Abstract
Purpose
The mandible is often portrayed as a primary structure of early babble production, but empiricists still need to specify (a) how mandibular motor control and kinematics vary among different types of multisyllabic babble, (b) whether chewing or jaw oscillation relies on a coordinative infrastructure that can be exploited for early types of multisyllables, and (c) whether the organization of motor control and associated kinematics varies across the nonspeech behaviors that are candidate motor stereotypies for speech.
Method
Electromyographic signals were obtained from mandibular muscle groups, and associated kinematics were measured longitudinally from a typically developing infant from 9 to 22 months during jaw oscillation, chewing, and several types of early multisyllabic babble.
Results
Measures of early motor control and mandibular kinematics for multisyllabic productions indicated task-dependent changes across syllable types and significant differences across babble and nonspeech behaviors. Differences in motor control were also observed across nonspeech behaviors.
Conclusions
Motor control for babble appears to be influenced by the balanced interaction between developing motor and linguistic systems, such that variation in linguistic complexity systematically evinces changes in motor organization apparently to meet these demands. This same effect was noted among chewing and jaw oscillation; task-dependent changes in mandibular control were noted across behaviors.
Keywords: speech, development, motor control, mandible, human
A theoretical framework of typical or disordered speech entails a robust representation of speech development; a crucial element in this representation is incorporating or segregating the influences of extant mechanisms of motor control. For example, one theoretical approach to speech development proposes that ingestive cyclicities, such as chewing or sucking (MacNeilage, 1998) or other extant motor stereotypies of jaw movement (e.g., Meier, McGarvin, Zakia, & Willerman, 1997; Thelen, 1991; variously called jaw oscillations, jaw wag, silent mandibular oscillations, silent babble, and mandibular oscillations), serve as precursors to the earliest appearing exemplars of mandibular motor control for speech. The underlying assumption is that early nonspeech behaviors rely on coordinative mechanisms that are later exploited for production of early speech vocalizations. Chewing and jaw oscillations are modeled as motor stereotypies (Thelen, 1991), which give rise to early mandibular motor control (supraglottal articulation) for babble (MacNeilage, 1998; Meier et al., 1997). More specifically, development supraglottal articulation has been suggested to occur separately from phonatory and respiratory speech motor control, with mature speech production emerging from the integration of control among these separate motor systems (MacNeilage, 1998; Meier et al., 1997). Though the relationship of mandibular motor control with babble and other behaviors remains unspecified, the conclusions of Meier et al. (1997) and MacNeilage (1998) suggest that mandibular motor control must be similar for these early speech and nonspeech behaviors and that this commonality is universally apparent in infants during speech development. This theoretical perspective has primarily relied on transcription studies of infant babble/speech, universal tendencies in phonetic development, and studies of adult speech errors.
A distinctly different theoretical perspective is supported by physiologic measures of mandibular motor control. These studies have provided evidence of parallel, but distinct, coordinative infrastructures for jaw movement during early alimentary behaviors, babble, and speech (Green et al., 1997; Moore & Ruark, 1996; Steeve, Moore, Green, Reilly, & Ruark McMurtrey, 2008), with each mechanism exhibiting a protracted course of development (Green et al., 1997; Steeve et al., 2008). These findings have not supported the notion that the coordinative mechanisms supporting “precursor” behaviors are exploited in the production of early vocalizations. Differences in mandibular motor control are apparent in the timing and amplitude of muscle activation across muscles, as well as in movement timing, extent, and trajectory.
Emerging Control of the Mandible Is Influenced by Distinct Neural Mechanisms
Investigations of mandibular motor control during the period of emergence of speech and nonspeech behaviors have failed to support the hypothesized transitional period during which developing speech motor control exploits the rhythmic motor infrastructures associated with alimentary behaviors, such as sucking or chewing (e.g., Moore & Ruark, 1996; Steeve et al., 2008). Rather, investigations using electromyography (EMG) to measure the emerging organization of muscle activity in human infants support the idea of parallel (distinct) neural infrastructures for motor control underlying mandibular coordination for speech and nonspeech behaviors. Findings in adults (Moore, 1993; Moore, Smith, & Ringel, 1988) and children (Green et al., 1997; Moore & Ruark, 1996) suggest that motor control underlying coordination of mandibular muscle groups is different across behaviors, such as chewing and speech. At a very early period when speech and nonspeech behaviors initially coexist, 9-month-old infants exhibit mandibular control that exhibits gross, but identifiable, similarities to the task-specific patterns seen in older children and adults (Steeve et al., 2008). These observations suggest that the coordinative organization is established differently among various mandibular behaviors and that the emergence of infrastructures for mandibular behaviors associated with sucking, chewing, and babble do not emerge sequentially or as precursors to other developing behaviors. Specifically, the organization of mandibular motor control in 9-month-olds for sucking, chewing, and babble (Steeve et al., 2008) resembles that seen in older toddlers (Green et al., 1997; Moore & Ruark, 1996), and the patterns associated with chewing and babble at 9 months appear to be fundamental behaviors of those seen for chewing and speech in adults (Moore, 1993; Moore et al., 1988). The essential properties of the coordinative organization of these speech and alimentary behaviors appear to be established very early in development, even though they are rather poorly specified. This finding of intertask differences coincident with parallel developmental changes suggests that the neural infrastructures supporting the motor development of sucking, chewing, and early speech are each continuously influenced by maturation, development, and use. However, the question remains as to whether a motor stereotypy, such as jaw oscillation, relies on a coordinative infrastructure that can be later exploited for early types of babble productions or whether motor control for jaw oscillation is distinct and develops in parallel with other mandibular behaviors.
Emerging Control of the Mandible: The Possible Role of Nonspeech Motor Stereotypies
Specialized neural mechanisms of pattern generation (i.e., central pattern generators [CPGs]) have been suggested as being primary infrastructures for motor control of alimentary behaviors (ingestive cyclicities; Grillner, 1985; MacNeilage, 1998), such as chewing or sucking. CPGs have only been observed directly in nonhuman preparations and have supported the suggestion that similar mechanisms are active in humans (Delcomyn, 1980, Marder & Bucher, 2001, Marder & Calabrese, 1996). In humans, brainstem-level motoneurons are presumed to be activated by CPGs composed of interneurons that contain specific motor programs for coordinating specific motor patterns. These specialized neural networks execute specific motor plans by activating or inhibiting target motoneurons in a sequence to produce patterned movement (Grillner, 2003b). The complexity of CPGs ranges from simple circuits that mediate very short latency reflexes to more complex structures underlying patterned generation, such as sucking or mastication. These specialized networks may also undergo the process of fractionation and recombination for more complex (skilled) motor behaviors, such as speech, with motor learning (use) and sensory feedback playing a critical role in the recombination of the complex motor acts (Grillner, 1985, 1991, 2003a, 2003b).
The influence of CPGs in the emergence of a motor control structure for early babble is unknown. Grillner (1982) hypothesized that in speech development, fractionations of the masticatory CPG are recombined to provide the necessary coordinative organization. This hypothesis, which has been further advanced by Lund and Kolta (2006), has been used to support prevailing linguistic models of speech development (frame/content theory; MacNeilage, 1998). According to this representation, differences in motor control of the mandible during chewing and speech arise from the reorganization (recombination) of subpopulations of neurons within the CPG to generate the observed range of mandibular behaviors and movement patterns.
CPG networks also influence coordination for vocalizations in animals. Jürgens (1998, response to MacNeilage) has described distinct neural mechanisms in animal models (Jürgens, 1998, 2002) by which underlying CPG networks differentially influence mandibular kinematics during vocalization and chewing. Data from humans provide further support to Jürgens’s argument in that mandibular kinematics during speech in adults is characterized by comparatively faster movements, more complex trajectories, and fewer total excursions than chewing (Gibbs & Masserman, 1972). Modulated EMG during chewing is typically cyclic; jaw elevators are reciprocally activated with jaw depressors to generate a pattern that is easily observed in children from 9 to 48 months of age (Green et al., 1997; Moore & Ruark, 1996; Steeve et al., 2008) and adults (e.g., Møller, 1966; Moore, 1993; Moore et al., 1988). Additionally, this modulated activity is highly correlated with consequential changes in jaw height (e.g., A. Smith, 2006). If central pattern generation influences the timing of muscle activity and associated movement of the jaw during chewing (e.g., Grillner, 1985; Luschei & Goldberg, 1981), then a linear relationship should be noted for frequency of modulated activity among jaw muscle groups and corresponding changes in jaw height; however, because more diffuse neural networks or task-related goals may differentially influence mandibular kinematics for vocalizations (e.g., Jürgens, 1998; Moore et al., 1988; A. Smith, 2006), this relationship may be weakened considerably.
Jaw oscillation is another mandibular behavior that has frequently been advanced as an extant motor platform for early babble (e.g., Meier et al., 1997; Thelen, 1991); these early appearing repetitive jaw movements have been variously called jaw oscillations, jaw wags, silent mandibular oscillations, silent babble, and mandibular oscillations. Jaw oscillation has been identified as a motor stereotypy that provides a motor infrastructure that can be exploited for early babble productions; this behavior has been claimed to exhibit mandibular kinematics that are similar to those observed during early babble (Meier et al., 1997). Physiologic investigations of mandibular motor control for jaw oscillation in adults fail to support these suggestions because the coordinative organization of mandibular muscle groups is distinct across behaviors associated with jaw oscillation, chewing, and speech (Moore, 1993; Moore et al., 1988). Unlike speech, jaw oscillation in adults exhibits modulated EMG activity that is highly correlated with jaw height (Moore, 1993; Moore et al., 1988). Among non-speech behaviors, differences in jaw kinematics have not been specifically addressed for an alimentary behavior, such as chewing, and another motor stereotypy not associated with feeding, such as jaw oscillation. Moreover, indices of mandibular coordination for jaw oscillation and babble in infants have not been compared.
Emerging Control of the Mandible: The Interface Between Speech and Language
An infant’s control of the mandible for prelinguistic vocalizations is likely shaped by the interaction among linguistic and vocalization systems, which is unique to each moment in development. A. Smith (2006) proposed a balanced, bidirectional influence in speech and language development for the hearing child (see review by Guenther, 2006) by which emerging linguistic objectives shape motor commands, and motor constraints delimit linguistic goals. These language and motor interfaces are apparent in 4- and 5-year-old children in that organized motor commands for speech are influenced by phonetic, phrase, and sentence-level goals. Multilayered mapping between language (linguistic units) and motor control for speech is already apparent in the preschooler (A. Smith, 2006). This process may be evident in differences observed in the organization of motor behaviors for mandibular movement in 15-month-olds during babble and true word productions (Moore & Ruark, 1996). Coordinative synergies among mandibular muscle groups organized distinctively for reduplicative multisyllabic utterances that differed only in terms of linguistic complexity (nonreferential vs. referential utterance; Moore & Ruark, 1996). Differences in mandibular kinematic and in the coordinative organization underlying muscle groups across categories of vocalizations differing in linguistic complexity (Stoel-Gammon, 1989) would support the idea that diffuse neural systems associated with language and motor control for speech interact dynamically during these varied productions; a disassociation between speech and nonspeech behaviors would indicate that the networking of neural systems is different among these behaviors.
Another factor influencing the motor control-language interface is that during the first years of life, the musculoskeletal system (e.g., Kent 1981, 1992; Kent & Vorperian, 1995) and the neuroanatomic/physiologic components (e.g., Netsell, 1981) underlying speech production undergo rapid growth and development, which influences the emergence of language processes. The linguistic complexity (phonemes and syllable shape) of early babble is often described as being less complex than those forms of babble that emerge later (B. L. Smith, Brown-Sweeny, & Stoel-Gammon, 1989; Stoel-Gammon, 1989). The infant’s vocal tract and related mechanisms undergo significant morphologic and histologic changes supporting the development of an infant’s phonetic capability to produce the breadth of vowels and consonants observed by 2 years of age (Kent, 1981, 1992). At around 6–9 months of life, the infant exhibits the ability to produce syllable sequences. This ability coincides with the general appearance of repetitive movement sequences in an infant’s limbs and other structures, often defined as rhythmic stereotypies (Kent, 1984; Thelen, 1991). It may be that the earliest appearing, least complex forms of babble represent rhythmic stereotypies from which more complex speech movements emerge. Early multisyllabic productions, such as vowel or canonical babble, may be the vocal manifestation of these repetitive or rhythmic movement patterns (Kent & Hodge, 1990; Thelen, 1991). Early mandibular kinematics for vowel or reduplicative babble, for example, may exhibit predominately stable, oscillatory movement patterns of the jaw with these movements becoming less oscillatory as the infant exerts greater control for producing the more complex articulatory gestures required for speech (Kent, 1984; Kent & Hodge, 1990; M. R. Smith, 1984). It might be anticipated that observations of reduced phonetic repertoire and oscillatory jaw movement would be characterized as cyclic (i.e., simple waveform) during early multisyllabic vocalizations (i.e., vowel and reduplicative babble; Stoel-Gammon, 1989), whereas changes in jaw height would be expected to be more complex (i.e., complex waveform) for later appearing, more linguistically complex syllable sequences, such as variegated babble (i.e., Stoel-Gammon, 1989).
