Abstract
Nicotinic acetylcholine receptors (nAChRs) that contain α6 and β4 subunits have been demonstrated functionally in human adrenal chromaffin cells, rat dorsal root ganglion neurons, and on noradrenergic terminals in the hippocampus of adolescent mice. In human adrenal chromaffin cells, α6β4* nAChRs (the asterisk denotes the possible presence of additional subunits) are the predominant subtype whereas in rodents, the predominant nAChR is the α3β4* subtype. Here we present molecular and pharmacological evidence that chromaffin cells from monkey (Macaca mulatta) also express α6β4* receptors. PCR was used to show the presence of transcripts for α6 and β4 subunits and pharmacological characterization was performed using patch-clamp electrophysiology in combination with α-conotoxins that target the α6β4* subtype. Acetylcholine-evoked currents were sensitive to inhibition by BuIA[T5A,P6O] and MII[H9A,L15A]; α-conotoxins that inhibit α6-containing nAChRs. Two additional agonists were used to probe for the expression of α7 and β2-containing nAChRs. Cells with currents evoked by acetylcholine were relatively unresponsive to the α7-selctive agonist choline but responded to the agonist 5-I-A-85380. These studies provide further insights into the properties of natively expressed α6β4* nAChRs.
Introduction
Nicotinic acetylcholine receptors (nAChRs) are ligand-gated ion channels that are ubiquitously expressed throughout the central and peripheral nervous systems as well as in non neuronal tissues. These channels are composed of five individual subunits that assemble around a central pore and open to allow the passage of ions across the plasma membrane when activated by an agonist [1]. There are seventeen nAChR genes that code for the different nAChR subunits and include ten α, four β, and one δ, ε, and γ subunits; receptors composed of α1β1δε/γ have only been found at the neuromuscular junction. These subunits combine in various combinations to form different receptor subtypes each having unique biophysical properties including permeability to calcium and sensitivity to ligands. Many of these receptor subtypes have been implicated in human conditions. For example, receptors containing α6 subunits are involved in disorders of the central nervous system including Parkinson's disease [2], [3], nicotine reward and withdrawal [4] and self-administration [5], [6].
There are two main α6-containing subtypes that generally contain β4 or β2 subunits and form the α6β4* and α6β2* subtypes, respectively. The α6β2* subtype is thought to be primarily expressed in the central nervous system while α6β4* receptors are primarily found in cells of the peripheral nervous system including rat dorsal root ganglion (DRG) neurons and human adrenal chromaffin cells but have also been found in mouse hippocampus [7], [8], [9]. Neither of these subtypes is widely expressed and consequently less is known about their biophysical properties and functional regulation than for other nAChR subtypes. Investigational studies of α6-containing receptors have been limited in part because they have proved difficult to express in heterologous systems [10], [11]. Nevertheless, functional expression in oocytes and some mammalian cell lines has been accomplished through the construction of subunit chimeras and concatamers [12], [13], [14], [15].
In the central nervous system, mRNA for the α6 subunit has been found in catecholaminergic nuclei of the rat brain [16], particularly in midbrain dopaminergic neurons [17], [18], and in the chick retina [19]. Functional studies have shown that the release of dopamine from dopaminergic terminals of rat [20], [21] and mouse striatal neurons [22], [23] is sensitive to α-Ctx MII, an α6- and α3-containing nAChR inhibitor. Subsequent studies using α6-selective α-Ctxs identified the α6β2* subtype as the nAChR responsible for modulating striatal dopamine release [24]. Additionally, in the ventral tegmental area α6-containing nAChRs have been shown to modulate the activity of dopaminergic neurons in the presence of ethanol or nicotine [25], [26] and α6β2* receptors have been shown to modulate the release of GABA from rat presynaptic GABAergic boutons [27]. In the peripheral nervous system, α6-containing nAChRs have been shown by electrophysiological and pharmacological analysis of recordings from rat DRG neurons [9] and human adrenal chromaffin cells [7] to contain the β4 subunit. In the present study we pharmacologically evaluated acetylcholine-evoked currents from monkey adrenal chromaffin cells and demonstrate that these catacholaminergic cells also express α6β4* nAChRs. This is the first report, to our knowledge, of electrophysiological recordings of nAChR-mediated currents from primary adrenal chromaffin cells isolated from M. mulatta.
Materials and Methods
Reagents and cRNA constructs
Stock solutions of all agonists and antagonist were made in water. ACh, choline, amphotericin-B, and N-(2-hydroxyethyl)piperazine-N’-(2-ethanesulfonic acid) (HEPES) were purchased from Sigma Aldrich (Madrid, Spain) and 5-I-A-85380 was purchased from Tocris Bioscience (Bristol, UK). The α-Ctxs were synthesized as previously described [28]. The human α3, α4, β2, β3, and β4 subunit clones were provided by J. Garrett (Cognetix Inc., Salt Lake City, UT, USA) and the α6/α3 chimera was provided by J. Lindstrom (University of Pennsylvania, Philadelphia, PA, USA) and subsequently subcloned into the pSGEM expression vector by Dr. Layla Azam (University of Utah, Salt Lake City, UT, USA). Construction of the α6/α3 subunit chimera has been previously described and consists of an α3 subunit where the first 207 amino acids of the ligand-binding domain were replaced with the corresponding α6 amino acids [10]. This chimera was used because injection of non-chimeric α6 with β2 results in few, if any, functional receptors [10], [13], [29]. However, injection of β2 and β3 cRNA in conjunction with the α6/α3 chimera produces sufficient numbers of receptors for electrophysiological recordings.
Ethics statement
Experimental procedures for obtaining monkey and mice adrenal glands were approved by the Committee for Research Ethics of the Universidad Autónoma de Madrid (registry # ES-280790000097) and conducted under the supervision of the Head of Animal Welfare and Health in accordance with Spanish and European guidelines (Boletín Oficial del Estado of March 18, 1988 and the 86/609/EEC European Council Directive). Monkey adrenal gland mRNA was purchased from Zyagen (San Diego, CA, USA). Oocytes were obtained from Xenopus laevis frogs. Procedures (protocol # 11-09008) for the care and use of Xenopus were approved by the University of Utah Institutional Animal Care and Use Committee.
Generation of single-stranded cDNA
Total mRNA (DNAse treated) isolated from M. mulatta adrenal gland was used to generate cDNA using the High Capacity cDNA Reverse Transcription Kit (cat. # 4368814, Ambion, Austin, TX, USA) following the manufacturer's instructions. Briefly, a reaction volume of 25 μl was used and contained 1.25 μg of mRNA. The cycling conditions for the reverse-transcription were as follows: step 1, 25°C for 10 min; step 2, 37°C for 120 min; and step 3, 85°C for 5 min and were achieved using a PTC-200 peltier thermal cycler (MJ Research, Waltham, MA, USA).
Analysis of nAChRs subunits using subunit-specific primers
Reverse-transcription polymerase chain reaction (PCR) analysis was performed using the Qiagen Taq PCR Master Mix Kit (Qiagen, Germantown, MD, USA) following the manufacturer's instructions. Briefly, 2.5 μl of cDNA template were added to the Master Mix solution containing sense and antisense primers (final concentration of 500 nM for each primer) for a total reaction volume of 50 μl. Negative controls for each reaction were performed by omission of the cDNA template and genomic DNA contamination was assessed by performing the reaction in the absence of the transcriptase. Primer sets for each nAChR subunit have been previously described and were designed to target Homo sapien sequences [30], [31], [32], [33]. Each primer pair was aligned with their respective nicotinic subunit target sequence to verify compatibility with M. mulatta sequences. Full length sequences for M. mulatta CHRNA3 and CHRNB4 were not available in NCBI and thus, a sequence comparison for these mRNAs was performed using the sequences of Papio anubis. The antisense primers for CHRNA10 and CHRNB3 were found to be mismatched and were corrected to the corresponding nucleotide found in the M. mulatta sequence. All primers were synthesized by the University of Utah Sequencing and Genomics Core Facility at the University of Utah (Salt Lake City, UT, USA). The PCR was performed in a PTC-200 thermal cycler using an initial 5 min denaturation step at 95°C followed by 35 cycles of denaturation at 94°C for 30 s, 55–60°C for 30 s for annealing, 72°C for 45 s for extension, with a final extension step at 72°C for 10 min. Following PCR, the reactions were analyzed by gel (1.5% agarose wt/vol) electrophoresis, stained and visualized with ethidum bromide.
Verification of the amplification products
To verify amplification of the correct sequence of interest, the bands were cut out from the gel, solubilized, and the products purified using a QIAquick PCR Purification Kit (cat. # 21804, Qiagen, Germantown, MD, USA) following the manufacture's instructions. Sequencing of the products was performed by the University of Utah Sequencing and Genomics Core Facility. The sequences were then subjected to BLAST analysis using NCBIs nucleotide BLAST program.
