Abstract
Purpose.
To quantify repeatability and reproducibility of thickness measurements and the effects of realignment and image quality on measurements of retinal thickness from optical coherence tomographic (OCT) imaging in the rat eye.
Methods.
Retinal imaging was performed in 16 Brown Norway rats (n = 16 eyes; x̄ = 372 g). Precision metrics: 95% limits of agreement (LoA), intraclass correlation coefficient (ICC), and the coefficient of variation (CV), were calculated using manual and combined manual + automated realignment procedures for nerve fiber and retinal ganglion cell layer (NFL/GCL), NFL/GCL and inner plexiform layer (NFL/GCL + IPL), and total retina thicknesses (excluding blood vessels). The influence of image quality on NFL thickness measurement was assessed by comparing high- and low-quality image data (real and simulated) from the rat as well as clinical data.
Results.
Mean NFL/GCL thickness was 26 ± 3 μm, NFL/GCL + IPL thickness was 70 ± 3 μm, and total retinal thickness was 192 ± 7 μm. Thickness difference between imaging sessions for NFL/GCL was 1 μm (95% LoA: −4 to 3 μm; ICC = 0.82; CV = 4.7%), for NFL/GCL + IPL was 0 μm (95% LoA: −4 to 4 μm; ICC = 0.88; CV = 1.4%), and total retinal thickness was 1 μm (95% LoA: −3 to 4 μm; ICC = 0.97; CV = 0.7%). Thickness differences were similar between realignment procedures (NFL/GCL: P = 0.43; NFL/GCL + IPL: P = 0.33; total retina: P = 0.62). Although NFL thickness measurements increased slightly in low-quality rat images (4 μm; P = 0.04), this was not true with clinical images (1.4 μm; P = 0.36).
Conclusions.
Precision of retinal layer thickness estimation from OCT imaging is excellent when manual and automated realignment procedures are combined, but may still be influenced by image quality and segmentation methods.
Repeatable retinal thickness measurements from SD-OCT in the rat were acquired by combining manual and automated realignment procedures. Despite these methods, thickness measurements were influenced by image quality and segmentation routines.
Introduction
Since it was first described in 1991, optical coherence tomography (OCT) imaging technology has evolved rapidly and has become one of the most important clinical imaging tools available.1 Current clinical spectral domain (SD)–OCT systems have sufficient speed and resolution that they have been successfully adapted for in vivo imaging in animal models of ocular disease, including a rat model of glaucoma2 and genetic retinal degenerations in mice.3,4 A benefit of adapting commercial clinical OCT imaging systems for use with these animal models is the ability to perform longitudinal studies through high-resolution in vivo ocular imaging without the need or expense to develop custom instrumentation. This capability will further encourage the use of OCT imaging as a tool for ocular disease research. The utility of OCT imaging in animal models of ocular disease will be determined in part by the system's resolution and sensitivity for detecting structural changes.
Precision of retinal thickness measurements from OCT data is influenced by factors that vary both within and between imaging sessions, including factors related to the examiner (alignment, stability, focus), the subject (motion, optical clarity), image hardware (e.g., acquisition speed, resolution), and postacquisition image processing routines (e.g., registration, segmentation). Despite these potential limitations, excellent repeatability of OCT thickness measurements has been reported in humans.5–15 There are a number of additional unique considerations for achieving repeatable scan-path alignment with nonfixating anesthetized animal subjects that can further influence reproducibility studies in the rodent eye.2 Previous studies reporting reproducibility of retinal thickness from OCT imaging limit analysis to only a few of the factors listed above and generally do not account for the influence of image quality on measurement precision. The purpose of this study was to quantify the effects of ocular-instrument realignment and image quality on measurements of retinal thickness from OCT imaging in the rat eye. The precision of retinal thickness measurements between and within imaging sessions was determined using a commercial OCT instrument with a manual and a combined manual + automated realignment procedure designed to account for scan location and ocular rotational orientation.
Methods
All experimental and animal care procedures were approved by the Institutional Animal Care and Use Committee of the University of Houston and were in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Sixteen healthy 8-month-old male Brown Norway rats (n = 16 eyes) were included in this study. Rats were anesthetized with an intraperitoneal injection of ketamine (50 mg/kg; Vedco, St. Joseph, MO) and xylazine (5 mg/kg; Vedco). Pupils were dilated using a topically applied drop of tropicamide (0.5%; Bausch & Lomb Pharmaceuticals, Inc., Tampa, FL).
Image Acquisition
Scanning laser ophthalmoscopic (SLO) fundus images and single horizontal OCT line scans were simultaneously captured using an SLO + OCT system (Spectralis, Acquisition Module Version 5.4.7; Heidelberg Engineering, Heidelberg, Germany). Images were acquired at a scan speed of 40,000 A-scans per second using a superluminescent diode light source, with a central wavelength of 870 nm and nominal axial resolution of 4 μm. A 30° line scan image comprised of 1536 A-scans per B-scan and at least 51 averaged frames. Repeatability was evaluated at several different retinal locations ranging from 0.3 to 1.1 mm away from the optic nerve head center in the superior and inferior retina; however, a single line scan position was evaluated for each eye. Scaled schematic illustrations of the rat retina and line-scan locations are shown in Figure 1. All images had an image quality > 25 dB (mean image quality value: 37 ± 3). The manufacturer considers image quality values > 25 dB to be good; image quality ranges from 0 dB (poor) to 40 dB (excellent).
