Abstract
Background
Screening overweight and obese children for non-alcoholic fatty liver disease (NAFLD) is recommended by paediatric and endocrinology societies. However, gastroenterology societies have called for more data before making a formal recommendation.
Aim
To determine whether the detection of suspected NAFLD in overweight and obese children through screening in primary care and referral to paediatric gastroenterology resulted in a correct diagnosis of NAFLD.
Methods
Information generated in the clinical evaluation of 347 children identified with suspected NAFLD through screening in primary care and referral to paediatric gastroenterology was captured prospectively. Diagnostic outcomes were reported. The diagnostic performance of two times the upper limit of normal (ULN) for alanine aminotransferase (ALT) was assessed.
Results
Non-alcoholic fatty liver disease was diagnosed in 55% of children identified by screening and referral. Liver disease other than NAFLD was present in 18% of those referred. Autoimmune hepatitis was the most common alternative diagnosis. Children with NAFLD had significantly (P < 0.05) higher screening ALT (98 ± 95) than children with liver disease other than NAFLD (86 ± 74). Advanced fibrosis was present in 11% of children. For the diagnosis of NAFLD, screening ALT two times the clinical ULN had a sensitivity of 57% and a specificity of 71%.
Conclusions
Screening of overweight and obese children in primary care for NAFLD with referral to paediatric gastroenterology has the potential to identify clinically relevant liver pathology. Consensus is needed on how to value the risk and rewards of screening and referral, to identify children with liver disease in the most appropriate manner.
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease in children.1 The diagnosis of NAFLD requires that 5% or more hepatocytes have macrovesicular steatosis, and that other liver diseases and/or clinical conditions, which may cause steatosis, are excluded.2 Approximately 25% of children with NAFLD have a progressive sub-phenotype known as non-alcoholic steatohepatitis (NASH).3 Some children with NASH will develop cirrhosis and end-stage liver disease.4–6 Thus, NAFLD is not a singular diagnosis, but a clinical–pathological diagnosis that encompasses a broad spectrum of liver disease ranging from isolated steatosis to steatohepatitis, fibrosis and cirrhosis.7
For the years 1993–2003, the prevalence of NAFLD in children aged 2–19 years was estimated to be 9.6%.1 Studies have consistently shown that obesity is one of the most important risk factors for paediatric NAFLD.1,8–11 An overweight or obese child with elevated alanine aminotransferase (ALT) is typically considered to have suspected NAFLD.12 A recent report from the National Health and Nutrition Examination Survey demonstrated that the prevalence of suspected NAFLD in children age 12–19 in the United States more than doubled from 1988–1994 to 2007–2010.13
In 2005, a report from UC San Francisco and Stanford University noted that general paediatricians were ‘underscreening’ overweight children for NAFLD.14 Beginning in 2007, major medical societies published guideline statements regarding screening overweight and obese children for NAFLD.15–17 The positions of these societies are summarised in Table1. Recommendations for screening have been made by paediatricians, endocrinologists and paediatric gastroenterologists. The paediatric guidelines state that overweight or obese children ≥10 years should be screened for NAFLD using serum ALT and aspartate aminotransferase (AST).18 There is, however, some controversy surrounding screening children for NAFLD. Using published literature through June 2011, the American Gastroenterology Association, American Association for the Study of Liver Diseases and the American College of Gastroenterology developed a Practice Guideline on the diagnosis and management of NAFLD, which was jointly published in Gastroenterology,19 Hepatology20 and American Journal of Gastroenterology21 in June 2012. The Practice Guideline states, ‘Due to a paucity of evidence, a formal recommendation cannot be made with regards to screening for NAFLD in overweight and obese children despite a recent expert committee recommendation for biannual screening for liver disease with liver enzyme measurements in this population’.
Table 1.
Society guidelines regarding screening overweight and obese children for NAFLD
| Society | Recommend screening children for NAFLD | |||
|---|---|---|---|---|
| Yes | No | Uncertain | Not stated | |
| American Academy of Family Physicians | X | |||
| American Academy of Pediatrics | X | |||
| American Association for the Study of Liver Disease | X | |||
| American College of Gastroenterology | X | |||
| American Gastroenterological Association | X | |||
| Endocrine Society | X | |||
| European Society for Pediatric Gastroenterology, Hepatology, and Nutrition | X | |||
| National Association of Pediatric Nurse Practitioners | X | |||
| North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition | X | |||
In the absence of uniform guidance, physicians must make their own decision whether or not to screen overweight children for NAFLD. Many primary care providers are screening overweight and obese children for NAFLD and many of these children identified as having suspected NAFLD are referred to paediatric gastroenterology for evaluation. The diagnostic outcomes for such children with suspected NAFLD who were referred to paediatric gastroenterology have not been reported. Therefore, we sought to address critical gaps in the knowledge base with the following study aims:
(i) To describe the population of children who were identified with suspected NAFLD through screening in primary care and referred to paediatric gastroenterology.
(ii) To determine whether the detection of suspected NAFLD in overweight and obese children through screening in primary care and referral to paediatric gastroenterology resulted in a correct diagnosis of NAFLD.
(iii) To determine the frequency of NASH amongst overweight and obese children who were identified with suspected NAFLD through screening in primary care and referred to paediatric gastroenterology.
(iv) To determine the frequency of advanced fibrosis amongst overweight and obese children who were identified with suspected NAFLD through screening in primary care and referred to paediatric gastroenterology.
(v) To determine the diagnostic performance of ALT two times the upper limit of normal (ULN) for the above outcomes amongst overweight and obese children who were identified with suspected NAFLD through screening in primary care and referred to paediatric gastroenterology.
