Abstract
Objective
The objective of this study was to compare the antimicrobial effect of mouthwashes containing Calendula officinalis L., Camellia sinensis (L.) Kuntze and 0.12% chlorhexidine digluconate on the adherence of microorganisms to suture materials after extraction of unerupted third molars.
Material and Methods
Eighteen patients with unerupted maxillary third molars indicated for extraction were selected (n=6 per mouthwash). First, the patients were subjected to extraction of the left tooth and instructed not to use any type of antiseptic solution at the site of surgery (control group). After 15 days, the right tooth was extracted and the patients were instructed to use the Calendula officinalis, Camellia sinensis or chlorhexidine mouthwash during 1 week (experimental group). For each surgery, the sutures were removed on postoperative day 7 and placed in sterile phosphate-buffered saline. Next, serial dilutions were prepared and seeded onto different culture media for the growth of the following microorganisms: blood agar for total microorganism growth; Mitis Salivarius bacitracin sucrose agar for mutans group streptococci; mannitol agar for Staphylococcus spp.; MacConkey agar for enterobacteria and Pseudomonas spp., and Sabouraud dextrose agar containing chloramphenicol for Candida spp. The plates were incubated during 24-48 h at 37ºC for microorganism count (CFU/mL).
Results
The three mouthwashes tested reduced the number of microorganisms adhered to the sutures compared to the control group. However, significant differences between the control and experimental groups were only observed for the mouthwash containing 0.12% chlorhexidine digluconate.
Conclusions
Calendula officinalis L. and Camellia sinensis (L.) Kuntze presented antimicrobial activity against the adherence of microorganisms to sutures but were not as efficient as chlorhexidine digluconate.
Keywords: Calendula officinalis L, Camellia sinensis (L.) Kuntze, Chlorhexidine, Antimicrobial activity
INTRODUCTION
The sutures used in dentoalveolar surgery, such as extraction of unerupted teeth, represent a risk factor for the healing of surgical wounds because they are prone to the adherence of pathogenic bacteria. The accumulation of microorganisms on sutures may serve as a focus of odontogenic infections. These infections are caused by both aerobic and anaerobic bacteria, including species of the genera Fusobacterium, Peptostreptococcus, Prevotella, Porphyromonas, Streptococcus, and Bacteroides. Recent studies suggest that odontogenic infections and bacteremia develop at the time of suture removal and are a possible risk for bacterial endocarditis4,23.
The use of oral antiseptics after surgery is an efficient method for microbial reduction and the consequent prevention of infections10. Chlorhexidine gluconate is currently the safest and most efficient antimicrobial agent used for the reduction of microorganisms in the oral cavity13,14,19,21,22. However, chlorhexidine is associated with a number of adverse effects, such as the formation of stains on teeth and dentures, dysgeusia, parotid enlargement, and desquamation of the oral mucosa. These factors have encouraged the search for other antimicrobial agents14.
Within this line of research, the use of medicinal plants indicated for the treatment of infectious processes has been investigated based on the results of ethno-pharmacobotanical studies. The use of plants for the treatment of different diseases is known since ancient times. The oldest studies on medicine and medicinal plants arose in China and Egypt11. Medicinal plants are valuable natural herbal products frequently used for the treatment of various diseases5 and represent an important source of new biologically active compounds11,24. The antimicrobial compounds found in plants inhibit the growth of bacteria and fungi by mechanisms that differ from those underlying the activity of commonly used antimicrobial agents and have a significant clinical value7,12,30.
Calendula officinalis L. is an annual plant of the family Asteraceae, which flourishes between May and October. Its flowers are used for medicinal preparations. This plant is native to Central Europe and the Mediterranean and grows naturally at sunny locations throughout North America and Europe27. The following chemical components are found in this species: sesquiterpenes, flavonoid glycosides, triterpene saponins, triterpene alcohols, flavonoids, carotenoids, xanthophylls, phenolic acids, steroids, mucilage, tocopherol, and calenduline28. The extract produced from C. officinalis has been widely used in Europe since the 12th century as a topical antiinflammatory agent. In vivo studies employing mouthwashes containing C. officinalis demonstrated the efficacy of this plant in the reduction of gingival bleeding18. According to Iauk, et al.15 (2003), C. officinalis also presents antimicrobial activity against periodontopathogenic bacteria.