Experimental Aims
Ontogenetically and phylogenetically primitive jaw movement stereotypies (MacNeilage, 1998; Meier et al., 1997; Thelen, 1991) have been proposed as coordinative mechanisms that are later exploited for production of early vocalizations. Mandibular oscillations, for example, may provide a control structure for jaw movement during early syllable productions apart from any linguistic or sensory input and have been advanced as a process underlying early vocalizations in infants with hearing loss (von Hapsburg, Davis, & MacNeilage, 2008). This perspective suggests that jaw muscle activation and movement for early speech and nonspeech behaviors are similar and are universally apparent in infants. Alternatively, dissociations in these indices of mandibular coordination observed among early motor stereotypies, such as chewing and jaw oscillation, and early multisyllabic productions, combined with prior findings (e.g., Moore & Ruark, 1996), would support the idea that distinct neural infrastructures for motor control underlie mandibular coordination for speech and nonspeech behaviors. Though distinct coordinative and kinematic patterns have been observed across behaviors for older children and larger samples, it may be that with sufficient temporal resolution (i.e., through frequent observation), a relatively brief period (e.g., weeks) of emergent speech coordination could be detected during which shared coordinative infrastructures for speech and nonspeech behaviors are evident.
Although mandibular coordination for motor stereotypies, such as chewing and jaw oscillation, most likely result from distinct coordinative mechanisms similar to that observed for chewing and sucking in infants (e.g., Moore & Ruark, 1996; Steeve et al., 2008), it is unknown whether early motor stereotypies, such as jaw oscillation, rely on a coordinative infrastructure that can be exploited for early types of babble productions and whether the organization of motor control and associated kinematics vary across the nonspeech behaviors that are candidate motor stereotypies for speech (Thelen, 1991; e.g., jaw oscillation, chewing). Furthermore, the motor-language interface influences the emergence of motor control for speech (A. Smith, 2006), which introduces the question of whether mandibular motor control and kinematics vary among types of multisyllabic babble that differ by level of linguistic complexity (as defined in the Methods section; Stoel-Gammon, 1989). The present investigation was designed to address these empirical lapses by making frequent longitudinal measurements of jaw movement and the associated muscle activity during jaw oscillation, chewing, and several types of early multisyllables in a single child. Dependent measures were designed to be sensitive to small changes in coordinative complexity and organization and were derived from (a) the spectral characteristics of mandibular movement, (b) the spectral complexity of mandibular movement, (c) the linear relationship between spectral compositions of both jaw movement and EMG activity, and (d) pairwise cross-correlation between mandibular muscle group activity.
Method
Participant
Longitudinal data were gathered from one typically developing male infant, at 4–6-week intervals from 8 to 22 months (i.e., 8.2, 9.1, 9.3, 10.2, 12.1 13.2, 14.3 16.3, 17.1, 19.2, and 21.3 months), raised in a monolingual, American-English home environment. The parents previously indicated scheduling availability and a willingness to have their infant participate over the course of the experiment, and the infant demonstrated the ability to participate in the experimental protocol. These factors were important because it is technologically challenging to obtain multiple channels of data from an infant and to perform this procedure successfully and repeatedly over a period of 14 months.
Informal screenings were conducted for each session by a certified speech-language pathologist. The infant passed screenings for oral motor function and speech development milestones. Speech development was evaluated on the basis of the participant’s observed production of age-appropriate phonemes and syllable structures. The infant passed all screenings administered at 6, 12, 18, and 24 months by his pediatrician for cognitive and motor development, and there were no symptoms or signs of middle ear infection and no other health problems. He passed hearing screenings administered by an audiologist at birth, 12 months, and 24 months. During each session, there were no indications of delay or pathology associated with hearing, communication skills, cognition, or motor development.
Experimental Protocol
Longitudinal observations were initiated by the infant’s first productions of multisyllabic, vowel babble, which were observed at the age of 8 months (Level 1; Stoel-Gammon, 1989). During each experimental session, physiologic data were recorded from the infant while he was seated in an adjustable chair, eating and vocalizing spontaneously. These experimental sessions typically lasted from 40 to 60 min. The parent and experimenter played with the infant to elicit vocalizations. Periodically, video recordings of an infant babbling or audio recordings of an adult producing repetitive bisyllabic vocalizations were presented to the child to elicit vocalizations; however, the audio recording only yielded three productions at the age of 19.2 months, and these productions were not included in the current data set. The infant tended to watch or listen silently during these tasks. The most common and effective elicitation procedure was for the adult to remain silent while engaged in play activities with the infant, during which the infant would vocalize during this activity. Although some infant vocalizations occurred while the parent or experimenter was talking as a part of the play activity, such as the adult vocalizing for a puppet, review of video recordings of these data verified that the infant’s productions were not imitative.
Event-Related Classification of Data
Nonspeech Data
Individual cycles of jaw movement for chewing and jaw oscillation were isolated and parsed prior to analysis. Delimiting each movement cycle relied on zero-crossing in the first-order derivative of the vertical displacement trace of the jaw (e.g., discussed in the Parsing Event-Related Data section). Chewing and jaw oscillation samples were included in the corpus if they contained at least two cycles of jaw movement (i.e., changes in vertical jaw excursion between subsequent zero-crossings, valley-to-peak, greater than 1 mm; see Figure 1, Panel B). Chewing samples only included active patterns of continuous mastication (Luschei & Goldberg, 1981); the initial bite, bolus positioning, and the final swallow movements were excluded. Food consistency for the infant included pureed (e.g., applesauce), semisolid (e.g., banana), and solid (e.g., cereal) foods. Jaw oscillation samples included at least two successive cycles of jaw excursion without concomitant vocalizations or food (Meier et al., 1997).
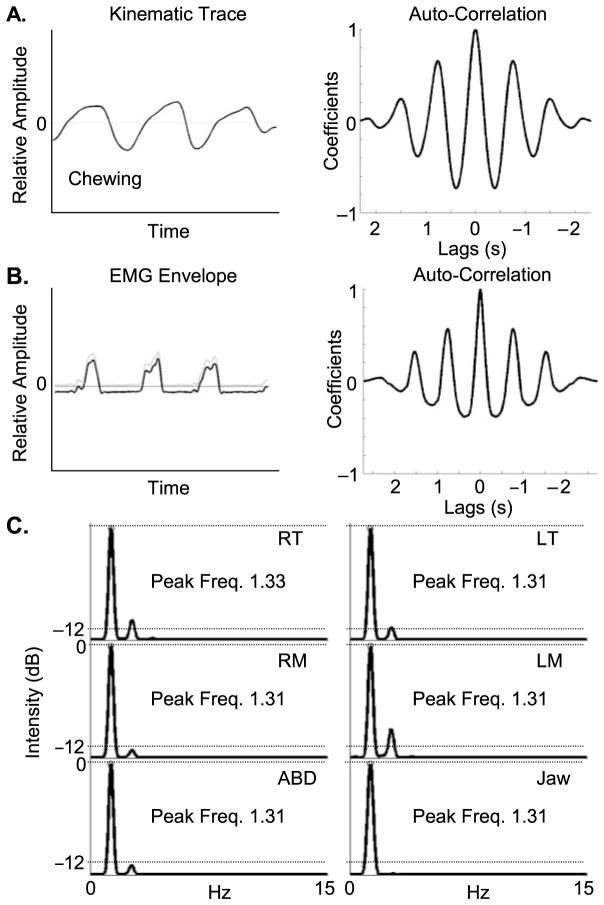
Figure 1.
Panel A illustrates placement of the kinematic markers, electromyography (EMG) electrodes, and microphone on the participant. Panel B illustrates that changes in jaw height between zero-crossings had to be greater than 1 mm; the original kinematic trace was detrended prior to spectral analyses. Panel C depicts the process for rectifying and filtering the raw EMG prior to further analysis.
Two observers used the above criteria and independently identified acceptable nonspeech samples in the video recordings and kinematic traces from the experimental sessions (discussed later in the article). Inter-observer agreement was perfect for all events subjected for further analysis.
Speech Data
An utterance was included in the corpus if it (a) was not coincident with food chewing, (b) included at least two syllables, (c) was produced with expiratory breath support, and (d) was speech-like (e.g., crying, screaming, and coughing were excluded). Each utterance was isolated from other events by at least 200 ms of silence. Speech data were categorized according to phonetic and syllabic complexity (linguistic complexity; Stoel-Gammon, 1989), which has been supported as a valid approach to distinguishing stages of speech motor development (e.g., Kent, 1992). The categories proposed by Stoel-Gammon (1989) are labeled as Level 1, Level 2, and Level 3.
Level 1 multisyllables consisted of syllable sequences that included a consonant vowel (CV) or vowel consonant (VC), with the consonant being a glide or glottal (i.e., not a true consonant); these vocalizations are commonly referred to as vowel babble. Level 2 multisyllables consisted of CV or VC sequences in which at least one consonant was a true consonant (i.e., excluding glide and glottal). If a sequence of at least two CV or VC syllables contained real consonants that had the same placement and manner (voicing could change), the syllables were considered reduplicative, even if a Level 1 syllable preceded or followed this sequence. Level 3 multisyllables contained at least two true consonants (i.e., excluding glide and glottal) that differed in place and manner of articulation (i.e., variegated syllables).
Two transcribers were trained to use the above procedures for classifying the vocalizations according to syllable length (i.e., monosyllabic and multisyllabic) as well as phonetic and syllabic complexity (i.e., Level 1, Level 2, and Level 3). Once trained, these transcribers independently reviewed the video clips of each vocal production. The presence of a referent (suggesting production of a true word) was not coded.
The infant produced a number of vocalizations that appeared to contain a referent (true words), especially when data were being acquired for the later age groups. The current protocol was not designed for reliably identifying and classifying these vocalizations; instead, vocalizations were classified according to linguistic complexity.
Acquisition of Kinematic Data
Mandibular position (i.e., vertical jaw position) was transduced using an infrared-sensitive, monochrome camera (Burle, TC351A) connected to a video recorder (Panasonic, AG-1980). A sample image of the infant shown in Figure 1, Panel A, was obtained from a video clip. Three flat, circular, reflective markers (~3 mm in diameter) were placed in the midline on the tip of the nose, the nasion, and superior to the protuberance of the mandible. The reference markers on the tip of the nose and the nasion were used to correct for head movement, and the nose marker served as the origin for the jaw marker. Target events were identified subsequently in reviewing the videotaped session, and these video clips were digitized using a commercially available video tracking system (Motus, Version 6.0; Peak Performance Technologies, 2000). This system used pattern recognition and tracking algorithms to extract position traces automatically from digital video recordings. Static calibration of this tracking system has indicated that jaw excursion in the ordinate plane (i.e., y axis) is accurate to at least 1 mm, which was equivalent to the precision used during the calibration procedure (Green, Moore, Higashikawa, & Steeve, 2000). The sampling rate for these kinematic data was 60 samples per second; data were subsequently low-pass filtered (flp = 8 Hz) using a zero-phase shift forward and reverse digital filter (Butterworth 8 pole filter, Matlab, Version 6.5; MathWorks, 2003).
