Abstract
There will be over half a million cancer-related deaths in the United States in 2012, with lung cancer being the leader followed by prostate in men and breast in women. There is estimated to be more than one and a half million new cases of cancer in 2012, making the development of effective therapies a high priority. As tumor immunologists, we are interested in the development of immunotherapies because the immune response offers exquisite specificity and the potential to target tumor cells without harming normal cells. In this review, we highlight the current advances in the field of immunotherapy and the current work being completed by laboratories at University of Colorado School of Medicine in multiple malignancies, including breast cancer, lung cancer, melanoma, thyroid cancer, and glioblastoma. This work focuses on augmenting the anti-tumor response of CD8 T cells in the blood, lymph nodes, and tumors of patients, determining biomarkers for patients who are more likely to respond to immunotherapy, and identifying additional anti-tumor and immunosuppressive cells that influence the overall response to tumors. These collaborative efforts will identify mechanisms to improve immune function, which may elucidate therapeutic targets for clinical trials to improve patient health and survival.
Keywords: Cancer, Human, Tumor immunology, Immunotherapy, Tumor-infiltrating lymphocyte
Introduction
Cancer is a significant public health problem; a total of 1,638,910 new cancer cases and 577,190 deaths from cancer are projected to occur in the United States in 2012 [1]. One in four deaths in the United States is due to cancer; it is the second leading cause of death after heart disease. Although data between 1998 and 2008 indicate that cancer death rates have declined by more than 1 % per year in men and women of almost every racial and ethnic group [1], there is profound interest in developing successful therapies to augment this decline.
The immune system and inflammation contribute to tumor elimination and progression. In fact, Hanahan and Weinberg highlight both “evading immune destruction” and “tumor-promoting inflammation” in their 2011 Hallmarks of Cancer [2]. However, traditional therapeutic drug development focused solely on the tumor itself, not on the other components of the tumor microenvironment. Although it was unknown when they were developed, many common treatments alter the immune system and its microenvironment. More recently, the field of immunotherapy has broadened to target immune infiltrates and evasion mechanisms. The ultimate goal of these therapies is to shift the balance from tumor escape to tumor destruction.
Cytotoxic T cells (CTL) have long been the focus of tumor immunotherapy. The first immunotherapies generated a non-specific increase in T-cell proliferation with the stimulatory cytokines IL-2 [3] or IFN-α [4]. Later, specific antibody therapies were developed that targeted the activating molecules expressed on T cells (41BB, OX40). Recently, an antibody against cytotoxic lymphocyte activation gene 4, CTLA-4, was approved by the FDA for metastatic melanoma [5]. Patients with different cancers in a phase I clinical trial of a programmed cell death-1, PD-1, antibody also showed evidence of improved anti-tumor activity [6]. These non-specific immunotherapies have shown response rates in clinical trials ranging from 5 to 20 % (reviewed in [7, 8]).
A strong correlation between tumor-infiltrating lymphocytes (TILs) and patient survival is reported in many cancers (Table 1). Other targeted immunotherapies for the stimulation of CD8 TILs include vaccines for identified tumor antigens and various adjuvants [9, 10] or the adoptive transfer of dendritic cells differentiated in vitro in the presence of tumor antigens [11]. Increased clinical response and improved production methods for the expansion of TILs from patients are leading to the use of adoptive transfer of TILs as a cancer treatment. Several immunotherapies that activate CD8 TIL responses against the tumor have demonstrated clinical benefit or even improved survival (Table 2).
Table 1.
CD8 tumor-infiltrating lymphocytes correlate with patient survival
| Cancer type | CD8 TIL counts | Patient survival (months) | Correlation | No. of patients | References |
|---|---|---|---|---|---|
| Colorectal | 100–400 cells per mm2 | 10–35 | Lower cell density correlates with tumor recurrence | 415 | [113] |
| Metastatic melanoma | 35 % of total T cells in tumor | 23–130 | Decrease in survival when CD8 TILs are around tumor versus throughout tumor | 147 | [114] |
| Glioblastoma | 1–10 cells per mm2 | 30–40 | Increase in CD8 TILs correlates with increase in malignancy | 93 | [115] |
| Lung | 0.4–45 cells per mm2 Median = 10.8 cells |
240 (20–40 % survival) | Higher survival in patients with CD8 TILs >median | 99 | [116] |
| Ovarian | 0–89.5 cells per field Median = 7.60 cells |
25.5–54.9 | Higher survival correlates with higher intraepithelial CD8 TILs | 117 | [117] |
| Breast | 90–557 cells per field | 240 (50–70 % survival) | Higher survival in patients with CD8 TILs and correlation with grade | 1334 | [118] |
Table 2.
Immunotherapy in the treatment for human cancer: a summary of recent successful clinical trials
| Cancer type | Trial phase | Compound name | Treatment description | No. of patients | Outcome | References |
|---|---|---|---|---|---|---|
| Numerous | I | MKC1106-PP-PRAME and PSMA antigens | Intralymph node injection of DNA & peptide vaccine | 26 | 7 patients with stable disease at 6 months | [119] |
| Numerous | I | MDX-1106-I.V. MDX-1106 | I.V. MDX-1106 | 39 | 1 CR and 2 PR at 3 months | [6] |
| Non-small cell lung cancer | IIB | L-BLP25-MUC1 antigen | I.V. cyclohexamide and multiple S.C. vaccine injections | 171 | 24 % (L-BLP25 + BSC) v. 12 % (BSC) survival at 3 years | [120] |
| Non-small cell lung cancer | I | Chemotherapy/CEA peptide-pulsed DC/cytokine-induced killer cells | Chemo followed by multiple I.V. injections of cells | 28 | Median progression-free survival 6.9 months v. 5.2 months (chemo only) | [121] |
| Glioblastoma multiforme | I/II | DC vaccine with autologous tumor lystates | Multiple S.C. injections near lymph node | 17 | 37.5 % (vaccine) v. 3.2 % (historical ctrl) survival at 3 years | [122] |
| Melanoma | II | Lymphodepletion, autologous TIL, and high-dose IL-2 | Infusion of TIL expanded in culture | 93 | 36 % survival at 3 years, 29 % survival at 5 years | [123] |
| Melanoma | III | Ipilimumab-anti-CTLA- 4 ± gp100 peptide vaccine | I.V. ipilimumab ± S.C. gp100 in IFA | 676 | Median overall survival 10.1 months v. 6.4 months (gp100 alone) | [5] |
| Melanoma | III | gp100 peptide and high-dose IL-2 | I.V. injection of IL-2 and S.C. injection of peptide in IFA | 185 | 17.8 months v. 11.1 months overall survival (IL-2 only) | [10] |
| Renal cell carcinoma | I/II | GM-CSF, IL-2, and IFN-γ | S.C. injection of cytokines | 60 | Median progression-free survival 6.0 months | [124] |
| Prostate cancer | III | Sipuleucel-T-GM-CSF/PAP antigen fusion protein | I.V. injection of activated PBMC | 512 | 31.7 v. 23 % (placebo) survival at 3 years | [11] |
PRAME preferentially expressed antigen in melanoma, PSMA prostate-specific membrane antigen, MUC1 Mucin 1, IV intravenous, SC subcutaneous, V versus, BSC best supportive care, DC dendritic cell, TIL tumor-infiltrating lymphocytes, GM-CSF granulocyte-macrophage colony-stimulating factor, IL-2 interleukin 2, IFN-γ interferon gamma, IFA incomplete Freund’s adjuvant, PAP prostatic acid phosphatase, PBMC peripheral blood mononuclear cells, CR complete response, PR partial response
Although these promising new therapies significantly improve overall survival of cancer patients and demonstrate that manipulation of the immune system can lead to successful cancer treatments, they rarely establish durable cures for cancer in most patients. One hypothesis for these inconsistent responses is that increased regulatory mechanisms in cancer patients overcome active immunity and may also need to be targeted for successful treatment of cancer. In many patients, regulatory T cells (Treg) create an immunosuppressive environment within the tumor and throughout the periphery. Human Treg express the forkhead box transcription factor (FOXP3) [12] and suppress autoreactive T cells. Treg are detected in TILs of many human solid tumors [13–16], and a decreased ratio between CD8 TILs and Treg in the microenvironment of the tumor correlates with poor survival [17, 18]. Treg suppress the function of tumor-specific T cells by secretion of the immunosuppressive cytokines TGF-β and IL-10 and engaging suppressive cell surface molecules (i.e., CTLA-4-CD80/86) [19].