Green tea is produced from the leaves of Camellia sinensis (L.) Kuntze (family Theaceae), and has been popularly used in Japan and China for many centuries3. Animal studies have shown that extracts of C. sinensis possess antimicrobial activity against Streptococcus mutans22. In addition, in vitro studies have demonstrated a strong antimicrobial action of C. sinensis against periodontal pathogens such as Porphyromonas gingivalis and Fusobacterium nucleatum29.
Several studies have tested the use of medicinal plants in mouthwashes. Groppo, et al.14 (2002) compared the effects of 0.12% chlorhexidine with mouthwashes containing Allium sativum L. and Melaleuca alternifolia Cheel. Chlorhexidine and Allium sativum L. showed antimicrobial activity against S. mutans, but not against other oral microorganisms seeded onto blood agar. In contrast, Melaleuca alternifolia Cheel presented strong antimicrobial activity against all oral microorganisms studied. Lauten, et al.18 (2005) tested a mouthwash containing essential oils and extracts from four plant species [Melaleuca alternifolia Cheel, Leptospermum scoparium Forst., Calendula officinalis L. and Camellia sinensis (L.) Kuntze] in 17 patients. No differences in plaque index or gingival bleeding index were observed between the mouthwash containing medicinal plants and the placebo group (mouthwash containing 12.8% ethanol in water).
The objective of the present study was to compare the effects of mouthwashes containing Calendula officinalis L. (Asteraceae), Camellia sinensis (L.) Kuntze (Theaceae) and 0.12% chlorhexidine digluconate on the adherence of microorganisms to suture materials after extraction of unerupted third molars.
MATERIAL AND METHODS
This study was conducted according to the guidelines for research involving humans and was approved by the Ethics Committee of the São José dos Campos Dental School (FOSJC), São Paulo State University (UNESP) (protocol 062/2007 - PH/CEP).
Preparation of the mouthwashes
Three mouthwashes were prepared for this study:
Oral solution of 0.12% chlorhexidine digluconate (Becker, São José dos Campos, SP, Brazil).
A 1% Calendula officinalis L. tincture mixed with mouthwash containing Hamposyl L®, aspartame, glycerine, and Nipagim® (Becker).
Mouthwash containing 25% (w/v) of a water-ethanol fluid extract (1:1) obtained from the leaves of Camellia sinensis (L.) Kuntze (5 g), glycerine (4 g), 70% sorbitol (3.5 g), sodium saccharin (0.5 g), sodium lauryl sulfate (0.6 g), lauryl polyglucoside (1 g), disodium phosphate (0.3 g), and distilled water (85.1g). Before preparation of the mouthwash, the alcohol of the fluid extract was completely evaporated. The mouthwash was prepared at the Laboratory of Pharmacognosy and Medicinal Plants (LAFAPLAM), Faculty of Pindamonhangaba (FAPI).
Patient selection
The patients were selected at the Clinic of Oral and Maxillofacial Surgery and Traumatology, FOSJC, UNESP. The patients were submitted to clinical and radiographic evaluation in order to exclude those who presented any pathological or infectious process. Eighteen patients ranging in age from 17 to 30 years with unerupted maxillary third molars indicated for extraction were selected. These patients presented good health conditions and satisfactory oral hygiene and were not using medications or any oral antiseptic solution. The 18 patients were randomly divided into 3 groups as shown in Figure 1.
Figure 1.
