Abstract
STUDY QUESTION
Are women's stress levels prospectively associated with fecundity and infertility?
SUMMARY ANSWER
Higher levels of stress as measured by salivary alpha-amylase are associated with a longer time-to-pregnancy (TTP) and an increased risk of infertility.
WHAT IS KNOWN ALREADY
Data suggest that stress and reproduction are interrelated; however, the directionality of that association is unclear.
STUDY DESIGN, SIZE, DURATION
In 2005–2009, we enrolled 501 couples in a prospective cohort study with preconception enrollment at two research sites (Michigan and Texas, USA). Couples were followed for up to 12 months as they tried to conceive and through pregnancy if it occurred. A total of 401 (80%) couples completed the study protocol and 373 (93%) had complete data available for this analysis.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Enrolled women collected saliva the morning following enrollment and then the morning following their first observed study menses for the measurement of cortisol and alpha-amylase, which are biomarkers of stress. TTP was measured in cycles. Covariate data were captured on both a baseline questionnaire and daily journals.
MAIN RESULTS AND THE ROLE OF CHANCE
Among the 401 (80%) women who completed the protocol, 347 (87%) became pregnant and 54 (13%) did not. After adjustment for female age, race, income, and use of alcohol, caffeine and cigarettes while trying to conceive, women in the highest tertile of alpha-amylase exhibited a 29% reduction in fecundity (longer TTP) compared with women in the lowest tertile [fecundability odds ratios (FORs) = 0.71; 95% confidence interval (CI) = (0.51, 1.00); P < 0.05]. This reduction in fecundity translated into a >2-fold increased risk of infertility among these women [relative risk (RR) = 2.07; 95% CI = (1.04, 4.11)]. In contrast, we found no association between salivary cortisol and fecundability.
LIMITATIONS, REASONS FOR CAUTION
Due to fiscal and logistical concerns, we were unable to collect repeated saliva samples and perceived stress questionnaire data throughout the duration of follow-up. Therefore, we were unable to examine whether stress levels increased as women continued to fail to get pregnant. Our ability to control for potential confounders using time-varying data from the daily journals, however, minimizes residual confounding.
WIDER IMPLICATIONS OF THE FINDINGS
This is the first US study to demonstrate a prospective association between salivary stress biomarkers and TTP, and the first in the world to observe an association with infertility.
STUDY FUNDING/COMPETING INTEREST(S)
This study was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (contracts #N01-HD-3-3355, N01-HD-3-3356, N01-HD-3358). There are no conflicts of interest to declare.
TRIAL REGISTRATION NUMBER
Not applicable.
Keywords: infertility, fecundity, stress, cortisol, alpha-amylase
Introduction
The role that stress plays in infertility remains controversial, largely because despite medical advances a large percentage of infertility remains unexplained (Kamath and Bhattacharya, 2012). While the causes of infertility have become arguably less relevant in many ways recently given that assisted reproduction technologies (ART) are successful in overcoming many fertility problems, continuing to elucidate the factors associated with optimizing natural fertility is extremely important as such knowledge could potentially lead to lower cost lifestyle modifications that could be recommended to patients experiencing conception delay prior to referring them for reproductive endocrinology analyses. Such interventions could have a major health impact as the use of ART is not without risk to the women and their offspring (Finnstrom et al., 2011; Zollner and Dietl, 2012).
Basic science has elucidated the linkages between the hypothalamic–pituitary axis (HPA) and hypothalamic–pituitary gonadal axis such that it is now accepted that physical stressors can perturb women's menstrual cycles (Chrousos et al., 1998). What is less clear is whether psychological stress can have the same effect. There are many reports in the literature of unassisted conception following adoption (Rock et al., 1965; Weir and Weir, 1966; Mai, 1971). Domar et al. (2000, 2011) have found higher pregnancy rates among IVF patients randomized to structured cognitive behavioral therapy groups and those randomized to a mind–body intervention program than women receiving usual care. Other investigators have reported increased pregnancy rates among women randomized to treatment with an antidepressant and psychotherapy compared with untreated women (Ramezanzadeh et al., 2011).
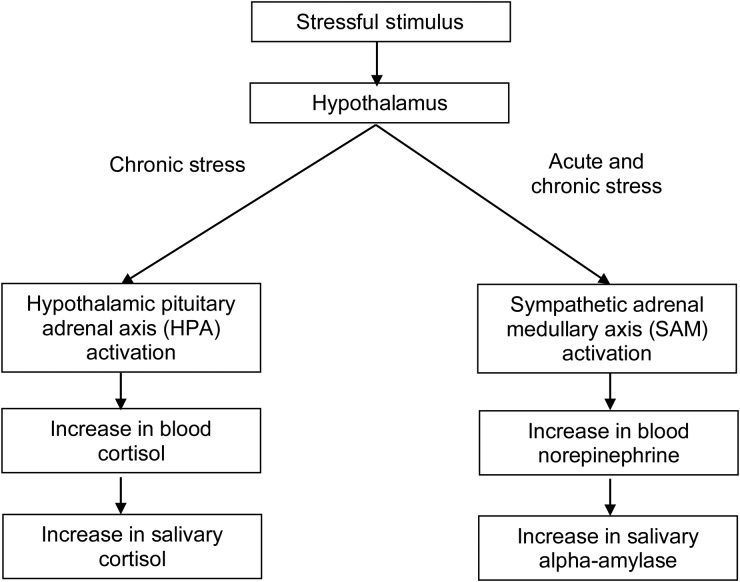
How does one measure the body's response to stressors objectively? First, one must understand the physiology of the human stress response (Fig. 1). When a stimulus is perceived as stressful, signals are sent to the hypothalamus, which then activates the sympathetic adrenomedullary (SAM) pathway; if the stress becomes chronic, the SAM can remain hyperactive and the HPA becomes activated as well. For the SAM system, norepinephrine is secreted into the bloodstream, which eventually results in an increase in salivary alpha-amylase production by the parotid gland. For the HPA axis, blood cortisol levels increase which subsequently results in an increase in salivary cortisol. The fact that both of these biomarkers can be detected in saliva makes them ideal for use in population-based studies (Rockett et al., 2004). Recent work suggests that psychological stressors produce a more pronounced alpha-amylase response than physical stressors (van Stegeren et al., 2008).
Figure 1.
