Abstract
Background
Club (formerly Clara) cell secretory protein (CC16) is produced mainly by bronchiolar club cells and has been shown to have protective effects against airway inflammation and oxidative stress from cigarette smoking and related carcinogens. The goal of this study was to determine whether serum CC16 levels predict all-cause and cancer-specific mortality in adults.
Methods
We used data from the population-based TESAOD study, a prospective cohort study of respiratory health initiated in Tucson, AZ in 1972. At baseline, participants completed standardized respiratory questionnaires and lung function tests. Serum CC16 was measured in cryopreserved serum samples. A review of vital status of participants as of January 1st, 2011 was completed through contact with next of kin, collection of death certificates, and linkage with the National Death Index.
Findings
A total of 1086 participants who were 21 to 70 years old at enrollment were included. Of these, 653 (60%) died by 2011 and cause of death was ascertained for 649 (99%). In Cox proportional hazards models adjusted for sex, age, education, body mass index categories, smoking and pack-years, and baseline levels of lung function, serum CC16 levels at the baseline survey were inversely associated with mortality risk over the study follow-up. Mortality risk increased by 16% for each standard deviation (SD) decrease in CC16 (Hazard Ratio (HR), 95% CI: 1.16, 1.06 – 1.26; p = 0.0007). When data on cause-specific mortality were analyzed, each SD decrease in serum CC16 was associated with >40% increased risk of dying of cancer (adjusted HR=1.41, 1.19 – 1.67; p < 0.0001). Among smokers, the corresponding adjusted HRs for mortality by lung cancer were 1.52 (1.14 – 2.03; p = 0.004).
Interpretation
Serum CC16 levels predict mortality risk in the general adult population. The excess risk associated with lower CC16 is largely explained by cancer, particularly lung cancer.
Introduction
Club (formerly Clara) cell secretory protein (CC16, also called CC10 or CCSP) is a 16kDa protein that belongs to the Secretoglobin superfamily and, although it can be also found in the urogenital tract, is mainly produced by bronchiolar club cells, which account for the majority of its levels in blood1–3. The biological functions of CC16 are not completely understood, but growing evidence indicates that this molecule has anti-inflammatory, anti-toxicant, and anti-tumoral properties1–3.
CC16 levels are substantially reduced in serum and bronchoalveolar lavage of smokers4, 5, possibly because of direct toxicity of cigarette smoking on club cells in the airways. In adults, serum CC16 levels are inversely correlated with lung function deficits5 and the presence of chronic obstructive pulmonary disease (COPD)6, suggesting that this molecule may be involved in protection from the pro-inflammatory effects and oxidative stress of cigarette smoking and other noxious exposures.
In line with these observations, expression of CC16 by bronchial epithelial cells has been shown to have strong protective effects against lung carcinogenesis7. CC16 levels in bronchoalveolar lavage were associated with regression of bronchial dysplasia in smokers at high risk for lung cancer8 and non-small cell lung cancer A549 cells transfected with CC16 showed reduced invasiveness and adhesiveness to fibronectin9. These results are in line with animal studies showing that CC16-knock-out mice are susceptible to airway epithelial hyperplasia and adenomas after exposure to 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone10, a smoking-related carcinogen, and that lung CC16 expression is inversely related to tumor development and progression11. Interestingly, the anti-tumoral effects of CC16 have been proposed to extend also to cancers originated from other organs either through direct effects on tumorigenesis or by reducing chronic inflammation and/or metastatic migration and invasion2, 12, 13.
The above in vivo and in vitro evidence implicates CC16 as a potential biomarker of cancer risk, particularly in smokers. To test this hypothesis, we used the long-term, population-based cohort of the Tucson Epidemiological Study of Airway Obstructive Disease (TESAOD) to determine whether baseline serum CC16 levels predicted all-cause and cancer-specific mortality of participants during the study follow-up.
Methods
TESAOD is a population-based, prospective cohort study of respiratory health initiated in Tucson, Arizona in 1972. Details of the enrollment process have been previously reported14. Participants completed a baseline and up to 12 follow-up surveys over 24 years. At baseline, they completed a standardized respiratory questionnaire that included specific questions on smoking habits, number of cigarettes smoked per day, and age at which they started and quit smoking. Lung function tests were also completed according to methods previously described15 and percent predicted values for lung function indices, including forced expiratory volume in one second (FEV1), were computed using reference equations generated by Knudson and colleagues16. Weight and height were measured by study nurses at the time of lung function tests and body mass index (BMI) computed and categorized into underweight (BMI<18.5 Kg/m2), normal weight (18.5≤BMI<25), overweight (25≤BMI<30), and obese (BMI≥30) according to the World Health Organization recommendations.