Distribution of the patients according to experimental group
| Number of patients | Extraction of unerupted third molar | |
|---|---|---|
| Left | Right | |
| 6 | Control | Chlorhexidine |
| 6 | Control | Calendula officinalis |
| 6 | Control | Camellia sinensis |
Surgery for unerupted maxillary third molar extraction
A panoramic radiograph was obtained from the patients before surgery for planning of the intervention. Tooth extraction was performed according to the following standardized surgical technique: first, post-tuberosity regional anesthesia of the greater palatine nerve was performed, complemented by infiltration of the anesthetic into the bottom of the buccal sulcus at the height of the first molar and on the palatine side of the gingival mucosa close to the tooth to be extracted. Next, an incision was made in the gingival mucosa of the alveolar bone crest from the curvature of the tuberosity to the mid-portion of the distal side of the second molar, complemented by an oblique incision in the direction of the bottom of the sulcus involving the distal side of the interdental papilla of the first molar. A mucoperiosteal flap was elevated and the tooth to be extracted was localized, followed by osteotomy and tooth sectioning. Extraction was performed with a Seldin and Potts elevator. The surgical pocket was irrigated with physiological saline. The wound was closed with interrupted 3-0 silk sutures (Ethicon, Johnson & Johnson, São José dos Campos, SP, Brazil) and the flap was repositioned in its original location.
The patients received 500 mg amoxicillin (Aché, São Paulo, SP, Brazil) and 50 mg sodium diclofenac (Novartis, São Paulo, SP, Brazil), and were instructed to brush their teeth using toothpaste only in the non-operated areas.
Control group
After the first surgery, i.e., extraction of the left tooth, all patients were instructed not to use any type of oral antiseptic solution.
Experimental groups
Fifteen days after the first surgery, the contralateral third molar was extracted following the same procedures as described above. However, the patients were instructed to use a mouthwash as shown in Table 1.
Table 1.
Mean number and standard deviation of colony-forming units (CFU)/mL (log10) of microorganisms isolated from suture materials and grown on Mitis Salivarius bacitracin sucrose agar obtained for the control groups and groups using Camellia sinensis, Calendula officinalis and chlorhexidine mouthwash
| Groups | Mouthwash | Control | p |
|---|---|---|---|
| Camellia sinensis | 1.49±1.57 | 2.19±1.36 | 0.098 |
| Calendula officinalis | 1.83±0.74 | 2.60±0.41 | 0.054 |
| Chlorhexidine | 2.10±1.17 | 3.68±0.63 | 0.081 |
The mouthwash was provided in an individual package (250 mL) accompanied by a measuring cup. The patients were asked to rinse their mouth with 15 mL of the product to be tested for 30 s according to the method of Metin, et al.20 (2006), twice a day (morning and night) after toothbrushing for 7 days.
Suture removal
The sutures were removed on postoperative day 7. After removal, one of the suture lines was standardized into a size of 15 mm using a sterile endodontic millimeter ruler. The suture line was transferred aseptically to a test tube containing 2 mL sterile 0.1 M phosphate-buffered saline (0.9% NaCl), pH 7.2. The samples were sent to the Laboratory of Microbiology, FOSJC, UNESP, for microbiological analysis.
Microbiological analysis
The test tube containing the suture was vortexed for 2 min (Fanem, São Paulo, SP, Brazil) in order to obtain a homogenous suspension. Decimal dilutions (10-1, 10-2 and 10-3) were then prepared from this suspension in sterile saline. Aliquots of 0.1 mL of the stock solution and of the dilutions were seeded in duplicate onto Petri dishes containing the following culture media: blood agar prepared with brain heart infusion agar (Difco, Detroit, MI, USA) supplemented with 5% sheep blood for total count of aerobic and facultative anaerobic microorganisms; Mitis Salivarius bacitracin sucrose (MSBS) agar prepared with Mitis Salivarius agar (Difco) supplemented with 0.2 IU/mL bacitracin and 15% sucrose for the growth of mutans group streptococci; mannitol agar (Difco) for the growth of Staphylococcus spp.; MacConkey agar (Difco) for the growth of enterobacteria and Pseudomonas spp., and Sabouraud dextrose agar (Difco) containing 0.1 mg/mL chloramphenicol (União Química, São Paulo, SP, Brazil) for the growth of Candida spp.