Figure 1. .
Scaled schematic illustration of the rat retina along with SD-OCT scan line locations. (A) Horizontal lines (labels 1–16) indicate the location where each OCT B-scan image was acquired and repeatability assessed. The optic nerve head center is marked by the asterisk and the radial lines represent blood vessels. Scale bar, 200 μm. (B) Baseline retinal layer thickness measurements (mean ± SD) for each scan location. Layers evaluated included: NFL/GCL, nerve fiber layer and retinal ganglion cell layer; NFL/GCL + IPL, nerve fiber layer and retinal ganglion cell layer plus inner plexiform layer, and total retina.
Adapting this commercial SLO/SD-OCT imaging system for use in the rat eye required several modifications to account for the shorter axial length and optical characteristics of the rat eye. The modifications for this study included adjustments to the spectrometer reference arm length, image scaling to correct for image lateral magnification, and compensation for some higher- and lower-order optical aberrations of the eye. The position of the reference arm was adjusted using the available OCT system software and no additional optics were added to the imaging system to compensate for the shorter axial length of the rat eye. The optical power of the rat eye is approximately 5-fold greater than that of the human eye, producing much greater image lateral magnification.16,17 This was corrected by calculating an average conversion factor (1.39 μm/pixel) using a three-surface schematic eye derived from ultrasonic measurements of axial length (n = 10 eyes; data not shown) and from published indices of refraction.17,18 Finally, a +3.50 diopter contact lens, with a 3.31-mm base curvature and 6.5-mm overall diameter, was placed on the eyes to preserve corneal hydration and to compensate for some optical aberrations of the eye.
Using a prealignment procedure, eyes were aligned perpendicular to the OCT scan path with a six-axis positioning stage and bite bar.19 This stage consists of a platform for rotation along the y-axis (vertical), and two stage goniometers for rotation along the x- (horizontal) and z-axes (imaging axis). Linear translation stages were used for fine positioning along the x-, y-, and z-axes. Next, the SLO image was optimized by making fine adjustments to the stage and camera position until there was minimal vignetting of the SLO image and the optic nerve head was located in the center of the image. Camera focus and spectrometer reference arm settings were adjusted until the best image contrast was achieved at the depth of the nerve fiber layer and there was even illumination and contrast across the entire SLO. OCT B-scans were acquired after final adjustments were made to minimize any residual vertical or horizontal tilt to the OCT image.
The OCT reference arm setting, camera focus, and positioning stage settings (translation and rotation) were recorded and were used to realign the rat eye during the follow-up imaging sessions. At the end of the first imaging session, the baseline SLO image and its corresponding OCT scan location were printed on transparencies to facilitate realignment during follow-up imaging sessions.
Two realignment procedures were evaluated: manual realignment and a combined manual + automated realignment procedure. The manual realignment procedure began with physical positioning of the animal and eye as described earlier in the prealignment procedures. The OCT reference arm and OCT camera focus were also set to the baseline imaging session values. Next, the SLO transparency was placed on top of the live SLO fundus image and the positioning stage further adjusted until the size and location of the retinal blood vessels and optic nerve head matched the baseline SLO image by visual inspection. Finally, the OCT scan line location was positioned at the baseline scan position; any residual vertical tilt in the OCT scan was minimized and the image was acquired. This realignment procedure was a balance between optimizing the SLO and OCT alignment and image contrast. In some cases, the optimized OCT image had some slight residual tilt. In these instances, the image was flattened using post hoc image processing to shift all A-scans until the OCT was leveled relative to the retinal pigment epithelium (RPE). The combined manual + automated realignment procedure began with the manual realignment procedure described earlier and further included use of the instrument's built-in automated realignment procedure (follow-up mode). The instrument and the animal were not repositioned between the manual and automated realignment procedures. Automated realignment alone (without manual realignment) was not sufficient because the length of time required for gross positioning and final realignment could exceed the instrument's maximum permitted scan duration of 5 minutes.
The reproducibility of retinal thickness measurements between imaging session for each realignment method was calculated from two images acquired during two different imaging sessions separated by an average of 10 ± 4 days. Within-session repeatability using the manual alignment procedure was calculated from thickness measurements of two images captured within a single imaging session, without adjusting the animal, OCT system, or repositioning the stage settings.
Quantifying Manual Realignment Precision
Raw image data were extracted from the binary output files and segmented for quantitative analysis with a customized commercial program (Matlab 2011b; The MathWorks, Natick, MA). The precision of OCT scan line placement was quantified from scan line position within the SLO fundus images. The follow-up SLO image was registered to the baseline SLO image using an affine transformation.20 The distance and angle between scan positions were calculated and used to quantify the precision of OCT scan line placement within imaging sessions and between imaging sessions using the manual realignment procedure. Figure 2 shows how the slope of the baseline and follow-up scans were used to calculate the angle between these two OCT scan lines. Small rotational and translational parameters translate to better agreement in scan placement between images. It was not possible to quantify the precision of scan line placement using the automated realignment procedure because image registration transformation parameters used by the OCT system software are not accessible.
Figure 2. .
