Abstract
Objective
Cyclin D1 plays a vital role in cancer cell cycle progression and is overexpressed in many human cancers, including colorectal cancer (CRC). However, the prognostic value of cyclin D1 overexpression in colorectal cancer is conflicting and heterogeneous. We conducted a meta-analysis to more precisely evaluate its prognostic significance.
Methods
A comprehensive literature search for relevant studies published up to January 2014 was performed using PubMed, EMBASE, and ISI Web of Science. The pooled hazard ratio (HR) with 95% confidence intervals (CI) was used to estimate the effects.
Results
22 studies with 4150 CRC patients were selected to evaluate the association between cyclin D1 and overall survival (OS), disease-free survival (DFS) and clinicopathological parameters. In a random-effects model, the results showed that cyclin D1 overexpression in CRC was significantly associated with both poor OS (HR = 0.73, 95% CI: 0.63–0.85, P<0.001) and DFS (HR = 0.60, 95% CI: 0.44–0.82, P = 0.001). Additionally, cyclin D1 overexpression was significantly associated with more relative older patients (≥60 years) (OR 0.62, 95% CI 0.44–0.89, P = 0.009), T3,4 tumor invasion (OR 0.70, 95% CI 0.57–0.85, P<0.001), N positive (OR 0.75, 95% CI 0.60–0.95, P = 0.016) and distant metastasis (OR 0.60, 95% CI 0.36–0.99, P = 0.047) of CRC.
Conclusion
The meta-analysis results indicated that cyclin D1 is an unfavorable prognostic factor for CRC. Cyclin D1 overexpression might be associated with poor clinical outcome and some clinicopathological factors such as age, T category, N category and distant metastasis in CRC patients.
Introduction
Colorectal cancer (CRC) is the third most frequent malignancy worldwide and the fourth most frequent cause of death from cancer in the world [1]. The incidence of CRC in China is lower than that in western countries, but has increased in recent years, particularly in more developed areas [2]. Despite the development of combined therapeutic modalities and the prolonged overall survival (OS) and disease-free survival (DFS) of CRC patients, CRC remains the second leading cause of overall cancer deaths [3]. It is valuable to identify molecular predictive markers for the prognosis, which would be helpful in the selection of therapeutic strategies and further improve patients' survival for CRC.
Much attention has been focused on the involvement of cyclin D1 in tumor development and progression [4]. Cyclin D1 has been considered to be an oncogene which could regulate progression from the G1 phase of the cell cycle to the S phase [5]. As known to us, the ability of cyclin D1 to drive the cell cycle forward can be blocked by cyclin D1-dependent kinase (CDK) inhibitors, such as p27 and p21. As key regulators of the G1 progression step within the cell cycle, cyclin D1 have been suspected to play a pivotal role in the process of carcinogenesis and cancer progression [6]. Cyclin D1 expression is known to be upregulated in a variety of tumor types and occurs in one-third or more of colorectal cancers [7]–[17]. Many studies have evaluated whether cyclin D1 overexpression may be a prognostic factor for survival in patients with CRC. However, the results of the studies are inconclusive and no consensus has been reached. Bahnassy et al. [10] and Maeda et al. [9] reported that cyclin D1 overexpression has been associated with poor prognosis, while Holland et al. [11] and Ogino et al. [18] draw a conclusion that the high level of cyclin D1 indicate good prognosis. A few studies have shown no prognostic value of cyclin D1 overexpression [8], [12], [19]. When it comes to the associations between cyclin D1 expression and clinicopathological parameters, the studies were also heterogeneous [10], [15]–[17], [20]–[25], [46]. It is necessary to establish whether cyclin D1 overexpression is a prognostic marker in CRC.
In this meta-analysis, we collected and combined all eligible published articles about the relation between cyclin D1 and survival in CRC. The aim of our study was to test the hypothesis that cyclin D1 overexpression would predict the clinical outcomes of patients with CRC. Additionally, the relation between cyclin D1 expression and clinicopathological parameters were examined.
Materials and Methods
Search Strategy
We searched PubMed, EMBASE and ISI Web of Science to identify studies assessing the cyclin D1 as prognostic factor in patients with CRC. The upper data limit of January 2014 was applied, with no lower date limit. The search strategy performed in PubMed combined the following terms: (colorectal OR colon OR rectum OR colorectum OR large bowel OR gut) AND (cancer* OR carcinoma* OR neoplasm* OR tumor* OR polyp*) AND (cyclin d1 protein OR CCND 1 OR cyclin D1 OR cyclin-D1) AND (prognosi*) (Table S1). The search was limited to human studies. The similar search strategy was used in other databases. The language of all publications was limited to English only. The title and abstract of each study identified in the search was scanned to exclude any clearly irrelevant ones. The reference lists of each identified study were also reviewed to identify the additional studies containing information on the topic of interest.
Study Selection
Criteria for eligibility of a study included in this meta-analysis were: (1) to assess cyclin D1 expression in the primary colorectal cancer tissues using immonohistochemistry (IHC) (not in metastatic tissue or mucosa adjacent to the tumor); (2) the endpoint investigated was OS, DFS; (3) the study reported a hazard ratio (HR) estimates with their 95% confidence intervals (CI) or the data sufficient allowing for estimation of the HR and 95% CI from survival analysis; (4) to be published as a full paper in the English language; (5) when the same author reported results from the same patient population, the most recent report or the most complete one was included. Articles that could not be identified based on title and abstract alone were retrieved for full-text review. To determine the issue of multiple publications from the same data sets, we checked all author names, institutions involved, and the time period of patient recruitment of the articles.
Data Extraction
Two authors (Li Y. and Wei J.) independently reviewed each eligible study and extracted data with a standardized protocol (Text S1) and predefined data collection form (Excel sheet). Disagreements were resolved with third author (You T. G.) through discussion. Information was carefully retrieved from the full publications, including the following items: the first author, year of publication, study location, number of participants, staining patterns of cyclin D1, the choice of cutoff scores for the definition of positive staining or staining intensity, antibody used, antibody working concentration, duration of follow-up, T category, N category, distant metastasis, histology, and prognostic outcomes of interest (DFS and/or OS). As the cutoff value for cyclin D1-high group varied with different studies, we defined cyclin D1-high expression values according to the original articles. Staging of CRC was based on the UICC classification revised in 2009 [26]. Tumor differentiation was graded by a pathologist according to the World Health Organization (WHO) classification system. The primary authors were contacted to provide additional information when necessary.
Assessment of Study Quality
Two authors (Li Y. and Xu C. H.) independently assessed the quality of all studies on the basis of a 9-scores system of the Newcastle-Ottawa Scale (NOS) [27]. Discrepancies in the score were resolved through discussion between the authors. Each study included in the meta-analysis was judged on three broad perspectives: (1) the selection of the groups of study (four items, one score each), (2) the comparability (one item, up to two scores) and (3) the ascertainment of either the exposure or outcome of interest (three items, one score each). A score presents a high quality choice of individual study. In this 9-scores system, studies scored equal or greater than 7 were considered as high quality.
Statistical Methods
Included studies were divided into two groups for analysis: OS and DFS. For the quantitative aggregation of the survival results, we measured the impact of cyclin D1 overexpression on survival by HR between the two survival distributions. HR and 95% confidence intervals (CI) were used to combine as the effective value. If these statistical variables were not given explicitly in an article, they were estimated from available data using methods reported by Tierney and colleagues [28]. Kaplan-Meier curves were read using Engauge Digitizer version 4.1 (http://digitizer.sourceforge.net/), and then the survival data read from Kaplan-Meier curves were entered in the spreadsheet based on Tierney [28]. For the pooled analysis of the relation between cyclin D1 overexpression and clinicopathological parameters (age, tumor size, T category, N category, distant metastasis, histological grade), OR and their 95% CI were combined to give the effective value. The individual HR estimates were pooled into a summary HR by using DerSimonian and Laird random-effects methods reported by Yusuf et al. [29]. The random-effects model, which not only weights each study by its inverse variance but also includes the within- and between-studies variances and thus is usually more conservative, was chosen. Statistical heterogeneity assessment between studies was performed by using a Chi-square heterogeneity statistic based Q test. Given the low test power, the significance level was defined as P<0.10. The effect of heterogeneity was also quantified using the inconsistency index (I 2). The I 2 statistic is defined as the percentage of total variance across studies attributable to heterogeneity rather than the chance [I 2 = (Q – df)/Q×100%]. As a guide, I 2 values of <25% may be considered “low”, values of 25–50% may be considered “moderate” and values of >50% may be considered “high” [30]. For OS, subgroup analyses were performed by treatment (single surgery and surgery as well as chemoradiation), geographic settings (Asian and non-Asian CRC patients), samples (whole tissue sections and tissue microarray), staining patterns (nuclear, nuclear & cytoplasmic and single cytoplasmic staining), study quality (≥7 and <7), study design (cohort studies and case-control studies). For DFS, subgroup analyses were performed by treatment, geographic settings, staining patterns, study design and study quality.
