Abstract
Objective
Higher circulating levels of tissue inhibitor of matrix metalloproteinases (TIMP)-1 at the time of severe sepsis diagnosis have been reported in nonsurviving than in surviving patients. However, the following questions remain unanswered: 1) Does TIMP-1/MMP-9 ratio differ throughout the first week of intensive care between surviving and non-surviving patients? 2) Is there an association between TIMP-1/MMP-9 ratio and sepsis severity and mortality during such period? 3) Could TIMP-1/MMP-9 ratio during the first week be used as an early biomarker of sepsis outcome? 4) Is there an association between TIMP-1/MMP-9 ratio and coagulation state and circulating cytokine levels during the first week of intensive care in these patients? The present study sought to answer these questions.
Methods
Multicenter, observational and prospective study carried out in six Spanish Intensive Care Units (ICUs) of 295 patients with severe sepsis. Were measured circulating levels of TIMP-1, MMP-9, tumour necrosis factor (TNF)-alpha, interleukin (IL)-10 and plasminogen activator inhibitor (PAI)-1 at day 1, 4 and 8. End-point was 30-day mortality.
Results
We found higher TIMP-1/MMP-9 ratio during the first week in non-surviving (n = 98) than in surviving patients (n = 197) (p<0.01). Logistic regression analyses showed that TIMP-1/MMP-9 ratio at days 1, 4 and 8 was associated with mortality. Receiver operating characteristic (ROC) curves showed that TIMP-1/MMP-9 ratio at days 1, 4 and 8 could predict mortality. There was an association between TIMP-1/MMP-9 ratio and TNF-alpha, IL-10, PAI-1 and lactic acid levels, SOFA score and platelet count at days 1, 4 and 8.
Conclusions
The novel findings of our study were that non-surviving septic patients showed persistently higher TIMP-1/MMP-9 ratio than survivors ones during the first week, which was associated with severity, coagulation state, circulating cytokine levels and mortality; thus representing a new biomarker of sepsis outcome.
Introduction
Sepsis represents a systemic response of the immune system to infection leading to high mortality and costs [1], [2]. Matrix metalloproteinases (MMPs) are a family of zinc-containing endoproteinases that are implicated in degradation and remodelling of the extracellular matrix (ECM) [3]; and also in proteolysis of intracellular protein [4]–[6], and are involved in innate immune defence and apoptosis. The regulation of MMP activity is complex and occurs at several levels [7], such as transcriptional (MMP gene expression in cells) which is modified by TNF-α, interleukin-1β and transforming growth factor (TGF)-β; post- transcriptional (stability of MMP transcripts in cells) which is influenced by glucocorticoids and TGF-β; translational (release of MMP from cells) which is regulated by plasmin and thrombin; and post-translational (activation of MMPs after the release) which is affected by oxidative stress, nitrosative stress, phosphorylation [8], proteolysis and tissue inhibitors of matrix metalloproteinases (TIMPs). MMPs and TIMPs help modulate inflammatory [9] and prothrombotic responses [10], [11].
Previous clinical studies including our own have shown higher circulating levels of MMP-9 and TIMP-1 in septic patients than in controls [12]–[22], and higher levels of TIMP-1 [15], [19], [20], [23] at the time of severe sepsis diagnosis in non-surviving than in surviving patients. In addition, an association between circulating TIMP-1 and plasminogen activator inhibitor (PAI)-1 levels in septic patients at severe sepsis diagnosis has been reported [23]. However, the following questions remain unanswered: 1) Does TIMP-1/MMP-9 ratio differ throughout the first week of intensive care between surviving and non-surviving septic patients? 2) Is there an association between TIMP- 1/MMP-9 ratio and sepsis severity during this period? 3) Is there an association between TIMP-1/MMP-9 ratio during the first week and sepsis mortality? 4) Could TIMP- 1/MMP-9 ratio be used as an early biomarker of sepsis outcome? 5) Is there an association between TIMP-1/MMP-9 ratio and coagulation state during the first week of intensive care in these patients? 6) Is there an association between TIMP-1/MMP-9 ratio and circulating cytokine levels during the first week of intensive care in septic patients? The present study sought to answer these questions. We focus on the determination of the TIMP-1/MMP-9 ratio because in previous studies we assessed serum TIMP-1 and MMP-9 levels [19], [23], and because MMP-9 is inhibited by TIMP-1.