Methods
Study population
Screening for NAFLD and referral to paediatric gastroenterology were performed clinically in primary care prior to participation in the study. Eligibility for this study was designed to mirror the paediatric screening guidelines for NAFLD.18 Therefore, children had to be at least 10 years old and either overweight or obese. In the clinical notes from the primary care office, there had to be documentation of screening for NAFLD with ALT and referral to paediatric gastroenterology for evaluation of ‘elevated ALT’ or ‘suspected NAFLD’ or ‘NAFLD’. There was no study inclusion threshold set for ALT; rather, the determination that the screening ALT was abnormal was made by the primary care provider. The parent(s) of all subjects provided written informed consent. Written assent was obtained for all children. The protocol was approved by the institutional review boards of the University of California, San Diego and Rady Children's Hospital San Diego.
Standard of care clinical evaluation of suspected NAFLD
The clinical evaluation of the children was at the discretion of the attending paediatric gastroenterologist and not dictated by research protocol. Information generated in the clinical evaluation of suspected NAFLD was captured prospectively. To provide clinical context for the study, details of this diagnostic process are provided.
Paediatric gastroenterology clinic
All children underwent a comprehensive history and physical as part of the clinical consultation for suspected NAFLD. The history included investigation of potential hepatotoxic medication intake as well as age-appropriate interviewing for relevant lifestyle factors including unprotected sexual activity, alcohol, tobacco and recreational drug use. Height and weight were measured. Physical examination also included assessment for signs of chronic liver disease.22–24 Initial laboratory studies performed in all children at least 1 month after screening labs included hepatic panel (ALT, AST, alkaline phosphatase, total protein, albumin, total bilirubin, direct bilirubin) and gamma glutamyl transferase (GGT) to assess the chronicity and nature of aminotransferase elevation; complete blood count to assess for anaemia and evidence of splenic sequestration related to portal hypertension; and coagulation studies to assess hepatic synthetic function. If this confirmatory testing showed continued evidence for liver disease, additional laboratory studies were performed as ordered by the paediatric gastroenterologist based on the clinical context. These labs included evaluation of both hepatic aetiologies [hepatitis A IgM, hepatitis B surface antigen, hepatitis B surface antibody, hepatitis C antibody, HIV ELISA, alpha-1 anti-trypsin phenotype, anti-nuclear antibody (ANA), anti-smooth muscle antibody (ASMA), anti-liver kidney microsomal antibody, quantitative IgG, ceruloplasmin, 24-h urinary copper measurement] and extra-hepatic aetiologies (tissue transglutaminase IgA, quantitative IgA, serum amino acids, urine organic acids, serum acylcarnitine, profile, creatine kinase, erythrocyte sedimentation rate, C-reactive protein, thyroid stimulating hormone and free thyroxine). In children who had no symptoms or signs of liver disease by history or physical examination, and all laboratory results were normal on confirmatory testing, further evaluation was not pursued, given low likelihood of liver disease. When there was evidence for chronic liver disease based on history, physical and/or laboratory testing, a percutaneous liver biopsy was offered for diagnosis.25
Liver biopsy
Children who underwent clinical liver biopsy did so according to the standard clinical protocol in use at our institution. Anaesthesia was provided by an attending paediatric anaesthesiologist. Children underwent a mask induction using a combination of gas ventilation with oxygen, nitrous oxide and sevoflurane. An intravenous line was placed and supplemental propofol and fentanyl was given according to the anaesthesiologist's discretion. Hemodynamic monitoring was placed. Most patients were mask ventilated or had laryngeal mask airways placed. Ventilation was spontaneous. A time out was performed to confirm the correct patient and procedure. Children were positioned supine, with the right hand raised. Limited ultrasonography of the liver was performed to identify the ideal biopsy path. Liver biopsy was performed by an experienced paediatric gastroenterologist using a 15-gauge Jamshidi needle. A portion of tissue was placed in saline and brought afresh to pathology and a portion was placed in formalin for standard processing. After the procedure was completed, patients were taken to the post-anaesthesia care unit (PACU), monitored and observed for 4 h prior to discharge.
Pathology
Pathology procedures were performed according to standard clinical protocol within our institution. Formalin-fixed paraffin-embedded liver sections were stained with haematoxylin and eosin, periodic acid Schiff with and without diastase, Masson trichrome, reticulin and iron histochemical stains. Fresh frozen sections were stained with oil-red-O. Slides were evaluated systematically (adequacy, overall architecture, portal tracts, and parenchyma) by an experienced, board-certified, paediatric pathologist. Additional stains were performed and reviewed as needed based on the clinical context. Fibrosis was staged using standard methods relevant to the specific pathologic findings (e.g. Kleiner for NAFLD, METAVIR for viral hepatitis, etc.).26,27 The pathological diagnosis was recorded.
