Abstract
This investigation was designed to quantify the coordinative organization of mandibular muscles in toddlers during speech and nonspeech behaviors. Seven 15-month-olds were observed during spontaneous production of chewing, sucking, babbling, and speech. Comparison of mandibular coordination across these behaviors revealed that, even for children in the earliest stages of true word production, coordination was quite different from that observed for other behaviors. Production of true words was predominantly characterized by relatively stronger coupling among all mandibular muscles compared with earlier-emerging chewing and sucking. Variegated babbling exhibited stronger coupling than reduplicated babbling, as well as chewing and sucking. The finding of coupled activation among mandibular antagonists during speech paralleled earlier comparisons of adult speech and nonspeech behaviors (Moore, Smith, & Ringel, 1988) and did not support the suggestion that speech coordination emerges from earlier appearing oral motor behaviors.
Keywords: speech, development, motor control, mandible, human
A persistent question in our understanding of the coordinative framework of speech production is the relationship of speech to other oral motor behaviors (Moore, Smith, & Ringel, 1988; Ostry, Feltham, & Munhall, 1984; Ostry & Munhall, 1994). Although recent investigations have provided strong support for the existence of separate and distinct mechanisms for speech and nonspeech coordination in adults (Moore et al., 1988), empirical physiologic studies of speech development are lacking. This lack of evidence leaves in doubt the essential foundations of speech motor control.
Two lines of reasoning can be taken to address the coordinative framework of speech during the early stages of development. The first draws on mechanisms of pattern generation, which have been directly observed in animals (see Grillner, 1981), as well as dynamical systems theory (e.g., Fentress, 1976; Kent & Hodge, 1990; Thelen & Cooke, 1987; Thelen & Fisher, 1983; Thelen, Skala, & Kelso, 1987; Thelen & Urich, 1991). A dynamic pattern perspective might suggest that speech movements emerge gradually through an interaction of context (i.e., external conditions) with intrinsically generated patterns stemming from the rhythmic movements of sucking, chewing, reduplicated babbling, and variegated babbling.
An alternative approach holds that speech develops independent of extant behaviors, emerging as a new and unique motor skill. Support for this position is drawn directly from observations of babbling rhythmicity (see summary by Kent, Mitchell, & Sancier, 1991) and further relies on findings that the coordinative organization of mature speech is distinct from that of any of the postulated precursors (Moore et al., 1988; Ostry & Munhall, 1994). The established orofacial coordination available to children from these behaviors does not appear to be well suited to speech. For example, kinematic and positional control characterize speech coordination, whereas force generation is probably one of the primary goals of coordination for chewing.
These two approaches to speech development are not necessarily incompatible, as discontinuities in speech development (e.g., changes in degree of speech rhythmicity, growth in the repertoire of speech and nonspeech movements) can be modeled as expressions of different “attractor states” of the dynamical systems involved. In any case, the considerations raised by these two dominant approaches may aid us in organizing our initial efforts, which at this stage rely heavily on the intuitive appeal of contemporary hypotheses.
The notion of speech as a successor to these earlier appearing behaviors may appear to be the more economical and parsimonious. This model appeals to our recognition of the child’s capacity to exploit redundancies across behaviors and to adapt his or her repertoire of skills and abilities to new and changing behavioral demands. Speech and prespeech behaviors may emerge as interactions of internal control states created by these extant mechanisms (see Fentress, 1976) with gradually changing external conditions (e.g., morphologic growth, behavior goals, cognitive and social development). Moreover, the reliance of speech and nonspeech behaviors on the same neurophysiologic infrastructure (i.e., shared musculoskeletal systems and neural connectivity) leads us to posit an organizational hierarchy based on a common coordinative organization. Empirical support can be drawn from investigations such as those demonstrating the dependence of early stepping and coordinated locomotion on earlier appearing rhythmic leg movement precursors (see Thelen, 1995).
Another source of support for this conceptualization can be drawn from models of speech production that incorporate the function of central pattern generators in speech production (e.g., Grillner, 1981). The essential assumption of these models is that there exist small neuronal populations, possibly central pattern generators like those observed in many nonprimate preparations, capable of establishing or influencing the motor organization required by such complex, rhythmic behaviors as mastication, respiration, phonation, swallowing, and sucking. It is further assumed that the coordinative organization afforded by these neural circuits can be brought to bear during speech production. One very attractive conceptualization of early speech movements is as a series of vocalizations, perhaps involving the organization of the respiratory, articulatory, and phonatory systems, mediated by the periaqueductal gray (Larson, 1985), superimposed on the rhythmic opening and closing (i.e., valving) of the vocal tract (Kent et al, 1991), perhaps generated by a masticatory central pattern generator. The parallels of the vocant-closant-vocant phonetic structure of early speech (Kent & Bauer, 1985; Kent & Murray, 1982) with these comparatively well understood behaviors (e.g., Lund, 1991; Luschei, 1991; Luschei & Goldberg, 1981; Luschei & Goodwin, 1974; Møller, 1966) are inescapable and promote the representation of speech development as a process of refinement of these gestures. Davis and MacNeilage (1995) focus attention explicitly on mandibular movement noting that “close and open phases of the (babbling) cycle often may have no associated neuromuscular activity other than movement of the mandible” (p. 1200). This view of the importance of the relationship between speech and nonspeech behaviors has been embraced, whether explicitly or tacitly, by many clinicians who train prespeech behaviors such as chewing, sucking, and swallowing as fundamentals to speech development (e.g., Fawcus, 1969).
The essential coordinative elements of speech production by adults separate and distinguish it from nonspeech behaviors (e.g., mandibular coordination for chewing, voluntary tracking movements; Moore et al., 1988). This dissimilarity of these behaviors in adults entails at a minimum their eventual divergent development. A more parsimonious account for this eventuality might model speech as unique, emerging independently of extant oral motor behaviors. According to this view the coordinative frameworks of nonspeech behaviors contribute little toward meeting the priorities of speech. A number of investigations of adults producing speech and nonspeech movements support this approach with rather definite findings (Moore, 1993; Moore & Scudder, 1989; Moore et al., 1988; Ostry & Flanagan, 1989; Ostry & Munhall, 1994; Wohlert & Goffman, 1994).
Of course these two representations need not be seen as mutually exclusive, nor as exhaustive with respect to the reasonable frameworks that might be applied to speech development. Nevertheless, these constructs can serve to unify many of the diverse results and predictions surrounding speech production research. For example, an expectation arising from the dynamical pattern perspective is that developmental processes comprise sequential attractor states that may arise from closely related organizing principles, yet manifest very different surface observables. In other words, observed differences do not preclude developmental contingencies. Instead, this orientation promotes a theoretical and empirical focus on the transitions between developmental states (Thelen, 1991), the challenge being to identify these attractor states and the associated periods of transition between them. What is of interest in this type of model are the putative driving forces, internal (e.g., general neurophysiologic and cognitive growth) and external (e.g., linguistic and social contexts), that promote developmental changes in coordinative states (Kelso & Schöner, 1988).
Even under controlled laboratory conditions with cooperative subjects, the explicit nature of stable and unstable periods will be difficult to reveal. During early development, the methodologic challenges confronting this domain of inquiry are far from trivial. Very young children are difficult to study, which makes the choice of method even more difficult because practical considerations will take priority over theoretical ones. Empirical descriptions of speech development comparing periods of relative stability and instability are unavoidably influenced by the domain selected to be observed, whether it is physiologic, kinematic, acoustic, or linguistic. Ultimately a more complete understanding of the forces driving the emergence of speech will be obtained by systematic integration of the disparate contributions afforded by each of these domains.