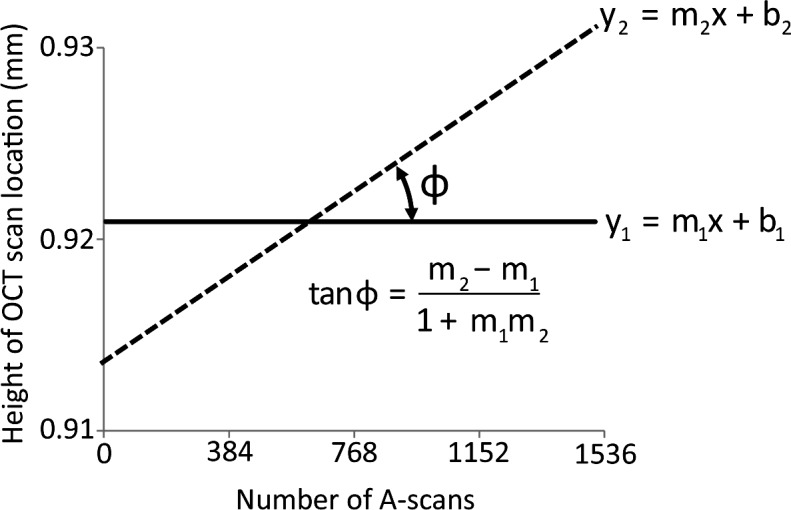
Method to determine the precision of SD-OCT scan line placement for repeated imaging. The precision of OCT scan line placement was quantified from scan line position within the SLO fundus images. The follow-up SLO image was registered with the baseline SLO image. The B-scan locations between images were then extracted from the registered SLO images and plotted against the baseline scan location. The x-axis represents the individual A-scan number (pixel column) in each OCT B-scan image; the y-axis is the scan location within the SLO retinal image relative to the optic nerve head center. The solid (y1) and dashed (y2) lines represent the locations of the OCT B-scans during the first and second imaging sessions. The slopes of these lines were used to calculate the angle between the two lines. An angle close to zero suggests minimal differences in retinal location between the two imaging sessions.
Thickness Measurement Precision
A customized segmentation protocol was developed to automatically delineate the nerve fiber layer/ganglion cell layer (NFL/GCL), whereas the inner plexiform layer (IPL) and RPE were manually segmented. Figure 3 shows a comparison between OCT and histologic retinal layers of the rat eye. This figure illustrates that the innermost layer of the rat retina is composed of the retinal ganglion cell axons along with their cell bodies and displaced amacrine cells.21 This corresponds with a prominent hyperreflective layer in the innermost region of the OCT image, followed by the less reflective IPL. The RPE is one of the last hyperreflective signals of the outer retina in the OCT image, deep to the photoreceptors, and before the structured choroid and hyperreflective sclera.22 Automated segmentation was performed using an edge-detection algorithm (after John F. Canny; Gaussian filter size of ). Intensity peaks were detected in the filtered image that corresponded to the boundary of the layers of interest. NFL/GCL thickness was defined as the distance between the inner limiting membrane (ILM) and outer boundary of the combined nerve fiber layer and ganglion cell layer. The NFL/GCL + IPL was defined as the distance from the ILM to the outer boundary of the IPL. Total retinal thickness was defined as the distance from the ILM to the RPE (Fig. 3). Mean NFL/GCL, NFL/GCL + IPL, and total retinal thicknesses were calculated for each OCT image (excluding blood vessel regions).
Figure 3. .
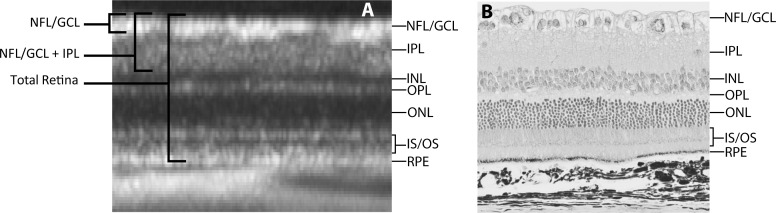
Comparison of rat retinal cross-section by OCT and histology. (A) Closeup of OCT rat retinal image indicating the various retinal layers as well as how thickness measurements were made for the repeatability study. INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; IS/OS, inner and outer segments. (B) Histologic image of the rat retina showing the various retinal layers and their correspondence with the OCT image.
Influence of Image Quality on Thickness
The impact of image quality on measurements of NFL/GCL thickness was evaluated by three methods. In the first method, NFL/GCL thickness was measured from simulated models of OCT images with varying degrees of image quality. These simulated models were generated by adding speckle noise to five reference OCT images (image quality = 37 ± 5 dB). Speckle noise is an inherent part of OCT images and results from backscatter light from multiple scatter points. Noise was modeled using a multiplicative approach (In = Io + Io × N), where Io is the original image, In is the noisy image, and N is random noise with zero mean and user-specified variance to generate a range of signal-to-noise ratios (SNRs) between 15 and 30 dB (in increments of 5 dB). The SNR was defined as 10 × log(Po/Pn), where Po is the original image power and Pn is the power of the image with added noise. The mean change in NFL/GCL thickness was calculated as a function of decreasing SNR. Image quality in the OCT system is calculated as 10 × log(S/N), where S is the maximum image intensity amplitude and N is the maximum noise intensity amplitude. This is a conventional definition of SNR used in physical optics. Figure 4 illustrates image quality over a range of SNRs. Statistical analysis was performed from thickness measurements taken across the entire OCT image at each SNR (Fig. 4B). The second evaluation of image quality on NFL/GCL thickness measurements was performed with a paired sample (n = 8 eyes; 16 images) of real images from normal adult rats that had high- and low-quality scans (Figs. 4C, 4D). A third evaluation of the effects of image quality on NFL thickness measurements was performed with clinical images from normal human subjects (Figs. 4E, 4F). All scans (12° circular scan pattern) were manually positioned on the optic nerve head center by a single examiner within an imaging session. Convenience samples (paired data; n = 26 images from 13 eyes) of high-quality (≥25 dB) and low-quality (<25 dB) scans were segmented using the native system software (Spectralis) and nerve fiber layer thickness was compared between the two groups.