Serum CC16 was measured in cryopreserved serum samples from the baseline survey using a commercially available enzyme-linked immunosorbent assay kit (BioVendor, Asheville, NC). In addition, we measured CC16 in serum samples from follow-up surveys in the subset of participants for whom they were available. Results of these secondary prospective analyses are presented in the online repository.
Because the distribution of CC16 values was skewed to the right, values were log-transformed before statistical analyses. Log-transformed CC16 values were analyzed as inverse standardized levels and as inverse tertiles to determine the effects of one SD or one tertile decrease, respectively, on mortality risk. Inverse standardized levels were calculated by subtracting the study population mean from raw log-transformed values and then dividing them by the study population standard deviation (SD). The resulting variable with mean 0 and SD 1 was then multiplied by −1.
A complete review of vital status of all TESAOD participants as of January 1st, 2011 was completed through direct contact with the family or designated next of kin of the participant and linkage with the National Death Index (NDI)17. Underlying causes of death were obtained directly from death certificates for events that occurred up to 1978 and from NDI records for events that occurred after 1978. The main cause of death was designed as 1) heart disease for records with underlying cause of death coded as ICD-9 390–398, 402, 404, or 410–429, or as ICD-10 I00–I09, I11, I13, or I20–I51; 2) cancer for records coded as ICD-9 140–208, or 238.6, or as ICD-10 C00–C97; 3) lung cancer for records coded as ICD-9 162 or ICD-10 C34. Cancer events were also classified as “tobacco-related” cancers based on review of existing evidence by the International Agency for Research on Cancer18. All cancers whose primary site was not on the list of tobacco-related cancers were classified as other types of cancer. A list of tobacco-related and other types of cancer from our study is provided in Table E1 in the online repository.
The relation of baseline serum CC16 to all-cause mortality risk was investigated in adjusted Cox proportional hazards models. Main analyses on cause-specific mortality were completed using Cox models with death events due to causes other than the specific cause of interest treated as censored observations and results were also confirmed using competing-risks survival regressions. In Cox models, proportional hazard assumptions were evaluated by both graphical and hypothesis testing approaches. Inverse standardized levels and tertiles of baseline CC16 were tested in separate Cox models and estimates of Hazard Ratios (HR) associated with medium or low CC16 as compared with high CC16 were obtained from linear trends across tertiles. Analyses on cause-specific mortality were powered only for the two most frequent causes of death: heart disease and cancer. Time to event was defined as the time from enrollment to the date of death for deceased participants and to January 1st, 2011 for subjects who were still alive as of that date.
The statistical packages IBM SPSS Statistics version 20 and Stata version 12 were used for all analyses.
Role of the funding source
The study sponsors had no role in study design, in the collection, analysis, and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
Results
Overall 2120 non-Hispanic whites between the ages of 21 and 70 years were enrolled at the baseline survey 1 in TESAOD. Of them, 1086 (51%) completed lung function tests at baseline and in at least one follow-up survey and had available serum samples of sufficient volume from survey 1 for CC16 measurements. Compared with the 1034 participants not included, the 1086 TESAOD subjects included in the present study were slightly older (44.8 vs 46.4, respectively; p = 0.02) and more likely to be females (53% vs 59%, p = 0.008). However, the two groups did not differ in terms of years of formal education, smoking status, and baseline lung function levels, and after taking into account the sex and age differences they had very comparable mortality risk (adjusted Hazard Ratio [HR] comparing included versus excluded participants: 1.00, p = 0.94).
Baseline serum CC16 levels had a geometric mean of 7.6 ng/ml and an inter-quartile range of 5.5–10.8 ng/ml. The baseline characteristics of participants and their association with serum CC16 levels are shown in Table I. Serum CC16 levels varied significantly with age, decreased with lower education, current smoking, and increasing pack-years, and correlated directly with levels of FEV1 % predicted (Spearman correlation coefficient: 0.135, p < 0.0001). The association of serum CC16 with sex and BMI categories was of borderline significance.
Table I.
Baseline characteristics and serum CC16 levels among the 1086 TESAOD participants.