Methods
Design and Subjects
A prospective, multicenter observational study was carried out in six Spanish intensive care units (ICUs). The Institutional Ethic Review Boards of the six hospitals approved this study: Hospital Universitario de Canarias (La Laguna. Santa Cruz de Tenerife. Spain), Hospital Universitario Nuestra Señora de Candelaria (Santa Cruz de Tenerife. Spain), Hospital Universitario Dr. Negrín (Las Palmas de Gran Canaria. Spain), Hospital Clínico Universitario de Valencia (Valencia. Spain), Hospital San Jorge (Huesca. Spain) and Hospital Insular (Las Palmas de Gran Canaria. Spain). Written informed consent from the patients or from their family members was obtained.
Patients admitted to the ICU of the participating hospitals and meeting the criteria for severe sepsis were included in the present study. The diagnosis of severe sepsis was established according to the International Sepsis Definitions Conference [24]. Exclusion criteria were: age <18 years, pregnancy, lactation, human immunodeficiency virus (HIV), white blood cell count <103/mm3, solid or hematological tumors or immunosuppressive, steroid or radiation therapy.
Variables recorded
The following variables were recorded for each patient: sex, age, diabetes mellitus, chronic obstructive pulmonary disease (COPD), ischemic heart disease, site of infection, microorganism responsible, bloodstream infection, antimicrobial treatment, pressure of arterial oxygen/fraction of inspired oxygen (PaO2/FIO2), creatinine, bilirubin, leukocytes, lactic acid, platelets, international normalized ratio (INR), activated partial thromboplastin time (aPTT), Sepsis-related Organ Failure Assessment [SOFA] score [25] and Acute Physiology and Chronic Health Evaluation (APACHE)-II score [26]. We assessed survival at 30 days as the endpoint.
Proteolytic, inflammatory and prothrombotic marker assays
Blood samples were collected at days 1, 4 and 8 of ICU admission. Serum separator tubes were used to determine MMP-9, TIMP-1, TNF-alpha and IL-10 concentrations, and venous citrated plasma tubes to determine PAI-1 concentration. Venous blood samples were taken and centrifuged within 30 minutes at 1000 g for 15 min, and frozen at −80°C until assayed.
The assay of MMP-9 and TIMP-1 was centralized in Atherosclerosis Research Laboratory of CIMA-University of Navarra (Pamplona, Spain). Specific ELISA (Quantikine, R&D Systems, Abingdon, United Kingdom) was used according to manufacturer's instructions with a serum dilution of 1∶80 and 1∶100 respectively. The interassay coefficients of variation (CV) were <8% (n = 20) and the detection limits for the assays were 0.156 ng/ml and 0.08 ng/ml respectively.
Assays for TNF-alpha, IL-10 and PAI-1 were performed at the Laboratory Department of the Hospital Universitario de Canarias (La Laguna, Santa Cruz de Tenerife, Spain). TNF-alpha and IL-10 were measured in serum by solid-phase chemiluminescent immunometric assays (Immulite, Siemens Healthcare Diagnostics Products, Llanberis, United Kingdom). The interassay CV were <6.5% (n = 20) and <9.9% (n = 40) respectively, and the detection limits for the assays were 1.7 pg/ml and 1 pg/ml respectively. PAI-1 antigen was assayed by specific ELISA (Imubind Plasma PAI-1 ElisaTM, American Diagnostica, Inc, Stanford, CT, USA). This assay detects latent (inactive) and active forms of PAI-1 and PAI-1 complexes. The interassay CV was <5% (n = 20) and the detection limit for the assay was 1 ng/ml.
Statistical Methods
Continuous variables are reported as medians and interquartile ranges. Categorical variables are reported as frequencies and percentages. Comparisons of continuous variables between groups were carried out using Wilcoxon, Mann-Whitney or Friedman tests. Comparisons between groups for categorical variables were carried out with chi-square test. Logistic regression analyses were applied to determine the independent contribution of TIMP-1/MMP-9 ratio at days 1, 4 and 8 on 30-day mortality, controlling for SOFA score and diabetes mellitus. To avoid the collinearity effect, we included only SOFA score and age as co-predictors. Odds ratio (OR) and 95% confidence intervals (CI) were calculated as measures of the clinical impact of the predictor variables. We plotted receiver operating characteristic (ROC) curves using survival at 30 days as the classification variable and TIMP-1/MMP-9 ratio at days 1, 4 and 8 as prognostic variables. The association between continuous variables was assessed using Spearman's rank correlation coefficient. A P value of less than 0.05 was considered statistically significant. Statistical analyses were performed with SPSS 17.0 (SPSS Inc., Chicago, IL, USA) and NCSS 2000 (Kaysville, Utah).
Results
Comparison of demographic and clinical parameters between non-surviving (n = 98) and surviving septic patients (n = 197) is shown in Table 1. Non-survivors were older and showed higher diabetes mellitus, creatinine, lactic acid, INR, aPTT, SOFA and APACHE-II, and lower platelet count than survivors. No differences were observed regarding sex, COPD, ischemic heart disease, site of infection, microorganism responsible, bloodstream infection and antimicrobial treatment.