Diagnosis
The final diagnosis was made by the paediatric gastroenterologist incorporating all available information from clinical interview, medical record, physical examination, laboratory testing and review of histopathology. A diagnosis of NAFLD was based on exclusion of other causes of steatosis by clinical history, laboratory studies and histology in addition to histologic demonstration of ≥5% of hepatocytes containing macrovesicular fat. Following the prevailing standard, for those biopsies indicative of NAFLD, the diagnosis of steatohepatitis was based on the pathologists' interpretation of the global histological features including steatosis, lobular and portal inflammation and ballooning degeneration of hepatocytes.26 The diagnoses of other liver diseases were made based on relevant society guidelines and standard gastroenterology, hepatology and pathology reference textbooks.24,28,29 For example, the diagnosis of autoimmune hepatitis was made following the recommendations of the AASLD Practice Guideline on the Diagnosis and Management of Autoimmune Hepatitis.30
Data collection
Information generated in the clinical evaluation of suspected NAFLD was captured prospectively. Age and sex were recorded. Because race and ethnicity influence the risk for NAFLD, each child's race and ethnicity were self-identified by the parent(s). Height and weight were recorded. From the primary care provider's office records, the screening ALT and AST values were recorded. In addition, from the paediatric gastroenterology clinical record, we prospectively recorded the values for laboratory assays performed for the evaluation of liver disease. Adverse events were recorded as problems in the operating room, patient complaints in the PACU, return to the hospital, calls to gastroenterology and by report at out-patient follow-up visit.
Data analysis
Calculated variables
Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared. BMI percentiles were determined from the Centers for Disease Control and Prevention 2000 growth curves. BMI Z scores (s.d.s from the national reference mean for a given age and gender of children's BMI values) also were determined.
Definitions
Subjects were classified as overweight (BMI 85–94th percentiles) or obese (BMI ≥95th percentile). The most common value used at children's hospitals in the United States for the ULN for ALT is 40 U/L.31 Therefore, two times the ULN was defined as 80 U/L. This value was used to assess the diagnostic performance of the paediatric guideline recommendation to use two times the ULN for ALT as the threshold for further evaluation. As a comparison, we also evaluated recently proposed biology-based thresholds for the ULN in children derived from the SAFETY study.31 These are gender-specific with ULN of 25 U/L in boys and 22 U/L in girls. Therefore, two times the biology-based ULN was defined as 50 U/L for boys and 44 U/L for girls. Advanced fibrosis was defined as bridging fibrosis or cirrhosis.
Statistics
Data were expressed as mean ± standard deviation (if not normally distributed, then geometric means were reported) or frequency and percentage. Continuous variables were analysed with Student's t-test; the Mann–Whitney U test was used for nonparametric measures. The Pearson χ2 test was used to test for differences in proportions. All hypothesis tests were two-tailed. Significance was defined a priori at α value of 0.05. Analyses were performed with Statistica 10 (StatSoft, Inc., Tulsa, OK, USA).
We performed a post-hoc analysis of published indices for NAFLD and advanced fibrosis using readily available clinical data points.32 For the detection of NAFLD, we tested the Fatty Liver Index (FLI).33 The FLI was calculated as (e0.953*loge (triglycerides) + 0.139*BMI + 0.718*loge (GGT) + 0.053*waist circumference−15.745)/(1 + e0.953*loge (triglycerides) + 0.139*BMI + 0.718*loge (GGT) + 0.053*waist circumference−15.745) × 100. The index produces a score from 1 to 100. Scores of <30 are considered negative for NAFLD, scores between 31 and 59 are considered indeterminate and scores >60 are considered positive for NAFLD. For the detection of advanced fibrosis, we tested the FIB-4 index.34,35 This was calculated as (Age × AST)/(Platelets × (sqr (ALT)). Values <1.3 are considered negative, values between 1.3 and 2.67 are considered indeterminate and values ≥2.67 are considered positive for advanced fibrosis. Finally, we tested the Paediatric NAFLD Fibrosis Index (PNFI).36 A linear predictor for PNFI was calculated as linear predictor (lp) = −6.539 × loge [age (years)] + 0.207 × waist (cm) + 1.957 × loge [triglycerides (mg/dl)] − 10.074. This linear predictor is transformed into a PNFI score: (1/1 + e-lp) × 10. PNFI scores ≥9 are considered positive for fibrosis. We tested for differences in the PNFI score between those children with and without advanced fibrosis.
Results
Aim 1: demographics and clinical features
Children were screened and referred by 58 primary care providers including 42 paediatricians, 9 family physicians and 7 paediatric nurse practitioners. A study flow chart is shown in Figure1. We enrolled 347 children who met eligibility criteria (were ≥10 years old, overweight or obese, identified by primary care screening as having elevated ALT and referred to paediatric gastroenterology for suspected NAFLD). As shown in Table2, the majority of children were boys (223/347, 64%). The mean age was 13.5 years. The majority of children were obese (93%), with the remaining children being overweight (7%). The mean screening ALT of children with suspected NAFLD was 99 U/L. The screening ALT was ≥40 U/L in 90% (313/347) of children referred. The screening ALT was higher than the gender-specific biological ULN for all children referred for suspected NAFLD.
Figure 1.
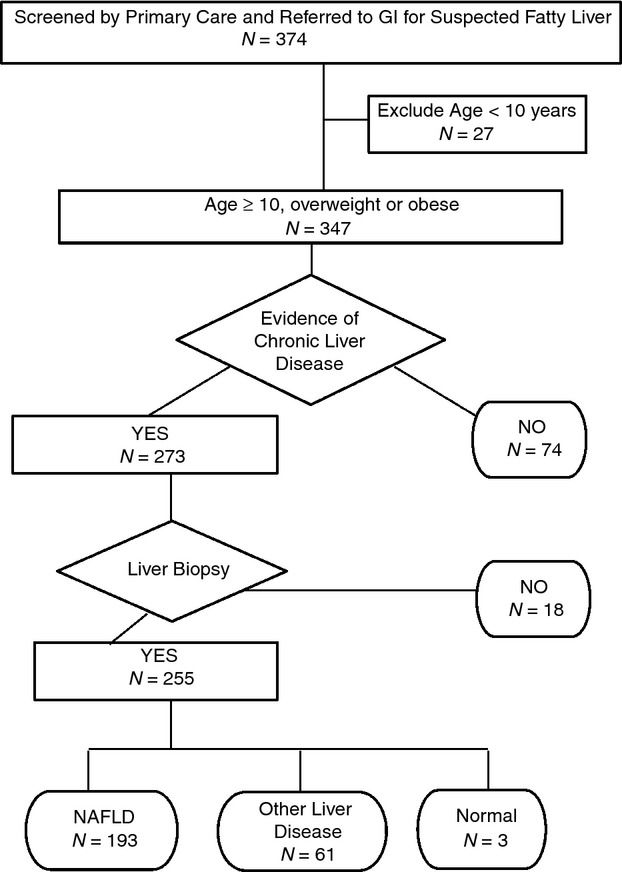
Flow chart shows study the application of inclusion and exclusion criteria along with progression to final diagnosis. In the terminal nodes for diagnosis, the cumulative number is greater than the number biopsied because two children had dual diagnosis.