We also carried out sensitivity analysis to evaluate the influence of a single study on the overall effect estimate by excluding one study at a time. The potential for publication bias was assessed by using the Begg rank correlation method and the Egger weighted regression method (P<0.05 was considered representatively of statistically significant publication bias). The meta-analysis including metan, metainf, and metabias command was performed by Stata 11.0 software (Stata Corp, College Station, TX, USA). A P value less than 0.05 was considered to be statistically significant except where otherwise specified.
Results
Search Results and Study Characteristics
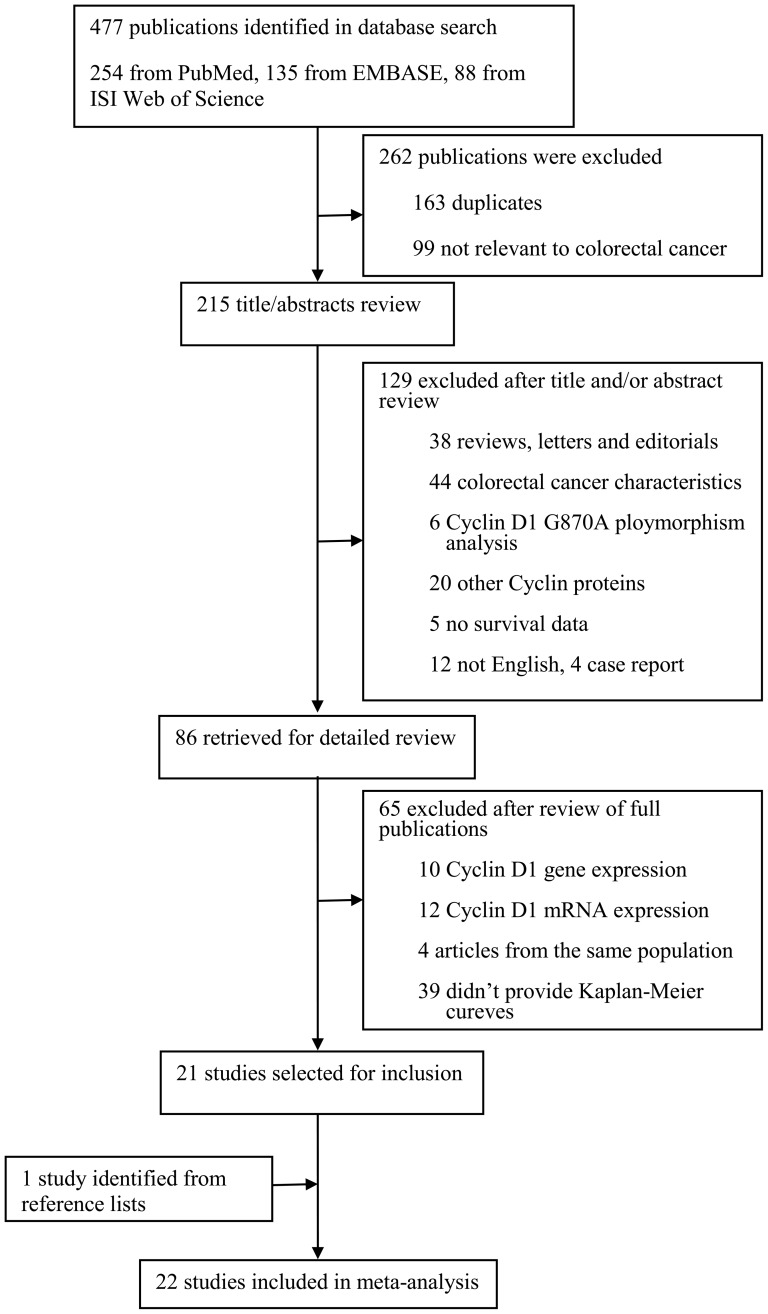
A total of 477 potentially relevant publications were retrieved after the initial database searches, and 22 observational studies met the predefined inclusion criteria comprising 4150 patients for final analysis [8]–[10], [13]–[25], [31]–[35], [47]. On the basis of full text review, we identified 21 studies [9]–[10], [13]–[25], [31]–[35], [47]. One study was identified from reference lists [8]. A flow diagram of the study selection process is presented in Figure 1. The major characteristics of the 22 eligible studies were reported in Table 1. The sample size of the included studies ranged from 39 to 602 patients (median sample size, 188.6 patients) and follow-up period vary from 30 to 107 months. The studies were conducted in 14 countries (China, Egypt, Finland, Germany, Greece, India, Japan, Korea, Netherlands, Norway, Poland, Sweden, United Kingdom and United States) and published between 1996 and 2013. Among the 22 studies, 8 studies (1395 patients, 33.6%) were performed in Asian populations [9], [13], [20], [22], [24], [31], [34], [47], and the remaining studies (2755 patients, 66.4%) followed non-Asian patients [8], [10], [14]–[19], [21], [23], [25], [32]–[33], [34].
Figure 1. Flow diagram of screened, excluded, and analyzed publications.
Table 1. Characteristics of studies included in the meta-analysis.
| First author of study [ref.] | Year | Country | Design | Number of patients | Duration of follow-up | Method to determine "high" Cyclin D1 cut-off level (high/low) | Scores of study quality | Adjusted confounders | Outcome | HR estimation |
| Bahnassy[10] | 2004 | Egypt | Case control | 60 | * | Staining index ≥6.1 (41/19) | 7 | Age, gender, the depth of invasion, stage, lymph node metastasis, cyclin A expression | OS, DFS | Data extrapolated |
| Wang[31] | 1996 | Japan | Case control | 39 | * | Tumor/mucosa ratio >1.3 (4/35) | 6 | _ | NA | _ |
| Balcerczak[25] | 2005 | Poland | Case control | 111 | * | >25% (69/42) | 7 | Age, gender, histological type, stage | OS, DFS | Reported in text |
| Tsai[22] | 2013 | Taiwan, China | Prospective cohort | 100 | Median 30.5 months | Score ≥2 (49/51) | 7 | Vascular invasion, stage, VEGF expression, postoperative CEA | OS, DFS | Data extrapolated |
| Mckay[15] | 2002 | UK | Prospective cohort | 249 | Median 35 months | >5% (137/112) | 8 | Age, stage | OS | Data extrapolated |
| Hilska[17] | 2005 | Finland | Prospective cohort | 363 | NA | >1% (99/264) | 7 | T stage, modified Dukes stage, histological differentiation, urgency of operation, Ki-67 labeling, CEA expression | OS | Reported in text |
| Theocharis[23] | 2007 | Greece | Prospective cohort | 86 | Median 43 months | >5% (56/30) | 7 | Age, gender, tumor location, histological stage and grade, lymph node and liver metastasis, venous invasion | OS | Reported in text |
| Von Wangenheim[21] | 2007 | Germany | Prospective cohort | 200 | At least 5 years | >5% (76/124) | 8 | Age | OS, DFS | Data extrapolated |
| Fang[34] | 2009 | China | Prospective cohort | 532 | Median 52 months | >10% (380/152) | 6 | Age, MMP7, Survivin, TROP2, pathology grade, bowel wall invasion, lymph node metastasis, during-operative chemotherapy | OS | Data extrapolated |
| Mao[24] | 2011 | China | Prospective cohort | 169 | 3 to 107 months | >5% (95/74) | 7 | T stage, lymph node metastasis, distant metastasis, TNM stage, P-Stat5 expression | OS | Data extrapolated |
| Saridaki[32] | 2010 | Greece | Prospective cohort | 144 | NA | ≥20% (26/118) | 6 | BRAF status, stage, metastasectomy, number of treatment lines | OS, DFS | Data extrapolated |
| Bhatavdekar[13] | 2001 | India | Prospective cohort | 98 | 5 years | Score ≥1 (30/68) | 7 | Dukes stage, CD44, CK-19, PRL | OS, DFS | Reported in text |
| Palmqvist[8] | 1998 | Sweden | Prospective cohort | 90 | Median 42 months | >50% (11/79) | 7 | Dukes stage, pRb expression, | OS | Data extrapolated |
| Belt[35] | 2012 | Netherlands | Prospective cohort | 379 | NA | Score ≥8 (168/211) | 8 | Age, gender, stage, tumor location, lymph node yield, MSI status, venous invasion, chemotherapy treatment | OS, DFS | Data extrapolated |
| Pasz-Walczak[14] | 2001 | Poland | Prospective cohort | 122 | Median 44.5 months | >50% (68/54) | 7 | Lymph node invasion, stage, hepatic metastasis, p21 | OS | Reported in text |
| Moore[33] | 2004 | USA | Prospective cohort | 40 | Median 69 months | >10% (6/34) | 8 | Lymph node invasion, the depth of tumor invasion, stage, p27, p53 | OS, DFS | Data extrapolated |
| Bondi[16] | 2005 | Norway | Prospective cohort | 219 | 5 years | >5% (24/195) | 6 | Age | OS, DFS | Data extrapolated |
| Maeda[9] | 1997 | Japan | Prospective cohort | 101 | 5 years | >50% (14/87) | 7 | Histological differentiation, stage, lymph node metastasis, the depth of invasion, lymphatic invasion, venous invasion, p53 | OS, DFS | Reported in text |
| Ogino[18] | 2009 | USA | Prospective cohort | 602 | Every 2 years | >50% (330/272) | 8 | Age, gender, year of diagnosis, BMI, family history of CRC in any first-degree relative, tumor location, stage, grade, status of MSI, CIMP, LINE-1, KRAS, BRAF, p53, p21, p27, COX-2, FASN | OS | Reported in text |
| Wang[20] | 2013 | China | Case control | 139 | * | >5% (83/56) | 6 | Differentiation, TNM stage, YAP expression | OS | Data extrapolated |
| Jang[47] | 2012 | Korea | Prospective cohort | 217 | NA | ≥30% (129/88) | 7 | Age, gender, tumor location, tumor size, differentiation, lymphovascular invasion, stage, preoperative CEA, CA19-9 level, β-catenin expression | OS | Reported in text |
| Lyall[19] | 2012 | UK | Prospective cohort | 90 | 60 to 100 months | >5% (46/44) | 8 | Age, gender, cluster group, tumor location, status of apical node, stage, nodal status, tumor differentiation | OS | Data extrapolated |
*these studies looked back at medical records and did not report the time of follow-up.
