Abstract
Bacteria of the genus Vibrio occur at a continuum from free-living to symbiotic life forms, including opportunists and pathogens, that can contribute to severe diseases, for instance summer mortality events of Pacific oysters Crassostrea gigas. While most studies focused on Vibrio isolated from moribund oysters during mortality outbreaks, investigations of the Vibrio community in healthy oysters are rare. Therefore, we characterized the persistence, diversity, seasonal dynamics, and pathogenicity of the Vibrio community isolated from healthy Pacific oysters. In a reciprocal transplant experiment we repeatedly sampled hemolymph from adult Pacific oysters to differentiate population from site-specific effects during six months of in situ incubation in the field. We characterized virulence phenotypes and genomic diversity based on multilocus sequence typing in a total of 70 Vibrio strains. Based on controlled infection experiments we could show that strains with the ability to colonize healthy adult oysters can also have the potential to induce high mortality rates on larvae. Diversity and abundance of Vibrio varied significantly over time with highest values during and after spawning season. Vibrio communities from transplanted and stationary oysters converged over time, indicating that communities were not population specific, but rather assemble from the surrounding environment forming communities, some of which can persist over longer periods.
Introduction
Bacteria of the genus Vibrio are highly abundant in the marine environment and include several pathogenic strains that can harm invertebrates and vertebrates, including humans [1]. In the aquaculture industry, mass mortalities resulting from Vibrio infections provoke severe production losses with great economic impacts in shrimp [2], [3], [4], [5], fish [6] and shellfish [7], [8], [9], [10] including the Pacific oyster Crassostrea gigas (Thunberg, 1793). The Pacific oyster is an important aquaculture species of great economic value with a production expanding more than six million tons per year [11]. Since the 1960's abnormal mass mortalities of the Pacific oyster, known as summer mortality syndrome (SMS), have been increasingly reported worldwide, including Japan [12], the United States [13], [14], France [15] and the Southern part of the North Sea [16]. Especially since 2008, reported mass mortalities of Pacific oyster spats increased dramatically in warmer seasons in some areas of France and New Zealand [17]. Multiple stressors such as elevated temperature, low dissolved oxygen, and limited energy resources after spawning were often associated with these reported mortality outbreaks [13], [18], [19], [20], [21], [22]. In combination with these factors bacteria of the genus Vibrio spp. have repeatedly been implicated as one causative agent [8], [9], [10], [13], [18], [19], [20], [21], [23], [24], [25], [26], [27] including recent adult mortalities in France associated with high abundance of V. aestuarianus [9], [26].
Vibrio spp. are ubiquitous in marine ecosystems and can utilize different lifestyles including free living, mutualistic, opportunistic or pathogenic forms [28], [29]. Pathogenicity of Vibrio is tightly linked to seawater temperature, which can on the one hand increase host susceptibility by weakening host's immune systems [30]. On the other hand high temperatures can result in a higher transmission and proliferation rate of Vibrio spp. or/and in an upregulation of several virulence factors involved in motility, host degradation, secretion, and antimicrobial resistance [31]. For instance, only at high temperatures, Vibrio shiloi can lyse the symbiotic zooxanthellae thereby causing severe coral bleaching events [32].
Bacteria of the genus Vibrio have been isolated and characterized from healthy and moribund C. gigas for more than 30 years [8], [9], [24], [27], [33], [34]. The predominant species associated with oyster mortality outbreaks were V. splendidus, V. anguillarum, V. tubiashii and V. aestuarianus [8], [9], [24], [27], [33], [34], [35]. The cultivable Vibrio flora harbored in healthy C. gigas is hypothesized to represent a bivalve specific community [36] with a high genetic diversity [37], [38], [39] ranging from 102 to 104 CFU/mg tissue with higher loads at higher temperatures [26].
Although, these findings have made great advances in characterizing pathogenic Vibrio spp. by isolating strains from moribund oysters, a comprehensive overview of the Vibrio community structure of healthy C. gigas, as well as an in-depth knowledge of its persistence over time is lacking.
Here, we describe the temporal dynamics of the Vibrio community structure in Pacific oysters stemming from two oyster beds that have been spared from mass mortalities thus far. Previous research showed that these oyster beds were genetically differentiated from each other and showed a bed specific response of the total bacterial community to disturbance [40]. Based on the genetic differentiation between beds we now wanted to disentangle the influences of host genetic bed affiliation and environmental site conditions on the Vibrio community by conducting a reciprocal transplant experiment. By means of repeated hemolymph sampling we were able to describe the persistence, dynamics and diversity of the in situ Vibrio community able to colonize C. gigas hemolymph during a six-month period. We further complement this data with an empirical assessment of the pathogenicity of local strains under elevated summer temperatures using controlled infection experiments on oyster larvae.
Materials and Methods
Permission to collect oysters was given by the Nationalparkamt Schleswig-Holstein; http://www.nationalpark-wattenmeer.de/.
Reciprocal transplant, hemolymph sampling, enumeration and isolation of Vibrio spp.
In May 2010 we collected 96 Pacific oysters C. gigas from two natural intertidal soft-bottom oyster reefs, i.e. Oddewatt (OW) (55°02′17″N, 08°26′32″E) and Diedrichsenbank (DB) (55°02′31″N, 08°26′53″E) from the Königshafen on the island of Sylt, North Sea, Germany. This area is characterized by a tidal regime (about 180 cm) and temperatures ranging from 0°C in winter to a maximum of 21.67°C in summer. We randomly collected healthy oysters with an average size of 7.41 cm±0.97 (mean ± SE). Oysters were brought to the lab and a hole was drilled with a small hand drill in the upper shell close to the adductor muscle through which a needle could be inserted to sample hemolymph from the adductor muscle. Afterwards oysters were individually labeled and deployed outside in the following manner: 48 oysters originating from each bed were equally deployed at OW and DB. This resulted in four different transplant groups, i.e. OW-OW, OW-DB, DB-OW, DB-DB (origin-site) containing 24 oysters each. Marked oysters were kept in plastic mesh bags (4 oysters per bag representing each transplant group) and anchored to sandy mud flats surrounded by the original oyster beds in the intertidal.
From May 2010 before deployment until October 2010 hemolymph was repeatedly sampled from each oyster once a month. At each time point we checked the oysters for mortality. Dead animals were denoted and removed from the batches. Hemolymph was withdrawn from each oyster with a 2-mL syringe attached to a needle of 23G×5/4″ that was inserted through the predrilled hole in the shell into the muscle tissue. During the sampling time in the field, withdrawn hemolymph was stored on ice. In the lab 4 µl of undiluted hemolymph was spread on Vibrio selective Thiosulfate Citrate Bile Sucrose Agar (TCBS) plates (Fluka Analytica, Sigma-Aldrich, Steinheim, Germany). Plates were incubated at 25°C for 24 hours. Afterwards colony forming units (CFU) were counted for each oyster and a random subset of 11–24 colonies (depending on growth of colonies) per month covering all transplant groups was taken for further analysis. Single colonies were randomly picked from the agar plates and resuspended in 3 mL Nutrient solution (5.0 g peptone, 3.0 g meat extract, 1.5 g NaCl in 1000 ml distilled water) and cultured at 25°C under constant shaking for 24 hours. We choose 25°C for isolation, because our main goal was to assess the pathogenicity of isolated strains at high summer temperatures and did not want to introduce bias by isolating strains at ambient temperatures observed in the field. This liquid bacteria culture was used for direct amplification of three different genes (16S rRNA, PyrH and GyrB) to determine the genetic affiliation (phylotype) based on multilocus sequence types (MLST) of the Vibrio cultures. The remaining cultures were cryopreserved at −80°C in 50% glycerol until further use.