Two-electrode voltage-clamp electrophysiology of Xenopus laevis oocytes
Detailed methods for conducting electrophysiological experiments of nAChRs heterologously expressed in Xenopus oocytes have been previously described [34]. Briefly, stage IV-V oocytes were injected at a 1∶1 ratio with cRNA encoding cloned human nAChR subunits α3, α4, α6/α3, β2, β3, and β4 and used 1–5 days after injection. The oocyte membranes were clamped at a holding potential of −70 mV and continuously gravity perfused with standard ND96 solution buffered with HEPES and stimulated with 1-sec pulses of 300 μM ACh or 100 μM 5-I-A-85380 once every min. The solution changes were controlled through a series of 3-way solenoid valves interfaced with a personal computer via a CoolDrive valve driver (Neptune Research & Development, West Caldwell, NJ, USA) and LabVIEW software (National Instruments, Austin, TX, USA). The agonist-gated currents were acquired using an Oocyte OC-725 series voltage-clamp amplifier (Warner Instruments, Hamden, CT, USA), filtered through a 5 Hz low-pass Bessel filter (model F1B1; Frequency Devices, Ottawa, IL, USA), and sampled at 50 Hz using a National Instruments USB-6009 digital to analog converter. The toxins were suspended in ND96 and either perfusion applied (for concentrations ≤1 μM) or applied in a static bath for 5 min (for concentrations ≥10 μM).
Cell culture
Monkey chromaffin cells were obtained from two adrenal glands of a nine years old male M. mulatta. The isolation and culture of monkey adrenal chromaffin cells was performed as previously described for human chromaffin cells [7]. Chromaffin cells were isolated from adrenal glands obtained from 1–2 month old male C57BLK6/J mice according to previously established procedures [35]. Electrophysiology experiments were started 48 h after plating, to allow recovery of the nicotinic receptor expression after collagenase treatment [36], and were continued for 5 days ex vivo. The inherent difficulties of obtaining M. mulatta glands limited the number of experiments that could be performed.
Electrophysiological recordings from adrenal chromaffin cells
Electrophysiology recordings were performed in the perforated-patch configuration using the patch-clamp technique. The external solution used to record nicotinic currents was (in mM): 2 CaCl2, 145 NaCl, 5.5 KCl, 1 MgCl2, 10 HEPES, and 10 D-glucose; the pH was adjusted to 7.4 with NaOH. The intracellular solution composition was (in mM): 145 K-glutamate, 8 NaCl, 1 MgCl2, 10 HEPES, and 0.5 mg/ml amphotericin B; the pH was adjusted to 7.2 with KOH. An amphotericin B stock solution was prepared daily at a concentration of 50 mg/ml in dimethylsulphoxide and kept protected from light. The final concentration of amphotericin B was prepared by sonicating 10 μl of stock amphotericin B in 1 ml of internal solution. Pipettes were dipped in amphotericin-free intracellular solution for several seconds and then back-filled with freshly mixed intracellular solution containing amphotericin. Patch pipettes were pulled from borosilicate glass capillary tubes, partially coated with wax then heat polished and had resistances of 2–3 MΩ when filled with the internal solution. After seal formation and perforation, only recordings in which the access resistance of the pipette and the leak current were ≤20 MΩ and ≤20 pA, respectively, were accepted for analysis; series resistance was compensated up to 80%. A HEKA EPC10 amplifier (HEKA Elektronic, Lambrecht, Germany) was used to record agonist-evoked currents. The recordings were acquired at holding potential of −80 mV and filtered through a 4-pole Bessel filter at 2.9 kHz. The sampling frequency used was 10 kHz.
The perfusion system for drug application consisted of a multi-barreled polyethylene pipette positioned close to the cell under study. The agonist used were ACh (300 μM), choline (10 mM), and 5-I-A-85380 (100 μM) and always delivered from separate tubes. The cells were stimulated with 200 ms pulses of agonists once every three minutes. The antagonists were continuously perfused between pulses and this flow was only interrupted during agonist perfusion.
Data analysis
Data analysis of electrophysiological recordings from monkey and mouse chromaffin cells was performed using using IGOR Pro software (Wavemetrics, Lake Oswego, OR, USA) and GraphPad Prism software (La Jolla, CA, USA). Concentration-response analyses of ACh-gated currents from X. laevis oocytes and all other statistical analyses were performed using GraphPad Prism. Concentration-response curves for inhibition of ACh-gated currents were generated by fitting the data to the Hill equation: % response = 100/(1 +([toxin]/IC50)nH). The confidence intervals for the α-Ctx IC50 values are given in parenthesis. All statistical analyses of current amplitudes and densities are shown as the mean ± S.E.M. unless otherwise specified. Each data set was assessed for Gaussian distribution and only those that passed normality tests (α = 0.05) were analyzed for significance by comparing the percent block by the toxins to a theoretical value of 100%, i.e. control values, using a one sample t-test. Data sets that did not pass tests for normality were subjected to a non parametric alternative where indicated.
Results
The nAChR agonists ACh and 5-I-A-85380 but not choline, evoke currents in monkey chromaffin cells
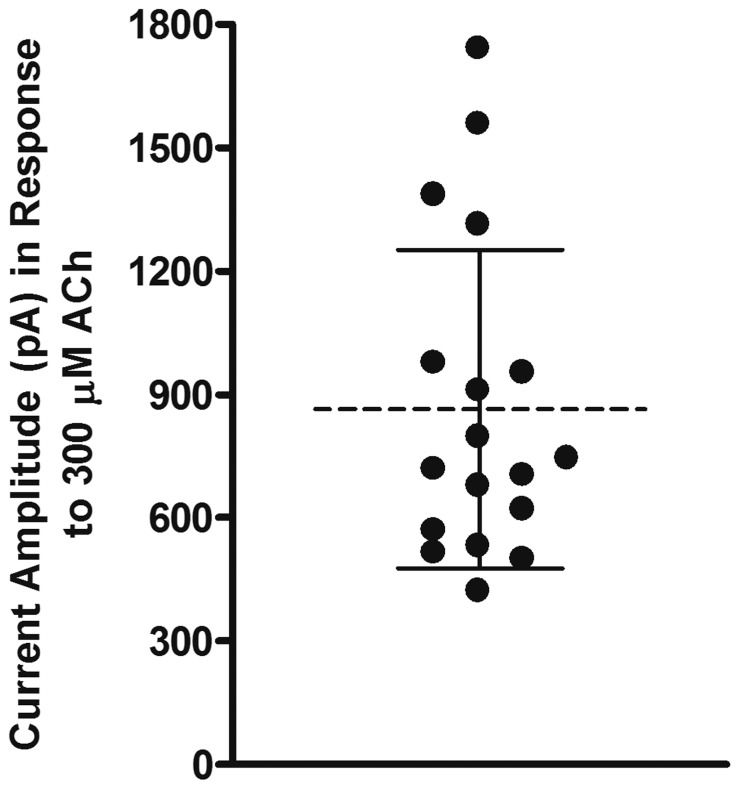
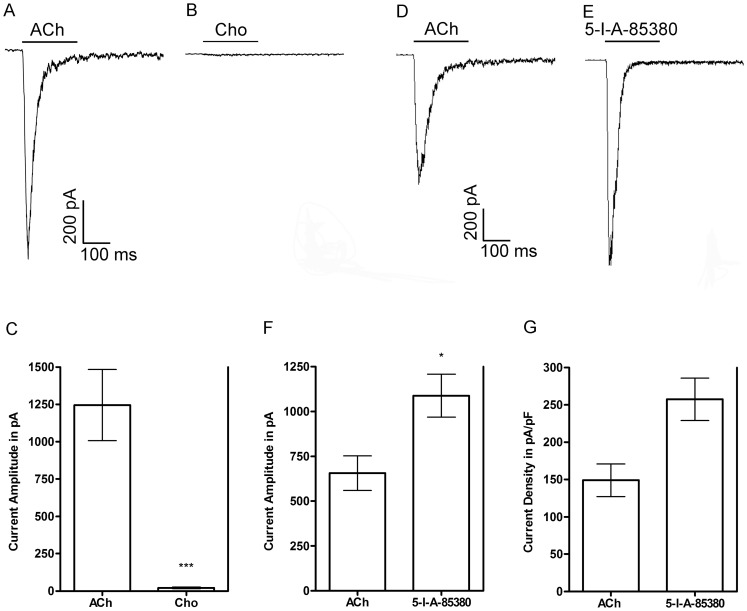
We began our investigation of isolated cells obtained from the adrenal glands of M. mulatta monkey by testing three different nAChR agonists, ACh, choline, and 5-I-A-85380. Choline is a full agonist of heterologously expressed M. mulatta α7 nAChRs [37] and is considered to be α7-selective and can be used to assay for the presence of α7 receptors when multiple nAChR subtypes are potentially expressed in a given cell. The compound 5-I-A-85380 has high affinity for heteromeric receptor subtypes that contain the β2 subunit [38] and was chosen with the expectation that receptors that contain this subunit would be preferentially activated. Acetylcholine (300 μM) evoked currents with average peak amplitudes of 865 ± 387 pA (n = 18;± = S.D.M; Fig. 1). In four of these cells, 10 mM choline evoked currents with amplitudes that were, on average, only 1.7±0.1% of the responses evoked by 300 μM ACh or 21±4 pA versus 1245±238 pA, respectively, in the same cells (Fig. 2A-C). The insensitivity to choline indicated that the ACh-evoked responses were mediated by a heteromeric nAChR subtype and that there were few nAChRs of the α7 subtype. Next we determined if the cells were responsive to the agonist 5-I-A-85380. As shown in Fig. 2D-F, 100 μM 5-I-A-85380 evoked currents with amplitudes that were, on average, 170±16% larger than the currents evoked by ACh or 1088±120 pA versus 657± 97 pA, respectively (n = 4). The current densities were also larger for those evoked by 5-I-A-85380 compared to those evoked by ACh or 256±28 pA/pF versus 149±22 pA/pF, respectively (Fig. 2G; n = 4). This difference was not statistically significant indicating that the effect was not due to differences in cell size. These results suggest that receptors containing the β2 subunit may also be present but at high concentrations of 5-I-A-85380 receptors containing the β4 subunit could potentially be activated [39]. The activation by both ACh and 5-I-A-85380, but not choline, suggested that the dominant nAChR subtype(s) expressed in monkey adrenal chromaffin cells contained either β2 and/or β4 subunits. However, based on the pharmacological profile of the receptors found in human and rodents, we hypothesized that these receptors were likely to be α3β4* or α6β4* nAChRs.