Table 1. Baseline clinical and biochemical characteristics of survivor and non-survivor patients.
| Survivors (n = 197) | Non-survivors (n = 98) | P | |
| Male sex– n (%) | 65 (23.0) | 35 (35.7) | 0.70 |
| Age - median years (percentile 25–75) | 59 (47–69) | 64 (55–74) | 0.004 |
| Diabetes Mellitus – n (%) | 49 (24.9) | 38 (38.8) | 0.01 |
| COPD - n (%) | 26 (13.2) | 13 (13.3) | 0.99 |
| Ischemic heart disease - n (%) | 21(11.0) | 10 (10.3) | 0.99 |
| Site of infection | 0.67 | ||
| Respiratory - n (%) | 111 (56.3) | 58 (59.2) | |
| Abdominal - n (%) | 55 (27.9) | 25 (25.5) | |
| Urinary - n (%) | 11 (5.6) | 4 (4.1) | |
| Skin - n (%) | 9 (4.6) | 4 (4.1) | |
| Endocarditis - n (%) | 6 (3.0) | 5 (5.1) | |
| Arthritis - n (%) | 0 | 1 (1.0) | |
| CNS- n (%) | 5 (2.5) | 1 (1.0) | |
| Microorganism responsibles | |||
| Unknown- n (%) | 97 (49.2) | 51 (52.0) | 0.71 |
| Gram-positive- n (%) | 50 (25.4) | 25 (25.5) | 0.99 |
| Gram-negative- n (%) | 50 (25.4) | 21 (21.4) | 0.47 |
| Fungii- n (%) | 4 (2.0) | 4 (4.1) | 0.45 |
| Anaerobe - n (%) | 2 (1.0) | 1 (1.0) | 0.99 |
| Bloodstream infection - n (%) | 30 (15.2) | 17 (17.3) | 0.74 |
| Empiric antimicrobial treatment adequate | 0.68 | ||
| Unknown due to negative cultures - n (%) | 97 (49.2) | 52 (53.1) | |
| Unknown due to diagnosis by antigenuria - n (%) | 14 (7.1) | 4 (4.1) | |
| Adequate - n (%) | 82 (41.6) | 39 (39.8) | |
| Inadequate - n (%) | 4 (2.0) | 3 (3.1) | |
| Treatment with betalactamic more aminoglycoside - n (%) | 43 (22.1) | 24 (24.5) | 0.66 |
| Treatment with betalactamic more quinolone - n (%) | 101 (51.8) | 51 (52.0) | 0.99 |
| Pa02/FI02 ratio - median (percentile 25–75) | 180 (122–270) | 168 (101–240) | 0.12 |
| Creatinine (mg/dl) - median (percentile 25–75) | 1.30 (0.84–2.10) | 1.60 (1.00–2.90) | 0.009 |
| Bilirubin (mg/dl) - median (percentile 25–75) | 0.90 (0.50–1.47) | 0.90 (0.50–2.30) | 0.41 |
| Leukocytes - median*103/mm3 (percentile 25–75) | 14.7 (9.2–20.0) | 15.0 (7.1–20.6) | 0.85 |
| Lactic acid - median mmol/L (percentile 25–75) | 2.00 (1.10–3.40) | 3.55 (1.60–6.00) | <0.001 |
| Platelets - median*103/mm3 (percentile 25–75) | 197 (131–273) | 132 (63–222) | <0.001 |
| INR - median (percentile 25–75) | 1.27 (1.10–1.51) | 1.42 (1.14–1.89) | 0.008 |
| aPTT - median seconds (percentile 25–75) | 32 (28–39) | 36 (29–45) | 0.008 |
| SOFA score - median (percentile 25–75) | 9 (7–11) | 11 (9–14) | <0.001 |
| APACHE-II score - median (percentile 25–75) | 19 (15–23) | 24 (19–29) | <0.001 |
| TIMP-1– median ng/ml (percentile 25–75) | 548 (372–732) | 750 (479–1020) | <0.001 |
| MMP-9 - median ng/ml (percentile 25–75) | 760 (359–1208) | 533 (231–952) | 0.003 |
| TIMP-1/MMP-9 ratio (percentile 25–75) | 0.71 (0.40–1.54) | 1.26 (0.64–3.86) | 0.003 |
| TNF-alpha - median pg/ml (percentile 25–75) | 30 (20–50) | 36 (18–74) | 0.24 |
| IL-10 - median pg/ml (percentile 25–75) | 11 (6–37) | 38 (9–119) | <0.001 |
| PAI-1 - median ng/ml (percentile 25–75) | 36 (21–62) | 58 (33–84) | <0.001 |
COPD = chronic obstructive pulmonary disease; CNS = Central Nervous System; PaO2/FIO2 = pressure of arterial oxygen/fraction inspired oxygen; INR = international normalized ratio; aPTT = activated partial thromboplastin time; SOFA = Sepsis-related Organ Failure Assessment score; APACHE II = Acute Physiology and Chronic Health Evaluation; MMP = matrix metalloproteinase; TIMP = tissue inhibitor of matrix metalloproteinase; TNF = tumour necrosis factor; IL = Interleukin; PAI = Plasminogen activator inhibitor.