Myeloid-derived suppressor cells (MDSCs) are also associated with immunosuppression in multiple cancers. MDSCs are potent inactivators of both CD4 and CD8 T cells. The mechanisms that MDSCs use to induce immunosuppression involve activity of amino acid-metabolizing enzymes (such as arginase I and nitric oxide synthase) and production of reactive oxygen species, which have debilitating effects on the T-cell receptor (TCR) [20].
Due to challenges in translating promising research discoveries into human cancer treatments, the use of immunotherapies in cancer patients has not yet reached its potential. However, recent successes described in this review and the current trend toward combinatorial therapies suggest that the immune system will ultimately play a pivotal role in future cancer treatments. This review highlights the progress and objectives of the clinical tumor immunology research being conducted at the University of Colorado School of Medicine (UCSOM) in the context of the rest of the field.
Activation of TILs from human solid tumors (Slansky laboratory)
Several studies demonstrate functional inertness of CD8 TILs in solid tumors [21–24]. One example is the CD8 TILs in prostate cancer [25, 26]. These CD8 TILs are antigen-experienced (CD45RO+) and do not proliferate when removed from the tumor microenvironment ([25], Bruno, unpublished). In addition, they are refractory to stimulation through either the TCR or downstream signaling pathways via PHA, or PMA and ionomycin ([25], Bruno, unpublished). One reason that the T cells may be tolerized is that they are desensitized due to constant exposure to self-antigen; removal from this stimulation does not fully restore function. However, these CD8 TILs do contain perforin granules and produce cytokines, which suggests some degree of prior stimulation ([25], Bruno, unpublished). The CD8 TILs may have been activated by matured antigen presenting cells (APCs) because of the inflammatory cues present in the prostate. Thus, intrinsic and extrinsic factors contribute to functional unresponsiveness or incomplete activation of the CD8 TILs.
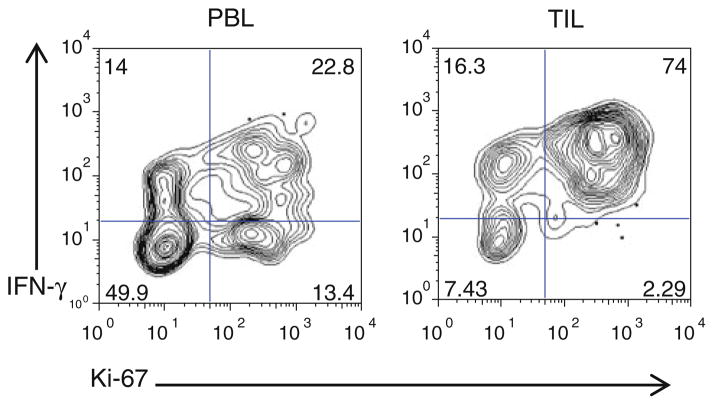
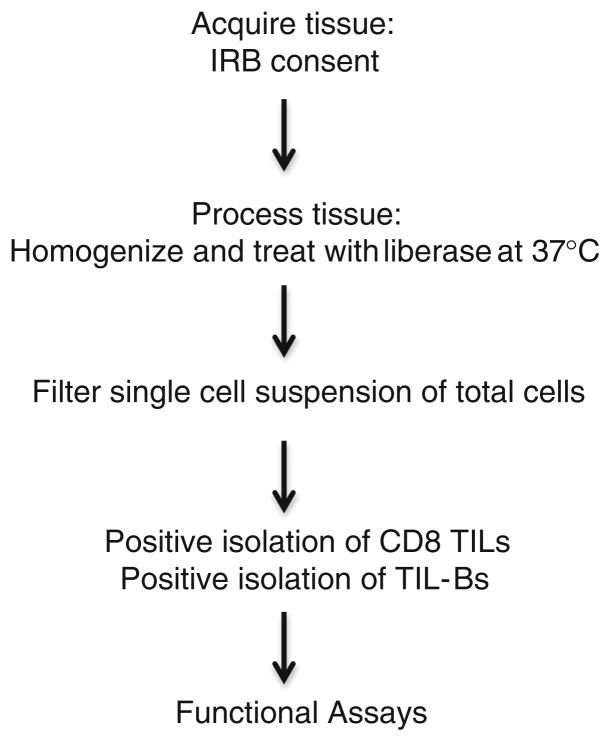
In multiple studies, the presence of CD8 TILs correlates with improved patient survival in various cancer types (Table 1). In human breast cancer, it has been reported that 11–57 % of tumors are infiltrated with CD8 TILs, which display an activated phenotype [27, 28]. Unlike prostate CD8 TILs, the Slansky laboratory has shown that CD8 TILs in human breast cancer tumors are functional when removed from the tumor microenvironment (Fig. 1). The laboratory is studying breast cancer CD8 TILs to determine the best way to enhance their cytolytic activity against tumors. One approach is to identify antigens that trigger a robust clonal proliferation of these CD8 TILs. To test methods of enhancing CTL activity of breast cancer TILs, we developed an effective protocol for isolating these cells from tumor tissue (Fig. 2). Determining the anti-tumor function and molecular characterization of these CD8 TILS will aid in the development of targeted immunotherapy for these patients. We ultimately envision an immunotherapy that targets the appropriate breast cancer antigen in combination with a blockade of one or multiple immune checkpoints, such as PD-1.
Fig. 1.
Breast cancer CD8 TILs proliferate (Ki-67) and produce IFN-γ in response to TCR stimulation. CD8 T cells were isolated from total PBMC post-Ficoll separation (left). CD8 TILs were isolated according to the protocol in Fig. 2 (right). Cells were stimulated postselection for 5 days with αCD3αCD28. On day five, the cells were restimulated with PMA, ionomycin, and brefeldin-A for 6 h, stained intracellularly for Ki-67 and IFN-γ, and analyzed by flow cytometry
Fig. 2.
TIL isolation protocol
B cells also infiltrate cancers. Tumor-infiltrating B cells (TIL-Bs) are present in about 25 % of breast cancers and comprise up to 40 % of the TIL population [29–31]. Detection of these cells correlates with positive survival rates in medullary breast cancer [30, 32, 33]; however, the function of these cells is unclear. There are several hypotheses offering explanation as to why these B cells are prevalent in tumors based on studies done in autoimmunity. Specifically, B cells are antigen-presenting cells and collaborate with T cells to generate immune responses with significant tissue damage. The B cells often enhance T-cell responses by producing antibodies, stimulatory cytokines, and chemokines and serve as a local antigen-presenting cell. Together, B cells and T cells often generate lymphoid structures at the site of the autoimmune reaction [34]. It has been hypothesized that B cells help generate a potent, long-term immune response against cancer. Although some B cells may amplify the immune response, regulatory B cells suppress the immune response via IL-10 and TGF-β-1 [35]. While these correlative data are interesting, there is not much known about the mechanism of TIL-Bs in cancer. Thus, we are also studying TIL-Bs to determine their function.
