Abstract
White matter hyperintensities (WMH) are associated with hypertension. We examined interactions between blood pressure (BP), internal carotid artery (ICA) flow velocity parameters and WMH. We obtained BP measurements from 694 community-dwelling subjects at mean ages 69.6 (±0.8) and again at 72.6 (±0.7) years, plus brain MRI and ICA ultrasound at age 73±1 years. Diastolic and mean BP decreased and pulse pressure increased but systolic BP did not change between 70 and 73 years. Multiple linear regression, corrected for vascular disease and risk factors, showed that WMH at age 73 were associated with history of hypertension (β=0.13, p<0.001) and with BP at age 70 (systolic β=0.08, mean β=0.09, diastolic β=0.08, all p<0.05); similar but attenuated associations were seen for BP at age 73. Lower diastolic BP and higher pulse pressure were associated with higher ICA pulsatility index at age 73 (diastolic BP: standardized β, age 70=−0.24, p<0.001; pulse pressure age 70 β=0.19, p<0.001). WMH were associated with higher ICA pulsatility index (β=0.13, p=0.002) after adjusting for BP and correction for multiple testing. Therefore falling diastolic BP and increased pulse pressure are associated with increased ICA pulsatility index, which in turn is associated with WMH. This suggests that hypertension and WMH may either associate indirectly because hypertension increases arterial stiffness which leads to WMH over time, or co-associate through advancing age and stiffer vessels, or both. Reducing vascular stiffness may reduce WMH progression and should be tested in randomised trials, in addition to testing antihypertensive therapy.
Keywords: blood flow velocity, blood pressure, pulse pressure, white matter hyperintensities, ageing, magnetic resonance imaging
Introduction
White matter hyperintensities (WMH) are indicators of cerebral small vessel disease1 and are implicated in the pathogenesis of cognitive impairment, stroke and dementia.2 WMH are associated with hypertension and increased risk of stroke,3-5 but the mechanism through which elevated blood pressure (BP) affects the brain is unclear. Advancing age is associated with loss of elasticity in the large arteries and muscular arterioles and increased arterial stiffness. Several risk factors, particularly hypertension, contribute to the stiffness.3,6-8 Arterial stiffening impairs the damping of the arterial waveform in large arteries and could lead to excessive transmission of BP pulsation to the brain.9,10 Increasing stiffness of the large central arteries is associated with WMH.8-12 One explanation for the association between arterial stiffness and WMH is that arterial stiffening exposes small vessels in the brain to high pulsatility, damaging the small vessel wall.7-9 Since this cyclic variation in BP is transmitted to the brain through the internal carotid arteries (ICA), an association between BP, ICA flow parameters and WMH might be expected.5 Few studies have compared BP, ICA or middle cerebral artery (MCA) blood flow velocity and WMH.9,13
Previous studies9,10 that investigated BP and/or ICA or MCA velocity parameters and WMH have focused on the pulse pressure component of BP and the pulsatility index component of the Doppler MCA or ICA waveform. However, pulse pressure is determined by diastolic and systolic BP and the relative contribution of these is a function of age: in young adults, both diastolic and systolic BP increase, whereas in the elderly systolic BP increases while diastolic BP reduces with age.14
Here we investigated the association between BP measured longitudinally, ICA blood flow velocity parameters and age-related WMH in a well characterised large community-dwelling cohort of older adults with a narrow age range. We hypothesised that as the ICAs are the main conduits of blood to the brain, that BP must exert its effects on the brain via the ICAs and therefore that we should find positive associations between BP and ICA velocity parameters, and in turn between ICA velocity parameters and WMH, if indeed there is a direct relationship between high blood pressure and WMH at older ages.
Methods
Subjects
Study participants were members of the Lothian Birth Cohort 1936 (LBC1936).15 They were all born in 1936, most undertook the Scottish Mental Survey of 1947,15 and most were living in the Lothian (Edinburgh) area of Scotland when first recruited into the LBC1936 between 2004 and 2007. At mean age 70 years (LBC1936 wave 1), 1091 participants undertook detailed medical and cognitive assessments.15 Three years later (wave 2), repeat medical and cognitive assessments were conducted (N=866); in addition, at wave 2 they underwent carotid Doppler ultrasound imaging and brain MRI (N=700, protocols detailed elsewhere).16
Subjects provided history of ischemic heart disease, diabetes, hypertension (diagnosed or on treatment), smoking (coded here as ever smoked previously or currently), hypercholesterolemia, peripheral vascular disease (PVD), clinically evident stroke and any other circulatory disease, and we calculated body mass index.
Written informed consent was obtained from all participants under protocols approved by the Lothian (REC 07/MRE00/58) and Scottish Multicentre (MREC/01/0/56) Research Ethics Committees; all procedures were conducted according to institutional guidelines and the Declaration of Helsinki.
BP Measurements
BP Measurements were taken from the brachial artery at wave 1 and 2 by trained research nurses in a Clinical Research Facility (http://www.wtcrf.ed.ac.uk) using an Omron 705IT monitor. Three readings of systolic and diastolic BP were taken, sitting and standing. We calculated average systolic and diastolic BP over the three sittings (or standings) and pulse pressure for each wave. Brachial pulse pressure closely reflects aortic pulse pressure – of five measures of arterial ‘stiffness’ outside the head, brachial pulse pressure showed the strongest correlation with, and explained the largest proportion of variance in, intracranial arterial stiffness.5 We also calculated mean BP (equation 1, SBP and DBP are the systolic and diastolic BP respectively).
| 1 |
We calculated BP variability using methods proposed previously:17-19 standard deviation (SD), coefficient of variation (standard deviation of successive measurements divided by their mean value), average real variability (average absolute difference between successive measurements) and successive variation (average squared difference between successive measurements), separately for systolic and diastolic BP (Supplementary Tables S1 and S2), and for each time point using the three sitting (or standing) BP measurements. Note the availability of three BP measurements for variability computation limits the strength of the metrics.