Table 2.
Characteristics of study population by diagnosis
| Variables | Children identified by screening, N = 347 | Children with liver diagnosis | |
|---|---|---|---|
| NAFLD, N = 193 | Other liver disease, N = 61 | ||
| Age, mean (s.d.)** | 13.5 (2.2) | 13.6 (2.2) | 14.5 (2.4) |
| Sex, N (%)** | |||
| Boys | 223 (64) | 140 (72) | 29 (48) |
| Girls | 124 (36) | 53 (28) | 32 (52) |
| Race and ethnicity, N (%) | |||
| Asian, non-Hispanic | 16 (5) | 8 (4) | 6 (10) |
| Hispanic | 252 (72) | 147 (76) | 38 (62) |
| White, non-Hispanic | 45 (13) | 21 (10) | 12 (20) |
| Other, non-Hispanic | 34 (10) | 17 (8) | 5 (8) |
| Weight, mean (s.d.) (kg) | 80.1 (20.7) | 81.5 (22.6) | 80.8 (17.3) |
| Height, mean (s.d.) (cm) | 157 (15.6) | 157(15.0) | 159 (12.6) |
| Body mass index (kg/m2) | |||
| Mean (s.d.) | 31.5 (6.0) | 31.5 (4.8) | 31.4 (4.4) |
| Percentile, mean (s.d.) | 98.1 (1.6) | 98.4 (1.5) | 97.4 (1.9) |
| Z-score, mean (s.d.) | 2.2 (0.36) | 2.2 (0.33) | 2.0 (0.34) |
| ALT, mean (s.d.) (U/L) | |||
| Screening* | 99 (89) | 98 (95) | 86 (74) |
| Confirmation** | 80 (68) | 89 (75) | 75 (57) |
| AST, mean (s.d.) (U/L) | |||
| Screening | 67 (50) | 63 (55) | 68 (57) |
| Confirmation | 57 (39) | 60 (43) | 56 (30) |
Statistical tests are for NAFLD vs. other liver disease.
P < 0.05
P < 0.001.
Following clinical evaluation by a paediatric gastroenterologist, 21% (74/347) of children identified by screening as having suspected NAFLD were determined not to have liver disease based on the absence of symptoms or signs of liver disease by history or physical examination, and normal laboratory results on confirmatory testing. The remaining 273 children were offered clinical liver biopsy. Liver biopsy was not performed in 6% of these children due to either parental refusal (n = 4) or insurance denial (n = 14). Notably, children whose parents refused liver biopsy were significantly (P < 0.05) younger (11 years-old) than the group overall (13 years-old). Moreover, children who had liver biopsy denied by insurance were significantly (P < 0.05) more likely to be Hispanic (92%) than the group overall (70%). The remaining 255 children successfully underwent liver biopsy. No child experienced bleeding or required post-procedure hospitalisation. While in the recovery room, 3% of patients reported symptoms that were successfully resolved prior to discharge (five complained of pain and two of nausea). After discharge to home, one patient's parent called about their child's pain which was successfully addressed over the telephone. All children were seen for clinical follow-up and review of biopsy results at a mean follow-up interval of 18 days (range 4–97). At follow-up, an additional nine children (3%) reported that they had experienced some degree of minor discomfort after discharge post-liver biopsy. No child was brought to urgent care nor required readmission.
Aim 2: diagnosis of NAFLD
The combination of histology, clinical and laboratory features yielded a diagnosis of NAFLD in 55% of those children identified by screening and referral (193/347) and in 75% of those who underwent liver biopsy (193/255). Liver histology was normal in three children (1%). Liver disease other than NAFLD was present in 18% of those referred (61/347) and 24% of those biopsied (61/255). Notably, there were two children who had both NAFLD and autoimmune hepatitis. As shown in Figure2, boys had significantly (P < 0.01) higher ALT than girls. Amongst subgroups, this was true both for children without liver disease and for children with NAFLD. However, ALT did not differ between boys and girls with liver disease other than NAFLD.
Figure 2.