The present investigation was designed to compare the coordinative organization of mandibular muscle activation during speech and nonspeech mandibular movements at a very early transitional period of speech development. Coordination of mandibular muscle activation was targeted for several reasons: the muscles of the mandible are very likely to be active during a wide variety of speech and nonspeech behaviors, the activity from these muscles can be recorded using noninvasive surface electrodes, and normative data from adults are available for comparison. Moreover, mandibular movement is primary to babbling (Davis & Mac-Neilage, 1995) and to those behaviors that might provide a coordinative organization for speech (e.g., mastication). Finally, rhythmicity, if it is to emerge as a characteristic of speech movements by young children, should be observable in the temporal features of jaw muscle activity.
Several additional assumptions guided the design of this investigation. Given the wide array and the potency of the potential neural inputs to mandibular motoneuron pools in children (i.e., the trigeminal motor nucleus; Smith, Weber, Newton, & Denny, 1991; Wood & Smith, 1992), it was essential that these behaviors be sampled over as much time and with as many repetitions as possible. Individual speech and nonspeech events are understood as being susceptible to varying contextual and sensorimotor effects, such that fundamental principles of motor organization can emerge only from multiple observations of the “same” event. Furthermore, it was assumed that the more general organizing principles of these behaviors can be extracted from, and defined by, the plasticity of coordinative relationships, which can be identified by variation in coupling of activity among biomechanically related muscles. Finally, we recognized that, even if it were practical in very young children, efforts to manipulate or control motor behaviors could obscure the naturally occurring (i.e., ecologically valid; Reed, 1982) behaviors we seek to observe. Accordingly the design of this investigation was guided by the assumption that initial attempts at description ought to minimize experimental manipulation, allowing the essential character of the phenomenon to reveal itself unencumbered by any a priori theoretical bias.
Method
Subjects
Subjects of this investigation included seven normally developing children, three females and four males, 15 months of age (± 12 days). Additional descriptors are included in Table 1. These children were serving in this experiment as part of an ongoing longitudinal study of speech development. Each child was free of any known neurologic deficit and passed otoscopic and tympanometric screening, which reduced the chance of including children with active middle ear pathology. An age-based subject-selection criterion was used, rather than age-equivalency indices based on lexical, phonological, or motor development. This criterion was the simplest compromise among competing indicators of physiologic, linguistic, anatomic, and cognitive growth, and was also adopted as the subject descriptor that would allow comparison with the largest part of the developmental literature. A home-based diary method provided an estimate of the size of each child’s lexicon, with six of the seven children shown to have acquired 10–35 real words. One child’s lexicon was well in excess of 100 words, although she produced no multiple word utterances. These estimates are included in Table 1. The children also produced reduplicated and variegated babbling sequences spontaneously, usually during self-directed play and object manipulation. The concurrent production of these nonspeech, prespeech, and speech behaviors by these children made them attractive candidates for exhibiting the contrasting coordinative organizations we were seeking.
TABLE 1.
Subject characteristics, including conditions for chewing and sucking behaviors.
| Subject number | Gender | Size of lexicon (No. of words) | Number of teeth | Food eaten | Sucking |
|---|---|---|---|---|---|
| 1 | F | >100 | 3 | pizza | straw |
| 2 | M | 10 | 6 | gummy bears | spout cup |
| 3 | M | 35 | 12 | cheese sandwich | straw |
| 4 | M | 10 | 3 | pretzels, raisins | feeding bottle |
| 5 | F | 10 | 9 | apple slices, cereal (Cheerios) | feeding bottle |
| 6 | F | 15 | 13 | apple slices, crackers | spout cup |
| 7 | M | 15 | 6 | apple slices, rice | feeding bottle |
Procedures
Each child was seen, accompanied by his or her mother, individually at a time near his or her usual lunch or snack time. Following a brief period of familiarization with the setting and the researchers involved, and application of surface electrodes, the child was seated in a highchair beside the mother, facing one of the researchers. Target behaviors were elicited from the child while continuous FM tape recordings were obtained. Target behaviors included sucking, chewing, reduplicated and variegated babbling, and production of real words. There was no attempt to control sampling order for these behaviors; rather, each child’s interest dictated the order in which target behaviors were observed.
Data selection and acquisition
Data channels included bipolar surface electromyographic (EMG) waveforms that targeted some of the primary muscles of mastication, including masseter, temporalis, and the anterior belly of the digastric. Because surface electrodes were used and the target muscles were comparatively small, it is possible that these EMG recordings were contaminated by other, nontarget muscles. Anatomic considerations would suggest less concern for the temporalis and masseter sites, which are large, superficial-layer muscles that are relatively distant from other jaw-moving muscles. The digastric site, approximately 1 cm posterior to the deep, midline surface of the mandible, may have been contaminated by activity in mylohyoid, another jaw-depressing muscle. Concern for this complication might be reduced by recognition of the fact that any jaw-depressing activity recorded at this site is treated equally in coordinative terms. For example, any activity at this site coincident with activity at the masseter or temporalis site was interpreted as antagonistic. Another consideration in connection with the digastric site was the use of bilateral electrode placement. Because this midline site is so small in toddlers, it was not possible to isolate left and right sides, and the anterior placement used provided the best access to this muscle, which tends to have a rather prominent layer of fat over it posteriorly.1
Skin preparation consisted of a light scrubbing with an alcohol-soaked gauze pad, followed by application of an antiperspirant skin electrode preparation (Prep N' Stay, Pharmaceutical Innovations, Inc.). Ag/AgCI mini-electrodes (InVivo Metric) were secured using adhesive collars, as well as surgical adhesive tape over the top of each pair. A high-quality wireless lapel microphone worn by the child provided the signal for the first of two audio channels. This channel provided simultaneous audio recordings of the child’s vocalizations along with an experimenter’s intermittent description and on-line gloss of the child’s behavior and speech. A second audio, recorded using the built-in edge track of the FM recorder, included a second experimenter’s commentary regarding ongoing changes in experimental conditions and preliminary data description. Data channels included: (a) right and (b) left masseter EMG, (c) right and (d) left temporalis EMG, (e) bilateral anterior belly of the digastric EMG (i.e., one electrode of the pair placed over each of the right and left members of the muscle pair), (f) child’s audio and Experimenter #1 audio on-line description of child’s behavior, and (g) Experimenter #2 audio experimenter observations and description of data and recording conditions.
The composition of the sampled data set is shown in Table 2. Although this table indicates eight discrete categories of behavior, these samples were far from being homogenous within each sample type. The children chewed a variety of foods, depending on each child’s normal diet; they drank from different styles of containers, ranging from bottles to spout cups; and babbling samples are extremely difficult to classify (i.e., as variegated or reduplicated), as are speech samples, given the imposition of adult perceptual classification on developing speech. Nevertheless, conservative classification procedures were used (e.g., independent, complete agreement among the investigators that a given token represented one category; see below), which makes the following comparisons possible.
TABLE 2.