Carotid Doppler Ultrasound Imaging
Carotid Doppler Ultrasound Imaging was performed at wave 2 on a Siemens Antares Premium Colour Doppler scanner (Siemens AG, Erlangen, Germany) with 7.5 MHz variable frequency probe by experienced neurovascular ultrasonographers. Blood flow velocity readings were obtained, after at least five minutes rest supine with head on pillow, from the left and right common, internal and external carotid arteries,20 including peak systolic and end diastolic blood flow velocities from all arteries and averaged the right and left velocities. We calculated ICA mean flow velocity, pulsatility index and resistivity index using average values of left and right ICAs in equations 2, 3 and 4 (ICAS=ICA systolic velocity and ICAD=ICA end diastolic velocity). We calculated mean velocity,20,21 rather than using the machine-derived time averaged mean, to avoid inaccurate machine calculations occurring secondary to signal drop out or artefact from the velocity waveform. ICA velocity parameters including pulsatility and resistivity indices, closely reflect intracranial arterial velocity parameters.5 Measuring blood velocity parameters in the ICAs avoids the problem of a) the ≥10% data loss due to acoustically dense skull and b) incorrect middle cerebral artery velocity calculations due to assumed angle of insonance that occur with transcranial Doppler ultrasound.
| 2 |
| 3 |
| 4 |
Magnetic Resonance Brain Imaging
We report the imaging findings according to the Standards for Reporting Vascular Changes in Neurodegeneration (STRIVE) criteria.1 All brain MRI data were acquired at wave 2 on a 1.5T GE Signa Horizon HDx scanner (General Electric, Milwaukee, WI, USA) with a self-shielding gradient set, maximum gradient strength 33 mT/m, and an 8-channel phased-array head coil. The image acquisition included: T1-weighted coronal, T2-weighted, T2*-weighted and FLAIR (Fluid Attenuated Inversion Recovery) sagittal whole brain scans (details in16). WMH were segmented and volumes measured using a validated multispectral image processing tool, MCMxxxVI (www.sourceforge.net/projects/bric1936).22 Intracranial volume (ICV) was measured using the Image Edit tool in the Analyze 9.0™.16 WMH were visually rated by an experienced, neuroradiologist on the FLAIR and T2-weighted images using the Fazekas scale,23 with deep and periventricular WMH first scored separately (0-3) and then the scores combined to give a total score out of 6.
Statistical Analysis
All statistical analyses were performed using SPSS version 19 (SPSS Inc. Chicago III, USA), all statistical tests being two-tailed, and p values <0.05 being considered significant. BP measures at wave 1 and 2 were compared using paired t-tests and health conditions at wave 1 and 2 were compared using Wilcoxon rank sum test.
Associations between BP measures, ICA blood velocity measures and WMH were investigated using multivariate linear regression models. The covariates which are known or proposed predictors of WMH, BP or blood velocity parameters were included in the analysis: age in days at MRI, sex, BMI, and self-reported history of ischemic heart disease, stroke, PVD, other circulatory disorders, diabetes, hypertension, smoking, and hypercholesterolemia. We modelled the association between BP, ICA blood velocity parameters and WMH in stages, each individually and then all three elements together. We tested associations with and without history of hypertension included in the models (to avoid over-fitting) – as there was little difference in the results whether hypertension was included or not, we report the results without hypertension as a covariate. All relevant covariates were included in the models and multiple testing was corrected for using the false-discovery rate (FDR). We tested both WMH volume and Fazekas score and whether the associations differed between hypertensive and non-hypertensive subjects using Pearson bivariate analysis. As WMH were not normally distributed, in sensitivity analyses we log transformed the WMH but found no difference in the models between the raw and transformed WMH. This was unsurprising because of our large sample size. In view of these and to simplify the interpretation of results, we report the results of the untransformed WMH.
Results
Subjects
Of the 700 subjects with brain MRI, six had incomplete data reducing the final sample to 694 (Table 1), mean ages 69.6±0.8 and 72.6±0.7 years for waves 1 and 2 respectively, with the same proportion of men (53%) at both waves. The proportions with vascular diagnoses increased significantly between wave 1 and wave 2: hypertension (37% to 48.7%), ischaemic heart disease (21.7% to 27.3%), diabetes (6.6% to 11.0%), stroke (4.4% to 6.9%), hypercholesterolemia (33.3% to 41.4%), PVD (37.5% to 42.1%) and other circulatory problems (13.6% to 17.6%, all p<0.00). There was no significant difference in BMI between wave 1 and 2. ICA stenosis >50% was only present (on either side) in 2.9% and internal carotid occlusion on either side in one patient each (0.3%).
Table 1. Descriptive statistics for measures of: BP, blood velocity in the ICA, WMH measures, demographic and health conditions.
