Abstract
Dental roots that have been exposed to the oral cavity and periodontal pocket environment present superficial changes, which can prevent connective tissue reattachment. Demineralizing agents have been used as an adjunct to the periodontal treatment aiming at restoring the biocompatibility of roots.
Objective
This study compared four commonly used demineralizing agents for their capacity of removing smear layer and opening dentin tubules.
Methods
Fifty fragments of human dental roots previously exposed to periodontal disease were scaled and randomly divided into the following groups of treatment: 1) CA: demineralization with citric acid for 3 min; 2) TC-HCl: demineralization with tetracycline-HCl for 3 min; 3) EDTA: demineralization with EDTA for 3 min; 4) PA: demineralization with 37% phosphoric acid for 3 min; 5)Control: rubbing of saline solution for 3 min. Scanning electron microscopy was used to check for the presence of residual smear layer and for measuring the number and area of exposed dentin tubules.
Results
Smear layer was present in 100% of the specimens from the groups PA and control; in 80% from EDTA group; in 33.3% from TC-HCl group and 0% from CA group. The mean numbers of exposed dentin tubules in a standardized area were: TC-HCl=43.8±25.2; CA=39.3±37; PA=12.1±16.3; EDTA=4.4±7.5 and Control=2.3±5.7. The comparison showed significant differences between the following pairs of groups: TC-HCl and Control; TC-HCl and EDTA; CA and Control; and CA and EDTA. The mean percentages of area occupied by exposed dentin tubules were: CA=0.12±0.17%; TC-HCl=0.08±0.06%; PA=0.03±0.05%; EDTA=0.01±0.01% and Control=0±0%. The CA group differed significantly from the others except for the TC-HCl group.
Conclusion
There was a decreasing ability for smear layer removal and dentin tubule widening as follows: AC>TC-HCl>PA>EDTA. This information can be of value as an extra parameter for choosing one of them for root conditioning.
Keywords: Demineralization, Root scaling, Smear layer, Scanning electron microscopy
INTRODUCTION
One of the goals of periodontal therapy is the predictable regeneration of the periodontium in areas previously affected by periodontal disease5,23,27. Histological and ultrastructural studies have demonstrated that dental roots that have been exposed to the oral cavity or to the periodontal pocket present reduced collagen fiber insertion1, changes in their mineral density27 and root contamination by bacteria and its products1. Scaling and root planing alone are not able to fully eliminate the etiological contaminants and produce a compact smear layer covering the instrumented surface2,5 which inhibits periodontal tissue reattachment5. These alterations have become the rationale for the use of demineralizing agents as adjunct to periodontal therapy due to their potential for removing smear layer and exposing the underlying radicular collagen fibrils, funneling dentin tubules and modifying dentin permeability, restoring the biocompatibility of the roots9,26.
It has been shown that demineralization of the root surface can exert neutralizing effects on endotoxins from periodontal pathogens in vitro, e.g., inhibition of fibroblast proliferation, synthesis and attachment11. Other studies have shown that, when compared to non-conditioned ones, acid conditioned dental roots are more effective in maintaining fibrin clot and exposing collagen fibrils and associated proteoglycans25.
In vivo animal22 and human histological studies12,13 have shown improved biological response when decalcifying agents are used to condition the root surface. The most used demineralizing agents for these purposes are citric acid9,14,17,28, phosphoric acid23, ethylenediaminetetraacetic acid (EDTA)18 and tetracycline hydrochloride17. Nevertheless, the great variability of protocols employed by clinicians and researchers has prevented consistent comparisons among them. Clinical trials have also provided insufficient evidence that acid conditioning of diseased dental roots present any additional new attachment when compared to non-conditioned ones. The only systematic review found on this subject was published by Mariotti19 (2006) who concluded that the use of citric acid, tetracycline or EDTA to modify the root surface provides no benefit of clinical significance to regeneration in patients with chronic periodontitis. On the other hand, the author identified that several factors as lack of controls, non-calibrated examiners, masked reference standards and small sample sizes, among others, reduced the observational quality of relevant studies. As a consequence, Mariotti19 (2006) stated that the overall conclusion of his review must be carefully considered.
Some authors have measured the number and diameter of dentin tubules exposed after root conditioning in order to relate these parameters with potential for cell adhesion and for intertubular collagen exposure13,15. Labahn, et al.17 (1992) have found a time-dependent increase in the mean dentin tubule orifice diameter after treating dentin surfaces with citric acid or tetracycline HCl and Ruggeri, et al.25 (2007) stressed that the exposure of the dentin matrix of root surface allows the formation of a proper fibrin clot, which is a determinant factor for the positive outcome of the early wound healing events25. This would facilitate the integration between the root surface and the connective tissue favoring migration and attachment of gingival fibroblasts4,6,7,18,20.