Figure 1. Acetylcholine evokes currents in monkey adrenal chromaffin cells.
Scatter plot showing the current amplitudes evoked by 300 μM ACh. The dashed line denotes the mean and the error bars denote the S.D.M; n = 18.
Figure 2. Representative trace recordings of agonist-evoked currents from monkey adrenal chromaffin cells.
A and B, Current traces of ACh- and choline-evoked currents from a single cell. C, Quantitative comparison of the current amplitudes evoked by each respective agonist. Responses to 10 mM choline were compared to the average responses evoked by 300 μM ACh in the same cells; the error bars denote the S.E.M. (n = 4) and the asterisks denote statistical significance (***p<0.0001) as determined by a one sample t-test. D and E, Current traces of ACh- and 5-I-A-85380-evoked currents from a single cell. F, Quantitative comparison of the current amplitudes evoked by each respective agonist. Responses to 100 μM 5-I-A-85380 were compared to the average responses evoked by 300 μM ACh in the same cells; the error bars denote the S.E.M. (n = 4) and asterisk denote statistical significance (*p<0.05) as determined by a one sample t-test. G, The current amplitudes shown in F were normalized to membrane capacitance and shown as an expression of current density in pA/pF. The differences between the current densities produced by the two agonists were not statistically significant as determined by a Wilcoxon signed rank test (p = 0.0625).
PCR analysis of monkey adrenal gland mRNA demonstrates the presence of multiple nAChR subunit transcripts
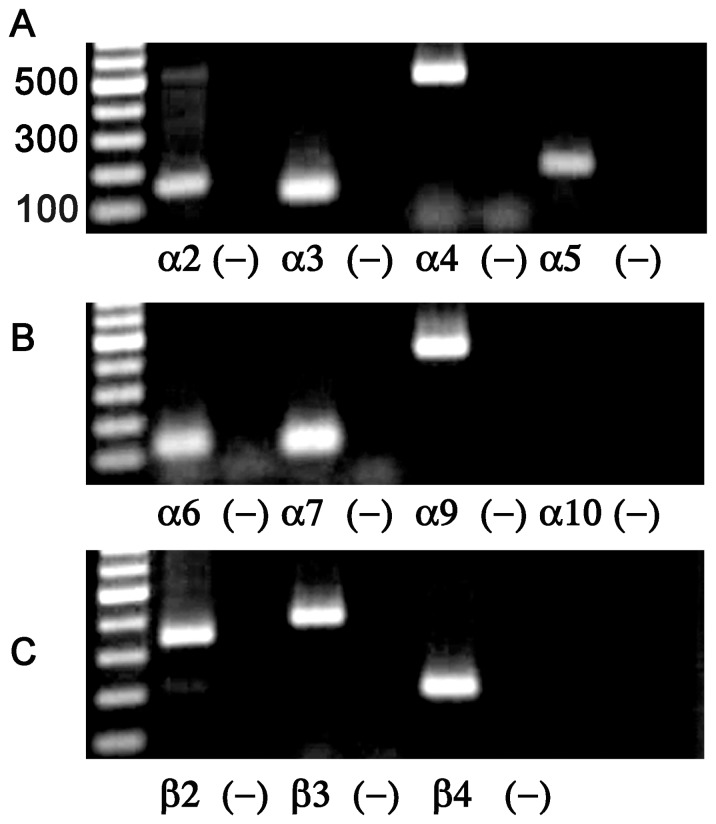
nAChRs vary significantly in terms of the number of possible subtypes that a given cell may express. In rodent chromaffin cells, the predominant nAChR is the α3β4* subtype [7], [40] but in contrast, in human the predominant subtype is an α6β4* nAChR [7]. Thus, in order to guide the pharmacological analysis of the nAChR currents present in monkey chromaffin cells, we performed PCR analysis of total mRNA obtained from M. mulatta adrenal gland. The primers used to assay for the various nAChR subunit transcripts are provided in Table 1. As shown in Fig. 3A-C, transcripts for α6, α3, β2 and β4 were detected as well as transcripts for other nAChR subunits. Reactions performed in the absence of cDNA template were always negative (Fig. 3A-C).
Table 1. PCR primers.
| Target | Primer Sequence | Size | Temp. | References |
| α2 | 5′-GTGGAGGAGGAGGACAGA-3′ (s) | 155 bp | 58 °C | [31] |
| 5′-CTTCTGCATGTGGGGTGATA-3′ (as) | ||||
| α3 | 5′-CAAGCAACGAGGGCAACG-3′ (s) | 121 bp | 60 °C | [32] |
| 5′-CCGTCCTGGCAGGGGTAG-3′ (as) | ||||
| α4 | 5′-GTTCCATGACGGGCGGGTGCAGTGGACT-3′ (s) | 482 bp | 60 °C | [32] |
| 5′-GGGATGACCAGTGAGGTGGACGGGATGAT-3′ (as) | ||||
| α5 | 5′-TTTCTTCACACGCTTCCCAAA-3′ (s) | 179 bp | 60 °C | [32] |
| 5′-TCACGGACATCATTTTCCTTCA-3′ (as) | ||||
| α6 | 5′-TCCATCGTGGTGACTGTGT-3′ (s) | 125 bp | 58 °C | [31] |
| 5′-AGGCCACCTCATCAGCAG-3′ (as) | ||||
| α7 | 5′-GGGAACCTGCTGTACATCGGC-3′ (s) | 115 bp | 60 °C | [32] |
| 5′-GGTGCTCATCGTGCGTGGG-3′ (as) | ||||
| α9 | 5′-GTCCAGGGTCTTGTTTGT-3′ (s) | 403 bp | 55 °C | [33] |
| 5′-ATCCGCTCTTGCTATGAT-3′ (as) | ||||
| α10 | 5′-CTGTTCCGTGACCTCTTCG-3′ (s) | 388 bp | 60 °C | Present Work |
| 5′-GAAGGCCGCCACGTCCA-3′ (as) | ||||
| β2 | 5′-CAGCTCATCAGTGTGCA-3′ (s) | 347 bp | 55 °C | [33] |
| 5′-GTGCGGTCGTAGGTCCA-3′ (as) | ||||
| β3 | 5′-TGGGGAGTACCTGCTGTTCA-3′ (s) | 385 bp | 58 °C | [30] Present Work |
| 5′-CGGCATAATTGGGAATACCA-3′ (as) | ||||
| β4 | 5′-AGCAAGTCATGCGTGACCAAG-3′ (s) | 210 bp | 60 °C | [32] |
| 5′-GCTGACACCTTCTAATGCCTCC-3′ (as) |
Figure 3. PCR analysis of the nAChR subunit transcripts present in monkey adrenal gland.