Our first analyses of TIL-Bs are in non-small cell lung cancer (NSCLC) patients. Like breast cancer, TIL-Bs correlate with positive survival in NSCLC patients [36, 37]. In collaboration with Dr. Jeffrey Kern, director of the Cancer Research Center at National Jewish Health, we obtain lung tumor and tumor-adjacent samples from primary NSCLC adenocarcinomas that undergo surgical resection at University of Colorado. We phenotype the memory and activation profile of TIL-Bs and dissect the function by isolating TIL-Bs from the tissue (Fig. 2) and monitoring proliferation and cytokine production. With a better understanding of the function of TIL-Bs, we hope to introduce them as a potential therapeutic target.
Immunotherapeutic biomarkers in the treatment for human melanoma (McCarter laboratory)
The recent successes in immunotherapy for cancer have largely been a result of research in melanoma patients (reviewed in [7]). Whether due to the antigens expressed by melanoma cells, the identification of these specific antigens, or the nature of the tumors themselves, melanoma uniquely generates detectable tumor antigen–specific T-cell responses [38]. In addition, melanoma is generally resistant to conventional chemotherapy and radiation, making it an ideal disease for studies of experimental immune therapies [39]. Several studies demonstrate that cells and molecules of the immune system correlate with survival and prognosis in melanoma patients; however, therapies designed to activate the immune system, such as IL-2 and IFN-α, rarely lead to long-term productive responses. To improve these treatments, there is a need for a better understanding of the immune response in treated patients and for biomarkers that predict which patients will respond.
A recent success in the treatment for melanoma is with the drug ipilimumab, an antibody therapy that targets CTLA-4. Although studies in knockout mice suggest that CTLA-4 controls effector T-cell function [40], CTLA-4 is constitutively expressed by Treg, which makes them a target of anti-CTLA-4 therapy [41]. One study found no differences in the number of peripheral Treg after anti-CTLA-4 treatment and no differences between patients that responded to the therapy and those that did not respond [42]. Others found that ipilimumab did not directly inhibit Treg function in vitro and that post-treatment Treg maintain suppressive function [43]. However, preclinical data suggest that blocking CTLA-4 on both effector and regulatory T cells is required for full benefit [44]. In stage IV melanoma patients, anti-CTLA-4 therapy increases the 1-year survival rate from 25 to 45 % and significantly extends overall survival [5, 45]. Further investigations into these mechanisms will identify why only some patients respond to this treatment.
An important lesson learned from the early clinical trials of anti-CTLA-4 treatment is that responses to drugs targeting the immune system are different from chemotherapy and radiation that directly and quickly kill tumor cells. Immunotherapies may result in an initial increase in tumor size, or they may take longer to generate a T-cell response required to decrease the tumor. Therefore, patients that initially fail to respond to these therapies using traditional Response Evaluation Criteria in Solid Tumors (RECIST) measurements may respond outside of the time limits imposed by the research study or may demonstrate an overall survival benefit in the absence of tumor shrinkage. Although not yet widely adopted, an important advance in immunotherapy was the development of new evaluation criteria, termed immune-related response criteria (irRC), which may prevent immune therapies from prematurely failing in clinical trials [46]. However, other response indicators, independent of tumor size, are actively under investigation and may be important for monitoring responses to immunotherapy. Several studies have found that changes in absolute lymphocyte and/or CD8 T-cell counts in the peripheral blood, particularly the rate at which these cell counts increase, correlate with positive responses to anti-CTLA-4 treatment [47]. A post-treatment increase in the expression of inducible costimulator (ICOS) on CD4 T cells correlates with overall survival [48]. Finally, one study found that CD4 memory T cells from patients responding to anti-CTLA-4 treatment develop resistance to suppression by Treg, while cells from non-responding patients maintain sensitivity to Treg inhibitory mechanisms [49].
Molecular biomarkers are currently in use for selecting targeted therapies in melanoma patients. For example, tumors expressing a BRAF mutation (40–60 % of all cutaneous melanoma) are more likely to respond to BRAF inhibitors [50, 51]. These types of mutational screens also help determine the mechanism of escape from drugs such as BRAF inhibitors, in which activation (or mutation) of other molecules in the MAPK pathway drives resistance [52]. In contrast, immune biomarkers are still exploratory, not standardized, and not currently directing patient care. Recent studies suggest that patients with increases in certain serum cytokines, such as IL-1α/β, TNF-α, IL-6, MIP-1α/β, are more likely to respond to IFN-α treatment [53]. Increased levels of serum C-reactive protein, fibronectin, and VEGF correlate with poor responses to IL-2 therapy [54, 55]. Finally, increased levels of intratumor Treg, the Treg-associated transcription factor FOXP3, and the Treg-associated enzyme indoleamine 2,3-dioxygenase (IDO) correlate with better responses to anti-CTLA-4 treatment [56]. These results suggest that preexisting immune responses against the tumor may predict responses to anti-CTLA-4 therapy ([57]; reviewed in [58]). With an understanding of the factors that distinguish responders from non-responders, immune biomarkers may help guide patient care and predict treatment outcomes.
In the McCarter laboratory, we are investigating several potential biomarkers of responses to ipilimumab. Using peripheral blood from stage IV melanoma patients, the number of T cells, expression of ICOS and PD-1 on T cells, Treg suppressive function, and plasma cytokine levels will be determined before and after treatment with 10 mg/kg ipilimumab. In agreement with previous studies [13, 59–61], the number of Treg in the peripheral blood of untreated stage IV melanoma patients is increased relative to normal donors, and the number of Treg in these patients correlates with the amount of TGF-β in the plasma (unpublished data). Important for this study and others, melanoma patients at UCSOM are also recruited to donate tumor tissue and serum samples to the Melanoma Tumor Bank, so that biomarker studies can be performed (Dr. William Robinson, Director). We are using live tumor cells isolated from tissue and human melanoma tumor lines from this bank for in vitro investigational studies to gain insight into identifying immunologic biomarkers that will help select patients that are most likely to benefit from specific immunotherapies.
Immune modulation in metastatic papillary thyroid cancer (French and Haugen laboratory)
Papillary thyroid cancer (PTC) commonly develops in patients with autoimmune thyroiditis. This association has led to much speculation about the role of inflammation in tumorigenesis and cancer progression [62]. Until recently, studies investigating the role of the immune system in PTC failed to distinguish between autoimmune thyroiditis and the tumor-directed immune response [63, 64]. Furthermore, these studies failed to fully characterize the types of leukocytes present in PTC. In a retrospective study, we assessed archived formalin-fixed paraffin-embedded primary thyroid tumors from PTC patients for evidence of lymphocytic infiltration. Patient samples were further distinguished based on the evidence of lymphocytic infiltration in normal thyroid tissue, a sign of autoimmune thyroiditis. While no significant difference was observed between patients without lymphocytic infiltration and those with concurrent autoimmune thyroiditis, patients with tumor-associated lymphocytes (TALs), from lymph nodes, displayed more severe disease [65]. Treg were detected among TALs, and increased Treg frequency correlated with a higher percentage of lymph node (LN) metastases [65]. These studies suggest that immune modulation in patients with PTC may play an important role in disease progression.
PTC is distinct from other types of cancer in that persistent metastatic disease is commonly confined to loco-regional LN. Analyses of central neck lymph node dissection (CNLD) specimens revealed that more than 60 % of patients with PTC develop LN metastases [66]. A total of 20–30 % of patients who have undergone standard therapies (i.e., primary thyroidectomy, CNLD, and radioactive iodine therapy) develop recurrent disease, most commonly in the locoregional LN [67, 68]. Metastatic LN in patients with PTC provide a unique model in which to study the interplay between the immune system and tumor. Our ongoing studies rely on postsurgical patient samples obtained by fine-needle biopsy from uninvolved (UILN) and tumor-involved lymph nodes (TILN). Live lymphocytes recovered from these samples are characterized using multicolor flow cytometry to assess cell surface activation markers, cytokine receptors, and intracellular expression of cytokines and transcription factors. These studies revealed that Treg are enriched in TILN compared with UILN and their frequency is even further increased in TILN from patients with recurrent metastatic disease [69]. In line with these findings, CD8 T cells expressing PD-1, a marker of T-cell exhaustion, are enriched in TILN [69–71]. Furthermore, high frequencies of PD-1+ CD8 T cells in TILN are associated with extranodal invasion [69]. A majority of PD-1+ CD8 T cells in TILN from patients with PTC are CD45RA−, suggesting recent antigen exposure; however, these cells fail to downregulate CD27 and are not actively proliferating [69]. Of note, the frequencies of Treg and PD-1+ CD8 T cells correlate directly in TILN [69]. Additional studies are necessary to determine the function of Treg and PD-1+ CD8 T cells, respectively, in PTC.
