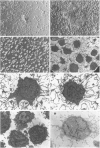
Abstract
Dendritic cells are potent antigen-presenting cells that initiate primary immune responses. Although dendritic cells derive from bone marrow stem cells, the intermediate stages in their development remain unknown. In this study, plastic-adherent blood monocytes (CD14+, CD1a-) cultured for 7 days with granulocyte-monocyte colony-stimulating factor, interleukin 4, and tumor necrosis factor alpha were shown to differentiate into CD1a+ CD83+ dendritic cells. These cells displayed all phenotypic and morphologic characteristics of mature dendritic cells and were the most potent stimulatory cells in allogeneic mixed leukocyte reactions. The identification of specific culture conditions that generate large numbers of dendritic cells from purified monocytes uncovers an important step in dendritic cell maturation that will allow the further characterization of their role in autoimmune diseases, graft rejection, and human immunodeficiency virus infection.
Full text
PDF
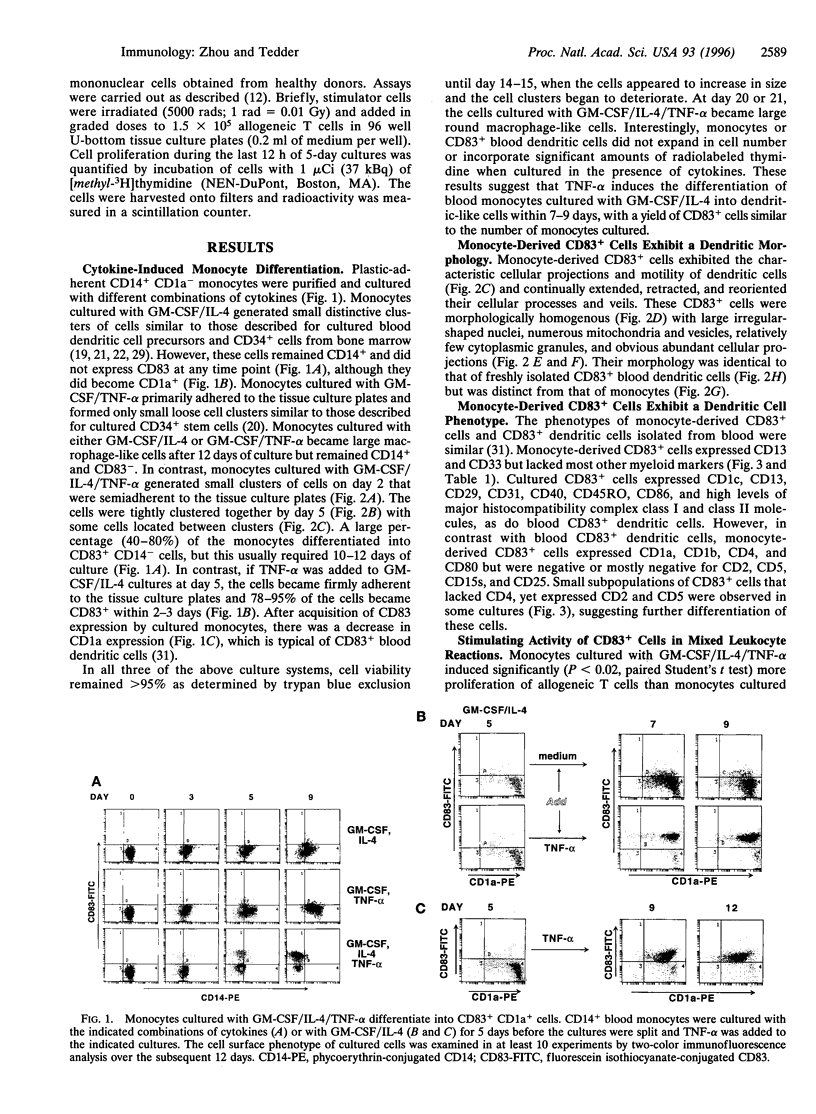
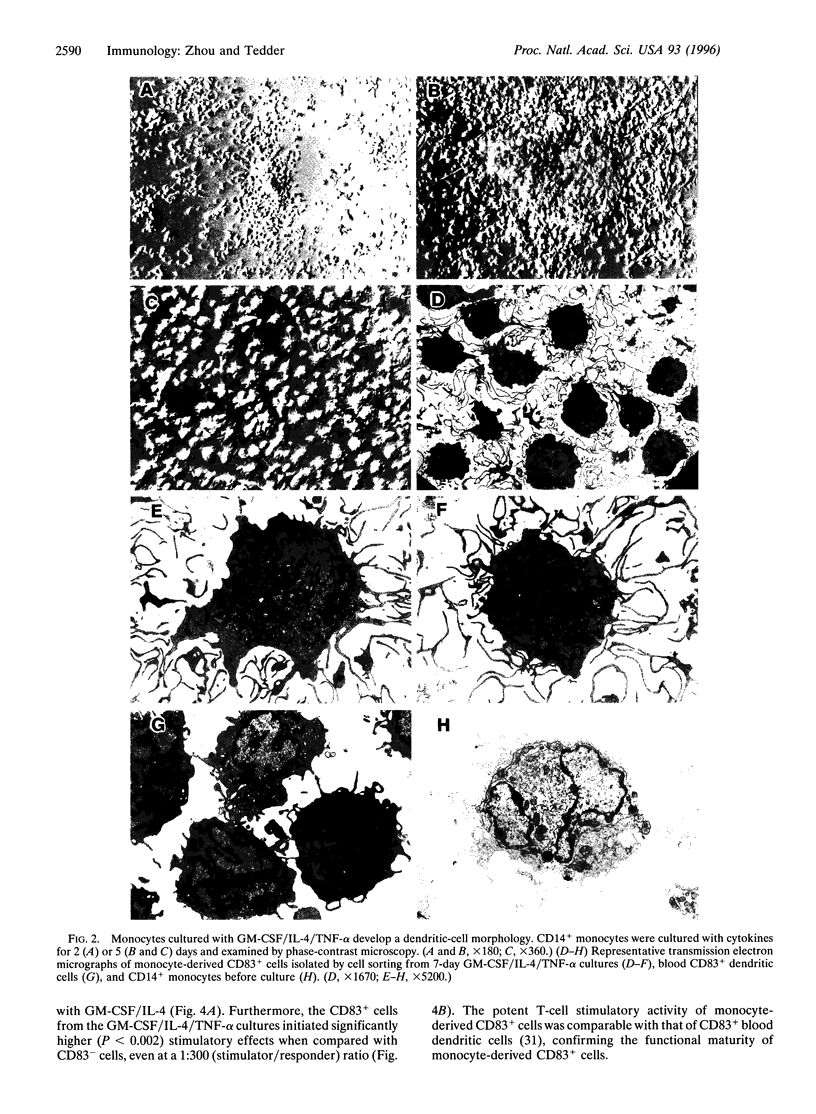
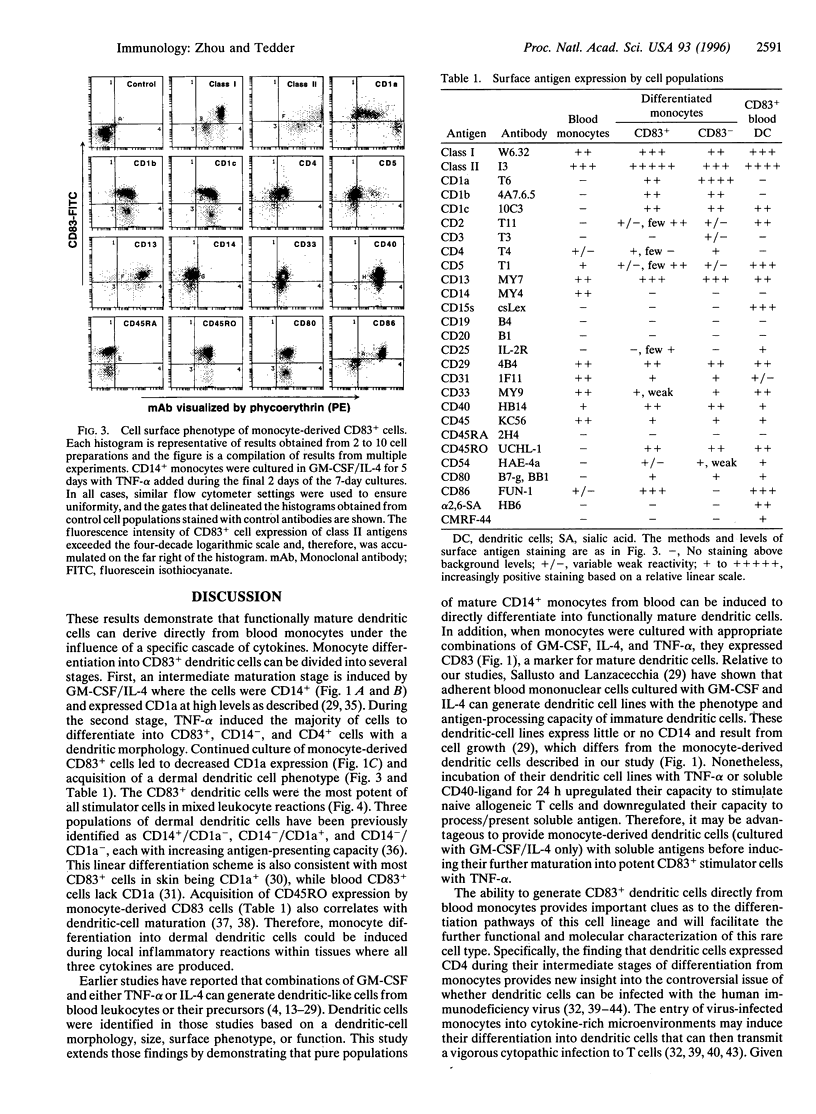
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cameron P. U., Freudenthal P. S., Barker J. M., Gezelter S., Inaba K., Steinman R. M. Dendritic cells exposed to human immunodeficiency virus type-1 transmit a vigorous cytopathic infection to CD4+ T cells. Science. 1992 Jul 17;257(5068):383–387. doi: 10.1126/science.1352913. [DOI] [PubMed] [Google Scholar]
- Caux C., Dezutter-Dambuyant C., Schmitt D., Banchereau J. GM-CSF and TNF-alpha cooperate in the generation of dendritic Langerhans cells. Nature. 1992 Nov 19;360(6401):258–261. doi: 10.1038/360258a0. [DOI] [PubMed] [Google Scholar]
- Crow M. K., Kunkel H. G. Human dendritic cells: major stimulators of the autologous and allogeneic mixed leucocyte reactions. Clin Exp Immunol. 1982 Aug;49(2):338–346. [PMC free article] [PubMed] [Google Scholar]
- Egner W., Andreesen R., Hart D. N. Allostimulatory cells in fresh human blood: heterogeneity in antigen-presenting cell populations. Transplantation. 1993 Oct;56(4):945–950. doi: 10.1097/00007890-199310000-00032. [DOI] [PubMed] [Google Scholar]
- Egner W., Prickett T. C., Hart D. N. Adhesion molecule expression: a comparison of human blood dendritic cells, monocytes, and macrophages. Transplant Proc. 1992 Oct;24(5):2318–2318. [PubMed] [Google Scholar]