There still is a remarkable controversy concerning to the type of chemical conditioner, time of its application and even the need of its use, which justifies the search for parameters that can support the option for this procedure in periodontal treatments. To the best of our knowledge, there is no standardized study comparing several chemical root conditioners for their ability of smear layer removing and dentin tubule widening. Therefore, the aim of this study was to contribute with reliable data to analyze and compare diseased dental root surfaces treated by manual scaling followed by conditioning with four commonly used demineralizing agents.
MATERIAL AND METHODS
Specimen preparation
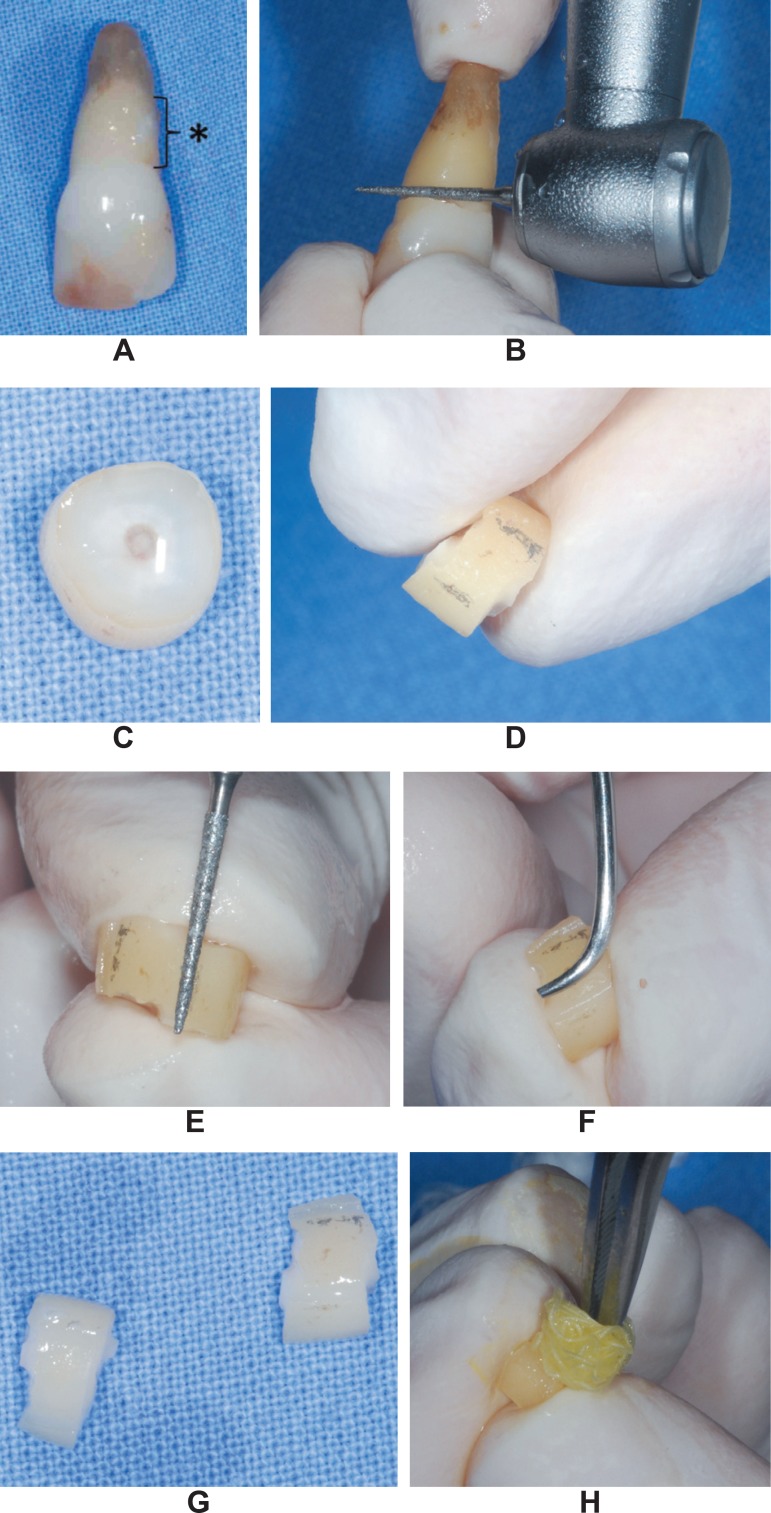
Twenty-five human single-rooted teeth scheduled for extraction due to advanced periodontal disease at the Bauru School of Dentistry, University of São Paulo, Brazil, were selected for this study after signing an informed consent form. The selected teeth had to meet the following inclusion criteria: 1) no history of scaling and root planing in the previous 6 months; 2) proximal attachment loss of 5 mm or more; 3) absence of decay lesions or restorations near the cementoenamel junction (CEJ). The freshly extracted teeth were cleaned from blood and other debris in saline solution and visible calculus was gently removed using manual scalers (Figure 1A). The teeth were stored in formalin at 10% for no more than 7 days. The crowns were removed by transversal sectioning at the CEJ with a water cooled high speed bur (Figure 1B). The diseased parts of the roots were visually identified with the aid of a magnifying glass (Lactona - 4× of magnification; Lactona, São Paulo, SP, Brazil) as the area showing absence of remnants of the periodontal ligament (Figure 1A). Then, each root received a second section made 2 mm away from the first in the apical direction, resulting in radicular dishes (Figure 1C). On the mesial and distal surfaces of the dishes, 2 grooves separated by a distance of 4 mm were made with the same previously used bur, determining an area measuring 2 mm x 4 mm (Figures 1D and 1E) approximately. This area was scaled by the same operator with 20 strokes24 of Gracey curettes (Hu-Friedy; Hu-Friedy do Brasil, Rio de Janeiro, RJ, Brazil) (Figure 1F) and then, the mesial and distal halves of the dishes were sectioned, producing 50 radicular fragments with an scaled area delimited by the grooves in which demineralizing agents were tested (Figure 1G). The radicular fragments were randomly divided into 5 groups of 10 fragments each according to the treatment given to the scaled surface as follows: 1) CA: demineralization with a liquid solution of citric acid at 50% and pH1 (Pharmácia Specífica; Farmácias e Drogarias, Bauru, SP, Brazil) for 3 min; 