Most patients with PTC have an excellent prognosis following standard therapies (97 % 5-year survival rate) [67]. However, patients with recurrent LN disease and distant metastatic lesions will benefit from innovative prognostic markers and adjuvant therapies. We are currently investigating the potential of Treg and PD-1+ CD8 T-cell frequencies in TILN as prognostic markers for patients with recurrent lymph node disease. Such markers will aid in determining the best therapeutic approach for each patient (i.e., compartment LN excision vs. focused LN dissection vs. radioiodine therapy vs. monitoring). Furthermore, regional or systemic immune-based therapies that target Treg and/or PD-1+ CD8 T cells may be viable options for patients with recurrent or advanced PTC that cannot be cured with surgery or standard therapies.
Identifying host factors that influence immune suppression in breast cancer (Borges laboratory)
Breast cancer is typically considered a disease of aging, as the dominant portion of cases is diagnosed in postmenopausal women. Similarly, the well-known risk factors for breast cancer are tightly tied to cumulative lifetime estrogen exposure, factors such as menarche, parity, late menopause, and exogenous hormone use, which only apply well to postmenopausal women. However, 25,000 cases of breast cancer are diagnosed each year in women under age 45 whose risk factors for the development of breast cancer are poorly understood. Diagnosis under age 40 is also associated with a significant increase in risk of metastasis and death for reasons that are not explained by known prognostic criteria [72–74].
Breast cancer has multiple subtypes that display distinct prognosis and potential for treatment response. These subtypes are based on genomic data, but are clinically identifiable through the expression of the estrogen receptor, progesterone receptor, and HER2/neu oncoprotein on tumor cells [75]. The nomenclature of these subtypes currently are Luminal A (ER+, PR+, HER2−), Luminal B (ER+ and either PR− or PR+ and HER2+), HER2 (ER−, PR− and HER2+), and triple negative (ER−, PR− and HER2−). To date, immunotherapy in breast cancer has been successful through targeting of the HER2 pathway. The monoclonal antibody trastuzumab has been standard of care for advanced breast cancer since 1998 and early stage breast cancer since 2005. HER2-targeted vaccine treatment also demonstrates very promising results in clinical trials [76, 77]. In our Young Women’s Breast Cancer Translational Program, we are studying the interaction of young age at diagnosis with the different biologic breast cancer subtypes. We are identifying the differences in the immune response to breast cancer depending on patient age and outcome. We are currently focused on the characterization of MDSCs and their function in newly diagnosed young women’s breast cancer, as well as potential mechanisms of reversal of their function that would be relevant in the treatment for breast cancer.
While MDSCs have been well defined in murine models, a clinical definition in humans remains more elusive. As recently reviewed by Montero et al., there are discrepancies over the phenotype of these cells between cancer types, with one of the only commonalities being their ability to suppress T-cell function, though through different mechanisms. In early descriptions, MDSCs were defined as a heterogeneous population made up of immature myeloid cells [78–83]. There have also been studies that show more differentiated MDSCs, most commonly expressing CD11b and CD33. These mature MDSCs can be broken down into two groups based on the markers CD14 and CD15. Granulocytic MDSCs tend to be CD15+ CD14− [84–89], while monocytic MDSCs are CD15− CD14+ [90–94]. To date, cancer patients, including breast cancer patients, have increased numbers of MDSCs intratumorally and in the blood [95]. In breast cancer, the number of MDSCs correlates with disease stage and T-cell suppressive function as well as adverse patient outcomes [96].
Through the identification of MDSCs and their suppressive function in newly diagnosed early-stage young women’s breast cancer, we hope to define their potential as a prognostic marker and potential immunotherapeutic target. As part of a larger prospective trial of stage I–IV newly diagnosed breast cancer patients and age-matched normal donors, we have quantitated the presence of MDSCs and their function in T-cell suppression in the peripheral blood (unpublished data). We are currently testing several potential drugs that may disrupt MDSC suppression with the goal of identifying adjunct treatment to improve standard breast cancer therapies.
Neutrophils and immunosuppression in glioblastoma (Waziri laboratory)
Glioblastoma (GBM) patients have suppression of cellular immunity within the tumor microenvironment as well as the systemic circulation, secondary to functional defects in both APCs and T cells (reviewed in [97–99]). Despite these defects, tumor antigen–specific T cells can be found within the circulation of affected patients [100]. This observation implies that tumor-specific immunity is blunted by tumor-mediated suppression of T-cell function, a consideration that is likely relevant to the success of immunotherapy. Therefore, targeting tumor-associated immunosuppression in patients with GBM will be critical for the development of meaningful immunotherapeutic strategies.
In Dr. Waziri’s laboratory, we have recently demonstrated that myeloid cells from GBM patients suppress normal donor T-cell function within a mixed lymphocyte reaction [101]. Through expression analysis of a range of classical myeloid lineage surface markers, we identified an expanded population of CD33lo cells within peripheral blood mononuclear cells (PBMC) from GBM patients that were not present in normal donors, or to a lesser extent, patients with other intracranial tumors. Further examination of the CD33lo population confirmed that these cells express the characteristic neutrophil markers CD15 and CD66 and exhibit the histological appearance of mature neutrophils. This finding was intriguing, as neutrophils are not normally found within PBMC following Ficoll density centrifugation, but rather separate with the flow-through fraction. The shift to the PBMC fraction suggested decreased density when compared to normal neutrophils, a phenomenon that could be induced through activation and degranulation. We confirmed this hypothesis by stimulating normal donor neutrophils within whole blood with formyl-methionyl-leucyl-phenylalanine (FMLP). Analysis of the PBMC from FMLP-stimulated whole blood confirmed the presence of increased numbers of neutrophils.
One of the major constituents of neutrophilic granules is the immunosuppressive enzyme arginase I (ArgI) [101], which is released upon neutrophil degranulation [102] and induces T-cell dysfunction through a well-described mechanism [103, 104]. To extend upon our prior phenotypic data suggesting the presence of activated neutrophils within the circulation of GBM patients, we provided confirmatory functional evidence by documenting increased levels of ArgI within in vitro immunofunctional assays and serum samples harvested from GBM patients. Critically, by targeting ArgI within in vitro immunofunctional assays, GBM patient T-cell function could be restored to the level of normal donors. As a direct result of this study, we have initiated a clinical trial to test the efficacy of oral L-argi-nine supplementation for improving T-cell function in newly diagnosed GBM patients. Through this work, we hope to confirm the role of targeting neutrophil-mediated immunosuppression as an adjuvant strategy to improve immunotherapy in GBM patients.
We continue to explore additional aspects of the biologic interactions between tumor and neutrophils. In general, neutrophils are not believed to release their granules outside the confines of inflamed tissue or move out of the tissue to reenter circulation once activated. Therefore, the presence of activated neutrophils within the peripheral circulation of GBM patients is of unclear etiology. Limited prior data suggests that activated neutrophils may exist in the circulation of other tumor patients [86, 87] although the mechanism responsible for this phenomenon remains to be described. Our initial comparative studies focusing on expression patterns of circulating activated neutrophils and intratumoral neutrophils have identified altered levels of several adhesion molecules necessary for transmigration on the circulating activated population (Sippel et al., manuscript in preparation). Further exploration of this mechanism may identify new targets for preventing the formation of activated neutrophils in circulation and the resulting suppression of cellular immunity.