Contributions by each subject to the composition of the parsed, raw data set, broken down by behavior type.
| Behavior | Subjects
|
||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Sucking (seconds) | 29 | 12 | 12 | 22 | 12 | 17 | 21 |
| Chewing (seconds) | 44 | 46 | 29 | 42 | 14 | 19 | 29 |
| Rbabble (tokens) | 2 | 9 | 4 | 5 | 3 | 8 | 2 |
| VBabble (tokens) | 10 | 2 | — | 13 | 5 | 2 | 1 |
| Rcvcv (tokens) | 4 | 11 | 18 | 5 | — | 17 | 1 |
| Vcvcv (tokens) | 3 | 2 | 10 | 1 | — | 9 | 1 |
| CVC (tokens) | — | 3 | 8 | 3 | 3 | — | — |
| CV (tokens) | 4 | 4 | 8 | 2 | 7 | 5 | 1 |
Note. Rbabble = reduplicated babbling; Vbabble = variegated babbling; Rcvcv = true words with a repeated syllable (e.g. /baba/); Vcvcv = true words consisting of two different syllables; CVC = single syllable true words with initial and final consonants; CV = single syllable true words with an initial consonant and a final vowel only.
At least 25 cycles of chewing were sampled for each child, the total sampling period consisting of at least 14 seconds per subject. The children chewed a variety of chewy foods, provided from the child’s usual diet by the mothers. In addition, between 12 and 29 seconds of sucking for each child were also sampled. The conditions of this behavior varied somewhat; two children used straws, three used infant feeding bottles, and two used cups with spouts. In each instance, however, we confirmed by inspection that the child was grasping the object with his or her lips and was exerting effortful, repetitive sucking activity.
Speech samples were obtained by a variety of methods, including picture naming, imitation, question and answer, and explicit requests for imitative productions (i.e., “Say _”). For example, /mama/ and /daede/ were easily elicited from most children with naming requests only. Less familiar true words (e.g., “Baby Bop”) were obtained imitatively. These productions were classified into CV, CVC, reduplicated CVCV, and variegated CVCV word forms.
Samples of babbling included both variegated and reduplicated productions from six of seven children (see Table 2). The children generated these samples most frequently during self-directed play. Mothers and/or the interacting experimenter confirmed that these vocalizations were neither meaningful nor referential in the experimental context. We adopted very conservative criteria for categorization of these children’s vocalizations, rejecting any utterances for which classification was unclear. Only those utterances that included clear, sequential repetition of one syllable were classified as reduplicated babbling; those with a clear change in the vocalic and/or the consonantal portion of the utterance were classified as variegated. Accordingly, the only tokens included were those for which there was agreement by both investigators that the tokens were representative of their designated classifications.
Selection and parsing of data for subsequent analysis
The epochs containing the target behaviors of interest were isolated from within the 45 minutes of continuous recording per session by use of a tape log that identified those intervals during which target behaviors occurred. These isolated portions across the six data channels (not including the second audio channel) were digitized (sampling rate: 1000 samples/s per channel) for subsequent analysis. These digitized periods contained only acceptable instances (e.g., free of movement artifact, uncontaminated audio signal) of target behaviors. Interrater reliability for identification and selection of target behaviors was assessed using a randomized selection of 20% of the samples.
Signal Processing
Following digitization of selected sample intervals (e.g., 30 seconds of chewing), analyses were completed for each record using algorithms custom designed for Matlab (v. 4.2c; The Mathworks, Inc.), a commercially available signal processing package. For continuous behaviors such as chewing and sucking, each 30-second sample was analyzed in 10 contiguous 3-second periods. For discrete behaviors such as speech and babbling, only those portions of the digitized sample associated with the target behavior were analyzed. For example, this procedure eliminated from the analysis those periods during which the child was not vocalizing, as well as those periods contaminated by movement artifact.
The computer-assisted analysis consisted of several stages. Initially the entire digitized sample (e.g., all five EMG channels and the audio channel obtained for 30 seconds of chewing) was displayed on a computer monitor. The experimenter placed cursors to delimit the period (3 seconds for continuous behaviors, less for discrete ones) selected for analysis. The EMG signals from this period were full-wave rectified and digitally low-pass filtered (8-pole Butterworth, f = 30 Hz), thus generating an activation “envelope” for each channel. All 10 possible pairwise crosscorrelation functions were computed for these five filtered and rectified EMG signals, each function consisting of about 6000 points (i.e., for a 3000 ms analysis period). These crosscorrelation functions yielded quantitative representations of two aspects of the relatedness of activity among muscles: the greatest predictability of one channel from another (i.e., peak coefficient) and the time delay associated with that strongest level of prediction (i.e., the lag to the peak coefficient). The simple correlation coefficient, obtained in the present analysis at a lag value of zero, was tabulated for comparison with earlier investigations (e.g., Moore et al., 1988). These three values were subjected to qualitative and statistical comparisons. Support for the use of these quantitative measures in this application was derived from the assumption that muscle synergies vary in strength of coupling and may be asynchronous (Cooke & Brown, 1990). Zero lag coefficients were included primarily to provide a basis for comparison with earlier investigations. These analyses yield values that can be used to compare coupling strength and synchrony of muscle pairs across behaviors. Coupling strength was quantified by differences among peak correlation coefficients. Comparisons of muscle activation synchrony were based on differences in the lags of the peak correlation coefficients.
To permit statistical description and evaluation across conditions, crosscorrelation coefficients (r) were converted to Fisher’s z values (Eq. 1) before averaging across repetitions within each subject.
| (1) |
Differences were evaluated statistically using a one-way analysis of variance (ANOVA) with a repeated measures design (BMDP-SV). Post hoc analysis of differences between pairs and between behaviors was judged to be inappropriate because of the small number of subjects and, further, because the primary focus of this investigation was to determine whether changes in coordinative organization occur across behaviors.
Results
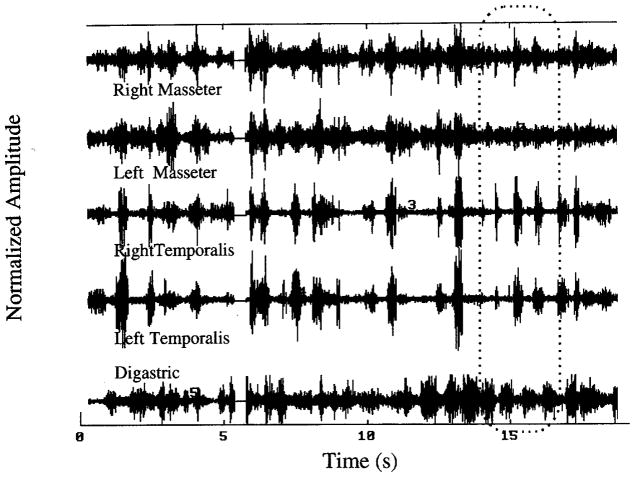
The experimental protocol and recording conditions proved to be more than sufficient for the purpose of gathering representative samples of the target behaviors from these young children. The combined interrater agreement for exclusion and inclusion of samples of these target utterances in the data corpus exceeded 95%. Additionally, the raw EMG waveforms proved to be well suited to the signal processing techniques applied. Figure 1 illustrates one set of EMGs obtained during two intervals of chewing separated by a brief recording pause at about 6 seconds. Although computational limits required analysis of sequential segments individually, all of the data in the figure were included in the analysis. The superimposed dotted line on this figure delimits one segment of the nonoverlapping segments subjected to analysis sequentially. Figure 1 also illustrates several of the challenges posed by orofacial surface EMG signals obtained from young children. For example, signal-to-noise ratios were usually relatively poor, which would tend to obscure correlated activity across muscles and would manifest lowered peak coefficients. In addition, these young children typically produced the target behaviors in short bursts. Nominally rhythmic activities, such as chewing or babbling, did not usually exhibit sustained rhythmicity, although characteristic cyclic patterns were sometimes observed for brief periods. Surprisingly, movement artifact in these very active children was a relatively rare problem.