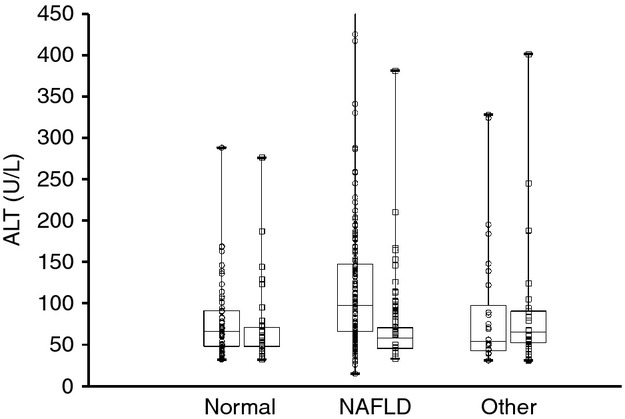
Box and whiskers plot for screening ALT separated by final diagnosis: no liver disease, NAFLD, or liver disease other than NAFLD. Within each diagnostic category, data are shown separately for boys (○) and girls (□).The horizontal lines inside the boxes represent the median, the box edges show the lower and upper quartiles and the whiskers show the minimum and maximum values. The Y axis was truncated at 450 U/L. Only the group of boys with NAFLD included outliers with screening ALT above 450 U/L.
For children with liver disease other than NAFLD, autoimmune hepatitis was the most common diagnosis (n = 11). Other forms of hepatitis included drug-induced (n = 6), eosinophilic (n = 4), granulomatous (n = 1), idiopathic (n = 8), viral (hepatitis B, hepatitis C, each n = 2), secondary to alcohol abuse (n = 2), associated with herbal supplement (n = 1) and associated with asymptomatic colitis (n = 1). Drug-induced hepatitis was associated with medications including aripiprazole, cetirizin, isotretinoin, minocycline and valproic acid. Microvesicular steatosis was present in 20 children and was idiopathic in 10. Specific causes identified for microvesicular steatosis included coeliac disease (n = 4), drug-induced (n = 5) and muscle disease (n = 1). Additional diagnoses included alpha-1-anti-trypsin deficiency (n = 1), sclerosing cholangitis (n = 1) and congestive hepatopathy (n = 1). As shown in Table1, children with liver disease other than NAFLD were significantly older, more likely to be female and had lower ALT at both screening and confirmation. Positive ANA was significantly more frequent in children with liver disease other than NAFLD (36%) than children with NAFLD (19%).
Aim 3: diagnosis of NASH
Non-alcoholic steatohepatitis was diagnosed in 105 children who represent 30% of children who were identified by screening and referred to paediatric gastroenterology, 41% of children who underwent liver biopsy, and 54% of those children with NAFLD. Children with NASH had significantly (P < 0.001) higher mean ALT at screening (100 ± 107 vs. 79 ± 79 U/L) and at confirmation (94 ± 89 vs. 71 ± 74 U/L) than children who did not have NASH. However, there was no significant difference in mean values of GGT, ceruloplasmin or frequency of positive ANA or ASMA between children with and without NASH.
Aim 4: diagnosis of advanced fibrosis
Advanced fibrosis was present in 11% (38/347) of all children in the study population. The rate of advanced fibrosis was greater in children with NAFLD (17%, 33/193) than in children with liver disease other than NAFLD (8%, 5/62). Advanced fibrosis was seen in autoimmune hepatitis (n = 3), sclerosing cholangitis (n = 1) and idiopathic hepatitis (n = 1). As a group, children with advanced fibrosis did not significantly differ by age, sex or severity of obesity. Children with advanced fibrosis did have significantly (P < 0.01) higher ALT (120 ± 140 U/L) and AST (79 ± 87 U/L) than children without advanced fibrosis (ALT 82 ± 78 U/L, AST 57 ± 45 U/L). Children with advanced fibrosis also had significantly (P < 0.001) higher GGT (58 ± 90) than children without advanced fibrosis (35 ± 41). Ceruloplasmin was also significantly (P < 0.05) higher in children with advanced fibrosis (38 ± 10) than children without advanced fibrosis (33 ± 8).
Aim 5: evaluation of ALT in the diagnostic process
Table3 shows the study population separated into those with screening ALT above and below two times the clinical ULN. As expected children with screening ALT ≥80 had significantly higher ALT and AST than children with screening ALT <80, but were otherwise not significantly different with regard to age, sex, race, ethnicity, height, weight or BMI. For children with screening ALT ≥80, NAFLD was diagnosed in 71% and liver disease other than NAFLD was present in 14%. For children with screening ALT that was elevated but <80, NAFLD was diagnosed in 43% and liver disease other than NAFLD in 22%. NASH was significantly (P < 0.01) more common in children with screening ALT ≥80 (41%, 64/155) than in children with screening ALT <80 (21%, 41/192). Similarly, advanced fibrosis was significantly (P < 0.01) more common in children with screening ALT ≥80 (19%, 29/155) than in children with screening ALT <80 (6%, 9/192). For the diagnosis of NAFLD in overweight and obese children ≥age 10, screening ALT of ≥80 had a sensitivity of 57% and a specificity of 71%. For the diagnosis of NASH, screening ALT ≥80 had a sensitivity of 61% and a specificity of 62%. For advanced fibrosis, screening ALT ≥80 had a sensitivity of 76% and a specificity of 59%.
Table 3.