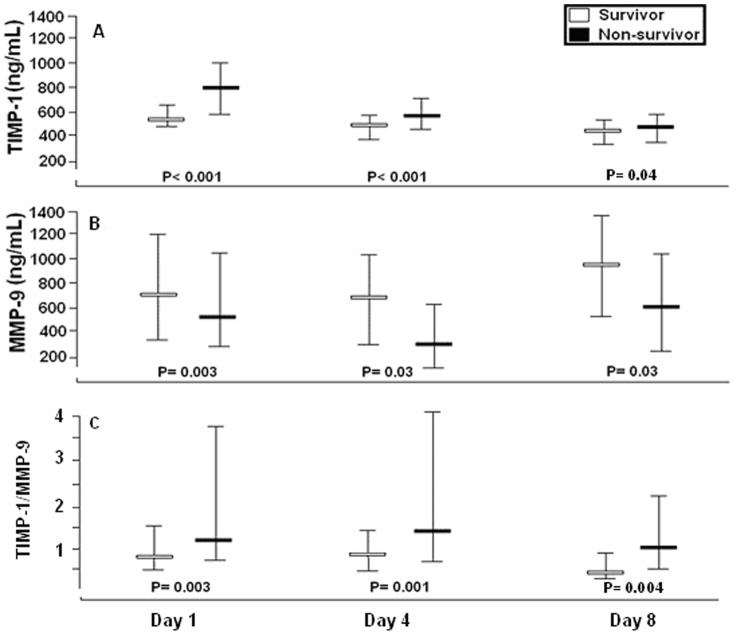
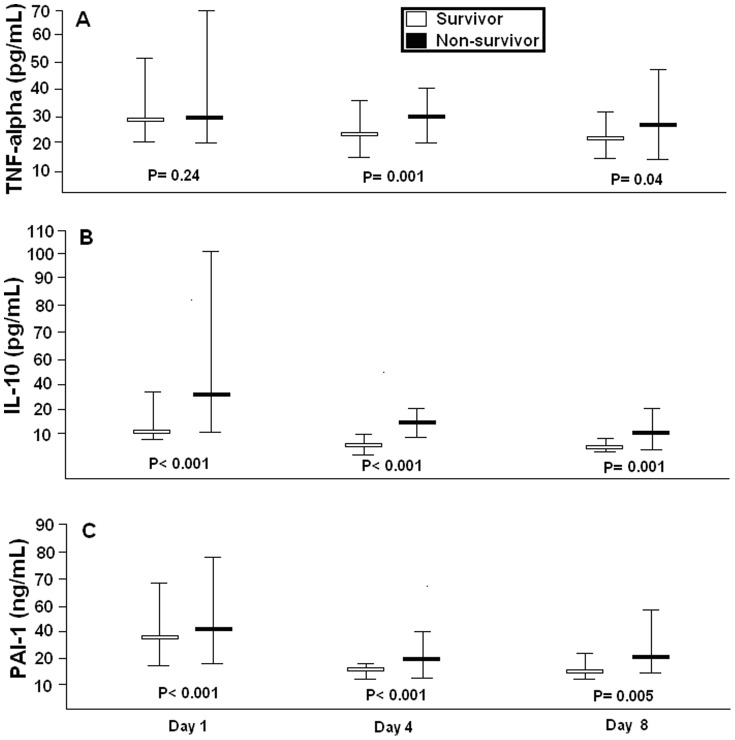
Non-surviving septic patients showed higher serum TIMP-1 levels, lower serum MMP-9 levels and higher TIMP-1/MMP-9 ratio than survivors at days 1, 4 and 8 (Table 1, Figure 1). Additionally, non-surviving septic patients exhibited higher PAI-1 and IL- 10 levels at days 1, 4 and 8, and higher TNF-alpha at day 4 and 8 than survivors (Table 1 and Figure 2).
Figure 1. Serum MMP-9, TIMP-1 and TIMP-1/MMP-9 ratio in survivor and non-survivor septic patient showed as median (percentiles 25–75).
MMP = Matrix metalloproteinase; TIMP = Tissue inhibitor of matrix metalloproteinase.