Acquisition of EMG Data
Electromyographic data were obtained using small (1-cm disk), neonatal/pediatric surface electrodes (Kendall KittyCat) coupled to a Grass, Model 15, Neurodata Amplifier System, where these signals were band-pass filtered between 10 and 1000 Hz before being digitized in real time at a sampling rate of 10,100 samples per second with a sample resolution of 14 bits (Windaq Acquisition software, Version 2.54; Dataq Instruments, 2001). Over-sampling of the EMG channels was necessitated by the frequency bandwidth requirements of the audio signal. Prior to electrode placement, the skin surface was prepared using an abrasive skin prep gel (Nuprep, D. O. Weaver & Co., Aurora, CO). A conductive paste (Ten 20, D. O. Weaver & Co., Aurora, CO) was applied to the skin to ensure electrical and mechanical connections between the electrode and skin surface. Bipolar electrode placement followed procedures previously described by Moore and colleagues (refer to Green et al., 1997; Moore & Ruark, 1996; Steeve et al., 2008). Electrode spacing was approximately 2 cm, parallel with the main mass of each target muscle (see Figure 1, Panel A). The target muscles included the (a) left temporalis (LT), (b) right temporalis (RT), (c) left masseter (LM), (d) right masseter (RM), and (e) anterior belly of the digastric (ABD), which was recorded bilaterally using a single electrode pair. A ground electrode was placed on the forehead.
Acquisition of Audio Data
A miniature, omni-directional microphone (ECM-77B, Sony) was adhered to the forehead-mounted ground EMG electrode for the purpose of maintaining a constant mouth-to-microphone distance during the recording session (see Figure 1, Panel A). The microphone signal was high-pass filtered at 50 Hz (1 pole, 6 dB/octave) and preamplified (Pro MPA, Applied Research Technology) with the microphone preamplifier coupled to both the video recorder (Panasonic, AG-1980), which recorded the kinematic data, and to the computer, which digitized the EMG data. Audio data digitally recorded to the computer were anti-alias filtered (flp = 5000 Hz) prior to digitization (sampling rate = 10100 Hz).
Postprocessing of Event-Related Data
Synchronization
Because the EMG and kinematic data were recorded using two independent setups, the resultant sets of data needed to be synchronized; a Matlab-based custom syncing routine accomplished this task (Matlab, Version 6.5; MathWorks, 2003). The kinematic data were synced with the EMG data by performing a cross-correlation across the common audio signal, which was recorded as part of each data set. The peak coefficient and lag value determined the amount of offset between these two data sets. The synchronization point was identified as the singular alignment that yielded a cross-correlation coefficient of 1.0.
Once the EMG and kinematic data were synchronized, the jaw kinematic data, sampled at 60 samples per second, were up-sampled to match the sampling rate of the EMG data, which were sampled at 10,100 samples per second. These operations were also completed using a custom Matlab algorithm (Matlab, Version 6.5; Math-Works, 2003). The kinematic, EMG, and audio data were then represented in a single, time-aligned data set.
Parsing Event-Related Data
The kinematic, EMG, and audio data were simultaneously parsed from the continuous, synced data set using boundaries operationally defined using the jaw movement trace. The first-order derivative of this displacement trace (i.e., velocity) was used to identify movement boundaries; parsing for the onset and offset boundaries were determined using zero-crossings identified in this velocity signal. Speech-related events were bounded by the zero-crossings immediately before and after the audio signal. Chewing and jaw oscillation events were bounded by the zero-crossings closest to the given event. Those jaw kinematic data for dependent measures that included spectral compositions of jaw movement, and EMG data for one dependent measure that included pairwise cross-correlation between mandibular muscle groups activity, were parsed on these zero-crossings. On the basis of the boundaries defined by the zero-crossings, the algorithm automatically parsed EMG data 200 ms before and after these boundaries for computing the power spectra for the activity of each muscle group. This 200-ms window was adopted to include any bursting activity in the EMG signal that occurred prior to and following mandibular movement. These EMG data were defined as being associated with the mandibular movement. This observation coincides with the empirical results of other investigators (e.g., Netsell & Daniel, 1974).
Inclusion Criteria for Kinematic and EMG Data
Each video recording from which kinematic data were measured was reviewed to ensure that the reflective markers were within the measurement space and that the infant was facing the camera. Furthermore, samples of kinematic data were free of movement artifact, which was defined as abrupt (high-frequency) changes in the waveform not associated with mandibular excursion. The sample had to contain at least two cycles of jaw movement, defined as changes in vertical jaw excursion between subsequent zero-crossings (velocity trace; e.g., valley to peak) that were greater than 1 mm (see Figure 1, Panel B). Any sample not reaching these criteria was removed from the kinematic data set.
Electromyographic data were evaluated for the presence of extremely low levels of task-related EMG modulation, line noise, and movement artifact. Task-related modulation of EMG was defined as being 3 dB or greater than baseline. Baseline noise was measured using a power spectrum computed across each EMG channel; the signal between 0 and 1000 Hz had to be 14 dB greater than the total noise measured at 60, 180, 300, 420, 540, 660, and 780 Hz. EMG signals were free of movement artifact, defined as spurious signals not associated with muscle activity, such as low-frequency modulation of the EMG signal, creating a DC offset from baseline. Any sample not reaching the above criteria was removed from the EMG data set.
Conditioning Jaw Kinematic and EMG Signals
Jaw kinematic data (vertical jaw movement) were low-pass filtered (flp = 8 Hz) and then high-pass filtered (fhp = .5 Hz) using a zero-phase shift, forward and reverse digital filter (Butterworth 3 pole filter, Matlab, Version 6.5; MathWorks, 2003). Figure 1 (Panel B) depicts how this filtering process detrended the DC offset of the kinematic trace and reduced the amplitude of the lower frequency components of the jaw trace while retaining its higher frequency components; this process served to roughly equalize the movement spectrum and facilitated the identification of the low-frequency peaks of interest in these analyses.
Figure 1 (Panel C) illustrates rectification and low-pass filtering (flp = 15 Hz; zero-phase shift forward and reverse digital Butterworth 3 pole filter; Matlab, Version 6.5; MathWorks, 2003) of each EMG signal. This process yielded an amplitude envelope for each EMG signal, which was subsequently de-meaned to have a mean of zero (see Figure 2, Panel B). These amplitude envelope traces were used in all subsequent EMG analyses.
Figure 2.
Panel A illustrates the auto-correlation function computed across a detrended kinematic waveform. Panel B depicts the demeaning of an EMG amplitude envelope and the associated auto-correlation function computed across this trace. Panel C illustrates spectral compositions for jaw kinematics and EMG. The power spectra was computed across each auto-correlation function for the right temporalis (RT), left temporalis (LT), right masseter (RM), left masseter (LM), anterior belly of the digastric (ABD), and vertical jaw position (Jaw). Freq. = frequency.
Indices of Mandibular Coordination
Jaw Kinematic Measures
Frequency of mandibular movement: Peak spectral frequency
The first analysis evaluated task differences among measures of peak spectral frequency for vertical jaw movement during chewing, jaw oscillation, and categories of vocalizations. It was hypothesized that the dominant frequency of sequential movements of the mandible (i.e., changes in jaw height) would be greater during production of multisyllables than nonspeech behaviors (e.g., Gibbs & Masserman, 1972; Jürgens’s, 1998, response to MacNeilage).
Figure 2 (Panel A) illustrates vertical jaw movement during chewing by the infant. Following detrending, an autocorrelation function was computed for the sample, and the power spectrum of the autocorrelation function was computed; this process revealed the dominant frequency for vertical mandibular movement. A custom spectral analysis routine was written for Matlab (Version 6.5; MathWorks, 2003); an example of the resultant power spectrum is shown in Figure 2 (Panel C, Jaw). The Hanning window of the spectrum function was set to equal the length of the entire autocorrelation function to maximize the frequency resolution; the window overlap per computation was 75%. The number of coefficients set for the Discreet Fourier Transform was 219, yielding a resolution of approximately 750 points across the spectral range of 0–15 Hz. The frequency associated with the peak spectral energy was extracted from each spectrum (e.g., 1.31 Hz in Figure 2, Panel C, Jaw). This spectral peak yielded the dominant jaw movement frequency for each sample of the target behaviors. This analysis yielded one dependent measure for each parsed sample.
Complexity of mandibular movement: Complexity of spectral composition
The second analysis evaluated task differences in spectral complexity for vertical jaw movement during chewing, jaw oscillation, and categories of vocalization. It was hypothesized that this general measure of complexity would be greater for multisyllables than nonspeech behaviors (e.g., Gibbs & Masserman, 1972; Jürgens’s, 1998, response to MacNeilage).
For each kinematic trace, this analysis simply counted the number of spectral peaks that were greater than a −12-dB threshold computed for each power spectrum. Figure 2 (Panel C, Jaw) illustrates this threshold at −12 dB (re: 10 × log10 [peak spectrum/minimum non-zero value]) below the maximum spectral peak within a given spectrum. The single spectral peak noted in this spectrum indicated that jaw movement during this sample of chewing was nearly sinusoidal. Higher counts of spectral peaks represented greater movement complexity. This analysis yielded one dependent measure for each parsed sample.
EMG With Jaw Kinematic Measures
The third analysis evaluated task differences among peak correlations for EMG spectra and the associated spectra for vertical jaw movement during chewing, jaw oscillation, and the several categories of vocalizations. It was hypothesized that the correlations between EMG modulation spectra and jaw motion spectra would be higher during the nonspeech behaviors of jaw oscillation and chewing than during multisyllables.
Figure 2, Panels A and B, illustrates the autocorrelation functions computed for each detrended kinematic trace and each demeaned EMG envelope. The autocorrelation function revealed the dominant frequency component in these data. The spectral energy was computed from these autocorrelation signals using the same custom spectral function previously described for the jaw kinematic data. The spectral compositions for all five EMG channels (i.e., RT, LT, RM, LM, and ABD) and the jaw kinematic waveform are shown at the bottom of Figure 2, Panel C, and the peak spectral frequencies are noted. EMG spectra revealed the burst frequency for each EMG channel, whereas the jaw spectrum reflected the frequency of vertical jaw movement.
Most of the measures in Figure 2, Panel C, revealed that jaw movement occurred at about 1.31 Hz and that the peak frequencies calculated from the EMG amplitude envelopes were very close to this value. The four measures from the jaw elevators were averaged and reduced to a single representative measure for these agonist muscle groups. There was one measure for the ABD EMG. The peak frequencies for jaw elevator and ABD EMG spectra were each separately compared with the associated peak frequency for vertical jaw movement. Three dependent measures were used for these comparisons: spectra for (a) combined jaw elevator EMG, (b) ABD EMG, and (c) jaw position.
EMG Measures
The fourth analysis evaluated task differences for the strength of coupling among mandibular muscle groups for chewing, jaw oscillation, and categories of vocalizations. Ten pairwise cross-correlations were computed among the five EMG channels, resulting in 10 peak coefficients. It was hypothesized that there would be task differences across these quantities as has been reported in previous investigations (e.g., Moore & Ruark, 1996; Steeve et al., 2008).
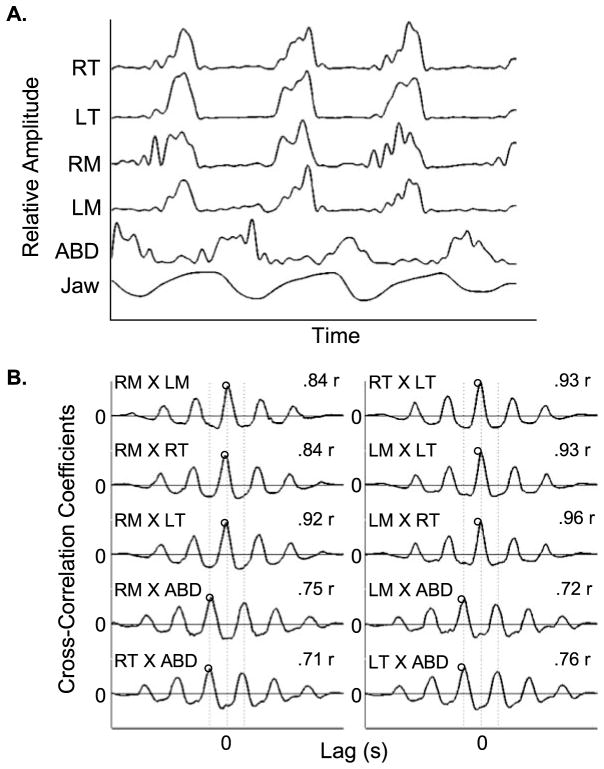
Figure 3, Panel A, illustrates the five EMG amplitude envelopes computed for a sample of chewing. For each parsed sample, pairwise cross-correlations were computed across the EMG amplitude envelopes, yielding a cross-correlation function for every combination of EMG waveforms (e.g., RM and LM EMG). Figure 3, Panel B, illustrates the results of each pairwise cross-correlation function for the EMG amplitude envelopes shown in Figure 3, Panel A. The peak coefficient for the cross-correlation function obtained for the RM and LM (i.e., RM × LM) EMG channels was .84. The coupling between the RM and LM appeared weaker than that of RT and LT (i.e., RT × LT), which yielded a peak coefficient of .93.