FIGURE 1.
Electromyograms of five mandibular muscles obtained during chewing by a 15-month-old child. Two intervals are combined to yield this sample of approximately 20 seconds.
Although crosstalk among recorded channels did not appear to be a concern in these records (i.e., high amplitude activity in left temporalis is not seen in the minimally activated left masseter channel), there is inescapable concern for contamination by nontarget muscles when using high gain amplification, typically a factor of 20,000 or 50,000 in this procedure. The digastric signal, which might be expected to be the most vulnerable to contamination by nearby nontarget muscles, exhibits in Figure 1 several characteristics that provide reasonable assurance that jaw-depressing muscle activity dominates the record. Even at the low levels of activity shown in the digastric trace, the well-documented pattern of reciprocal activation with the jaw elevating muscles is evident. Periods of large, phasic activity unrelated to jaw elevating EMG in this channel were not observed, although before recording there had been the concern that genioglossus or mylohyoid activity would be seen during swallowing or manipulation of the food bolus during chewing.
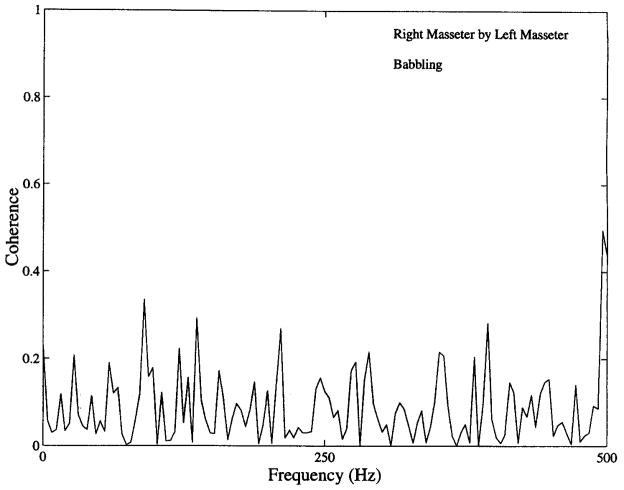
Crosstalk among recorded EMG channels is also a source of concern with respect to crosscorrelational analyses such as those used in the present investigation. Accordingly, coherence functions among all 10 possible pairs were computed to evaluate signal isolation. Calculation of coherence, which is based on the correlation of spectral energy at each frequency in two signals, is especially effective at detecting frequency-specific contamination and shared signal content across channels, effectively distinguishing between shared contamination and unshared, channel-specific noise (a complete discussion of interpretation of coherence functions of orofacial EMG is found in Wohlert & Goffman, 1994). These measures were computed for three conditions for each subject: high-level activity in all muscles (e.g., during chewing), inactivity or low-level activity in all muscles, and periods of high activity in one muscle paired with low-level activity in another (as seen in Figure 1 for temporalis versus masseter channels). None of these coherence functions revealed evidence of cross-channel contamination. A typical result is shown in Figure 2, which illustrates the coherence function obtained for right and left masseter channels from Subject 3 during babbling. The absence of consistent coherent energy across a broad frequency range minimized concerns of crosstalk. The appearance of isolated peaks (e.g., in Figure 2, one peak of .32 at 90 Hz) was of no concern, as crosstalk would be evidenced by a broad range of coherent frequencies (Goffman & Smith, 1994). This analysis also provided an indication of the lack of line noise interference at 60 Hz, because there was no evidence of highly coherent energy at this frequency.
FIGURE 2.
This coherence function, obtained from right and left masseter EMGs obtained during babbling, shows no evidence of cross-channel contamination.
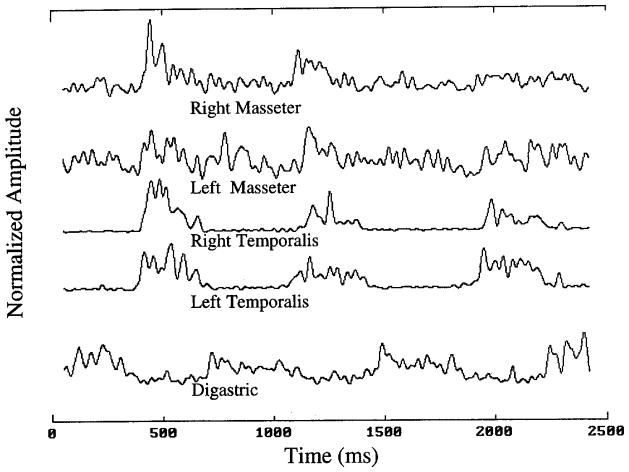
Figure 3 illustrates the parsed raw EMG signal indicated in Figure 1 following full-wave rectification and lowpass filtering. The data in this figure illustrate a typical reciprocal organization pattern, which characterizes the well-known mandibular coordinative organization for chewing (Møller, 1966). Left and right temporalis channels show clear synchrony in this figure, as do the somewhat lower amplitude signals of left and right masseters. Reciprocity of antagonists is observed here with peaks in digastric coinciding with less active periods in the four jaw-elevating muscles.
FIGURE 3.
Rectified and filtered EMGs obtained from the indicated section in Figure 1. These processed signals were subjected to crosscorrelational analysis to obtain indices of coupling and synchrony.
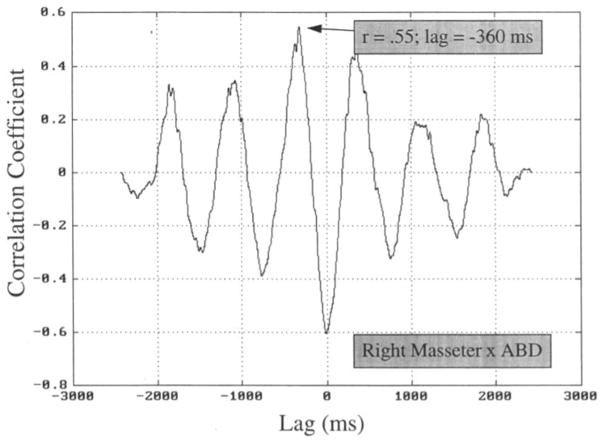
Quantification of patterns of muscle activation was based on pairwise crosscorrelational analysis of timing in the EMG signals obtained. Each crosscorrelation function contributed only two data points to the present description: the peak correlation coefficient, which provided an index of coupling for the pair, and the lag associated with that peak coefficient, which provided an index of the delay between coupled activation of the pair. In this way, strongly coupled activity, such as the reciprocal activation of jaw depressing and jaw elevating muscles during chewing, can be quantified in such a way as to reveal the delay (typically 200 ms for chewing) between correlated activity in opposing muscles. Figure 4 illustrates this pairwise computation for the right masseter and digastric channels included in the data illustrated in Figures 1 and 3. (Nine other paired comparisons were made for these data; this single comparison is shown for illustration purposes only.) Visual inspection of Figure 3 suggests that digastric activity can be predicted partly by right masseter activity; this suggestion was addressed using the peak correlation coefficient. The asynchrony of these related (i.e., coupled) periods of activity is seen in Figure 3 to be approximately 300–400 ms (e.g., from the peak of right masseter activity to the corresponding peak of activity in the digastric); this temporal relationship was evaluated using the lag to the peak correlation coefficient. Figure 4 illustrates how computation of the crosscorrelation function confirms and quantifies this impression. A peak coefficient of .55 suggests that the EMG signals from right masseter and ABD are somewhat related, with activation of right masseter being most predictive of digastric activity 360 ms later. A related observation, which was not included in the present analysis, can be seen in the periodicity of the crosscorrelation function (about 720 ms in Figure 4), which stems from the periodicity of the EMG amplitude envelopes themselves. For example, the frequency of chewing in the records shown was about 1.4 Hz.