Figure 2. Serum TNF-alpha and IL-10 levels and plasma PAI-1 levels in survivor and non-survivor septic patients showed as median (percentile 25–75).
TNF = tumour necrosis factor; IL = Interleukin; PAI = Plasminogen activator inhibitor.
Logistic regression analysis showed that TIMP-1/MMP-9 ratios at day 1 (OR = 1.11; 95% CI = 1.011–1.223; p = 0.03), day 4 (OR = 1.27; 95% CI = 1.004–1.617; p = 0.047) and day 8 (OR = 1.76; 95% CI = 1.041–2.977; p = 0.03) were associated with higher mortality at 30 days, controlling for SOFA score (Table 2).
Table 2. Logistic regression analyses to predict 30-day mortality.
| Odds Ratio | 95% Confidence IntervalP | ||||
| First model: | |||||
| TIMP-1/MMP-9 ratio at day 1 | 1.11 | 1.011–1.223 | 0.03 | ||
| SOFA score at day 1 | 1.17 | 1.083–1.268 | <0.001 | ||
| Diabetes mellitus | 1.99 | 1.137–3.489 | 0.02 | ||
| Second model: | |||||
| TIMP-1/MMP-9 ratio at day 4 | 1.27 | 1.004–1.617 | 0.047 | ||
| SOFA score at day 4 | 1.15 | 1.050–1.267 | 0.003 | ||
| Diabetes mellitus | 2.76 | 1.182–6.425 | 0.02 | ||
| Third model: | |||||
| TIMP-1/MMP-9 ratio at day 8 | 1.76 | 1.041–2.977 | 0.03 | ||
| SOFA score at day 8 | 1.19 | 1.064–1.334 | 0.002 | ||
| Diabetes mellitus | 1.93 | 0.652–5.721 | 0.23 | ||
TIMP = tissue inhibitor of matrix metalloproteinase; MMP = matrix metalloproteinase; SOFA = Sepsis-related Organ Failure Assessment score.
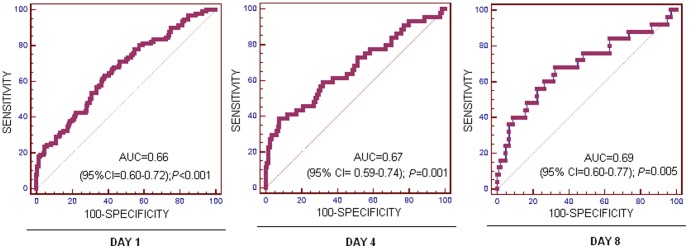
On ROC curve analysis, the area under the curve of TIMP-1/MMP-9 ratios at day 1, 4 and 8 to predict 30-day survival were 0.66 (95% CI = 0.60–0.72; p<0.001), 0.67 (95% CI = 0.59–0.74; p = 0.001) and 0.69 (95% CI = 0.60–0.77; p = 0.005) respectively, (Figure 3).
Figure 3. Receiver operation characteristic analyses using TIMP-1/MMP-9 ratio at day 1, 4 and 8 after admission as predictor of mortality at 30 days in severe septic patients.
As shown in Table 3, TIMP-1/MMP-9 ratios at days 1, 4 and 8 correlated with circulating TNF-alpha, IL-10, PAI-1 and lactic acid levels, SOFA score, and platelet count, and at days 1 and 4 with INR and aPTT.
Table 3. Correlations between circulating TIMP-1/MMP-9 ratio levels and TNF-alpha, IL-10, PAI-1, lactic acid, SOFA score, platelet, INR and aPTT at days 1, 4 and 8.
| Day 1 | Day 4 | Day 8 | |
| TNF-alpha | rho = 0.45; p<0.001 | rho = 0.20; p = 0.04 | rho = 0.25; p = 0.01 |
| IL-10 | rho = 0.51; p<0.001 | rho = 0.33; p<0.001 | rho = 0.19; p = 0.05 |
| PAI-1 | rho = 0.36; p<0.001 | rho = 0.34; p<0.001 | rho = 0.23; p = 0.01 |
| Lactic acid | rho = 0.45; p<0.001 | rho = 0.31; p<0.001 | rho = 0.21; p = 0.02 |
| SOFA score | rho = 0.51; p<0.001 | rho = 0.31; p<0.001 | rho = 0.36; p<0.001 |
| Platelet | rho = −0.46; p<0.001 | rho = −0.34; p<0.001 | rho = −0.26; p = 0.004 |
| INR | rho = 0.49; p<0.001 | rho = 0.23; p = 0.01 | rho = 0.11; p = 0.26 |
| aPTT | rho = 0.34; p<0.001 | rho = 0.22; p = 0.01 | rho = 0.08; p = 0.41 |
| Age | rho = 0.12; p = 0.02 | rho = 0.19; p = 0.01 | rho = 0.25; p = 0.003 |
TIMP = tissue inhibitor of matrix metalloproteinase; MMP = matrix metalloproteinase; TNF = tumour necrosis factor; IL = Interleukin; PAI = plasminogen activator inhibitor; SOFA = Sepsis-related Organ Failure Assessment score; INR = international normalized ratio; aPTT = activated partial thromboplastin time.