A-C, PCR products for the nAChR subunits α2–α7, α9, α10, and β2–β4 were analyzed by agarose gel electrophoresis and visualized using ethidum bromide fluorescence. Negative controls for each reaction were performed by omission of the cDNA template and the results are shown in the lanes immediately to the right for each respective subunit.
α-Contoxins MII[H9A,L15A] and BuIA[T5A,P6O] distinguish among nAChR subtypes
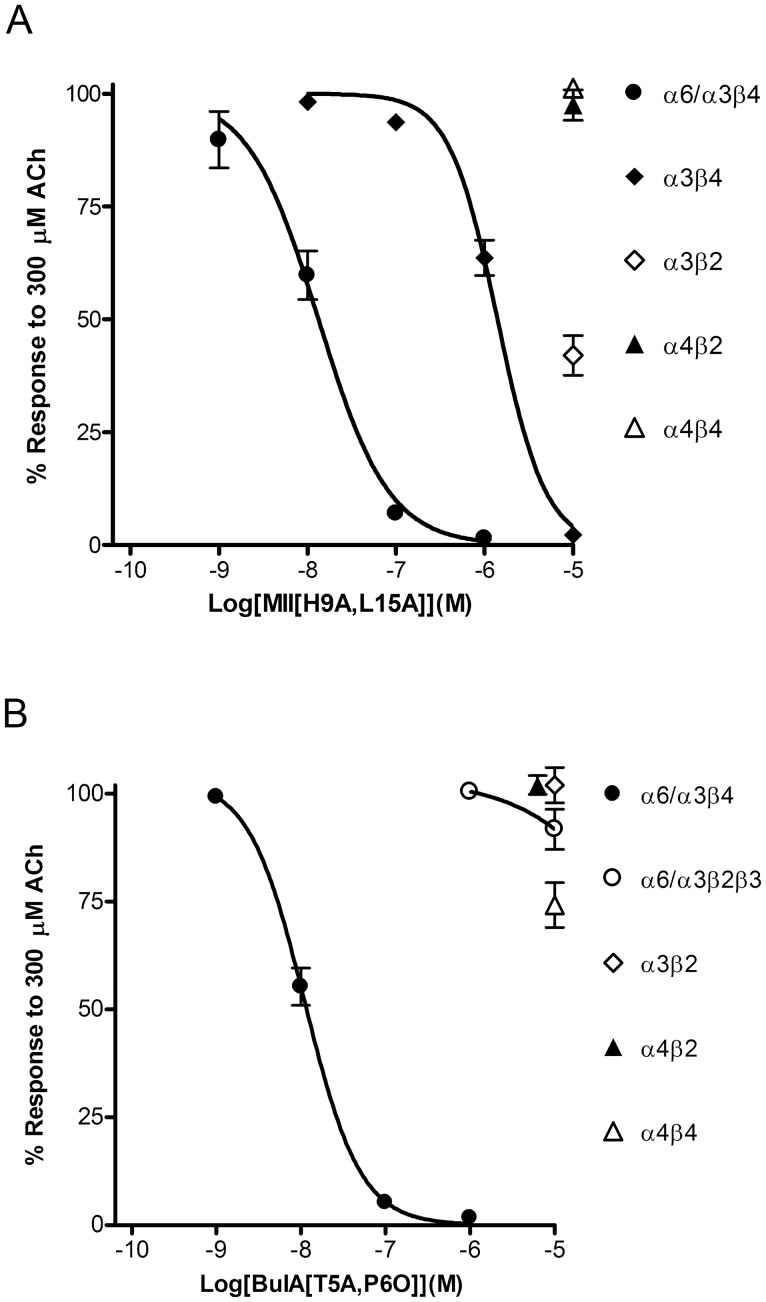
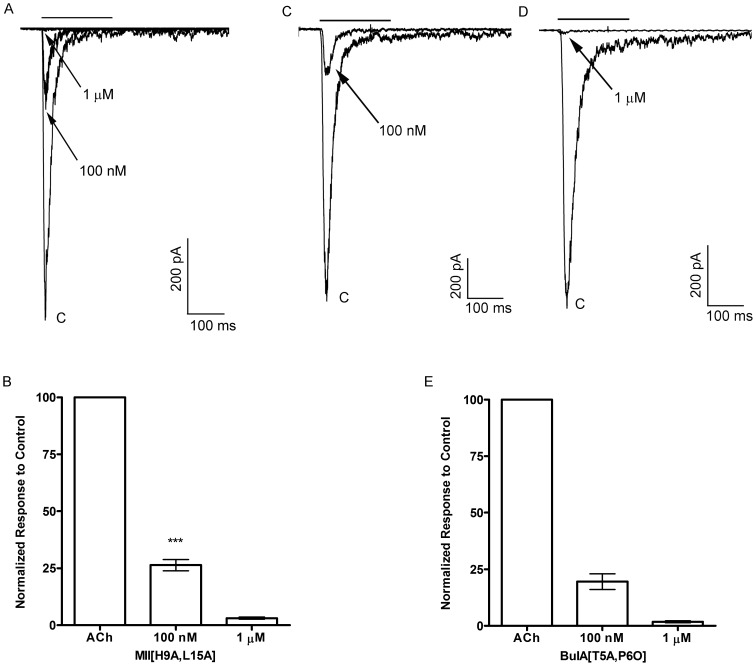
Some α-Ctxs and their synthetic analogs show a remarkable ability to distinguish among the various nAChR subtypes. These small peptides are derived from the venom of marine cone snails that use them to capture their prey. Two of these peptides, α-Ctxs MII [28] and BuIA [41], are widely used to identify native receptors. Synthetic analogs of these α-Ctxs have been developed with increased specificity for α6-containing over α3-containing nAChRs. An analog of MII, MII[H9A,L15A], distinguishes between rat α6/α3β4 and α3β4, being more potent on the α6/α3β4 subtype [42], and BuIA[T5A,P6O], an analog of BuIA, distinguishes between rat and mouse α6/α3β4 and α6/α3β2β3 receptors, being more potent on the α6/α3β4 subtype [8]. Since the activity of these α-Ctx analogs on primate receptors had not been previously quantified, we tested them on heterologously expressed human receptors in X. laevis oocytes and determined their IC50 values. MII[H9A,L15A] inhibited ACh-evoked responses mediated by α6/α3β4 receptors with an IC50 value of 13.3 (9.7–18.1) nM (Fig. 4A). This value was 100-fold lower than the 1.4 (1.1–1.7) μM value obtained for α3β4 nAChRs (Fig. 4B). Next we tested BuIA[T5A,P6O] on the α6/α3β4 and α6/α3β2β3 subtypes. This BuIA analog was >900-fold more potent at inhibiting α6/α3β4 versus α6/α3β2β3 nAChRs (IC50 values of 11.1 (9.1–13.6) nM versus >10 μM, respectively). Thus, MII[H9A,L15A] can be used to distinguish between α6β4* and α3β4* nAChRs and BuIA[T5A,P6O] can be used to distinguish between α6β4* and α6β2* nAChRs. We also tested MII[H9A,L15A] and BuIA[T5A,P6O] on non α6-containing human nAChR subtypes including α3β2, α4β2, α4β4 and found that both toxins showed very limited activity on these receptors subtypes (Fig. 4A,B).
Figure 4. Concentration-response analysis of the inhibition of cloned human nAChRs expressed in X. laevis oocytes by α-Ctxs MII[H9A,L15A] and BuIA[T5A,P6O].
Oocytes expressing the indicated nAChRs were subjected to TEVC as described in “Materials and Methods” and the IC50 values for inhibition of the responses to ACh by each α-Ctx analog determined by fitting the data to the Hill equation. A, MII[H9A,L15A] inhibited α6/α3β4 with an IC50 value of 13.3 (9.7–18.1) nM (n = 4) and α3β4 with an IC50 value of 1.4 (1.1–1.7) μM (n = 4). Compared to controls, the responses after a 5 min exposure to 10 μM toxin were 42±4% (n = 7) for α3β2, 93±5% (n = 7) for α4β2, and 101±1% (n = 4) for α4β4 receptors. B, BuIA[T5A,P6O] inhibited α6/α3β4 with an IC50 value of 11.1 (9.1–13.6) nM and α6/α3β2β3 with an IC50 value >10 μM. The responses after a 5 min exposure to 10 μM toxin were 102±4% (n = 4) for α3β2, 102±2% (n = 4) for α4β2, and 74±5% (n = 5) for α4β4 receptors. For clarity, the symbols for inhibition of α3β2 and α4β2 receptors are shown staggered to avoid overlap.