In addition to a potential immunosuppressive role, neutrophils may also exert other significant effects (either advantageous or detrimental) on tumor growth [105–107]. Neutrophils may promote tumor growth by initiating angiogenesis, aiding in invasion and metastasis, and enhancing proliferative activity. In contrast, they have been associated with anti-tumor effects through direct cytotoxicity as well as promotion of the inflammatory response. We and others have observed the presence of neutrophils within areas of active necrosis in GBM [101, 108] although the overarching effects of these cells remain indeterminate. Interestingly, neutrophils have been associated with increased necrosis, ischemic tissue, and edema in brain injury and stroke [109, 110]. As necrosis is a distinguishing feature in GBM [111] and edema significantly contributes to morbidity in these patients [112], it is possible that neutrophils may be intimately involved in these processes in GBM. Our ongoing work continues to focus upon the involvement of neutrophils in GBM, with the hope of identifying new therapeutic targets for this disease.
Conclusion
While important strides have been made to improve detection and treatment, thousands of patients succumb to cancer. The tumor’s ability to escape immune destruction remains a constant variable that may be central to the host’s inability to eradicate the transformed cells. To this end, improving the immune system’s response to eliminate these malignant cells has become the focus of many research laboratories. In this review, we have focused on the recent efforts of multiple laboratories at UCSOM and in the field to improve immune function and identify targetable immunosuppressive mechanisms in multiple malignancies including breast cancer, lung cancer, melanoma, thyroid cancer, and glioblastoma.
The ongoing, collaborative efforts of these laboratories focus on mechanisms to improve CD8 TIL responses, determine biomarkers for patients who are more likely to respond to immunotherapy, and identify cells within the tumor, surrounding lymph nodes and peripheral blood that are immunosuppressive. These efforts will identify mechanisms to improve immune function, which may elucidate therapeutic targets that could directly translate to the design of clinical trials and directly impact patient health and overall survival.
Contributor Information
Tullia C. Bruno, National Jewish Health, Integrated Department of Immunology, University of Colorado School of Medicine, 1400 Jackson Street, Room K511, Denver, CO 80206, USA
Jena D. French, Division of Endocrinology, Metabolism, and Diabetes, Department of Medicine, University of Colorado School of Medicine, Aurora, CO 80045, USA. University of Colorado School of Medicine, Aurora, CO 80045, USA
Kimberly R. Jordan, Department of Surgery, University of Colorado School of Medicine, Aurora, CO 80045, USA
Oscar Ramirez, Department of Medical Oncology, University of Colorado School of Medicine, Aurora, CO 80045, USA.
Trisha R. Sippel, Department of Neurosurgery, University of Colorado School of Medicine, Aurora, CO 80045, USA
Virginia F. Borges, Department of Medical Oncology, University of Colorado School of Medicine, Aurora, CO 80045, USA
Bryan R. Haugen, Division of Endocrinology, Metabolism, and Diabetes, Department of Medicine, University of Colorado School of Medicine, Aurora, CO 80045, USA. Department of Pathology, University of Colorado School of Medicine, Aurora, CO 80045, USA
Martin D. McCarter, Department of Surgery, University of Colorado School of Medicine, Aurora, CO 80045, USA
Allen Waziri, Department of Neurosurgery, University of Colorado School of Medicine, Aurora, CO 80045, USA.
Jill E. Slansky, Email: Jill.Slansky@ucdenver.edu, National Jewish Health, Integrated Department of Immunology, University of Colorado School of Medicine, 1400 Jackson Street, Room K511, Denver, CO 80206, USA
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Smith FO, Downey SG, Klapper JA, Yang JC, Sherry RM, Royal RE, et al. Treatment of metastatic melanoma using interleukin-2 alone or in conjunction with vaccines. Clin Cancer Res. 2008;14(17):5610–8. doi: 10.1158/1078-0432.CCR-08-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mocellin S, Pasquali S, Rossi CR, Nitti D. Interferon alpha adjuvant therapy in patients with high-risk melanoma: a systematic review and meta-analysis. J Natl Cancer Inst. 2010;102(7):493–501. doi: 10.1093/jnci/djq009. [DOI] [PubMed] [Google Scholar]
- 5.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexandrescu DT, Ichim TE, Riordan NH, Marincola FM, Di Nardo A, Kabigting FD, et al. Immunotherapy for melanoma: current status and perspectives. J Immunother. 2010;33(6):570–90. doi: 10.1097/CJI.0b013e3181e032e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber J. Immunotherapy for melanoma. Curr Opin Oncol. 2011;23(2):163–9. doi: 10.1097/CCO.0b013e3283436e79. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10(9):909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364(22):2119–27. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–22. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 12.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 13.Ahmadzadeh M, Felipe-Silva A, Heemskerk B, Powell DJ, Jr, Wunderlich JR, Merino MJ, et al. FOXP3 expression accurately defines the population of intratumoral regulatory T cells that selectively accumulate in metastatic melanoma lesions. Blood. 2008;112(13):4953–60. doi: 10.1182/blood-2008-06-163048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169(5):2756–61. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 15.Miller AM, Lundberg K, Ozenci V, Banham AH, Hellstrom M, Egevad L, et al. CD4+ CD25 high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol. 2006;177(10):7398–405. doi: 10.4049/jimmunol.177.10.7398. [DOI] [PubMed] [Google Scholar]
- 16.Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005;65(6):2457–64. doi: 10.1158/0008-5472.CAN-04-3232. [DOI] [PubMed] [Google Scholar]
- 17.Chen KJ, Zhou L, Xie HY, Ahmed TE, Feng XW, Zheng SS. Intratumoral regulatory T cells alone or in combination with cytotoxic T cells predict prognosis of hepatocellular carcinoma after resection. Med Oncol. 2012;29:1817–26. doi: 10.1007/s12032-011-0006-x. [DOI] [PubMed] [Google Scholar]
- 18.Shen Z, Zhou S, Wang Y, Li RL, Zhong C, Liang C, et al. Higher intratumoral infiltrated Foxp3+ Treg numbers and Foxp3+/CD8+ ratio are associated with adverse prognosis in resectable gastric cancer. J Cancer Res Clin Oncol. 2010;136(10):1585–95. doi: 10.1007/s00432-010-0816-9. [DOI] [PubMed] [Google Scholar]
- 19.von Boehmer H. Mechanisms of suppression by suppressor T cells. Nat Immunol. 2005;6(4):338–44. doi: 10.1038/ni1180. [DOI] [PubMed] [Google Scholar]
- 20.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–79. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 21.Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat Med. 2001;7(10):1118–22. doi: 10.1038/nm1001-1118. [DOI] [PubMed] [Google Scholar]
- 22.Radoja S, Frey AB. Cancer-induced defective cytotoxic T lymphocyte effector function: another mechanism how antigenic tumors escape immune-mediated killing. Mol Med. 2000;6(6):465–79. [PMC free article] [PubMed] [Google Scholar]
- 23.Whiteside TL. Immune cells in the tumor microenvironment. Mechanisms responsible for functional and signaling defects. Adv Exp Med Biol. 1998;451:167–71. [PubMed] [Google Scholar]
