s disease pathogenesis. J Neurosci. 2010;30:14946–54. doi: 10.1523/JNEUROSCI.4305-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Patton RL, Kalback WM, Esh CL, Kokjohn TA, Van Vickle GD, Luehrs DC, et al. Amyloid-beta peptide remnants in AN-1792-immunized Alzheimer’s disease patients: a biochemical analysis. Am J Pathol. 2006;169:1048–63. doi: 10.2353/ajpath.2006.060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Boche D, Zotova E, Weller RO, Love S, Neal JW, Pickering RM, et al. Consequence of Abeta immunization on the vasculature of human Alzheimer’s disease brain. Brain. 2008;131:3299–310. doi: 10.1093/brain/awn261. [DOI] [PubMed] [Google Scholar]
- 116.Uro-Coste E, Russano de PG, Guilbeau-Frugier C, Sastre N, Ousset PJ, da Silva NA, et al. Cerebral amyloid angiopathy and microhemorrhages after amyloid beta vaccination: case report and brief review. Clin Neuropathol. 2010;29:209–16. doi: 10.5414/npp29209. [DOI] [PubMed] [Google Scholar]
- 117.Ferrer I, Boada RM, Sanchez Guerra ML, Rey MJ, Costa-Jussa F. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer’s disease. Brain Pathol. 2004;14:11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wilcock DM, Colton CA. Immunotherapy, vascular pathology, and microhemorrhages in transgenic mice. CNS Neurol Disord Drug Targets. 2009;8:50–64. doi: 10.2174/187152709787601858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kerchner GA, Boxer AL. Bapineuzumab. Expert Opin Biol Ther. 2010;10:1121–30. doi: 10.1517/14712598.2010.493872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jack CR, Jr, Wiste HJ, Vemuri P, Weigand SD, Senjem ML, Zeng G, et al. Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer’s disease. Brain. 2010;133:3336–48. doi: 10.1093/brain/awq277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Grammas P, Ottman T, Reimann-Philipp U, Larabee J, Weigel PH. Injured brain endothelial cells release neurotoxic thrombin. J Alzheimers Dis. 2004;6:275–81. doi: 10.3233/jad-2004-6308. [DOI] [PubMed] [Google Scholar]
- 122.Cortes-Canteli M, Paul J, Norris EH, Bronstein R, Ahn HJ, Zamolodchikov D, et al. Fibrinogen and beta-amyloid association alters thrombosis and fibrinolysis: a possible contributing factor to Alzheimer’s disease. Neuron. 2010;66:695–709. doi: 10.1016/j.neuron.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Merkle DL, Cheng CH, Castellino FJ, Chibber BA. Modulation of fibrin assembly and polymerization by the beta-amyloid of Alzheimer’s disease. Blood Coagul Fibrinolysis. 1996;7:650–658. doi: 10.1097/00001721-199609000-00011. [DOI] [PubMed] [Google Scholar]
- 124.Roher AE, Esh CL, Kokjohn TA, Castano EM, Van Vickle GD, Kalback WM, et al. Amyloid beta peptides in human plasma and tissues and their significance for Alzheimer’s disease. Alzheimers Dement. 2009;5:18–29. doi: 10.1016/j.jalz.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M, et al. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73:2061–70. doi: 10.1212/WNL.0b013e3181c67808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Spencer FA, Emery C, Lessard D, Anderson F, Emani S, Aragam J, et al. The Worcester Venous Thromboembolism study: a population-based study of the clinical epidemiology of venous thromboembolism. J Gen Intern Med. 2006;21:722–27. doi: 10.1111/j.1525-1497.2006.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Oger E. Incidence of venous thromboembolism: a community-based study in Western France. EPI-GETBP Study Group. Groupe d’Etude de la Thrombose de Bretagne Occidentale. Thromb Haemost. 2000;83:657–60. [PubMed] [Google Scholar]
- 128.Luo F, Rustay NR, Seifert T, Roesner B, Hradil V, Hillen H, et al. Magnetic resonance imaging detection and time course of cerebral microhemorrhages during passive immunotherapy in living amyloid precursor protein transgenic mice. J Pharmacol Exp Ther. 2010;335:580–588. doi: 10.1124/jpet.110.172932. [DOI] [PubMed] [Google Scholar]
- 129.Van Nostrand WE, Schmaier AH, Farrow JS, Cunningham DD. Protease nexin-II (amyloid beta-protein precursor): a platelet alpha-granule protein. Science. 1990;248:745–48. doi: 10.1126/science.2110384. [DOI] [PubMed] [Google Scholar]