PCR and Gene sequencing
To increase the resolution of Vibrio isolate identification, we chose a MLST approach based on three different genes, i.e. 16S rRNA, GyrB and PyrH (Primer details listed in supporting information: Table S1). Partial 16S rRNA gene of representative isolates was amplified directly from the liquid bacteria culture without prior extraction using universal primers 16S-27f and 16S-1392R. PCR mixtures were composed of 4 µL PCR buffer (Promega, Mannheim, Germany), 1.2 µL MgCl2(25 mM), 1.0 µL dNTPs (10 mM), 1.0 µL each forward and reverse primers (50 µM), 0.05 µL Taq polymerase (Promega, Mannheim, Germany) and 1 µL template DNA in a total volume of 20 µL. The amplification program used was one cycle at 95°C for 5 min, 30 cycles at 95°C for 30 s, 50°C for 30 s, 72°C for 90 s and one final cycle at 72°C for 10 min. Prior to amplification of the protein encoding genes (PyrH and GyrB), Vibrio cells were lysed for 3 min at 100°C. DNA amplification of PyrH and GyrB followed the protocol described by [41]. The PCR was conducted in a final reaction volume of 50 µL containing 5 µL Buffer (Peqlab, Erlangen, Germany), 1.0 µL dNTPs (10 mM), 1.0 µL each forward and reverse primers (50 µM), 0.5 µL Taq DNA polymerase (Peqlab, Erlangen, Germany), and 5 µL template DNA. The thermal program consisted of: (i) 5 min at 95°C; (ii) 3 cycles of 1 min at 95°C, 2 min 15 s at 55°C and 1 min 15 s at 72°C; (iii) 30 cycles of 35 s at 95°C, 1 min and 15 s at 55°C and 1 min and 15 s at 72°C; and (iv) a final extension for 10 min at 72°C. PCR purification and standard Sanger sequencing was performed by the Institute for Clinical Molecular Biology (IKMB), Christian-Albrechts-University Kiel.
Controlled infection experiments
Larvae rearing
In order to estimate the pathogenic potential of isolated Vibrio strains we conducted controlled infection experiments on 11 day old larvae. Parental oysters were randomly collected from the Oddewatt (OW). Crosses were performed by strip-spawning 3 females and 3 males, resulting in three independent full-sib families. Gametes were stripped directly from the gonads and collected into 0.45 µm filtered, UV treated seawater. Fertilization was performed at a ratio of 200 spermatozoa per oocyte, with 4×105 oocytes per family. After 20 min, spermatozoa were removed by collecting oocytes on a 20 µm mesh screen. One hour after fertilization embryos were transferred to the rearing tanks at a concentration of 5 embryos/ml. Larvae were kept at 21°C with salinity of 28 psu in 2L rearing tanks filled with 0.45 µm filtered, UV treated seawater and fed with Isochrysis galbana at a concentration ranging from 10 to150 cells/µL depending on larval age. Water was exchanged every second day. At day 10 after fertilization, families were pooled with equal contribution of each family.
Experimental infections
Out of the 93 sampled isolates, we were able to successfully sequence all genes needed for MLST genotyping in 74 isolates. Out of those we were able to grow 70 isolates under agitation at 25°C in Nutrient solution 1.5% NaCl for 20 h. We determined the bacterial concentration by optical density (OD) at 550 nm. We assessed the correlation between OD at 550 nm and colony forming units of 10 selected strains (details listed in supporting information: Figure S1) and observed that an OD of 1 at 550 nm corresponded to 4.63–5.24×108 CFU/ml. Therefore, we used an approximation of an OD value of 1 to correspond to 5×108 CFU/ml as has been previously done by [42]. Bacteria cells were centrifuged at 5500 rpm at 25°C for 5 min and resuspended in Nutrient agar at 1×109 cells/mL. Controlled infection experiments on larvae were carried out using sterile 96-well culture plates, following a modification of the protocol described by [33]. Briefly, 10–15 larvae per family were placed in one micro-well containing 0.45 µm filtered and UV treated seawater. Larvae were challenged by bathing in a bacterial suspension containing 107 cells/mL of each respective Vibrio isolate. We added PBS to 4 additional wells, serving as controls. Each experimental group was assayed in duplicates. Infection experiments were carried out in a constant climate chamber at 21°C. We considered 21°C as an optimal temperature to test Vibrio pathogenicity on oyster larvae for two reasons (1) Vibrio induced mass mortalities as well as oyster larvae only occur at warm summer temperatures (above 19°C), (2) 21°C is the maximum temperature found in the study area and projections of future sea surface temperatures do suggest that similar temperatures can be expected on average during summer months in the study area. Survival was observed at day 3 after inoculation using an inverted microscope by counting the amount of dead larvae, i.e. empty shells or closed larvae without velum movement and signs of necrosis.
Data Analysis
Dynamics
There was no effect of oyster origin (factorial repeated measures ANOVA: χ2 (df = 6) = 0.43, p = 0.51), transplanted site (χ2 (df = 7) = 0.18, p = 0.67), as well as no significant interaction of origin and site (χ2 (df = 8) = 1.27, p = 0.26) on the amount of colony forming units (CFU). Therefore we pooled the data and conducted a multilevel linear model to assess differences in CFU between months, with CFU as the dependent variable and months as the independent variable. Significant effects were further analyzed with a Tukey HSD post-hoc test. Potential associations between CFU and temperature were analyzed with Pearson's correlation analysis.
Phylogenetic Analysis
Sequences were manually edited and automatically assembled using Sequencher™ 4.8 software. Edited gene sequences obtained from each single gene were compared against published sequences in NCBI GenBank using BLASTN algorithm with default settings based on 99% sequence identity to identify putatively close phylogenetic relatives of isolated Vibrio. Multiple alignment of concatenated sequences was created by MUSCLE, version 3.8.31 [43]. This included 2,560 positions after manual removal of ambiguous positions using JalView, version 2.8 [44]. We used jModelTest [45], [46] to select the best fitting substitution model of sequence evolution following recent recommendations [47]. Based on the concatenated sequences a phylogenetic tree was constructed using maximum-likelihood phylogenies PhyML v.3.0 [46], [48] by using the General Time Reversible model plus a discrete γ-distribution to account for rate heterogeneity among sites plus invariant sites (GTR+G+I) [49] as suggested by jModelTest. The following parameter settings were used for phylogenetic analysis: Transition/transversion ratio was estimated by PhyML; four gamma rate categories were used; a BIONJ tree was initially used; branch length and tree topology were optimized by PhyML using Allovibrio fischerii as an outgroup. Additionally we included 14 reference strains (Supporting information: Table S2) that were accessed from NCBI GenBank. Reliability of topology was assessed by bootstrapping with 500 replicates [50]. A radial cladogram was drawn using the Interactive Tree of Life web service at http://itol.embl.de/ [51], [52]. All sequences were deposited at GenBank with Accession Numbers KJ507408–KJ507477 (16 s), KJ507478–KJ507547 (GyrB), KJ507548–KJ507617 (PyrH).
Diversity analysis
To describe the diversity of the Vibrio community we first built a distance matrix based on the concatenated sequences using the DNADIST program within the PHYLIP software package based on the Kimura-2-parameter distance method with all other options set to default [53]. This distance matrix was imported into the MOTHUR software version 1.23.6 [54] to assign operational taxonomic units (OTUs) defined by 99% sequence similarity using the average neighbour algorithm. We calculated non-parametric OTU-based richness estimators (Chao1 and Shannon-Wiener Index) to estimate the diversity of the Vibrio community for the different sampling sites and sampling months. We further conducted a Pearson's correlation analysis to identify possible associations between diversity and temperature. Because Vibrio diversity correlated positively with mean temperature per sampling month we conducted a lag analysis to assess whether the Vibrio community composition reacts to quick temperature shifts or is stable over longer time periods. We sequentially extended the time window by 4 days in which temperature was measured starting from sampling date for each month, calculated the average temperature for each time window and correlated it with the respective diversity index. The highest correlation coefficient over all months can then give an idea whether community composition is integrated over longer time periods or reacts quickly to shifts in temperature.
Persistence
Temporal and spatial differences as well as the persistence of the Vibrio community structure between the different sampling months, as well as between transplanted and original oysters were analysed using UniFrac distances, a metric of the unique phylogenetic distance between communities, available as a web application at http://bmf.colorado.edu/unifrac [55], [56], [57]. UniFrac was applied to cluster the sequences from the different transplant groups by months as well as by site and origin and to test which months and transplant groups were significantly different from each other using UniFrac-test and Principal Coordinate Analysis (PCoA). P-values have been corrected for multiple comparisons using Bonferroni correction.
Pathogenicity
Virulence of a given strain was assessed as induced mortality at day 3 after inoculation. Virulence was not normally distributed as determined by the Shapiro-Wilk test. Differences in induced virulence between the different clades as well as differences in average virulence per month were analyzed with non-parametric Kruskal-Wallis rank sum test. Possible influences of temperature and diversity (Chao1 and Shannon-Wiener Index) on mean and maximum virulence were assessed with a Spearman rank correlation.