FIGURE 4.
The crosscorrelation function obtained for the right masseter and digastric records in Figure 3 (2.5 s of chewing). The presence of a peak coefficient of .55 indicates moderate coupling of activity in these two antagonistic muscles, and the lag of −360 rns suggests a high degree of asynchrony.
One additional feature extracted from the crosscorrelation function was the coefficient when the lag value was zero milliseconds. At this zero lag point the crosscorrelation function yields the result of a simple correlation (i.e., with no sensitivity to asynchronous coupling). This value is useful in the present context for comparison to previous quantification of EMG activation patterns, which used only the zero lag coefficient as the coordinative index. For example, the zero lag coefficient in Figure 4 is about −. 60, which corresponds well to the values reported by Moore et al. (1988, Table 1).
Differences in coupling exhibited within behaviors across comparison pairs
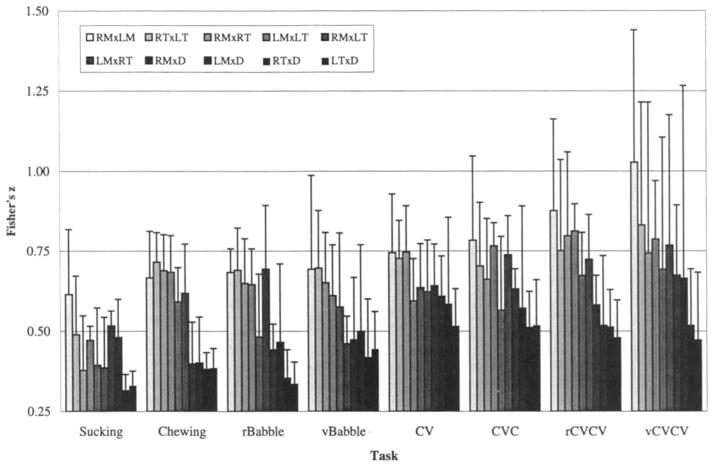
One of the central questions addressed in the present analysis was whether there are differences in coupling among mandibular muscles within each of the target behaviors. Figure 5 displays the average Fisher’s z values, grouped by target behavior, across paired comparisons of EMG recording sites. This figure is formatted generally with the earliest emerging behaviors to the left, latest to the right, although no linearity is meant to be suggested by their spacing along the abcissa. Within each behavior, group results of comparisons of homologous muscles (shown with the lightest shading) are positioned to the left, synergists toward the center, and antagonists (shown with darkest shading) to the right. The obvious pattern within each behavior is the tendency for the homologous pairs to exhibit the highest coupling and the antagonists to exhibit the lowest coupling. A one-way analysis of variance with repeated measures revealed that the within-behavior differences (i.e., across comparison pairs) were statistically significant (df = 9; p < .05) for each of the eight conditions. Subject effects within behaviors or within paired comparisons were not evaluated because of the small sample size and the use of repeated measures. These results demonstrated varying strength of coupling across mandibular muscles, irrespective of the behavior.
FIGURE 5.
Fisher’s z means and standard deviations, across all subjects, grouped by behavior. Within each behavior group, homologous muscle pairs are to the left, antagonists to the right, and synergists are toward the center. Differences within the group were statistically significant for each behavior (e.g., within the Chewing grouping, there was a significant effect for comparison pair).
Differences in coupling exhibited across behaviors by each comparison pair
The second issue of interest was the difference in coupling exhibited by a given comparison pair across behaviors. The results shown in Figure 5 also illustrate the differences in the peaks of the crosscorrelation function within each comparison pair across behaviors (e.g., comparison of the leftmost member, RMxLM, across behaviors). The clearest trend in this comparison is the increase in coupling strength (i.e., increasing peak coefficients) for the later-emerging (i.e., speech) behaviors; this is apparent in most of the 10 paired comparisons. This apparent within pair effect across each of the eight behaviors was supported using a second one-way ANOVA with repeated measures, which revealed that differences within each of the 10 muscle pairs compared (i.e., across behaviors) were statistically significant (df = 7; p < .05), with the exception of the RTxLT comparison pair which yielded no significant effect (p = .30) across behaviors.
Differences in timing of muscle activity within behaviors
Results for the analysis of timing differences also revealed marked differences across behaviors and across compared muscle pairs. The lag to the peak coefficient for each crosscorrelation function was extracted and converted to its absolute value (i.e., the sign of the lag was discarded) to permit averaging. Figure 6 presents the means and associated standard deviations for the paired comparisons of all muscles within each behavior. Most lag values were found to be below 200 ms, although there were several expected exceptions. Chewing was characterized by long lags for antagonistic pairs and very short lags for synergists, because synergistic muscles coactivate during chewing (i.e., lags tend toward 0 ms) and antagonists activate 1800 out of phase with each other (i.e., lags tend toward 50% of the chewing period-about 300–600 ms). CV words, on the other hand exhibited uniformly brief lags across synergistic and antagonistic pairs. Statistical comparison of these averages, using one-way analysis of variance with repeated measures, revealed significant differences across comparison pairs within chewing, sucking, and reduplicated babbling (df = 9; p < .05) but not within variegated babbling or any of the true word productions. These results demonstrated that the coordinative synergies exhibited during chewing, sucking, and reduplicated babbling include more than one pair of asychronously coupled muscle activation groups. Variegated babbling and true word production, on the other hand, were characterized by a lack of difference in the timing of activity across both antagonistic and synergistic muscle pairs.
FIGURE 6.
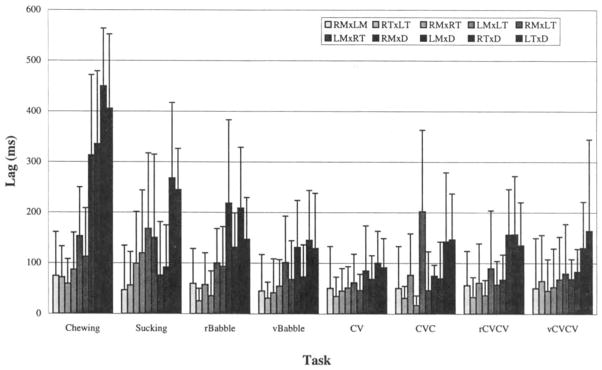
Absolute lag values averaged across all repetitions and all subjects, grouped by behavior, with earlier-emerging behaviors to the left. Within each grouping, homologous pair comparisons are to the left, antagonists to the right, and synergists are toward the middle. The statistical effect of comparison pair was significant only for chewing, sucking, and reduplicated babbling–the three earliest emerging behaviors.
Differences in timing of muscle activity across behaviors
The variations in coupling across behaviors within each muscle group were also evaluated by examining timing differences across behaviors within each of the 10 comparison pairs. It is clear from Figure 6 that homologous muscle pairs (i.e., RMxLM and RTxLT, the leftmost two bars in each of the task groups across the graph) yielded very brief lags regardless of the behavior and that the timing of activity within these pairs appeared to be consistently coupled. This rigid coupling was in sharp contrast to that exhibited by the antagonistic pairs (e.g., RMxD, the fourth bar from the right in each group), which involved very long lags for chewing and much shorter lags for speech. Statistical analysis, again employing one-way analysis of variance with repeated measures, revealed that none of the synergistic pairs yielded a statistically significant behavior effect, whereas each of the antagonistic pairs did yield this effect (df = 7; p < .05). In general these results can be summarized by noting that differences in timing among muscle pairs were observed within and across behaviors for antagonistic pairs, but not for synergists.