2) TC-HCl: demineralization with a liquid solution of tetracycline hydrochloride at 50 mg/mL (Laboratório Teuto, Anápolis, GO, Brazil) for 3 min; 3) EDTA: demineralization by a gel of EDTA at 24% (PrefGel; BIORA AB, Malmö, Sweden) for 3 min; 4) PA: demineralization with a liquid solution of 37% phosphoric acid (Pharmácia Specífica; Farmácias e Drogarias) for 3 min; 5) Control: treatment with saline solution for 3 min. All the agents were applied to the roots surfaces by burnishing them with a sterile cotton pellet changed every 30 s (Figure 1H); the surfaces were then flushed profusely with distilled water. After the treatments, the dental fragments were immediately processed for analysis by conventional scanning electron microscopy (SEM).
Figure 1.
Specimen preparation. A: extracted teeth in which the diseased part of the root was identified by the absence of periodontal ligament remnants (*); B: tooth crown being transversely cut at the cementoenamel with a water-cooled high-speed bur; C: radicular dish obtained by sectioning the root 2 mm away from the first cut in the apical direction; D: pencil marks made 4 mm apart from each other on the mesial and distal surfaces of the dishes where grooves were made (E), delimiting an area of 2 mm x 4 mm approximately; F: scaling of the area determined in E; G: mesial and distal halves of the dishes separated before receiving the burnishing of the demineralizing agents with a sterile cotton pellet (H)
SEM analysis
The dental fragments were prepared for SEM analysis as described by Braidotti, et al.8 (2000) and observed at a JSM-5600 LV scanning electron microscope (JOEL, Tokyo, Japan). Digital images were taken at 1,000× and 2,000× magnification and at zero tilt angle.
Quantitative and Qualitative Measurements
The SEM micrographs were transferred to a computer and analyzed by Image J software (available from http://rbs.info.nih.gov/ij/). The core of the groove-delimited area on each specimen was chosen for analysis. The roots surfaces were examined for general morphologic characteristics and for the presence of smear layer at magnification of 1,000×. The field shown at magnification of 1,000× was also taken as reference for the area in which the number of exposed dentin tubules was counted. The field shown at magnification of 2,000× was taken as reference for the total area (266, 240 µm2) in which the percentage of area occupied by the enlarged dentin tubules was calculated. All the morphometric measurements were performed by a single investigator who was unaware of the origin of the specimens. This investigator was calibrated with repeated measurements until a 90% level of reproducibility was attained.
The data were analyzed statistically by one-way ANOVA, Kruskal-Wallis and Dunn post-tests at significance level of 0.05.