Discussion
A novel finding of our study was that non-surviving septic patients showed persistently higher circulating levels TIMP-1 levels, lower circulating MMP-9 levels and higher TIMP-1/MMP-9 ratio than survivors during the first week of ICU stay. In addition, we found for the first time that the TIMP-1/MMP-9 ratio during the first week was associated with mortality according to the results of logistic regression analyses. Two previous studies exploring MMP levels during the course of sepsis found no significant differences between surviving and non-surviving patients regarding TIMP-1 [21] and MMP-9 levels [21], [22]. The higher sample size of our study (295 patients) compared with the smaller size (20 and 44 patients respectively) of previous studies [21], [22] could explain the significant differences reported between surviving and non- surviving patients.
In a previous study by our team we found that TIMP-1 levels at the time of severe sepsis diagnosis could be used as a biomarker of sepsis outcome [19]. A novel finding of the current study was that TIMP-1/MMP-9 ratio during the first week could be used as a biomarker of sepsis outcome according to the results of ROC curve analysis. In addition, we previously reported that circulating TIMP-1 levels were associated with TNF-alpha, IL-10, PAI-1, SOFA score, lactic acid levels, platelet count, aPTT and INR [19]. Another novel finding of the present study was a significant association between TIMP-1/MMP-9 ratio and circulating cytokine levels, coagulation parameters and SOFA score during the first week.
Taken together, these results indicate that TIMP-1 and MMP-9 levels could play a pathophysiological role in septic patients. MMP-9 inhibits platelet aggregation [27], [28] and an association between circulating TIMP-1 and PAI-1 levels has been found [29], [30]. In addition, an association between PAI-1 levels and sepsis mortality has been reported [31]–[33]. Thus, it may be that the higher circulating levels TIMP-1 levels, lower circulating MMP-9 levels and higher TIMP-1/MMP-9 ratio found in non-surviving patients during the first week could favour a prothrombotic state and contribute to capillary thrombosis, multiple organ dysfunction, and death in septic patients.
From a therapeutic perspective, the development of modulators of MMP/TIMP activity could be used as a new class of drugs for the treatment of severe sepsis [34], [35].
The strengths of our study were the large sample size and the follow-up of circulating TIMP-1, MMP-9, TNF-alpha, IL-10, and PAI-1 levels throughout the first week after diagnosis of severe sepsis. However, our study has certain limitations. First, we did not analyze other MMPs and TIMPs; and it could be interesting to describe levels of other MMPs and TIMPs, and the ratios between them. Second, measurement of other cytokines and coagulation markers would be desirable in order to better evaluate the relationship between proteolysis, inflammation and coagulation in these patients. Third, we analyzed serum levels of MMP-9 and TIMP-1 but plasma determination is preferable because there could be a release of several MMPs or TIMPs from platelets in serum [36]–[39].
In conclusion, the novel findings of our study were that non-surviving septic patients showed persistently higher TIMP-1/MMP-9 ratio than survivors during the first week, which was associated with severity, coagulation state, circulating cytokine levels and mortality, thus representing a new biomarker of sepsis outcome.