Abbreviations: BMI: body mass index; CA19-9: carbohydrate antigen 19-9; CEA: carcinoembryonic antigen; CIMP: the CpG island methylator phenotype; CK-19: cytokeratin-19; COX-2: cyclooxygenase-2; CRC: colorectal cancer; DFS, disease-free survival; FASN: fatty acid synthase; HR: hazard ratio; LINE-1: long interspersed nucleotide element-1; MMP-7: matrix metalloproteinase-7; MSI: microsatellite instability; NA: not available; OS: overall survival; pRb: retinoblastoma protein; PRL: prolactin; P-Stat5: phosphorylated signal transducer and activator of transcription-5; VEGF: vascular endothelial growth factor; YAP: Yes-associated protein.
Of the 22 studies, 21 studies reported the prognostic value of cyclin D1 expression for OS in patients with colorectal cancer [8]–[10], [13]–[25], [32]–[35], [47]. Regarding treatment, colorectal cancer can be treated with either surgery, radiotherapy, chemotherapy or a combination of these treatments. The study could be classified two subgroups according to whether the patients received chemoradiation in addition to surgical operation. In subgroup analysis, 16 studies were treated by single surgery [8]–[10], [14]–[25], [35], while 5 studies were treated with surgery as well as chemoradiation treatment [13], [32]–[34], [47]. 7 studies (1346 patients, 32.7%) were performed in Asian populations [9], [13], [20], [22], [24], [34], [47], and the remaining 14 studies (2765 patients, 67.3%) followed non-Asian patients [8], [10], [14]–[19], [21], [23], [25], [32]–[33], [34]. 17 studies used whole tissue sections to detect cyclin D1 antigen [8]–[10], [13]–[17], [19]–[25], [32]–[33], while 4 studies used tissue microarray [18], [34]–[35], [47]. With respect to the staining patterns, 13 studies detected the cyclin D1 with nuclear cyclin D1 staining [9], [14], [16], [18]–[20], [22], [23], [25], [32]–[33], [35], [47], 7 studies with combined nuclear and cytoplasmic cyclin D1 staining [8], [10], [15], [17], [21], [24], [34], and 1 study with cytoplasmic only [13]. 4 studies were classified into low quality group [16], [20], [32], [34], and 17 studies were high quality group [8]–[10], [13]–[15], [17]–[19], [21]–[25], [33], [35], [47]. 3 studies were case-control studies [10], [20], [25] and 18 were prospective cohort studies [8]–[9], [13]–[19], [21]–[24], [32]–[35], [47]. DFS was obtained in ten studies [9]–[10], [13], [16], [21]–[22], [25], [32]–[33], [35]. In subgroup analysis defined by geographic settings, 3 studies (299 patients, 20.6%) were performed in Asian populations [9], [13], [22], and 7 studies (1153 patients, 79.4%) followed non-Asian populations [10], [16], [21], [25], [32]–[33], [35]. Among these studies, seven were treated by single surgery [9]–[10], [16], [21]–[22], [25], [35], while three were undergone surgery and chemoradiation therapies [13], [32]–[33]. When grouped according to the staining patterns, cyclin D1 was detected by nuclear staining in 7 studies [9], [16], [22], [25], [32]–[33], [35], by nuclear and cytoplasmic staining in 2 studies [10], [21], and by single cytoplasmic staining only in 1 study [13]. With respect to the study design, two were case-control studies [10], [25] and eight were prospective cohort studies [9], [13], [16], [21]–[22], [32]–[33], [35]. According to the Newcastle-Ottawa quality assessment scale, 2 studies were classified into low quality group [16], [32], and 8 studies were high quality group [9]–[10], [13], [21]–[22], [25], [33], [35].
Methodological Quality of the Studies
To assess the quality of the included studies, two authors (Li Y. and Xu C. H.) independently extracted data and assessed the methodological quality using the Newcastle-Ottawa quality assessment scale. The scores of the included studies ranged from 6 to 8 (with a mean of 7.05), which were shown in Table 1. The studies included in our meta-analysis had moderate or high levels of methodological quality. Table S2 summarizes the quality scores of each item of cohort studies and case-control studies.
Quantitative synthesis
Impact of cyclin D1 expression on OS of CRC
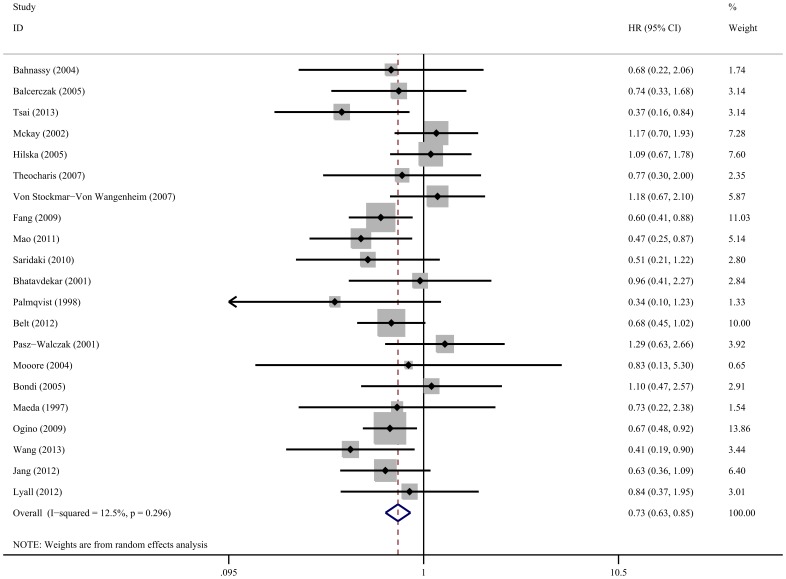
Meta analysis of 21 studies on the prognostic value of cyclin D1 expression showed that high cyclin D1 levels were associated with poor OS (HR obtained from random-effects model: 0.73, 95% CI: 0.63–0.85, P<0.001), without significant heterogeneity between studies (I 2 = 12.5%, P = 0.296) (Figure 2). Subgroup analysis indicated that when grouped according to geographic settings of individual studies, the pooled HR of Asian studies and non-Asian studies were 0.56 (95% CI: 0.45–0.72, P<0.001, without significant heterogeneity) and 0.83 (95% CI: 0.70–0.98, P = 0.026, and without significant heterogeneity), respectively, indicating that cyclin D1 is an indicator of poor OS both in Asian patients and non-Asian patients (Table 2). Cyclin D1 overexpression was related markedly with poor OS in colorectal cancer patients treated by single surgery (HR: 0.77, 95% CI: 0.63–0.93, P = 0.006) and surgery as well as chemoradiation (HR: 0.63, 95% CI: 0.48–0.83, P = 0.001), without significant heterogeneity between studies. Patients with high cyclin D1 expression based on the whole tissue sections seemed to have worse OS than those with cyciln D1 low expression group (HR: 0.79, 95% CI: 0.64–0.98, P = 0.032, and I 2 = 18.9%, P = 0.233 for heterogeneity). Cyclin D1 overexpression detected by tissue microarray was also associated with a worse OS (HR: 0.64, 95% CI: 0.53–0.79, P<0.001, without significant heterogeneity). When grouped according to staining patterns, cyclin D1 overexpression had significant impact on OS with nuclear staining (HR: 0.68, 95% CI: 0.57–0.81, P<0.001, without significant heterogeneity) but not for nuclear combining with cytoplasmic staining (HR: 0.79, 95% CI: 0.57–1.10, P = 0.159, I 2 = 51.2%, P = 0.056 for heterogeneity) or only cytoplasmic staining (HR: 0.96, 95% CI: 0.41–2.27, P = 0.922). Subgroup analysis by study design suggested that cyclin D1 overexpression predicted poor OS in both case-control studies (HR: 0.57, 95% CI: 0.35–0.94, P = 0.028, without significant heterogeneity) and prospective cohort studies (HR: 0.75, 95% CI: 0.64–0.89, P = 0.001, I2 = 17.8%, P = 0.241 for heterogeneity). The study quality subgroup analysis indicated a significant relation between cyclin D1 overexpression and poor OS, which was exhibited in both low-quality studies (HR: 0.60, 95% CI: 0.44–0.81, P = 0.001, without significant heterogeneity) and high-quality studies (HR: 0.77, 95% CI: 0.65–0.91, P = 0.002, I2 = 9.7%, P = 0.341 for heterogeneity).