Figure 3.
Panel A illustrates the EMG amplitude envelope for RT, LT, RM, LM, ABD, and changes in vertical jaw position recorded from the participant during chewing. Panel B illustrates each pairwise cross-correlation computed across the EMG amplitude envelopes depicted in Panel A. The peak coefficient value represents the degree of coupling between pairwise comparisons of EMG.
Figure 3, Panel B, illustrates the extraction of ten peak coefficients for each parsed sample. These 10 values were reduced to five dependent measures representing (a) homologous pairs (RM × LM; RT × LT), (b) ipsilateral synergists (RM × RT; LM × LT), (c) contralateral synergists (RM × LT; LM × RT), (d) masseter antagonists (RM × ABD; LM × ABD), and (e) temporalis antagonists (RT × ABD; LT × ABD). This reduction entailed averaging of the coefficient values obtained for each pairwise comparisons for a given muscle group. For example, the homologous muscle group average was derived from the simple mean of RM × LM and RT × LT values.
Statistical Treatment: Planned Comparisons
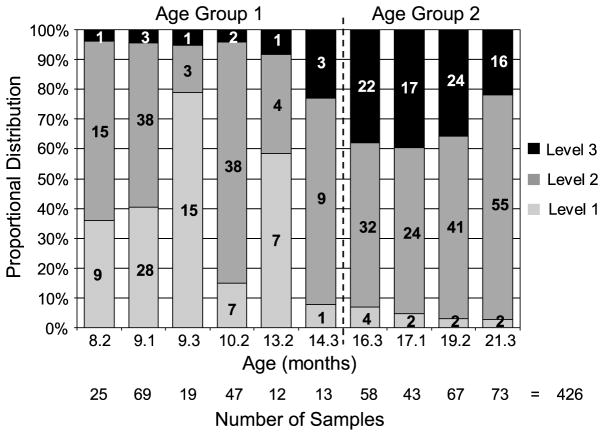
Nonparametric statistical analysis across sets of planned comparisons was necessary because the distributions of these data did not meet the required assumptions of normality and/or equal variance. Statistical analyses further stipulated that each cell in a comparison contained at least 10 samples. Chewing and multisyllabic vocalization data were reduced into two main age groups because at 16.3, 17.1, 19.2, and 21.3 months the vocalization data contained significantly more Level 3 and fewer Level 1 utterances than observed at younger ages of observation (see Figure 4). Data derived from jaw oscillation samples were not grouped by age of observation because these tokens were produced sporadically across ages, though they were observed most often for the younger ages (see Table 1).
Figure 4.
An illustration of the dispersion across age for multisyllabic vocalizations varying by three levels of linguistic complexity, with the total number of samples below each age and the grand total. Age was combined into two main groups: age 1 and age 2. For Age 1, there were significantly more Level 1 productions, whereas for Age 2, there were significantly more Level 3 vocalizations.
Table 1.
Distribution of chewing and jaw oscillation samples across age.
| Task | Age (months)
|
Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8.2 | 9.1 | 9.3 | 10.2 | 12.0 | 13.2 | 14.3 | 16.3 | 17.1 | 19.2 | 21.3 | ||
| Chewing | 24 | 19 | 67 | 30 | 38 | 164 | 51 | 69 | 21 | 38 | 20 | 541 |
| Jaw oscillation | 4 | 2 | 0 | 0 | 9 | 9 | 0 | 2 | 2 | 0 | 0 | 28 |
There were a total of 10 dependent measures. The jaw kinematic analyses included two dependent measures: spectra for (a) rate and (b) complexity of jaw position. EMG with jaw kinematic analysis included three dependent measures: spectra for (a) combined jaw elevator EMG, (b) ABD EMG, and (c) jaw position. The EMG analysis for coupling included five dependent measures: average peak coefficients for (a) homologous contralateral synergist muscle groups, (b) ipsilateral contralateral synergist muscle groups, (c) contralateral synergist muscle groups, (d) Masseter × ABD antagonist muscle groups, and (e) Temporalis × ABD antagonist muscle groups. The EMG with jaw kinematic analysis was statistically tested using a linear regression. The jaw kinematic spectra analyses (rate and complexity) and the EMG analysis for coupling across muscle groups were statistically tested using planned comparisons. For each dependent measure, planned comparisons were first performed across age groups for chewing and multisyllables. The next planned comparisons were across multisyllabic utterances differing by three levels of linguistic complexity (i.e., Levels 1, 2, and 3; Stoel-Gammon, 1989). Finally, planned comparisons were made among the nonspeech and speech behaviors.
Testing across Age Groups 1 and 2 composed the first planned comparisons. To determine whether developmental changes were apparent, a Mann–Whitney rank sum test was used to compare each dependent measure across age, within the behaviors of chewing and multisyllabic vocalizations. For this comparison, the error rate was .05. If an age effect was significant, then this dependent measure within a given behavior was further categorized into Age Groups 1 and 2 for subsequent planned comparisons.
To evaluate the significance of differences among multisyllabic utterances, each dependent measure was compared across the three levels of linguistic complexity using a Kruskal–Wallis one-way analysis of variance (ANOVA) on ranks, and the error rate was .05. If an age effect was obtained initially for a given dependent measure, then the main effect within Age Groups 1 and 2 was separately tested. If the Kruskal–Wallis ANOVAyielded a significant main effect for a dependent measure, then a Mann–Whitney rank sum test was used to compare this measure across the three planned comparisons for linguistic complexity (i.e., Level 1 × Level 2; Level 1 × Level 3; Level 2 × Level 3) using an alpha error rate of .05. If a dependent measure did not reach significance for a main effect or planned comparison, then it was not further subdivided according to level of linguistic complexity. However, significant differences among these comparisons of multisyllables were further subdivided according to level of linguistic complexity (i.e., Levels 1, 2, and 3) for the subsequent planned comparisons with nonspeech behaviors. The alpha error rate was then set to .01 because these comparisons were made between subsets of the multisyllabic data (Levels 1, 2, and 3) and nonspeech behaviors, either collapsed across age or within a given age group.
Following these across-age and within-behavior (for linguistic complexity) comparisons, planned comparisons for each dependent measure were performed among the nonspeech and speech behaviors using a Mann–Whitney rank sum test. If a dependent measure had reached significance across levels of linguistic complexity, then the alpha error rate was set to .01 because subsequent comparisons were made between nonspeech behaviors and subsets of the multisyllabic vocalizations (e.g., Level 1, Level 2, and/or Level 3); otherwise, the alpha error rate was set to .05. Each dependent measure was compared between jaw oscillation and multisyllabic vocalizations, which were categorized according to age group and/or level of complexity. This process was also performed across chewing and multisyllabic vocalizations. Each dependent measure was also subjected to planned comparisons across nonspeech behaviors using a Mann–Whitney rank sum test with an alpha error rate of .05; jaw oscillation was compared with the chewing task(s).
Results
Categorization of Data
A total of 757 vocalization samples were obtained from these longitudinal observations. These samples were categorized according to syllable length (i.e., monosyllable or multisyllable) and level of phonetic and syllabic (linguistic) complexity (i.e., three levels). The kappa level for category agreement between the two transcribers was .86 for syllable length and .88 for level of linguistic complexity (N = 757). Only those vocalizations that both transcribers agreed were multisyllabic were considered for this investigation. These samples of multisyllabic vocalizations had to be unambiguously assigned to a level of linguistic complexity; therefore, the two transcribers and a third judge classified those few tokens in which there was disagreement and consensus was required. If one of the three perceptual judges did not agree with either classification or judged that the token could not be reliably classified, then the token remained unclassified and was not included in the study. Figure 4 shows the distribution of 426 multisyllabic vocalizations that were perceptually classified with 100% agreement. Table 1 reports the distribution of tokens across age for chewing and jaw oscillation.
Figure 4 illustrates the distribution of multisyllabic utterances categorized according to linguistic complexity across age. A Spearman rank order test indicated a significant, negative correlation (r = −.85, p < .001) for the proportion of Level 1 utterances distributed across age, and a positive correlation (r = .80, p = .003) for Level 3 utterances distributed across age. These data were grouped into one earlier (i.e., Age 1; 8.2–14.3 months) and one later (i.e., Age 2; 16.3–21.3 months) age group for statistical comparisons because these age ranges exhibited a marked change in the distribution of Level 1 and Level 3 utterances. Those data recorded at 14.3 months were combined with Age 1 because the recording interval was closest to this age group. Because jaw oscillation samples were sporadically produced across sessions and few samples occurred within Age 2, these data were collapsed across all ages (see Table 1).
Within multisyllabic Level 2 vocalizations for Age 1, Judges 1 and 2, respectively, perceived 74% and 70% of these samples as reduplicative syllables; within Age 2, 90% and 91% of the samples were perceived as reduplicative. Reduplicative syllables were defined as containing repeated true consonants as judged by place and manner (voicing could change); the vowel was not perceptually judged.
The duration of each sample for a given behavior was measured. These measures of duration were statistically compared for behavior across age group (e.g., Chewing × Age) and compared across behaviors (e.g., Chewing × Jaw Oscillation). A Mann–Whitney rank sum test did not reveal significant differences for behavior across age group comparisons (e.g., chewing across age groups), and a main effect was not obtained for comparisons across behaviors using a Kruskal–Wallis one-way ANOVA on ranks (i.e., jaw oscillation; chewing within each age group; and multisyllables Levels 1, 2, and 3 within each age group). The average duration for samples of jaw oscillation was 1.68 s (SD = 0.58); the average duration for samples of chewing was 1.55 s (SD = 0.47); the average duration for samples of Level 1 multisyllables was 1.41 s (SD = 0.75); the average duration for samples of Level 2 multisyllables was 1.58 s (SD = 0.73); and the average duration for samples of Level 3 multisyllables was 1.66 s (SD = 0.63).
Statistical Comparisons: Indices of Coordination
The following tables show the results of the planned comparisons across measures for jaw kinematics and EMG coupling. Even though some of these comparisons did not reach significance, the findings are still tabulated for consistency and completeness. The relatively simple results for linear regression of EMG and jaw spectra are summarized in a single table.
Jaw Kinematic Measures
Frequency of mandibular movement: Peak spectral frequency
The first set of comparisons for jaw movement was for peak frequency (i.e., predominant rate) during nonspeech behaviors and multisyllables. Peak frequency of jaw movement in the frontal plane was the single dependent measure for this comparison. A developmental trend was not observed for these data as shown in Table 2; a Mann–Whitney rank sum test did not reach significance for within behavior differences across age for frequency of jaw movement. Multisyllabic vocalizations differing by level of linguistic complexity did not differ significantly either; a Kruskal–Wallis one-way ANOVA did not reveal a main effect among multisyllabic vocalizations differing by level of linguistic complexity. The remaining between behavior planned comparisons were made between jaw oscillation, chewing, and multisyllabic vocalizations using the Mann–Whitney rank sum test (see Table 3). Jaw oscillation and chewing were significantly slower than multisyllabic utterances (T = 4,105.5, p = .011; T = 199,368, p < .001). No other across behavior comparisons were statistically significant. The mean frequency of jaw motion for jaw oscillation was 1.65 Hz (SD = 0.56); the mean frequency of jaw motion for chewing was 1.57 Hz (SD = 0.34); and the mean frequency of jaw motion for multisyllables was 1.99 Hz (SD = 0.69).
Table 2.
Planned comparisons using a Mann–Whitney rank sum test to compare behavior by age group for differences in peak frequency of jaw movement.
| Comparison | Medians
|
T | p | |
|---|---|---|---|---|
| Age 1 | Age 2 | |||
| Chewing × Age Group | 1.53 | 1.52 | 41,285.5 | .412 |
| Multisyllabic × Age Group | 1.86 | 1.76 | 28,331.5 | .473 |
Note. α = .05.
Table 3.
Planned comparisons using a Mann–Whitney rank sum test to compare between-behavior differences in peak frequency of jaw movement.
| Comparison | Medians
|
T | p | |
|---|---|---|---|---|
| Task 1 | Task 2 | |||
| Jaw Oscillation × Chewing | 1.68 | 1.53 | 4,422.5 | .145 |
| Jaw Oscillation < Multisyllabic | 1.68 | 1.80 | 4,105.5 | .011a |
| Chewing < Multisyllabic | 1.53 | 1.80 | 199,368 | < .001a |
Note. α = .05.