Figure 4. .
The effect of image quality on NFL/GCL segmentation. (A) Original reference OCT image; NFL/GCL is delineated by white lines. For illustrative purposes here, the original reference OCT image was divided into four regions (indicated by the arrows above [B]). (B) Images were degraded by adding speckle noise across the entire image to yield SNRs ranging from 15 to 30 dB. (C) NFL/GCL segmentation of a real rat image with an image quality of 19 dB. Segmentation errors were prominent in low-quality images (<25 dB). Automatic blood vessel localization was more difficult to discern in low-quality images. (D) NFL/GCL segmentation of a real rat scan with image quality of 39 dB, where blood vessels were successfully located and excluded from thickness analysis. (E) NFL segmentation errors in human eyes using the native Spectralis segmentation routines were also prominent in circular scans with an image quality of 19 dB. (F) NFL segmentation of a circular scan of a human eye that had an image quality of 32 dB.
Statistical Analysis
Mean NFL/GCL, NFL/GCL + IPL, and total retinal thicknesses were calculated within imaging sessions and between imaging sessions. The measures of precision that were assessed included: the difference in thickness measurements between imaging sessions and within imaging sessions, as well as the 95% limits of agreement (LoA), intraclass correlation coefficients (ICC), and coefficient of variation (CV).23 Statistical comparisons of mean thicknesses between (for both manual and the combined manual + automated realignment procedures) and within imaging sessions (manual alignment) were assessed using paired t-test. The methods described by Shrout and Fleiss24 were used to calculate the appropriate ICC, for assessing precision of a single rater (the OCT) performing multiple measurements (averaged) from a random sample of animals. The method introduced by Wolf-Schnurrbusch et al.14 was used to calculate the CV (Equation 1). In Equation 1, xi and xj are thickness measurements from the first and second imaging sessions, respectively, and n is the number of eyes included in the study.
 |
Dunnett's test (multiple comparisons to a single reference standard) was used to compare NFL/GCL thickness values between the original reference OCT image (Fig. 4A) against the images with added noise (Fig. 4B). A paired t-test was used to compare mean NFL/GCL thickness between high- and low-quality images from rat eyes, as well as for comparing NFL thickness for the clinical human images.
Results
Quantifying Manual Realignment Precision
The mean and SD of the residual distance and angle between scan locations captured between two imaging sessions was 5 ± 10 μm and 0.34 ± 0.59° (rotation). Figure 5A illustrates the follow-up image aligned and superimposed on top of the baseline SLO image for one animal. The checkerboard patterns at the bottom and left margins of the SLO images highlight the nonoverlapping regions of the two SLO images and Figure 5A also shows that the residual difference in scan location for this particular animal and between these two images was 0 μm. Similar analysis was conducted from SLO images captured within imaging sessions, and the residual difference in scan position was 1 ± 0.4 μm and the averaged angle between these two lines was 0.04 ± 0.03°.
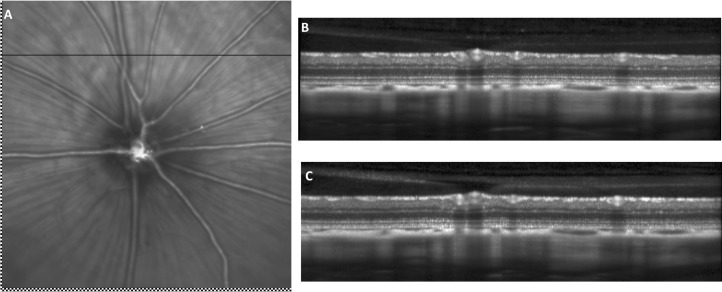
Figure 5. .
Illustration of SLO and OCT repeatability in the rat eye. Retinal fundus images and their associated OCT images for one animal captured at two different time points. (A) SLO retinal fundus image captured during the second imaging session was registered and superimposed on baseline SLO retinal fundus image. The residual difference between the baseline and follow-up scan locations was 0 μm for this particular animal. Checkerboard pattern highlights the nonoverlapping regions of the baseline and repeated SLO images. The horizontal line represents the OCT B-scan position. (B) Baseline OCT image. (C) OCT image captured during the second imaging sessions with the manual realignment procedure.
Thickness Measurement Precision
Baseline NFL/GCL thickness (mean ± SD) was 26 ± 3 μm, whereas mean NFL/GCL+IPL was 70 ± 3 μm, and total retinal thickness was 192 ± 7 μm (Fig. 1). There was no significant difference between these baseline and follow-up measurements using the manual realignment procedure: NFL/GCL (26 ± 3; P = 0.24), NFL/GCL + IPL (70 ± 4; P = 0.81), and total retinal thickness (192 ± 7; P = 0.18).