Funding Statement
This study was supported, in part, by grants from Instituto de Salud Carlos III (FIS-PI-10-01572, I3SNS-INT-11-063 and I3SNS-INT-12-087) (Madrid, Spain) and co-financed by Fondo Europeo de Desarrollo Regional (FEDER), CIMA (University of Navarra, Pamplona, Spain), and Sociedad Española de Trombosis y Hemostasia (SETH). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Vincent JL, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, et al. (2006) Sepsis in European intensive care units: results of the SOAP study. Crit Care Med 34: 344–353. [DOI] [PubMed] [Google Scholar]
- 2. Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, et al. (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29: 1303–1310. [DOI] [PubMed] [Google Scholar]
- 3. Brinckerhoff CE, Matrisian LM (2002) Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol 3: 207–214. [DOI] [PubMed] [Google Scholar]
- 4. Cauwe B, Opdenakker G (2010) Intracellular substrate cleavage: a novel dimension in the biochemistry, biology and pathology of matrix metalloproteinases. Crit Rev Biochem Mol Biol 45: 351–423. [DOI] [PubMed] [Google Scholar]
- 5. Wang W, Schulze CJ, Suarez-Pinzon WL, Dyck JR, Sawicki G, et al. (2002) Intracellular action of matrix - metalloproteinase-2 accounts for acute myocardial ischemia and reperfusion injury. Circulation 106: 1543–1549. [DOI] [PubMed] [Google Scholar]
- 6. Schulz R (2007) Intracellular targets of matrix metalloproteinase-2 in cardiac disease: rationale and therapeutic approaches. Annu Rev Pharmacol Toxicol 47: 211–242. [DOI] [PubMed] [Google Scholar]
- 7. Sternlicht MD, Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17: 463–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sariahmetoglu M, Crawford BD, Leon H, Sawicka J, Li L, et al. (2007) Regulation of matrix metalloproteinase-2 (MMP-2) activity by phosphorylation. FASEB J 21: 2486–2495. [DOI] [PubMed] [Google Scholar]
- 9. Elkington PT, O'Kane CM, Friedland JS (2005) The paradox of matrix metalloproteinases in infectious disease. Clin Exp Immunol 142: 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lijnen H (2002) Matrix metalloproteinases and cellular fibrinolytic activity. Biochemistry (Mosc) 67: 92–98. [DOI] [PubMed] [Google Scholar]
- 11. Santos-Martínez MJ, Medina C, Jurasz P, Radomski MW (2008) Role of metalloproteinases in platelet function. Thromb Res 121: 535–542. [DOI] [PubMed] [Google Scholar]
- 12. de Lizarrondo SM, Roncal C, Calvayrac O, Rodríguez C, Varo N, et al. (2012) Synergistic effect of thrombin and CD40 ligand on endothelial matrix metalloproteinase-10 expression and microparticle generation in vitro and in vivo. Arterioscler Thromb Vasc Biol 32: 1477–1487. [DOI] [PubMed] [Google Scholar]
- 13. Pugin J, Widmer MC, Kossodo S, Liang CM, Preas H, et al. (1999) Human neutrophils secrete gelatinase B in vitro and in vivo in response to endotoxin and proinflammatory mediators. Am J Respir Cell Mol Biol 20: 458–464. [DOI] [PubMed] [Google Scholar]
- 14. Yassen KA, Galley HF, Webster NR (2001) Matrix metalloproteinase-9 concentrations in critically ill patients. Anaesthesia 56: 729–732. [DOI] [PubMed] [Google Scholar]
- 15. Hoffmann U, Bertsch T, Dvortsak E, Liebetrau C, Lang S, et al. (2006) Matrix-metalloproteinases and their inhibitors are elevated in severe sepsis: prognostic value of TIMP-1 in severe sepsis. Scand J Infect 38: 867–872. [DOI] [PubMed] [Google Scholar]
- 16. Nakamura T, Ebihara I, Shimada N, Shoji H, Koide H (1998) Modulation of plasma metalloproteinase-9 concentrations and peripheral blood monocyte mRNA levels in patients with septic shock: effect of fiber-immobilized polymyxin B treatment. Am J Med Sci 316: 355–360. [DOI] [PubMed] [Google Scholar]
- 17. Ricou B, Nicod L, Lacraz S, Welgus HG, Suter PM, et al. (1996) Matrix metalloproteinases and TIMP in acute respiratory distress syndrome. Am J Respir Crit Care Med 154: 346–352. [DOI] [PubMed] [Google Scholar]
- 18. Hartog CM, Wermelt JA, Sommerfeld CO, Eichler W, Dalhoff K, et al. (2003) Pulmonary matrix metalloproteinase excess in hospital-acquired pneumonia. Am J Respir Crit Care Med 167: 593–598. [DOI] [PubMed] [Google Scholar]
- 19. Lorente L, Martin MM, Labarta L, Diaz C, Sole-Violan J, et al. (2009) Matrix metalloproteinase-9, -10, and tissue inhibitor of matrix metalloproteinases-1 blood levels as biomarkers of severity and mortality in sepsis. Crit Care 13: R158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lauhio A, Hästbacka J, Pettilä V, Tervahartiala T, Karlsson S, et al. (2011) Serum MMP-8, -9 and TIMP-1 in sepsis: High serum levels of MMP-8 and TIMP-1 are associated with fatal outcome in a multicentre, prospective cohort study. Hypothetical impact of tetracyclines. Pharmacol Res 64: 590–594. [DOI] [PubMed] [Google Scholar]