RESULTS
Group CA
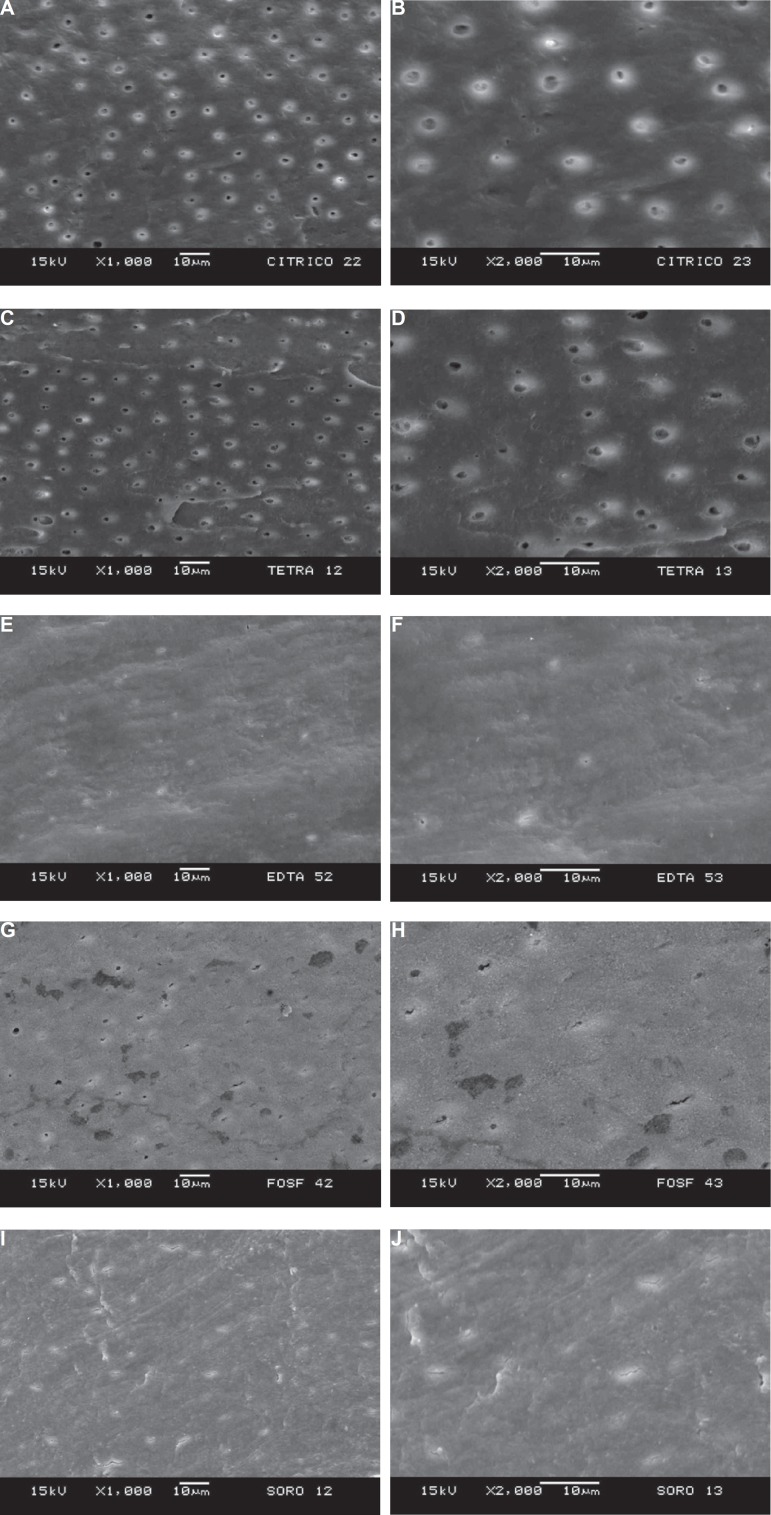
None of the specimens of CA group showed smear layer. Debris or other particles were not observed on the examined surfaces (Figure 2A). Dentin tubules were totally exposed at the 1,000× field and the average percentage of the area occupied by tubules was 0.12% (ranging from 8.67 µm2 to 159 µm2 at the magnification of 2,000× (Figure 2B).
Figure 2.
Panel of scanning electron microscopy (SEM) micrographs. A: representative specimen of the Ca group in which no smear layer can be noted and dentin tubules are totally exposed (1,000×); B: 2,000× magnification of the central part of A showing dentin tubules widened; C: representative specimen of the TC-HCl group at 1,000× showing absence of smear layer and exposed dentin tubules; D: 2,000× magnification of the central part of C showing widened dentin tubules; E: representative specimen of EDTA group presenting smear layer and few exposed tubules (1,000×); F: 2,000× magnification of the central part of E; G: representative specimen of PA group at 1,000× of magnification covered by smear layer and some exposed tubules; H: 2,000× magnification of the central part of G showing discrete widening of the tubules; I: representative specimen of the control group at 1,000× showing smear layer without evident tubule exposure; J: 2,000× magnification of the central part of I in which the tubules are not enlarged enough to measure their corresponding area
Group TC-HCl
Even though all the tubules were exposed in 8 specimens of the TC-HCl group at the 1,000× field (Figure 2C), residual smear layer and some debris were present in 33.3% of them. Two specimens did not show any exposed tubule. The mean percentage of area occupied by exposed tubules was 0.08% (ranging from 0 µm2 to 48.18 µm2) at the magnification of 2,000× (Figure 2D).