Measuring the human stress response.
Until recently, much of the data regarding the association between stress and infertility have been derived from cross-sectional studies of couples seeking infertility treatment, in which the directionality of the association could not be determined. There have been very few prospective cohort studies to examine this issue due to the complexities associated with identifying the at-risk population (e.g. couples trying to become pregnant) (Buck et al., 2004). Recently, we published a series of papers from a study that we conducted in the UK, in which we prospectively examined stress in relation to time-to-pregnancy (TTP) among women who were followed for up to 6 months as they tried to conceive. In that study, we found no association between self-reported stress and TTP (Lynch et al., 2012). We did, however, report lower day-specific probabilities of conception among those with the highest levels of alpha-amylase in comparison with women with the lowest levels; yet, this effect did not translate into a longer TTP for these women, possibly due to the short 6 month follow-up period (Louis et al., 2011). In an attempt to further address this critical data gap, we introduced a novel stress component into the Longitudinal Investigation of Fertility and the Environment (LIFE) study's overall protocol in keeping with its focus on environmental and lifestyle factors and human fecundity and fertility. The intent was to provide a first look at the prospective association between stress and TTP and infertility in a cohort of US women.
Materials and Methods
Population and eligibility
This study was conducted using data collected from 2005 to 2009 as part of the LIFE study (Buck Louis et al., 2011). In brief, we enrolled 501 couples in two states who were discontinuing contraception for purposes of becoming pregnant. The primary aim was to assess the association between environmental chemicals in the context of lifestyle including stress and couple fecundity. Given the absence of established sampling frameworks for identifying couples planning a pregnancy in the near future, we utilized a marketing and fish/hunting license registry in 16 counties in Michigan and Texas, respectively, for recruitment and observed few differences in the characteristics of couples by sampling framework (Buck Louis et al., 2011). Eligibility included the following: (1) non-pregnant females aged 18–40 years; (2) married or in a committed relationship; (3) male partner age 18+ years; (4) self-reported menstrual cycle length of 21–42 days (to comply with the fertility monitor specifications); (5) ability to communicate in English or Spanish; (6) and no use of hormonal birth control injections in the prior 12 months; (7) woman and her partner have never been told by a healthcare provider that they could not get pregnant without medical help and (8) actively trying to get pregnant and off contraception for ≤2 months at study entry. We chose to exclude women with a history of hormonal birth control injections given the uncertainty regarding the time required for return to normal fertility. Identified couples were followed for up to 12 months or through pregnancy if pregnancy occurred.
Data collection
Once an eligible and interested couple was identified via telephone screen, the study team visited the couple's home. The research team enrolled and interviewed both partners of the couple separately using standardized baseline questionnaires and trained the couple in the use of their daily journals and other study elements such as the study-provided fertility monitor (Clearblue® Fertility Monitor, SPD Development Co.) and pregnancy tests. Additional information regarding LIFE study procedures can be found elsewhere (Buck Louis et al., 2011). The protocol was approved by the Institutional Review Boards at each participating institution and all participants provided written informed consent before enrollment.
Exposure assessment
To objectively assess stress, females collected a first morning (basal) saliva sample using a Salivette® collection device at two time points: (i) the morning following enrollment and (ii) the morning following their first observed menses in the study. Women were told to collect the sample immediately upon awakening before eating, drinking, smoking or brushing their teeth. Samples were returned via prepaid overnight shipping and stored at −20°C until analyzed.
Salivary cortisol and alpha-amylase were quantified in a laboratory with extensive expertise in this area (Salimetrics, LLC, State College, PA, USA), as biomarkers of stress using established protocols inclusive of quality control procedures. Cortisol (μg/dl) was measured using a highly sensitive enzyme immunoassay (Raff et al., 2003). Salivary alpha-amylase (U/ml) was quantified using a commercially available kinetic reaction assay (Granger et al., 2007). Only salivary specimens collected prior to pregnancy were considered for this analysis.
Outcomes assessment
The first outcome of interest was fecundity as measured by TTP (Joffe, 1997). We used study journals supplemented with fertility monitors to define menstrual cycles distinct from episodic bleeding. Specifically, a menstrual cycle denoted the interval (in days) from the onset of bleeding that increased in intensity and lasted ≥2 days to the onset of the next similar bleeding episode. A unique feature of the study design was the ability to capture couples enrolling mid-cycle who immediately became pregnant. We define this as cycle 0 to differentiate it from cycle 1, which denotes one fully observed menstrual cycle. Pregnancy was defined as a positive study-provided home pregnancy test, which is sensitive for 25 mIU/ml hCG.
The second outcome of interest was the day-specific probabilities of pregnancy during the fertile window. The fertile window for each menstrual cycle was defined as −5 to +1 days from the peak day of LH as detected by the fertility monitor (Lynch et al., 2006).
The final outcome that we considered was clinical infertility, which was defined as a failure to achieve pregnancy despite 12 months of regular appropriately timed unprotected intercourse consistent with American Society of Reproductive Medicine guidelines (The Practice Committee of the American Society of Reproductive Medicine, 2012).
Covariate information
Covariate data were captured via the baseline interviews that collected information regarding general and reproductive health, lifestyle and demographics. Of note, the 4-item Cohen's perceived stress scale (PSS-4) was also administered as part of the baseline interview (Cohen and Williamson, 1998). Time-varying covariate data were collected via the daily journals. The women's journal recorded bleeding, intercourse, periodic use of contraception, fertility monitor results, pregnancy test results and other lifestyle data, such as smoking, drinking and consumption of caffeinated beverages. Periodic use of contraception was quantified as the authors' previous work has suggested that some couples who are trying to conceive use contraception on occasion (e.g. in March in an effort to avoid a holiday baby). We did not, however, observe this phenomenon in the LIFE study. There was one daily stress question on the journal which read, ‘Please tell us your overall stress level each day: ‘1’ = almost no stress; ‘2’ = relatively little stress; ‘3’ = a moderate amount of stress and ‘4’ a lot of stress.’