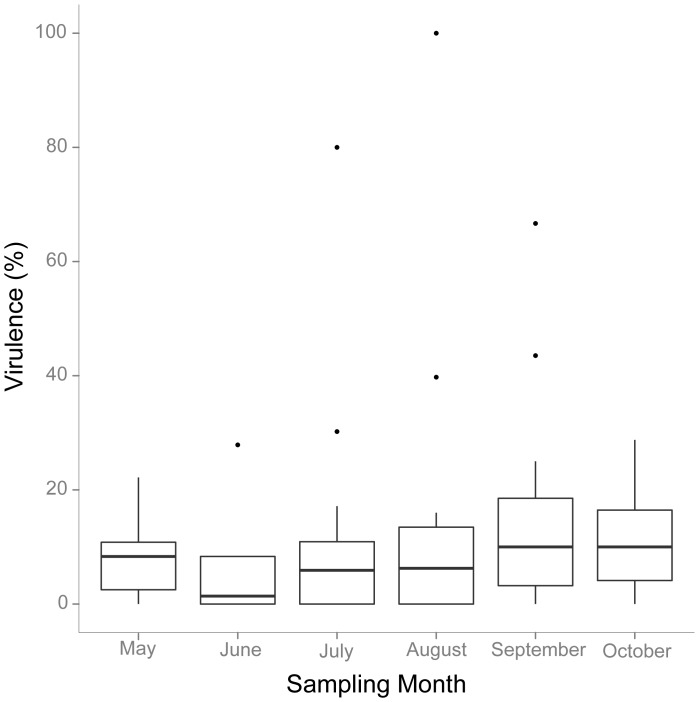
Maximum virulence per sampling month correlated positively with temperature (Spearman rank correlation: r = 0.77, p = 0.08). To predict the probability of encountering a highly virulent strain per month we used a logistic regression, predicting the occurrence of strains with higher than average virulence (outliers in Fig. 5) by mean monthly temperature.
Figure 5. Distribution of induced virulence (%) from May to October 2010.
Shown are median virulence values (%) per sampling month, with an interquartile box and a 5 to 95% range. Outliers are marked by black dots.
We estimated the variability within the duplicates per strain by calculating the mean difference between all duplicates and the variance (mean = 4.61, variance = 29.38). In addition we estimated the mean difference of a total of 1000 permutations of randomly 70 chosen differences between the two datasets and calculated the average variance (mean = 16.81, variance = 40.13). With that we could show, that variability within the experimental treatment was low, and results are reliable.
All statistical analyses were performed in the R 2.15.2 statistical language (R Development Core Team, 2011).
Results
Abundance
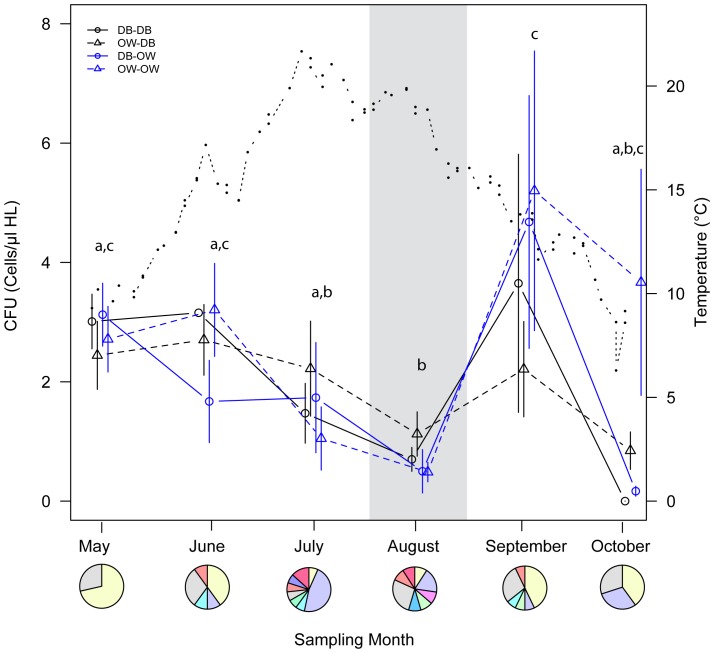
Temporal variation in the amount of Vibrio bacteria in oyster hemolymph was monitored from May to October 2010 by counting colony forming units (CFU) on a monthly basis. Cultivation of Vibrio spp. from oyster hemolymph revealed that Vibrio are consistently present as a member of the oyster hemolymph community. Vibrio abundances varied significantly over time (multilevel linear model: χ2 (df = 9) = 23.05, p<0.001) but showed similar patterns in all transplant groups. The number of cultivable Vibrio in the oyster hemolymph decreased from May to August, followed by a drastic increase after the spawning period (i.e. in September with a maximum value of 5.2 CFU µl−1) and a decrease towards autumn when temperature dropped down (Figure 1). Total bacteria load was not significantly correlated to temperature (Pearson's correlation: r = −0.19, t(df = 4) = −0.39, p = 0.72) indicating that temperature was not the main driver for Vibrio load in oyster hemolymph.
Figure 1. Temporal variation in the amount of Vibrio bacteria in oyster hemolymph (HL).
Quantification of total Vibrio spp. (open circles and triangles) isolated from oyster hemolymph stemming from the four transplant groups (origin_site), i.e. DB_DB, DB_OW, OW_DB, OW_OW. Blue lines represent oysters assayed in site OW and black lines represent oysters assayed in site DB. Solid lines show oyster origin DB, while dashed lines show oyster origin OW. Water temperature is shown on the secondary y-axis (full circles, dotted line). The area shaded in grey marks the spawning period. Different letters indicate significant differences according to Tukey HSD post hoc test. Pi charts show relative proportion of Vibrio isolates by phylogenetic association based on 99% similarity by BLASTN analysis in each sampling month. Color codes correspond to those in Figure 2.
Diversity
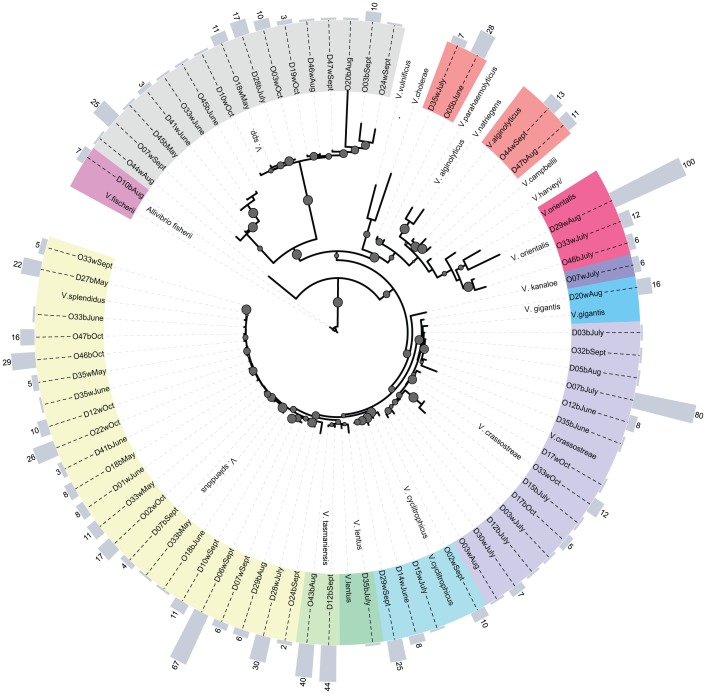
From April to October 2010 we isolated a total of 93 strains from the hemolymph of C. gigas. Out of these we successfully determined the virulence and MLST genotype for 74 strains. Based on BLASTN comparison of each single gene the phylogenetic affiliation of 16 strains could not be assigned uniquely to one clade within the genus Vibrio and are therefore named as Vibrio sp. All other strains were assigned to 10 different Vibrio species that fell into four distinct clades as defined by [58], [59] (Figure 2). Strains of the Splendidus clade were the most frequently observed (64.3%) and were represented by seven different species: V. splendidus: 23 isolates, V. crassostreae: 14 isolates, V. cyclitrophicus: 3 isolates, V. tasmaniensis: 2 isolates, V. gigantis, V. lentus and V. kanaloae: 1 isolate each. The second most common clade was represented by members of the Vibrio core (Harveyi clade), with a total of 4 isolates (i.e. 5.7%), all represented by V. alginolyticus. We further assigned three isolates to the Orientalis clade (4.29%), and another one single isolate to the Fischeri clade, identified as Allivibrio fischerii. Of all isolated species, V. splendidus was the only one that was consistently present in oyster hemolymph in every month.