- 24.Zippelius A, Batard P, Rubio-Godoy V, Bioley G, Lienard D, Lejeune F, et al. Effector function of human tumor-specific CD8 T cells in melanoma lesions: a state of local functional tolerance. Cancer Res. 2004;64(8):2865–73. doi: 10.1158/0008-5472.can-03-3066. [DOI] [PubMed] [Google Scholar]
- 25.Bronte V, Kasic T, Gri G, Gallana K, Borsellino G, Marigo I, et al. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J Exp Med. 2005;201(8):1257–68. doi: 10.1084/jem.20042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karja V, Aaltomaa S, Lipponen P, Isotalo T, Talja M, Mokka R. Tumour-infiltrating lymphocytes: a prognostic factor of PSA-free survival in patients with local prostate carcinoma treated by radical prostatectomy. Anticancer Res. 2005;25(6C):4435–8. [PubMed] [Google Scholar]
- 27.Georgiannos SN, Renaut A, Goode AW, Sheaff M. The immunophenotype and activation status of the lymphocytic infiltrate in human breast cancers, the role of the major histo-compatibility complex in cell-mediated immune mechanisms, and their association with prognostic indicators. Surgery. 2003;134(5):827–34. doi: 10.1016/s0039-6060(03)00292-7. [DOI] [PubMed] [Google Scholar]
- 28.Ruffell B, Au A, Rugo HS, Esserman LJ, Hwang ES, Coussens LM. Leukocyte composition of human breast cancer. Proc Natl Acad Sci USA. 2012;109(8):2796–801. doi: 10.1073/pnas.1104303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chin Y, Janseens J, Vandepitte J, Vandenbrande J, Opdebeek L, Raus J. Phenotypic analysis of tumor-infiltrating lymphocytes from human breast cancer. Anticancer Res. 1992;12(5):1463–6. [PubMed] [Google Scholar]
- 30.Coronella-Wood JA, Hersh EM. Naturally occurring B-cell responses to breast cancer. Cancer Immunol Immunother. 2003;52(12):715–38. doi: 10.1007/s00262-003-0409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marsigliante S, Biscozzo L, Marra A, Nicolardi G, Leo G, Lobreglio GB, et al. Computerised counting of tumour infiltrating lymphocytes in 90 breast cancer specimens. Cancer Lett. 1999;139(1):33–41. doi: 10.1016/s0304-3835(98)00379-6. [DOI] [PubMed] [Google Scholar]
- 32.Lim KH, Telisinghe PU, Abdullah MS, Ramasamy R. Possible significance of differences in proportions of cytotoxic T cells and B-lineage cells in the tumour-infiltrating lymphocytes of typical and atypical medullary carcinomas of the breast. Cancer Immun. 2010;10:1–5. [PMC free article] [PubMed] [Google Scholar]
- 33.Ridolfi RL, Rosen PP, Port A, Kinne D, Mike V. Medullary carcinoma of the breast: a clinicopathologic study with 10 year follow-up. Cancer. 1977;40(4):1365–85. doi: 10.1002/1097-0142(197710)40:4<1365::aid-cncr2820400402>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 34.Nelson BH. CD20+ B cells: the other tumor-infiltrating lymphocytes. J Immunol. 2010;185(9):4977–82. doi: 10.4049/jimmunol.1001323. [DOI] [PubMed] [Google Scholar]
- 35.Lund FE. Cytokine-producing B, lymphocytes-key regulators of immunity. Curr Opin Immunol. 2008;20(3):332–8. doi: 10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund LT. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res. 2008;14(16):5220–7. doi: 10.1158/1078-0432.CCR-08-0133. [DOI] [PubMed] [Google Scholar]
- 37.Riemann D, Wenzel K, Schulz T, Hofmann S, Neef H, Lautenschlager C, et al. Phenotypic analysis of T lymphocytes isolated from non-small-cell lung cancer. Int Arch Allergy Immunol. 1997;114(1):38–45. doi: 10.1159/000237640. [DOI] [PubMed] [Google Scholar]
- 38.Topalian SL, Solomon D, Avis FP, Chang AE, Freerksen DL, Linehan WM, et al. Immunotherapy of patients with advanced cancer using tumor-infiltrating lymphocytes and recombinant interleukin-2: a pilot study. J Clin Oncol. 1988;6(5):839–53. doi: 10.1200/JCO.1988.6.5.839. [DOI] [PubMed] [Google Scholar]
- 39.Garbe C, Eigentler TK, Keilholz U, Hauschild A, Kirkwood JM. Systematic review of medical treatment in melanoma: current status and future prospects. Oncologist. 2011;16(1):5–24. doi: 10.1634/theoncologist.2010-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3(5):541–7. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 41.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116(7):1935–45. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Attia P, Phan GQ, Maker AV, Robinson MR, Quezado MM, Yang JC, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23(25):6043–53. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maker AV, Attia P, Rosenberg SA. Analysis of the cellular mechanism of antitumor responses and autoimmunity in patients treated with CTLA-4 blockade. J Immunol. 2005;175(11):7746–54. doi: 10.4049/jimmunol.175.11.7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206(8):1717–25. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Korn EL, Liu PY, Lee SJ, Chapman JA, Niedzwiecki D, Suman VJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol. 2008;26(4):527–34. doi: 10.1200/JCO.2007.12.7837. [DOI] [PubMed] [Google Scholar]
- 46.Wolchok JD, Hoos A, O’Day S, Weber JS, Hamid O, Lebbe C, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–20. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 47.Ku GY, Yuan J, Page DB, Schroeder SE, Panageas KS, Carvajal RD, et al. Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer. 2010;116(7):1767–75. doi: 10.1002/cncr.24951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carthon BC, Wolchok JD, Yuan J, Kamat A, Ng Tang DS, Sun J, et al. Preoperative CTLA-4 blockade: tolerability and immune monitoring in the setting of a presurgical clinical trial. Clin Cancer Res. 2010;16(10):2861–71. doi: 10.1158/1078-0432.CCR-10-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Menard C, Ghiringhelli F, Roux S, Chaput N, Mateus C, Grohmann U, et al. Ctla-4 blockade confers lymphocyte resistance to regulatory T-cells in advanced melanoma: surrogate marker of efficacy of tremelimumab? Clin Cancer Res. 2008;14(16):5242–9. doi: 10.1158/1078-0432.CCR-07-4797. [DOI] [PubMed] [Google Scholar]
- 50.Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467(7315):596–9. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rubinstein JC, Sznol M, Pavlick AC, Ariyan S, Cheng E, Bacchiocchi A, et al. Incidence of the V600 K mutation among melanoma patients with BRAF mutations, and potential therapeutic response to the specific BRAF inhibitor PLX4032. J Transl Med. 2010;8:67. doi: 10.1186/1479-5876-8-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sznol M. Molecular markers of response to treatment for melanoma. Cancer J. 2011;17(2):127–33. doi: 10.1097/PPO.0b013e318212dd5a. [DOI] [PubMed] [Google Scholar]
- 53.Yurkovetsky ZR, Kirkwood JM, Edington HD, Marrangoni AM, Velikokhatnaya L, Winans MT, et al. Multiplex analysis of serum cytokines in melanoma patients treated with interferon-alpha2b. Clin Cancer Res. 2007;13(8):2422–8. doi: 10.1158/1078-0432.CCR-06-1805. [DOI] [PubMed] [Google Scholar]
- 54.Sabatino M, Kim-Schulze S, Panelli MC, Stroncek D, Wang E, Taback B, et al. Serum vascular endothelial growth factor and fibronectin predict clinical response to high-dose interleukin-2 therapy. J Clin Oncol. 2009;27(16):2645–52. doi: 10.1200/JCO.2008.19.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tartour E, Blay JY, Dorval T, Escudier B, Mosseri V, Douillard JY, et al. Predictors of clinical response to interleukin-2–based immunotherapy in melanoma patients: a French multiinstitutional study. J Clin Oncol. 1996;14(5):1697–703. doi: 10.1200/JCO.1996.14.5.1697. [DOI] [PubMed] [Google Scholar]
- 56.Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci USA. 2008;105(8):3005–10. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weber JS, Hamid O, Chasalow SD, Wu DY, Parker SM, Galbraith S, et al. Ipilimumab increases activated T cells and enhances humoral immunity in patients with advanced melanoma. J Immunother. 2012;35(1):89–97. doi: 10.1097/CJI.0b013e31823aa41c. [DOI] [PubMed] [Google Scholar]