| Mean (SD) |
|||
|---|---|---|---|
| Parameter Assessed | Measures | Wave 1 (N=1091) | Wave 2 (N=694) |
| BP measures | Peak Systolic BP (mmHg) | 149.45 (18.96) | 148.69 (18.95) |
| Mean BP (mmHg) | 104.00 (11.81) | 102.00 (11.27)** | |
| End diastolic BP (mmHg) | 81.45 (10.17) | 78.1 (9.68)** | |
| Pulse pressure (mmHg) | 68.00 (14.91) | 71.34 (18.94)** | |
| Measures of blood velocity in the ICA | Peak Systolic velocity (cm s−1) | 59.91 (20.33) | |
| Mean velocity (cm s−1) | 32.53 (10.59) | ||
| End Diastolic velocity (cm s−1) | 18.85 (7.09) | ||
| Pulsatility index | 1.27 (0.26) | ||
| Resistivity index | 0.66 (0.40) | ||
| WMH and related measures | White matter hyperintensities volume (cm3) | 12.05 (12.84) | |
| Intracranial volume (cm3) | 1450.97 (140.57) | ||
| Percentage of White matter lesions in ICV | 0.83 (0.90) | ||
| Total Fazekas scores, median (IQR) | 2.00 (1.00) | ||
| Deep Fazekas scores, median (IQR) | 1.00 (0) | ||
| Periventricular Fazekas scores, median (IQR) | 1.00 (1) | ||
| Demographic and health conditions | % men | 53 | 53 |
| Age in years, mean (SD) | 69.57 (0.83) | 72.55 (0.71) | |
| Body mass index, mean (SD) | 27.83 (4.38) | 27.98 (4.50) | |
| History of hypertension (%) | 37.10 | 48.70 † | |
| History of ischemic heart disease (%) | 21.70 | 27.30 † | |
| History of diabetes (%) | 8.60 | 11.00 † | |
| History of stroke (%) | 4.40 | 6.90 † | |
| History of smoking (%) | 56.10 | ||
| History of hypercholesterolemia (%) | 33.30 | 41.40 † | |
| History of peripheral vascular diseases (%) | 37.50 | 42.10 * | |
| Problems with blood circulation (%) | 13.60 | 17.60 * | |
Measures changed significantly from wave 1 to 2:
p<0.05,
p<0.001
We found similar changes from wave 1 to wave 2 for BP taken while sitting or standing, therefore all subsequent analyses refer to sitting BP (data for standing available on request). There was no significant change in systolic BP (Table 1), but mean and diastolic BP fell significantly (p<0.001) and thus pulse pressure increased significantly (p<0.001) from wave 1 to 2. The mean absolute WMH volume was 12.05±12.84 mm3, or 0.83±0.90% of ICV. The total median and interquartile range Fazekas score was 2.0±1.0, range 0 to 6.
BP and ICA blood velocity parameters
For brevity, only the summary results of the regression analyses are presented here (Figure 1, Table 2, Supplementary Figure S1). Full results, including covariate effects, are reported in Supplementary Table S3. There were numerous relatively weak associations between BP and ICA velocities, but in general, these were strongest and most consistent for lower diastolic BP and higher ICA pulsatility index (with few associations for systolic BP), and for BP measured at wave 2 (results in text) than at wave 1 (Table 2). Thus, higher ICA systolic and mean velocities were associated with lower diastolic BP (all p<0.001) and higher pulse pressure (all p<0.004). Higher ICA diastolic velocity was associated with lower diastolic BP (β=−0.09, p=0.024) and lower mean BP (β=−0.08, p=0.029), but no other BP measure. Higher ICA pulsatility index was associated with higher systolic BP (β=0.08, p=0.04), lower diastolic BP (β=−0.19, p<0.001) and higher pulse pressure (β=0.10, p=0.008). Higher ICA resistivity index was associated with lower diastolic BP (β=−0.18, p<0.001) and higher pulse pressure (β=0.17, p<0.001). All the significant associations remained significant after a correction for false-discovery rate was applied. There were no associations for BP variability parameters (Supplementary Table S1).
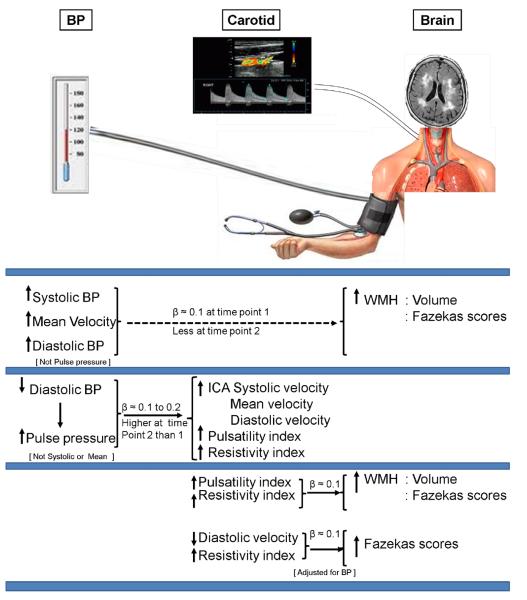
Figure 1. Summary of the associations between measures of BP, of ICA blood velocity parameters and of WMH (volume and Fazekas scores).
BPs were measured at waves 1 (time point 1), mean age 69.6±0.83, and 2 (time point 2), mean age 72.6±0.71; ICA velocity parameters and WMH were measured at wave 2. Models accounted for all covariates.
Table 2. Association between measures of BP and ICA blood velocity parameters.
Values are standardized β (p value, FDR correctedpvalues) for the sitting BP measures predicting ICA blood velocity measures after accounting for covariates. See Supplementary Table S3 for full covariate effects.