Figure 2. Phylogenetic relationships of Vibrio isolates sampled from oyster hemolymph.
Maximum likelihood method (GTR+G+I substitution model) using concatenated sequences of three genes (16S rRNA, GyrB, PyrH) (2560 bp). Bootstrap percentages above 80% are represented by grey dots at the parent nodes. Virulence of each strain is depicted by the grey bars on the outer ring (a value of 100 corresponds to 100% mortality during inoculation experiments). Assignment to the different clades (see legend) based on 99% sequence similarity by BLASTN analysis is depicted in color on the inner ring.
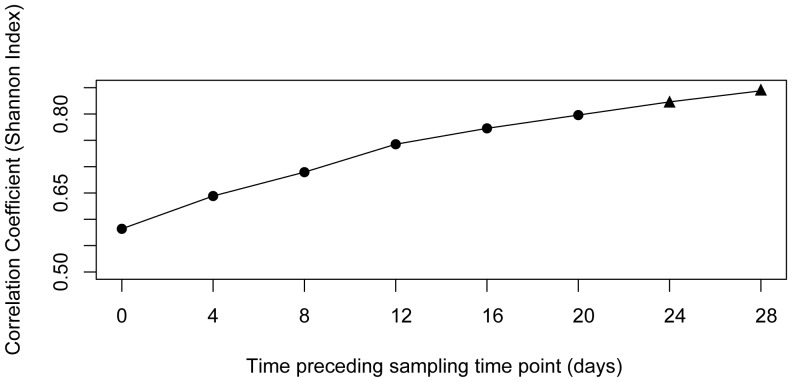
Analysis of operational taxonomic units (OTUs) based on concatenated MLST sequences resulted in 41 OTUs on the basis of 99% sequence similarity (Table 1). The Vibrio community structure differed significantly between months (Figure 1, UniFrac significant test after Bonferroni correction: p<0.001). Total number and diversity was highest during summer (July–September) and lowest in May (Table 1). Vibrio communities showed a clear differentiation between spring/autumn (May, June/October) and the warm summer months (July, August, September, Figure 1). Vibrio diversity was positively correlated with mean temperature per sampling month (Chao1 index: r = 0.81, t(df = 4) = 2.78 p = 0.05; Shannon-Wiener Index: r = 0.87, t(df = 4) = 3.45 p = 0.03) and lag analysis revealed that this correlation was strongest when integrated over longer time periods as opposed to immediate responses to temperature shifts (Figure 3). Both sampling sites (DB and OW) had almost equal numbers of OTUs and shared nine OTUs. Richness estimators indicated that both sampling sites had a similar diversity (Table 1).
Table 1. Number of OTUs and richness estimators (Chao1 and Shannon-Wiener Index) for each sampling month (May–Oct) as well as at both sampling sites (Dietrichsenbank and Oddewatt) on the basis of 99% identity cut-off.
| Group | No. of sampled colonies | No. of sequences | Richness estimators | ||
| No. of observed OTUs | Chao1 | Shannon | |||
| DB | 55 | 33 | 24 | 94 | 2.9 |
| OW | 58 | 37 | 26 | 73.5 | 3.1 |
| May | 11 | 7 | 4 | 7 | 1.15 |
| June | 16 | 12 | 9 | 19.5 | 2.09 |
| July | 24 | 14 | 12 | 27 | 2.44 |
| Aug | 24 | 11 | 11 | 66 | 2.39 |
| Sept | 23 | 15 | 12 | 24.5 | 2.39 |
| Oct | 15 | 11 | 7 | 12 | 1.77 |
Figure 3. Lag analyses for the correlation between diversity (Shannon Index) and mean temperature integrated over 28 days prior to the sampling event.
Day 0 corresponds to the temperature measured at the sampling day representing an immediate response, while day 28 uses the mean temperature of the previous month representing a slower response to temperature shifts. Significant correlations between diversity and temperature are depicted by black triangles.
Persistence
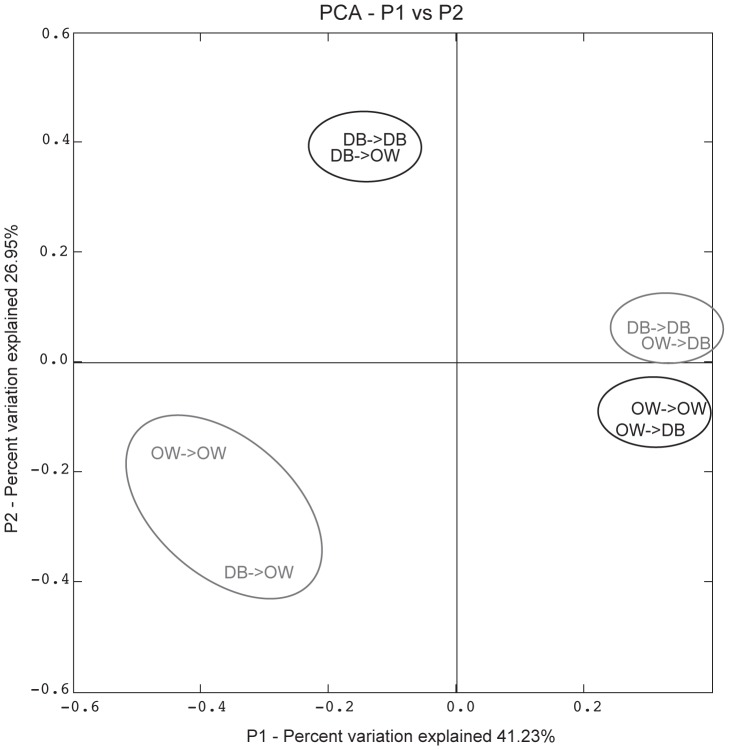
To assess temporal and spatial variation in the Vibrio community structure between original and transplanted oyster samples we used June as the starting month to give the Vibrio communities in the transplanted oysters one month to stabilize, and we used September as the final month, because we lost all samples at the Diedrichsenbank– site in October. The first two principal coordinates of our ordination explained a cumulative percentage of 68.18% and showed a clear grouping of Vibrio communities by origin in June. In other words, Vibrio communities maintained the signature of their collection site, although oysters were exposed to different site conditions for more than a month, indicating that Vibrio communities are somewhat stable on the short term. However, the grouping by origin converged to a clear grouping by sampling site in September (Figure 4), indicating that oyster associated Vibrio communities were not population- or genotype-specific for extended periods of time but rather recruit from the surrounding environment, which has also been described for mussels [60]. Overall there was a significant differentiation between the different transplant groups from June and September reflecting temporal changes in community composition (UniFrac significant test after Bonferroni correction: p = 0.02).
Figure 4. Ordination of Vibrio communities from all different transplants.
Origin_Transplant site: DB->DB, DB->OW, OW->DB, OW->OW at the beginning (June in Black) and end (September in Grey) of the experiment. Shown are the first two principle coordinates from PCoA implemented in web-based UniFrac analysis.
Pathogenicity
Based on controlled infection experiments we were able to assess the pathogenic potential of 70 different Vibrio isolates on oyster larvae. There was no mortality in all control treatments. The tested isolates harbored high variation in pathogenicity, ranging from 0 to 100% with a median virulence of 6.25% (Figure 2). About one third of the tested isolates did not induce any mortality. Strain O29w_Aug, identified as member of the Orientalis clade, induced 100% mortality. Based on only 16S rRNA sequence similarity this isolate was identified as V. tubiashii, which has often been shown to be highly pathogenic to bivalves [61], [62]. In total we found 12 strains that induced more than 30% mortality. In addition, the three most pathogenic strains, that induced mortalities above 50%, were isolated during spawning season, i.e. July–September.