- 58.Callahan MK, Wolchok JD, Allison JP. Anti-CTLA-4 antibody therapy: immune monitoring during clinical development of a novel immunotherapy. Semin Oncol. 2010;37(5):473–84. doi: 10.1053/j.seminoncol.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jandus C, Bioley G, Speiser DE, Romero P. Selective accumulation of differentiated FOXP3(+) CD4(+) T cells in metastatic tumor lesions from melanoma patients compared to peripheral blood. Cancer Immunol Immunother. 2008;57(12):1795–805. doi: 10.1007/s00262-008-0507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCarter MD, Baumgartner J, Escobar GA, Richter D, Lewis K, Robinson W, et al. Immunosuppressive dendritic and regulatory T cells are upregulated in melanoma patients. Ann Surg Oncol. 2007;14(10):2854–60. doi: 10.1245/s10434-007-9488-3. [DOI] [PubMed] [Google Scholar]
- 61.Vence L, Palucka AK, Fay JW, Ito T, Liu YJ, Banchereau J, et al. Circulating tumor antigen-specific regulatory T cells in patients with metastatic melanoma. Proc Natl Acad Sci USA. 2007;104(52):20884–9. doi: 10.1073/pnas.0710557105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guarino V, Castellone MD, Avilla E, Melillo RM. Thyroid cancer and inflammation. Mol Cell Endocrinol. 2010;321(1):94–102. doi: 10.1016/j.mce.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 63.Hirabayashi RN, Lindsay S. The relation of thyroid carcinoma and chronic thyroiditis. Surg Gynecol Obstet. 1965;121:243–52. [PubMed] [Google Scholar]
- 64.Matsubayashi S, Kawai K, Matsumoto Y, Mukuta T, Morita T, Hirai K, et al. The correlation between papillary thyroid carcinoma and lymphocytic infiltration in the thyroid gland. J Clin Endocrinol Metab. 1995;80(12):3421–4. doi: 10.1210/jcem.80.12.8530576. [DOI] [PubMed] [Google Scholar]
- 65.French JD, Weber ZJ, Fretwell DL, Said S, Klopper JP, Haugen BR. Tumor-associated lymphocytes and increased FoxP3+ regulatory T cell frequency correlate with more aggressive papillary thyroid cancer. J Clin Endocrinol Metab. 2010;95(5):2325–33. doi: 10.1210/jc.2009-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ito Y, Jikuzono T, Higashiyama T, Asahi S, Tomoda C, Takamura Y, et al. Clinical significance of lymph node metastasis of thyroid papillary carcinoma located in one lobe. World J Surg. 2006;30(10):1821–8. doi: 10.1007/s00268-006-0211-5. [DOI] [PubMed] [Google Scholar]
- 67.Howlader NNA, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA, Edwards BK, editors. Surveillance epidemiology and end results (SEER): Cancer Statistics Review 1975–2008. Bethesda, MD: National Cancer Institute; 2011. Posted to the SEER web site. [Google Scholar]
- 68.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97(5):418–28. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 69.French JD, Kotnis GR, Said S, Raeburn CD, McIntyre JRC, Klopper JP, et al. Programmed death-1+ T cells and regulatory T Cells are enriched in tumor-involved lymph nodes and associated with aggressive features in papillary thyroid cancer. J Clin Endocrinol Metab. 2012 Mar 30; doi: 10.1210/jc.2011-3428. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ahmadzadeh M, Johnson LA, Heemskerk B, Wunderlich JR, Dudley ME, White DE, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood. 2009;114(8):1537–44. doi: 10.1182/blood-2008-12-195792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci USA. 2010;107(17):7875–80. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anders CK, Johnson R, Litton J, Phillips M, Bleyer A. Breast cancer before age 40 years. Semin Oncol. 2009;36(3):237–49. doi: 10.1053/j.seminoncol.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bleyer WA. Cancer in older adolescents and young adults: epidemiology, diagnosis, treatment, survival, and importance of clinical trials. Med Pediatr Oncol. 2002;38(1):1–10. doi: 10.1002/mpo.1257. [DOI] [PubMed] [Google Scholar]
- 74.Fredholm H, Eaker S, Frisell J, Holmberg L, Fredriksson I, Lindman H. Breast cancer in young women: poor survival despite intensive treatment. PLoS ONE. 2009;4(11):e7695. doi: 10.1371/journal.pone.0007695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98(19):10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375(9712):377–84. doi: 10.1016/S0140-6736(09)61964-4. [DOI] [PubMed] [Google Scholar]
- 77.Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109–19. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166(1):678–89. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 79.Kusmartsev S, Su Z, Heiser A, Dannull J, Eruslanov E, Kubler H, et al. Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2008;14(24):8270–8. doi: 10.1158/1078-0432.CCR-08-0165. [DOI] [PubMed] [Google Scholar]
- 80.Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, et al. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;66(18):9299–307. doi: 10.1158/0008-5472.CAN-06-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pak AS, Wright MA, Matthews JP, Collins SL, Petruzzelli GJ, Young MR. Mechanisms of immune suppression in patients with head and neck cancer: presence of CD34(+) cells which suppress immune functions within cancers that secrete granulocyte-macrophage colony-stimulating factor. Clin Cancer Res. 1995;1(1):95–103. [PubMed] [Google Scholar]
- 82.Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14+ HLA-DR-/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. 2010;70(11):4335–45. doi: 10.1158/0008-5472.CAN-09-3767. [DOI] [PubMed] [Google Scholar]
- 83.Young MR, Wright MA, Lozano Y, Prechel MM, Benefield J, Leonetti JP, et al. Increased recurrence and metastasis in patients whose primary head and neck squamous cell carcinomas secreted granulocyte-macrophage colony-stimulating factor and contained CD34+ natural suppressor cells. Int J Cancer. 1997;74(1):69–74. doi: 10.1002/(sici)1097-0215(19970220)74:1<69::aid-ijc12>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 84.Eruslanov E, Neuberger M, Daurkin I, Perrin GQ, Algood C, Dahm P, et al. Circulating and tumor-infiltrating myeloid cell subsets in patients with bladder cancer. Int J Cancer. 2012;130(5):1109–19. doi: 10.1002/ijc.26123. [DOI] [PubMed] [Google Scholar]
- 85.Liu CY, Wang YM, Wang CL, Feng PH, Ko HW, Liu YH, et al. Population alterations of L-arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14/CD15+/CD33+ myeloid-derived suppressor cells and CD8+ T lymphocytes in patients with advanced-stage non-small cell lung cancer. J Cancer Res Clin Oncol. 2010;136(1):35–45. doi: 10.1007/s00432-009-0634-0. [DOI] [PubMed] [Google Scholar]
- 86.Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, et al. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69(4):1553–60. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001;61(12):4756–60. [PubMed] [Google Scholar]