- Freudenthal P. S., Steinman R. M. The distinct surface of human blood dendritic cells, as observed after an improved isolation method. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7698–7702. doi: 10.1073/pnas.87.19.7698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grage-Griebenow E., Lorenzen D., Fetting R., Flad H. D., Ernst M. Phenotypical and functional characterization of Fc gamma receptor I (CD64)-negative monocytes, a minor human monocyte subpopulation with high accessory and antiviral activity. Eur J Immunol. 1993 Dec;23(12):3126–3135. doi: 10.1002/eji.1830231213. [DOI] [PubMed] [Google Scholar]
- Inaba K., Inaba M., Deguchi M., Hagi K., Yasumizu R., Ikehara S., Muramatsu S., Steinman R. M. Granulocytes, macrophages, and dendritic cells arise from a common major histocompatibility complex class II-negative progenitor in mouse bone marrow. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):3038–3042. doi: 10.1073/pnas.90.7.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Inaba M., Romani N., Aya H., Deguchi M., Ikehara S., Muramatsu S., Steinman R. M. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992 Dec 1;176(6):1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba K., Steinman R. M., Pack M. W., Aya H., Inaba M., Sudo T., Wolpe S., Schuler G. Identification of proliferating dendritic cell precursors in mouse blood. J Exp Med. 1992 May 1;175(5):1157–1167. doi: 10.1084/jem.175.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabel P. J., de Haan-Meulman M., Voorbij H. A., Kleingeld M., Knol E. F., Drexhage H. A. Accessory cells with a morphology and marker pattern of dendritic cells can be obtained from elutriator-purified blood monocyte fractions. An enhancing effect of metrizamide in this differentiation. Immunobiology. 1989 Oct;179(4-5):395–341. doi: 10.1016/S0171-2985(89)80044-0. [DOI] [PubMed] [Google Scholar]
- Kasinrerk W., Baumruker T., Majdic O., Knapp W., Stockinger H. CD1 molecule expression on human monocytes induced by granulocyte-macrophage colony-stimulating factor. J Immunol. 1993 Jan 15;150(2):579–584. [PubMed] [Google Scholar]
- Knight S. C., Farrant J., Bryant A., Edwards A. J., Burman S., Lever A., Clarke J., Webster A. D. Non-adherent, low-density cells from human peripheral blood contain dendritic cells and monocytes, both with veiled morphology. Immunology. 1986 Apr;57(4):595–603. [PMC free article] [PubMed] [Google Scholar]