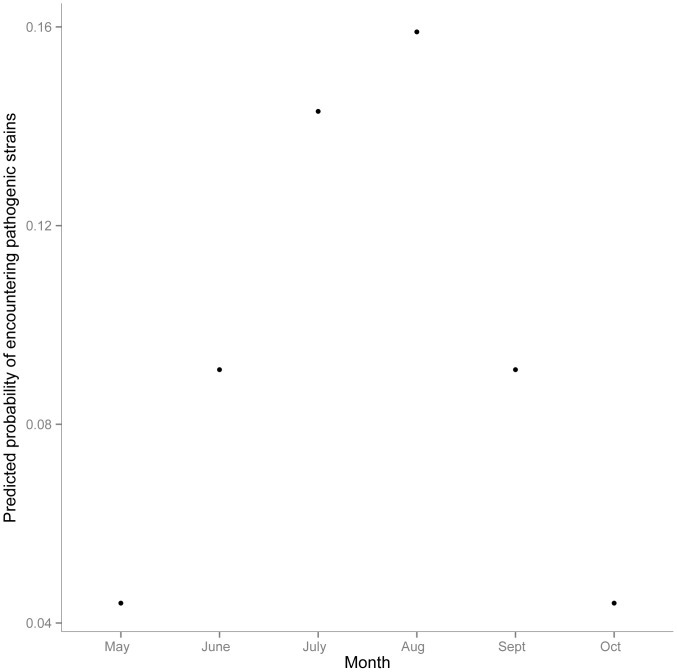
On average, the majority of strains within each sampling month displayed low pathogenicity (Figure 5) and there was no significant association between mean virulence per month and temperature (Spearman rank correlation: r = 0.65, p = 0.16). Although diversity correlated well with mean induced virulence per month, we could not find a significant relationship (Chao1 index: Spearman rank correlation: r = 0.77, p = 0.10; Shannon-Wiener Index: Spearman rank correlation: r = 0.64, p = 0.17), which is probably owed to low statistical power associated with N = 6. However, highly virulent strains were exclusively found in summer months especially during spawning season (Figure 5) and we observed a significant correlation between maximum virulence per sampling month and diversity (Chao1 index: Spearman rank correlation: r = 0.89, p = 0.03; Shannon-Wiener Index: Spearman rank correlation: r = 0.75, p = 0.08), which was also reflected in a nearly significant correlation of maximum virulence per sampling month with temperature (Spearman rank correlation: r = 0.77, p = 0.08). The predicted probability of encountering a highly virulent strain increased during summer and was highest in August, i.e. during spawning season where it is on average four times higher than in spring (Figure 6).
Figure 6. Predicted probability to encounter highly pathogenic strains (i.e. induced mortality >25%) for each sampling month.
Discussion
The Vibrio community in the hemolymph of healthy Pacific oysters C. gigas showed strong seasonal variation in diversity, prevalence and virulence of Vibrio, with highest values of all parameters coinciding with the spawning season in warmer summer months when mass mortalities usually occur. Environmental temperature therefore seemed to be a major driver for Vibrio community dynamics in oyster hemolymph, reflecting patterns observed for open water communities [28], [63]. Although Vibrio community dynamics did not immediately react to temperature shifts, we could show with a reciprocal transplant experiment that the Vibrio community associated to oyster hemolymph is not persistent over extended periods of time but seems to be recruited from environmental reservoirs (Figure 4).
Diversity
The structure and diversity of the Vibrio community was largely driven by temperature. The diversity of the Vibrio community increased with increasing temperatures from May (minimum sea water temperature: 5.9°C) to August (maximum sea water temperature: 21.67°C), followed by a decrease as temperatures dropped in autumn. Low diversities in colder months could have underestimated the true diversity due to our isolation procedure at 25°C, which may have excluded psychotropic strains. We were however mainly interested in sources and reservoirs of potential pathogens that usually impose risk on oysters during warmer summer months.
Members of the Splendidus clade (V. splendidus and V. crassostreae) were predominating and present in every month, followed by members of the Vibrio core and the Orientalis clade, which were only present in the warm summer months. In general, bacterial communities of Pacific oysters [26] as well as coastal waters [64] are often dominated by V. splendidus related strains when temperatures are below 20°C, whereas members of the Vibrio core are most prevalent when sea water temperatures are above 20°C [65]. Similarly, Vibrio communities in the hemolymph of the spider crab Maja brachydactyla were dominated by species belonging to the Splendidus clade in areas with water temperatures below 20°C, while members of the Vibrio core, the Orientalis and the Splendidus clade dominated equally in areas where water temperatures were around 21°C [38]. This suggests that temperature dependent composition of different Vibrio communities follows similar rules in host associated microbiota as well as open water communities [28], [65], [66]. Our lag-analysis, on the other hand showed that Vibrio communities in Pacific oysters are largely inert to quick temperature shifts and that the effect of temperature on community composition is strongest when integrated over longer periods of time, indicating that these communities are stable for relatively long periods of time.
Abundance
The abundance of cultivatable Vibrio decreased throughout the summer season before it peaked just after the spawning season, indicating that abundance within oyster associated communities did not react directly to water temperatures (Figure 1). This is in contrast to open water abundances of Vibrio that usually correlate positively with water temperature [28], [65], [67]. Unfortunately, we do not have any data on Vibrio abundance in the open water column here, but Vibrio abundance in the North Sea were also shown to correlate with temperature [63], indicating that there may be a mismatch between open water abundance and abundance within oyster hemolymph.
Deviations from temperature correlated Vibrio population sizes may also be caused by several other environmental factors, such as salinity or nutrients [63]. In algal blooms (March–May), Vibrio abundances were correlated to Chlorophyll a, indicating that high algal abundance might provide high amounts of nutrients [63]. Consumption of Vibrio-loaded algae might therefore explain the high Vibrio abundances observed at the beginning of our study (May). Similarly, the steep increase after the spawning season might be attributable to high take-up of larvae that were previously colonized by Vibrio.
Additionally, part of the mismatch between environmental and oyster hemolymph abundances can be caused by the interaction between Vibrio and host condition or host immune response. In a previous infection experiment we observed that there was no difference in mortality between gravid and pre-spawning oysters when infected with several Vibrio strains [22]. Post-spawning oysters, on the other hand, suffered from high mortality rates resulting from insufficient clearance of pathogens due to an impaired phagocytic ability of hemocytes. Therefore, the strong increase in CFU in oyster hemolymph may reflect the impaired ability of oysters to keep Vibrio abundance low just after spawning.
Persistence
The natural microbiota of oysters can be resistant to environmental perturbation and can persist during depuration processes [68]. Similarly, under ambient conditions the total microbial community composition depended on host genotype in the oyster beds investigated here [40] and the genetic differentiation observed among the oyster beds, may suggest that also Vibrio communities of oyster hemolymph assemble in a bed specific manner resembling host genetic differentiation. Our reciprocal transplant experiment could disentangle effects of spatial origin or host genetic factors from environmental effects. The Principal Coordinate Analysis (PCoA) revealed a clear grouping of the different transplant groups separating communities based on origin, and thus potentially host genotype, in June. At the end of the sampling period in September oyster bacterial communities converged by experimental site rather than origin (Figure 4). These findings are in concert with a study on the metapopulation structure of Vibrionaceae of blue mussels and crabs suggesting a neutral population structure without host preferences [60]. The spatial convergence over time observed in the present study therefore suggests that similarly oyster associated Vibrio community is not stable within hosts, and rather depends on Vibrio spp. taken up from the surrounding environment.
Pathogenicity
Our infection experiment demonstrated that Vibrio strains isolated from healthy adult Pacific oysters have the potential to kill oyster larvae under controlled experimental conditions. In total we found 12 pathogenic strains killing more than 30% of the oyster larvae out of which the most virulent strain O29w_Aug, identified as member of the Orientalis clade, killed 100% of oyster larvae. Although we are not aware of any study on the pathogenicity of V. orientalis, there are reports of several strains from the Orientalis clade (e.g. V. tubiashii) that have a high pathogenic potential for bivalves [61], [62]. The majority (8 out of 12) of the strains that induced high mortalities were isolated during spawning season, i.e. July–September when the probability of encountering such strains was highest (Figure 6). Since highly virulent strains may have the potential to cause epidemic mass mortalities, the observed increase in virulence during summer reflects the worldwide summer mortality patterns of larvae, spats and adult oysters [13], [14], [15], [16], [69].