Figure 2. Forest plot of the hazard ratio (HR) for the association of cyclin D1 expression with overall survival (OS).
Horizontal lines represent 95% CI. Each box represents the HR point estimate, and its area is proportional to the weight of the study. The diamond (and broken line) represents the overall summary estimate, with CI represented by its width. The unbroken vertical line indicates the null value (HR = 1).
Table 2. Results of overall and subgroup analyses for effects of cyclin D1 expression on overall and disease-free survival in colorectal cancer.
| Categories | N | Patients | References | Random-effects model | Heterogeneity | |||
| HR (95% CI) | P | Q | I 2 | P | ||||
| Overall survival (OS) | 21 | 4111 | 8–10, 13–25, 32–35, 47 | 0.73 (0.63–0.85) | <0.001 | 22.85 | 12.5% | 0.296 |
| Subgroup 1: Single surgery | 16 | 3080 | 8–10, 14–25, 35 | 0.77 (0.63–0.93) | 0.006 | 20.09 | 25.3% | 0.169 |
| Surgery and chemoradiation | 5 | 946 | 13, 32–34, 47 | 0.63 (0.48–0.83) | 0.001 | 1.30 | 0% | 0.861 |
| Subgroup 2: Asian | 7 | 1362 | 9, 13, 20, 22, 24, 34, 47 | 0.56 (0.45–0.72) | <0.001 | 3.82 | 0% | 0.700 |
| Non-Asian | 14 | 2749 | 8, 10, 14–19, 21, 23, 25, 32–34 | 0.83 (0.70–0.98) | 0.026 | 12.21 | 0% | 0.511 |
| Subgroup 3: Whole tissue sections | 17 | 2381 | 8–10, 13–17, 19–25, 32–33 | 0.79 (0.64–0.98) | 0.032 | 19.73 | 18.9% | 0.233 |
| Tissue microarray | 4 | 1730 | 18, 34–35, 47 | 0.64 (0.53–0.79) | <0.001 | 0.26 | 0% | 0.968 |
| Subgroup 4: Nuclei | 13 | 2350 | 9, 14, 16, 18–20, 22, 23, 25, 32–33, 35, 47 | 0.68 (0.57–0.81) | <0.001 | 8.90 | 0% | 0.712 |
| Nuclei and cytoplasm | 7 | 1663 | 8, 10, 15, 17, 21, 24, 34 | 0.79 (0.57–1.10) | 0.159 | 12.30 | 51.2% | 0.056 |
| Cytoplasm | 1 | 98 | 13 | 0.96 (0.41–2.27) | 0.922 | 0 | - | - |
| Subgroup 5: Case control studies | 3 | 310 | 10, 20, 25 | 0.57 (0.35–0.94) | 0.028 | 1.15 | 0% | 0.563 |
| Prospective cohort studies | 18 | 3801 | 8– 9, 13–19, 21–24, 32–35, 47 | 0.75 (0.64–0.89) | 0.001 | 20.68 | 17.8% | 0.241 |
| Subgroup 6: Low quality studies | 4 | 1034 | 16, 20, 32, 34 | 0.60 (0.44–0.81) | 0.001 | 2.98 | 0% | 0.395 |
| High quality studies | 17 | 3077 | 8–10, 13–15, 17–19, 21–25, 33, 35, 47 | 0.77 (0.65–0.91) | 0.002 | 17.72 | 9.7% | 0.341 |
| Disease-free survival (DFS) | 10 | 1452 | 9–10, 13, 16, 21–22, 25, 32–33, 35 | 0.60 (0.44–0.82) | 0.001 | 11.80 | 23.7% | 0.225 |
| Subgroup 1: Single surgery | 7 | 1170 | 9–10, 16, 21–22, 25, 35 | 0.69 (0.51–0.92) | 0.011 | 6.56 | 8.5% | 0.364 |
| Surgery and chemoradiation | 3 | 282 | 13, 32–33 | 0.33 (0.17–0.63) | 0.001 | 1.17 | 0% | 0.556 |
| Subgroup 2: Asian | 3 | 299 | 9, 13, 22 | 0.41 (0.24–0.72) | 0.002 | 0.44 | 0% | 0.804 |
| Non-Asian | 7 | 1153 | 10, 16, 21, 25, 32–33, 35 | 0.67(0.46–0.98) | 0.041 | 8.92 | 32.7% | 0.178 |
| Subgroup 3: Nuclei | 7 | 1094 | 9, 16, 22, 25, 32–33, 35 | 0.54 (0.38–0.77) | 0.001 | 7.66 | 21.7% | 0.264 |
| Nuclei and cytoplasm | 2 | 260 | 10, 21 | 1.00 (0.57–1.77) | 0.997 | 0.56 | 0% | 0.453 |
| Cytoplasm | 1 | 98 | 13 | 0.46 (0.18–1.18) | 0.107 | 0 | - | - |
| Subgroup 4: Case control studies | 2 | 171 | 10, 25 | 0.72 (0.37–1.40) | 0.329 | 0.02 | 0% | 0.885 |
| Prospective cohort studies | 8 | 1281 | 9, 13, 16, 21–22, 32–33, 35 | 0.57 (0.39–0.83) | 0.004 | 11.54 | 39.3% | 0.117 |
| Subgroup 5: Low quality studies | 2 | 363 | 16, 32 | 0.52 (0.12–2.24) | 0.381 | 4.34 | 77.0% | 0.037 |
| High quality studies | 8 | 1089 | 9–10, 13, 21–22, 25, 33, 35 | 0.61 (0.46–0.82) | 0.001 | 7.46 | 6.1% | 0.383 |
Abbreviations: HR: hazard ratio; 95% CI: 95% confidence interval; N: number of studies.