Statistical analysis
To assess differences in exposure and covariates by outcome we used χ2 tests and t-tests for categorical and continuous variables, respectively. Given the lack of statistically significant within-woman variation in the stress biomarker levels between the first and second saliva collection, the values were averaged. For women who had only one available saliva sample (n = 2), the biomarker levels from that sample were used. Cortisol and alpha-amylase values were then divided into tertiles based on the distributions of data among study participants. We used discrete-time survival analysis to estimate the association between the biomarkers and TTP while adjusting for relevant covariates that were chosen based on the review of the literature and analysis of a directed acyclic graph (see Supplementary data, Fig. S1). Care was taken to ensure that the lifestyle factors for which we adjusted were in fact confounders and not in the causal pathway. We calculated fecundability odds ratio (FORs) and 95% confidence intervals (CIs) with estimates below one denoting decreased fecundability (longer TTP) and those above one indicative of increased fecundability (shorter TTP). To examine the association between cortisol and salivary alpha-amylase and the day-specific probabilities of pregnancy, we used a survival analysis-based fecundity model (Sundaram et al., 2012). We also assessed the RR for infertility using Poisson regression with robust standard errors (Zou, 2004). For that model, our analysis was restricted to women for whom the outcome of interest (i.e. TTP > 12 months or not) was known. All models were adjusted for time off contraception prior to enrollment (left truncation in survival analysis). Analyses were conducted using SAS software (version 9.2) and R software (version 2.12.1). Statistical significance was set at P < 0.05.
Results
Among the 501 couples who were enrolled into the LIFE study, 100 (20%) withdrew over the course of the study, most commonly due to a lack of continued interest in participation (Buck Louis et al., 2011). Among the 401 (80%) women who completed the protocol, 347 (87%) became pregnant and 54 (13%) did not. Among those 401 women, 373 women (93%) had complete saliva data for this analysis. As mentioned previously, there were no statistically significant differences in the salivary stress biomarkers over those first two cycles. The mean cortisol values were 0.41 mg/dl in cycle 1 and 0.50 mg/dL in cycle 2, while for alpha-amylase, the mean values were 24.7 and 25.6 U/ml, respectively.
Table I presents selected characteristics of couples by study outcome. Non-Hispanic black and Hispanic women were more likely to withdraw from the study than non-Hispanic white women. Women who became pregnant were more likely to be parous, have higher educational attainment, have a higher family income and to be a non-smoker (or smoke less on average if they did smoke) when compared with women who did not get pregnant. There were no important differences by pregnancy status in the average number of acts of intercourse during the fertile window, salivary cortisol, salivary alpha-amylase or number of months trying to conceive prior to enrollment.
Table I.
Selected female and male characteristics by study outcome (n = 373).
| Pregnant, n (%), 247 (66) | Not pregnant, n (%), 51 (14) | Withdrew, n (%), 75 (20) | |
|---|---|---|---|
| Female age (years)a,b | 29.9 (3.9) | 30.2 (4.0) | 30.6 (4.7) |
| Male age (years)a,b | 31.7 (4.4) | 32.0 (5.2) | 32.3 (5.6) |
| Female race/ethnicity** | |||
| Non-Hispanic white | 204 (83.3) | 38 (74.5) | 52 (69.3) |
| Non-Hispanic black | 5 (2.0) | 3 (5.9) | 8 (10.7) |
| Hispanic | 20 (8.2) | 8 (15.7) | 10 (13.3) |
| Other | 16 (6.5) | 2 (3.9) | 5 (6.7) |
| Graviditya,**,b | 1.1 (1.3) | 0.8 (1.4) | 1.3 (1.6) |
| Nulligravid | 101 (41.2) | 33 (64.7) | 31 (41.3) |
| 1 | 71 (29.0) | 8 (15.7) | 18 (24.0) |
| 2+ | 73 (29.8) | 10 (19.6) | 26 (34.7) |
| Parity a,b** | 0.7 (0.8) | 0.2 (0.5) | 0.7 (0.9) |
| Nulliparous | 117 (48.0) | 44 (86.3) | 44 (58.7) |
| 1 | 89 (36.5) | 5 (9.8) | 16 (21.3) |
| 2+ | 38 (15.6) | 2 (3.9) | 15 (20.0) |
| Female BMIb | |||
| Underweight | 3 (1.2) | 1 (2.0) | 0 (0.0) |
| Healthy | 117 (47.4) | 22 (43.1) | 24 (32.0) |
| Overweight | 64 (25.9) | 11 (21.6) | 23 (30.7) |
| Obese | 63 (25.5) | 17 (33.3) | 28 (37.3) |
| Educational attainment**,b | |||