Oyster summer mortalities affecting all life-stages of C. gigas are the result of a complex interaction of adverse environmental factors (e.g. high temperature), host physiological condition and genetics, and pathogens, such as Ostreid herpesvirus 1 and Vibrio spp. [8], [9], [10], [18], [20], [21], [22], [33], [70], [71]. This complex interaction affecting all life-stages makes it difficult to assess the risk of mortality. Here, we concentrated on Vibrio strains that showed the ability to colonize the hemolymph of adult oysters, and its pathogenic potential to larvae. Our interest on oyster larvae was due to several reasons. First, larvae occur only at high temperatures during summer month, which coincides with a peak in Vibrio abundance in the study area [63], [72]. Hence, any time larvae occur, they are at high risk of Vibrio infection. Therefore we also isolated the Vibrio strains at 25°C, representing high summer temperatures, and hence allow the detection of pathogenic Vibrio that are present in warm waters. Second, larvae mortality is one of the main drivers for stock recruitment. Therefore, assessing the risk of mortality on early life-stages is essential to draw conclusions about the impact of potential disease outbreaks on local oyster populations only if the pathogenicity of local strains is known. Third, although oyster hemolymph seems to be relatively isolated from the surrounding waters, we demonstrated in the present study, that oyster associated Vibrio community is to a large extend influenced by the surrounding environment. Therefore we could show that the isolated strains are likely to be encountered by larvae, which are in direct contact with the water column and in close proximity to the adult oysters.
By characterizing the Vibrio community of healthy Pacific oysters, we were indeed able to isolate highly pathogenic strains to larvae and could show, that the probability to encounter those strains is highest in summer, during spawning season. However, overall the amount of highly pathogenic strains as well as the probability to encounter them was relatively low. Nevertheless, the chance of observing highly virulent strains that could cause such mass mortalities was higher in more diverse communities suggesting that diversity can be used partly as an indicator for the maximum virulence to be expected at a given sampling time point. Since diversity was correlated to mean monthly temperature, temperature driven shifts in the composition of the Vibrio community, as could be expected with a projected increase in mean water temperature in the future, could increase the risk of the presence of highly pathogenic strains during warm summer months contributing to potential mass mortalities. On the other hand, larval mortality rate induced by certain Vibrio strains may be a weak predictor of juveniles and adults mortality. Indeed, some strains from the present experiment have also been tested on adults [73] and virulence differed substantially between life-stages with an overall higher impact on adult oysters than on larvae. Nevertheless, adults may act as reservoir of pathogenic Vibrio strains to earlier life stages and are hence important for the transmission and spreading of these pathogens.
How will rising temperatures affect the oyster associated Vibrio community?
Vibrio diversity and abundance increased substantially during and after spawning. A single shellfish can contain between 100 to 1000 strains [74], however mortality can result from a single strain in a heterogeneous population found in shellfish [75]. Containing highly diverse repertoires of Vibrio spp. therefore seems to increase the chance of any such highly virulent strain being present in the animal. Furthermore studies using simultaneous injection of several strains observed increased virulence [10]. Such synergistic effects between Vibrio strains are yet more likely to happen when Vibrio diversity is high like in summer months. Although oysters in the northern parts of the North Sea never experienced summer disease outbreaks, pathogenic Vibrio strains are present in the ambient microbiota and could react to changing environmental conditions. Average water temperatures during summer months in the investigated area are about 18°C, while oyster summer mortality appears to occur when temperatures exceed a threshold of 19°C to 20°C [21]. We performed the experimental infections with the isolated Vibrio strains at 21°C, which is higher than the average water temperature observed during our study. The most virulent strains found here have been assigned to the Orientalis clade and the Vibrio core, but we found substantially fewer Vibrio strains associated to the Orientalis clade and the Vibrio core than similar studies in France during an episode of extensive mass mortalities [26]. Since members of the Vibrio core dominate when sea water temperatures are above 20°C [65], we might expect a shift from a V. splendidus dominated community to a community that is dominated by members of the Vibrio core and the Orientalis clade, when temperatures rise as predicted by current climate change models. Such a temperature dependent community shift can then result in an increase of diversity, abundance and virulence of the oyster associated Vibrio community given that predicted probability to encounter pathogenic Vibrio spp. also increases with increasing temperature.
Conclusion
With the present study we could demonstrate that persistence, dynamics, and diversity of the Vibrio community in the hemolymph of Pacific oysters underlies strong seasonal effects. The likelihood to encounter pathogenic strains that represented a considerable proportion (∼18%) of the strains with the ability to colonize oyster hemolymph was highest in warm summer month during and after spawning season. By means of a reciprocal transplant experiment we could show that Vibrio spp. were permanently present in hemolymph microbiota, but formed an unstable, dynamic community that was recruited from the surrounding environment.
As summer mortalities of C. gigas have been observed solely 130 km southward, ongoing research focusing on the study of the Vibrio ecology including methods to predict the pathogenic potential of a strain are pivotal in this area, where oyster aquaculture is prominent. Continuously monitoring the key parameters of diversity, abundance and pathogenic potential of Vibrio from all potential reservoirs (sediments and open water) as well as their affected targets (oyster hemolymph and larvae), can therefore help to detect first signs of summer mortality heralded by a change in the natural community structure. The ability to identify epidemiological patterns linked to bacterial community structure and specific dynamics of candidate pathogens will be a high priority, especially when infectious diseases are predicted to increase with global warming [76].
Supporting Information
Correlation between colony forming units and OD values at 550 nm for 10 selected Vibrio strains of low, intermediate, and high virulence. An OD value of 1 at 550 nm corresponds to 4.63–5.24×108 CFU/ml.
(TIF)
Sequences of all primers used for genotyping the bacteria of the genus Vibrio spp. ( pyrH , GyrB , 16S rRNA).
(DOCX)
Reference Vibrio sequences used for the phylogeny. The references sequences were aligned to the sequences of the particular gene (16S rDNA, pyrH, GyrB).
(DOCX)
Acknowledgments
Jenny Moehler and Rene Gerrits helped with fieldwork. We would like to thank Tatyana Romanova for providing the temperature data. We thank the Institute of Clinical Molecular Biology in Kiel for providing Sanger Sequencing as supported in part by the DFG Cluster of Excellence “Inflammation at Interfaces” and “Future Ocean”. We thank the technicians S. Greve and I. Baumgartner for technical support.
Funding Statement
Funding was provided by Deutsche Forschungsgesellschaft, Emmy-Noether Grant WE464-1 given to KMW. http://www.dfg.de/foerderung/Programme/einzelfoerderung/emmy_noether/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Pruzzo C, Hug A, Colwell RR, Donelli G (2005) Pathogenic Vibrio species in the marine and estuarine environment. In: S B, RR C, editors. Ocean and health pathogens in the marine environment. Heidelberg: Springer. pp. 217–252. [Google Scholar]
- 2. Lavilla-Pitogo CR, Baticados CL, Cruz-Lacierda ER, Pena de la LD (1990) Occurrence of luminous bacterial disease of Penaeus monodon larvae in the Philippines. Aquaculture 91: 1–13. [Google Scholar]
- 3. Leano EM, Lavilla-Pitogo CR, Paner MG (1998) Bacterial flora in the hepatopancreas of pond-reared Penaeus monodon juveniles with luminous Vibriosis . Aquaculture 164: 367–374. [Google Scholar]
- 4. Haldar S, Chatterjee S, Sugimoto N, Das S, Chowdhury N, et al. (2011) Identification of Vibrio campbellii isolated from diseased farm-shrimps from south India and establishment of its pathogenic potential in an Artemia model. Microbiology-Sgm 157: 179–188. [DOI] [PubMed] [Google Scholar]
- 5. Lavilla-Pitogo CR, Leano EM, Paner MG (1998) Mortalities of pond-cultured juvenile shrimp, Penaeus monodon, associated with dominance of luminescent vibrios in the rearing environment. Aquaculture 164: 337–349. [Google Scholar]
- 6. Austin B, Austin DA, Blanch AR, Cerda M, Grimont F, et al. (1997) A comparison of methods for the typing of fish-pathogenic Vibrio spp. Systematic and Applied Microbiology 20: 89–101. [Google Scholar]
- 7. DiSalvo LH, Blecka J, Zebal R (1978) Vibrio anguillarum and larval mortality in a California coastal shellfish hatchery. Applied and Environmental Microbiology 35: 219–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lacoste A, Jalabert F, Malham S, Cueff A, Gelebart F, et al. (2001) A Vibrio splendidus strain is associated with summer mortality of juvenile oysters Crassostrea gigas in the Bay of Morlaix (North Brittany, France). Diseases of Aquatic Organisms 46: 139–145. [DOI] [PubMed] [Google Scholar]
- 9. Garnier M, Labreuche Y, Garcia C, Robert A, Nicolas JL (2007) Evidence for the involvement of pathogenic bacteria in summer mortalities of the Pacific oyster Crassostrea gigas . Microbial Ecology 53: 187–196. [DOI] [PubMed] [Google Scholar]
- 10. Gay M, Renault T, Pons A-M, LeRoux F (2004) Two Vibrio splendidus related strains collaborate to kill Crassostrea gigas: taxonomy and host alterations. Diseases of Aquatic Organisms 62: 65–74. [DOI] [PubMed] [Google Scholar]
- 11.FAO (2011) Yearbook of fisheries statistics summary tables. Available: ftp://ftpfaoorg/fi/STAT/summary/defaulthtm#aqua. Accessed 2013 Oct 01.