Statistically significant.
Complexity of mandibular movement: Complexity of spectral composition
Complexity of jaw movement was evaluated in a second analytic approach, which used the simple count of spectral peaks as its single dependent variable. Each distinct peak rising above the −12-dB noise floor within the spectrum for each jaw kinematic trace was counted, yielding a broad index of jaw movement complexity. Table 4 shows that a developmental trend was observed for multisyllabic vocalizations. A Mann–Whitney rank sum test was significant for multisyllabic vocalizations across age (T = 29,752, p = .034), with complexity of jaw movement being greater for Age 1 than for Age 2 (see Table 4).
Table 4.
Planned comparisons using a Mann–Whitney rank sum test to compare behavior by age group for differences in complexity of jaw movement.
| Comparison | Spectral energy description | T | p |
|---|---|---|---|
| Chewing × Age Group | Often 1 peak of energy | 42,336 | .141 |
| Multisyllabic × Age Group: Age 1 > Age 2 | Age 1 had more samples of 1+ peaks | 29,752 | .034a |
Note. α = .05.
Statistically significant.
A post hoc analysis was performed separately on multisyllabic Level 2 and Level 3 (i.e., variegated syllables) vocalizations across age groups to determine whether jaw complexity within each syllable type significantly changed with development. A Mann–Whitney rank sum test indicated that multisyllabic Level 2 and Level 3 vocalizations significantly changed across Ages 1 and 2 (T = 12,964, p = .006; T = 758.5, p = .006), with jaw movement becoming simpler with increasing age.
Comparisons among multisyllables are depicted in Table 5. A Kruskal–Wallis one-way ANOVA on ranks indicated a significant difference for complexity of mandibular movement across multisyllabic utterances differing by level of linguistic complexity; main effects were observed at Age 1 ( p = .002) and at Age 2 ( p = .004). Planned comparisons within each age group were performed among these levels of syllabic complexity using a Mann–Whitney rank sum test; however, fewer than 10 tokens were noted for Level 1 within Age 2, so these data were excluded from further tests. Within each age group, jaw movement was more complex for multisyllabic Level 3 vocalizations than for Level 2 vocalizations (T = 910.5, p = .015; T = 9,126, p = .045). Within Age 1, jaw movement for Level 3 multisyllables was more complex than for Level 1 multisyllables (T = 454.5, p = .002). Level 3 multisyllables are composed of variegated syllables, whereas Levels 1 and 2 are consistent with vowel babble and reduplicative syllable production.
Table 5.
Within-behavior comparisons for complexity of jaw movement across different classifications of multisyllabic vocalizations.
| Comparison | Spectral energy description | T | p |
|---|---|---|---|
| Multisyllabic × Level: Age Group 1 | .002a | ||
| Multisyllabic × Level: Age Group 2 | .004a | ||
| Within Age Group 1 Comparisons | |||
| Multisyllabic 1 × Multisyllabic 2 | Multisyllabic 3 often had samples of 2 or 3 peaks of energy | 2,352.5 | .125 |
| Multisyllabic 1 < Multisyllabic 3 | 454.5 | .002a | |
| Multisyllabic 2 < Multisyllabic 3 | 910.5 | .015a | |
| Within Age Group 2 Comparisons | |||
| Multisyllabic 1 × Multisyllabic 2 | NT | NT | |
| Multisyllabic 1 × Multisyllabic 3 | NT | NT | |
| Multisyllabic 2 < Multisyllabic 3 | 9,126 | .045a | |
Note. Within-behavior main effects were tested using a Kruskal–Wallis one-way analysis of variance (ANOVA) on ranks. A main effect was obtained in each age group, and a Mann–Whitney rank sum test was used to test for differences of jaw movement complexity across a set of planned comparisons. NT = not tested; α = .05.
Statistically significant.
Significant differences for complexity of jaw movement were measures among speech and nonspeech behaviors. Planned comparisons across behaviors were tested using a Mann–Whitney rank sum test, and these results are presented in Table 6. Jaw movement during chewing was simpler than that noted for multisyllabic Level 2 and Level 3 utterances within Age 1 (T = 39,580.5, p < .001; T = 5,941, p < .001) and multisyllabic Level 3 utterances within Age 2 (T = 29,370.5, p < .001). Movement for jaw oscillation was significantly less complex than that noted for Level 3 multisyllables within Age 1 (T = 356, p = .001).
Table 6.
Planned comparisons using a Mann–Whitney rank sum test to compare between behaviors for differences in complexity of jaw movement.
| Comparison | Spectral energy description | T | p |
|---|---|---|---|
| Jaw Oscillation × Chewing | 9,035 | .200 | |
| Multisyllabic Age Group 1 Comparisons | |||
| Jaw Oscillation × Multisyllabic 1 | 942.5 | .909 | |
| Jaw Oscillation × Multisyllabic 2 | Nonspeech behaviors often had 1 peak of energy | 1,502.5 | .122 |
| Jaw Oscillation < Multisyllabic 3 | 356 | .001a | |
| Chewing × Multisyllabic 1 | 12,914.5 | .107 | |
| Chewing < Multisyllabic 2 | 39,580.5 | < .001a | |
| Chewing < Multisyllabic 3 | Multisyllables had more samples of 2 or more peaks of energy | 5,941 | < .001a |
| Multisyllabic Age Group 2 Comparisons | |||
| Jaw Oscillation × Multisyllabic 2 | 2,403 | .876 | |
| Jaw Oscillation × Multisyllabic 3 | 1,312.5 | .249 | |
| Chewing × Multisyllabic 2 | 52,239 | .025 | |
| Chewing < Multisyllabic 3 | 29,370.5 | < .001a | |
Note. α = .05.
Statistically significant.
Jaw kinematic measures: Summary
Frequency of jaw movement was significantly lower for nonspeech behaviors than for early multisyllabic productions. Complexity of mandibular movement decreased from 1 to 2 years of age for multisyllabic vocalizations for Level 2 and Level 3 multisyllables. Variegated multisyllables (i.e., Level 3) exhibited greater mandibular movement complexity than Level 1 multisyllables within Age 1 and than Level 1 and Level 2 multisyllables at both age levels. Mandibular movement was less complex for chewing than multisyllabic Level 2 and Level 3 vocalizations within Age 1 and Age 2, and it was also less complex for jaw oscillation than for multisyllabic Level 3 vocalizations within Age 1.
EMG With Jaw Kinematic Measures
This third measure evaluated the linear relationship of peak frequencies of EMG amplitude modulation with the associated jaw movement. For each parsed sample, there were three dependent measures: the peak spectral frequencies for (a) jaw movement, (b) jaw elevator muscle activity (bilateral masseter and temporalis EMG), and (c) jaw depressor muscle activity (ABD and other suprahyoids EMG). Linear regressions were computed for EMG elevator and depressor muscle groups with the peak frequency of associated jaw movement to yield two regressions: (a) peak frequency of EMG modulation and (b) peak frequency of jaw movement.
As already discussed and presented in Table 2, no age effect was obtained for jaw movement frequency during chewing or multisyllabic productions, as tested using a Mann–Whitney rank sum test. Similarly, no developmental trends were noted for peak frequency of EMG modulation for jaw elevator or depressor muscles groups during chewing or multisyllabic productions. Accordingly, data for chewing and multisyllables were collapsed across age. Comparisons among spectral peak measures (EMG and kinematic) within behaviors were also made across productions of multisyllables differing in level of linguistic complexity. A Kruskal–Wallis one-way ANOVA on ranks did not yield a main effect; data for multisyllables were not categorized according to level of complexity.
As noted in Table 7, jaw oscillation and chewing exhibited significant linear relationships between peak frequencies of EMG modulation (both elevator and depressor muscle groups) and associated jaw movement (p < .001), whereas multisyllables did not. This result is consistent with the finding that multisyllabic utterances exhibited greater spectral complexity than the other behaviors studied.
Table 7.
Linear regression between peak frequency of electromyography (EMG) modulation for jaw elevator and depressor muscle groups across associated jaw movement for jaw oscillation, chewing, and multisyllabic vocalizations.
| Task | r | r2 | m(slope) | b(intercept) | p |
|---|---|---|---|---|---|
| EMG Elevators × Jaw Kinematics | |||||
| Jaw Oscillation | .635 | .403 | .798 | 0.013 | < .001a |
| Chewing | .497 | .247 | .843 | 0.273 | < .001a |
| Multisyllabic | .071 | .005 | .119 | 1.04 | .183 |
| EMG Depressors × Jaw Kinematics | |||||
| Jaw Oscillation | .724 | .403 | .798 | 0.013 | < .001a |
| Chewing | .194 | .038 | .523 | 0.606 | < .001a |
| Multisyllabic | .013 | .000 | .030 | 1.21 | .812 |
Note. α = .05.
Statistically significant.
EMG Measures
Coupling of EMG activity: Pairwise cross-correlation between EMG amplitude envelopes
The coupling of activity in mandibular muscles was evaluated in a fourth measure of these developing behaviors. Five dependent correlational measures were obtained: (a) homologous synergistic muscle groups, (b) ipsilateral synergistic muscle groups, (c) contralateral synergistic muscle groups, (d) Masseter × ABD antagonistic muscle groups, and (e) Temporalis × ABD antagonistic muscle groups. As depicted in Table 8, chewing and multisyllabic productions each exhibited increased coupling (i.e., higher peak coefficient values) with age for homologous (chewing—not significant; multisyllables, T = 28,112, p = .012), ipsilateral (chewing, T = 66,072, p < .001; multisyllables—not significant), and contralateral (chewing, T = 67,467, p < .001; multisyllables, T = 24,385, p < .001) synergistic muscle groups, as tested by a Mann–Whitney rank sum test. For antagonist muscle groups, chewing revealed decreased coupling across age for masseter antagonists (T = 43,716.5, p = .026), whereas multisyllables showed an increase in coupling for masseter antagonists (T = 31,235.5, p = .006) and temporalis antagonists (T = 28,096, p < .001). These developmental changes are consistent with prior investigations (e.g., Steeve et al., 2008).
Table 8.
Planned comparisons using a Mann–Whitney rank sum test to compare behavior by age group differences in coupling between pairwise comparisons of EMG modulation.
| Muscle group comparisons | Medians (r)
|
T | p | |
|---|---|---|---|---|
| Age 1 | Age 2 | |||
| Within Homologous Comparisons | ||||
| Chewing × Age Group | .850 | .870 | 49,723 | .395 |
| Multisyllabic × Age Group: Age 1 < Age 2 | .605 | .650 | 28,112 | .012a |
| Within Ipsilateral Comparisons | ||||
| Chewing × Age Group: Age 1 < Age 2 | .730 | .860 | 66,072 | < .001a |
| Multisyllabic × Age Group | .625 | .600 | 31,515.5 | .668 |
| Within Contralateral Comparisons | ||||
| Chewing × Age Group: Age 1 < Age 2 | .680 | .860 | 67,467 | < .001a |
| Multisyllabic × Age Group: Age 1 < Age 2 | .515 | .630 | 24,385 | < .001a |
| Within Masseter Antagonist Comparisons | ||||
| Chewing × Age Group: Age 1 > Age 2 | .450 | .420 | 43,716.5 | .026a |
| Multisyllabic × Age Group: Age 1 < Age 2 | .490 | .570 | 31,235.5 | .006a |
| Within Temporalis Antagonist Comparisons | ||||
| Chewing × Age Group | .430 | .440 | 49,401.5 | .715 |
| Multisyllabic × Age Group: Age 1 < Age 2 | .420 | .490 | 28,096 | < .001a |
Note. α = .05.
Statistically significant.
Significant differences in coupling were observed across multisyllables differing in linguistic complexity. These results are presented in Table 9. Because of the developmental effects described above, within-age comparisons were made for homologous and contralateral synergists as well as for masseter and temporalis antagonists. A Kruskal–Wallis one-way ANOVA on ranks revealed a main effect for homologous ( p = .040) and contralateral ( p = .002) comparisons within Age 1, and for masseter ( p = .032) and temporalis ( p = .012) antagonist comparisons within Age 2. A Mann–Whitney rank sum test indicated that coupling was greater for multi-syllabic Level 1 (i.e., vowel babble) than for multisyllabic Level 2 (e.g., reduplicative syllables) vocalizations for homologous and contralateral synergists within Age 1 (T = 4,772, p = .012; T = 4,827, p < .001) and that coupling was greater for multisyllabic Level 2 than for multi-syllabic Level 3 (e.g., variegated syllables) vocalizations for masseter and temporalis antagonists within Age 2 (T = 7,185.5, p < .001; T = 7,057, p = .004).