- Langhoff E., Haseltine W. A. Infection of accessory dendritic cells by human immunodeficiency virus type 1. J Invest Dermatol. 1992 Nov;99(5):89S–94S. doi: 10.1111/1523-1747.ep12669964. [DOI] [PubMed] [Google Scholar]
- Langhoff E., Kalland K. H., Haseltine W. A. Early molecular replication of human immunodeficiency virus type 1 in cultured-blood-derived T helper dendritic cells. J Clin Invest. 1993 Jun;91(6):2721–2726. doi: 10.1172/JCI116512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine T. P., Chain B. M. Endocytosis by antigen presenting cells: dendritic cells are as endocytically active as other antigen presenting cells. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8342–8346. doi: 10.1073/pnas.89.17.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najar H. M., Bru-Capdeville A. C., Gieseler R. K., Peters J. H. Differentiation of human monocytes into accessory cells at serum-free conditions. Eur J Cell Biol. 1990 Apr;51(2):339–346. [PubMed] [Google Scholar]
- Nestle F. O., Zheng X. G., Thompson C. B., Turka L. A., Nickoloff B. J. Characterization of dermal dendritic cells obtained from normal human skin reveals phenotypic and functionally distinctive subsets. J Immunol. 1993 Dec 1;151(11):6535–6545. [PubMed] [Google Scholar]
- O'Doherty U., Peng M., Gezelter S., Swiggard W. J., Betjes M., Bhardwaj N., Steinman R. M. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology. 1994 Jul;82(3):487–493. [PMC free article] [PubMed] [Google Scholar]
- O'Doherty U., Steinman R. M., Peng M., Cameron P. U., Gezelter S., Kopeloff I., Swiggard W. J., Pope M., Bhardwaj N. Dendritic cells freshly isolated from human blood express CD4 and mature into typical immunostimulatory dendritic cells after culture in monocyte-conditioned medium. J Exp Med. 1993 Sep 1;178(3):1067–1076. doi: 10.1084/jem.178.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson S., Gross J., English N., Stackpoole A., Bedford P., Knight S. C. CD4 expression on dendritic cells and their infection by human immunodeficiency virus. J Gen Virol. 1995 May;76(Pt 5):1155–1163. doi: 10.1099/0022-1317-76-5-1155. [DOI] [PubMed] [Google Scholar]
- Peters J. H., Ruppert J., Gieseler R. K., Najar H. M., Xu H. Differentiation of human monocytes into CD14 negative accessory cells: do dendritic cells derive from the monocytic lineage? Pathobiology. 1991;59(3):122–126. doi: 10.1159/000163628. [DOI] [PubMed] [Google Scholar]