- 12. Iami T, Numachi K, Oizumi J, Sato S (1965) Studies on the Mass Mortality of the Oyster in Matsushima BayII. Search for Cause of Mass Mortality and the Possibility to Prevent it by Transplantation Experiment. Bulletin of the Tohoku National Fisheries Research Institute 25: 27–38. [Google Scholar]
- 13. Cheney DP, MacDonald BF, Elston RA (2000) Summer mortality of Pacific oysters, Crassostrea gigas (Thunberg): Initial findings on multiple environmental stressors in Puget Sound, Washington, 1998. Journal of Shellfish Research 19: 353–359. [Google Scholar]
- 14. Perdue JA, Beattie JH, Chew KK (1981) Some relationships between gametogenic cycle and summer mortality phenomenon in the Pacific oyster (Crassostrea gigas) in Washington State. Journal of Shellfish research 1: 9–16. [Google Scholar]
- 15.Goulletquer P, Soletchnik P, Le Moine O, Razet D, Geairon P, et al. (1998) Summer mortality of the Pacific cupped oyster Crassostrea gigas in the Bay of Marennes-Oléron (France). ICES Mariculture Committee CM, Copenhagen. [Google Scholar]
- 16. Watermann BT, Herlyn M, Daehne B, Bergmann S, Meemken M, et al. (2008) Pathology and mass mortality of Pacific oysters, Crassostrea gigas (Thunberg), in 2005 at the East Frisian coast, Germany. Journal of Fish Disease 31: 621–630. [DOI] [PubMed] [Google Scholar]
- 17. ESFA (2011) Scientific opinion of the panel on animal health and welfare on a request from the European Commission on the increased mortality events in Pacific oysters Crassostrea gigas . European Food Safety Association 8: 1894–1953. [Google Scholar]
- 18. Li Y, Qin JG, Abbott CA, Li XX, Benkendorff K (2007) Synergistic impacts of heat shock and spawning on the physiology and immune health of Crassostrea gigas: an explanation for summer mortality in Pacific oysters. American Journal of Physiology-Regulatory Integrative and Comparative Physiology 293: 2353–2362. [DOI] [PubMed] [Google Scholar]
- 19. Li Y, Qin JG, Li XX, Benkendorff K (2009) Spawning-dependent stress response to food deprivation in Pacific oyster Crassostrea gigas . Aquaculture 286: 309–317. [Google Scholar]
- 20. Malham SK, Cotter E, O'Keeffe S, Lynch S, Culloty SC, et al. (2009) Summer mortality of the Pacific oyster, Crassostrea gigas, in the Irish Sea: The influence of temperature and nutrients on health and survival. Aquaculture 287: 128–138. [Google Scholar]
- 21. Soletchnik P, Lambert C, Costil K (2005) Summer Mortality of Crassostrea gigas (Thunberg) in relation to environmental rearing conditions. Journal of Shellfish Research 24: 197–207. [Google Scholar]
- 22. Wendling CC, Wegner KM (2013) Relative contribution of reproductive investment, thermal stress and Vibrio infection to summer mortality phenomena in Pacific oytsers. Aquaculture 412–413: 88–96. [Google Scholar]
- 23. Gómez-León J, Villamil L, Lemos M, Novoa B, Figueras A (2005) Isolation of Vibrio alginolyticus and Vibrio splendidus from Aquacultured Carpet Shell Clam (Ruditapes decussatus) Larvae Associated with Mass Mortalities. Applied and Environmental Microbiology 71: 98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jeffries V (1982) Three Vibrio strains pathogenic to larvae of Crassostrea gigas and Ostrea edulis . Aquaculture 29: 201–226. [Google Scholar]
- 25. Le Roux F, Gay M, Lambert C, Waechter M, Poubalanne S, et al. (2002) Comparative analysis of Vibrio splendidus-related strains isolated during Crassostrea gigas mortality events. Aquatic Living Resources 15: 251–258. [Google Scholar]
- 26. Saulnier D, Decker SD, Haffner P, Cobret L, Robert M, et al. (2010) A Large-Scale Epidemiological Study to Identify Bacteria Pathogenic to Pacific Oyster Crassostrea gigas and Correlation Between Virulence and Metalloprotease Activity. Microbial Ecology 59: 787–798. [DOI] [PubMed] [Google Scholar]
- 27. Sugumar G, Nakai T, Hirata Y, Matsubara D, Muroga K (1998) Vibrio splendidus biovar II as the causative agent of bacillary necrosis of Japanese oyster Crassostrea gigas larvae. Diseases of Aquatic Organisms 33: 111–118. [DOI] [PubMed] [Google Scholar]
- 28. Thompson J, Randa M, Marcelino L, Tomita-Mitchell A (2004) Diversity and Dynamics of a North Atlantic Coastal Vibrio Community. Applied and Environmental Microbiology 70: 4103–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hunt DE, David LA, Gevers D, Preheim SP, Alm EJ, et al. (2008) Resource partitioning and sympatric differentiation among closely related bacterioplankton. Science 320: 1081–1085. [DOI] [PubMed] [Google Scholar]
- 30. Harvell D, Altizer S, Cattadori IM, Harrington L, Weil E (2009) Climate change and wildlife diseases: When does the host matter the most? Ecology 90: 912–920. [DOI] [PubMed] [Google Scholar]
- 31. Kimes NE, Grim CJ, Johnson WR, Hasan NA, Tall BD, et al. (2012) Temperature regulation of virulence factors in the pathogen Vibrio coralliilyticus . Isme Journal 6: 835–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rosenberg E, Ben-Haim Y (2002) Microbial diseases of corals and global warming. Environmental Microbiology 4: 318–326. [DOI] [PubMed] [Google Scholar]
- 33. Estes RM, Friedman CS, Elston RA, Herwig RP (2004) Pathogenicity testing of shellfish hatchery bacterial isolates on Pacific oyster Crassostrea gigas larvae. Diseases of Aquatic Organisms 58: 223–230. [DOI] [PubMed] [Google Scholar]
- 34. Waechter M, Le Roux F, Nicolas JL, Marissal E, Berthe F (2002) Characterisation of Crassostrea gigas spat pathogenic bacteria. Comptes Rendus Biologies 325: 231–238. [DOI] [PubMed] [Google Scholar]
- 35. Tubiash HS, Colwell RR, Sakazaki R (1970) Marine Vibrios Associated with Bacillary Necrosis a Disease of Larval and Juvenile Bivalve Mollusks. Journal of Bacteriology 103: 271–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pruzzo C, Gallo G, Canesi L (2005) Persistence of Vibrios in marine bivalves: the role of interactions with haemolymph components. Environmental Microbiology 7: 761–772. [DOI] [PubMed] [Google Scholar]
- 37. Beaz-Hidalgo R, Balboa S, Romalde J, Figueras M (2010) Diversity and pathogenicity of Vibrio species in cultured bivalve molluscs. Environmental Microbiology Reports 2: 34–43. [DOI] [PubMed] [Google Scholar]
- 38. Gomez-Gil B, Roque A, Lacuesta B, Rotllant G (2010) Diversity of Vibrios in the haemolymph of the spider crab Maja brachydactyla . Journal of Applied Microbiology 109: 918–926. [DOI] [PubMed] [Google Scholar]
- 39. Guisande J, Lago E, Prado S, Nieto T, Seguín R (2008) Genotypic Diversity of Culturable Vibrio Species Associated with the Culture of Oysters and Clams in Galicia and Screening of Their Pathogenic Potential. Journal of Shellfish Research 27: 801–809. [Google Scholar]
- 40. Wegner KM, Volkenborn N, Peter H, Eiler A (2013) Disturbance induced decoupling between host genetics and composition of the associated microbiome. BMC Microbiology 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pascual J, Macian MC, Arahal DR, Garay E, Pujalte MJ (2010) Multilocus sequence analysis of the central clade of the genus Vibrio by using the 16S rRNA, recA, pyrH, rpoD, gyrB, rctB and toxR genes. International Journal of Systematic and Evolutionary Microbiology 60: 154–165. [DOI] [PubMed] [Google Scholar]
- 42. Gueguen Y, Cadoret JP, Flament D, Barreau-Roumiguiere C, Girardot AL, et al. (2003) Immune gene discovery by expressed sequence tags generated from hemocytes of the bacteria-challenged oyster, Crassostrea gigas . Gene 303: 139–145. [DOI] [PubMed] [Google Scholar]