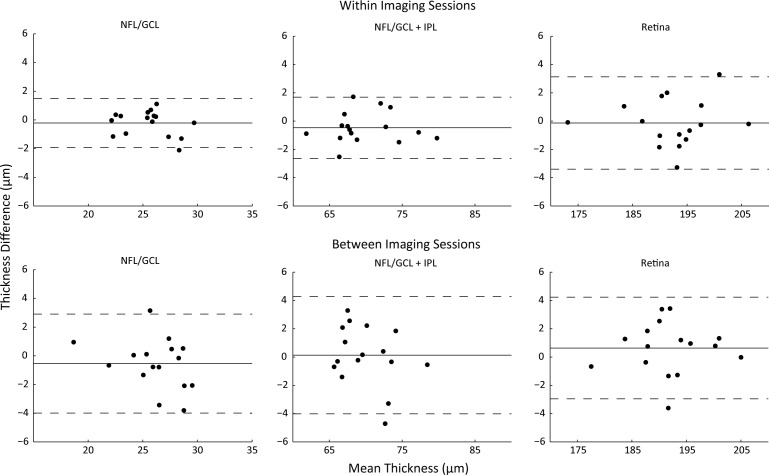
The limits of agreement for measurements of retinal thickness between imaging sessions using the manual realignment procedure are shown in Figure 6 for NFL/GCL, NFL/GCL + IPL, and total retinal thickness. These plots demonstrate that the thickness difference is close to zero and that the limits of agreement are between −4 and 4 μm or better for all measurements. ICCs between imaging sessions with the manual realignment were all >0.82 (Table). The CV values were <4.7% for all thickness measurements.
Figure 6. .
Precision of thickness measurements taken within and between imaging session with manual realignment. Residual difference in measured thickness versus mean thickness. Each point represents the difference in mean thickness calculated for each animal. Mean difference is indicated by the solid line and the upper and lower 95% LoA are indicated by dashed lines. NFL/GCL, NFL/GCL + IPL, and total retinal thicknesses between and within imaging sessions for all subjects.
Table. .
Precision Metrics for Retinal OCT Measurements in the Normal Brown Norway Rat
|
|
Mean Difference, μm |
95% Limits of Agreement, μm |
ICC |
CV, % |
| Between session | ||||
| Manual realignment | ||||
| NFL/GCL | 1 | −4 to 3 | 0.82 | 4.70 |
| NFL/GCL + IPL | 0 | −4 to 4 | 0.88 | 1.40 |
| Total retina | 1 | −3 to 4 | 0.97 | 0.70 |
| Manual + automated realignment | ||||
| NFL/GCL | 0 | −3 to 4 | 0.98 | 4.20 |
| NFL/GCL + IPL | 0 | −3 to 4 | 0.95 | 3.30 |
| Total retina | 2 | −2 to 6 | 0.98 | 1.00 |
| Within session | ||||
| NFL/GCL | 0 | −2 to 2 | 0.97 | 2.40 |
| NFL/GCL + IPL | 1 | −3 to 2 | 0.99 | 1.20 |
| Total retina | 0 | −3 to 3 | 0.99 | 0.60 |
The 95% limits of agreement were not significantly different between manual and combined (manual + automated) realignment procedures.
The mean difference and limits of agreement for measurements of retinal thickness within an imaging session using the manual realignment procedure are also shown in Figure 6. There was no significant difference in NFL/GCL (25 ± 2; P = 0.33), NFL/GCL + IPL (70 ± 5; P = 0.11), or total retinal thickness (192 ± 8; P = 0.75) measured from two images captured within the same imaging session. Over all of the retinal layers considered, the widest calculated limits of agreement observed within an imaging session (−3 to 3 μm: total retina) were similar to the widest limits of agreement between imaging sessions (−4 to 4 μm: NFL/GCL + IPL). In summary, there were no significant differences in retinal thickness measurements when comparing within and between imaging sessions with manual realignment: NFL/GCL (P = 0.52), NFL/GCL + IPL (P = 0.33), and total retinal thickness (P = 0.22).
Using the combined manual + automated realignment procedure, the mean difference in NFL/GCL thickness was 0 μm (95% LoA: −3 to 4 μm). The mean difference for the NFL/GCL + IPL was 0 μm and the 95% LoA were −3 to 4 μm. The mean difference for total retinal thickness was 2 μm and the 95% LoA were −2 to 6 μm. The mean differences calculated between imaging session with the combined manual + automated realignment procedures were not statistically different from those calculated with the manual realignment procedure alone: NFL/GCL (P = 0.38), NFL/GCL + IPL (P = 0.27), and total retina (P = 0.10).
Influence of Image Quality on Thickness
Using simulated data, NFL/GCL thickness measurements increased as image quality was degraded. In the five high-quality reference images (SNR = 37 ± 5 dB), NFL/GCL thickness ranged between 20 and 26 μm. In the degraded images, NFL/GCL thickness at the lowest SNR was, on average, 2 ± 2 μm thicker than that at the highest SNR. On average, NFL/GCL thickness increased by 1.3 μm per 10 dB decrease in SNR.
The NFL/GCL thickness for real rat images with high quality (31 ± 5 dB) was 30 μm (95% CI = 22–39 μm) and 34 μm (95% CI: 27–41 μm) for low-quality images (22 ± 2 dB). The 4-μm increase in thickness between high- and low-quality images was statistically significant (P = 0.04). The NFL thickness measurements for high-quality (30 ± 4 dB) clinical images in human eyes was 99 μm (95% CI = 95–103 μm). The NFL thickness measurements for low-quality (22 ± 2 dB) clinical images in human eyes was 101 μm (95% CI = 97–104 μm). These differences were not statistically significant (1.4 ± 5.3 μm; P = 0.36).