| High school or less | 13 (5.3) | 2 (3.9) | 7 (9.3) |
| Some college | 29 (11.9) | 11 (21.6) | 23 (30.7) |
| College graduate | 202 (82.8) | 38 (74.5) | 45 (60.0) |
| Income level**,b | |||
| <$29 999 | 6 (2.5) | 3 (5.9) | 7 (9.5) |
| $30 000–$49 999 | 20 (8.3) | 13 (25.5) | 11 (14.9) |
| $50 000–$69 999 | 36 (14.9) | 7 (13.7) | 14 (18.9) |
| At least $70 000 | 179 (74.3) | 28 (54.9) | 42 (56.8) |
| Average cigarettes per cycle**,c | 14.7 (66.9) | 55.7 (153.9) | 62.7 (188.9) |
| None | 201 (81.4) | 32 (62.7) | 52 (70.3) |
| 0 < Cigarettes < 11 | 22 (8.9) | 8 (15.7) | 7 (9.5) |
| 11 ≤ Cigarettes < 41 | 8 (3.2) | 2 (3.9) | 1 (1.4) |
| 41 ≤ Cigarettes | 16 (6.5) | 9 (17.6) | 14 (18.9) |
| Average alcoholic beverages per cyclec | 12.1 (15.1) | 12.1 (20.7) | 13.3 (18.2) |
| None | 30 (12.1) | 4 (7.8) | 8 (10.8) |
| 0 < Alcohol < 4 | 73 (29.6) | 16 (31.4) | 21 (28.4) |
| 4 ≤ Alcohol < 21 | 90 (36.4) | 26 (51.0) | 31 (41.9) |
| 21 ≤ Alcohol | 54 (21.9) | 5 (9.8) | 14 (18.9) |
| Average caffeinated beverages per cyclec | 37.4 (27.1) | 32.6 (26.1) | 41.8 (32.9) |
| None | 4 (1.6) | 1 (2.0) | 2 (2.7) |
| 0 < Caffeine < 14 | 42 (17.0) | 14 (27.5) | 15 (20.3) |
| 14 ≤ Caffeine < 41 | 102 (41.3) | 24 (47.1) | 24 (32.4) |
| 41 ≤ Caffeine | 99 (40.1) | 12 (23.5) | 33 (44.6) |
| Average acts of intercourse during the fertile window**,c | 2.8 (1.4) | 2.6 (1.6) | 2.6 (1.2) |
| None | 0 (0.0) | 0 (0.0) | 2 (3.1) |
| 0 < Intercourse < 4 | 191 (81.3) | 47 (94.0) | 54 (83.1) |
| 4 ≤ Intercourse | 44 (18.7) | 3 (6.0) | 9 (13.8) |
| Cortisol (μg/dl)*,c | 0.4 (0.6) | 0.3 (0.2) | 0.4 (0.3) |
| Alpha-amylase (U/ml)a,c | 22.7 (29.5) | 32.9 (34.1) | 25.4 (33.5) |
| Female average total of daily stress per cyclea,c | 54.8 (13.7) | 54.3 (15.6) | 56.5 (14.7) |
| Number of months trying prior to study entryb | |||
| 0 | 188 (76.1) | 32 (62.7) | 51 (68.0) |
| 1 | 24 (9.7) | 5 (9.8) | 9 (12.0) |
| 2 | 35 (14.2) | 14 (27.5) | 15 (20.0) |
| Form of contraception used at study entry | |||
| None | 158 (64.0) | 39 (76.5) | 54 (72.0) |
| Barrier method | 48 (19.4) | 7 (13.7) | 15 (20.0) |
| Hormonal method | 15 (6.1) | 1 (2.0) | 1 (1.3) |
| Natural method | 22 (8.9) | 4 (7.8) | 5 (6.7) |
| Intrauterine device | 3 (1.2) | 0 (0.0) | 0 (0.0) |
| Spermicide | 1 (0.4) | 0 (0.0) | 0 (0.0) |
Note: variable totals do not always equal the column totals due to missing values.
aMean (SD).
bAt baseline.
cDuring the study.
**P < 0.05.
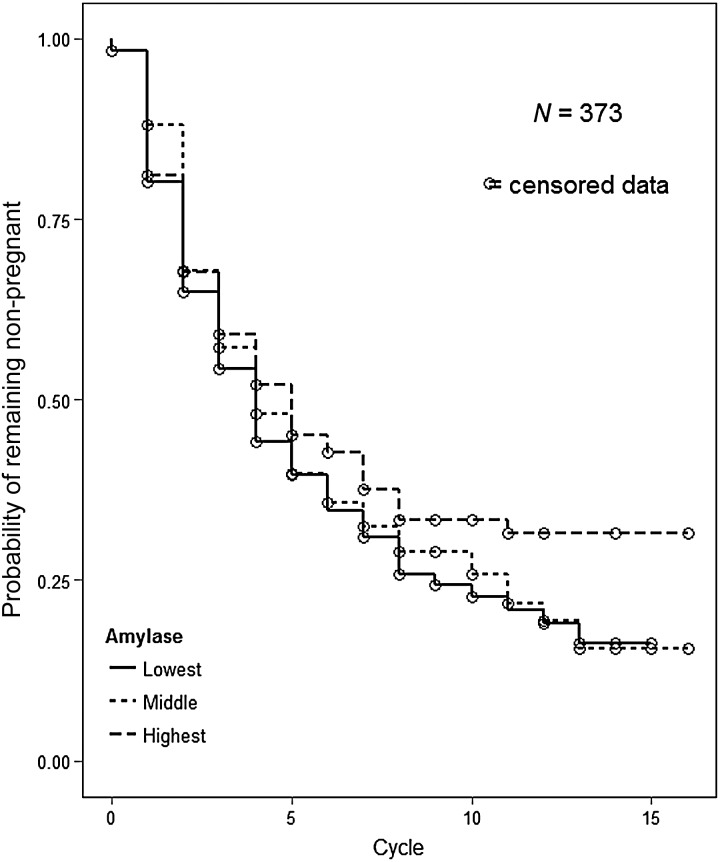
Table II presents the FORs for the salivary stress biomarkers and TTP, first with the biomarkers modeled continuously and next with them modeled in tertiles. All of the FORs are <1 indicative of reduced couple fecundity. We found that women in the highest tertile of salivary alpha-amylase had a 29% decreased odds of pregnancy (longer TTP) after adjustment for covariates compared with women in the lowest tertile [FOR = 0.71; 95% CI = (0.51, 1.00)]. Women in the middle tertile of salivary alpha-amylase had a 7% decreased odds of pregnancy after adjustment [FOR = 0.93; 95% CI = (0.68, 1.29)]. The magnitude of the differences in TTP among women in the various tertiles of salivary alpha-amylase is depicted in Fig. 2. Women had similar TTP until around cycle 5 at which time women in the highest tertile of alpha-amylase began to demonstrate lower probabilities of pregnancy. We also examined the adjusted association between alpha-amylase and the FOR using a five knot spline and the results were similar (see Supplementary data, Fig. S2).
Table II.
Association between average stress biomarker level among females in the first two cycles of participation and time to pregnancy (n = 373).
| FOR | 95% CI | Adjusted FORb | 95% CI | |
|---|---|---|---|---|
| Alpha-amylasea | 0.92 | [0.79, 1.07] | 0.87 | [0.75, 1.02] |
| Lowest | — | — | — | — |
| Middle | 0.99 | [0.73, 1.36] | 0.93 | [0.68, 1.29] |
| Highest | 0.82 | [0.59, 1.13] | 0.71 | [0.51, 1.00]‡ |
| Cortisola | 0.89 | [0.41, 1.91] | 0.96 | [0.43, 2.12] |
| Lowest | — | — | — | — |
| Middle | 0.86 | [0.63, 1.19] | 0.77 | [0.56, 1.07] |
| Highest | 0.97 | [0.71, 1.33] | 0.95 | [0.69, 1.30] |
FOR, fecundability odds ratio.
aModeled as a continuous variable then in tertiles (separate models).
bAdjusted for age of female, difference in age between male and female, income of female (dichotomized), race of female (dichotomized), female's cigarette use, female's caffeine use and female's alcohol use.
‡P < 0.05.