| Blood velocity in the ICA |
|||||
|---|---|---|---|---|---|
| BP Measures | Systolic velocity | Mean velocity | Diastolic velocity | Pulsatility index | Resistivity index |
| Wave 1 | |||||
| Systolic BP | 0.06 (0.141,0.256) | 0.04 (0.285,0.407) | 0.01 (0.76,0.80) | 0.04 (0.335,0.447) | 0.04 (0.256,0.394) |
| Mean BP | −0.04 (0.245,0.394) | −0.02 (0.647,0.711) | 0.02 (0.533,0.627) | −0.11 (0.003,0.009) * | −0.08 (0.054,0.111) |
| Diastolic BP | −0.13 (0.001,0.003) * | −0.07 (0.068,0.120) | 0.03 (0.423,0.529) | −0.24 (<0.0001,<0.0005) * | −0.18 (<0.0001,<0.0005) * |
| Pulse pressure | 0.15 (<0.0001,<0.0005) * | 0.09 (0.011,0.028) * | −0.01 (0.887,0.88) | 0.19 (<0.0001,<0.0005) * | 0.17 (<0.0001,<0.0005) * |
| Wave 2 | |||||
| Systolic BP | 0.02 (0.536,0.596) | −0.01 (0.757,0.850) | −0.06 (0.114,0.147) | 0.11 (0.004,0.014) * | 0.10 (0.013,0.022) * |
| Mean BP | −0.10 (0.009,0.018) * | −0.10(0.008,0.018) * | −0.08 (0.029,0.031) * | −0.04 (0.349,0.400) | −0.01 (0.722,0.75) |
| Diastolic BP | −0.20 (<0.0001,<0.0005) * | −0.17 (<0.0001,<0.0005) * | −0.09 (0.024,0.029) * | −0.18 (<0.0001,<0.005) * | −0.12 (0.002,0.003) * |
| Pulse pressure | 0.13 (<0.0001,<0.004) * | 0.11 (0.004,0.010) * | 0.05 (0.217,0.271) | 0.12 (0.002,0.007) * | 0.10 (0.012,0.022) * |
represent associations that remained significant after applying a correction for false discovery rate.
BP measures and WMH measures
Associations between BP variables and WMH were generally stronger for BP assessed at wave 1 and for Fazekas scores. At wave 1, higher mean BP (β=0.09, p=0.02, Figure 1, Table 3, Supplementary Figure S1) and diastolic BP (β=0.08, p=0.04) were weakly associated with larger WMH volume, with similar but weaker associations at wave 2. Higher Fazekas scores (Supplementary Table S4) were significantly associated with higher systolic BP (wave 1: β=0.12, p=0.002), mean BP (wave 1: β=0.13, p=0.001) and diastolic BP (wave 1: β=0.11, p=0.003), with similar but weaker associations at wave 2. No association was found between WMH measures (WMH volume or Fazekas) and pulse pressure or variability (Supplementary Table S2). All the significant associations remained significant after correction for FDR.
Table 3. Association between WMH volume and measures of BP and ICA blood velocity (standardized β (p value, FDR correctedpvalues)).
Models accounted for all covariates.
| Predicting WMH volume from measures of BP | |||||
|---|---|---|---|---|---|
| Covariates |
|||||
| Measures of BP at waves 1 or 2 | BP Measures | ICV | Sex | Age in days | |
| Systolic BP, wave 1 | 0.08 (0.043,0.057) | 0.12 (0.021) | 0.08 (0.126) | 0.15 (<0.0001) | |
| Systolic BP, wave 2 | 0.06 (0.113,0.248) | 0.12 (0.019) | 0.08 (0.123) | 0.14 (<0.0001) | |
| Mean BP, wave 1 | 0.09 (0.021,0.057) | 0.11 (0.023) | 0.08 (0.118) | 0.15 (<0.0001) | |
| Mean BP, wave 2 | 0.06 (0.144,0.248) | 0.12 (0.02) | 0.08 (0.131) | 0.14 (<0.0001) | |
| Diastolic BP, wave 1 | 0.08 (0.036,0.057) | 0.11 (0.025) | 0.08 (0.121) | 0.15 (<0.0001) | |
| Diastolic BP, wave 2 | 0.04 (0.306,0.306) | 0.12 (0.021) | 0.08 (0.123) | 0.14 (<0.0001) | |
| Pulse pressure, wave 1 | 0.04 (0.249,0.249) | 0.12 (0.02) | 0.07 (0.142) | 0.14 (<0.0001) | |
| Pulse pressure, wave 2 | 0.05 (0.186,0.248) | 0.12 (0.021) | 0.08 (0.131) | 0.14 (<0.0001) | |
| Predicting WMH volume from measures of ICA velocity | |||||
|---|---|---|---|---|---|
| Covariates |
|||||
| Measure of ICA velocity at wave 2 ICA velocity measures | ICV | Sex | Age in days | Hypertension | |
| Systolic velocity | 0.04 (0.343,0.428) | 0.13 (0.011) | 0.09 (0.085) | 0.14 (<0.0001) | 0.13 (<0.0001) |
| Mean velocity | 0.00(0.990,0.990) | 0.13 (0.011) | 0.09 (0.086) | 0.13 (<0.0001) | 0.13 (<0.0001) |
| Diastolic velocity | −0.05 (0.178,0.283) | 0.13 (0.012) | 0.09 (0.075) | 0.13 (0.001) | 0.13 (0.001) |
| Pulsatility index | 0.09 (0.016,0.08) | 0.12 (0.014) | 0.10 (0.047) | 0.13 (<0.0001) | 0.12 (0.002) |
| Resistivity index | 0.07 (0.054,0.135) | 0.12 (0.015) | 0.09 (0.066) | 0.14 (<0.0001) | 0.13 (0.001) |
Note: Dependent variables were measures of WMH while independent variables were measures of ICA velocities and of BP. Each row represents a separate model which controlled for ICV and demographic variables. Health variables’ inclusion used stepwise method and only those that passed the Akaike Information Criterion test appeared in the final model above.
ICA blood velocity measures and WMH measures
Without accounting for BP measures (Figure 1, Table 3), larger WMH volume was associated with higher ICA pulsatility index (β=0.09, p=0.016), and higher Fazekas scores (Supplementary Table S4) were associated with lower ICA diastolic velocity (β=−0.11, p=0.005) and higher resistivity index (β=0.08, p=0.04) but no other ICA blood velocity measures. Accounting for BP measures (Figure 1, Supplementary Table S5 and S7) resulted in marginal adjustments to these associations: larger WMH volume (β=0.13, p=0.002) and higher Fazekas scores (β=0.12, p=0.003) were associated with higher ICA pulsatility index; higher Fazekas scores were also associated with lower ICA diastolic velocity (β=−0.11, p=0.005) and higher resistivity index (β=0.11, p=0.005). The associations between ICA pulsatility and resistivity indices and WMH remained after FDR correction. No association was found between WMH and other ICA blood velocity measures, but those with history of hypertension had larger WMH volumes (Table 3, β=0.13, p<0.001) and higher Fazekas scores (Supplementary Table S4, β=0.16, p<0.001).