The blood agar, mannitol agar, MacConkey agar and Sabouraud agar plates were incubated at 37ºC for 24-48 h. The MSBS agar plates were incubated at 37ºC for 48-72 h in a 5% CO2 atmosphere. After the time of incubation, plates containing 30 to 300 colonies were counted and the number of colony-forming units per milliliter (CFU/mL) obtained was log transformed (log10).
Statistical analysis
The results were analyzed statistically by the paired Student's t-test using the Minitab software (Minitab, Inc., State College, PA, USA), with the level of significance set at 5%.
RESULTS
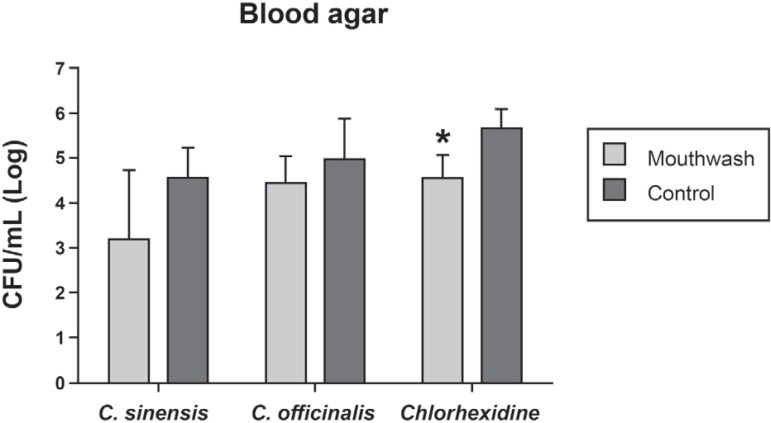
The mean number of CFU/mL (log10) of aerobic and facultative anaerobic microorganisms grown on blood agar obtained for the control and experimental groups is shown in Figure 2. The 3 mouthwashes caused microbial reduction when compared to the control group. However, this reduction was only significant for sutures from the groups using 0.12% chlorhexidine digluconate (p=0.008).
Figure 2.
Mean number and standard deviation of colony-forming units (CFU)/mL (log10) of microorganisms isolated from suture materials and grown on blood agar obtained for the control groups and groups using Camellia sinensis, Calendula officinalis and chlorhexidine mouthwash *Statistically significant difference between the mouthwash and control groups of cholorhexidine (Student's t-test)
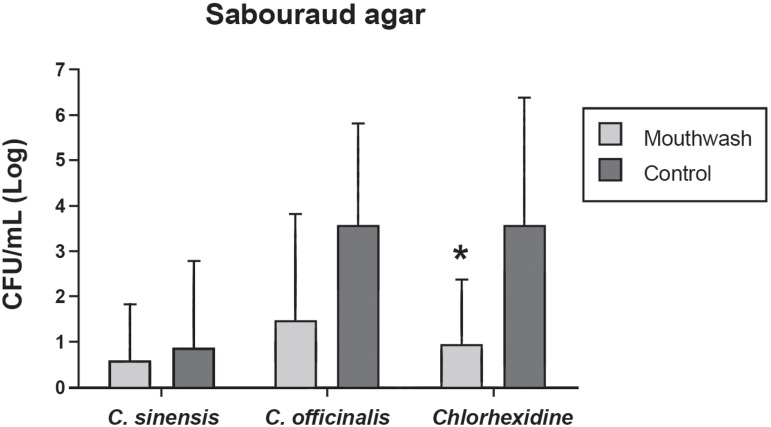
The 3 mouthwashes also reduced the growth of Candida spp. on Sabouraud agar (Figure 3). However, statistically significant difference in mean CFU/mL (log10) was only observed between the control and chlorhexidine groups (p=0.048).
Figure 3.