| N | CC16 geom mean (95% CI) in ng/ml | ANOVA p | |
|---|---|---|---|
| Sex | 0.06 | ||
| M | 448 | 7.89 (7.51, 8.29) | |
| F | 638 | 7.41 (7.09, 7.74) | |
|
| |||
| Age | <0.0001 | ||
| 21 ≤ years < 30 | 253 | 8.28 (7.71, 8.89) | |
| 30 ≤ years < 45 | 230 | 6.66 (6.19, 7.17) | |
| 45 ≤ years < 60 | 323 | 7.05 (6.65, 7.48) | |
| 60 ≤ years ≤ 70 | 280 | 8.55 (8.05, 9.09) | |
|
| |||
| Years of formal education | 0.002 | ||
| ≤ 12 years | 589 | 7.24 (6.91, 7.59) | |
| > 12 years | 497 | 8.05 (7.70, 8.43) | |
|
| |||
| BMI (kg/m2) categories | 0.06 | ||
| Underweight (BMI<18.5) | 21 | 5.83 (4.20, 8.10) | |
| Normal weight (18.5 ≤ BMI < 25) | 611 | 7.73 (7.40, 8.07) | |
| Overweight (25 ≤ BMI < 30) | 343 | 7.56 (7.13, 8.02) | |
| Obese (BMI ≥ 30) | 75 | 6.94 (6.06, 7.94) | |
|
| |||
| Smoking status and intensity | <0.0001 | ||
| Never smokers | 430 | 8.83 (8.44, 9.23) | |
| Smokers < 1 pack-year | 23 | 9.60 (8.28, 11.3) | |
| Former smokers 1 ≤ pkyrs < 20 | 138 | 8.21 (7.54, 8.93) | |
| Former smokers 20 ≤ pkyrs < 50 | 71 | 7.81 (7.04, 8.66) | |
| Former smokers pkyrs ≥ 50 | 38 | 7.49 (6.18, 9.08) | |
| Current smokers 1 ≤ pkyrs < 20 | 182 | 6.49 (5.91, 7.12) | |
| Current smokers 20 ≤ pkyrs < 50 | 159 | 5.94 (5.43, 6.49) | |
| Current smokers pkyrs ≥ 50 | 43 | 5.62 (4.59, 6.89) | |
|
| |||
| % predicted FEV1 | 0.0003 | ||
| FEV1 ≥ 100% | 428 | 8.28 (7.88, 8.70) | |
| 80% ≤ FEV1 < 100% | 465 | 7.32 (6.93, 7.72) | |
| 50% ≤ FEV1 < 80% | 174 | 6.95 (6.42, 7.52) | |
| FEV1 < 50% | 19 | 6.44 (5.03, 8.24) | |
Of the 1086 participants, 653 (60%) died by January 1, 2011, and cause of death was ascertained for 649 (99%) of them. The two main underlying causes of death were heart disease and cancer, with 220 and 141 death events, respectively. In the total population, baseline serum CC16 levels were inversely associated with mortality risk over the study follow-up, with mortality risk increasing by 13% for each standard deviation (SD) decrease in CC16 (HR, 95% CI: 1.13, 1.05 – 1.21; p = 0.002) and by 19% for each lower CC16 tertile (1.19, 1.09 – 1.31; p = 0.0003). After adjusting for sex, age, education, BMI categories, smoking and pack-years, and baseline levels of FEV1 % predicted, lower serum CC16 levels at baseline were still significantly associated with all-cause mortality, both when used on a continuous scale and as tertiles (Table II).
Table II.
Adjusted hazard ratios (AdjHR) for all-cause and cause-specific mortality associated with baseline CC16 levels in TESAOD.
| Total Population (N = 1084) adjHR* (95% CI); p value |
Never smokers (N = 430) adjHR** (95% CI); p value |
Smokers (N = 654) adjHR* (95% CI); p value |
|
|---|---|---|---|
|
All Causes (N events = 651)
| |||
| AdjHR associated with 1-SD decrease in log CC16 | 1.16 (1.06 – 1.26) | 1.07 (0.90 – 1.26) | 1.17 (1.06 – 1.29) |
| P value | p = 0.0007 | p = 0.45 | p = 0.002 |
|
| |||
| Estimated∘ adjHR associated with High CC16 (ref) | 1 | 1 | 1 |
| Medium CC16 | 1.16 (1.05 – 1.28) | 1.03 (0.87 – 1.22) | 1.22 (1.08 – 1.39) |
| Low CC16 | 1.34 (1.10 – 1.63) | 1.06 (0.76 – 1.49) | 1.49 (1.16 – 1.93) |
| P value for trend | p = 0.004 | p = 0.73 | p = 0.002 |
|
| |||
|
Heart Disease (N events = 220)
| |||
| AdjHR associated with 1-SD decrease in log CC16 | 1.12 (0.96– 1.30) | 1.11 (0.84 – 1.46) | 1.10 (0.92 – 1.32) |
| P value | p = 0.14 | p = 0.47 | p = 0.27 |
|
| |||
| Estimated∘ adjHR associated with High CC16 (ref) | 1 | 1 | 1 |
| Medium CC16 | 1.14 (0.96 – 1.35) | 1.08 (0.81 – 1.42) | 1.17 (0.93 – 1.46) |
| Low CC16 | 1.29 (0.92 – 1.82) | 1.16 (0.66 – 2.01) | 1.36 (0.86 – 2.14) |
| P value for trend | p = 0.14 | p = 0.61 | p = 0.19 |
|
| |||
|
Cancer (N events = 141)
| |||
| AdjHR associated with 1-SD decrease in log CC16 | 1.41 (1.19 – 1.67) | 1.31 (0.92 – 1.85) | 1.43 (1.18 – 1.73) |
| P value | p < 0.0001 | p = 0.13 | p = 0.0003 |