Comparison of children with ALT above or below 2 times upper limit of normal
| Variables | Screening ALT <80, (N = 192) | Screening ALT ≥80, (N = 155) |
|---|---|---|
| Age, mean (s.d.) | 13.5 (2.2) | 13.6 (2.2) |
| Sex, N (%) | ||
| Boys | 111 (58) | 112 (72) |
| Girls | 81 (42) | 43 (28) |
| Race and ethnicity, N (%) | ||
| Asian, non-Hispanic | 9 (4) | 7 (4) |
| Hispanic | 133 (69) | 119 (76) |
| White, non-Hispanic | 31 (16) | 14 (9) |
| Other, non-Hispanic | 19 (10) | 15 (10) |
| Weight, mean (s.d.), (kg) * | 77.8 (19.9) | 83 (21.4) |
| Height, mean (s.d.) (cm) | 157 (12.2) | 158 (19.1) |
| Body mass index (kg/m2) | ||
| Mean (s.d.) | 31.3 (6.9) | 31.8 (4.5) |
| Z-score, mean (s.d.) | 2.1 (0.3) | 2.2 (0.3) |
| ALT, mean (s.d.) (U/L) | ||
| Screening** | 54 (14) | 156 (95) |
| Confirmation | 59 (43) | 107 (83) |
| AST, mean (s.d.) (U/L) | ||
| Screening | 46 (20) | 93 (63) |
| Confirmation* | 45 (22) | 71 (49) |
| Diagnosis | ||
| NAFLD, N (%) | 83 (43) | 110 (71) |
| Other liver disease, N (%) | 42 (22) | 21 (14) |
P < 0.05
P < 0.001.
As a comparison, we also analysed the diagnostic accuracy of using two times the gender-specific, biology-based, ALT thresholds. For the diagnosis of NAFLD in overweight and obese children ≥age 10, ALT ≥50 for boys and ≥44 for girls had a sensitivity of 88% and a specificity of 26%. For the diagnosis of NASH, two times the gender-specific biology-based thresholds had a sensitivity of 90% and a specificity of 22%. For advanced fibrosis, screening ALT ≥50 for boys and ≥44 for girls had a sensitivity of 92% and a specificity of 19%.
Predictive indices
There were 270 children with FLI <30, of whom 163 (60%) had NAFLD. There were 75 children with indeterminate values (30–59) of whom 29 (39%) had NAFLD. There were only two children who had a score >60; one with NAFLD and one without NAFLD. Because FLI only detected 1 of 193 children with NAFLD, further diagnostic analysis was not performed. The mean FIB-4 score among children with advanced fibrosis was 0.34. None of the 38 children with advanced fibrosis had a positive FIB-4 score. For PNFI, there was no significant (P = 0.92) difference between children with advanced fibrosis (mean = 6) and children without advanced fibrosis (mean = 6).
Discussion
We studied a large clinical sample of overweight and obese children who were identified as having suspected NAFLD by screening in primary care following paediatric guidelines and referred to paediatric gastroenterology. Children were evaluated by a paediatric gastroenterologist and those with evidence of chronic liver disease underwent liver biopsy, which was well-tolerated. NAFLD was the most common diagnosis established. However, many children with suspected NAFLD were shown to have liver disease other than NAFLD. Amongst children with NAFLD, approximately half had steatohepatitis. Furthermore, many overweight and obese children were determined to have previously unrecognised advanced fibrosis.
Society recommendations to screen overweight and obese children for NAFLD were based in part on the asymptomatic nature of chronic liver disease that evades diagnosis without a screening effort. The paediatric guidelines as applied by primary care providers identified many children with liver disease, most commonly NAFLD. In addition, the current data demonstrated that not all overweight and obese children with a positive screening ALT will have liver disease. Thus, one major challenge is the interpretation of ALT values. As shown in the SAFETY study, there is wide institution-to-institution variability in the definition of the normal range for ALT and controversy over whether or not to use multipliers of the ULN.31 This creates confusion for paediatricians, gastroenterologists and endocrinologists as well as for the children themselves and their families. The current data show the strengths and limitations of various thresholds for ALT in children. The paediatric guidelines suggest using two times the ULN as the criterion for referral to paediatric gastroenterology. However, our data suggest that primary care providers vary greatly in their choice of threshold used for referral. Although the use of two times the ULN would improve the specificity for NAFLD, many children with NAFLD would be missed including some with NASH and advanced fibrosis. In addition, contrary to conventional wisdom, children with liver disease other than NAFLD had lower ALT than children with NAFLD. Thus, national standardisation of ALT thresholds is needed, but no single ALT threshold will be sufficient to be considered diagnostic.
Once the possibility of liver disease has been detected by screening, it is important to make an accurate diagnosis. One important lesson from this study is that physicians should not tell children that they have fatty liver based solely on the finding of elevated ALT in the context of obesity. Determining whether a child has NAFLD or another form of liver disease has important therapeutic implications, as many of the possible aetiologies have specific therapies. Although it is true that some diseases, such as hepatitis C, can be detected by serologic testing, many other diseases, such as autoimmune hepatitis, require liver biopsy to distinguish from NAFLD. In fact, the screening test for autoimmune hepatitis, auto-antibodies, has been reported to be positive in approximately 20% of patients with NAFLD.37 Indeed, in this study, a positive ANA did not sufficiently distinguish between those with NAFLD and those with autoimmune hepatitis. Because there are no alternative tests with satisfactory diagnostic accuracy, liver biopsy remains the clinical standard to determine the aetiology and stage of liver disease. Liver biopsy is not without risk; however, the current data show that when performed by experienced personnel, it can be performed with minimal adverse events.
The rationale for detecting NAFLD, and especially NASH, is based in part on the risk for progression to cirrhosis. In a national multi-centre study, advanced fibrosis was reported at the time of diagnostic liver biopsy in nearly one of seven children with NAFLD.38 Our study had similar findings, with 17% of children with NAFLD having advanced fibrosis. The detection of advanced fibrosis is important because these are the children who in the short-term are at risk for portal hypertension and its consequences, and in the long-term may require liver transplant and/or develop hepatocellular carcinoma.39 Beyond the hepatic consequences, obese children with NAFLD are phenotypically distinct from obese children without NAFLD. NAFLD is an independent risk factor for diabetes and cardiovascular disease.40 In addition, children with NAFLD also have substantially lower bone mineralisation than age- and adiposity-matched peers.41 Thus, the early identification of NAFLD has the potential to be clinically important.