α-Ctxs MII[H9A,L15A] and BuIA[T5A,P6O] potently inhibit ACh-evoked currents in monkey chromaffin cells
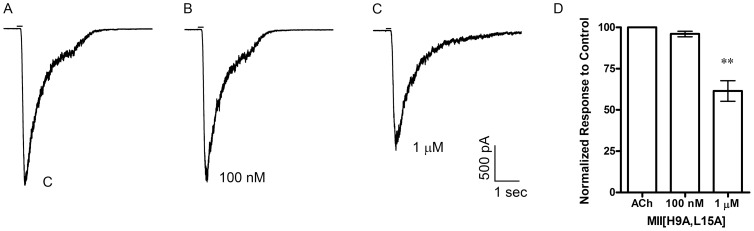
To pharmacologically determine the nAChR subtype mediating the ACh-evoked currents in monkey chromaffin cells, we started by using MII[H9A,L15A] at 100 nM, a concentration where little, if any, inhibition of α3β4* receptors would be expected, followed by a concentration of 1 μM. At a concentration of 100 nM, this MII analog inhibited the ACh-evoked responses by 74±2% (n = 5) and at 1 μM the responses were almost completely abolished (97±0.4% inhibition; n = 4) (Fig. 5A,B). Next we tested BuIA[T5A,P6O], also at 100 nM and 1 μM, to selectively inhibit α6β4* versus α6β2* nAChRs. The ACh-evoked responses were inhibited by 81±4% (n = 2) at 100 nM and by 98±0.4% (n = 4) at 1 μM (Fig. 5 C-E). As an additional test of the specificity of MII[H9A,L15A] for the α6β4 subtype over the α3β4 subtype, we tested the toxin on mouse chromaffin cells that have been reported to lack mRNA for the α6 subunit [43] and thus the receptors expressed by these cells are likely to be mainly α3β4* nAChRs. As shown in Fig. 6, in the presence of 100 nM MII[H9A,L15A], the average response to ACh was 96±2% (n = 6) of control responses and in the same cells the responses in the presence of 1 μM were on average 62±6% (n = 6) of controls. These data indicate that the nAChRs expressed in M. mulatta adrenal chromaffin cells are α6β4* nAChRs.
Figure 5. α-Ctx MII[H9A,L15A] and BuIA[T5A,P6O] potently inhibit the ACh-evoked currents in monkey chromaffin cells.
A, Representative trace recordings of ACh-evoked currents and the inhibition by 100 nM and 1 μM MII[H9A,L15A]. B, Quantitative analysis of the inhibition by 100 nM (n = 5) and 1 μM MII[H9A,L15A] (n = 4). Error bars show average values ± S.E.M (n = 5); asterisks denote statistical significance (***p<0.001) as determined by a one sample t-test. C,D, Trace recordings of ACh-evoked currents and the inhibition by 100 nM and 1 μM BuIA[T5A,P6O]. E, Quantitative analysis of the inhibition by 100 nM (n = 2) and 1 μM BuIA[T5A,P6O] (n = 4); error bars show average values ± S.E.M.
Figure 6. α-Ctx MII[H9A,L15A] inhibits ACh-evoked responses less potently in mouse adrenal chromaffin cells.
A, Representative trace recordings of ACh-evoked currents and the inhibition by 100 nM and 1 μM MII[H9A,L15A]. B, Quantitative analysis of the inhibition by 100 nM (n = 6) and 1 μM MII[H9A,L15A] (n = 6). Statistical analysis of the inhibition by 100 nM indicated no statistical significance compared to control responses (p>0.05) but significance for 1 μM (p<**0.01); error bars show average values ± S.E.M.
5-I-A-85380 activates monkey chromaffin cell α6β4* nAChRs
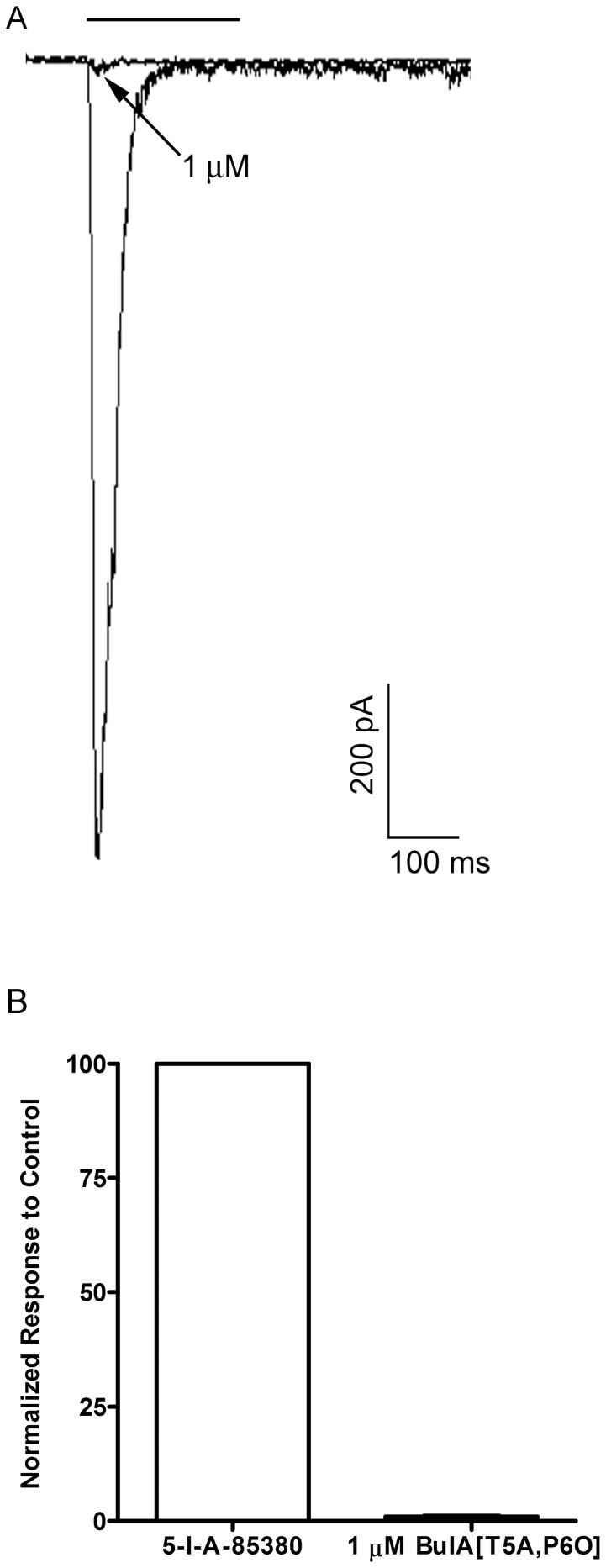
As shown in Fig. 2D-F, the efficacy of 100 μM 5-I-A-85380 in evoking currents was greater than 300 μM ACh in cells that were sequentially stimulated with both agonists. Although 5-I-A-85380 has high affinity for receptors containing the β2 subunit, at 100 μM receptors containing the β4 subunit may also be activated. To determine if 100 μM 5-I-A-85380 could activate α6β4* nAChRs, we tested this compound on heterologously expressed human α6/α3β4 receptors in Xenopus oocytes and found that at 100 μM this compound activated currents that were on average 100±12.1% (n = 4; Fig. S1) of the amplitude produced by 300 μM ACh in the same cells. Additionally, inhibition of 5-I-A-85380-evoked currents in monkey chromaffin cells by BuIA[T5A,P6O] would also suggest that this agonist activates α6β4* nAChRs. Indeed, perfusion with 1 μM BuIA[T5A,P6O] almost completely abolished the 5-I-A-85380-evoked currents (95±0.4% inhibition; n = 4) (Fig. 7A,B). Thus 5-I-A-85380 shows high efficacy in activating the nAChRs in monkey chromaffin cells and the inhibition of these currents by BuIA[T5A,P6O] indicated that they were mediated by the α6β4* subtype and not by nAChRs containing two αxβ2 ligand binding sites.
Figure 7. BuIA[T5A,P6O] inhibits 5-I-A-85380-evoked currents in monkey chromaffin cells.
A, Representative trace recordings of 5-I-A-85380-evoked currents and the inhibition by 1 μM BuIA[T5A,P6O]. B, Quantitative analysis of the inhibition by 1 μM BuIA[T5A,P6O] (n = 4); error bars show average values ± S.E.M.