Mean number and standard deviation of colony-forming units (CFU)/mL (log10) of microorganisms isolated from suture materials and grown on Sabouraud agar obtained for the control groups and groups using Camellia sinensis, Calendula officinalis and chlorhexidine mouthwash *Statistically significant difference between the mouthwash and control groups of cholorhexidine (Student's t-test)
Tables 1-3 show the mean number of CFU/mL (log10) of microorganisms grown on MSBS, Mannitol and MacConkey agar obtained for the control and experimental groups using mouthwash containing 25% C. sinensis fluid extract, 1% C. officinalis tincture and 0.12% chlorhexidine digluconate. No significant differences in microbial reduction were observed between the control groups and the groups using either mouthwash.
Table 3.
Mean number and standard deviation of colony-forming units (CFU)/mL (log10) of microorganisms isolated from suture materials and grown on MacConkey agar obtained for the control groups and groups using Camellia sinensis, Calendula officinalis and chlorhexidine mouthwash
| Groups | Mouthwash | Control | p |
|---|---|---|---|
| Camellia sinensis | 0.96±1.53 | 1.50±1.68 | 0.343 |
| Calendula officinalis | 0.12±0.29 | 0.22±0.53 | 0.363 |
| Chlorhexidine | 0.12±0.29 | 0.48±0.76 | 0.177 |
DISCUSSION
Microorganisms of the oral microbiota can adhere to suture materials, a fact favoring their passage into the surgical wound and causing odontogenic infections and bacteremia. This situation is favored or prevented, depending on the adsorption properties of the suture material and oral hygiene care during the postoperative period23.
The results of the present study demonstrated the adherence of a large number of microorganisms to the suture material after third molar extraction. According to Banche, et al.2 (2001), contamination of suture material in the oral cavity mainly originates from the saliva which contains approximately 7.5x108 microorganisms/mL.
The largest mean number of CFU/mL (log10) of microorganisms adhered to the suture material was observed when the isolates were grown on blood agar, followed by MSBS, whereas smaller numbers of microorganisms grew on mannitol and MacConkey agar, which are selective for Staphylococcus and enterobacteria, respectively. These results agree with literature data reporting that staphylococci and enteric bacilli usually are not found in the oral cavity8,31; if present, they occur in smaller numbers and are part of the transitory microbiota.
The smallest number of microorganisms adhered to the suture material was observed when the isolates were grown on Sabouraud agar, which is selective for the growth of Candida spp. According to Appleton1 (2000), yeast of the genus Candida colonize the mucosal membranes of approximately 75% of the healthy population and are usually present in small numbers.
Thus, the use of an antiseptic for the control of microorganism adherence to suture material is an extremely important procedure, reducing the risks of transitory bacteremia, especially in patients with a serious predisposition to bacterial endocarditis and those with immunological dysfunction6. Several plant-derived mouthwashes are used for oral hygiene, but studies investigating the action of these phytotherapeutic agents on microbial adherence to suture materials are scarce.
In the present study, the 3 mouthwashes tested showed a tendency for microbial reduction, but significant differences were only observed for the mouthwash containing 0.12% chlorhexidine digluconate when the microorganisms were grown on blood and Sabouraud agar. These findings agree with the literature on the action of chlorhexidine on aerobic and anaerobic Gram-positive and Gram-negative bacteria and yeast. Chlorhexidine has high affinity for the cell wall of microorganisms and induces cell surface alterations that lead to the loss of osmotic balance and cytoplasmic precipitation9.
In the present study, although the antimicrobial activity of chlorhexidine was more efficient than that of the C. sinensis and C. officinalis mouthwashes, these plants also showed a tendency for reduction of the number of microorganisms. The antimicrobial activity of C. sinensis has been attributed to its main component, catechin, which acts directly on the bacterial plasma membrane, causing irreversible damage and bacterial death16. In addition to its antimicrobial activity, C. officinalis also possesses high topical antiinflammatory activity due to the presence of saponins and flavonoids in its phytochemical composition10. Zitterl-Eglseer, et al.33 (1997) observed that C. officinalis extracts exerted antiinflammatory activity similar to that of prostaglandin inhibitors.