The current study is notable for its large sample size of overweight and obese children identified by screening in primary care as having suspected NAFLD based on prevailing national clinical guidelines and referred to paediatric gastroenterology. In addition, data were available for detailed diagnostic outcomes based on history, physical examination, laboratory evaluation, liver biopsy and histology. These data represent children identified by screening and not those tested based on symptoms, thus may not reflect all overweight or obese children with elevated liver chemistry. Moreover, given the influence of race and ethnicity, there are likely to be differences in findings depending upon the demographics of the community being considered.
Conclusion
In conclusion, screening of overweight and obese children in primary care for NAFLD with referral to paediatric gastroenterology has the potential to identify clinically relevant liver pathology. NAFLD was the most common explanation for elevated ALT in children detected by screening, but almost as common was either the absence of liver disease or an alternative form of chronic liver disease. The magnitude of ALT elevation was associated with worse disease in group aggregate, but was not an effective discriminate tool on the individual patient level. Importantly, the screening and referral process followed by liver biopsy was able to identify many obese children with advanced fibrosis that would have otherwise remained undiagnosed. Proper treatment of these children rests upon an accurate and definitive diagnosis. Important next steps will include the assessment of the cost and benefits of screening. These can be evaluated at multiple different decision points including initial screening for liver disease, referral for further evaluation of liver disease and the decision to perform liver biopsy for definitive diagnosis and disease staging. The current data can be used for efforts to develop a consensus on how to value these risk and rewards. In turn, with larger observational data, clinical practice guidelines can be refined to best identify overweight children with liver disease in the most appropriate manner.
Authorship
Guarantor of article: Jeffrey B. Schwimmer, M.D.
Author contributions: Jeffrey B. Schwimmer conceptualised and designed the study, acquired data, interpreted data, drafted the manuscript and approved the final manuscript as submitted. Kimberly P. Newton designed the study, acquired data, critically reviewed and revised the manuscript and approved the final manuscript as submitted. Hannah I. Awai, Lillian J. Choi, Mary Abigail Garcia and Linda L. Ellis acquired data, critically reviewed and revised the manuscript and approved the final manuscript as submitted. Karen Vanderwall acquired data, drafted the manuscript and approved the final manuscript as submitted. John Fontanesi contributed to study design, performed statistical analysis, interpreted data, drafted the manuscript and approved the final manuscript as submitted. All authors approved the final version of the article, including the authorship list.
Acknowledgments
Declaration of personal interests: Dr Schwimmer was supported by NIH grants DK61734, DK088925, DK088831 and DK090350. Dr Newton was supported by NIH grants DK61734, DK088831 and DK090350. Dr Awai was supported by NIH grants DK088925-02S1 and DK088831. Dr Fontanesi was supported by NSF grant 414916 and NIH DK088831.
Declaration of funding interests: This study was supported in part by UL1RR031980 from the NCRR for the Clinical and Translational Research Institute at UCSD, DK088925-02S1 and NSF GRANT #414916. The funders did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or the National Science Foundation.
References
- 1.Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–93. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 2.Lindback SM, Gabbert C, Johnson BL, et al. Pediatric nonalcoholic fatty liver disease: a comprehensive review. Adv Pediatr. 2010;57:85–140. doi: 10.1016/j.yapd.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 3.Pardee PE, Lavine JE, Schwimmer JB. Diagnosis and treatment of pediatric nonalcoholic steatohepatitis and the implications for bariatric surgery. Semin Pediatr Surg. 2009;18:144–51. doi: 10.1053/j.sempedsurg.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rubinstein E, Lavine JE, Schwimmer JB. Hepatic, cardiovascular, and endocrine outcomes of the histological subphenotypes of nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:380–5. doi: 10.1055/s-0028-1091982. [DOI] [PubMed] [Google Scholar]
- 5.Schwimmer JB, Behling C, Newbury R, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005;42:641–9. doi: 10.1002/hep.20842. [DOI] [PubMed] [Google Scholar]
- 6.Molleston JP, White F, Teckman J, Fitzgerald JF. Obese children with steatohepatitis can develop cirrhosis in childhood. Am J Gastroenterol. 2002;97:2460–2. doi: 10.1111/j.1572-0241.2002.06003.x. [DOI] [PubMed] [Google Scholar]
- 7.Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202–19. doi: 10.1053/jhep.2003.50193. [DOI] [PubMed] [Google Scholar]
- 8.Guzzaloni G, Grugni G, Minocci A, Moro D, Morabito F. Liver steatosis in juvenile obesity: correlations with lipid profile, hepatic biochemical parameters and glycemic and insulinemic responses to an oral glucose tolerance test. Int J Obes Relat Metab Disord. 2000;24:772–6. doi: 10.1038/sj.ijo.0801224. [DOI] [PubMed] [Google Scholar]
- 9.Strauss RS, Barlow SE, Dietz WH. Prevalence of abnormal serum aminotransferase values in overweight and obese adolescents. J Pediatr. 2000;136:727–33. [PubMed] [Google Scholar]
- 10.Rashid M, Roberts EA. Nonalcoholic steatohepatitis in children. J Pediatr Gastroenterol Nutr. 2000;30:48–53. doi: 10.1097/00005176-200001000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Schwimmer JB, Deutsch R, Rauch JB, Behling C, Newbury R, Lavine JE. Obesity, insulin resistance, and other clinicopathological correlates of pediatric nonalcoholic fatty liver disease. J Pediatr. 2003;143:500–5. doi: 10.1067/S0022-3476(03)00325-1. [DOI] [PubMed] [Google Scholar]