Discussion
In this work we provide the first report of the molecular and pharmacological characterization of the nAChRs expressed by monkey adrenal chromaffin cells. Initial studies demonstrated that the nAChRs in these cells could be activated by ACh and 5-I-A-85380 but not choline (Fig. 2A-F). These results suggested that the dominant receptor expressed was a heteromeric nAChR subtype. PCR analysis of monkey adrenal gland mRNA demonstrated the presence of transcripts for multiple nAChR subunits including α2–α7, α9, and β2–β4 but not α10 (Fig 3A-C). Subsequent pharmacological analysis of the agonist-evoked currents was performed using α-Ctxs that selectively target the α6/α3β4 receptor over the α6/α3β2β3 and α3β4 subtypes as determined in Xenopus oocytes (Fig. 4A, B). In monkey chromaffin cells, substantial inhibition of the ACh-evoked currents was achieved using a submaximal concentration (100 nM) of MII[H9A,L15A] (Fig. 5A,B), an antagonist that, as shown in Figure 4A, is >100-fold more potent on human α6/α3β4 receptors than α3β4 receptors. Substantial inhibition was also achieved using 100 nM BuIA[T5A,P6O] (Fig. 5C,E) which is >900-fold more potent on α6/α3β4 receptors than α6/α3β2β3 receptors (Fig. 4B). Complete inhibition of the ACh-evoked currents was achieved using a concentration of 1μM MII[H9A,L15A] or BuIA[T5A,P6O] (Fig. 5A,B and 5D,E). In mouse chromaffin cells, that likely only express α3β4*, much less inhibition was observed by MII[H9A,L15A] lending further support to our conclusion that monkey chromaffin cells predominantly express α6β4* nAChRs. Thus, when taken together the molecular and pharmacological evidence presented here support the presence of a nAChR in monkey chromaffin cells that contains α6 and β4 subunits and that the receptor composed of these subunits, the α6β4* subtype, predominates. We note, however, that these experiments do not rule out the possible presence of additional subunits such as α5, β2, or β3, that are known to assemble with the α6 subunit [44], or homomeric α7 nAChRs. Previously in human chromaffin cells, we found that α7 nAChRs contribute only ∼7% to the whole-cell current under electrophysiological conditions similar to those used in the current study and that positive identification was only possible using selective α7 agonists and positive allosteric modulators to prevent desensitization [45]. These compounds may also be useful in future studies to determine if α7 nAChRs are also functionally expressed in monkey chromaffin cells.
We also observed that the nAChR agonist 5-I-A-85380 at 100 μM was more efficacious than 300 μM ACh at activating monkey chromaffin cell nAChRs (Fig 2D-G) and as efficacious as 300 μM ACh when tested on human α6/α3β4 nAChRs expressed in Xenopus oocytes (Fig. S1). This ligand was initially described as an αxβ2-selective ligand but was later shown to also activate other non β2-containing nAChRs [38], [39]. In this report we have shown that 5-I-A-85380 also activates heterologously expressed human α6/α3β4 as well as native monkey α6β4* nAChRs as evidenced by the fact that currents evoked by this compound in monkey cells could be fully inhibited by 1 μM BuIA[T5A,P6O] (Fig. 6A,B). The presence of multiple discrete agonist and/or antagonist binding sites in a single nAChR complex can be challenging to detect in functional assays. There are two putative agonist binding sites in a given αxβx receptor complex and gating of the channel is thought to require the binding of two agonist molecules [1], [46]. Thus, an agonist that shows selectivity for αx-β2 binding sites would not be expected to activate receptors that contain both αx-β4 and αx-β2 binding sites. In contrast, a single antagonist ligand of nAChRs is sufficient for inhibition of agonist-evoked responses therefore, the simplest interpretation of the data presented here is that the nAChRs in monkey chromaffin cells contain at least one α6-β4 ligand binding interface. Nevertheless, future studies using radiolabeled ligands and immunoprecipitation to assay for the presence of additional subunits is warranted.
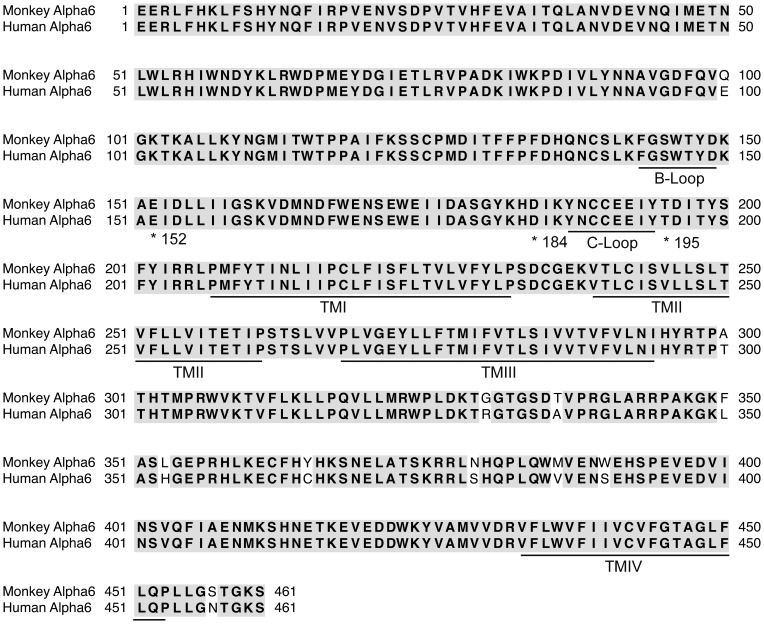
Although the α6 and α3 subunits are highly homologous and receptors composed of these subunits often have similar pharmacological properties with respect to agonist and antagonist sensitivities in some cases large differences can be observed. For example, it was recently reported that some analogs of α-Ctx PeIA show a 15,000-fold difference in the IC50 values for heterologously expressed rat α6/α3β2β3 versus α3β2 nAChRs [47]. This difference was shown to be attributed to three amino acids that differ between the α6 and α3 ligand binding domains. Differences in amino acid composition of the same subunit between species can also affect ligand potencies. A comparative study between human and monkey showed that there are minor differences in the sensitivity of heterologously expressed α7 receptors to agonists but not to antagonists even though the ligand binding domains between the two species differ by only two amino acids [37]. We performed a sequence alignment between monkey and human α6 subunits and found that in the ligand-binding domain there is a single amino acid difference at position 100 (Fig. 8); in monkey this residue is a glutamine whereas in human it is a glutamate. This single amino acid difference is not likely to affect the binding of the α-Ctxs used in this study, however, because the critical residues of the α6 subunits that interact with the α-Ctxs are strictly conserved across monkey, human, and rodent species. These residues, E152, D184, and T195, have been previously identified as the critical residues that confer high affinity binding of MII- and BuIA-related α-Ctxs to heterologously expressed α6-containing nAChRs [47], [48], [49]. Thus, the potencies obtained in this report for heterologously expressed human α6-containing nAChRs are likely to match those of native monkey α6-containing nAChRs. Indeed, the values obtained for inhibition of monkey α6β4* nAChRs by 100 nM and 1 μM MII[H9A,L15A] and BuIA[T5A,P6O] closely match the values obtained for human α6/α3β4 nAChRs in oocytes (Fig. 5A-E, and 4A,B, respectively). We also performed a sequence alignment of other human and monkey nAChR subunits and note that the sequences of the β2 extracellular ligand binding domains differ by a single amino acid at position 165 where there is a conservative aspartate to glutamate difference between monkey and human, respectively (data not shown). Unfortunately, complete sequences for M. mulatta α3 and β4 subunits are not available in NCBI for analysis. However, a comparison between human and baboon (Papio anubis) α3 revealed that the amino acids in the positions important for α-Ctx binding are also shared between these two species.
Figure 8. Sequence alignment of monkey and human α6 nAChR subunits.
A, Sequence alignment of monkey and human α6 subunits identified a single residue in the extracellular ligand-binding domain at position 100 that differed between the two species. The asterisks identify residues that have previously shown to be important for α-Ctx binding [47], [48], [49]. Note that these residues are strictly conserved between the two species. TM indicates the transmembrane domains.
Coexpression of α6 and β4 subunits appears to be limited to a few discrete areas in the nervous system. In rodents, immunoprecipitation studies have demonstrated that α6 and β4 subunits are present in the retina [50] and studies that utilized PCR analysis showed that α6 and β4 subunits may be expressed in neurons of the ventral tegmental area and DRG neurons [9], [27], [51], [52]. In M. mulatta, in situ hybridization studies of brain slices found α6 and β4 coexpression in the lateral part of the medial habenula [53]. Electrophysiological recordings of α6β4* nAChR currents have only been demonstrated in rat DRG neurons [9], in human adrenal chromaffin cells [7], and in M. mulatta chromaffin cells as shown in the present study. In human chromaffin cells, α6β4* nAChRs are the predominant nAChR subtype expressed in contrast to what is found in rodent chromaffin cells where the nAChRs that predominate are α3β4* nAChRs [7], [40]. As in human chromaffin cells, α6β4* nAChRs also appear to dominate in monkey chromaffin cells. Thus, there is a species difference between primates and rodents in terms of nAChR expression by chromaffin cells.