Group EDTA
The majority of the specimens of EDTA group presented with smear layer (80%) and there were no exposed tubules 5 of them. The mean number of exposed tubules in this group was 4.4 at the 1,000× field (Figure 2E), occupying an area equivalent to 0.01% of the total (ranging from 0 µm2 to 5.82 µm2) at the 2,000× of magnification (Figure 2F).
Group PA
Smear layer was seen in all specimens of this group. All of them also showed a foamy surface (Figure 2G) probably due to the precipitation of an insoluble calcium phosphate layer. The mean number of exposed tubules at 1,000× of magnification was 12.1 (Figure 2G) and these tubules accounted for a mean area of 0.03% (ranging from 0 µm2 to 46.25 µm2) at 2,000× of magnification (Figure 2H).
Control group
Although all specimens of this group showed smear layer, open tubules could be seen in 2 of them which were responsible for the mean number of 2.3 tubules for this group at the 1,000× magnification field (Figure 2I). Nevertheless, most of them were not enlarged enough to measure their corresponding area and the mean value for this group was 0% of the total area at 2,000× of magnification (Figure 2J).
The mean number of exposed dentin tubules by the different treatments decreased according to the following order: TC-HCl>CA>PA>EDTA>Control. Nevertheless, statistically significant differences were only found when compared the groups TC-HCl, and C; TC-HCl and EDTA; CA and C and CA and EDTA (Table 1).
Table 1.
Mean number of exposed dentin tubules and corresponding area after conditioning with the four different demineralizing agents
| Citric acid | Tetracycline-HCl | Phosphoric acid | EDTA | Control group (saline) | |
|---|---|---|---|---|---|
| Mean number of exposed tubules | 39.3±37.0A | 43.8±25.2A | 12.1±16.3Aa | 4.4±7.5Ba | 2.3±5.7Ba |
| Mean area occupied by enlarged tubules (%) | 0.12±0.17A | 0.08±0.06Aa | 0.03±0.05Ba | 0.01±0.01Ba | 0.00±0.00B |
Same uppercase or lowercase letter in the same line means no statistically significant difference (p>0.05).
When comparing the area occupied by dentin tubule openings, CA produced the greatest tubule widening followed by TC-HCl, PA, EDTA and saline (Table 1). There was no statistically significant difference between CA and TC-HCl groups, and CA was different from all other groups. TC-HCl group differed only from group C. The exposure of dentin tubules in group C did not result in widening.
DISCUSSION
This study compared the 4 most commonly used chemical agents for root conditioning as adjunctive therapy for teeth affected by periodontitis. The presented data suggest that citric acid and tetracycline-HCl, in this particular way of use, are more effective in removing smear layer and in exposing and widening dentin tubules than phosphoric acid and EDTA.
Since Register and Burdick23 (1975) compared root conditioning with citric acid (pH 1 for 2-3 min) and other chemical substances and found optimal cementogenesis and connective tissue new attachment, several investigators have devoted considerable time studying conditioning agents to improve periodontal regeneration. Unfortunately, numerous and often uncontrolled histological and clinical studies have created controversy and confusion about the positive or negative effects of those agents19. The inconsistency of these studies may be due to differences in experimental systems and techniques. Nevertheless, there is a common acceptance that it is not possible to decontaminate periodontitis-affected root surfaces by mechanical means alone. It is also well documented that hand or ultrasonic scaling of root surface produces a nonbiocompatible smear layer that must be removed to expose the underlying collagen in order to favor fibroblast migration, attachment, and orientation4,5,9,11,13,14,16-18,25,27.
Tetracycline became one of the most widely used and studied demineralizing agents since in vitro studies of Terranova, et al.29 (1986) suggested its potential usefulness in regenerative procedures. Many concentrations and application times were tested ranging from 0.5% to 200% and from 0.5 to 10 min16,30. Most of the studies, however, found the best results with concentrations between 50 mg/mL and 125 mg/mL during 3 to 4 min of application by burnishing technique. Isik, et al.16 (2000) concluded that concentrations between 50 mg/mL and 125 mg/mL might alter dentin surfaces by removing the smear layer and also maximize tubule openings in a short period if repeated applications are performed. Based on these studies, we adopted the concentration of 50 mg/mL for TC-HCl. It has also been shown that burnishing the demineralizing agent on the root surface results in efficient removal of smear layer and exposure of the underlying tubules due to demineralization action of fresh acid solution28. This was the rationale for the use of burnishing technique and for changing the cotton pellet at every 30 s in this study.