Cyclin D1 expression and DFS in CRC
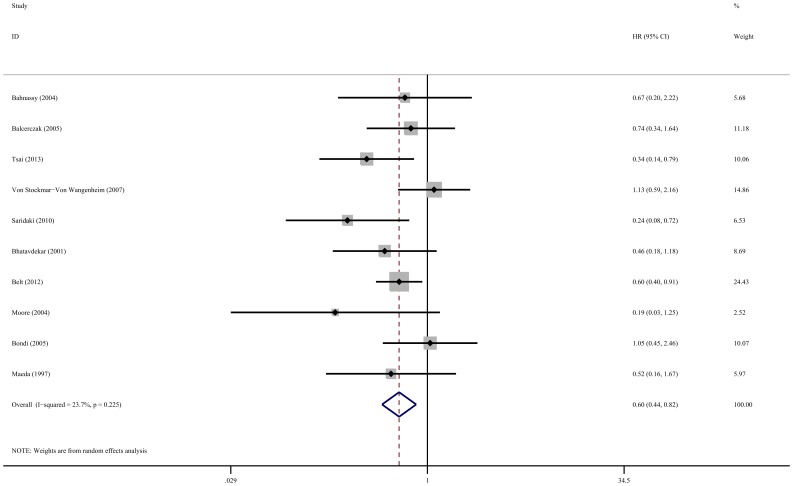
Meta-analysis of 10 studies showed that high cyclin D1 expression was associated with poor DFS in colorectal cancer patients (HR: 0.60, 95% CI: 0.44–0.82, P = 0.001, and I 2 = 23.7%, P = 0.225 for heterogeneity) (Figure 3). When grouped according to geographic settings, the pooled HR of Asian studies and non-Asian studies were 0.41 (95% CI: 0.24–0.72, P = 0.002, without significant heterogeneity) and 0.67 (95% CI: 0.46–0.98, P = 0.041, and I 2 = 32.7%, P = 0.178 for heterogeneity), respectively (Table 2). The results showed that cyclin D1 overexpression were markedly associated with poor DFS in both Asian and non-Asian CRC patients. Restricting the analysis to studies that treated by single surgery (HR: 0.69, 95% CI: 0.51–0.92, P = 0.011, and I 2 = 8.5%, P = 0.364 for heterogeneity) and surgery as well as chemoradiation subgroups (HR: 0.33, 95% CI: 0.17–0.63, P = 0.001, without significant heterogeneity) also indicated the difference in DFS between cyclin D1-high and low level groups. When grouped according to staining patterns, cyclin D1 overexpression had significant impact on DFS with only nuclear staining (HR: 0.54, 95% CI: 0.38–0.77, P = 0.001, I2 = 21.7%, P = 0.264 for heterogeneity) but not for nuclear and cytoplasmic staining (HR: 1.00, 95% CI: 0.57–1.77, P = 0.997, without significant heterogeneity) and single cytoplasmic staining (HR: 0.46, 95% CI: 0.18–1.18, P = 0.107). Furthermore, subgroup analysis revealed that the significant correlation between cyclin D1 overexpression and worse DFS in prospective cohort studies (HR: 0.57, 95% CI: 0.39–0.83, P = 0.004, I2 = 39.3%, P = 0.117 for heterogeneity) but not in case-control studies (HR: 0.72, 95% CI: 0.37–1.40, P = 0.329, without significant heterogeneity). The study quality subgroup analysis shown that a significant relation between high level cyclin D1 and poor DFS in high quality studies (HR: 0.61, 95% CI: 0.46–0.82, P = 0.001, I2 = 6.1%, P = 0.383 for heterogeneity) but not in low quality studies (HR: 0.52, 95% CI: 0.12–2.24, P = 0.381, I2 = 77.0%, P = 0.037 for heterogeneity).
Figure 3. Forest plot of the hazard ratio (HR) for the association of cyclin D1 expression with disease-free survival (DFS).
Cyclin D1 expression and clinicopathological parameters
The association between cyclin D1 and several clinicopathological parameters was illustrated in Figure S1, S2, S3, S4, S5, and S6. Eleven studies reported data on the correlation between cyclin D1 expression and colorectal cancer patients' age [13]–[14], [17], [20], [22], [24], [31]–[32], [34]–[35], [47]. The pooled OR was 0.62 (95% CI: 0.44–0.89, P = 0.009), suggesting elder patients (≥60 years) had significantly higher cyclin D1 expression than younger patients (<60 years) (Table 3). Furthermore, six studies reported data on tumor size [10], [20], [22], [24], [35], [47], sixteen studies reported data on T category [8]–[10], [14]–[15], [17]–[18], [20]–[22], [24]–[25], [31], [34]–[35], [47], fourteen studies reported data on N category [9]–[10], [14]–[15], [20]–[25], [31], [34]–[35], [47], nine studies reported data on distant metastasis [14], [20], [22]–[25], [31], [34], [47], eighteen studies reported data on histology and their relationship with cyclin D1 expression [8]–[10], [13]–[15], [17]–[18], [20]–[24], [31]–[32], [34]–[35], [47]. When the data was pooled, there were significant association between high cyclin D1 expression and the clinicopathological parameters except tumor size and histology. Specifically, the pooled OR were as follows: 0.64 (0.35–1.16, P = 0.139) for tumor size (<5 cm vs. ≥5 cm), 0.70 (0.57–0.85, P<0.001) for T category (T1,2 vs. T3,4), 0.75 (0.60–0.95, P = 0.016) for N category (negative vs. positive), 0.60 (0.36–0.99, P = 0.047) for distant metastasis (M0 vs. M1), 0.87 (0.71–1.07, P = 0.178) for histology (Well, moderate vs. poor).
Table 3. Meta-analysis of the association between cyclin D1 expression and clinicopathological features of colorectal cancer.
| Categories | N | Patients | References | Random-effects model | Heterogeneity | |||
| OR (95% CI) | P | Q | I 2 | P | ||||
| Age in years (<60 vs. ≥60) | 11 | 2302 | 13–14, 17, 20, 22, 24, 31–32, 34–35, 47 | 0.62 (0.44–0.89) | 0.009 | 32.79 | 69.5% | <0.001 |
| Tumor size (cm) (<5 vs. ≥5) | 6 | 1064 | 10, 20, 22, 24, 35, 47 | 0.64 (0.35–1.16) | 0.139 | 23.81 | 79.0% | <0.001 |
| T category (T1,2 vs. T3,4) | 16 | 3473 | 8–10, 14–15, 17–18, 20–22, 24–25, 31, 34–35, 47 | 0.70 (0.57–0.85) | <0.001 | 21.24 | 29.4% | 0.129 |
| N category (negative vs. positive) | 14 | 2504 | 9–10, 14–15, 20–25, 31, 34–35, 47 | 0.75 (0.60–0.95) | 0.016 | 20.55 | 36.7% | 0.082 |
| Distant metastasis (M0 vs. M1) | 9 | 1376 | 14, 20, 22–25, 31, 34, 47 | 0.60 (0.36–0.99) | 0.047 | 23.03 | 65.3% | 0.003 |
| Histology (Well,mod vs. por) | 18 | 3688 | 8–10, 13–15, 17–18, 20–24, 31–32, 34–35, 47 | 0.87 (0.71–1.07) | 0.178 | 19.12 | 11.1% | 0.321 |
Abbreviations: OR: odds ratio; 95% CI: 95% confidence interval; mod: moderate; por: poor; N, number of studies.
Sensitivity analyses
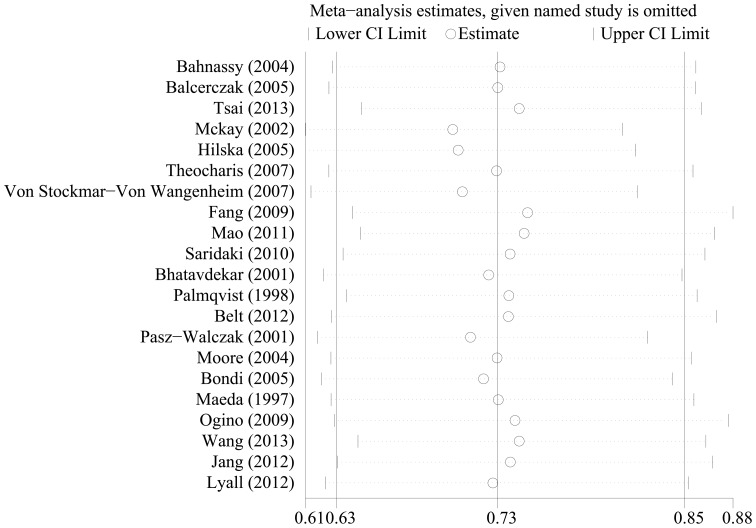
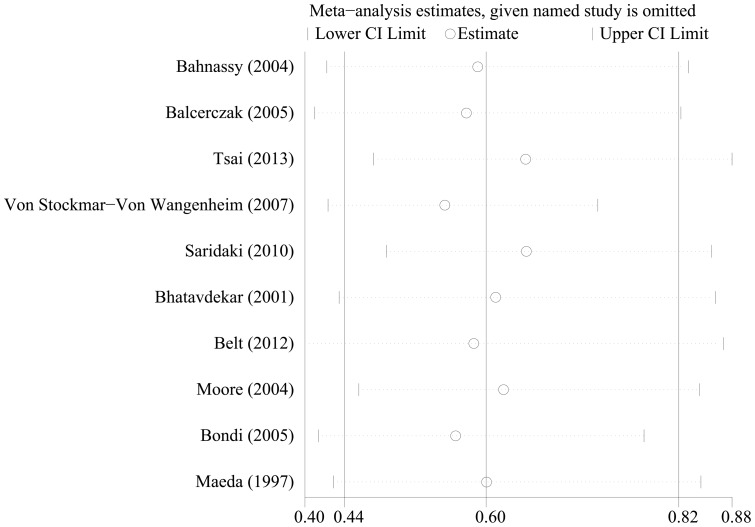
To test the robustness of association between cyclin D1 expression and survival outcome (OS and DFS) and characterize possible sources of statistical heterogeneity, sensitivity analyses were performed by excluding studies one-by-one and analyzing the homogeneity and effect size for all of rest studies. Sensitivity analyses indicated that no significant variation in combined HR by excluding any of the study, confirming the stability of present results (Figure 4 and Figure 5).