Ligands that target α6β2* nAChRs have been proposed as potential pharmacological agents for the treatment of several human conditions including Parkinson's and nicotine dependence [54], [55]. However, ligands that target α6β2* would need to be devoid of activity on the α6β4* subtype to avoid potential cardiovascular side effects caused by alternations in the release of catecholamines from the adrenal gland. Our study provides valuable insights into the pharmacology of α6β4* nAChRs in catecholaminergic cells and validate chromaffin cells as a particularly promising system for the study of native α6β4* nAChRs.
Supporting Information
The activity of 5-I-A-85380 on α6/α3β4 nAChRs. Human α6/α3β4 nAChRs were expressed in Xenopus laevis oocytes and subjected to two-electrode voltage-clamp electrophysiology as described in “Materials and Methods”. The responses to 100 μM 5-I-A-85380 were normalized to the responses to 300 μM ACh obtained in the same cells. The responses to 5-I-A-85380 were on average 100±12% of those evoked by 300 μM ACh. The error bars denote the S.D.M from four oocytes.
(TIF)
Acknowledgments
The authors thank Dr. Carmen Cavada for the supply of the M. mulatta adrenal glands and Dr. Carmen Fernández and Marta Miró for the extraction of these glands (Universidad Autónoma de Madrid, Madrid, Spain). We also thank Dr. Layla Azam (University of Utah, Salt Lake City, Utah, USA) for generating the α6/α3 pSGEM construct. AHV holds a fellowship award from the Universidad Autónoma de Madrid. AJH holds a Marie Curie International Fellowship from the European Commission.
Funding Statement
This work was supported by grants from the Ministerio de Ciencia e Innovación No. BFU2011-27690 and BFU2012-30997 awarded to AA and by grants from the U.S. National Institutes of Health GM103801 and GM48677 to JMM. AHV holds a fellowship award from the Universidad Autónoma de Madrid. AJH hold a Marie Curie International Fellowship from the European Commission. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Albuquerque EX, Pereira EF, Alkondon M, Rogers SW (2009) Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev 89: 73–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Quik M, Bordia T, Forno L, McIntosh JM (2004) Loss of alpha-conotoxinMII- and A85380-sensitive nicotinic receptors in Parkinson's disease striatum. J Neurochem 88: 668–679. [DOI] [PubMed] [Google Scholar]
- 3. Bordia T, Grady SR, McIntosh JM, Quik M (2007) Nigrostriatal damage preferentially decreases a subpopulation of alpha6beta2* nAChRs in mouse, monkey, and Parkinson's disease striatum. Mol Pharmacol 72: 52–61. [DOI] [PubMed] [Google Scholar]
- 4. Jackson KJ, McIntosh JM, Brunzell DH, Sanjakdar SS, Damaj MI (2009) The role of alpha6-containing nicotinic acetylcholine receptors in nicotine reward and withdrawal. J Pharmacol Exp Ther 331: 547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pons S, Fattore L, Cossu G, Tolu S, Porcu E, et al. (2008) Crucial role of alpha4 and alpha6 nicotinic acetylcholine receptor subunits from ventral tegmental area in systemic nicotine self-administration. J Neurosci 28: 12318–12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brunzell DH, Boschen KE, Hendrick ES, Beardsley PM, McIntosh JM (2010) Alpha-conotoxin MII-sensitive nicotinic acetylcholine receptors in the nucleus accumbens shell regulate progressive ratio responding maintained by nicotine. Neuropsychopharmacology 35: 665–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perez-Alvarez A, Hernandez-Vivanco A, McIntosh JM, Albillos A (2012) Native alpha6beta4* nicotinic receptors control exocytosis in human chromaffin cells of the adrenal gland. FASEB J 26: 346–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Azam L, Maskos U, Changeux JP, Dowell CD, Christensen S, et al. (2010) alpha-Conotoxin BuIA[T5A;P6O]: a novel ligand that discriminates between alpha6beta4 and alpha6beta2 nicotinic acetylcholine receptors and blocks nicotine-stimulated norepinephrine release. FASEB J 24: 5113–5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hone AJ, Meyer EL, McIntyre M, McIntosh JM (2012) Nicotinic acetylcholine receptors in dorsal root ganglion neurons include the alpha6beta4* subtype. FASEB J 26: 917–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kuryatov A, Olale F, Cooper J, Choi C, Lindstrom J (2000) Human alpha6 AChR subtypes: subunit composition, assembly, and pharmacological responses. Neuropharmacology 39: 2570–2590. [DOI] [PubMed] [Google Scholar]
- 11. Gerzanich V, Kuryatov A, Anand R, Lindstrom J (1997) “Orphan” alpha6 nicotinic AChR subunit can form a functional heteromeric acetylcholine receptor. Mol Pharmacol 51: 320–327. [PubMed] [Google Scholar]
- 12. Capelli AM, Castelletti L, Chen YH, Van der Keyl H, Pucci L, et al. (2011) Stable expression and functional characterization of a human nicotinic acetylcholine receptor with alpha6beta2 properties: discovery of selective antagonists. Br J Pharmacol 163: 313–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuryatov A, Lindstrom J (2011) Expression of functional human alpha6beta2beta3* acetylcholine receptors in Xenopus laevis oocytes achieved through subunit chimeras and concatamers. Mol Pharmacol 79: 126–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rasmussen AH, Strobaek D, Dyhring T, Jensen ML, Peters D, et al. (2014) Biophysical and pharmacological characterization of alpha6-containing nicotinic acetylcholine receptors expressed in HEK293 cells. Brain Res 1542: 1–11. [DOI] [PubMed] [Google Scholar]
- 15. Papke RL, Dwoskin LP, Crooks PA, Zheng G, Zhang Z, et al. (2008) Extending the analysis of nicotinic receptor antagonists with the study of alpha6 nicotinic receptor subunit chimeras. Neuropharmacology 54: 1189–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Le Novere N, Zoli M, Changeux JP (1996) Neuronal nicotinic receptor alpha 6 subunit mRNA is selectively concentrated in catecholaminergic nuclei of the rat brain. Eur J Neurosci 8: 2428–2439. [DOI] [PubMed] [Google Scholar]
- 17. Klink R, de Kerchove d'Exaerde A, Zoli M, Changeux JP (2001) Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci 21: 1452–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Azam L, Winzer-Serhan UH, Chen Y, Leslie FM (2002) Expression of neuronal nicotinic acetylcholine receptor subunit mRNAs within midbrain dopamine neurons. J Comp Neurol 444: 260–274. [DOI] [PubMed] [Google Scholar]
- 19. Fucile S, Matter JM, Erkman L, Ragozzino D, Barabino B, et al. (1998) The neuronal alpha6 subunit forms functional heteromeric acetylcholine receptors in human transfected cells. Eur J Neurosci 10: 172–178. [DOI] [PubMed] [Google Scholar]
- 20. Kulak JM, Nguyen TA, Olivera BM, McIntosh JM (1997) Alpha-conotoxin MII blocks nicotine-stimulated dopamine release in rat striatal synaptosomes. J Neurosci 17: 5263–5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kaiser SA, Soliakov L, Harvey SC, Luetje CW, Wonnacott S (1998) Differential inhibition by alpha-conotoxin-MII of the nicotinic stimulation of [3H]dopamine release from rat striatal synaptosomes and slices. J Neurochem 70: 1069–1076. [DOI] [PubMed] [Google Scholar]
- 22. Grady SR, Salminen O, Laverty DC, Whiteaker P, McIntosh JM, et al. (2007) The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem Pharmacol 74: 1235–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, et al. (2004) Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol 65: 1526–1535. [DOI] [PubMed] [Google Scholar]
- 24. Azam L, McIntosh JM (2005) Effect of novel alpha-conotoxins on nicotine-stimulated [3H]dopamine release from rat striatal synaptosomes. J Pharmacol Exp Ther 312: 231–237. [DOI] [PubMed] [Google Scholar]