Sensitivity analyses
In hypertensive subjects, the associations between BP, ICA parameters and WMH were slightly stronger than in normotensive subjects, but there were no differences in direction of association or other features (Supplementary Table S6). After converting standardized to unstandardized betas, for every 1 year increase in age there was approximately a 2.43 cm3 increase in WMH volume. Additionally, for every additional individual diagnosis of hypertension, there was approximately a 3.47 cm3 increase in WMH volume.
Discussion
We investigated associations between BP parameters measured longitudinally, ICA velocity parameters and WMH in about 700 community-dwelling individuals aged around 73. Higher systolic, mean and diastolic BPs were weakly associated with WMH, especially for BP measured several years previously. Considering the route by which BP effects reach the brain, higher concurrent ICA pulsatility index, largely the result of falling diastolic BP, was associated with WMH (Figure 1). All associations remained significant after correcting for multiple testing and whether or not ‘hypertension’ was included in the model. Thus the association between BP measures and WMH is different to that between BP measures and WMH when the route between the heart and the brain via the ICAs is accounted for meaning that BP and WMH either associate indirectly through BP elevation earlier in life leading to stiffer vessels which in turn lead to WMH, or hypertension and WMH co-associate through advancing age and stiffer vessels. In either case, the data suggest that the route from BP to WMH is indirect in community-dwelling generally healthy older subjects. Notably, even within this narrow age-range, as little as a one year increase in age was associated with 2.43 ml increase in WMH volume, and ‘hypertension’ (vs no hypertension) was associated with 3.47 ml increase in WMH volume. This novel finding provides important quantitative information on the effect of age and hypertension on WMH.
Comparison with literature
Our large sample of subjects (694) with longitudinal BP assessments, fell within a narrow age range in their late 60s to early 70s at the two waves, and were living in the community. The proportion with cardiovascular conditions and risk factors increased over the three years, with hypertension increasing from 38% to 50% consistent with previous studies.8-10,24-28 The association between higher systolic, mean and diastolic BP and WMH is consistent with many previous studies.3,4,8,29-32
Some studies9,10 have reported associations between WMH and ICA or MCA pulsatility index, but no previous studies examined associations between BP, ICA parameters and WMH simultaneously or longitudinally, or the role of falling diastolic BP identified in this study. Increased arterial stiffness (measured in various ways, in various arteries) and WMH are emerging: 167 patients with hypertension,12 363 community-dwelling subjects,11 in hypertensive subjects amongst 1460 community-dwelling subjects,8 in 1587 Framingham subjects,33 in 1800 subjects in the 3C-Dijon Study34 and in 1270 Dallas Heart Study35 but none of these studies dissected the complete path from BP via carotid to brain and the subjects’ ages covered several decades. Pulse wave velocity assessed 10 years later in 303 elderly subjects, but only in one white matter tract.36 Lower aortic diastolic BP, increased aortic pulse pressure and increased MCA pulsatility index were associated with WMH in 100 stroke patients of wide age range,10 similar to our findings and suggesting a co-association rather than a direct association. The powerful effects of age on many biological processes is difficult to correct statistically: in addition to our 2.43 ml/year increase in WMH, MCA flow velocity falls by 0.2 cms−1 per year increase in age (p=0.045) and by 3.75 cms−1 per point increase in WMH Fazekas score (p=0.004). Consequently, MCA PI has even been suggested as an office screening tool for WMH.37
We did not find associations of WMH with BP variability, although our data were limited for assessing variability, but this is consistent with two other large recent prospective studies,31,32 which disagreed with previous cross sectional studies showing BP variability-WMH associations.38,39
BP, ICA velocities and potential pathophysiological effects on WMH
In our study, systolic BP did not change between waves 1 and 2 but diastolic BP fell and consequently pulse pressure increased. Lower diastolic BP, higher pulse pressure and higher ICA pulsatility index, mean and diastolic velocity were consistently associated, but systolic BP associations were generally inconsistent and weak. After adjusting for BP, larger WMH volume was associated with higher ICA pulsatility index and lower diastolic BP. The pathway from BP to WMH is therefore through falling diastolic BP, rising pulse pressure and ICA pulsatility index. Increased vessel stiffness would fit with emerging evidence that WMH associate more with BP levels taken years earlier than with concurrent readings. Associations between BP and WMH when ICA parameters are not considered, in which systolic BP is most prominent, are contrary to the direct path of BP transmission to the brain via the carotid arteries where lower diastolic BP is the associated variable. This might suggest that diastolic BP was falling below an acceptable perfusion pressure to result in WMH, but the diastolic BP values (mean 78.1, SD 9.68, Table 1) do not suggest that that is likely.
Strengths
Strengths include using well validated image processing tools, accounting for all necessary covariates in the statistical models, and comprehensive assessment of: several BP measures at sitting and standing positions, at ages 70 and 73; five ICA blood velocity measures averaged across right and left and two measures of WMH recorded at mean age 73. ICA and MCA pulsatility index are closely related; brachial pulse pressure (as measured here) showed the strongest correlation with MCA pulsatility index and explained the largest variance in MCA pulsatility index.5
Limitations
We cannot comment on longitudinal WMH or ICA velocity parameters. The LBC193615,16 participants are currently undergoing repeat MRI to provide longitudinal data. Our variability measures were limited, but other studies with comprehensive longitudinal visit-to-visit variability measures have not found associations.32,40 We did not account for medical treatment, but the risk factor diagnoses and BP measures encompass treatment. Others have shown that BP levels are more important than treatment per se in relation to WMH.31 We calculated mean velocity to avoid errors in machine-calculated values which may have under-or over-estimated the time averaged mean; however pulsatility index (the strongest covariate) is the same whether calculated by hand or machine.