|
| |||
| Estimated∘ adjHR associated with High CC16 (ref) | 1 | 1 | 1 |
| Medium CC16 | 1.46 (1.17 – 1.81) | 1.33 (0.91 – 1.94) | 1.50 (1.15 – 1.96) |
| Low CC16 | 2.12 (1.38 – 3.28) | 1.76 (0.82 – 3.76) | 2.25 (1.32 – 3.86) |
| P value for trend | p = 0.0007 | p = 0.15 | p = 0.003 |
|
| |||
|
Tobacco-related Cancer (N events = 88)
| |||
| AdjHR associated with 1-SD decrease in log CC-16 | 1.50 (1.22–1.84) | 1.48 (0.89–2.43) | 1.49 (1.19 – 1.86) |
| P value | p = 0.0001 | p = 0.127 | p = 0.0006 |
|
| |||
| Estimated∘ adjHR associated with High CC16 (ref) | 1 | ||
| Medium CC16 | 1.58 (1.19–2.09) | 1.37 (0.77–2.44) | 1.62 (1.17 – 2.24) |
| Low CC16 | 2.49 (1.42–4.36) | 1.89 (0.60–5.97) | 2.64 (1.37 – 5.05) |
| P value for trend | p = 0.001 | p = 0.280 | p = 0.004 |
|
| |||
|
Lung Cancer (N events = 42)
| |||
| AdjHR associated with 1-SD decrease in log CC-16 | N/A*** | N/A*** | 1.52 (1.14 – 2.03) |
| P value | p = 0.004 | ||
|
| |||
| Estimated∘ adjHR associated with High CC16 (ref) | N/A*** | N/A*** | 1 |
| Medium CC16 | 1.63 (1.07 – 2.48) | ||
| Low CC16 | 2.66 (1.15 – 6.16) | ||
| P value for trend | P = 0.03 | ||
|
| |||
adjusted for sex, age, years of formal education (≤ 12 versus > 12), BMI categories (normal, under-weight, over-weight, obese, missing), smoking and pack-years categories, baseline levels of FEV1 % predicted (used as a continuous variable)
adjusted for sex, age, years of formal education (≤ 12 versus > 12), BMI categories (normal, under-weight, over-weight, obese, missing), baseline levels of FEV1 % predicted (used as a continuous variable)
estimated with linear contrasts from Cox models including CC16 tertiles as an ordinal variable
N/A: not applicable because no death events by lung cancer were recorded among never smokers
Total N is 1084 because two subjects had smoking history missing
When cause-specific mortality was analyzed, baseline CC16 was not associated significantly with heart disease but the association with cancer was highly significant (Table II). After full adjustment, each SD decrease in serum CC16 was associated with >40% increased risk of dying of cancer (1.41, 1.19 – 1.67; p < 0.0001) and subjects in the lowest CC16 tertile were more than twice as likely to die of cancer as were subjects in the highest tertile (p = 0.0007). The corresponding adjusted HRs tended to be stronger for the 88 mortality events by tobacco-related cancers (as shown in Table II) than for mortality by other types of cancer (HR for each SD decrease in CC16: 1.31, 0.96 – 1.80, p = 0.09). In the subset of 555 participants with available serum samples from the follow-up, further inclusion of slopes of CC16 change over the follow-up in the Cox models did not change the estimates of the effects of baseline CC16 on either all-cause or cancer-specific mortality risk (Tables E2 and E3). In these models, evidence for a U-shaped association with CC16 slope was found both for all-cause and cancer-specific mortality, although results from these secondary analyses should be interpreted with caution (see online repository for a full discussion).
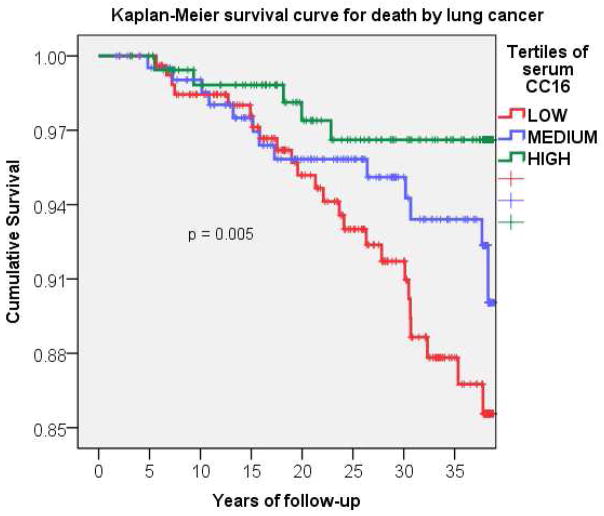
When analyses on baseline CC16 were stratified by smoking, the above associations of CC16 with all-cause and cancer-specific mortality were confirmed in ever smokers, while they were weaker and non significant among never smokers. However, only the effects of the lowest CC16 tertile on all-cause mortality were significantly different between never and ever smokers (p for interaction between low CC16 tertile and smoking = 0.026). Among smokers, associations with serum CC16 were particularly strong for mortality by lung cancer (Figure 1), with the lowest CC16 tertile being associated with a nearly 170% increased risk of dying of lung cancer as compared with the highest tertile (Table II).