Figure 2.
Adjusted* probability of remaining not pregnant by tertile of salivary alpha-amylase.*Adjusted for age of female, difference in age between male and female, income of female (dichotomized), race of female (dichotomized), female's cigarette use, female's caffeine use, and female's alcohol use.
The presence of time-varying covariates in the model presented in Table II allows the odds function to vary in a non-proportional way with respect to the baseline odds function over time. So, the overall proportionality assumption cannot be tested. However, we did inspect the functional form for assessing the effect of the stress markers by looking at the regression coefficients against the tertiles of the stress biomarkers, and the direction of the coefficients did not change indicating that the linear form for the stress biomarker effect was reasonable.
We assessed the differential effect of the alpha-amylase after cycle 5 by introducing a separate coefficient after cycle 5. We found the effect to be significant when alpha-amylase was modeled as a continuous covariate with FOR (95%CI) as follows: for cycles ≤5, the effect of alpha-amylase was: FOR = 0.94 [95% CI = (0.78, 1.14)] and for cycles >5: FOR = 0.72 [95% CI = (0.54, 0.97)] after adjusting for the rest of the covariates as in the adjusted model for Table II.
We then assessed the association between the stress biomarkers and the day-specific probabilities of pregnancy (data not shown). We found an association between alpha-amylase and fecundity with women in the highest tertile having 5–6% lower daily probabilities of pregnancy across the fertile window in the first cycle when compared with women in the lowest tertile after adjustment for confounders. We again did not find an association between salivary cortisol and fecundity.
Finally, to assess whether the observed decreases in fecundity were clinically relevant, we calculated the RR of infertility as a function of alpha-amylase and cortisol while adjusting for covariates. As shown in Table III, women in the highest tertile of alpha-amylase had a >2-fold increased risk of infertility in comparison with women in the lowest tertile [RR = 2.07; 95% CI = (1.04, 4.11)]. Women in the middle tertile of alpha-amylase had no increased risk of infertility in comparison with women in the lowest tertile [RR = 1.02; 95% CI = (0.47, 2.19)]. We again found no association with cortisol.
Table III.
RR of infertility by average stress biomarker level in the first two cycles of participation (n = 299).
| RR | 95% CI | Adjusted RRb | 95% CI | |
|---|---|---|---|---|
| Alpha-amylasea | 1.41 | [1.04, 1.90] | 1.46 | [1.08, 1.98] |
| Lowest | — | — | — | — |
| Middle | 0.92 | [0.44, 1.93] | 1.02 | [0.47, 2.19] |
| Highest | 1.75 | [0.91, 3.35] | 2.07 | [1.04, 4.11] |
| Cortisola | 0.24 | [0.03, 2.20] | 0.36 | [0.05, 2.77] |
| Lowest | — | — | — | — |
| Middle | 1.12 | [0.60, 2.08] | 1.33 | [0.69, 2.55] |
| Highest | 0.72 | [0.36, 1.43] | 0.84 | [0.40, 1.76] |
aModeled as a continuous variable then in tertiles (separate models).
bAdjusted for age of female, difference in age between male and female, income of female (dichotomized), race of female (dichotomized), female's cigarette use, female's caffeine use and female's alcohol use.
Among the 1708 cycles among 373 women in this analysis, 146 (8.5%) did not indicate an act of intercourse during the fertile window. However, we performed a standard discrete time survival analysis, in which TTP was defined as the number of cycles until pregnancy regardless of whether the monitor and daily journal data indicated the cycle was at risk. We performed a sensitivity analysis to assess what impact, if any, including all cycles had on our analyses. Specifically, we examined Kaplan–Meier survival curves for TTP as calculated above versus TTP as calculated using only the cycles at risk. The log-rank test for the comparison of these curves was not significant (P = 0.32). So, in both the TTP and infertility analysis, we used the TTP in cycles regardless of whether they were calculated to be at risk.
Discussion
To our knowledge, this is the first US cohort study to demonstrate a prospective temporal association between a SAM biomarker of stress and both TTP and infertility. After adjustment, we found a 29% decrease in fecundity among women in the highest tertile of alpha-amylase when compared with women in the lowest tertile. We also found a >2-fold increased risk of infertility among those women. We found no association between salivary cortisol and either measure of fecundity.
This work corroborates and extends our previous work in which we reported a prospective association between increased alpha-amylase and a 12% reduction in the day-specific probabilities of pregnancy in the first cycle among UK women (Louis et al., 2011). This effect, however, did not translate into a longer TTP among stressed women likely due to our limited sample size (n = 274) and short six cycle follow-up period (Louis et al., 2011). Consistent with the present study, no association between salivary cortisol and fecundity was observed.
The current study differed from our UK study in several ways. First, women in the current study collected their saliva the morning following enrollment and then on the morning of their first study-observed menses in contrast to collection on Day 6 of each cycle in the UK study (Louis et al., 2011). Moreover, we followed women for a full 12 months in this study, in contrast to only six cycles in the UK study, which allowed us to examine the impact of stress throughout the full spectrum of fecundity.
While cortisol is thought to be the classical biomarker of stress, we did not see any association between salivary cortisol and fecundity in this study. Cortisol has a marked circadian rhythm with levels peaking in the morning and decreasing throughout the day (Weitzman et al., 1971). While it has been suggested that a single basal saliva sample is sufficient (Yehuda et al., 2003), others have argued that cortisol reactivity (requiring multiple samples per day) might be more important in relation to human health effects (Pruessner et al., 2003). Indeed, several studies have failed to find an association between salivary cortisol and self-reported stress in studies of reproductive outcomes (Harville et al., 2009; Lynch et al., 2012). Further, we are not the first to demonstrate an asymmetric response in salivary cortisol and alpha-amylase (Chatterton et al., 1997; O'Donnell et al., 2009). In fact, some authors have argued that the asymmetry itself is of interest, as chronic stress might affect the HPA axis and SAM pathway differently, thereby, leading to asymmetry (Tarullo and Gunnar, 2006; Gordis et al., 2008).