- 12.Schwimmer JB, McGreal N, Deutsch R, Finegold MJ, Lavine JE. Influence of gender, race, and ethnicity on suspected fatty liver in obese adolescents. Pediatrics. 2005;115:e561–5. doi: 10.1542/peds.2004-1832. [DOI] [PubMed] [Google Scholar]
- 13.Welsh JA, Karpen S, Vos MB. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988-1994 to 2007-2010. J Pediatr. 2013;162:496–500 e1. doi: 10.1016/j.jpeds.2012.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riley MR, Bass NM, Rosenthal P, Merriman RB. Underdiagnosis of pediatric obesity and underscreening for fatty liver disease and metabolic syndrome by pediatricians and pediatric subspecialists. J Pediatr. 2005;147:839–42. doi: 10.1016/j.jpeds.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 15.Barlow SE, Expert C. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl. 4):S164–92. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 16.August GP, Caprio S, Fennoy I, et al. Prevention and treatment of pediatric obesity: an endocrine society clinical practice guideline based on expert opinion. J Clin Endocrinol Metabol. 2008;93:4576–99. doi: 10.1210/jc.2007-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vajro P, Lenta S, Socha P, et al. Diagnosis of nonalcoholic fatty liver disease in children and adolescents: position paper of the ESPGHAN Hepatology Committee. J Pediatr Gastroenterol Nutr. 2012;54:700–13. doi: 10.1097/MPG.0b013e318252a13f. [DOI] [PubMed] [Google Scholar]
- 18.Krebs NF, Himes JH, Jacobson D, Nicklas TA, Guilday P, Styne D. Assessment of child and adolescent overweight and obesity. Pediatrics. 2007;120(Suppl. 4):S193–228. doi: 10.1542/peds.2007-2329D. [DOI] [PubMed] [Google Scholar]
- 19.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142:1592–609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–23. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 21.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Am J Gastroenterol. 2012;107:811–26. doi: 10.1038/ajg.2012.128. [DOI] [PubMed] [Google Scholar]
- 22.D'Agata ID, Balistreri WF. Evaluation of liver disease in the pediatric patient. Pediatr Rev. 1999;20:376–90. [PubMed] [Google Scholar]
- 23.Tsouka A, McLin VA. Complications of chronic liver disease. Clin Res Hepatol Gastroenterol. 2012;36:262–7. doi: 10.1016/j.clinre.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Suchy FJ, Sokol RJ, Balistreri WF. Liver Disease in Children. 3rd ed. New York: Cambridge University Press; 2007. [Google Scholar]
- 25.Ovchinsky N, Moreira RK, Lefkowitch JH, Lavine JE. Liver biopsy in modern clinical practice: a pediatric point-of-view. Adv Anat Pathol. 2012;19:250–62. doi: 10.1097/PAP.0b013e31825c6a20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 27.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349:825–32. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 28.Kleinman R, Goulet O, Mieli-Vergani G, Sanderson I, Sherman P, Shneider B. Walker's Pediatric Gastrointestinal Disease. 5th ed. Shelton: People's Medical Publishing House; 2008. [Google Scholar]
- 29.Burt AD, Portmann BC, Ferrell LD. MacSween's Pathology of the Liver. 6th ed. London: MacSween's Pathology of the Liver; 2011. [Google Scholar]
- 30.Manns MP, Czaja AJ, Gorham JD, et al. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:2193–213. doi: 10.1002/hep.23584. [DOI] [PubMed] [Google Scholar]
- 31.Schwimmer JB, Dunn W, Norman GJ, et al. SAFETY study: alanine aminotransferase cutoff values are set too high for reliable detection of pediatric chronic liver disease. Gastroenterology. 2010;138:1357–64. doi: 10.1053/j.gastro.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dowman JK, Tomlinson JW, Newsome PN. Systematic review: the diagnosis and staging of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2011;33:525–40. doi: 10.1111/j.1365-2036.2010.04556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bedogni G, Bellentani S, Miglioli L, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–25. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 35.Shah AG, Lydecker A, Murray K, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104–12. doi: 10.1016/j.cgh.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nobili V, Alisi A, Vania A, Tiribelli C, Pietrobattista A, Bedogni G. The pediatric NAFLD fibrosis index: a predictor of liver fibrosis in children with non-alcoholic fatty liver disease. BMC Med. 2009;7:21. doi: 10.1186/1741-7015-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patton HM, Lavine JE, Van NattaML, et al. Clinical correlates of histopathology in pediatric nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1961–71, e2. doi: 10.1053/j.gastro.2008.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kistler KD, Molleston J, Unalp A, Abrams SH, Behling C, Schwimmer JB. Symptoms and quality of life in obese children and adolescents with nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2009;31:396–406. doi: 10.1111/j.1365-2036.2009.04181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, Benson JT, Enders FB, Angulo P. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut. 2009;58:1538–44. doi: 10.1136/gut.2008.171280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwimmer JB, Pardee PE, Lavine JE, Blumkin AK, Cook S. Cardiovascular risk factors and the metabolic syndrome in pediatric nonalcoholic fatty liver disease. Circulation. 2008;118:277–83. doi: 10.1161/CIRCULATIONAHA.107.739920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pardee PE, Dunn W, Schwimmer JB. Non-alcoholic fatty liver disease is associated with low bone mineral density in obese children. Aliment Pharmacol Ther. 2012;35:248–54. doi: 10.1111/j.1365-2036.2011.04924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