Figure 1.
Kaplan-Meier survival curve for death by lung cancer among TESAOD smokers.
P value for trend across tertiles
| enrollment | 5 yrs follow-up | 10 yrs follow-up | 15 yrs follow-up | 20 yrs follow-up | 25 yrs follow-up | 30 yrs follow-up | 35 yrs follow-up | |
|---|---|---|---|---|---|---|---|---|
| High CC16 | 363 | 359 | 328 | 304 | 271 | 241 | 205 | 183 |
| Medium CC16 | 361 | 352 | 340 | 309 | 278 | 247 | 204 | 174 |
| Low CC16 | 362 | 356 | 334 | 305 | 262 | 222 | 176 | 122 |
The above significant associations were confirmed after additional adjustment for number of cigarettes smoked per day and when pack-years were modeled on a continuous scale. The effects of CC16 on mortality risk were also confirmed after restricting analyses to the 388 subjects who were current smokers at baseline (HRs associated with 1-SD decrease in CC16: 1.15, 1.02 – 1.30, p = 0.03 for all-cause mortality; 1.34, 1.07 – 1.67, p = 0.01 for cancer mortality; 1.51, 1.11 – 2.05, p = 0.009 for lung cancer mortality, respectively). Among the 381 participants who were current smokers at baseline and had follow-up information on smoking habits, no differences in baseline serum CC16 levels were found between subjects who consistently reported current smoking at all completed follow-up surveys (N=182) and those who did not (N=199), suggesting that the relation of CC16 at baseline to subsequent mortality was not due to differential persistence of cigarette smoking during the study follow-up.
Discussion
Using the long-term TESAOD cohort, to our knowledge we report here for the first time that serum levels of CC16 predict all-cause and cancer-specific mortality risk in the general population. These relations were confirmed after full adjustment for covariates and were particularly strong among smokers and for mortality by tobacco-related cancers including lung cancer, which has now become the fifth leading cause of death worldwide19. In contrast, the association of baseline serum CC16 was not significant for mortality by heart disease, although the HRs for this cause-specific outcome were comparable to those for all-cause mortality and therefore the lack of statistical significance may be related to the smaller number of death events and, in turn, statistical power.
Serum CC16 is a lung-specific biomarker whose levels increase in response to acute exposure to irritants and augmented bronchial epithelial permeability. However, chronic exposure to noxious inhalants, including cigarette smoking, is associated with a decrease in serum levels, possibly in response to airway damage of club cells. Consistent with this scenario, both CC16 levels in serum and broncho-alveolar lavage (BAL) and the percentage of CC16-positive bronchiolar epithelial cells were found to be lower in smokers than non-smokers, even in the presence of conserved lung function levels4. The positive correlation between circulating and BAL levels of CC164, 20 also indicates that deficits in serum CC16 are likely to reflect similar CC16 deficits in the airways. In this framework, decreased serum CC16 has been proposed as a potential systemic biomarker of obstructive lung disease and found to be related to coexisting lung function impairment in the general population5 and accelerated decline of lung function among smokers with COPD21. In line with these observations, we found that subjects who smoked and those with lung function deficits had lower levels of serum CC16 at baseline in TESAOD. Of note, full adjustment for smoking status at baseline, pack-years, number of cigarettes smoked per day, and persistence of smoking during follow-up did not reduce the relation of low CC16 to increased mortality risk, implicating CC16 not only as a biomarker for exposure but also for susceptibility to increased mortality from cigarette smoking. The above associations held true also after adjustment for lung function deficits, which have been shown to be an additional independent risk factor for lung cancer22.
Our results are in apparent contradiction with those from the ECLIPSE study in which baseline serum CC16 among 1843 COPD patients was not related with risk of all-cause mortality for the 168 patients who died over the 3-year study follow-up23. The different findings in ECLIPSE may be related to insufficient cancer mortality over the 3-year follow-up because all participants were required not to have had any cancer in the five years prior to recruitment to be eligible for the study. In addition, at the beginning of the study patients had moderate to severe COPD and therefore they may have had already substantial deficits in serum CC16 levels, which could bias the results towards the null for mortality risk.