- Pinchuk L. M., Polacino P. S., Agy M. B., Klaus S. J., Clark E. A. The role of CD40 and CD80 accessory cell molecules in dendritic cell-dependent HIV-1 infection. Immunity. 1994 Jul;1(4):317–325. doi: 10.1016/1074-7613(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Pope M., Betjes M. G., Romani N., Hirmand H., Cameron P. U., Hoffman L., Gezelter S., Schuler G., Steinman R. M. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell. 1994 Aug 12;78(3):389–398. doi: 10.1016/0092-8674(94)90418-9. [DOI] [PubMed] [Google Scholar]
- Reid C. D., Fryer P. R., Clifford C., Kirk A., Tikerpae J., Knight S. C. Identification of hematopoietic progenitors of macrophages and dendritic Langerhans cells (DL-CFU) in human bone marrow and peripheral blood. Blood. 1990 Sep 15;76(6):1139–1149. [PubMed] [Google Scholar]
- Reid C. D., Stackpoole A., Meager A., Tikerpae J. Interactions of tumor necrosis factor with granulocyte-macrophage colony-stimulating factor and other cytokines in the regulation of dendritic cell growth in vitro from early bipotent CD34+ progenitors in human bone marrow. J Immunol. 1992 Oct 15;149(8):2681–2688. [PubMed] [Google Scholar]
- Romani N., Gruner S., Brang D., Kämpgen E., Lenz A., Trockenbacher B., Konwalinka G., Fritsch P. O., Steinman R. M., Schuler G. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994 Jul 1;180(1):83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppert J., Friedrichs D., Xu H., Peters J. H. IL-4 decreases the expression of the monocyte differentiation marker CD14, paralleled by an increasing accessory potency. Immunobiology. 1991 Aug;182(5):449–464. doi: 10.1016/S0171-2985(11)80209-3. [DOI] [PubMed] [Google Scholar]
- Sallusto F., Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994 Apr 1;179(4):1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Schwarz F., Belilos E., Diamond B., Carsons S. E. TNF in combination with GM-CSF enhances the differentiation of neonatal cord blood stem cells into dendritic cells and macrophages. J Leukoc Biol. 1992 Sep;52(3):274–281. [PubMed] [Google Scholar]
- Santiago-Schwarz F., Divaris N., Kay C., Carsons S. E. Mechanisms of tumor necrosis factor-granulocyte-macrophage colony-stimulating factor-induced dendritic cell development. Blood. 1993 Nov 15;82(10):3019–3028. [PubMed] [Google Scholar]