The low pH of the saturated solutions of citric acid and tetracycline-HCl were suggested as one of the reasons for the reduced cellular insertion and for the unpredictability of the results, once it could denature the organic matrix of dentin14. It was also suggested that acid etching would interfere on periodontal healing by its necrotizing effect on the surrounding progenitor cells13. Thus, EDTA at 12%-24%, neutral pH for 30 s to 3 min was introduced aiming at removing smear layer and widening dentin tubules without damaging biological structures7. Notwithstanding, Ruggeri, et al.25 (2007) presented reliable findings using monoclonal antibodies and field emission in-lens scanning electron microscopy (FEISEM) showing that both citric acid and EDTA treatments are able to etch and expose collagen fibrils and proteoglycans without any degradation of dentin collagen matrix. Our findings demonstrated that EDTA, in this way of use, failed to properly remove smear layer and expose dentin tubules. Five specimens (50%) from the EDTA group had none of their dentin tubules exposed and only 2 were totally clean from smear layer.
It must be emphasized that even though CA and TC-HCl did not differ significantly on tubule number and widening evaluations, TC-HCl could not be considered different from the other groups. This result was the responsible for classifying TC-HCl in second place in our analysis. Another interesting finding was that CA and PA exposed similar numbers of dentin tubules, but CA produced greater enlargement as reflected by the area measurements. EDTA and PA had the same behavior as saline solution on both evaluations, suggesting that these agents failed on efficiently conditioning the roots surfaces. As EDTA showed lower numerical results, it was classified in fourth place.
All specimens from the PA group showed a foamy surface, probably due to a chemical acid/basic reaction between a strong acid and the hydroxyapatite, leading to calcium phosphate deposition on the roots surfaces. This occurrence can be attributed to the extended time of acid contact with the roots (3 min). It may produce an insoluble form of that salt, which precipitates, blocking tubule openings and annulling its demineralizing effect10,21. As the superficial composition of these deposits was not characterized in this study, this interpretation is speculative. However, it is suggestive that phosphoric acid is not appropriate for root conditioning in this particular way of using.
Relevant findings from other researchers have reinforced that acid etching plays a decisive role in the establishment of new connective tissue attachment influencing the early healing events, i.e., adsorption and adhesion of blood elements and fibrin to the root surface3. In this aspect, Baker, et al.3 (2005) have clearly demonstrated the superiority of citric acid in comparison to EDTA when applied for 5 min on planed dentin root surfaces. Fibrin clot adhesion was better supported by the CA-treated than EDTA-treated dentin surfaces and forces produced by three 5-min rinses in PBS under agitation on a rotary shaker table partially removed the fibrin clot from EDTA-treated surfaces but not from CA-treated surfaces. The authors stressed that a fibrin network was firmly attached directly on dentin surface were the tubules were exposed and widened by the citric acid treated fragments. These findings led us to believe that the more exposed and widened the dentin tubules are, the more retained the fibrin clot will be on the root surface, perhaps within limits still to be determined. Citric acid and tetracycline behaved very similarly in this particular aspect, suggesting that both can be equally effective as conditioning agents.
The number and diameter of exposed dentin tubules in laser-conditioned roots were calculated in SEM micrographs by Herrero, et al.15 (2009) who related increased numbers and diameters to better root conditioning, once widening tubules openings causes higher exposure of the underlying dentin favoring connective tissue attachment. Gamal and Mailhot12 (2003) also observed that periodontal ligament fibroblasts adhere and differentiate on EDTA-conditioned roots surfaces that were free from smear layer and presented exposed round to oval dentin tubule orifices. Nevertheless, there is no study comparing the number of exposed dentin tubules and their corresponding area after conditioning with several conditioners for comparison with our data.
The findings of this study corroborate previous data stressing the advantages of using conditioning agents on diseased root surfaces and provide additional relevant information when choosing an agent for root conditioning. Before extrapolation of data to the clinical conditions can be done, the correspondence between capacity for tubule exposure/widening and intensity of connective tissue attachment should be further investigated in in vivo surveys.
CONCLUSION
The comparison among four of the most frequently used chemical root conditioners according to their efficiency on smear layer removal and dentin tubule widening showed that citric acid was the most effective followed by tetracycline-HCl, phosphoric acid and EDTA. This information can be of value as an extra parameter for choosing one of them for root conditioning.
