Abstract
Electromyographic activity of bilateral mandibular muscle pairs in humans was studied during several tasks: mastication, voluntary oscillation of the jaw, and speech production, as a replication and extension of an earlier investigation by Moore, Smith, and Ringel (1988). The synchrony of activity within and across these paired muscles (masseter, medial pterygoid, and the anterior belly of the digastric) was evaluated by statistical comparison of zero-lag cross-correlation coefficients between all possible pairs. Paired comparisons were classified and combined according to anatomical and biomechanical properties into comparisons of homologous pairs (e.g., synchrony of activity in right masseter with left masseter), ipsilateral synergists (e.g., right masseter with right medial pterygoid), contralateral synergists (e.g., right masseter with left medial pterygoid), ipsilateral antagonists (e.g., right masseter with right digastric), and contralateral antagonists (e.g., right masseter with left digastric). Statistical comparison of the coactivation within muscle groups (across tasks) and across these muscle groups (within tasks) revealed significantly different groups of coactivated groups for each of the three tasks studied. The grouping of these muscles into coactivated groups always included homologous pairs among those most synchronously active. During mastication, homologous pairs and ipsilateral synergists were coactivated to a degree significantly greater than either of the antagonistic groups or the contralateral synergists. During voluntary oscillation of the jaw, coactive muscle groups were shown to be primarily the homologous pairs; synergists were coactivated to a significantly lesser degree, and antagonistic muscles were reciprocally active. During speech production, only homologous pairs emerged as a highly coactive group, although synergists and antagonistic pairs were coactive to a lesser degree. This finding was interpreted as a further indication of the coordinative plasticity among mandibular muscles, and as a demonstration of the vast differences in the apparent coordinative strategies for speech and nonspeech tasks. Speculation regarding the root of these differences is focused on the differences in kinematic and force-generating requirements of each task.
Keywords: jaw, electromyography, speech, chewing, human
Like other motor behaviors, motor control of orofacial movement can be modulated by a variety of neural mechanisms, including brain stem and cortical influences, which may vary in relative importance across and perhaps within behaviors. Of critical importance to our understanding of speech production is the relationship of speech motor control mechanisms and neural pathways to those of other orofacial motor behaviors. Some of the best understood mechanisms include those associated with rhythmic behaviors such as mastication, which has been shown to be controlled by a brain stem central pattern generator (CPG) (see review by Lund, 1991). The appeal of applying low-level mechanisms such as these to models of speech motor control is in the representation of speech production as the “product of a coordinated multilevel motor process” (Smith, 1992), and the notion that speech production might exploit various control mechanisms at different levels, including “fractionations” (Grillner, 1981) of central pattern generators. Accordingly, one reasonable experimental goal might be to seek evidence of these mechanisms during speech and related behaviors.
The assembly of individual muscles into functional units reduces the number of degrees of freedom controlled by the motor control system at the expense of control flexibility, and to the extent that various mandibular movements share common goals, it would be reasonable to expect similarities in control mechanisms. Because of the relatively unusual mechanical coupling created by the bilaterally symmetric articulation of the mandible (as compared to limb structures), a compelling model of the coordinative organization of the mandibular system might reduce degrees of freedom by linking activity in bilateral muscle pairs (e.g., right and left masseter muscles) at a very low level in the nervous system, especially for tasks lacking a lateral movement component. Alternatively for tasks involving a significant degree of lateral mandibular displacement, ipsilateral (but not bilateral) synergists might be constrained to act as a functional unit. Furthermore, it is possible that at least for some tasks, specification of functional units includes asynchronous, phasically modulated activity among antagonists for which parameters of timing and amplitude are tightly coupled (Cooke & Brown, 1990). The present investigation was designed to quantify the symmetry of mandibular muscle activation patterns across tasks as a means of assessing the degree of shared coordinative organization. An earlier study by Moore, Smith, and Ringel (1988) similarly evaluated task dependence of mandibular muscle activity ipsilaterally. This investigation of bilateral symmetry partially replicates and further extends these earlier findings using a new data set to include measurement of effects across the midline.
An example of a model for mastication that might accommodate some of the proposed coordinative characteristics of speech production has been proposed by Lund (1991). This model of mandibular control, which is similar to a model of respiration proposed by Feldman, Smith, McCrimmon, Ellen-berger, and Speck (1988), specifies that the observed motor rhythm of a centrally patterned behavior is generated by a system with two separate components. Lund represents the control systems for mastication as including (a) a rhythm generator located in the medial bulbar reticular formation between the trigeminal motor nucleus and the inferior olive, and (b) jaw elevator and jaw depressor burst generators, which are premotor neurons close to the trigeminal motor nucleus. The burst generators described by this model include interneurons projecting directly to jaw elevators and depressors, as well as inhibitory premotor neurons responsible for inhibition of jaw elevator motor neurons immediately prior to jaw depression. An important aspect of this model is that essential elements of jaw muscle coordination, rhythmic motor output and reciprocal inhibition, are present in brain stem structures, and are subject to the influence of known contralateral corticobulbar projections (Nozaki, Iriki, & Nakamura, 1986; Tal, 1987), which must be anticipated for many mandibular behaviors.