Our data are in line with experimental evidence that supports a protective role of CC16 against oxidative stress and chronic inflammation in the lungs and, in turn, against carcinogenesis1–3, 7–13. We found serum CC16 to be also associated with protection against other cancers whose primary site was not in the lung. These CC16 associations may be mediated by effects in the airways that result in reduced absorption of tobacco carcinogens in circulation, by a reduction in systemic inflammation, or by inhibition of neoplastic cell metastatic migration and invasion2, 12, 13. However, prediction of risk does not imply causality, and whether CC16 is etiologically involved in resistance to cancer or only a marker of that resistance remains to be determined. Also, we cannot completely rule out that residual confounding (e.g., by under-reported and unmeasured smoking behavior or occupational exposures) may at least partially explain the effects of CC16 on mortality risk. However, reports from nationally representative samples of the adult population indicate a lower than 1% expected under-report of smoking status in face-to-face interviews in the US24, 25 and the fact that we observed similar trends in non-smokers (albeit not significant) as in smokers (Table II) argues against the possibility that our findings can be completely explained by confounding by smoking. In addition, in our study CC16 associations with mortality by all causes, cancer, and lung cancer did not change after adjustment for education, which is known to be at least partly related to occupational exposures associated with lung cancer risk26. In any case, our results support serum CC16 as an informative biomarker that may improve risk prediction over traditional demographic and behavioral risk factors.
Information on vital status was obtained from several sources and cause of death was assigned based on direct review of death certificates for events that occurred up to 1978 and based on NDI reports for events that occurred after 1978. Although this has been shown to be a reliable and sensitive method to assess mortality17, 27, some level of misclassification (alive subjects classified as deceased and vice versa) is likely to occur. However, this could have biased our results towards spurious associations only if a significant proportion of misclassification was differential (i.e., if the likelihood of participants to have their vital status misclassified was dependent on their baseline CC16 levels). We consider this scenario unlikely. Based on available data, we cannot determine whether the association of low CC16 with cause-specific mortality risk is related to increased disease incidence or decreased survival once the disease has occurred. In this context, the substantial improvements that have occurred in cancer treatment over the 40 years of follow-up of the TESAOD study need to be taken into account as they may have differential effects on the CC16 associations with cancer mortality over time. However, it is noteworthy that the risk for cancer mortality associated with low CC16 did not change relevantly over the follow-up and that the strongest CC16 effects were seen with lung cancer, whose 5-year relative survival rate in the US white population was lower than 13% in the 1970s and about 17% by 200828. These observations suggest that at least part of the CC16 effects were related to protection against incidence. Finally, no information was available on renal function in our study and we could not adjust serum CC16 for this known determinant of its serum levels29. The increase of serum CC16 with age that was found in our study is consistent with previous reports5, 30 and may indeed reflect the reduction of glomerular filtration rate associated with aging29. However, these age effects are very unlikely to explain our results because both serum CC16 and mortality risk increase with aging, whereas in this study we found an inverse association between CC16 and mortality.
In conclusion, serum CC16 levels predict mortality risk in the general adult population and the excess mortality risk associated with low CC16 is largely explained by cancer, particularly tobacco-related and lung cancer in smokers. Studies are warranted to establish the potential use of this biomarker in risk prediction and targeted prevention.
Supplementary Material
Systematic review.
Serum levels of CC16 have been long known to be reduced in smokers and patients with chronic lung disease. In addition, growing experimental evidence supports possible protective effects of this molecule in the lungs against airway inflammation, oxidative stress, and smoking-related carcinogens. However, whether serum CC16 predicts all-cause and cancer-specific mortality remains unknown. While a PubMed search returned multiple experimental and cross-sectional studies linking this molecule to smoking, lung function deficits, and cancer and prospective studies addressing this molecule for mortality risk in clinical cohorts, no previous prospective study has evaluated serum CC16 in relation to cancer mortality in the general population.
Interpretation.
By showing that serum CC16 levels predict mortality risk by all causes and by cancer in the general adult population, our study supports the potential value of this molecule in risk prediction and targeted prevention. The most valuable and novel component of our results refers to their longitudinal cohort-based nature because cross-sectional case-control studies cannot fully resolve the temporal sequence between reduced CC16 and cancer onset and may be affected by changes in biological and behavioral factors (including smoking habits) that are likely to occur once a cancer diagnosis is established. The potential clinical implications of CC16 in cancer and lung disease prevention should be exploited in future research.
Acknowledgments
Funding. Grant HL-095021 and CADET award HL-107188 by the National Heart, Lung, and Blood Institute.
This study was supported by CADET award HL107188 and R01 award HL095021 from the National Heart, Lung, and Blood Institute.
Footnotes
Contributors
SG and FDM designed the study. SG and MV analyzed the data. AS analyzed the TESAOD samples with supervision by MH. SG drafted the manuscript with input from all authors. All authors approved the final version of the manuscript.