Figure 4. Sensitivity analysis based on stepwise omitting one study at a time for overall survival (OS).
Figure 5. Sensitivity analysis based on stepwise omitting one study at a time for disease-free survival (DFS).
Publication bias
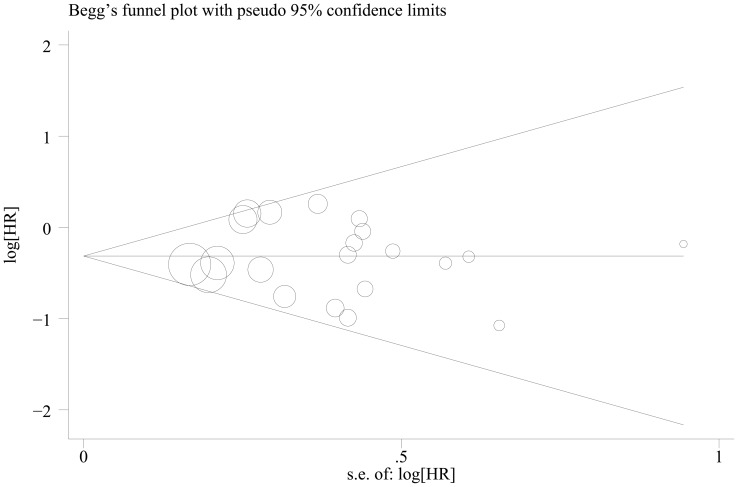
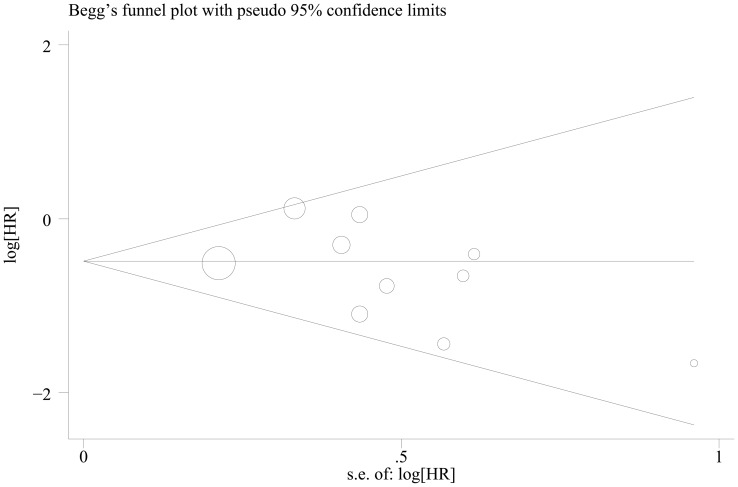
Begg's funnel plot and Egger's test were performed to investigate the publication bias of the eligible studies on the summary OS and DFS. The shape of the funnel plot did not reveal any evidence of obvious asymmetry. Then the Egger's test was used to provide statistical evidence of funnel plot symmetry. Twenty one and ten studies investigating cyclin D1 overexpression on OS and DFS yielded an Egger's test score of P = 0.886 and P = 0.260, respectively, indicating the absence of publication bias in the studies (Figure 6 and Figure 7).
Figure 6. Begg's funnel plot for the evaluation of potential publication bias on overall estimate of overall survival (OS).
Figure 7. Begg's funnel plot for the evaluation of potential publication bias on overall estimate of disease-free survival (DFS).
Discussion
Recently, attention has been drawn at a meta-analytical level on the prognostic marker. Potential roles of cyclin D1 overexpression have been presumed in various types of cancers, including CRC. Zhao et al. [36] and Xu et al. [37] demonstrated that cyclin D1 overexpression was associated with worst clinicopathological features and prognosis for esophageal squamous cell carcinoma and ER-positive breast cancer. Cyclin D1 overexpression has been reported to occur in 40–70% of colorectal tumors [7], [11], [12], [38]–[40], [47]. Despite a well-established role of cyclin D1 in cell cycle progression, previous data on cyclin D1 and clinical outcome in colorectal cancer have been conflicting. The presence of both significant and non-significant studies addressing the importance of cyclin D1 overexpression in CRC made it necessary to perform a quantitative aggregation of the survival results.
To our knowledge, this is the first meta-analysis on the association between cyclin D1 expression and OS, DFS and the clincopathological parameters in CRC. The present meta-analysis has combined 22 publications including 4150 patients to yield statistics, indicating the cyclin D1 expression is significantly associated with the CRC patients OS and DFS. Subgroup analysis indicated that high cyclin D1 expression was related significantly with poor OS in CRC treated by single surgery and surgery as well as chemoradiation. Cyclin D1 overexpression was also related significantly with poor OS in Asian and non-Asian CRC patients. Besides, high cyclin D1 expression detected by whole tissue sections and tissue microarray was associated with poor OS in CRC patients. Cyclin D1 overexpression based on the nuclear staining was related with a poor OS in CRC patients. In study quality subgroup analysis, both the low quality and high quality studies showed that cyclin D1 overexpresssion had a worse OS. There were also significant relation between cyclin D1-high groups and poor OS in case-control studies and prospective cohort studies. In addition, cyclin D1 overexpression was related significantly with poor DFS not only in patients who received surgery, but also in patients who received surgery and chemoradiation therapies. High cyclin D1 expression was also associated with poor DFS in both Asian and non-Asian patients. High cyclin D1 expression based on nuclear staining was associated with a poor DFS in CRC patients. In study design subgroup analysis, there were significant association between cyclin D1 overexpression and poor DFS in prospective cohort studies but not in case-control studies. A significant relation was also found between cyclin D1 high level and poor DFS in high quality studies but not in low quality studies. Besides, cyclin D1 high expression was related with more older patients (≥60 years), T3,4 category, N positive, distant metastasis patients.
Three patterns of cyclin D1 expression by immunohistochemical method had been found in CRC specimens. Previous studies reported that there existed differences in nuclear cyclin D1 overexpression for colorectal cancer (11–30%) [7], [9], [41]. Cytoplasmic cyclin D1 expression has been shown to be common in non-small lung cancer [42]. Lucas et al. suggested that intracellular localization of cyclin D1 is changed during progress through the cell cycle and from the G1-S transition the protein becomes more soluble, reflecting the loss of nuclear cyclin D1 proteins as part reason for cytoplasmic cyclin D1 [43]. Arber et al. considered that cytoplasmic localization of cyclin D1 is probably not caused by leakage of protein from the nucleus, since cytoplasmic staining was observed in the total absence of nucleus staining [7]. Bhatavdekar et al. showed that cyclin D1 antigen was detected in the cytoplasm of the colorectal cells [13]. Other studies assessed cyclin D1 expression only in the nuclei [9], [14], [16], [18]–[20], [22], [23], [25], [32]–[33], [35], [47]. Nevertheless, some studies demonstrated that cyclin D1 could be detected both in nuclei and cytoplasm in CRC [8], [10], [15], [17], [21], [24], [34]. This present results suggested that only nuclear staining patterns of cyclin D1 overexpression were correlated with the OS and DFS in CRC patients. Thus, large prospective studies taking combinations of three staining patterns to evaluate the OS and DFS in CRC patients into accounts are needed.