Discussion
The purpose of this study was to analyze factors that influence repeatability of SD-OCT thickness measurements in the rat. Ocular and instrument realignments constituted the first factor analyzed. The mean differences in retinal thickness measurements between and within imaging sessions were <2 μm and the 95% limits of agreement were within −4 to 6 μm, near the axial resolution of the OCT system (4 μm). Thickness measurements from the manual realignment procedure were not significantly different from the combined manual + automated realignment procedure. Similar results were published by Fortune and colleagues,25 where they reported that manual and the Spectralis built-in automated realignment procedures yield similar thickness results. However, they did not provide details about the realignment procedures used to quantify the similarity in thickness measurements that they reported. Although there may be advantages to using the automated realignment procedure, it cannot be used alone in nonfixating anesthetized animal subjects without some prealignment procedure. In some cases, the length of time required for gross positioning and final realignment can exceed the instrument's maximum permitted scan duration of 5 minutes. Both realignment methods evaluated in this study performed well and did not contribute significant variability to retinal thickness measurements between imaging sessions.
Nagata and colleagues26 reported an NFL/GCL thickness of 27.9 ± 1.8 μm, 500 μm away from the optic nerve head center, which agrees with the measurements reported in this study (26 ± 3 μm) that were collected at an average distance of 617 μm away from the optic nerve head center. NFL/GCL + IPL thickness measurements in the current study are thinner (70 ± 4 μm) than those reported by Guo et al.2 (84.87 μm), who also used the Spectralis (Heidelberg Engineering), but they made their measurements closer to the optic nerve head center (∼300 μm) and used a different rat strain (Dark Agouti). Srinivasan and colleagues27 used a custom-built SD-OCT system to measure total retinal thickness in Long–Evans rats. They generated thickness maps over a 2.6-mm2 region and reported a mean total retinal thickness of 189.3 μm. Total retinal thickness measurements in the current study were thicker (192 ± 7 μm) than those reported by Guo et al.2 (172.19 ± 5.17 μm) and Srinivasan et al.27 Total retinal thickness in this study was defined as the distance from the internal limiting membrane to the RPE, whereas the previous two studies2,27 defined retinal thickness as the distance from the NFL/GCL to the outer segments of photoreceptors. There is disagreement about how to assign anatomic correspondence between histology and OCT imaging.28–30 This is especially true in the outer retina where distinctions between RPE and photoreceptor outer segments have similar contrast. This disagreement between studies can account for some of the differences in retinal thickness reported.
The ability to scan the same retinal location between imaging sessions was quantified by registering the follow-up SLO image with its corresponding baseline SLO image and measuring the distance between scan locations. The average residual difference between scan locations was 5 μm. NFL/GCL measurements were made at 0.3 to 1.1 mm away from the optic nerve head center in 11 Brown Norway rats and were used to calculate the average rate of change in NF/GCL thickness. NFL/GCL decreased by 18 μm/mm, and this translates to a 0.09-μm difference in NFL/GCL thickness when scan locations are separated by 5 μm. These results show that a small portion (<1 μm thickness difference) of the calculated thickness difference is attributable to the variability in scan location between imaging sessions with the manual realignment procedure. The image registration and transformation parameters used in the automated realignment procedure are proprietary and limited the ability to evaluate the precision of scan line placement and its contribution to the observed variations in retinal thickness in the combined manual + automated realignment procedure. Nevertheless, it was still possible to quantify the repeatability of retinal thickness measurements with the manual + automated realignment procedure and that they were not significantly different from thickness measurements made from images captured with the manual realignment procedure alone.
Gabriele and colleagues31 collected volumetric data with a different SD-OCT system (Bioptigen, Inc., Durham, NC) and measured total retinal thickness in the C57Bl/6 mouse. Their global CV for total retinal thickness was greater (1.6%) than the CV calculated in the current study (0.7%). This may be due in part to the fact that the authors used mice that have thinner retinas (178 μm) than those of the rat. The authors also did not account for out-of-plane rotational alignment in their analysis. They performed postacquisition image realignment, which cannot account for variations in the OCT beam angle that can influence thickness measurements. In this study, a positioning stage was used to systematically control the amount of rotation and translation used during an imaging session. Also, the vertical and horizontal scan alignments were checked before image capture to ensure that the scan beam was as normal to the retinal surface as possible. This minimized the variation in OCT beam angle and maximized the ability to repeatedly scan the same retinal location both within and between imaging sessions.
The native segmentation routines of the Spectralis failed to automatically segment the layers of the rat retina. This required development of customized segmentation routines specifically for this purpose. Results show that retinal boundaries and blood vessel localizations are more difficult to be correctly discerned from images with poor image quality (Fig. 4). Using an image-processing approach to simulate degraded image quality, NFL/GCL thickness increased by 1.3 μm per 10 dB decrease in SNR. This increase in thickness was also observed in a paired analysis of in vivo high- and low-quality images from the rat eye (+4 μm). These differences in thickness were similar to the nominal resolution of the instrument (4 μm) and are unlikely to have an important impact on the repeatability of retinal thickness measurements. Paired comparisons of clinical images of human eyes using the native segmentation routines of the Spectralis, showed no detectable difference in thickness between high-quality (≥25 dB) and low-quality OCT scans, suggesting that the native segmentation routines are robust over a range of image quality. More detailed and systematic studies of the effects of image quality on retinal thickness measurements are needed.