Perspectives
That the association between BP and WMH may be a co-association acting through increased arterial stiffness has implications for strategies to prevent WMH progression, their cognitive and physical consequences. Treatment of hypertension is important for stroke prevention, but there is less evidence that it reduces WMH progression41,42 (but BP lowering may have been too little or not for long enough) and mixed information about effects on cognition (results of the Secondary Prevention of Small Subcortical Stroke (SPS3) trial on BP lowering in 3000+ patients with lacunar stroke are awaited). Perhaps therapies to reduce arterial/arteriolar stiffness, by acting more directly on the suggested pathophysiological pathway to WMH, might have valuable impacts on preventing WMH progression. Our data suggest that lifestyle or pharmacological methods to reduce arterial stiffness preferentially would be worth evaluating in case some antihypertensive therapies alone are insufficient to restore normal vascular tone and cerebral vasoreactivity.
Conclusion
The association between BP and WMH at older ages, when considering the path via the carotid arteries, is most closely aligned with increased ICA pulsatility index which was a consequence of falling diastolic BP, questioning the ‘directness’ of the link between BP and WMH. Longitudinal studies with narrow age range subjects help to differentiate potentially causal relationships from shared, age-related co-associations. Determining if it is falling or rising BP in later life that increases risk of WMH, and differential age effects, is important for future prevention of the stroke and dementia consequences of small vessel disease.
Supplementary Material
Novelty and Significance.
What is new?
Blood pressure (BP) and brain vascular damage seen as white matter hyperintensities (WMH) appear to be linked indirectly through a shared co-association with increasing arterial stiffness.
The complete pathophysiological pathway from BP via internal carotid artery (ICA) velocity parameters to WMH has not been studied before.
Narrow age-range sample allows differentiation of direct from indirect BP effects.
What is relevant?
Hypertension increases with age and is a major risk factor for WMH.
Advancing age strongly influences WMH.
Summary.
Increased pulse pressure, secondary to falling diastolic BP, is associated with increased ICA pulsatility index, which in turn is associated with WMH at age 72. Further research is required to determine if methods to reduce arterial stiffness, as well as to reduce BP, prevent WMH formation or progression and their cognitive and physical consequences.
Acknowledgements
The imaging was performed in the Brain Research Imaging Centre, University of Edinburgh (http://www.bric.ed.ac.uk), a SINAPSE Centre. The DICOM to Analyze image format conversion tools used in the analysis were written by Dr. Paul A. Armitage.
Sources of funding
This work was funded by Age UK and the UK Medical Research Council in the Disconnected Mind (http://www.disconnectedmind.ed.ac.uk), The Centre for Cognitive Aging and Cognitive Epidemiology (CCACE; http://www.ccace.ed.ac.uk), The Row Fogo Charitable Trust and the Scottish Founding Council through the SINAPSE collaboration (http://www.sinapse.ac.uk). Funding (for CCACE; G0700704/84698) from BBSRC, EPSRC, ESRC and MRC is gratefully acknowledged.
Footnotes
Conflict of Interest/Disclosure: None.
References
- 1.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O’Brien JT, Barkhof F, Benavente OR, Black S, Brayne C, Breteler M, Chabriat H, DeCarli C, de Leeuw F-E, Doubal F, Duering M, Fox N, Greenberg S, Hachinski V, Kilimann I, Mok V, van Oostengbrugge R, Pantoni L, Speck O, Stephan BC, Teipel S, Viswanathan A, Werring D, Chen C, Smith C, van Buchem M, Norrving B, Gorelick PB, Dichgans M. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol. 2007;50:1–13. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 4.Shrestha I, Takahashi T, Nomura E, Ohtsuki T, Ohshita T, Ueno H, Kohriyama T, Matsumoto M. Association between central systolic blood pressure, white matter lesions in cerebral MRI and carotid atherosclerosis. Hypertens Res. 2009;32:869–874. doi: 10.1038/hr.2009.121. [DOI] [PubMed] [Google Scholar]
- 5.Xu TY, Staessen JA, Wei FF, Xu J, Li FH, Fan WX, Gao PJ, Wang JG, Li Y. Blood flow pattern in the middle cerebral artery in relation to indices of arterial stiffness in the systemic circulation. Am J Hypertens. 2012;25:319–324. doi: 10.1038/ajh.2011.223. [DOI] [PubMed] [Google Scholar]
- 6.Benetos A, Waeber B, Izzo J, Mitchell G, Resnick L, Asmar R, Safar M. Influence of age, risk factors, and cardiovascular and renal disease on arterial stiffness: clinical applications. Am J Hypertens. 2002;15:1101–1108. doi: 10.1016/s0895-7061(02)03029-7. [DOI] [PubMed] [Google Scholar]
- 7.O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005;46:200–204. doi: 10.1161/01.HYP.0000168052.00426.65. [DOI] [PubMed] [Google Scholar]