Conflicts of interest
SG has received speaker’s honorarium from MedImmune in 2011
In the past five years FDM has participated as a paid member of the Merck Advisory Board and as a consultant for MedImmune. He has also received honoraria/travel expenses as a guest lecturer for events sponsored by Merck and Abbott.
No other conflicts exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Broeckaert F, Bernard A. Clara cell secretory protein (CC16): characteristics and perspectives as lung peripheral biomarker. Clin Exp Allergy. 2000;30(4):469–75. doi: 10.1046/j.1365-2222.2000.00760.x. [DOI] [PubMed] [Google Scholar]
- 2.Mukherjee AB, Zhang Z, Chilton BS. Uteroglobin: a steroid-inducible immunomodulatory protein that founded the Secretoglobin superfamily. Endocr Rev. 2007;28(7):707–25. doi: 10.1210/er.2007-0018. [DOI] [PubMed] [Google Scholar]
- 3.Lakind JS, Holgate ST, Ownby DR, Mansur AH, Helms PJ, Pyatt D, et al. A critical review of the use of Clara cell secretory protein (CC16) as a biomarker of acute or chronic pulmonary effects. Biomarkers. 2007;12(5):445–67. doi: 10.1080/13547500701359327. [DOI] [PubMed] [Google Scholar]
- 4.Shijubo N, Itoh Y, Yamaguchi T, Shibuya Y, Morita Y, Hirasawa M, et al. Serum and BAL Clara cell 10 kDa protein (CC10) levels and CC10-positive bronchiolar cells are decreased in smokers. Eur Respir J. 1997;10(5):1108–14. doi: 10.1183/09031936.97.10051108. [DOI] [PubMed] [Google Scholar]
- 5.Rava M, Tares L, Lavi I, Barreiro E, Zock JP, Ferrer A, et al. Serum levels of Clara cell secretory protein, asthma, and lung function in the adult general population. J Allergy Clin Immunol. 2013;132(1):230–2. doi: 10.1016/j.jaci.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 6.Lomas DA, Silverman EK, Edwards LD, Miller BE, Coxson HO, Tal-Singer R. Evaluation of serum CC-16 as a biomarker for COPD in the ECLIPSE cohort. Thorax. 2008;63(12):1058–63. doi: 10.1136/thx.2008.102574. [DOI] [PubMed] [Google Scholar]
- 7.Linnoila RI, Szabo E, DeMayo F, Witschi H, Sabourin C, Malkinson A. The role of CC10 in pulmonary carcinogenesis: from a marker to tumor suppression. Ann N Y Acad Sci. 2000;923:249–67. doi: 10.1111/j.1749-6632.2000.tb05534.x. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Lam S, Pilon A, McWilliams A, Macaulay C, Szabo E. Higher levels of the anti-inflammatory protein CC10 are associated with improvement in bronchial dysplasia and sputum cytometric assessment in individuals at high risk for lung cancer. Clin Cancer Res. 2008;14(5):1590–7. doi: 10.1158/1078-0432.CCR-07-4066. [DOI] [PubMed] [Google Scholar]
- 9.Szabo E, Goheer A, Witschi H, Linnoila RI. Overexpression of CC10 modifies neoplastic potential in lung cancer cells. Cell Growth Differ. 1998;9(6):475–85. [PubMed] [Google Scholar]
- 10.Yang Y, Zhang Z, Mukherjee AB, Linnoila RI. Increased susceptibility of mice lacking Clara cell 10-kDa protein to lung tumorigenesis by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, a potent carcinogen in cigarette smoke. J Biol Chem. 2004;279(28):29336–40. doi: 10.1074/jbc.C400162200. [DOI] [PubMed] [Google Scholar]
- 11.Hicks SM, Vassallo JD, Dieter MZ, Lewis CL, Whiteley LO, Fix AS, et al. Immunohistochemical analysis of Clara cell secretory protein expression in a transgenic model of mouse lung carcinogenesis. Toxicology. 2003;187(2–3):217–28. doi: 10.1016/s0300-483x(03)00060-x. [DOI] [PubMed] [Google Scholar]
- 12.Saha A, Lee YC, Zhang Z, Chandra G, Su SB, Mukherjee AB. Lack of an endogenous anti-inflammatory protein in mice enhances colonization of B16F10 melanoma cells in the lungs. J Biol Chem. 2010;285(14):10822–31. doi: 10.1074/jbc.M109.083550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z, Kundu GC, Panda D, Mandal AK, Mantile-Selvaggi G, Peri A, et al. Loss of transformed phenotype in cancer cells by overexpression of the uteroglobin gene. Proc Natl Acad Sci U S A. 1999;96(7):3963–8. doi: 10.1073/pnas.96.7.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lebowitz MD, Knudson RJ, Burrows B. Tucson epidemiologic study of obstructive lung diseases. I: Methodology and prevalence of disease. Am J Epidemiol. 1975;102(2):137–52. doi: 10.1093/oxfordjournals.aje.a112141. [DOI] [PubMed] [Google Scholar]