Other studies with human subjects have found that poor image quality leads to differences in thickness measurements (both greater and less have been reported).32–36 Reported differences in retinal thickness arising from poor image quality may be related to the OCT imaging system as well as the methods of image processing.37 For example, two studies33,36 used the Stratus OCT (a time-domain OCT system) to evaluate the influence of signal strength, a metric used to quantify image quality, on retinal nerve fiber layer (RNFL) thickness measurements. These two studies found that RNFL increased by approximately 10 μm with higher signal strengths. Balasubramanian and colleagues32 evaluated the effect of image quality on thickness measurements by three SD-OCT systems. They reported that total retinal thickness increased by 20 to 40 μm with decreasing image quality (average image quality of 24 to 11 dB) on the Spectralis. It should be noted that Balasubramanian and colleagues made a deliberate effort to obtain poor quality images. These low-quality scans may be similar to the simulated image degradation performed in the present study. The likelihood of acquiring these low-quality images in a clinical setting is low and their relevance therefore questionable. It is unlikely that the image quality plays an important role in retinal thickness measurements with moderate- to high-quality images.
In summary, the combined manual + automated realignment procedure helped to repeatedly image the same retinal location between imaging sessions. In future studies, the combined manual + automated realignment procedure will be used to longitudinally track the retinal structural changes associated with elevated intraocular pressure in the rat eye.
Acknowledgments
The authors thank Nimesh Patel for providing the clinical image data set and Tian Siva for statistical guidance.
Footnotes
Supported by National Eye Institute/National Institutes of Health Grants P30 EY07551 and T32 EY07024.
Disclosure: D.C. Lozano, None; M.D. Twa, None
References
- 1.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo L, Normando EM, Nizari S, Lara D, Cordeiro MF. Tracking longitudinal retinal changes in experimental ocular hypertension using the cSLO and spectral domain-OCT. Invest Ophthalmol Vis Sci. 2010;51:6504–6513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huber G, Beck SC, Grimm C, et al. Spectral domain optical coherence tomography in mouse models of retinal degeneration. Invest Ophthalmol Vis Sci. 2009;50:5888–5895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim KH, Puoris'haag M, Maguluri GN, et al. Monitoring mouse retinal degeneration with high-resolution spectral-domain optical coherence tomography. J Vis. 2008;8:17.1–17.11 [DOI] [PubMed] [Google Scholar]
- 5.Baumann M, Gentile RC, Liebmann JM, Ritch R. Reproducibility of retinal thickness measurements in normal eyes using optical coherence tomography. Ophthalmic Surg Lasers. 1998;29:280–285 [PubMed] [Google Scholar]
- 6.Blumenthal EZ, Williams JM, Weinreb RN, Girkin CA, Berry CC, Zangwill LM. Reproducibility of nerve fiber layer thickness measurements by use of optical coherence tomography. Ophthalmology. 2000;107:2278–2282 [DOI] [PubMed] [Google Scholar]
- 7.Budenz DL, Chang RT, Huang X, Knighton RW, Tielsch JM. Reproducibility of retinal nerve fiber thickness measurements using the stratus OCT in normal and glaucomatous eyes. Invest Ophthalmol Vis Sci. 2005;46:2440–2443 [DOI] [PubMed] [Google Scholar]
- 8.González-García AO, Vizzeri G, Bowd C, Medeiros FA, Zangwill LM, Weinreb RN. Reproducibility of RTVue retinal nerve fiber layer thickness and optic disc measurements and agreement with Stratus optical coherence tomography measurements. Am J Ophthalmol. 2009;147:1067–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gürses-Ozden R, Teng C, Vessani R, Zafar S, Liebmann JM, Ritch R. Macular and retinal nerve fiber layer thickness measurement reproducibility using optical coherence tomography (OCT-3). J Glaucoma. 2004;13:238–244 [DOI] [PubMed] [Google Scholar]
- 10.Kim JS, Ishikawa H, Sung KR, et al. Retinal nerve fibre layer thickness measurement reproducibility improved with spectral domain optical coherence tomography. Br J Ophthalmol. 2009;93:1057–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langenegger SJ, Funk J, Töteberg-Harms M. Reproducibility of retinal nerve fiber layer thickness measurements using the eye tracker and the retest function of Spectralis SD-OCT in glaucomatous and healthy control eyes. Invest Ophthalmol Vis Sci. 2011;52:3338–3344 [DOI] [PubMed] [Google Scholar]
- 12.Massin P, Vicaut E, Haouchine B, Erginay A, Paques M, Gaudric A. Reproducibility of retinal mapping using optical coherence tomography. Arch Ophthalmol. 2001;119:1135–1142 [DOI] [PubMed] [Google Scholar]
- 13.Paunescu LA, Schuman JS, Price LL, et al. Reproducibility of nerve fiber thickness, macular thickness, and optic nerve head measurements using StratusOCT. Invest Ophthalmol Vis Sci. 2004;45:1716–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolf-Schnurrbusch UEK, Ceklic L, Brinkmann CK, et al. Macular thickness measurements in healthy eyes using six different optical coherence tomography instruments. Invest Ophthalmol Vis Sci. 2009;50:3432–3437 [DOI] [PubMed] [Google Scholar]