Evidence suggests that stress is not the only factor that may affect salivary alpha-amylase levels (Stegmann, 2011). The current literature suggests that alpha-amylase levels may be impacted by smoking, caffeine intake, food consumption and exercise (Rohleder and Nater, 2009). Specifically, tobacco acutely inhibits salivary alpha-amylase activity, whereas caffeine intake has been shown to stimulate alpha-amylase activity, as have food intake and exercise. However, our protocol was designed to minimize these issues by instructing women to collect their saliva samples upon awakening before any cigarette smoking, drinking, eating or teeth brushing. Further, we adjusted all of our multivariable models for prospectively measured smoking, caffeine intake and alcohol use. While we did not collect time-varying information on exercise, alpha-amylase levels are shown to return to normal within 2.5 h of vigorous physical activity, making confounding unlikely given our protocol (Walsh et al., 1999). Smoking in the day prior to collection has been shown to have no effect on alpha-amylase levels nor have differences in BMI (Nater et al., 2007). Thus, we believe the alpha-amylase levels that we report are unlikely to have been systematically affected by known confounders.
So how do the stress levels in our population compare with other women of reproductive age? First, the mean salivary alpha-amylase level upon awakening that we report is markedly lower than that reported in a study of healthy German volunteers despite identical sample collection procedures, 25.0 versus 106.1 U/ml, respectively (Nater et al., 2007). Further, the mean score on the 4-item PSS collected at baseline among the participants in our current study was 3.6 [normative sample of US women on PSS-4 = 4.7, SD = 3.1]. Therefore, it seems that our population was likely less stressed than other study populations, which may reflect research findings reporting that stressed women are less likely to participate in intensive study protocols (Domar et al., 2011).
Could our findings be a result of reverse causality or bias? First, and most importantly, it is important to establish that couples did not enter our study because they were worried about their ability to conceive. To examine this issue, we looked at the salivary alpha-amylase and cortisol levels by the time off contraception prior to study entry. If the stress biomarker levels were higher among those women who had been off contraception for 2 months (couples were excluded if they were trying >2 months), then that would be evidence of potential reverse causation (i.e. taking longer to conceive prior to study entry induced stress). When we investigated this issue, however, we found absolutely no evidence of a difference in stress biomarker levels by time off contraception. The mean salivary alpha-amylase levels were 22.5, 29.7 and 21.0 U/ml for those off contraception 0, 1 or 2 months prior to study entry, respectively. Another issue of concern is how the stress levels of individuals who remained under study might have varied from those who dropped out. As shown in Table I, the stress levels of women who withdrew were similar to those who remained under study, thereby, suggesting that differential attrition by exposure status was not a concern.
This cohort study has many strengths, although it has important limitations given its observational design. First, the outcomes of interest in this study, TTP and infertility, are highly accurate in that they were measured prospectively based on longitudinal fertility monitor and daily journal data required for defining menstrual cycles. In addition, we had sensitive biomarkers for our stress exposures and study outcome—TTP by home hCG testing. Further, we were able to reduce the likelihood of residual confounding by measuring all known potential confounders, most in a time-varying fashion in the participant journals. With regard to limitations, due to fiscal and logistical concerns, we were unable to obtain repeated saliva samples and administer additional psychosocial questionnaires. As such, we are unable to comment on whether failing to get pregnant each month increased women's stress level over time. Moreover, while parity (nulliparous versus parous) could theoretically modify the association between stress and TTP, we were underpowered to examine effect modification. In an effort to ensure that all participants collected at least one salivary specimen prior to becoming pregnant, we asked that women collect their first specimen the morning following enrollment. Therefore, the first saliva samples were collected at varying points in time in the women's menstrual cycles. We are unaware of any data suggesting that salivary alpha-amylase levels differ throughout the menstrual cycle. Nater et al., (2007) finding of no sex differences in the diurnal patterns of salivary alpha-amylase supports our assumption. Similarly, a study examining the effects of oral contraceptive use on whole saliva found no differences in salivary amylase concentrations (Laine et al., 1991). This was an epidemiologic study looking at factors related to achieving an unassisted pregnancy in a community-based sample. Therefore, we did not make any effort to exclude couples based on clinical criteria such as tubal occlusion. Examining factors associated with natural conception among subfertile couples or those with a pre-existing biologic reason for fertility problems is a different research question and is beyond the scope of this work. Finally, this was a study conducted among an apparently lower stress population of pregnancy planners and a recent RCT suggests that women using the fertility monitor that was used in this study were more likely to get pregnancy within two cycles compared with those who did not use the monitor (Robinson et al., 2007); as such, the results might not generalize to all women of reproductive age.
One question that remains unanswered is the biologic mechanism by which stress might impact fecundity. In the current study, we found no differences in acts of intercourse during the fertile window between women who did and did not become pregnant, nor did we see a decreased coital frequency among women with the highest salivary alpha-amylase levels (i.e. 2.8, 2.7, and 2.6 acts of intercourse in the fertile window for women in the low, middle and highest tertiles of alpha-amylase, respectively). As such, we have no evidence to suggest that stressed couples have more/less intercourse relative to others. Another hypothesized mechanism is that high levels of stress could lead to a delay or inhibition of the LH surge (Ferin, 1999). In fact, in our study, we did not see a difference in the peak LH day as measured by the fertility monitor between women with high and low levels of alpha-amylase (data not shown). Additional theories that we could not examine include stress-induced alterations in gamete transport or the development of an autoimmune state unfavorable for implantation (Schenker et al., 1992; Makrigiannakis et al., 2001).
We have now shown in two populations, one in the USA and one in the UK, that stress as measured by increased salivary alpha-amylase is associated with lower fecundity among affected women. While this study certainly does not give a definitive answer regarding causation, it provides further evidence of the independent adverse role that stress might play. Future work should focus first on affirming the lack of variation in salivary alpha-amylase response during the menstrual cycle. It will also be important to identify a valid and reliable questionnaire measure of stress (perhaps chronic psychological stress) that is highly correlated with salivary alpha-amylase levels such that women for whom stress might be a problem can be quickly identified.