Differences in timing of muscle activity across comparison pairs
A summary of the present findings for chewing can be seen in Figure 5, which shows moderate coupling of homologous and synergistic muscles and weaker coupling among antagonists. Lag values for chewing were typically well under 100 ms for homologous and synergistic pairs, indicating near-synchrony of activation among these pairs, whereas antagonistic pairs yielded lags as high as 300–400 ms. Rigid specification of timing, sometimes seen as a hallmark of centrally patterned behaviors, was not characteristic of the present data set. This lack of rigid temporal specification was probably due to the fluctuating task demands of chewing, which initially require generation of occlusal and incisal force, but gradually give way to grinding and jaw depression to permit repositioning of the food bolus. Therefore these fluctuations are entirely consistent with fluctuations observed in adult chewing (Meller, 1966).
In contrast, the results of analyses of speech revealed relatively rigid coupling. This tighter coupling was reflected by high Fisher’s z values (see Figure 5) and was further characterized by short lag values, typically less than 100 ms on average (Figure 6). The highest mean Fisher’s z values obtained were from the latest emerging speech form: variegated CVCV true words.
Discussion
The present description of the coordinative organization underlying mandibular movement by young children has shown it to be, as it is in adults, quite plastic; there was no evidence of a simple, dominant pattern of reciprocal activation across behaviors. These toddlers exhibited roughly the same range and variety of muscle activation patterns as adults, generating reciprocal activity for chewing and cocontraction of antagonistic muscles during speech (Møller, 1966; Moore et al., 1988). Moreover the observed coordinative variations may reflect a continuum, with later developing, more complex behaviors exhibiting coordinative organization that was markedly different from that of earlier emerging ones. This continuum may represent a developmental progression or adjustments to differences in task complexity. Changes were observed in coupling differences (i.e., observed across peak correlation coefficients) as well as in relative timing of activity (i.e., differences in lag values). Although the sample size and design were not intended to test statistically these differences as developmental progressions or ordered effects (i.e., chronological/developmental emergence), Figures 5 and 6 leave the clear impression of a developmental effect, at least to the extent that the later developing tasks were different from the earlier emerging ones. If these developmental trends were to be confirmed in more extensive analyses, including longitudinal observations, they would represent a progression of coordinative organization coincident with changing communicative, physiologic, and anatomic contexts. The interactions of these diverse developmental pressures would be of special interest in such a case.
Specifically, Figure 5 yields the impression of stronger coupling with later developing behaviors (i.e., behaviors toward the left side of the figure yielded lower peak coefficients than those to the right). Figure 5 suggests further that the strongest coupling observed was for homologous muscle pairs and synergists, with antagonistic coupling appearing somewhat weaker (i.e., the leftmost six bars in each group generally appear to be higher than the rightmost four, especially for the Chewing group). The overall impression of Figure 6 is one of gradually decreasing lags for later-emerging behaviors (i.e., coupling–especially among antagonistic pairs, the leftmost four bars in each group–tended toward synchrony and away from reciprocity). This figure also leaves the clear impression that adjustments in timing occur primarily among the antagonistic pairs. In most cases lower peak correlation coefficients were paralleled by higher lag values. The mirrored trends of Figures 5 and 6 support the suggestion of looser coupling (lower peak coefficients) with greater asynchrony (longer lags) within as well as across behaviors. Finally, as might have been expected, a prominent, general finding was that activation of homologous pairs was uniformly synchronous (i.e., very short lags) and appeared to be most rigidly coupled (i.e., exhibited the highest coefficients).
Implications
The contemporaneous observation of significantly different coordinative strategies for earlier- and later-emerging behaviors may be taken to argue against a developmental process in which speech relies on, or emerges from, extant coordinative mechanisms. The present results are equivocal, however, with respect to support for specific mechanisms of coordination of mandibular muscle activity in the framework of prevailing accounts of the development of motor control for speech production. Although it is quite clear that, with respect to the present analysis, the coordinative organization for even very early speech production was distinct from that of earlier emerging nonspeech behaviors, it remains to be determined how and when these differences emerged. Nevertheless, the similarities of results for reduplicated babbling and earlier emerging behaviors provide support for some level of shared control during an earlier developmental period. This contrast must be reconciled in future investigations, because a model requiring two distinct, apparently unrelated (with respect to motor development) processes for babbling and emergent speech production would be poorly supported by the existing empirical data, with none of the parsimony of contemporary models.
The finding of task-specific coordinative organization in adults has been taken to support the suggestion that the neurophysiologic infrastructure for speech is distinct from that of nonspeech behaviors (Moore et al., 1988). Such a suggestion also arises from the present findings, although a very different inference must also be considered. Support for a developmental continuum for these tasks could be based on the observation that 15-month-old children are in a stage of rapid transition and may manifest the earlier established stabilities of chewing and sucking, as well as the less stable attractor states of babbling and speech (Kent & Hodge, 1990). The challenge that remains for this interpretation is to observe these longitudinal changes in motor organization so that specific stabilities and transitions can be described.
One distinctive contrast arose from the different results for reduplicated and variegated babbling, although the difficulties in firmly distinguishing these utterance types warrant a cautious interpretation. Lags for reduplicated babbling were significantly different across compared pairs, a finding shared only with chewing and sucking and not with variegated babbling or any of the speech behaviors. This distinction implies that only reduplicated babbling manifests the kinds of rhythmicity and coordinative organization seen in earlier emerging behaviors. Moreover, this difference in coordinative organization, occurring between two early emerging prespeech behaviors may provide evidence of a transition in oral motor coordination from established to emergent structures dependent upon specific task demands. Schöner and Kelso (1988) referred to this type of observation explicitly, suggesting that the goal of describing motor development is primarily addressed by identifying periods of stability (attractor states) and their associated transitions, recognized and defined by their increased instability. In the present design, sampling the behaviors of children at an early stage of speech development was meant to enhance the potential for observing these transitions between speech states, each with its own precursors, successors, and variants. It is possible, given the similarity of the results for variegated babbling and speech behaviors, that 15-month-old children may have already developed beyond the transition period during which stable mandibular coordinative organization is generated. In fact, studies evaluating the relative developmental pressure of motoric constraints and linguistic context have shown that during this very early stage of lexical acquisition, motor constraints may dominate. This hypothesis is supported by the tendency of a child to generate new word forms consistent with his or her preferred babbling patterns (e.g., Vihman, Ferguson, & Elbert, 1986).
Methodologic concerns and limits
One important aspect of the present investigation was the extension of crosscorrelational analyses in the quantification of coordinative organization, specifically with respect to reliance on peak coefficients and lag values. Because this is a new application for these methods it is important to note explicitly what limitations and concerns exist. The design of this analysis was intended to yield very broad descriptors of coordinative tendencies, at the expense of detailing fine structure in these patterns. Consistently strong coupling of muscle activity across multiple muscles was taken as an indication of behavior-specific constraint and simplification of the motor control problem for mandibular movement. It might be concluded that, where the activity of one muscle in a group is highly predictive of that of another, there is support for the suggestion that the number of degrees of freedom is sharply reduced for those elements, further suggesting the action, at some level, of a common control signal. One strength of this method is the implicit recognition of the fact that these coordinative groups may be coupled asynchronously, a consideration not inherent in most other analytic tools applied to muscle activation patterns.