- Steinman R. M., Gutchinov B., Witmer M. D., Nussenzweig M. C. Dendritic cells are the principal stimulators of the primary mixed leukocyte reaction in mice. J Exp Med. 1983 Feb 1;157(2):613–627. doi: 10.1084/jem.157.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Szabolcs P., Moore M. A., Young J. W. Expansion of immunostimulatory dendritic cells among the myeloid progeny of human CD34+ bone marrow precursors cultured with c-kit ligand, granulocyte-macrophage colony-stimulating factor, and TNF-alpha. J Immunol. 1995 Jun 1;154(11):5851–5861. [PubMed] [Google Scholar]
- Thomas R., Davis L. S., Lipsky P. E. Isolation and characterization of human peripheral blood dendritic cells. J Immunol. 1993 Feb 1;150(3):821–834. [PubMed] [Google Scholar]
- Van Voorhis W. C., Hair L. S., Steinman R. M., Kaplan G. Human dendritic cells. Enrichment and characterization from peripheral blood. J Exp Med. 1982 Apr 1;155(4):1172–1187. doi: 10.1084/jem.155.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman D., Li Y., Ananworanich J., Zhou L. J., Adelsberger J., Tedder T. F., Baseler M., Fauci A. S. Three populations of cells with dendritic morphology exist in peripheral blood, only one of which is infectable with human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):826–830. doi: 10.1073/pnas.92.3.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood G. S., Freudenthal P. S. CD5 monoclonal antibodies react with human peripheral blood dendritic cells. Am J Pathol. 1992 Oct;141(4):789–795. [PMC free article] [PubMed] [Google Scholar]
- Xu H., Friedrichs U., Gieseler R. K., Ruppert J., Ocklind G., Peters J. H. Human blood dendritic cells exhibit a distinct T-cell-stimulating mechanism and differentiation pattern. Scand J Immunol. 1992 Nov;36(5):689–696. doi: 10.1111/j.1365-3083.1992.tb03129.x. [DOI] [PubMed] [Google Scholar]
- Young J. W., Steinman R. M. Accessory cell requirements for the mixed-leukocyte reaction and polyclonal mitogens, as studied with a new technique for enriching blood dendritic cells. Cell Immunol. 1988 Jan;111(1):167–182. doi: 10.1016/0008-8749(88)90061-5. [DOI] [PubMed] [Google Scholar]
- Zhou L. J., Schwarting R., Smith H. M., Tedder T. F. A novel cell-surface molecule expressed by human interdigitating reticulum cells, Langerhans cells, and activated lymphocytes is a new member of the Ig superfamily. J Immunol. 1992 Jul 15;149(2):735–742. [PubMed] [Google Scholar]
- Zhou L. J., Tedder T. F. A distinct pattern of cytokine gene expression by human CD83+ blood dendritic cells. Blood. 1995 Nov 1;86(9):3295–3301. [PubMed] [Google Scholar]
- Zhou L. J., Tedder T. F. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J Immunol. 1995 Apr 15;154(8):3821–3835. [PubMed] [Google Scholar]