In humans, a low-level common neural drive to the muscles of mastication has been supported by the observation of coherent high-frequency oscillations in mandibular muscles during chewing, but not during speech (Smith & Denny, 1990). In addition, task-specific variation of orofacial coordination has been demonstrated among ipsilateral mandibular muscles (Moore, Smith, & Ringel, 1988), showing that a variety of activation patterns is employed over a range of task demands. These findings suggest that different neural mechanisms underlie various behaviors, although the degree of shared organizational resources is unknown. The findings of Moore, Smith, and Ringel would not support, for example, the idea that speech and mastication employ redundant mechanisms of motor control. For example, the burst generators described by Lund’s model (1991) promote reciprocal inhibition as well as bursts of activity in the agonist muscle. Speech exhibits a consistent lack of reciprocal inhibition, which would fail to support modulation of muscle activation by masticatory burst generators.
In the study of ipsilateral synergistic and antagonistic mandibular muscles, Moore, Smith, and Ringel (1988) demonstrated that the coordinative organization of this system exhibits changing patterns of muscle activity with changing task demands. During mastication, activation of ipsilateral jaw-elevating synergists is synchronous and is reciprocal with activity in the jaw-depressing anterior belly of the digastric. This characteristic reciprocity of chewing represents one extreme in the overall coordinative organization of jaw muscle activity, and contrasts markedly with the patterns observed during speech production. During continuous production of a passage designed to elicit large and frequent jaw excursions (from Zimmermann & Hanley, 1983), mandibular antagonists (i.e., ipsilateral jaw-elevating muscles and the anterior belly of the digastric) were most frequently coactive. This plasticity of observed muscle synergies was attributed to varying task demands and behavioral goals; masticatory movements can be assumed to be optimized for high interdental force generation, whereas speech production requires rapid movement with frequent reversals in direction. The mechanical stiffening afforded by coactivation of antagonistic muscles enables these rapid reversals and results in mandibular positioning that is more resistant to intrinsic mechanical perturbation (Fel’dman, 1980b).
The task-dependence of mandibular muscle activation patterns has gained recent support by a very precise investigation of single motor unit activity in masseter in humans during a variety of static activation tasks. McMillan and Hannam (1992) demonstrated that, in contrast with the size principle of motor unit recruitment (Henneman, Somjen, & Carpenter, 1965), masseter motor units were differentially recruited for different tasks such as jaw clenching, protrusion, and retrusion. Furthermore, motor units in different quadrants of the masseter tended to be recruited for different tasks. The task dependence shown in these results suggests a very high degree of control specificity for activation of masseter motor units.
Given a continuum of reciprocity and antagonistic coactivation among muscle synergies of the mandibular motor control system, it is possible to construct a comparative representation of the coordinative organization of these muscles. The coactivation among jaw-elevating muscles during mastication provides one point of reference; the activity of these synergists is so tightly coupled that despite neural noise and local variations in the EMG interference patterns, the timing and amplitude variation of their concurrent electromyographic signals yield zero-lag cross-correlation coefficients usually in excess of .90. At the opposite extreme, reciprocally activated muscles yield coefficients on the order of −.40 (Moore, Smith, & Ringel, 1988). Not only do these correlation coefficients provide a description of mandibular coordination for these particular motor behaviors, they also provide a quantitative reference to which other muscle synergies can be comparatively described and examined.
The coactivation of the homologous members of a single muscle pair and of other contralateral muscles has not been described quantitatively for speech production. At first consideration, it might be reasonable to expect the activation of bilateral members of a muscle pair (e.g., right and left masseter) to be very closely related, because of their symmetric biomechanical effects and muscle geometry, and because of some assumed degree of shared neural drive. This expectation would be especially strong for mandibular movements occurring predominantly in the vertical plane with minimal lateral displacement. Deviations from complete symmetry in activation patterns might be derived from several sources, some of which include structural differences (including normal anatomical asymmetries), neural “noise” (i.e., random fluctuations in activation level, which are within the presumed “tolerance” of the neuromuscular system), and differences in the underlying control signal to each muscle, the last of these three factors being of primary interest in the current investigation. A greater proportion of symmetry might be taken to suggest a reduction in the degrees of freedom managed by the control system by constraining muscle groups to synchronous activation, for example, by the action of burst generators. Less synchronous activation, in contrast, might suggest greater movement flexibility at the expense of managing a larger number of degrees of freedom. By observing the relative bilateral coupling of muscles in this system, it may be possible to generate a more detailed description of the shifting coordinative relationships among synergistic and antagonistic muscles, to better define the demands of various oromotor tasks, and to assess the capacity of orofacial systems to employ a range of coordinative organizations and constraint.