Table 9.
Within-behavior comparisons for coupling measured among EMG muscle groups across different classifications of multisyllabic vocalizations.
| Muscle group comparisons | Medians (r)
|
T | p | |
|---|---|---|---|---|
| Task 1 | Task 2 | |||
| Within Homologous Comparisons | ||||
| Multisyllabic × Level: Age Group 1 | .040a | |||
| Multisyllabic × Level: Age Group 2 | .098 | |||
| Within Ipsilateral Comparisons | ||||
| Multisyllabic × Level | .982 | |||
| Within Contralateral Comparisons | ||||
| Multisyllabic × Level: Age Group 1 | .002a | |||
| Multisyllabic × Level: Age Group 2 | .393 | |||
| Within Masseter Antagonist Comparisons | ||||
| Multisyllabic × Level: Age Group 1 | .738 | |||
| Multisyllabic × Level: Age Group 2 | .032a | |||
| Within Temporalis Antagonist Comparisons | ||||
| Multisyllabic × Level: Age Group 1 | .955 | |||
| Multisyllabic × Level: Age Group 2 | .012a | |||
|
| ||||
| Within Homologous Comparisons | ||||
| Multisyllabic 1 > Multisyllabic 2: Age Group 1 | .670 | .540 | 4,772 | .012a |
| Within Contralateral Comparisons | ||||
| Multisyllabic 1 > Multisyllabic 2: Age Group 1 | .570 | .460 | 4,827 | < .001a |
| Within Masseter Antagonist Comparisons | ||||
| Multisyllabic 2 > Multisyllabic 3: Age Group 2 | .600 | .500 | 7,185.5 | .010a |
| Within Temporalis Antagonist Comparisons | ||||
| Multisyllabic 2 > Multisyllabic 3: Age Group 2 | .510 | .425 | 7,057 | .004a |
Note. Within-behavior main effects were tested using a Kruskal–Wallace one-way ANOVA on ranks. For main effects, a Mann–Whitney rank sum test was used to test for differences in coupling across a set of planned comparisons. α = .05.
Statistically significant.
Across behavior differences were tested using a Mann–Whitney rank sum test, which revealed significant differences between the nonspeech behaviors and multisyllables and between chewing and jaw oscillation. Homologous muscle group comparisons are shown in Table 10; ipsilateral comparisons are shown in Table 11; and contralateral comparisons are shown in Table 12. Chewing exhibited greater coupling than multisyllables across each of the dependent measures associated with synergistic muscle groups ( p < .001), except for one comparison. Homologous comparisons (see Table 10) included chewing (collapsed across age) and multisyllables within Age 1 (Level 1, T = 28,479.5; Level 2, T = 13,145.5) and within Age 2 (collapsed across level, T = 63,217.5); ipsilateral comparisons (see Table 11) included chewing within Age 1 and Age 2 compared with multisyllables collapsed across level (T = 158,686; T = 57,322.5); and contralateral comparisons (see Table 12) included chewing and Level 2 multisyllables within Age 1 (T = 15,552.5) and chewing and multisyllables collapsed across level within Age 2 (T = 38,218). Differences were noted among the nonspeech behaviors; chewing within Age 2 had significantly greater coupling than jaw oscillation among ipsilateral (see Table 11; T = 1,424, p < .001) and contralateral (see Table 12; T = 1,168.5, p < .001) comparisons.
Table 10.
Planned comparisons between behaviors using a Mann–Whitney rank sum test to measure differences in coupling among pairwise comparisons of EMG modulation for homologous synergists.
| Within homologous comparisons | Medians (r)
|
T | p | |
|---|---|---|---|---|
| Task 1 | Task 2 | |||
| Jaw Oscillation × Chewing | .840 | .850 | 7,654.5 | .976 |
| Multisyllabic Age Group 1 Comparisons | ||||
| Jaw Oscillation > Multisyllabic Level 1 | .840 | .600 | 3,553 | < .001a |
| Jaw Oscillation > Multisyllabic Level 2 | .840 | .540 | 2,451 | < .001a |
| Chewing > Multisyllabic Level 1 | .850 | .600 | 28,479.5 | < .001a |
| Chewing > Multisyllabic Level 2 | .850 | .540 | 13,145.5 | < .001a |
| Multisyllabic Age Group 2 Comparisons | ||||
| Jaw Oscillation > Multisyllabic | .840 | .650 | 5,225 | < .001a |
| Chewing > Multisyllabic | .850 | .650 | 63,217.5 | < .001a |
Note. α = .01.
Statistically significant.
Table 11.
Planned comparisons between behaviors using a Mann–Whitney rank sum test to measure differences in coupling among pairwise comparisons of EMG modulation for ipsilateral synergists.
| Within ipsilateral comparisons | Medians (r)
|
T | p | |
|---|---|---|---|---|
| Task 1 | Task 2 | |||
| Jaw Oscillation × Chewing Age 1 | .720 | .730 | 7,276 | .616 |
| Jaw Oscillation < Chewing Age 2 | .720 | .860 | 1,424 | < .001a |
| Jaw Oscillation × Multisyllabic | .720 | .610 | 7,075 | .086 |
| Chewing Age 1 > Multisyllabic | .730 | .610 | 158,686 | < .001a |
| Chewing Age 2 > Multisyllabic | .860 | .610 | 57,322.5 | < .001a |
Note. α = .01.
Statistically significant.
Table 12.
Planned comparisons between behaviors using a Mann–Whitney rank sum test to measure differences in coupling among pairwise comparisons of EMG modulation for contralateral synergists.
| Within contralateral comparisons | Medians (r)
|
T | p | |
|---|---|---|---|---|
| Task 1 | Task 2 | |||
| Jaw Oscillation × Chewing Age 1 | .695 | .680 | 7,228 | .680 |
| Jaw Oscillation < Chewing Age 2 | .695 | .860 | 1,168.5 | < .001a |
| Within Multisyllabic Age Group 1 Comparisons | ||||
| Jaw Oscillation × Multisyllabic Level 1 | .695 | .570 | 1,330 | .139 |
| Jaw Oscillation > Multisyllabic Level 2 | .695 | .460 | 2,287.5 | < .001a |
| Chew Age 1 × Multisyllabic Level 1 | .680 | .570 | 12,888 | .019 |
| Chew Age 1 > Multisyllabic Level 2 | .680 | .460 | 15,552.5 | < .001a |
| Within Multisyllabic Age Group 2 Comparisons | ||||
| Jaw Oscillation × Multisyllabic | .695 | .630 | 3,908 | .688 |
| Chew Age 2 > Multisyllabic | .860 | .630 | 38,218 | < .001a |
Note. α = .01.
Statistically significant.
Comparisons among the antagonistic muscle groups revealed weaker coupling for chewing than for the other target behaviors. Comparisons for masseter antagonists are presented in Table 13, and temporalis antagonists are presented in Table 14. For masseter antagonists, chewing within Age 2 exhibited weaker coupling than Level 2 and Level 3 multisyllables (see Table 13; Level 2, T = 26,019, p < .001; Level 3, T = 10,211.5, p < .001), and chewing with Age 1 and Age 2 exhibited weaker coupling than jaw oscillation (see Table 13; T = 10,801.5, p < .001; T = 3,579, p < .001). For temporalis antagonists, chewing (collapsed across age) exhibited weaker coupling than Level 2 multisyllables within Age 2 (see Table 14; T = 59,377, p < .001) and jaw oscillation (see Table 14; T = 11,171, p < .001).
Table 13.
Planned comparisons between behaviors using a Mann–Whitney rank sum test to measure differences in coupling among pairwise comparisons of EMG modulation for masseter antagonists.
| Within masseter antagonist comparisons | Medians (r)
|
T | p | |
|---|---|---|---|---|
| Task 1 | Task 2 | |||
| Jaw Oscillation > Chewing Age 1 | .580 | .450 | 10,801 | < .001a |
| Jaw Oscillation > Chewing Age 2 | .580 | .420 | 3,579 | < .001a |
| Within Multisyllabic Age Group 1 Comparisons | ||||
| Jaw Oscillation × Multisyllabic | .580 | .490 | 3,500 | .016 |
| Chewing Age 1 × Multisyllabic | .450 | .490 | 64,464 | .032 |
| Within Multisyllabic Age Group 2 Comparisons | ||||
| Jaw Oscillation × Multisyllabic Level 2 | .580 | .600 | 2,568 | 446 |
| Jaw Oscillation × Multisyllabic Level 3 | .580 | .500 | 1,775 | .026 |
| Chewing Age 2 < Multisyllabic Level 2 | .420 | .600 | 26,019 | < .001a |
| Chewing Age 2 < Multisyllabic Level 3 | .420 | .500 | 10,211.5 | < .001a |
Note. α = .01.
Statistically significant.
Table 14.
Planned comparisons between behaviors using a Mann–Whitney rank sum test to measure differences in coupling among pairwise comparisons of EMG modulation for temporalis antagonists.
| Within temporalis antagonist comparisons | Medians (r)
|
T | p | |
|---|---|---|---|---|
| Task 1 | Task 2 | |||
| Jaw Oscillation > Chewing | .545 | .430 | 11,171 | < .001a |
| Within Multisyllabic Age Group 1 Comparisons | ||||
| Jaw Oscillation > Multisyllabic | .545 | .420 | 3,696.5 | < .001a |
| Chewing × Multisyllabic | .430 | .420 | 56,084.5 | .824 |
| Within Multisyllabic Age Group 2 Comparisons | ||||
| Jaw Oscillation × Multisyllabic Level 2 | .545 | .510 | 2,563.5 | .478 |
| Jaw Oscillation > Multisyllabic Level 3 | .545 | .425 | 1,840.5 | .007a |
| Chewing < Multisyllabic Level 2 | .430 | .510 | 59,377 | < .001a |
| Chewing × Multisyllabic Level 3 | .430 | .425 | 24,375 | .290 |
Note. α = .01.
Statistically significant.
Jaw oscillation exhibited greater coupling than multisyllables for both muscle synergists and antagonist comparisons, which was unlike that observed for chewing. These differences were observed for homologous comparisons (see Table 10; multisyllables, Age 1, Level 1, T = 3,553, p < .001; Level 2, T = 2,451, p < .001; multisyllables, Age 2, collapsed across level, T = 5,225, p < .001), contralateral comparisons (see Table 12; multisyllabic Age 1, Level 2, T = 2,287.5, p < .001), and temporalis antagonists comparisons (see Table 14; multisyllabic Age 1, collapsed across level, T = 3,696.5, p < .001; multisyllabic Age 2, Level 3, T = 1,840.5, p = .007).
EMG measures: Summary
For chewing and multi-syllabic vocalizations, coupling increased from Age 1 to Age 2 for homologous, ipsilateral, and contralateral synergists for chewing and multisyllabic vocalizations; for antagonists, coupling weakened with development of these behaviors. Similarly, homologous and contralateral synergists showed greater coupling among vocalizations for Level 1 than for Level 2 multisyllables within Age 1; masseter and temporalis antagonists showed greater coupling for Level 2 than for Level 3 multisyllables within Age 2.
During chewing, more rigid coupling was observed among homologous, ipsilateral, and contralateral synergists; weaker coupling was observed among antagonists. Compared with multisyllabic utterances, jaw oscillation revealed stronger coupling among homologous and contralateral synergists and among masseter and temporalis antagonists. Chewing exhibited weaker coupling than for jaw oscillation for ipsilateral and contralateral synergists only within Age 2, with greater coupling for chewing among masseter and temporalis antagonists, regardless of age.
Discussion
Data from several observational domains in this infant from 9 to 22 months of age failed to support the hypothesis that mandibular control for early babble exploits the putative motor stereotypy underlying chewing or jaw oscillation. All indices of coordination were categorically different among multisyllabic productions and nonspeech behaviors, which suggest that distinct coordinative infrastructures provide the frameworks for task-related goals of the behaviors studied: (a) jaw oscillation, (b) chewing, and (c) multisyllabic vocalizations. Motor control for multi-syllabic utterances appears to be influenced by the balanced interaction between developing motor and linguistic systems (A. Smith, 2006), such that variation in linguistic complexity systematically evinces changes in motor organization and jaw kinematics to meet these demands. This same effect was noted even among the nonspeech behaviors; task-dependent changes in mandibular control were noted across chewing and jaw oscillation.