- 25. Liu L, Zhao-Shea R, McIntosh JM, Tapper AR (2013) Nicotinic acetylcholine receptors containing the alpha6 subunit contribute to ethanol activation of ventral tegmental area dopaminergic neurons. Biochem Pharmacol 86: 1194–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Engle SE, Shih PY, McIntosh JM, Drenan RM (2013) alpha4alpha6beta2* nicotinic acetylcholine receptor activation on ventral tegmental area dopamine neurons is sufficient to stimulate a depolarizing conductance and enhance surface AMPA receptor function. Mol Pharmacol 84: 393–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang K, Hu J, Lucero L, Liu Q, Zheng C, et al. (2009) Distinctive nicotinic acetylcholine receptor functional phenotypes of rat ventral tegmental area dopaminergic neurons. J Physiol 587: 345–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cartier GE, Yoshikami D, Gray WR, Luo S, Olivera BM, et al. (1996) A new alpha-conotoxin which targets alpha3beta2 nicotinic acetylcholine receptors. J Biol Chem 271: 7522–7528. [DOI] [PubMed] [Google Scholar]
- 29. Dash B, Bhakta M, Chang Y, Lukas RJ (2011) Identification of N-terminal extracellular domain determinants in nicotinic acetylcholine receptor (nAChR) alpha6 subunits that influence effects of wild-type or mutant beta3 subunits on function of alpha6beta2*- or alpha6beta4*-nAChR. J Biol Chem 286: 37976–37989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carlisle DL, Hopkins TM, Gaither-Davis A, Silhanek MJ, Luketich JD, et al. (2004) Nicotine signals through muscle-type and neuronal nicotinic acetylcholine receptors in both human bronchial epithelial cells and airway fibroblasts. Respir Res 5: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lips KS, Bruggmann D, Pfeil U, Vollerthun R, Grando SA, et al. (2005) Nicotinic acetylcholine receptors in rat and human placenta. Placenta 26: 735–746. [DOI] [PubMed] [Google Scholar]
- 32. Liu J, McGlinn AM, Fernandes A, Milam AH, Strang CE, et al. (2009) Nicotinic acetylcholine receptor subunits in rhesus monkey retina. Invest Ophthalmol Vis Sci 50: 1408–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. West KA, Brognard J, Clark AS, Linnoila IR, Yang X, et al. (2003) Rapid Akt activation by nicotine and a tobacco carcinogen modulates the phenotype of normal human airway epithelial cells. J Clin Invest 111: 81–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hone AJ, Whiteaker P, Christensen S, Xiao Y, Meyer EL, et al. (2009) A novel fluorescent alpha-conotoxin for the study of alpha7 nicotinic acetylcholine receptors. J Neurochem 111: 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Perez-Alvarez A, Hernandez-Vivanco A, Caba-Gonzalez JC, Albillos A (2011) Different roles attributed to Cav1 channel subtypes in spontaneous action potential firing and fine tuning of exocytosis in mouse chromaffin cells. J Neurochem 116: 105–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Almazan G, Aunis D, Garcia AG, Montiel C, Nicolas GP, et al. (1984) Effects of collagenase on the release of [3H]-noradrenaline from bovine cultured adrenal chromaffin cells. Br J Pharmacol 81: 599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Papke RL, McCormack TJ, Jack BA, Wang D, Bugaj-Gaweda B, et al. (2005) Rhesus monkey alpha7 nicotinic acetylcholine receptors: comparisons to human alpha7 receptors expressed in Xenopus oocytes. Eur J Pharmacol 524: 11–18. [DOI] [PubMed] [Google Scholar]
- 38. Mukhin AG, Gundisch D, Horti AG, Koren AO, Tamagnan G, et al. (2000) 5-Iodo-A-85380, an alpha4beta2 subtype-selective ligand for nicotinic acetylcholine receptors. Mol Pharmacol 57: 642–649. [DOI] [PubMed] [Google Scholar]
- 39. Xiao Y, Meyer EL, Thompson JM, Surin A, Wroblewski J, et al. (1998) Rat alpha3/beta4 subtype of neuronal nicotinic acetylcholine receptor stably expressed in a transfected cell line: pharmacology of ligand binding and function. Mol Pharmacol 54: 322–333. [DOI] [PubMed] [Google Scholar]
- 40. Di Angelantonio S, Matteoni C, Fabbretti E, Nistri A (2003) Molecular biology and electrophysiology of neuronal nicotinic receptors of rat chromaffin cells. Eur J Neurosci 17: 2313–2322. [DOI] [PubMed] [Google Scholar]
- 41. Azam L, Dowell C, Watkins M, Stitzel JA, Olivera BM, et al. (2005) Alpha-conotoxin BuIA, a novel peptide from Conus bullatus, distinguishes among neuronal nicotinic acetylcholine receptors. J Biol Chem 280: 80–87. [DOI] [PubMed] [Google Scholar]
- 42. McIntosh JM, Azam L, Staheli S, Dowell C, Lindstrom JM, et al. (2004) Analogs of alpha-conotoxin MII are selective for alpha6-containing nicotinic acetylcholine receptors. Mol Pharmacol 65: 944–952. [DOI] [PubMed] [Google Scholar]
- 43. Wu PC, Fann MJ, Kao LS (2012) Characterization of Ca2+ signaling pathways in mouse adrenal medullary chromaffin cells. J Neurochem 112: 1210–1222. [DOI] [PubMed] [Google Scholar]
- 44. Quik M, Vailati S, Bordia T, Kulak JM, Fan H, et al. (2005) Subunit composition of nicotinic receptors in monkey striatum: effect of treatments with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine or L-DOPA. Mol Pharmacol 67: 32–41. [DOI] [PubMed] [Google Scholar]
- 45.Perez-Alvarez A, Hernandez-Vivanco A, Gregorio SA, Tabernero A, McIntosh JM, et al. (2011) Pharmacological characterization of native alpha7 nAChRs and their contribution to depolarization-elicited exocytosis in human chromaffin cells. Br J Pharmacol. [DOI] [PMC free article] [PubMed]
- 46. Sine SM, Taylor P (1980) The relationship between agonist occupation and the permeability response of the cholinergic receptor revealed by bound cobra alpha-toxin. J Biol Chem 255: 10144–10156. [PubMed] [Google Scholar]
- 47.Hone AJ, Ruiz M, Scadden M, Christensen S, Gajewiak JB, et al. (2013) Positional-scanning mutagenesis of alpha-conotoxin PeIA identifies critical residues that confer potency and selectively for alpha6/alpha3beta2beta3 and alpha3beta2 nicotinic acetylcholine receptors. J Biol Chem. [DOI] [PMC free article] [PubMed]
- 48. Azam L, Yoshikami D, McIntosh JM (2008) Amino acid residues that confer high selectivity of the alpha6 nicotinic acetylcholine receptor subunit to alpha-conotoxin MII[S4A,E11A,L15A]. J Biol Chem 283: 11625–11632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kim HW, McIntosh JM (2012) alpha6 nAChR subunit residues that confer alpha-conotoxin BuIA selectivity. FASEB J 26: 4102–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Marritt AM, Cox BC, Yasuda RP, McIntosh JM, Xiao Y, et al. (2005) Nicotinic cholinergic receptors in the rat retina: simple and mixed heteromeric subtypes. Mol Pharmacol 68: 1656–1668. [DOI] [PubMed] [Google Scholar]
- 51. Genzen JR, Van Cleve W, McGehee DS (2001) Dorsal root ganglion neurons express multiple nicotinic acetylcholine receptor subtypes. J Neurophysiol 86: 1773–1782. [DOI] [PubMed] [Google Scholar]
- 52. Haberberger RV, Bernardini N, Kress M, Hartmann P, Lips KS, et al. (2004) Nicotinic acetylcholine receptor subtypes in nociceptive dorsal root ganglion neurons of the adult rat. Auton Neurosci 113: 32–42. [DOI] [PubMed] [Google Scholar]
- 53. Han ZY, Le Novere N, Zoli M, Hill JA Jr, Champtiaux N, et al. (2000) Localization of nAChR subunit mRNAs in the brain of Macaca mulatta. Eur J Neurosci 12: 3664–3674. [DOI] [PubMed] [Google Scholar]
- 54. Wooters TE, Smith AM, Pivavarchyk M, Siripurapu KB, McIntosh JM, et al. (2011) bPiDI: a novel selective alpha6beta2* nicotinic receptor antagonist and preclinical candidate treatment for nicotine abuse. Br J Pharmacol 163: 346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Huang LZ, Campos C, Ly J, Ivy Carroll F, Quik M (2011) Nicotinic receptor agonists decrease L-dopa-induced dyskinesias most effectively in partially lesioned parkinsonian rats. Neuropharmacology 60: 861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The activity of 5-I-A-85380 on α6/α3β4 nAChRs. Human α6/α3β4 nAChRs were expressed in Xenopus laevis oocytes and subjected to two-electrode voltage-clamp electrophysiology as described in “Materials and Methods”. The responses to 100 μM 5-I-A-85380 were normalized to the responses to 300 μM ACh obtained in the same cells. The responses to 5-I-A-85380 were on average 100±12% of those evoked by 300 μM ACh. The error bars denote the S.D.M from four oocytes.
(TIF)