Cyclin D1 is the significant prognostic factor for predicting CRC patients' survival. However, co-expression of cyclin D1, p21, PCNA and p53 was previously observed in a subset of patient population [44]; Co-expression of cyclin D1, p21 and PCNA was contributed to the role of cyclin D1 for tumor proliferation, while p53 was inversely associated with cyclin D1 levels, suggesting that overexpression p53 protein is acting to inhibit cellular proliferation [45]. Co-expression of cyclin D1, p21, PCNA and p53, may be an independent prognostic factor for predicting survival. The prognostic value of cyclin D1 in patients with CRC should be examined in the context of other proposed molecular markers such as EGFR, Bcl-2, p21, p53, PCNA, pRb [15], [17], [33], [35]. Only two studies [22], [24] included in the meta-analysis had included a multivariate analysis of co-expression of cyclin D1 and one or several biomarkers. Therefore, large prospective studies taking combinations of cyclin D1 and other most promising markers into account are needed.
Other than displaying cyclin D1 molecule in situ by immunohistochemical staining, some studies have examined cyclin D1 gene or mRNA expression using Southern blot or reverse transcriptase-polymerase chain reaction (RT-PCR) method. Bahnassy et al. detected cyclin D1 gene amplification in 50 colorectal cancer cases and found cyclin D1 gene amplification was significantly associated with an advanced disease stage since amplification was detected in 10/15 (66.7%) of stage IV tumors compared to 12/45 (26.7%) of stageI–III tumors. Balcerczak et al. used RT-qPCR to quantify cyclin D1 mRNA levels in the investigated colorectal cancers and he found that CCND1 expression was significantly related to lymph nodes and distant metastases. There was also a significant statistical correlation between the presence of CCND1 gene expression and high stages C1, C2, D according to Astler-Cooler's classification [25]. Oda et al. assessed cyclin D1 mRNA levels by qRT-PCR in surgically resected specimens of colorectal cancers and observed that the rate of cyclin D1 mRNA expression was significantly higher in patients with venous invasion. Besides, the overexpression of cyclin D1 mRNA was correlated with poor prognosis in CRC patients.
The results should be interpreted cautiously since some limitations exist in this meta-analysis. First, the number of studies and patients classified into the surgery and chemoradiation subgroup of the OS and DFS analysis were limited, respectively. The results upon treatment subgroup analysis should be interpreted with caution. Second, although immunohistochemistry was the most commonly used method for detecting cyclin D1 in situ, RT-qPCR method has also been used for the evaluation of the levels of cyclin D1 gene or mRNA expression in tumor tissue. Studies measuring cyclin D1 gene or mRNA level by RT-qPCR was not yet included in this meta-analysis. Third, another potential source of bias is the variable length of follow-up amongst studies and the differently defined cutoff value. Fourth, the method of obtaining survival data is a potential source of bias. If these statistics were not reported directly by the authors, we calculated from the data available in the article or by extrapolating them from the survival curves, which seemed to be less reliable than when HR was obtained directly from published statistics. These results should be confirmed by well designed prospective studies. Finally, although we did not detect significant heterogeneity or publication bias between studies evaluating the prognostic role of cyclin D1, it is important to note that when the sample size of the studies or the number of primary studies is small, the power to detect potentially important differences is limited. Some important studies had to be excluded from our analysis, for reasons of small size, insufficient survival data, etc. It is known that negative studies are less frequently published or, if they are, with less detailed results, making them less assessable. The missing information reflected “negative” association of cyclin D1 with survival that could decrease the significance of cyclin D1 expression as a predictor of survival outcome. Language bias should not be completed avoided, because of restricted only in English.
In summary, as determined in our meta-analysis, we concluded that cyclin D1 overexpression was significantly associated with poor OS as well as DFS in CRC patients. Cyclin D1 might be an unfavorable prognostic factor for CRC patients. To strengthen our findings, well-designed prospective studies with better standardized assessment of prognostic markers should help to explore the relation between cyclin D1 expression and the CRC patients' outcome.
Supporting Information
Forest plot of the odds ratio (OR) for the association of cyclin D1 expression with years of age.
(TIF)
Forest plot of the odds ratio (OR) for the association of cyclin D1 expression with tumor size.
(TIF)
Forest plot of the odds ratio (OR) for the association of cyclin D1 expression with T category.
(TIF)
Forest plot of the odds ratio (OR) for the association of cyclin D1 expression with N category.
(TIF)
Forest plot of the odds ratio (OR) for the association of cyclin D1 expression with distant metastasis.
(TIF)
Forest plot of the odds ratio (OR) for the association of cyclin D1 expression with histological grade.
(TIF)
Search strategy in PubMed.
(DOCX)
Quality assessment of included studies based on the Newcastle-Ottawa Scale.
(DOCX)
Prognostic significance of cyclin D1 expression in colorectal cancer (Protocol).
(DOCX)
PRISMA checklist.
(DOC)
Acknowledgments
We are indebted to the authors of the primary studies.
Funding Statement
This work was an investigator-led study. The authors have no support or funding to report.
References
- 1. Weitz J, Koch M, Debus J, Hohler T, Galle PR, et al. (2005) Colorectal cancer. Lancet 365: 153–165. [DOI] [PubMed] [Google Scholar]
- 2. Xu AG, Yu ZJ, Jiang B, Wang XY, Zhong XH, et al. (2010) Colorectal cancer in Guangdong Province of China: a demographic and anatomic survey. World journal of gastroenterology: WJG 16: 960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62: 10–29. [DOI] [PubMed] [Google Scholar]
- 4. Hunter T, Pines J (1994) Cyclins and cancer. II: Cyclin D and CDK inhibitors come of age. Cell 79: 573–582. [DOI] [PubMed] [Google Scholar]
- 5. Nevins JR (1992) E2F: a link between the Rb tumor suppressor protein and viral oncoproteins. Science 258: 424–429. [DOI] [PubMed] [Google Scholar]
- 6. Besson A, Dowdy SF, Roberts JM (2008) CDK inhibitors: cell cycle regulators and beyond. Dev Cell 14: 159–169. [DOI] [PubMed] [Google Scholar]
- 7. Arber N, Hibshoosh H, Moss SF, Sutter T, Zhang Y, et al. (1996) Increased expression of cyclin D1 is an early event in multistage colorectal carcinogenesis. Gastroenterology 110: 669–674. [DOI] [PubMed] [Google Scholar]
- 8. Palmqvist R, Stenling R, Oberg A, Landberg G (1998) Expression of cyclin D1 and retinoblastoma protein in colorectal cancer. Eur J Cancer 34: 1575–1581. [DOI] [PubMed] [Google Scholar]
- 9. Maeda K, Chung YS, Kang SM, Ogawa M, Onoda N, et al. (1997) Overexpression of cyclin D1 and p53 associated with disease recurrence in colorectal adenocarcinoma. Int J Cancer 74: 310–315. [DOI] [PubMed] [Google Scholar]
- 10. Bahnassy AA, Zekri AR, El-Houssini S, El-Shehaby AM, Mahmoud MR, et al. (2004) Cyclin A and cyclin D1 as significant prognostic markers in colorectal cancer patients. BMC Gastroenterol 4: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Holland TA, Elder J, McCloud JM, Hall C, Deakin M, et al. (2001) Subcellular localisation of cyclin D1 protein in colorectal tumours is associated with p21(WAF1/CIP1) expression and correlates with patient survival. Int J Cancer 95: 302–306. [DOI] [PubMed] [Google Scholar]
- 12. Bukholm IK, Nesland JM (2000) Protein expression of p53, p21 (WAF1/CIP1), bcl-2, Bax, cyclin D1 and pRb in human colon carcinomas. Virchows Arch 436: 224–228. [DOI] [PubMed] [Google Scholar]
- 13. Bhatavdekar JM, Patel DD, Chikhlikar PR, Shah NG, Vora HH, et al. (2001) Molecular markers are predictors of recurrence and survival in patients with Dukes B and Dukes C colorectal adenocarcinoma. Dis Colon Rectum 44: 523–533. [DOI] [PubMed] [Google Scholar]