Regarding the effects of mouthwashes on healing process, chlorhexidine, in a concentration-and time-dependent manner, affects negatively fibroblasts and keratinocyte cell proliferation in vitro and can impair wound healing17,32. Kozlovsky, et al.17 (2007) demonstrated that application of 0.1% and 0.2% chlorhexidine solution on an excisional wound in rats did not have a negative effect on the rate of wound closure. On the other hand, C. sinensis and C. officinalis showed potent wound healing activity. Qin, et al.26 (2010) verified that chitosan green tea polyphenol complex has enhanced the healing of incision wounds in rats by increasing the breaking strength of the wounds. Preethi and Kuttan25 (2009) evaluated the effects of C. officinalis flower extract on excision wounds made in rats and observed that the percentage of wound closure was 90.0% in the extract-treated group, whereas the control group showed only 51.1% on eighth day of wounding. The days needed for reepithelization were 17.7 for the control animals and 13.0 for the extract-treated group. These data suggest the use of mouthwashes containing C. sinensis and C. officinalis during the postoperative period after unerupted third molar extraction.
CONCLUSION
It may be concluded that antisepsis with mouthwashes containing Calendula officinalis L. and Camellia sinensis (L.) Kuntze showed a tendency for reducing the number of microorganisms adhered to suture materials after extraction of unerupted third molars. However, the antimicrobial activity of the plant extracts was not as efficient as that of chlorhexidine.
Table 2.
Mean number and standard deviation of colony-forming units (CFU)/mL (log10) of microorganisms isolated from suture materials and grown on Mannitol agar obtained for the control groups and groups using Camellia sinensis, Calendula officinalis and chlorhexidine mouthwash
| Groups | Mouthwash | Control | p |
|---|---|---|---|
| Camellia sinensis | 0.08±0.95 | 1.77±1.52 | 0.174 |
| Calendula officinalis | 0.17±0.41 | 0.23±0.57 | 0.363 |
| Chlorhexidine | 0.28±0.69 | 1.05±0.83 | 0.081 |
REFERENCES
- 1.Appleton SS. Candidiasis: pathogenesis, clinical characteristics and treatment. J Calif Dent Assoc. 2000;28:942–948. [PubMed] [Google Scholar]
- 2.Banche G, Roana J, Mandras N, Amasio M, Gallesio C, Allizond V, et al. Microbial adherence on various intraoral suture materials in patients undergoing dental surgery. J Oral Maxillofac Surg. 2001;65:1503–1507. doi: 10.1016/j.joms.2006.10.066. [DOI] [PubMed] [Google Scholar]
- 3.Bandyopadhyay D, Chatterjee TK, Dasgupta A, Lourduraja J, Dastidar SG. In vitro and in vivo antimicrobial action of tea: the commonest beverage of Asia. Biol Pharm Bull. 2005;28:2125–2127. doi: 10.1248/bpb.28.2125. [DOI] [PubMed] [Google Scholar]
- 4.Brown AR, Papasian CJ, Shultz P, Theisen FC, Shultz RE. Bacteremia and intraoral suture removal: can an antimicrobial rinse help? J Am Dent Assoc. 1998;129:1455–1461. doi: 10.14219/jada.archive.1998.0081. [DOI] [PubMed] [Google Scholar]
- 5.Chandrasekaran M, Venkatesalu V. Antibacterial and antifungal activity of Syzygium jambolanum seeds. J Ethnopharmacol. 2004;91:105–108. doi: 10.1016/j.jep.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Clark WB, Magnusson I, Walker CB, Marks RG. Efficacy of Perimed antibacterial system on established gingivitis. (I). Clinical results. J Clin Periodontol. 1989;16:630–635. doi: 10.1111/j.1600-051x.1989.tb01031.x. [DOI] [PubMed] [Google Scholar]