- 21. Yazdan-Ashoori P, Liaw P, Toltl L, Webb B, Kilmer G, et al. (2011) Elevated plasma matrix metalloproteinases and their tissue inhibitors in patients with severe sepsis. J Crit Care 26: 556–565. [DOI] [PubMed] [Google Scholar]
- 22. Gäddnäs FP, Sutinen MM, Koskela M, Tervahartiala T, Sorsa T, et al. (2010) Matrix-metalloproteinase-2, -8 and -9 in serum and skin blister fluid in patients with severe sepsis. Crit Care 14: R49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lorente L, Martín MM, Plasencia F, Solé-Violán J, Blanquer J, et al. (2013) The 372 T/C genetic polymorphism of TIMP-1 is associated with serum levels of TIMP-1 and survival in patients with severe sepsis. Crit Care 17: R94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, et al. (2003) International Sepsis Definitions Conference. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med 29: 530–538. [DOI] [PubMed] [Google Scholar]
- 25. Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, et al. (1996) The Sepsis-related Organ Failure Assessment (SOFA) score to describe organ dysfunction/failure. Intensive Care Med 22: 707–710. [DOI] [PubMed] [Google Scholar]
- 26. Knaus WA, Draper EA, Wagner DP, Zimmerman JE (1985) APACHE II: a severity of disease classification system. Crit Care Med 13: 818–829. [PubMed] [Google Scholar]
- 27. Sheu JR, Fong TH, Liu CM, Shen MY, Chen TL, et al. (2004) Expression of matrix metalloproteinase-9 in human platelets: regulation of platelet activation in in vitro and in vivo studies. Br J Pharmacol 143: 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lee YM, Lee JJ, Shen MY, Hsiao G, Sheu JR (2006) Inhibitory mechanisms of activated matrix metalloproteinase-9 on platelet activation. Eur J Pharmacol 537: 52–58. [DOI] [PubMed] [Google Scholar]
- 29. Aznaouridis K, Vlachopoulos C, Dima I, Vasiliadou C, Ioakeimidis N, et al. (2007) Divergent associations of tissue inhibitors of metalloproteinases-1 and -2 with the prothrombotic/fibrinolytic state. Atherosclerosis 195: 212–215. [DOI] [PubMed] [Google Scholar]
- 30. Cavusoglu E, Ruwende C, Chopra V, Yanamadala S, Eng C, et al. (2006) Tissue inhibitor of metalloproteinase-1 (TIMP-1) is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction. Am Heart J 151: 1101.e1–8. [DOI] [PubMed] [Google Scholar]
- 31. Madoiwa S, Nunomiya S, Ono T, Shintani Y, Ohmori T, et al. (2006) Plasminogen activator inhibitor 1 promotes a poor prognosis in sepsis-induced disseminated intravascular coagulation. Int J Hematol 84: 398–405. [DOI] [PubMed] [Google Scholar]
- 32. Hermans PW, Hibberd ML, Booy R, Daramola O, Hazelzet JA, et al. (1999) 4G/5G promoter polymorphism in the plasminogen-activator-inhibitor-1 gene and outcome of meningococcal disease. Meningococcal Research Group. Lancet 354: 556–560. [DOI] [PubMed] [Google Scholar]
- 33. Pralong G, Calandra T, Glauser MP, Schellekens J, Verhoef J, et al. (1989) Plasminogen activator inhibitor 1: a new prognostic marker in septic shock. Thromb Haemost 61: 459–462. [PubMed] [Google Scholar]
- 34. Zhu S, Ashok M, Li J, Li W, Yang H, et al. (2009) Spermine protects mice against lethal sepsis partly by attenuating surrogate inflammatory markers. Mol Med 15: 275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ho CH, Hsu SP, Yang CC, Lee YH, Chien CT (2009) Sialic acid reduces acute endotoxemia-induced liver dysfunction in the rat. Shock 32: 228–235. [DOI] [PubMed] [Google Scholar]
- 36. Wu CY, Wu MS, Chiang EP, Chen YJ, Chen CJ, et al. (2007) Plasma matrix metalloproteinase-9 level is better than serum matrix metalloproteinase-9 level to predict gastric cancer evolution. Clin Cancer Res 13: 2054–2060. [DOI] [PubMed] [Google Scholar]
- 37. Jung K, Lein M, Laube C, Lichtinghagen R (2001) Blood specimen collection methods influence the concentration and the diagnostic validity of matrix metalloproteinase 9 in blood. Clin Chim Acta 314: 241–244. [DOI] [PubMed] [Google Scholar]
- 38. Sawicki G, Salas E, Murat J, Miszta-Lane H, Radomski MW (1997) Release of gelatinase A during platelet activation mediates aggregation. Nature 386: 616–619. [DOI] [PubMed] [Google Scholar]
- 39. Radomski A, Jurasz P, Sanders EJ, Overall CM, Bigg HF, et al. (2002) Identification, regulation and role of tissue inhibitor of metalloproteinases-4 (TIMP-4) in human platelets. Br J Pharmacol 137: 1330–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]