- 15.Wu H, de Boer JF, Chen TC. Reproducibility of retinal nerve fiber layer thickness measurements using spectral domain optical coherence tomography. J Glaucoma. 2011;20:470–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell MC, Hughes A. An analytic, gradient index schematic lens and eye for the rat which predicts aberrations for finite pupils. Vision Res. 1981;21:1129–1148 [DOI] [PubMed] [Google Scholar]
- 17.Hughes A. A schematic eye for the rat. Vision Res. 1979;19:569–588 [DOI] [PubMed] [Google Scholar]
- 18.Block MT. A note on the refraction and image formation of the rat's eye. Vision Res. 1969;9:705–711 [DOI] [PubMed] [Google Scholar]
- 19.Kocaoglu OP, Uhlhorn SR, Hernandez E, et al. Simultaneous fundus imaging and optical coherence tomography of the mouse retina. Invest Ophthalmol Vis Sci. 2007;48:1283–1289 [DOI] [PubMed] [Google Scholar]
- 20.Evangelidis GD, Psarakis EZ. Parametric image alignment using enhanced correlation coefficient maximization. IEEE Trans Pattern Anal Mach Intell. 2008;30:1858–1865 [DOI] [PubMed] [Google Scholar]
- 21.Perry VH. Evidence for an amacrine cell system in the ganglion cell layer of the rat retina. Neuroscience. 1981;6:931–944 [DOI] [PubMed] [Google Scholar]
- 22.Ruggeri M, Wehbe H, Jiao S, et al. In vivo three-dimensional high-resolution imaging of rodent retina with spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2007;48:1808–1814 [DOI] [PubMed] [Google Scholar]
- 23.Huang J, Savini G, Feng Y, Wang Q. Retinal nerve fiber layer thickness measurements in rats with spectral domain-optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53:749–750 [DOI] [PubMed] [Google Scholar]
- 24.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–428 [DOI] [PubMed] [Google Scholar]
- 25.Fortune B, Choe TE, Reynaud J, et al. Deformation of the rodent optic nerve head and peripapillary structures during acute intraocular pressure elevation. Invest Ophthalmol Vis Sci. 2011;52:6651–6661 [DOI] [PubMed] [Google Scholar]
- 26.Nagata A, Higashide T, Ohkubo S, Takeda H, Sugiyama K. In vivo quantitative evaluation of the rat retinal nerve fiber layer with optical coherence tomography. Invest Ophthalmol Vis Sci. 2009;50:2809–2815 [DOI] [PubMed] [Google Scholar]
- 27.Srinivasan VJ, Ko TH, Wojtkowski M, et al. Noninvasive volumetric imaging and morphometry of the rodent retina with high-speed, ultrahigh-resolution optical coherence tomography. Invest Ophthalmol Vis Sci. 2006;47:5522–5528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abbott CJ, McBrien NA, Grünert U, Pianta MJ. Relationship of the optical coherence tomography signal to underlying retinal histology in the tree shrew (Tupaia belangeri). Invest Ophthalmol Vis Sci. 2009;50:414–423 [DOI] [PubMed] [Google Scholar]
- 29.Gloesmann M, Hermann B, Schubert C, Sattmann H, Ahnelt PK, Drexler W. Histologic correlation of pig retina radial stratification with ultrahigh-resolution optical coherence tomography. Invest Ophthalmol Vis Sci. 2003;44:1696–1703 [DOI] [PubMed] [Google Scholar]
- 30.Huang Y, Cideciyan AV, Papastergiou GI, et al. Relation of optical coherence tomography to microanatomy in normal and rd chickens. Invest Ophthalmol Vis Sci. 1998;39:2405–2416 [PubMed] [Google Scholar]
- 31.Gabriele ML, Ishikawa H, Schuman JS, et al. Reproducibility of spectral-domain optical coherence tomography total retinal thickness measurements in mice. Invest Ophthalmol Vis Sci. 2010;51:6519–6523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balasubramanian M, Bowd C, Vizzeri G, Weinreb RN, Zangwill LM. Effect of image quality on tissue thickness measurements obtained with spectral domain-optical coherence tomography. Opt Express. 2009;17:4019–4036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheung CY, Leung CK, Lin D, Pang CP, Lam DS. Relationship between retinal nerve fiber layer measurement and signal strength in optical coherence tomography. Ophthalmology. 2008;115:1347–1351 [DOI] [PubMed] [Google Scholar]
- 34.Huang J, Liu X, Wu Z, Sadda S. Image quality affects macular and retinal nerve fiber layer thickness measurements on Fourier-domain optical coherence tomography. Ophthalmic Surg Lasers Imaging. 2011;42:216–221 [DOI] [PubMed] [Google Scholar]
- 35.Stein DM, Ishikawa H, Hariprasad R, et al. A new quality assessment parameter for optical coherence tomography. Br J Ophthalmol. 2006;90:186–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Z, Vazeen M, Varma R, et al. Factors associated with variability in retinal nerve fiber layer thickness measurements obtained by optical coherence tomography. Ophthalmology. 2007;114:1505–1512 [DOI] [PubMed] [Google Scholar]
- 37.Patel NB, Wheat JL, Rodriguez A, Tran V, Harwerth RS. Agreement between retinal nerve fiber layer measures from Spectralis and Cirrus spectral domain OCT. Optom Vis Sci. 2012;89:652–666 [DOI] [PMC free article] [PubMed] [Google Scholar]