Along with these advantages, several cautions are associated with the use of crosscorrelational analysis as an index to coordinative organization. These computations are very sensitive to sampling procedures. Sampling of pre- and postbehavior intervals must be avoided, as these periods can yield spuriously high coefficients as silent intervals will be crosscorrelated with periods of activity. Elimination of exogenous artifact (e.g., movement artifact, line noise) is essential for the same reason; large deviations from the levels of activity seen during the behavior performance will, when observed across channels, dominate the crosscorrelation function. Thus, the use of coherence analysis before computing crosscorrelation functions narrows the interpretability of those functions to targeted relationships. Finally, generation of the EMG “envelope” must be accomplished with attention to minimization or elimination of differential phase shifts across frequencies (e.g., by forward and reverse digital filtering or use of a linear phase filter) and to selection of a lowpass cutoff frequency.
A less significant limitation of this method in the present application is its inability to reveal coordinative stabilities relative to dynamic rate changes, such as those that must be observed in demonstrations of coordinative structures (Fowler, Rubin, Remez, & Turvey, 1980). For example, the shorter lags observed for the later-emerging speech behaviors were partly a consequence of faster movement cycles, although lags were not statistically significantly different across muscle pairs within any of the speech tasks. One solution to this issue is to represent lags with values standardized to the period of the movement cycle (e.g., a lag of 180° or 50% would be anticipated for antagonistic pairs during chewing), although identification of a movement cycle is not always possible for speech movements. We have had marginal success with this technique in the analysis of the isolated speech utterances included in the present data set.
The present results yielded the counterintuitive finding of generally more rigid coupling (i.e., higher peak coefficients) for speech than for mastication. Tighter coupling for chewing might have been expected for at least three reasons. First, especially in very young children, it might be reasoned that, relative to speech, chewing involves neural mechanisms that reside at a level closer to the final common pathway. Second, chewing probably entails more narrowly specified force, displacement, and rhythmic timing demands. Third, a more advanced level of mastery might be presumed at this age for chewing. As such, control mechanisms for chewing might be expected to exert a more constant and predictable influence on muscle activation patterns (i.e., which would be observed in the present methodology as higher peak coefficients). Certainly for centrally patterned behaviors such as locomotion one would expect a very high degree of predictability within and across motor systems. Speech movement, on the other hand, is a behavior that these children are far from mastering. Consistent with most representations of motor learning, studies of speech timing (Tingley & Allen, 1975) and kinematics (Smith, 1995; Smith & McClean-Muse, 1986) have uniformly demonstrated significantly greater variability in younger speakers. Thus, the present findings are in stark contrast to expectations that speech, or any other emergent behavior, will exhibit relatively greater variability.
One interpretation of this counterintuitive finding is that children adopt a motor learning strategy whereby they initially sharply reduce the number of controlled elements by linking them together. The uniformly high Fisher’s z values shown in Figure 5 for speech behaviors is consistent with this suggestion. Alternatively, it is possible that representations of chewing as an “extant” behavior are false, and that chewing is the emerging, more poorly mastered behavior. Fifteen-month-old children are beset by rapid skeletal, dental, and dietary changes, each of which complicates task demands with respect to mandibular movement during chewing. Chewing may itself be an emerging behavior among children of this age, which would make it an even less attractive candidate to provide a coordinative infrastructure for speech. Unfortunately we cannot draw upon existing data to address this question because descriptive data on the coordinative development of chewing are lacking. We are currently exploring in our laboratories this issue of learning stages in mastication.
Conclusions
The present investigation of 15-month-old children during early speech development has suggested that the coordinative organization for speech and nonspeech behaviors is task-specific and distinct. Given their early stage of speech development, it was taken as somewhat remarkable that these children exhibited muscle activation patterns similar to those found in complex utterances by adults. The data also supported the suggestion of less rigidly specified coordinative organization for reduplicated babbling than for variegated babbling. These findings may be taken to support the suggestion that mandibular coordination follows a developmental continuum from earlier emerging behaviors, such as chewing and sucking, through babbling, to speech, which emerges latest among the behaviors studied. Alternatively, it may be that unique task demands give rise to distinct coordinative constraints (e.g., requisite coactivation of antagonistic muscles during speech production) that develop independently of other behaviors. In any case, it is clear that assumptions regarding the relationship of speech to earlier emerging behaviors are unsupported by the present findings. Longitudinal studies are currently underway to explicate these relations.
Acknowledgments
This work was supported by research grant number 7R29DC00822 from the National Institute on Deafness and Other Communication Disorders, National Institutes of Health, and from the University of Pittsburgh, Pittsburgh, PA. Both authors are formerly of the Department of Communication Sciences and Disorders at the University of Pittsburgh. They would like to acknowledge the valued contributions to this work by Tammy Jo Caulfield and Jordan Green.
Footnotes
Questions have arisen frequently regarding the practicality of collecting this type of data from very young children. It is not a simple procedure and there are some children who will not complete protocols of this sort (e.g., those who will not tolerate others touching their faces, those with extreme anxiety around strangers, and those who are unable to sit still for an entire experimental session). Most children adapt rapidly to these methods, quickly becoming absorbed in the experimental tasks and forgetting about the leads extending from their faces. Optimal recording conditions for EMG are not attainable for young children. Uncomfortable or painful procedures (e.g., hooked-wire electrodes) are, of course, not permissable. Moreover, the presence of a fatty layer over the face and neck attenuates the EMG signal obtained at the skin surface and introduces the greater potential for volume conduction of signal from nontarget muscles. These problems are addressed in the present experiment partly by using signal processing techniques applied to very large samples.