- 7.Cruz FG, Roque NF, Giesbrecht AM, Davino SC. Antibiotic activity of diterpenes from Mikania triangularis. Fitoterapia. 1996;67:189–190. [Google Scholar]
- 8.Dahlén G, Wikström M. Occurrence of enteric rods, staphylococci and Candida in subgingival samples. Oral Microbiol Immun. 2003;10:42–46. doi: 10.1111/j.1399-302x.1995.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 9.Delilbasi C, Saracoglu U, Keskin A. Effects of 0.2% chlorhexidine gluconate and amoxicillin plus clavulanic acid on the prevention of alveolar osteitis following mandibular third molar extractions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:301–304. doi: 10.1067/moe.2002.125200. [DOI] [PubMed] [Google Scholar]
- 10.Della Loggia R, Tubaro A, Sosa S, Becker H, Saar S, Isaac O. The role of triterpenoids in the topical anti-inflammatory activity of Calendula officinalis flowers. Planta Med. 1994;60:516–520. doi: 10.1055/s-2006-959562. [DOI] [PubMed] [Google Scholar]
- 11.Duarte MCT, Figueira GM, Sartoratto A, Rehder VLG, Delarmelina C. Anti-Candida activity of Brazilian medicinal plants. J Ethnopharmacol. 2005;97:305–311. doi: 10.1016/j.jep.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 12.Eloff JN. Which extractant should be used for the screening and isolation of antimicrobial components from plants? J Ethnopharmacol. 1988;60:1–8. doi: 10.1016/s0378-8741(97)00123-2. [DOI] [PubMed] [Google Scholar]
- 13.Franco CA, Neto, Parolo CCF, Rösing CK, Maltz M. Comparative analysis of the effect of two chlorhexidine mouthrinses on plaque accumulation and gingival bleeding. Braz Oral Res. 2008;22:139–144. doi: 10.1590/s1806-83242008000200008. [DOI] [PubMed] [Google Scholar]
- 14.Groppo FC, Ramacciato JC, Simões RP, Flório FM, Sartoratto A. Antimicrobial activity of garlic, tea tree oil, and chlorhexidine against oral microorganisms. Int Dent J. 2002;52:433–437. doi: 10.1111/j.1875-595x.2002.tb00638.x. [DOI] [PubMed] [Google Scholar]
- 15.Iauk L, Lo Bue AM, Milazzo I, Rapisarda A, Blandino G. Antibacterial activity of medicinal plant extracts against periodontopathic bacteria. Phytother Res. 2003;17:599–604. doi: 10.1002/ptr.1188. [DOI] [PubMed] [Google Scholar]
- 16.Ikigai H, Nakae T, Hara Y, Shimamura T. Bactericidal catechins damage the lipid bilayer. Biochim Biophys Acta. 1993;1147:132–136. doi: 10.1016/0005-2736(93)90323-r. [DOI] [PubMed] [Google Scholar]
- 17.Kozlovsky A, Artzi Z, Hirshberg A, Israeli-Tobias C, Reich L. Effect of local antimicrobial agents on excisional palatal wound healing: a clinical and histomorphometric study in rats. J Clin Periodontol. 2007;34:164–171. doi: 10.1111/j.1600-051X.2006.01033.x. [DOI] [PubMed] [Google Scholar]
- 18.Lauten JD, Boyd L, Hanson MB, Lillie D, Gullion C, Madden TE. A clinical study: Melaleuca, Manuka, Calendula and green tea mouth rinse. Phytother Res. 2005;19:951–957. doi: 10.1002/ptr.1763. [DOI] [PubMed] [Google Scholar]
- 19.Meiller TF, Kelley JI, Jabra-Rizk MA, DePaola LG, Baqui AAM, Baqui AAMA, et al. In vitro studies of the efficacy of antimicrobials against fungi. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;91:663–670. doi: 10.1067/moe.2001.113550. [DOI] [PubMed] [Google Scholar]