- 14. Pasz-Walczak G, Kordek R, Faflik M (2001) P21 (WAF1) expression in colorectal cancer: correlation with P53 and cyclin D1 expression, clinicopathological parameters and prognosis. Pathol Res Pract 197: 683–689. [DOI] [PubMed] [Google Scholar]
- 15. McKay JA, Douglas JJ, Ross VG, Curran S, Loane JF, et al. (2002) Analysis of key cell-cycle checkpoint proteins in colorectal tumours. J Pathol 196: 386–393. [DOI] [PubMed] [Google Scholar]
- 16. Bondi J, Husdal A, Bukholm G, Nesland JM, Bakka A, et al. (2005) Expression and gene amplification of primary (A, B1, D1, D3, and E) and secondary (C and H) cyclins in colon adenocarcinomas and correlation with patient outcome. J Clin Pathol 58: 509–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hilska M, Collan YU, VJ OL, Kossi J, Hirsimaki P, et al. (2005) The significance of tumor markers for proliferation and apoptosis in predicting survival in colorectal cancer. Dis Colon Rectum 48: 2197–2208. [DOI] [PubMed] [Google Scholar]
- 18. Ogino S, Nosho K, Irahara N, Kure S, Shima K, et al. (2009) A cohort study of cyclin D1 expression and prognosis in 602 colon cancer cases. Clin Cancer Res 15: 4431–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lyall MS, Dundas SR, Curran S, Murray GI (2006) Profiling markers of prognosis in colorectal cancer. Clin Cancer Res 12: 1184–1191. [DOI] [PubMed] [Google Scholar]
- 20. Wang Y, Xie C, Li Q, Xu K, Wang E (2013) Clinical and prognostic significance of Yes-associated protein in colorectal cancer. Tumour Biol 34: 2169–2174. [DOI] [PubMed] [Google Scholar]
- 21. Von Stockmar-Von Wangenheim CA, Monig SP, Schneider PM, Landsberg S, Drebber U, et al. (2008) p16, cyclin D1 and Rb expression in colorectal carcinomas: Correlations with clinico-pathological parameters and prognosis. Mol Med Rep 1: 27–32. [PubMed] [Google Scholar]
- 22. Tsai HL, Yeh YS, Chang YT, Yang IP, Lin CH, et al. (2013) Co-existence of cyclin D1 and vascular endothelial growth factor protein expression is a poor prognostic factor for UICC stage I-III colorectal cancer patients after curative resection. J Surg Oncol 107: 148–154. [DOI] [PubMed] [Google Scholar]
- 23. Theocharis S, Giaginis C, Parasi A, Margeli A, Kakisis J, et al. (2007) Expression of peroxisome proliferator-activated receptor-gamma in colon cancer: correlation with histopathological parameters, cell cycle-related molecules, and patients' survival. Dig Dis Sci 52: 2305–2311. [DOI] [PubMed] [Google Scholar]
- 24. Mao Y, Li Z, Lou C, Zhang Y (2011) Expression of phosphorylated Stat5 predicts expression of cyclin D1 and correlates with poor prognosis of colonic adenocarcinoma. Int J Colorectal Dis 26: 29–35. [DOI] [PubMed] [Google Scholar]
- 25. Balcerczak E, Pasz-Walczak G, Kumor P, Panczyk M, Kordek R, et al. (2005) Cyclin D1 protein and CCND1 gene expression in colorectal cancer. Eur J Surg Oncol 31: 721–726. [DOI] [PubMed] [Google Scholar]
- 26.Sobin LH, Gospodarowicz MK, Wittekind C (2010) TNM classification of malignant tumours, 7th edition.
- 27.Wells G, Shea B, O'Connell D, Peterson P, Welch V, et al.. (2011) The Newcatsle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis. Ottawa: Ottawa Hospital Research Institute.
- 28. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR (2007) Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yusuf S, Peto R, Lewis J, et al. (1985) Beta blockade during and after myocardial infarction: an overview of the randomized trials. Prog Cardiovasc Dis 27: 335–371. [DOI] [PubMed] [Google Scholar]
- 30. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang A, Yoshimi N, Suzui M, Yamauchi A, Tarao M, et al. (1996) Different expression patterns of cyclins A, D1 and E in human colorectal cancer. J Cancer Res Clin Oncol 122: 122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Saridaki Z, Papadatos-Pastos D, Tzardi M, Mavroudis D, Bairaktari E, et al. (2010) BRAF mutations, microsatellite instability status and cyclin D1 expression predict metastatic colorectal patients' outcome. Br J Cancer 102: 1762–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moore HG, Shia J, Klimstra DS, Ruo L, Mazumdar M, et al. (2004) Expression of p27 in residual rectal cancer after preoperative chemoradiation predicts long-term outcome. Ann Surg Oncol 11: 955–961. [DOI] [PubMed] [Google Scholar]
- 34. Fang YJ, Lu ZH, Wang GQ, Pan ZZ, Zhou ZW, et al. (2009) Elevated expressions of MMP7, TROP2, and survivin are associated with survival, disease recurrence, and liver metastasis of colon cancer. Int J Colorectal Dis 24: 875–884. [DOI] [PubMed] [Google Scholar]
- 35. Belt EJ, Brosens RP, Delis-van Diemen PM, Bril H, Tijssen M, et al. (2012) Cell cycle proteins predict recurrence in stage II and III colon cancer. Ann Surg Oncol 19 Suppl 3S682–692. [DOI] [PubMed] [Google Scholar]
- 36. Zhao J, Li L, Wei S, Gao Y, Chen Y, et al. (2012) Clinicopathological and prognostic role of cyclin D1 in esophageal squamous cell carcinoma: a meta-analysis. Diseases of the Esophagus 25: 520–526. [DOI] [PubMed] [Google Scholar]
- 37. Xu XL, Chen SZ, Chen W, Zheng WH, Xia XH, et al. (2013) The impact of cyclin D1 overexpression on the prognosis of ER-positive breast cancers: a meta-analysis. Breast Cancer Res Treat 139: 329–339. [DOI] [PubMed] [Google Scholar]
- 38. Utsunomiya T, Doki Y, Takemoto H, Shiozaki H, Yano M, et al. (2001) Correlation of beta-catenin and cyclin D1 expression in colon cancers. Oncology 61: 226–233. [DOI] [PubMed] [Google Scholar]
- 39. Sutter T, Doi S, Carnevale KA, Arber N, Weinstein IB (1997) Expression of cyclins D1 and E in human colon adenocarcinomas. J Med 28: 285–309. [PubMed] [Google Scholar]
- 40. Kristt D, Turner I, Koren R, Ramadan E, Gal R (2000) Overexpression of cyclin D1 mRNA in colorectal carcinomas and relationship to clinicopathological features: an in situ hybridization analysis. Pathol Oncol Res 6: 65–70. [DOI] [PubMed] [Google Scholar]
- 41. Bartkova J, Lukas J, Strauss M, Bartek J (1994) The PRAD-1/cyclin D1 oncogene product accumulates aberrantly in a subset of colorectal carcinomas. Int J Cancer 58: 568–573. [DOI] [PubMed] [Google Scholar]
- 42. Betticher DC, Heighway J, Hasleton PS, Altermatt HJ, Ryder WD, et al. (1996) Prognostic significance of CCND1 (cyclin D1) overexpression in primary resected non-small-cell lung cancer. Br J Cancer 73: 294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lukas J, Bartkova J, Welcker M, Petersen OW, Peters G, et al. (1995) Cyclin D2 is a moderately oscillating nucleoprotein required for G1 phase progression in specific cell types. Oncogene 10: 2125–2134. [PubMed] [Google Scholar]
- 44. McKay JA, Douglas JJ, Ross VG, Curran S, Murray GI, et al. (2000) Cyclin D1 protein expression and gene polymorphism in colorectal cancer. Aberdeen Colorectal Initiative. Int J Cancer 88: 77–81. [DOI] [PubMed] [Google Scholar]
- 45. Hall PA, Meek D, Lane DP (1996) p53—integrating the complexity. J Pathol 180: 1–5. [DOI] [PubMed] [Google Scholar]
- 46. Oda K, Okabayashi T, Kataoka M, Takeda A, Shibuya Y, et al. (1999) Evaluation of cyclin D1 mRNA expression in gastric and colorectal cancers. Res Commun Mol Pathol Pharmacol 105: 237–252. [PubMed] [Google Scholar]
- 47. Jang KY, Kim YN, Bae JS, Chung MJ, Moon WS, et al. (2012) Expression of cyclin D1 is associated withβ-catenin expression and correlates with good prognosis in colorectal adenocarcinoma. Translational Oncology 5: 370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Forest plot of the odds ratio (OR) for the association of cyclin D1 expression with years of age.
(TIF)
Forest plot of the odds ratio (OR) for the association of cyclin D1 expression with tumor size.
(TIF)
Forest plot of the odds ratio (OR) for the association of cyclin D1 expression with T category.
(TIF)
Forest plot of the odds ratio (OR) for the association of cyclin D1 expression with N category.
(TIF)
Forest plot of the odds ratio (OR) for the association of cyclin D1 expression with distant metastasis.
(TIF)
Forest plot of the odds ratio (OR) for the association of cyclin D1 expression with histological grade.
(TIF)
Search strategy in PubMed.
(DOCX)
Quality assessment of included studies based on the Newcastle-Ottawa Scale.
(DOCX)
Prognostic significance of cyclin D1 expression in colorectal cancer (Protocol).
(DOCX)
PRISMA checklist.
(DOC)