Until such data become available, it seems prudent to consider stress as a potential factor among couples who have failed to get pregnant despite 6 months of targeted intercourse (given that the effect of stress becomes apparent after the fifth cycle). While there is a dearth of information regarding effective stress reduction techniques among women of reproductive age who are trying to conceive but have not yet sought treatment from a reproductive endocrinologist, stress reduction modalities, such as yoga, meditation and mindfulness, that have been shown to be helpful in reducing stress in studies of other health outcomes, might be relevant for further consideration (Carmody and Baer, 2008; Balaji et al., 2012; Yadav et al., 2012). While one must be careful to avoid any potential ‘blame’, by pointing out that high levels of stress are clearly neither the only nor the most important factor predicting one's ability to get pregnant, the suggestion that a woman considers participating in an effort to reduce her stress level is certainly unlikely to cause harm.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors' roles
C.D.L. assisted in the design of the study, conceptualized paper and wrote first draft and took the lead on subsequent edits. R.S.: oversaw statistical analysis and provided substantive edits on the manuscript. J.M.M. performed statistical analyses and provided substantive edits on the manuscript. A.M.S. assisted in the design of the study, oversaw data collection at the Texas site, and provided substantive edits on the manuscript. G.M.B.L. designed the study and provided substantive edits on the analytic plan and manuscript.
Funding
This study was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (contracts #N01-HD-3-3355, N01-HD-3-3356, N01-HD-3358).
Conflict of interest
The authors have no conflicts of interest to declare in relation to this manuscript.
Supplementary Material
Acknowledgements
None.
References
- Balaji PA, Varne SR, Ali SS. Physiological effects of yogic practices and transcendental meditation in health and disease. N Am J Med Sci. 2012;4:442–448. doi: 10.4103/1947-2714.101980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck GM, Lynch CD, Stanford JB, Sweeney AM, Schieve LA, Rockett JC, Selevan SG, Schrader SM. Prospective pregnancy study designs for assessing reproductive and developmental toxicants. Environ Health Perspect. 2004;112:79–86. doi: 10.1289/ehp.6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Schisterman EF, Sweeney AM, Wilcosky TC, Gore-Langton RE, Lynch CD, Boyd BD, Schrader SM, Kim S, Chen Z, et al. Designing prospective cohort studies for assessing reproductive and developmental toxicity during sensitive windows of human reproduction and development—the LIFE Study. Paediatr Perinat Epidemiol. 2011;25:413–424. doi: 10.1111/j.1365-3016.2011.01205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody J, Baer RA. Relationships between mindfulness practice and levels of mindfulness, medical and psychological symptoms and well-being in a mindfulness-based stress reduction program. J Behav Med. 2008;31:23–33. doi: 10.1007/s10865-007-9130-7. [DOI] [PubMed] [Google Scholar]
- Chatterton RT, Jr, Vogelsong KM, Lu YC, Hudgens GA. Hormonal responses to psychological stress in men preparing for skydiving. J Clin Endocrinol Metab. 1997;82:2503–2509. doi: 10.1210/jcem.82.8.4133. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Torpy DJ, Gold PW. Interactions between the hypothalamic-pituitary-adrenal axis and the female reproductive system: clinical implications. Ann Intern Med. 1998;129:229–240. doi: 10.7326/0003-4819-129-3-199808010-00012. [DOI] [PubMed] [Google Scholar]
- Cohen S, Williamson G. Perceived stress in a probability sample of the United States. In: Spacapan S, Scamp S, editors. The Social Psychology of Health: Claremont Symposium on Applied Social Psychology. Newbury Park, CA: Sage; 1998. [Google Scholar]
- Domar AD, Clapp D, Slawsby EA, Dusek J, Kessel B, Freizinger M. Impact of group psychological interventions on pregnancy rates in infertile women. Fertil Steril. 2000;73:805–811. doi: 10.1016/s0015-0282(99)00493-8. [DOI] [PubMed] [Google Scholar]
- Domar AD, Rooney KL, Wiegand B, Orav EJ, Alper MM, Berger BM, Nikolovski J. Impact of a group mind/body intervention on pregnancy rates in IVF patients. Fertil Steril. 2011;95:2269–2273. doi: 10.1016/j.fertnstert.2011.03.046. [DOI] [PubMed] [Google Scholar]
- Ferin M. Clinical review 105: stress and the reproductive cycle. J Clin Endocrinol Metab. 1999;84:1768–1774. doi: 10.1210/jcem.84.6.5367. [DOI] [PubMed] [Google Scholar]
- Finnstrom O, Kallen B, Lindam A, Nilsson E, Nygren KG, Olausson PO. Maternal and child outcome after in vitro fertilization—a review of 25 years of population-based data from Sweden. Acta Obstet Gynecol Scand. 2011;90:494–500. doi: 10.1111/j.1600-0412.2011.01088.x. [DOI] [PubMed] [Google Scholar]
- Gordis EB, Granger DA, Susman EJ, Trickett PK. Salivary alpha amylase-cortisol asymmetry in maltreated youth. Horm Behav. 2008;53:96–103. doi: 10.1016/j.yhbeh.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, el-Sheikh M, Gordis EB, Stroud LR. Salivary alpha-amylase in biobehavioral research: recent developments and applications. Ann N Y Acad Sci. 2007;1098:122–144. doi: 10.1196/annals.1384.008. [DOI] [PubMed] [Google Scholar]
- Harville EW, Savitz DA, Dole N, Herring AH, Thorp JM. Stress questionnaires and stress biomarkers during pregnancy. J Womens Health (Larchmt) 2009;18:1425–1433. doi: 10.1089/jwh.2008.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe M. Time to pregnancy: a measure of reproductive function in either sex. Asclepios Project. Occup Environ Med. 1997;54:289–295. doi: 10.1136/oem.54.5.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath MS, Bhattacharya S. Demographics of infertility and management of unexplained infertility. Best Pract Res Clin Obstet Gynaecol. 2012;26:729–738. doi: 10.1016/j.bpobgyn.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Laine M, Pienihakkinen K, Ojanotko-Harri A, Tenovuo J. Effects of low-dose oral contraceptives on female whole saliva. Arch Oral Biol. 1991;36:549–552. doi: 10.1016/0003-9969(91)90151-j. [DOI] [PubMed] [Google Scholar]