- 88.Srivastava MK, Bosch JJ, Thompson JA, Ksander BR, Edelman MJ, Ostrand-Rosenberg S. Lung cancer patients’ CD4(+) T cells are activated in vitro by MHC II cell-based vaccines despite the presence of myeloid-derived suppressor cells. Cancer Immunol Immunother. 2008;57(10):1493–504. doi: 10.1007/s00262-008-0490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65(8):3044–8. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 90.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based anti-tumor vaccine. J Clin Oncol. 2007;25(18):2546–53. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 91.Gustafson MP, Lin Y, New KC, Bulur PA, O’Neill BP, Gastineau DA, et al. Systemic immune suppression in glioblastoma: the interplay between CD14+ HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro Oncol. 2010;12(7):631–44. doi: 10.1093/neuonc/noq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+) CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135(1):234–43. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 93.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203(12):2691–702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vuk-Pavlovic S, Bulur PA, Lin Y, Qin R, Szumlanski CL, Zhao X, et al. Immunosuppressive CD14+ HLA-DRlow/− monocytes in prostate cancer. Prostate. 2010;70(4):443–55. doi: 10.1002/pros.21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Montero AJ, Diaz-Montero CM, Kyriakopoulos CE, Bronte V, Mandruzzato S. Myeloid-derived suppressor cells in cancer patients: a clinical perspective. J Immunother. 2012;35(2):107–15. doi: 10.1097/CJI.0b013e318242169f. [DOI] [PubMed] [Google Scholar]
- 96.Diaz-Montero C, Salem M, Nishimura M, Garrett-Mayer E, Cole D, Montero A. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin–cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58(1):49–59. doi: 10.1007/s00262-008-0523-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dix AR, Brooks WH, Roszman TL, Morford LA. Immune defects observed in patients with primary malignant brain tumors. J Neuroimmunol. 1999;100(1–2):216–32. doi: 10.1016/s0165-5728(99)00203-9. [DOI] [PubMed] [Google Scholar]
- 98.Gomez GG, Kruse CA. Mechanisms of malignant glioma immune resistance and sources of immunosuppression. Gene Ther Mol Biol. 2006;10A:133–46. [PMC free article] [PubMed] [Google Scholar]
- 99.Waziri A. Glioblastoma-derived mechanisms of systemic immunosuppression. Neurosurg Clin N Am. 2010;21(1):31–42. doi: 10.1016/j.nec.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 100.Dunn GP, Dunn IF, Curry WT. Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human glioma. Cancer Immun. 2007;7:12. [PMC free article] [PubMed] [Google Scholar]
- 101.Sippel TR, White J, Nag K, Tsvankin V, Klaassen M, Kleinschmidt-DeMasters BK, et al. Neutrophil degranulation and immunosuppression in patients with GBM: restoration of cellular immune function by targeting Arginase I. Clin Cancer Res. 2011;17(22):6992–7002. doi: 10.1158/1078-0432.CCR-11-1107. [DOI] [PubMed] [Google Scholar]
- 102.Munder M, Mollinedo F, Calafat J, Canchado J, Gil-Lamaignere C, Fuentes JM, et al. Arginase I is constitutively expressed in human granulocytes and participates in fungicidal activity. Blood. 2005;105(6):2549–56. doi: 10.1182/blood-2004-07-2521. [DOI] [PubMed] [Google Scholar]
- 103.Munder M, Schneider H, Luckner C, Giese T, Langhans CD, Fuentes JM, et al. Suppression of T-cell functions by human granulocyte arginase. Blood. 2006;108(5):1627–34. doi: 10.1182/blood-2006-11-010389. [DOI] [PubMed] [Google Scholar]
- 104.Rodriguez PC, Zea AH, DeSalvo J, Culotta KS, Zabaleta J, Quiceno DG, et al. L-arginine consumption by macrophages modulates the expression of CD3 zeta chain in T lymphocytes. J Immunol. 2003;171(3):1232–9. doi: 10.4049/jimmunol.171.3.1232. [DOI] [PubMed] [Google Scholar]
- 105.Gregory AD, Houghton AM. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res. 2011;71(7):2411–6. doi: 10.1158/0008-5472.CAN-10-2583. [DOI] [PubMed] [Google Scholar]
- 106.Houghton AM. The paradox of tumor-associated neutrophils: fueling tumor growth with cytotoxic substances. Cell Cycle. 2010;9(9):1732–7. doi: 10.4161/cc.9.9.11297. [DOI] [PubMed] [Google Scholar]
- 107.Piccard H, Muschel RJ, Opdenakker G. On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Crit Rev Oncol Hematol. 2012;82:296–309. doi: 10.1016/j.critrevonc.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 108.Fossati G, Ricevuti G, Edwards SW, Walker C, Dalton A, Rossi ML. Neutrophil infiltration into human gliomas. Acta Neuropathol. 1999;98(4):349–54. doi: 10.1007/s004010051093. [DOI] [PubMed] [Google Scholar]
- 109.Buck BH, Liebeskind DS, Saver JL, Bang OY, Yun SW, Starkman S, et al. Early neutrophilia is associated with volume of ischemic tissue in acute stroke. Stroke. 2008;39(2):355–60. doi: 10.1161/STROKEAHA.107.490128. [DOI] [PubMed] [Google Scholar]
- 110.Whalen MJ, Carlos TM, Wisniewski SR, Clark RS, Mellick JA, Marion DW, et al. Effect of neutropenia and granulocyte colony stimulating factor-induced neutrophilia on blood-brain barrier permeability and brain edema after traumatic brain injury in rats. Crit Care Med. 2000;28(11):3710–7. doi: 10.1097/00003246-200011000-00029. [DOI] [PubMed] [Google Scholar]
- 111.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 112.Preusser M, de Ribaupierre S, Wohrer A, Erridge SC, Hegi M, Weller M, et al. Current concepts and management of glioblastoma. Ann Neurol. 2011;70(1):9–21. doi: 10.1002/ana.22425. [DOI] [PubMed] [Google Scholar]
- 113.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 114.Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, et al. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 2012;72(5):1070–80. doi: 10.1158/0008-5472.CAN-11-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lohr J, Ratliff T, Huppertz A, Ge Y, Dictus C, Ahmadi R, et al. Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-beta. Clin Cancer Res. 2011;17(13):4296–308. doi: 10.1158/1078-0432.CCR-10-2557. [DOI] [PubMed] [Google Scholar]
- 116.Dai F, Liu L, Che G, Yu N, Pu Q, Zhang S, et al. The number and microlocalization of tumor-associated immune cells are associated with patient’s survival time in non-small cell lung cancer. BMC Cancer. 2010;10:220. doi: 10.1186/1471-2407-10-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102(51):18538–43. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29(15):1949–55. doi: 10.1200/JCO.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 119.Weber JS, Vogelzang NJ, Ernstoff MS, Goodman OB, Cranmer LD, Marshall JL, et al. A phase 1 study of a vaccine targeting preferentially expressed antigen in melanoma and prostate-specific membrane antigen in patients with advanced solid tumors. J Immunother. 2011;34(7):556–67. doi: 10.1097/CJI.0b013e3182280db1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Butts C, Murray RN, Smith CJ, Ellis PM, Jasas K, Maksymiuk A, et al. A multicenter open-label study to assess the safety of a new formulation of BLP25 liposome vaccine in patients with unresectable stage III non-small-cell lung cancer. Clin Lung Cancer. 2010;11(6):391–5. doi: 10.3816/CLC.2010.n.101. [DOI] [PubMed] [Google Scholar]
- 121.Zhong R, Teng J, Han B, Zhong H. Dendritic cells combining with cytokine-induced killer cells synergize chemotherapy in patients with late-stage non-small cell lung cancer. Cancer Immunol Immunother. 2011;60(10):1497–502. doi: 10.1007/s00262-011-1060-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chang CN, Huang YC, Yang DM, Kikuta K, Wei KJ, Kubota T, et al. A phase I/II clinical trial investigating the adverse and therapeutic effects of a postoperative autologous dendritic cell tumor vaccine in patients with malignant glioma. J Clin Neurosci. 2011;18(8):1048–54. doi: 10.1016/j.jocn.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 123.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17(13):4550–7. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Garcia JA, Mekhail T, Elson P, Wood L, Bukowski RM, Dreicer R, et al. Phase I/II trial of subcutaneous interleukin-2, granulocyte-macrophage colony-stimulating factor and interferon-alpha in patients with metastatic renal cell carcinoma. BJU Int. 2012;109(1):63–9. doi: 10.1111/j.1464-410X.2010.10011.x. [DOI] [PubMed] [Google Scholar]