- 43. Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucelic Acids Research 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Waterhouse AM, Procter JB, Martin DMA, Clamp M, Barton GJ (2009) Jalview Version 2-a multiple sequence alignment editor and analysis workbench. Bioinformatics 25: 1189–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Posada D (2008) jModelTest: Phylogenetic model averaging. Molecular Biology and Evolution 25: 1253–1256. [DOI] [PubMed] [Google Scholar]
- 46. Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology 52: 696–704. [DOI] [PubMed] [Google Scholar]
- 47. Posada D, Buckley TR (2004) Model selection and model averaging in phylogenetics: Advantages of akaike information criterion and Bayesian approaches over likelihood ratio tests. Systematic Biology 53: 793–808. [DOI] [PubMed] [Google Scholar]
- 48. Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, et al. (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology 59: 307–321. [DOI] [PubMed] [Google Scholar]
- 49. Rodriguez F, Oliver JL, Marin A, Medina JR (1990) The General Stochastic-Model of Nucleotide Substitution. Journal of Theoretical Biology 142: 485–501. [DOI] [PubMed] [Google Scholar]
- 50. Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783–791. [DOI] [PubMed] [Google Scholar]
- 51. Letunic I, Bork P (2011) Interactive Tree Of Life v2: online annotation and display of phylogenetic trees made easy. Nucleic Acids Research 39: W475–W478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Letunic I, Bork P (2007) Interactive Tree Of Life (iTOL): an online tool for phylogenetic tree display and annotation. Bioinformatics 23: 127–128. [DOI] [PubMed] [Google Scholar]
- 53.Felsenstein J (2005) PHYLIP (Phylogeny Inference Package) version 3.6. Distributed by the author Department of Genome Sciences, University of Washington, Seattle. [Google Scholar]
- 54. Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, et al. (2009) Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Applied and Environmental Microbiology 75: 7537–7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lozupone C, Hamady M, Knight R (2006) UniFrac - An online tool for comparing microbial community diversity in a phylogenetic context. Bmc Bioinformatics 7: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lozupone C, Knight R (2005) UniFrac: a new phylogenetic method for comparing microbial communities. Applied and Environmental Microbiology 71: 8228–8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lozupone C, Lladser ME, Knights D, Stombaugh J, Knight R (2011) UniFrac: an effective distance metric for microbial community comparison. Isme Journal 5: 169–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Beaz-Hidalgo R, Cleenwerck I, Balboa S, Wachter MD, Thompson F, et al. (2008) Diversity of Vibrios associated with reare clams in Galicia (NW Spain). Systematic and Applied Microbiology 31: 215–222. [DOI] [PubMed] [Google Scholar]
- 59. Sawabe T, Kita-Tsukamoto K, Thompson FL (2007) Inferring the evolutionary history of Vibrios by means of multilocus sequence analysis. Journal of Bacteriology 189: 7932–7936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Preheim SP, Boucher Y, Wildschutte H, David LA, Veneziano D, et al. (2011) Metapopulation structure of Vibrionaceae among coastal marine invertebrates. Environmental Microbiology 13: 265–275. [DOI] [PubMed] [Google Scholar]
- 61. Elston RA, Hasegawa H, Humphrey KL, Polyak IK, Hase CC (2008) Re-emergence of Vibrio tubiashii in bivalve shellfish aquaculture: severity, environmental drivers, geographic extent and management. Diseases of Aquatic Organisms 82: 119–134. [DOI] [PubMed] [Google Scholar]
- 62. Hada HS, West PA, Lee JV, Stemmler J, Colwell RR (1984) Vibrio tubiashii Sp–Nov, a Pathogen of Bivalve Mollusks . International Journal of Systematic Bacteriology 34: 1–4. [Google Scholar]
- 63. Oberbeckmann S, Fuchs BM, Meiners M, Wichels A, Wiltshire KH, et al. (2012) Seasonal Dynamics and Modeling of a Vibrio Community in Coastal Waters of the North Sea. Microbial Ecology 63: 543–551. [DOI] [PubMed] [Google Scholar]
- 64. Thompson J, Pacocha S, Pharino C, Klepac-Ceraj V, Hunt D, et al. (2005) Genotypic Diversity Within a Natural Coastal Bacterioplankton Population. Science 307: 1311–1313. [DOI] [PubMed] [Google Scholar]
- 65. Maeda T, Matsuo Y, Furushita M, Shiba T (2003) Seasonal dynamics in a coastal Vibrio community examined by a rapid clustering method based on 16S rDNA. Fisheries Science 69: 385–394. [Google Scholar]
- 66. Vezzulli L, Previati M, Pruzzo C, Marchese A, Bourne DG, et al. (2010) Vibrio infections triggering mass mortality events in a warming Mediterranean Sea. Environmental Microbiology 12: 2007–2019. [DOI] [PubMed] [Google Scholar]
- 67. Buroker NE, Hershberger WK, Chew KK (1979) Population-Genetics of the Family Ostreidae .1. Intraspecific Studies of Crassostrea gigas and Saccostrea commercialis . Marine Biology 54: 157–169. [Google Scholar]
- 68. Groubert TN, Oliver JD (1994) Interaction of Vibrio vulnificus and the Eastern Oyster, Crassostrea virginica . Journal of Food Protection 57: 224–228. [DOI] [PubMed] [Google Scholar]
- 69. Imai T, Numachi K, Oizumi J, Sato S (1965) Studies on the Mass Mortality of the Oyster in Matsushima Bay II. Search for Cause of Mass Mortality and the Possibility to Prevent it by Transplantation Experiment. Bulletin of the Tohoku National Fisheries Research Institute 25: 27–38. [Google Scholar]
- 70. Li Y, Qin JG, Li XX, Benkendorff K (2009) Spawning-dependent stress responses in pacific oysters Crassostrea gigas: A simulated bacterial challenge in oysters. Aquaculture 293: 164–171. [Google Scholar]
- 71. Renault T, Le Deuff RM, Cochennec N, Maffart P (1994) Herpesviruses associated with mortalities among Pacific oyster, Crassostrea gigas, in France-Comparative study. Revue Méd Vél 145: 735–742. [Google Scholar]
- 72. Boer SI, Heinemeyer EA, Luden K, Erler R, Gerdts G, et al. (2013) Temporal and Spatial Distribution Patterns of Potentially Pathogenic Vibrio spp. at Recreational Beaches of the German North Sea. Microbial Ecology 65: 1052–1067. [DOI] [PubMed] [Google Scholar]
- 73. Thieltges DW, Engelsma MY, Wendling CC, Wegner KM (2012) Parasites in the Wadden Sea food web. Journal of Sea Research 82: 122–133. [Google Scholar]
- 74. Buchrieser C, Gangar VV, Murphree RL, Tamplin ML, Kaspar CW (1995) Multiple Vibrio vulnificus Strains in Oysters as Demonstrated by Clamped Homogeneous Electric-Field Gel-Electrophoresis. Applied and Environmental Microbiology 61: 1163–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jackson JK, Murphree RL, Tamplin ML (1997) Evidence that mortality from Vibrio vulnificus infection results from single strains among heterogeneous populations in shellfish. Journal of Clinical Microbiology 35: 2098–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Harvell CD, Kim K, Burkholder JM, Colwell RR, Epstein PR, et al. (1999) Emerging marine diseases–climate links and anthropogenic factors. Science 285: 1505–1510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlation between colony forming units and OD values at 550 nm for 10 selected Vibrio strains of low, intermediate, and high virulence. An OD value of 1 at 550 nm corresponds to 4.63–5.24×108 CFU/ml.
(TIF)
Sequences of all primers used for genotyping the bacteria of the genus Vibrio spp. ( pyrH , GyrB , 16S rRNA).
(DOCX)
Reference Vibrio sequences used for the phylogeny. The references sequences were aligned to the sequences of the particular gene (16S rDNA, pyrH, GyrB).
(DOCX)