Mandibular Kinematics Vary Among Types of Babble
Perceptual observations of phonetic and syllabic complexity reflected distinct stages of speech development (Kent, 1992; Stoel-Gammon, 1989, 1998) as the distribution of a metric of complexity changed significantly across age groups, with Age 1 containing few Level 3 tokens (i.e., variegated syllables) and Age 2 containing few Level 1 tokens (i.e., vowel babble; refer to Figure 4). Proportionally more reduplicative syllables (i.e., with true consonants) were produced within Level 2 for the later age group, which replicates previous findings (B. L. Smith et al., 1989; Stoel-Gammon, 1985). Comparable proportions of utterances within each age group were categorized as Level 2.
These stages of developing speech are probably influenced deterministically by the interaction of emergent linguistic and motor capacities (e.g., Kent, 1992; A. Smith, 2006); it would be difficult to model emergent speech and language otherwise. The infant’s evolving linguistic abilities were especially evident in the later age range when fewer productions of vowel babble (i.e., Level 1) and more variegated syllable productions (i.e., Level 3) were observed along with increased productions of reduplicative, canonical syllables (i.e., Level 2, true syllable productions; refer to Figure 4). This distribution was consistent with the maturation and development of the linguistic system as well as the reduced constraints imposed on the linguistic system by the capacities of the motor control system for speech (e.g., Kent, 1992; A. Smith, 2006). Growth and development of the musculoskeletal system and neuroanatomical/physiologic components underlying speech production influenced the infant’s capacity to produce more varied phonetic productions and to string these productions into syllable sequences (e.g., Kent, 1992; Kent & Vorperian, 1995; Netsell, 1981). This claim was supported by the finding that mandibular kinematics exhibited more complex movement patterns for variegated syllable productions (Level 3; see Table 5), which were more frequently observed for the later age group (refer to Figure 4).
Empirical evidence for the primary role of rhythmic stereotypies supporting the earliest appearing, least complex forms of babble (e.g., Kent, 1984; MacNeilage, 1998; Thelen, 1991) remains stubbornly equivocal. For example, the infant produced roughly the same proportion of Level 2 syllables during the earlier and later age groups, but the frequency of reduplicative syllable productions (i.e., two true consonants) increased across age groups (Age 1 = 74% and 70%; Age 2 = 90% and 91%). If Level 2 vocalizations presented roughly equal coordinative challenges, then the increasing frequency of production of true consonants at the later age should signal the influence of a maturing linguistic system. Fortunately, the cross-domain observations afforded by the present protocol permit an intuitively and ecologically more appealing interpretation. Because the observed jaw kinematics across age were less complex for both Level 2 (e.g., reduplicative) and Level 3 (i.e., variegated) syllables (see Table 4 and post hoc comparisons), it seems more likely that the child’s spoken word production was exhibiting the bidirectional interaction of the linguistic and speech motor systems. It seems reasonable that the interaction of these systems evolves into a multilayered mapping of the linguistic and motor control systems for speech to provide a richly and narrowly prescribed goal, which would entail equivalently well-specified and consistent movement parameters for mandibular movement (A. Smith, 2006).
Mandibular Motor Control Varies Among Types of Babble
Early motor control of multisyllabic productions does not appear to be constrained to a specific coordinative organization. Rather, these utterances exhibit plasticity across syllable types. Multisyllables differing by linguistic complexity require specific capabilities of the motor system for successful production of the given utterance type. Physiologic differences across multisyllabic utterances are consistent with results reported by Moore and Ruark (1996), in which mandibular muscle group activity was seen to be organized differently across occurrences of babble and true words. In the present investigation, the motor output varied across utterance types; this finding was exhibited in the production of Level 1 and Level 2 multisyllables for the early age group (Age 1; see Table 9) and in the production of Level 2 and Level 3 multisyllables for the later age group (Age 2; see Table 9). For the early age group, multisyllabic Level 1 vocalizations (vowel babble) exhibited significantly greater, more consistent (i.e., more highly correlated) EMG activity for homologous and ipsilateral synergist muscle groups than that observed for Level 2 productions; however, for the later age group, multisyllabic Level 2 productions exhibited greater coupling of EMG activity for masseter and temporalis antagonists than for Level 3 productions.
These relative differences in coupling across multi-syllabic utterances possibly reflected the child’s coordinative response to differences in task demands (e.g., Thelen, 1991), which reflected gradually narrowing linguistic goals. Greater coupling during vowel babble, for example, may be partially explained by the simplified syllable structure for these multisyllables, which is solely composed of glottals and glides; narrowly defined jaw movement patterns may characterize these productions. Level 3 multisyllables, in contrast, entail greater linguistic variation (e.g., including at least two different true consonants), which would similarly entail greater physiologic latitude (see Table 9). The same applies for the later age group comparison; for example, at least 90% of the Level 2 multisyllables were reduplicative babble, which may have relied on more consistent motor patterns than those supporting the variegated syllable structures of Level 3 vocalizations (see Table 9). Further support for this idea comes from the observed jaw movement complexity, which was greater for Level 3 than Level 2 multisyllables.
Emerging Mandibular Control: Differences Between Nonspeech Motor Stereotypies and Multisyllables
Theoretical questions persist regarding the postulated primary or supportive role of early alimentary behaviors (e.g., chewing, sucking; MacNeilage, 1998) and other nonspeech behaviors (e.g., jaw oscillation; Meier et al., 1997) as precursors for early speech development. The assumptions underlying these theories suggest that early nonspeech behaviors use coordinative mechanisms that are later exploited for production of early vocalizations, such as canonical babble. Prior physiologic investigations, like the current study, have consistently failed to support these assumptions (Green et al., 1997; Moore & Ruark, 1996; Steeve et al., 2008). The current comprehensive findings show that the emergence of mandibular control for nonspeech behaviors is significantly different from that for the production of multisyllables across all physiologic levels: jaw kinematics properties (see Tables 3 and 6), timing of EMG activity with respect to jaw movement (see Table 7), and EMG activation patterns (see Tables 10–14).
Jaw kinematic properties
The commonly expressed proposition that early mandibular behaviors are uniformly mediated by a common control structure is supported primarily by indirect observations and inferences of oromandibular movement (e.g., acoustic transcription). Chewing and jaw oscillation exhibited rates of mandibular movement that were significantly slower than during multisyllabic productions (see Table 3). Similarly, complexity of jaw movement during chewing was significantly less than during multisyllables that contained true consonants (i.e., Levels 2 and 3) at both the earlier and later age groups (see Table 6). Jaw oscillation was similarly less complex than variegated syllable (i.e., Level 3) productions for the earlier age group (see Table 6). During the 1st year of life, rate and/or complexity of jaw kinematics are distinctively different between nonspeech behaviors and early multisyllabic productions. The present kinematic findings are consistent with those of Gibbs and Masserman (1972), who reported significant differences of trajectory and frequency of jaw movement in humans (adults) during mastication and speech. These data also support Jürgens’s (1998, 2002) argument that neural systems associated with mastication and articulation are located in different regions of the brain and operate at very different frequencies, with jaw movement during chewing appearing as a categorically distinct cyclic movement pattern produced at a significantly slower frequency than during vocalization (Jürgens, 1998; see open peer commentary in MacNeilage, 1998).
Frequency of EMG activity and associated changes in jaw height
Significant differences were also obtained for early nonspeech behaviors and multisyllabic productions on spectral comparisons between EMG modulation and changes in jaw height. Modulated EMG activity is highly correlated with consequential changes in jaw height for chewing (e.g., A. Smith, 2006), and this observation is apparent for jaw oscillation measured in adults (Moore, 1993; Moore et al., 1988). This causal relationship of muscle activity and movement is not evident during speech. Co-contraction of jaw elevators and depressors, which yields dramatic increases in mandibular stiffness during speech behaviors, has been well documented for early, multisyllabic babble and true words in 9–15-month-olds (Moore & Ruark, 1996; Steeve et al., 2008) and in adults (Moore, 1993; Moore et al., 1988). Consistent with these prior investigations, linear regressions across the spectral peaks of EMG modulation for jaw elevators and depressors and associated jaw movement were significant only for chewing and jaw oscillation (see Table 7). Modulation of EMG activity was observed to be more phasic for jaw oscillation and chewing, whereas this type of modulation was not as apparent for multisyllabic productions.
Motor control: EMG activation patterns
A significant difference in muscle synergies for speech and nonspeech behaviors has been reported for adults (Moore, 1993; Moore et al., 1988) and for infants from 12 to 48 months (Green et al., 1997; 15 months, Moore & Ruark, 1996; 9–15 months, Steeve et al., 2008). This investigation extended these findings to a different motor stereotypy and jaw oscillation, and comparisons were made across multisyllables that were categorized according to linguistic complexity. The current results are consistent with prior suggestions that parallel (distinct) neural infrastructures for motor control underlie mandibular coordination for speech and nonspeech behaviors. These coordinative differences were further distinguished by differences between multisyllables and nonspeech behaviors for jaw kinematics and for the relationship between EMG activity and associated jaw movement.
Cross-correlations computed for pairs of EMG amplitude envelopes revealed stronger coupling among synergist muscle groups during chewing (see Tables 10–12) and jaw oscillation (see Tables 10 and 12) than during multisyllabic productions. Similarly, cross-correlation coefficients across antagonist pairs revealed stronger coupling during jaw oscillation than during production of multisyllables but weaker coupling during chewing than during production of multisyllables (see Tables 13 and 14). Modulation of EMG activity was qualitatively more phasic for jaw oscillation and chewing, whereas this type of modulation was not as apparent for multisyllabic productions. These physiologic measures do not support the suggestion that the coordinative organization of early nonspeech behaviors is exploited in the development of mandibular control for early multisyllabic utterances.
Emerging Mandibular Control: Differences Between Nonspeech Motor Stereotypies
Distinct coordinative patterns were also obtained for the two nonspeech behaviors observed. The typically reciprocal relationship among antagonist muscle groups during chewing has been described for both adults (Moore, 1993; Moore et al., 1988) and very young children (Green et al., 1997; Moore & Ruark, 1996; Steeve et al., 2008). Jaw oscillation as measured in adults (Moore, 1993; Moore et al., 1988) was found to exhibit a combination of reciprocity and coactivity across antagonists, depending on rate and extent of movement, and even on idiosyncratic subject effects. The infant studied in the present experiments exhibited similarly distinct coordinative infrastructures during chewing and jaw oscillation. Differences in motor control were obtained for coupling measured within EMG pairs.
Consistent with prior physiologic investigations (e.g., Moore & Ruark, 1996; Moore et al., 1988), chewing by this infant was characterized by reciprocal timing of cyclic muscle activity among jaw elevator and depressor muscle groups. Compared with chewing (and regardless of age), the coupling across antagonist muscle groups during jaw oscillation appeared to be more rigidly specified; cross-correlation coefficients revealed more rigid coupling for jaw oscillation among these muscle groups (see Tables 13 and 14). Coupling for chewing among ipsilateral and contralateral muscle groups increased across comparisons for age (see Table 8); this change resulted in more rigid specification among these synergist muscle groups during chewing than during jaw oscillation (see Tables 11 and 12). These findings suggest that emerging mandibular motor control for nonspeech behaviors develop as distinct coordinative infrastructures. These measures of mandibular motor control for chewing resembled those observed for infants, young children, and adults (Green et al., 1997; Moore, 1993; Moore & Ruark, 1996; Moore et al., 1988; Steeve et al., 2008), and emerging control for jaw oscillation resembled that observed for adults (Moore, 1993; Moore et al., 1988).
Developmental Changes
Prior investigations of oral motor behaviors and the emergence of mandibular control have provided empirical evidence of task specificity coincident with parallel developmental changes, suggesting that the development of the neural infrastructures underlying mandibular coordination for babble/speech and other nonspeech behaviors are influenced by maturation, development, and use (Green et al., 1997; Moore & Ruark, 1996; Steeve et al., 2008). Development of mandibular control across distinct oral motor behaviors entails sensorimotor experiences that differ categorically across behaviors. For example, models of speech development cite the importance of the auditory (e.g., Guenther, 2006) and linguistic (e.g., A. Smith, 2006) systems, whereas these systems are probably less influential in the development of chewing or jaw oscillation. This investigation revealed similar findings, in that intertask differences persisted across age, and developmental changes within each behavior were evident.