- 15.Knudson RJ, Slatin RC, Lebowitz MD, Burrows B. The maximal expiratory flow-volume curve. Normal standards, variability, and effects of age. Am Rev Respir Dis. 1976;113(5):587–600. doi: 10.1164/arrd.1976.113.5.587. [DOI] [PubMed] [Google Scholar]
- 16.Knudson RJ, Lebowitz MD, Holberg CJ, Burrows B. Changes in the normal maximal expiratory flow-volume curve with growth and aging. Am Rev Respir Dis. 1983;127(6):725–34. doi: 10.1164/arrd.1983.127.6.725. [DOI] [PubMed] [Google Scholar]
- 17.Davis KB, Fisher L, Gillespie MJ, Pettinger M. A test of the National Death Index using the Coronary Artery Surgery Study (CASS) Control Clin Trials. 1985;6(3):179–91. doi: 10.1016/0197-2456(85)90001-7. [DOI] [PubMed] [Google Scholar]
- 18.Secretan B, Straif K, Baan R, Grosse Y, El Ghissassi F, Bouvard V, et al. A review of human carcinogens--Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10(11):1033–4. doi: 10.1016/s1470-2045(09)70326-2. [DOI] [PubMed] [Google Scholar]
- 19.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Lam S, Pilon A, McWilliams A, Melby J, Szabo E. The association between the anti-inflammatory protein CC10 and smoking status among participants in a chemoprevention trial. Cancer Epidemiol Biomarkers Prev. 2007;16(3):577–83. doi: 10.1158/1055-9965.EPI-06-0923. [DOI] [PubMed] [Google Scholar]
- 21.Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, et al. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365(13):1184–92. doi: 10.1056/NEJMoa1105482. [DOI] [PubMed] [Google Scholar]
- 22.Brenner DR, Boffetta P, Duell EJ, Bickeboller H, Rosenberger A, McCormack V, et al. Previous lung diseases and lung cancer risk: a pooled analysis from the International Lung Cancer Consortium. Am J Epidemiol. 2012;176(7):573–85. doi: 10.1093/aje/kws151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Celli BR, Locantore N, Yates J, Tal-Singer R, Miller BE, Bakke P, et al. Inflammatory biomarkers improve clinical prediction of mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185(10):1065–72. doi: 10.1164/rccm.201110-1792OC. [DOI] [PubMed] [Google Scholar]
- 24.Yeager DS, Krosnick JA. The validity of self-reported nicotine product use in the 2001–2008 National Health and Nutrition Examination Survey. Med Care. 2010;48(12):1128–32. doi: 10.1097/MLR.0b013e3181ef9948. [DOI] [PubMed] [Google Scholar]
- 25.West R, Zatonski W, Przewozniak K, Jarvis MJ. Can we trust national smoking prevalence figures? Discrepancies between biochemically assessed and self-reported smoking rates in three countries. Cancer Epidemiol Biomarkers Prev. 2007;16(4):820–2. doi: 10.1158/1055-9965.EPI-06-0679. [DOI] [PubMed] [Google Scholar]
- 26.Menvielle G, Boshuizen H, Kunst AE, Vineis P, Dalton SO, Bergmann MM, et al. Occupational exposures contribute to educational inequalities in lung cancer incidence among men: Evidence from the EPIC prospective cohort study. Int J Cancer. 2010;126(8):1928–35. doi: 10.1002/ijc.24924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doody MM, Hayes HM, Bilgrad R. Comparability of national death index plus and standard procedures for determining causes of death in epidemiologic studies. Ann Epidemiol. 2001;11(1):46–50. doi: 10.1016/s1047-2797(00)00177-0. [DOI] [PubMed] [Google Scholar]
- 28.Howlader N, Noone A, Krapcho M, Neyman N, Aminou R, Waldron W, et al., editors. SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) National Cancer Institute; Bethesda, MD: 2012. [Google Scholar]
- 29.Hermans C, Bernard A. Lung epithelium-specific proteins: characteristics and potential applications as markers. Am J Respir Crit Care Med. 1999;159(2):646–78. doi: 10.1164/ajrccm.159.2.9806064. [DOI] [PubMed] [Google Scholar]
- 30.Bernard AM, Roels HA, Buchet JP, Lauwerys RR. Serum Clara cell protein: an indicator of bronchial cell dysfunction caused by tobacco smoking. Environ Res. 1994;66(1):96–104. doi: 10.1006/enrs.1994.1047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.