ACKNOWLEDGMENTS
We would like to thank Professor José Roberto Pereira Lauris, from Bauru School of Dentistry, University of São Paulo, for the statistical analysis, Mr. Adriano Luis Martins from Laboratory of Electron Microscopy of the Faculty of Dentistry of Piracicaba (UNICAMP) for helping us with SEM analysis and the National Council for Scientific and Technological Development (CNPq) for providing financial support to this research.
REFERENCES
- 1.Adriaens PA, Adriaens LM. Effects of nonsurgical periodontal therapy on hard and soft tissues. Periodontol 2000. 2004;36:121–145. doi: 10.1111/j.1600-0757.2004.03676.x. [DOI] [PubMed] [Google Scholar]
- 2.Babay N. Attachment of human gingival fibroblasts to periodontally involved root surface following scaling and/or etching procedures: a scanning electron microscopy study. Braz Dent J. 2001;12:17–21. [PubMed] [Google Scholar]
- 3.Baker DL, Stanley Pavlow SA, Wikesjö UME. Fibrin clot adhesion to dentin conditioned with protein constructs: an in vitro proof-ofprinciple study. J Clin Periodontol. 2005;32:561–566. doi: 10.1111/j.1600-051X.2005.00714.x. [DOI] [PubMed] [Google Scholar]
- 4.Blomlöf JP, Blomlöf LB, Lindskog SF. Smear removal and collagen exposure after non-surgical root planning followed by etching with an EDTA gel preparation. J Periodontol. 1996;67:841–845. doi: 10.1902/jop.1996.67.9.841. [DOI] [PubMed] [Google Scholar]
- 5.Blomlöf JP, Lindskog S. Periodontal tissue-vitality after different etching modalities. J Clin Periodontol. 1995;22:464–468. [PubMed] [Google Scholar]
- 6.Blomlöf JP, Lindskog S. Root surface texture and early cell and tissue colonization after different etching modalities. Eur J Oral Sci. 1995;103:17–24. doi: 10.1111/j.1600-0722.1995.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 7.Bogle G, Garrett S, Crigger M, Egelberg J. New connective tissue attachment in beagles with advanced natural periodontitis. J Periodontal Res. 1983;18:220–228. doi: 10.1111/j.1600-0765.1983.tb00355.x. [DOI] [PubMed] [Google Scholar]
- 8.Braidotti P, Bemporad E, D'Alessio T, Sciuto SA, Stagni L. Tensile experiments and SEM fractography on bovine subchondral bone. J Biomech. 2000;33:1153–1157. doi: 10.1016/s0021-9290(00)00074-9. [DOI] [PubMed] [Google Scholar]
- 9.Crigger M, Renvert S, Bogle G. The effect of topical citric acid application of surgically exposed periodontal attachment. J Periodont Res. 1983;18:303–305. doi: 10.1111/j.1600-0765.1983.tb00363.x. [DOI] [PubMed] [Google Scholar]
- 10.Di Renzo M, Ellis TH, Sacher E, Stangel I. A photoacoustic FTIRS study of the chemical modifications of human dentin surfaces: I. Demineralization. Biomaterials. 2001;22:787–792. doi: 10.1016/s0142-9612(00)00240-4. [DOI] [PubMed] [Google Scholar]
- 11.Fardal O, Lowenberg BF. A quantitative analysis of the migration, attachment, and orientation of human gingival fibroblasts to human dental root surfaces in vitro. J Periodontol. 1990;61:529–535. doi: 10.1902/jop.1990.61.8.529. [DOI] [PubMed] [Google Scholar]
- 12.Gamal AY, Mailhot JM. The effects of EDTA gel conditioning exposure time on periodontitis-affected human root surfaces: surface topography and PDL cell adhesion. J Int Acad Periodontol. 2003;5:11–22. [PubMed] [Google Scholar]
- 13.Gamal AY, Mailhot JM, Garnick JJ, Newhouse R, Sharawy MM. Human periodontal ligament fibroblast response to PDGF-BB and IGF-1 application on tetracycline HCI conditioned root surfaces. J Clin Periodontol. 1998;25:404–412. doi: 10.1111/j.1600-051x.1998.tb02463.x. [DOI] [PubMed] [Google Scholar]
- 14.Hanes P, Polson A, Frederick T. Citric acid treatment of periodontitis-affected cementum. A scanning electron microscopic study. J Clin Periodontol. 1991;18:567–575. doi: 10.1111/j.1600-051x.1991.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 15.Herrero A, García-Kass AI, Gómez C, Sanz M, García-Nuñez JA. Effect of two kinds of Er:YAG laser systems on root surface in comparison to ultrasonic scaling: an in vitro study. Photomed Laser Surg. 2010;28:497–504. doi: 10.1089/pho.2009.2527. [DOI] [PubMed] [Google Scholar]