Across age group comparisons for multisyllabic utterances revealed lower movement complexity for the jaw for Level 2 and Level 3 productions at the later age level (Age 2; see Table 4 and post hoc comparisons in the Results section), which may reflect increased stabilization of this system with increased interaction among developing linguistic and motor systems. Muscle synergies for mandibular control stabilized with age to the extent that cross-correlations of EMG among synergists showed increased coupling for both chewing and multisyllables (see Table 8; within homologous, ipsilateral, and contralateral), whereas antagonists muscle groups exhibited less coupling during chewing but greater coupling for multisyllables (see Table 8; with masseter and temporalis antagonists). These results are consistent with prior investigations demonstrating that maturation paths of chewing and babble and speech behaviors are each characterized primarily by the refinement and rescaling of their own existing mandibular coordinative patterns rather than by newly emergent structures or generalizations of earlier established skills (Green et al., 1997; Moore & Ruark, 1996; Steeve et al., 2008).
Conclusion
Mandibular motor control relies on distinct coordinative infrastructures during the early development of jaw oscillation, chewing, and multisyllabic vocalizations, and this task-dependent, parallel organization of motor control persists throughout maturation. A motor stereotypy, such as jaw oscillation, does not exhibit motor patterns or associated kinematics that resemble those task-dependent, coordinative infrastructures required for multisyllabic utterances. Similarly, motor control for jaw oscillation, for example, does not appear to be the result of generalizations from an earlier precursor skill, such as chewing.
With respect to the validity of the observations of this single developing child, it is important to note that the organization of motor control for chewing in this infant closely resembled that previously observed in other infants (Moore & Ruark, 1996; Steeve et al., 2008), children (Green et al., 1997), and adults (Moore, 1993; Moore et al., 1988); motor control for jaw oscillation resembled that noted for adults (Moore, 1993; Moore et al., 1988); and motor control for early multisyllabic vocalizations resembled that noted in other infants (Moore & Ruark, 1996; Steeve et al., 2008) and in speech for adults (Moore, 1993; Moore et al., 1988).
Although the influences of the developing phonetic, phonological, and lexical systems remain to be revealed, it seems likely, even unavoidable, that the instantiation of multisyllabic utterances is the product of the developing motor and linguistic systems (A. Smith, 2006). The evident motor organization and kinematics of the mandible varied systematically across multisyllabic utterances differing in linguistic complexity. Biologic and linguistic growth and development (e.g., Kent, 1981, 1992; Netsell, 1981) provided the foundation for the infant to produce more complex linguistic structures, composed of reduplicative and variegated multisyllables, relying on stabilized motor abilities and relatively simplified movement patterns. These results suggest that with maturation, the multilayered mapping of linguistic and motor control capacities to evolving speech skills generates a richly and narrowly prescribed behavioral goal, which were observed in the present results as well-specified and consistent speech movements by the mandible (A. Smith, 2006).
Acknowledgments
This work was supported, in part, by the National Institutes of Health Idea Network of Biomedical Research Excellence Program (INBRE), National Center for Research Resources Grant P20 RR16474 as well as by National Institute on Deafness and Other Communication Disorders Grants R01 DC00822, T32 DC00033, and F31 DC00295; the University of Wyoming in Laramie; and the University of Washington in Seattle. We would like to acknowledge Katey Connaghan, Lakshmi Venkatesh, Jennell Vick, and Adam Politis for their assistance with data collection and analysis.
Contributor Information
Roger W. Steeve, University of Wyoming, Laramie
Christopher A. Moore, National Institute on Deafness and Other Communication Disorders, Bethesda, MD
References
- DataqInstruments. Windaq Acquisition (Version 2.54) [Computer software] Akron, OH: Author; 2001. [Google Scholar]
- Delcomyn F. Neural basis of rhythmic behavior in animals. Science. 1980 Oct 31;210:492–498. doi: 10.1126/science.7423199. [DOI] [PubMed] [Google Scholar]
- Gibbs CH, Masserman T. Jaw motion during speech. ASHA Reports. 1972;7:R104–R112. [Google Scholar]
- Green JR, Moore CA, Higashikawa M, Steeve RW. The physiologic development of speech motor control: Lip and jaw coordination. Journal of Speech, Language, and Hearing Research. 2000;43:239–255. doi: 10.1044/jslhr.4301.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JR, Moore CA, Ruark JL, Rodda PR, Morvée WT, VanWitzenburg MJ. Development of chewing in children from 12 to 48 months: Longitudinal study of EMG patterns. Journal of Neurophysiology. 1997;77:2704–2727. doi: 10.1152/jn.1997.77.5.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S. Possible analogies in the control of innate motor acts and the production of sound in speech. In: Grillner S, Lindblom B, Lubker J, Persson A, editors. Speech motorcontrol. New York, NY: Pergamon Press; 1982. pp. 217–230. [Google Scholar]
- Grillner S. Neurobiological bases of rhythm motor acts in vertebrates. Science. 1985 Apr 12;228:143–149. doi: 10.1126/science.3975635. [DOI] [PubMed] [Google Scholar]
- Grillner S. Recombination of motor pattern generators: Simple neuronal networks combine to produce complex versatile motor patterns. Current Biology. 1991;1:231–233. doi: 10.1016/0960-9822(91)90066-6. [DOI] [PubMed] [Google Scholar]
- Grillner S. Fundamentals of motor systems. In: Squire LR, Bloom FE, McConnell SK, Roberts JL, Spitzer NC, Zigmond MJ, editors. Fundamental neuroscience. 2. San Diego, CA: Academic Press; 2003a. pp. 753–766. [Google Scholar]
- Grillner S. The motor infrastructure: From ion channels to neuronal networks. Nature Reviews Neuroscience. 2003b;4:573–586. doi: 10.1038/nrn1137. [DOI] [PubMed] [Google Scholar]
- Guenther FH. Cortical interactions underlying the production of speech sounds. Journal of Communication Disorders. 2006;39:350–365. doi: 10.1016/j.jcomdis.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Jürgens U. Neuronal control of mammalian vocalization, with specific reference to the squirrel monkey. Naturwissenschaften. 1998;85:376–388. doi: 10.1007/s001140050519. [DOI] [PubMed] [Google Scholar]
- Jürgens U. Neural pathways underlying vocal control. Neuroscience and Biobehavioral Reviews. 2002;26:235–258. doi: 10.1016/s0149-7634(01)00068-9. [DOI] [PubMed] [Google Scholar]
- Kent RD. Articulatory-acoustic perspectives on speech development. In: Stark RE, editor. Language behavior in infancy and early childhood. New York, NY: Elsevier; 1981. pp. 105–126. [Google Scholar]
- Kent RD. Psychobiology of speech development: Coemergence of language and a movement system. American Journal of Physiology. 1984;246:R888–R894. doi: 10.1152/ajpregu.1984.246.6.R888. [DOI] [PubMed] [Google Scholar]
- Kent RD. The biology of phonologic development. In: Ferguson CA, Menn L, Stoel-Gammon C, editors. Phonological development: Models, research, and implications. Timonium, MD: York Press; 1992. pp. 65–90. [Google Scholar]
- Kent RD, Hodge MM. The biogenesis of speech: Continuity and process of early speech and language development. In: Miller JF, editor. Progress in research on child language disorders. Austin, TX: Pro-Ed; 1990. pp. 25–53. [Google Scholar]
- Kent RD, Vorperian MA. Development of the craniofacial-oral-laryngeal anatomy: A review. Journal of Medical Speech-Language Pathology. 1995;3:145–190. [Google Scholar]
- Lund JP, Kolta A. Brainstem circuits that control mastication: Do they have anything to say during speech? Journal of Communication Disorders. 2006;39:381–390. doi: 10.1016/j.jcomdis.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Luschei ES, Goldberg LJ. Neural mechanisms of mandibular control: Mastication and voluntary biting. In: Brooks VB, editor. Handbook of physiology—Section I: The nervous system, Volume II, motor control. Bethesda, MD: American Physiological Society; 1981. pp. 1237–1274. [Google Scholar]
- MacNeilage PF. The frame/content theory of evolution of speech production. Behavioral and Brain Sciences. 1998;21:499–546. doi: 10.1017/s0140525x98001265. [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D. Central pattern generators and the control of rhythmic movements. Current Biology. 2001;11:R986–R996. doi: 10.1016/s0960-9822(01)00581-4. [DOI] [PubMed] [Google Scholar]
- Marder E, Calabrese RL. Principles of rhythmic motor pattern generation. The American Physiological Society. 1996;76:687–717. doi: 10.1152/physrev.1996.76.3.687. [DOI] [PubMed] [Google Scholar]
- MathWorks. Matlab (Version 6.5) [Computer software] Natick, MA: Author; 2003. [Google Scholar]
- Meier RP, McGarvin L, Zakia RAE, Willerman R. Silent mandibular oscillations in vocal babbling. Phonetica. 1997;54:153–171. doi: 10.1159/000262219. [DOI] [PubMed] [Google Scholar]
- Møller E. The chewing apparatus: An electromyographic study of the action of muscles of mastication and its correlation to facial morphology. Acta Physiologica Scandinavica. 1966;69(Suppl 280):1–229. [PubMed] [Google Scholar]
- Moore CA. Symmetry of mandibular muscle activity as an index of coordinative strategy. Journal of Speech and Hearing Research. 1993;36:1145–1157. doi: 10.1044/jshr.3606.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CA, Ruark JL. Does speech emerge from earlier appearing motor behaviors? Journal of Speech and Hearing Research. 1996;39:1034–1047. doi: 10.1044/jshr.3905.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CA, Smith A, Ringel RL. Task-specific organization of activity in human jaw muscles. Journal of Speech and Hearing Research. 1988;31:670–680. doi: 10.1044/jshr.3104.670. [DOI] [PubMed] [Google Scholar]
- Netsell R. The acquisition of speech motor control: A perspective with direction for research. In: Stark RE, editor. Language behavior in infancy and early childhood. New York, NY: Elsevier; 1981. pp. 127–156. [Google Scholar]
- Netsell R, Daniel B. Neural and mechanical response time for speech production. Journal of Speech and Hearing Research. 1974;17:608–618. doi: 10.1044/jshr.1704.608. [DOI] [PubMed] [Google Scholar]
- Peak Performance Technologies. Motus (Version 6.0) [Computer software] Englewood, CO: Author; 2000. [Google Scholar]
- Smith A. Speech motor development: Integrating muscles, movements, and linguistic units. Journal of Communication Disorders. 2006;39:331–349. doi: 10.1016/j.jcomdis.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Smith BL, Brown-Sweeny S, Stoel-Gammon C. A quantitative analysis of reduplicative and variegated babbling. First Language. 1989;9:175–190. [Google Scholar]
- Smith MR. Intrinsic characteristics of activity: A comment on Kent’s psychobiology. American Journal of Physiology. 1984;246:R895–R896. doi: 10.1152/ajpregu.1984.246.6.R895. [DOI] [PubMed] [Google Scholar]
- Steeve RW, Moore CA, Green JR, Reilly KJ, Ruark McMurtrey JL. Babbling, chewing, and sucking: Oromandibular coordination at 9-months. Journal of Speech, Language, and Hearing Research. 2008;51:1390–1404. doi: 10.1044/1092-4388(2008/07-0046). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoel-Gammon C. Phonetic inventories, 15–24 months: Longitudinal study. Journal of Speech and Hearing Research. 1985;28:505–512. doi: 10.1044/jshr.2804.505. [DOI] [PubMed] [Google Scholar]
- Stoel-Gammon C. Prespeech and early speech development of two late talkers. First Language. 1989;9:207–224. [Google Scholar]
- Stoel-Gammon C. The role of babbling and phonology in early linguistic development. In: Wetherby AM, Warren SF, Reichle J, editors. Transitions in prelinguistic communication. Baltimore, MD: Brookes; 1998. pp. 87–110. [Google Scholar]
- Thelen E. Motor aspects of emergent speech: A dynamic approach. In: Krasnegor NA, Rumbaugh DM, Schiefelbusch RL, Studdert-Kennedy M, editors. Biological and behavioral determinants of language development. Hillsdale, NJ: Erlbaum; 1991. pp. 339–362. [Google Scholar]
- von Hapsburg D, Davis BL, MacNeilage PF. Frame dominance in infants with hearing loss. Journal of Speech, Language, and Hearing Research. 2008;51:306–320. doi: 10.1044/1092-4388(2008/023). [DOI] [PubMed] [Google Scholar]