Contributor Information
Christopher A. Moore, Department of Speech and Hearing Sciences, University of Washington, Seattle
Jacki L. Ruark, Department of Audiology and Speech Pathology, University of Tennessee, Knoxville
References
- Cooke JD, Brown SH. Movement-related phasic muscle activation. II. Generation and functional role of the triphasic pattern. Journal of Neurophysiology. 1990;63:465–472. doi: 10.1152/jn.1990.63.3.465. [DOI] [PubMed] [Google Scholar]
- Davis BL, MacNeilage PF. The articulatory basis of babbling. Journal of Speech and Hearing Research. 1995;38:1199–1211. doi: 10.1044/jshr.3806.1199. [DOI] [PubMed] [Google Scholar]
- Fawcus B. Oropharyngeal function in relation to speech. Developmental Medicine and Child Neurology. 1969;11:556–560. doi: 10.1111/j.1469-8749.1969.tb01484.x. [DOI] [PubMed] [Google Scholar]
- Fentress JC. Dynamic boundaries of patterned behavior: Interaction and self-organization. In: Bateson PP, Hinde RA, editors. Growing points in ethology. Cambridge, MA: Cambridge University Press; 1976. pp. 135–169. [Google Scholar]
- Fowler CA, Rubin P, Remez RE, Turvey MT. Implications for speech production of a general theory of action. In: Butterworth B, editor. Language production. I. New York: Academic Press; 1980. pp. 373–420. [Google Scholar]
- Goffmah L, Smith A. Motor unit territories in the human perioral musculature. Journal of Speech and Hearing Research. 1994;37:975–984. doi: 10.1044/jshr.3705.975. [DOI] [PubMed] [Google Scholar]
- Grillner S. Possible analogies in the control of innate motor acts and the production of sound in speech. In: Grillner S, Lindblom B, Lubker J, Persson A, editors. Speech motor control. New York: Pergamon Press; 1981. pp. 217–230. [Google Scholar]
- Kelso JA, Schöner G. Self-organization of coordinate movement patterns. Human Movement Science. 1988;7:27–46. [Google Scholar]
- Kent RD, Bauer HR. Vocalizations of one-year-olds. Journal of Child Language. 1985;12:491–526. [Google Scholar]
- Kent RD, Hodge M. The biogenesis of speech: Continuity and process in early speech and language. In: Miller JF, editor. Progress in research on child language disorders. Austin, TX: Pro-Ed; 1990. [Google Scholar]
- Kent RD, Mitchell PR, Sanicer M. Evidence and role of rhythmic organization in early vocal development in human infants. In: Fagard J, Wolff PH, editors. The development of timing control and temporal organization in coordinated action. New York: Elsevier; 1991. pp. 135–139. [Google Scholar]
- Kent RD, Murray AD. Acoustic features of infant vocalic utterances at 3, 6, & 9 months. Journal of the Acoustical Society of America. 1982;72:353–365. doi: 10.1121/1.388089. [DOI] [PubMed] [Google Scholar]
- Larson CR. The midbrain periaqueductal gray: A brainstem structure involved in vocalization. Journal of Speech and Hearing Research. 1985;28:241–249. doi: 10.1044/jshr.2802.241. [DOI] [PubMed] [Google Scholar]
- Lund JP. Mastication and its control by the brain stem. Critical Reviews in Oral Biology and Medicine. 1991;2:33–64. doi: 10.1177/10454411910020010401. [DOI] [PubMed] [Google Scholar]
- Luschei ES. Development of objective standards of nonspeech oral strength and performance: An advocate’s views. In: Moore CA, Yorkston KM, Beukelman DR, editors. Dysarthria and apraxia of speech: Perspectives on management. Baltimore, MD: Brookes; 1991. [Google Scholar]
- Luschei ES, Goldberg LJ. Neural mechanisms of mandibular control: Mastication and voluntary biting. In: Brooks VB, editor. Handbook of physiology-section I: The nervous system, volume II, motor control. Bethesda, MD: American Physiological Society; 1981. pp. 1237–1274. [Google Scholar]
- Luschei ES, Goodwin G. Patterns of mandibular movement and jaw muscle activity during mastication in the monkey. Journal of Neurophysiology. 1974;37:954–966. doi: 10.1152/jn.1974.37.5.954. [DOI] [PubMed] [Google Scholar]
- Møller E. The chewing apparatus. An electromyographic study of the action of muscles of mastication and its correlation to facial morphology. Acta Physiologica Scandinavica. 1966;69(Supplement 280) [PubMed] [Google Scholar]
- Moore CA. Symmetry of mandibular muscle activity as an index of coordinative strategy. Journal of Speech and Hearing Research. 1993;36:1145–1157. doi: 10.1044/jshr.3606.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CA, Smith A, Ringel RL. Behavior-specific organization of activity in human jaw muscles. Journal of Speech and Hearing Research. 1988;31:670–680. doi: 10.1044/jshr.3104.670. [DOI] [PubMed] [Google Scholar]
- Moore CA, Scudder RR. Coordination of jaw muscle activity in parkinsonian movement: Description and response to traditional treatment. In: Yorkston KM, Beukelman DM, editors. Recent advances in clinical dysarthria. Boston, MA: College-Hill; 1989. [Google Scholar]
- Ostry DJ, Feltham RF, Munhall KG. Characteristics of speech motor development in children. Developmental Psychology. 1984;20:859–871. [Google Scholar]
- Ostry DJ, Flanagan JR. Human jaw movement in mastication and speech. Archives of Oral Biology. 1989;34:685–693. doi: 10.1016/0003-9969(89)90074-5. [DOI] [PubMed] [Google Scholar]
- Ostry DJ, Munhall KG. Control of jaw orientation and position in mastication and speech. Journal of Neurophysiology. 1994;71:1528–1545. doi: 10.1152/jn.1994.71.4.1528. [DOI] [PubMed] [Google Scholar]
- Reed ES. An outline of a theory of action systems. Journal of Motor Behavior. 1982;14:98–134. doi: 10.1080/00222895.1982.10735267. [DOI] [PubMed] [Google Scholar]
- Schöner G, Kelso JAS. Dynamic pattern generation in behavioral and neural systems. Science. 1988;239:1513–1520. doi: 10.1126/science.3281253. [DOI] [PubMed] [Google Scholar]
- Smith A, Weber CM, Newton J, Denny M. Developmental and age-related changes in reflexes of the human jaw-closing system. Electroencephalography and Clinical Neurophysiology. 1991;81:118–128. doi: 10.1016/0168-5597(91)90005-i. [DOI] [PubMed] [Google Scholar]
- Smith BL. Variability of lip and jaw movements in the speech of children and adults. Phonetica. 1995;52:307–316. doi: 10.1159/000262184. [DOI] [PubMed] [Google Scholar]
- Smith BL, McClean-Muse A. Articulatory movement characteristics of labial consonant productions by children and adults. Journal of the Acoustical Society of America. 1986;80:1321–1328. doi: 10.1121/1.394383. [DOI] [PubMed] [Google Scholar]
- Thelen E. Monographs of the Society for Research in Child Development. 1991. Hidden skills: A dynamic systems analysis of treadmill stepping during the first year; p. 56. [PubMed] [Google Scholar]
- Thelen E. Motor development: A new synthesis. American Psychologist. 1995;50:79–95. doi: 10.1037//0003-066x.50.2.79. [DOI] [PubMed] [Google Scholar]
- Thelen E, Cooke D. Relationship between newborn stepping and later walking: A new interpretation. Developmental Medicine and Child Neurology. 1987;29:380–393. doi: 10.1111/j.1469-8749.1987.tb02492.x. [DOI] [PubMed] [Google Scholar]
- Thelen E, Fisher DM. From spontaneous to instrumental behavior: Kinematic analysis of movement changes during very early learning. Child Development. 1983;54:129–140. [PubMed] [Google Scholar]
- Thelen E, Skala KD, Kelso JS. The dynamic nature of early coordination: Evidence from bilateral leg movements in young infants. Developmental Psychology. 1987;23:179–186. [Google Scholar]
- Thelen E, Ulrich BD. Hidden skills: A dynamic systems analysis of treadmill stepping during the first year. Monographs of the Society for Research in Child Development. 1991:56. [PubMed] [Google Scholar]
- Tingley BM, Allen GD. Development of speech timing control in children. Child Development. 1975;46:186–194. [Google Scholar]
- Vihman MM, Ferguson CE, ELbert M. Phonological development from babbling to speech: Common tendencies and individual differences. Applied Psycholinguistics. 1986;7:3–40. [Google Scholar]
- Wohlert AB, Goffman L. Human perioral muscle activation patterns. Journal of Speech and Hearing Research. 1994;37:1032–1040. doi: 10.1044/jshr.3705.1032. [DOI] [PubMed] [Google Scholar]
- Wood JL, Smith A. Cutaneous oral-motor reflexes of children with normal and disordered speech. Developmental Medicine and Child Neurology. 1992;34:797–812. doi: 10.1111/j.1469-8749.1992.tb11518.x. [DOI] [PubMed] [Google Scholar]