- 8.Poels MM, Zaccai K, Verwoert GC, Vernooij MW, Hofman A, van der Lugt A, Witteman JC, Breteler MM, Mattace-Raso FU, Ikram MA. Arterial stiffness and cerebral small vessel disease: the Rotterdam Scan Study. Stroke. 2012;43:2637–2642. doi: 10.1161/STROKEAHA.111.642264. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson O, Garcia M, Aspelund T, Harris TB, Gudnason V, Launer LJ. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility--Reykjavik study. Brain. 2011;134:3398–3407. doi: 10.1093/brain/awr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Webb AJ, Simoni M, Mazzucco S, Kuker W, Schulz U, Rothwell PM. Increased cerebral arterial pulsatility in patients with leukoaraiosis: arterial stiffness enhances transmission of aortic pulsatility. Stroke. 2012;43:2631–2636. doi: 10.1161/STROKEAHA.112.655837. [DOI] [PubMed] [Google Scholar]
- 11.Hatanaka R, Obara T, Watabe D, Ishikawa T, Kondo T, Ishikura K, Aikawa T, Aono Y, Hara A, Metoki H, Asayama K, Kikuya M, Mano N, Ohkubo T, Izumi S, Imai Y. Association of arterial stiffness with silent cerebrovascular lesions: the Ohasama study. Cerebrovasc Dis. 2011;31:329–337. doi: 10.1159/000322599. [DOI] [PubMed] [Google Scholar]
- 12.Henskens LH, Kroon AA, van Oostenbrugge RJ, Gronenschild EH, Fuss-Lejeune MM, Hofman PA, Lodder J, de Leeuw PW. Increased aortic pulse wave velocity is associated with silent cerebral small-vessel disease in hypertensive patients. Hypertension. 2008;52:1120–1126. doi: 10.1161/HYPERTENSIONAHA.108.119024. [DOI] [PubMed] [Google Scholar]
- 13.Pase MP, Grima NA, Stough CK, Scholey A, Pipingas A. Cardiovascular disease risk and cerebral blood flow velocity. Stroke. 2012;43:2803–2805. doi: 10.1161/STROKEAHA.112.666727. [DOI] [PubMed] [Google Scholar]
- 14.Wills AK, Lawlor DA, Matthews FE, Sayer AA, Bakra E, Ben-Shlomo Y, Benzeval M, Brunner E, Cooper R, Kivimaki M, Kuh D, Muniz-Terrera G, Hardy R. Life course trajectories of systolic blood pressure using longitudinal data from eight UK cohorts. PLoS Med. 2011;8:e1000440. doi: 10.1371/journal.pmed.1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deary IJ, Gow AJ, Taylor MD, Corley J, Brett C, Wilson V, Campbell H, Whalley LJ, Visscher PM, Porteous DJ, Starr JM. The Lothian Birth Cohort 1936: a study to examine influences on cognitive ageing from age 11 to age 70 and beyond. BMC Geriatr. 2007;7:28. doi: 10.1186/1471-2318-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wardlaw JM, Bastin ME, Valdes Hernandez MC, Maniega SM, Royle NA, Morris Z, Clayden JD, Sandeman EM, Eadie E, Murray C, Starr JM, Deary IJ. Brain aging, cognition in youth and old age and vascular disease in the Lothian Birth Cohort 1936: rationale, design and methodology of the imaging protocol. Int J Stroke. 2011;6:547–559. doi: 10.1111/j.1747-4949.2011.00683.x. [DOI] [PubMed] [Google Scholar]
- 17.Howard SC, Rothwell PM. Reproducibility of measures of visit-to-visit variability in blood pressure after transient ischaemic attack or minor stroke. Cerebrovasc Dis. 2009;28:331–340. doi: 10.1159/000229551. [DOI] [PubMed] [Google Scholar]
- 18.Muntner P, Joyce C, Levitan EB, Holt E, Shimbo D, Webber LS, Oparil S, Re R, Krousel-Wood M. Reproducibility of visit-to-visit variability of blood pressure measured as part of routine clinical care. J Hypertens. 2011;29:2332–2338. doi: 10.1097/HJH.0b013e32834cf213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lagro J, Claassen JA, Rikkert MG. Prognostic significance of blood-pressure variability. Lancet. 2010;376:413–414. doi: 10.1016/S0140-6736(10)61215-9. [DOI] [PubMed] [Google Scholar]
- 20.Alexandrov AV. Section II. Cerebral vessels. In: Zwiebel WJ, Pellerito JS, editors. Introduction to Vascular Ultrasonography. 5th ed. Elsevier Saunders; Philadelphia: 2005. pp. 107–131. [Google Scholar]
- 21.Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J Neurosurg. 1982;57:769–774. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez Mdel C, Ferguson KJ, Chappell FM, Wardlaw JM. New multispectral MRI data fusion technique for white matter lesion segmentation: method and comparison with thresholding in FLAIR images. Eur Radiol. 2010;20:1684–1691. doi: 10.1007/s00330-010-1718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5T in Alzheimer’s dementia and normal aging. AJR Am J Roentgenol. 1987;149:351–356. doi: 10.2214/ajr.149.2.351. [DOI] [PubMed] [Google Scholar]
- 24.McDaniel MA. Big-brained people are smarter: a meta-analysis of the relationship between in vivo brain volume and intelligence. Intelligence. 2005;33:337–346. [Google Scholar]
- 25.Meaume S, Benetos A, Henry OF, Rudnichi A, Safar ME. Aortic pulse wave velocity predicts cardiovascular mortality in subjects >70 years of age. Arterioscler Thromb Vasc Biol. 2001;21:2046–2050. doi: 10.1161/hq1201.100226. [DOI] [PubMed] [Google Scholar]
- 26.Miura K, Soyama Y, Morikawa Y, Nishijo M, Nakanishi Y, Naruse Y, Yoshita K, Kagamimori S, Nakagawa H. Comparison of four blood pressure indexes for the prediction of 10-year stroke risk in middle-aged and older Asians. Hypertension. 2004;44:715–720. doi: 10.1161/01.HYP.0000145108.23948.7b. [DOI] [PubMed] [Google Scholar]