- 16.Isik AG, Tarim B, Hafez AA, Yalçin FS, Onan U, Cox CF. A comparative scanning electron microscopic study on the characteristics of demineralized dentin root surface using different tetracycline HCl concentrations and application times. J Periodontol. 2000;71:219–225. doi: 10.1902/jop.2000.71.2.219. [DOI] [PubMed] [Google Scholar]
- 17.Labahn R, Fahrenbach WH, Clark SM, Lie T, Adams DF. Root dentin morphology after different modes of citric acid and tetracycline hydrochloride conditioning. J Periodontol. 1992;63:303–309. doi: 10.1902/jop.1992.63.4.303. [DOI] [PubMed] [Google Scholar]
- 18.Lasho DJ, O'Leary TJ, Kafrawy AH. A scanning electron microscope study of the effects of various agents on instrumented periodontally involved root surfaces. J Periodontol. 1983;54:210–220. doi: 10.1902/jop.1983.54.4.210. [DOI] [PubMed] [Google Scholar]
- 19.Mariotti A. Efficacy of chemical root surface modifiers in the treatment of periodontal disease. A systematic review. Ann Periodontol. 2006;8:205–226. doi: 10.1902/annals.2003.8.1.205. [DOI] [PubMed] [Google Scholar]
- 20.Metzerg Z, Weinstock B, Dotan M, Narayanan AS, Pitaru S. Differential chemotactic effect of cementum attachment protein on periodontal cells. J Periodontal Res. 1998;33:126–129. doi: 10.1111/j.1600-0765.1998.tb02301.x. [DOI] [PubMed] [Google Scholar]
- 21.Misra DN. Interaction of citric acid with hydroxyapatite: surface exchange of ions and precipitation of calcium citrate. J Dent Res. 1996;75:1418–1425. doi: 10.1177/00220345960750061401. [DOI] [PubMed] [Google Scholar]
- 22.Polson A, Proye MP. Fibrin linkage: a precursor for new attachment. J Periodontol. 1983;54:141–147. doi: 10.1902/jop.1983.54.3.141. [DOI] [PubMed] [Google Scholar]
- 23.Register AA, Burdick FA. Accelerated reattachment with cementogenesis to dentin demineralized in situ. I. Optimun range. J Periodontol. 1975;46:646–655. doi: 10.1902/jop.1975.46.11.646. [DOI] [PubMed] [Google Scholar]
- 24.Rezende MLR, Campos A, Jr, Nahás D, Consolaro A, Araújo MG. Histologic response analysis for three types of mechanical radicular treatment. In: 68th General Session of the International Association for Dental Research. J Dent Res. 1990;70:641–641. [Google Scholar]
- 25.Ruggeri A, Prati C, Mazzoni A, Nucci C, Di Lenarda R, Mazzotti G, et al. Effects of citric acid and EDTA conditioning on exposed root dentin: an immunohistochemical analysis of collagen and proteoglycans. Arch Oral Biol. 2007;52:1–8. doi: 10.1016/j.archoralbio.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 26.Sampaio JEC, Rached RSGA, Pilatti GL, Theodoro LH, Batista LHC. Effectiveness of EDTA and EDTA-T brushing on the removal of root surface smear layer. Pesqui Odontol Bras. 2003;17:319–325. doi: 10.1590/s1517-74912003000400005. [DOI] [PubMed] [Google Scholar]
- 27.Selvig KA, Hals E. Periodontally diseased cementum studied by correlated microradiography, electron probe analysis and electron microscopy. J Periodontal Res. 1977;12:419–429. doi: 10.1111/j.1600-0765.1977.tb00137.x. [DOI] [PubMed] [Google Scholar]
- 28.Sterret JD, Murphy HJ. Citric acid burnishing of dentinal root surfaces. A scanning electron microscopy report. J Clin Periodontol. 1989;16:98–104. doi: 10.1111/j.1600-051x.1989.tb01621.x. [DOI] [PubMed] [Google Scholar]
- 29.Terranova VP, Franzetti LC, Hic S, DiFlorio RM, Lyall RM, Wikesjö UM, et al. A biochemical approach to periodontal regeneration: tetracycline treatment of dentin promotes fibroblast adhesion and growth. J Periodontal Res. 1986;21:330–337. doi: 10.1111/j.1600-0765.1986.tb01467.x. [DOI] [PubMed] [Google Scholar]
- 30.Wikesjö UM, Baker PJ, Christersson LA, Genco RJ, Lyall RM, Hic S, et al. A biochemical approach to periodontal regeneration: tetracycline treatment conditions dentin surfaces. J Periodontal Res. 1986;21:322–329. doi: 10.1111/j.1600-0765.1986.tb01466.x. [DOI] [PubMed] [Google Scholar]