- 20.Metin M, Tek M, Sener I. Comparison of two chlorhexidine rinse protocols on the incidence of alveolar osteitis following the surgical removal of impacted third molars. J Contemp Dent Pract. 2006;7:79–86. [PubMed] [Google Scholar]
- 21.Nascimento AP, Tanomaru JMG, Matoba-Júnior F, Watanabe E, Tanomaru-Filho M, Ito IY. Maximum inhibitory dilution of mouthwashes containing chlorhexidine and polyhexamethylene biguanide against salivary Staphylococcus aureus. J Appl Oral Sci. 2008;16:336–339. doi: 10.1590/S1678-77572008000500006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Otake S, Makimura M, Kuroki T, Nishihara Y, Hirasawa M. Anticaries effects of polyphenolic compounds from Japanese green tea. Caries Res. 1991;25:438–443. doi: 10.1159/000261407. [DOI] [PubMed] [Google Scholar]
- 23.Otten JE, Wiedmann-Al-Ahmad M, Jahnke H, Pelz K. Bacterial colonization on different suture materials - a potential risk for intraoral dentoalveolar surgery. J Biomed Mater Res B Appl Biomater. 2005;74:627–635. doi: 10.1002/jbm.b.30250. [DOI] [PubMed] [Google Scholar]
- 24.Portillo A, Vila R, Freixa B, Adzet T, Cañigueral S. Antifungal activity of Paraguayan plants used in traditional medicine. J Ethnopharmacol. 2001;76:93–98. doi: 10.1016/s0378-8741(01)00214-8. [DOI] [PubMed] [Google Scholar]
- 25.Preethi KC, Kuttan R. Wound healing activity of flower extract of Calendula officinalis. J Basic Clin Physiol Pharmacol. 2009;20:73–79. doi: 10.1515/jbcpp.2009.20.1.73. [DOI] [PubMed] [Google Scholar]
- 26.Qin Y, Wang H, Karuppanapandian T, Kim W. Chitosan green tea polyphenol complex as a released control compound for wound healing. Chin J Traumatol. 2010;13:91–95. [PubMed] [Google Scholar]
- 27.Radulescu V, Doneanu C, Loloiu T. CGC investigation of chemical composition of Calendula officinalis. Revue Roumaine de Chimie. 2000;45:271–275. [Google Scholar]
- 28.Ramos A, Edreira A, Vizoso A, Betancourt J, López M, Décalo M. Genotoxicity of an extract of Calendula officinalis L. J Ethnopharmacol. 1988;61:49–55. doi: 10.1016/s0378-8741(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 29.Saeki Y, Ito Y, Shibata M, Sato Y, Takazoe I, Okuda K. Antimicrobial action of green tea extract, flavono flavor and copper chlorophyll against oral bacteria. Bull Tokyo Dent Coll. 1993;34:33–37. [PubMed] [Google Scholar]
- 30.Sartoratto A, Machado ALM, Delarmelina C, Figueira GM, Duarte MCT, Rehder VLG. Composition and antimicrobial activity of essential oils from aromatic plants used in Brazil. Braz J Microbiol. 2004;35:275–280. [Google Scholar]
- 31.Slots J, Ramss TE, Listgarten MA. Yeasts, enteric rods and pseudomonads in the subgingival flora of severe adult periodontitis. Oral Microbiol Immunol. 1988;3:47–52. doi: 10.1111/j.1399-302x.1988.tb00080.x. [DOI] [PubMed] [Google Scholar]
- 32.Thomas GW, Rael LT, Bar-Or R, Shimonkevitz R, Mains CW, Slone DS, et al. Mechanisms of delayed wound healing by commonly used antiseptics. J Trauma. 2009;66:82–90. doi: 10.1097/TA.0b013e31818b146d. [DOI] [PubMed] [Google Scholar]
- 33.Zitterl-Eglseer K, Sosa S, Jurenitsch J. Anti-oedematous activities of the main triterpendiol esters of marigold (Calendula officinalis L) J Ethnopharmacol. 1997;57:139–144. doi: 10.1016/s0378-8741(97)00061-5. [DOI] [PubMed] [Google Scholar]