- Louis GM, Lum KJ, Sundaram R, Chen Z, Kim S, Lynch CD, Schisterman EF, Pyper C. Stress reduces conception probabilities across the fertile window: evidence in support of relaxation. Fertil Steril. 2011;95:2184–2189. doi: 10.1016/j.fertnstert.2010.06.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch CD, Jackson LW, Buck Louis GM. Estimation of the day-specific probabilities of conception: current state of the knowledge and the relevance for epidemiological research. Paediatr Perinat Epidemiol. 2006;20(Suppl. 1):3–12. doi: 10.1111/j.1365-3016.2006.00765.x. [DOI] [PubMed] [Google Scholar]
- Lynch CD, Sundaram R, Buck Louis GM, Lum KJ, Pyper C. Are increased levels of self-reported psychosocial stress, anxiety, and depression associated with fecundity? Fertil Steril. 2012;98:453–458. doi: 10.1016/j.fertnstert.2012.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai FM. Conception after adoption: an open question. Psychosom Med. 1971;33:509–514. doi: 10.1097/00006842-197111000-00004. [DOI] [PubMed] [Google Scholar]
- Makrigiannakis A, Zoumakis E, Kalantaridou S, Coutifaris C, Margioris AN, Coukos G, Rice KC, Gravanis A, Chrousos GP. Corticotropin-releasing hormone promotes blastocyst implantation and early maternal tolerance. Nat Immunol. 2001;2:1018–1024. doi: 10.1038/ni719. [DOI] [PubMed] [Google Scholar]
- Nater UM, Rohleder N, Schlotz W, Ehlert U, Kirschbaum C. Determinants of the diurnal course of salivary alpha-amylase. Psychoneuroendocrinology. 2007;32:392–401. doi: 10.1016/j.psyneuen.2007.02.007. [DOI] [PubMed] [Google Scholar]
- O'Donnell K, Kammerer M, O'Reilly R, Taylor A, Glover V. Salivary alpha-amylase stability, diurnal profile and lack of response to the cold hand test in young women. Stress. 2009;12:549–554. doi: 10.3109/10253890902822664. [DOI] [PubMed] [Google Scholar]
- Pruessner M, Hellhammer DH, Pruessner JC, Lupien SJ. Self-reported depressive symptoms and stress levels in healthy young men: associations with the cortisol response to awakening. Psychosom Med. 2003;65:92–99. doi: 10.1097/01.psy.0000040950.22044.10. [DOI] [PubMed] [Google Scholar]
- Raff H, Homar PJ, Skoner DP. New enzyme immunoassay for salivary cortisol. Clin Chem. 2003;49:203–204. doi: 10.1373/49.1.203. [DOI] [PubMed] [Google Scholar]
- Ramezanzadeh F, Noorbala AA, Abedinia N, Rahimi FA, Naghizadeh MM. Psychiatric intervention improved pregnancy rates in infertile couples. Malays J Med Sci. 2011;18:16–24. [PMC free article] [PubMed] [Google Scholar]
- Robinson JE, Wakelin M, Ellis JE. Increased pregnancy rate with use of the Clearblue Easy Fertility Monitor. Fertil Steril. 2007;87:329–334. doi: 10.1016/j.fertnstert.2006.05.054. [DOI] [PubMed] [Google Scholar]
- Rock J, Tietze C, McLaughlin HB. Effect of adoption on infertility. Fertil Steril. 1965;16:305–312. doi: 10.1016/s0015-0282(16)35583-2. [DOI] [PubMed] [Google Scholar]
- Rockett JC, Buck GM, Lynch CD, Perreault SD. The value of home-based collection of biospecimens in reproductive epidemiology. Environ Health Perspect. 2004;112:94–104. doi: 10.1289/ehp.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohleder N, Nater UM. Determinants of salivary alpha-amylase in humans and methodological considerations. Psychoneuroendocrinology. 2009;34:469–485. doi: 10.1016/j.psyneuen.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Schenker JG, Meirow D, Schenker E. Stress and human reproduction. Eur J Obstet Gynecol Reprod Biol. 1992;45:1–8. doi: 10.1016/0028-2243(92)90186-3. [DOI] [PubMed] [Google Scholar]
- Stegmann BJ. Other nonstress influences can alter salivary alpha-amylase activity. Fertil Steril. 2011;95:2190–2191. doi: 10.1016/j.fertnstert.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Sundaram R, McLain AC, Buck Louis GM. A survival analysis approach to modeling human fecundity. Biostatistics. 2012;13:4–17. doi: 10.1093/biostatistics/kxr015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Horm Behav. 2006;50:632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- The Practice Committee of the American Society of Reproductive Medicine. Diagnostic evaluation of the infertile female: a committee opinion. Fertil Steril. 2012;98:302–307. doi: 10.1016/j.fertnstert.2012.05.032. [DOI] [PubMed] [Google Scholar]
- van Stegeren AH, Wolf OT, Kindt M. Salivary alpha amylase and cortisol responses to different stress tasks: impact of sex. Int J Psychophysiol. 2008;69:33–40. doi: 10.1016/j.ijpsycho.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Walsh NP, Blannin AK, Clark AM, Cook L, Robson PJ, Gleeson M. The effects of high-intensity intermittent exercise on saliva IgA, total protein and alpha-amylase. J Sports Sci. 1999;17:129–134. doi: 10.1080/026404199366226. [DOI] [PubMed] [Google Scholar]
- Weir WC, Weir DR. Adoption and subsequent conceptions. Fertil Steril. 1966;17:283–288. doi: 10.1016/s0015-0282(16)35897-6. [DOI] [PubMed] [Google Scholar]
- Weitzman ED, Fukushima D, Nogeire C, Roffwarg H, Gallagher TF, Hellman L. Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J Clin Endocrinol Metab. 1971;33:14–22. doi: 10.1210/jcem-33-1-14. [DOI] [PubMed] [Google Scholar]
- Yadav RK, Magan D, Mehta N, Sharma R, Mahapatra SC. Efficacy of a short-term yoga-based lifestyle intervention in reducing stress and inflammation: preliminary results. J Altern Complement Med. 2012;18:662–667. doi: 10.1089/acm.2011.0265. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Halligan SL, Yang RK, Guo LS, Makotkine I, Singh B, Pickholtz D. Relationship between 24-hour urinary-free cortisol excretion and salivary cortisol levels sampled from awakening to bedtime in healthy subjects. Life Sci. 2003;73:349–358. doi: 10.1016/s0024-3205(03)00286-8. [DOI] [PubMed] [Google Scholar]
- Zollner U, Dietl J. Perinatal risks after IVF and ICSI. J Perinat Med. 2013;41:17–22. doi: 10.1515/jpm-2012-0097. [DOI] [PubMed] [Google Scholar]
- Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.