- 27.Nair GV, Chaput LA, Vittinghoff E, Herrington DM. Pulse pressure and cardiovascular events in postmenopausal women with coronary heart disease. Chest. 2005;127:1498–1506. doi: 10.1378/chest.127.5.1498. [DOI] [PubMed] [Google Scholar]
- 28.Nichols WW, O’Rourke MF. Mcdonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. Hodder Arnold Publication; London: 1993. [Google Scholar]
- 29.MacLullich AMJ, Ferguson KJ, Reid LM, Deary IJ, Starr JM, Seckl JR, Bastin ME, Wardlaw JM. Higher systolic blood pressure is associated with increased water diffusivity in normal-appearing white matter. Stroke. 2009;40:3869–3871. doi: 10.1161/STROKEAHA.109.547877. [DOI] [PubMed] [Google Scholar]
- 30.Waldstein SR, Wendell CR, Lefkowitz DM, Siegel EL, Rosenberger WF, Spencer RJ, Manukyan Z, Katzel LI. Interactive relations of blood pressure and age to subclinical cerebrovascular disease. J Hypertens. 2012;30:2352–2356. doi: 10.1097/HJH.0b013e3283595651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verhaaren BF, Vernooij MW, de Boer R, Hofman A, Niessen WJ, van der Lugt A, Ikram MA. High blood pressure and cerebral white matter lesion progression in the general population. Hypertension. 2013;61:1354–1359. doi: 10.1161/HYPERTENSIONAHA.111.00430. [DOI] [PubMed] [Google Scholar]
- 32.Liu W, Liu R, Sun W, Peng Q, Zhang W, Xu E, Cheng Y, Ding M, Li Y, Hong Z, Wu J, Zeng J, Yao C, Huang Y. Different impacts of blood pressure variability on the progression of cerebral microbleeds and white matter lesions. Stroke. 2012;43:2916–2922. doi: 10.1161/STROKEAHA.112.658369. [DOI] [PubMed] [Google Scholar]
- 33.Tsao CW, Seshadri S, Beiser AS, Westwood AJ, DeCarli C, Au R, Himali JJ, Hamburg NM, Vita JA, Levy D, Larson MG, Benjamin EJ, Wolf PA, Vasan RS, Mitchell GF. Relations of arterial stiffness and endothelial function to brain aging in the community. Neurology. 2013;81:984–991. doi: 10.1212/WNL.0b013e3182a43e1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brisset M, Boutouyrie P, Pico F, Zhu Y, Zureik M, Schilling S, Dufouil C, Mazoyer B, Laurent S, Tzourio C, Debette S. Large-vessel correlates of cerebral small-vessel disease. Neurology. 2013;80:662–669. doi: 10.1212/WNL.0b013e318281ccc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.King KS, Chen KX, Hulsey KM, McColl RW, Weiner MF, Nakonezny PA, Peshock RM. White matter hyperintensities: use of aortic arch pulse wave velocity to predict volume independent of other cardiovascular risk factors. Radiology. 2013;267:709–717. doi: 10.1148/radiol.13121598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosano C, Watson N, Chang Y, Newman AB, Aizenstein HJ, Du Y, Venkatraman V, Harris TB, Barinas-Mitchell E, Sutton-Tyrrell K. Aortic pulse wave velocity predicts focal white matter hyperintensities in a biracial cohort of older adults. Hypertension. 2013;61:160–165. doi: 10.1161/HYPERTENSIONAHA.112.198069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mok V, Wong KK, Xiong Y, Wong A, Schmidt R, Chu W, Hu X, Leung EY, Chen S, Chen Y, Tang WK, Chen X, Ho CL, Wong KS, Wong ST. Cortical and frontal atrophy are associated with cognitive impairment in age-related confluent white-matter lesion. J Neurol Neurosurg Psychiatry. 2011;82:52–57. doi: 10.1136/jnnp.2009.201665. [DOI] [PubMed] [Google Scholar]
- 38.Brickman AM, Reitz C, Luchsinger JA, Manly JJ, Schupf N, Muraskin J, DeCarli C, Brown TR, Mayeux R. Long-term blood pressure fluctuation and cerebrovascular disease in an elderly cohort. Arch Neurol. 2010;67:564–569. doi: 10.1001/archneurol.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Havlik RJ, Foley DJ, Sayer B, Masaki K, White L, Launer LJ. Variability in midlife systolic blood pressure is related to late-life brain white matter lesions: the Honolulu-Asia Aging study. Stroke. 2002;33:26–30. doi: 10.1161/hs0102.101890. [DOI] [PubMed] [Google Scholar]
- 40.Sabayan B, Wijsman LW, Foster-Dingley JC, Stott DJ, Ford I, Buckley BM, Sattar N, Jukema JW, van Osch MJ, van der Grond J, van Buchem MA, Westendorp RG, de Craen AJ, Mooijaart SP. Association of visit-to-visit variability in blood pressure with cognitive function in old age: prospective cohort study. BMJ. 2013;347:f4600. doi: 10.1136/bmj.f4600. [DOI] [PubMed] [Google Scholar]
- 41.Dufouil C, Chalmers J, Coskun O, Besancon V, Bousser MG, Guillon P, MacMahon S, Mazoyer B, Neal B, Woodward M, Tzourio-Mazoyer N, Tzourio C. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Substudy. Circulation. 2005;112:1644–1650. doi: 10.1161/CIRCULATIONAHA.104.501163. [DOI] [PubMed] [Google Scholar]
- 42.Weber R, Weimar C, Blatchford J, Hermansson K, Wanke I, Moller-Hartmann C, Gizewski ER, Forsting M, Demchuck AM, Sacco RL, Saver JL, Warach S, Diener HC, Diehl A, PRoFESS Imaging Substudy Group. Telmisartan on top of antihypertensive treatment does not prevent progression of cerebral white matter lesions in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) MRI substudy. Stroke. 2012;43:2336–2342. doi: 10.1161/STROKEAHA.111.648576. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.