The present investigation was designed to provide a comparative description of relative bilateral coordination among muscles of the mandible. The coactivity among mandibular muscles was evaluated in order to provide a descriptive reference for the coordinative organization of various jaw movements. This approach entails the assumption that the commonalities (e.g., neural, biomechanical) of homologous muscle pairs lead to patterns of coactivation that define the limit of neuromuscular coupling, and furthermore that the relative strength of coupling among other members of the system can be validly expressed relative to these homologous pairs. It should be recognized that the rigid, bilaterally symmetric structure of the mandible and the bilateral corticobulbar innervation of the trigeminal nuclei make this system somewhat unusual and might represent the strong case of coupling across members of muscle pairs. The essential principles underlying variations in coordinative organization within this musculoskeletal system would not be expected to be unique; the properties described for the jaw may be generalizable to other systems. It is possible, however, that systems such as the lips, with greater mechanical independence and distinct corticobulbar pathways, would exhibit much more diversity in coordinative strategies.
There are of course alternative a priori hypotheses to be considered regarding the differences in coactivation that might be observed, particularly those cases in which homologous pairs do not yield the highest correlation coefficients. It is possible, for example, that the relatively small amplitude, vertical mandibular movements characteristic of speech (including rotation about the temporomandibular joint and anteroposterior displacement) (Baragar & Osborn, 1984; Gentil & Gay, 1983) might exhibit symmetry in muscle activation in excess of that seen during mastication, perhaps because of the characteristic lateral displacement of the mandible toward the working side during the elevation phase of chewing (Luschei & Goodwin, 1974). However, generation of mastication by a brain stem central pattern generator might be expected to yield EMG patterns with near-maximum levels of symmetry in homologous muscles (e.g., right and left masseter). In either case, the experimental results obtained during mastication provide a reference with a postulated neural mechanism against which other muscle synergies can be evaluated.
Asymmetry during rudimentary, brain stem-mediated behavior is not, of course, precluded by the presence of all or part of a CPG, as CPGs have been directly observed for a wide range of rhythmic behaviors (e.g., respiration, feeding, locomotion, swimming), many of which demonstrate asymmetric, cyclic activation (Delcomyn, 1980). Asymmetric phase differences are an essential characteristic of the chewing pattern (Luschei & Goodwin, 1974). It is only suggested that, as has been shown earlier (Moore, Smith, & Ringel, 1988), that the most consistent coactivation observable in electromyographic patterns are anticipated for simultaneously active paired muscles during behaviors mediated by very low-level neural mechanisms. For the rigid mandible, composed of a single, bilaterally articulating, bone, the distribution of biomechanical conditions across all members of the system might make this coupling appear even more rigid than in biomechanically separate systems such as the limbs. Alternating, or reciprocal, activity among muscles will involve time-varying effects of, for example, physiologic noise levels, afferent effects on the motorneuron pool, and reflex gain, and would be expected to exhibit patterns of activity with less interdependence or covariation. Analysis of bilateral muscle activity during a variety of behaviors makes possible the construction of a representation of coordinative organization based on the relative degree of coactivity in muscle pairs. The present investigation focused on electromyographic patterns during speech and nonspeech mandibular movements in order to provide this comparison.
Methods
Subjects
Subjects were 6 adult volunteers (3 female, 3 male) with negative histories of neuropathology, speech pathology, dental abnormalities (including temporomandibular joint disorders and current use of dental appliances), or current use of medication. Subjects ranged in age from 19 to 45 years. Four subjects completed the protocol with only one electrode configuration. Subjects A and B, however, completed the protocol twice and three times, respectively, with differing electrode configurations to yield a total number of experimental runs of nine. The various EMG electrode configurations are described below.
EMG Recording Sites and Procedures
Gross, bipolar EMG recordings were obtained bilaterally from three pairs of mandibular muscles: masseter, medial (internal) pterygoid, and the anterior belly of the digastric (ABD). These three pairs were selected so that several different muscle relationships could be studied: homologous pairs (e.g., right and left masseter), ipsilateral and contralateral synergistic pairs (e.g., right masseter with right medial pterygoid, or right masseter with left medial pterygoid, respectively), and ipsilateral and contralateral antagonistic pairs (e.g., right masseter with right ABD, or right masseter with left ABD, respectively). Masseter activity was obtained using miniature surface Ag-AgCl electrodes. Medial pterygoid activity was obtained using intramuscular bipolar hooked-wire electrodes (Basmajian & Stecko, 1962). ABD recordings were obtained from four subjects (four runs) using surface electrodes, and from 2 subjects (Subjects A and B; five runs) using intramuscular recordings. Electrodes within the masseter electrode pair were placed directly over the main mass (determined by palpation) of the muscle, aligned longitudinally on the muscle, and separated by about 3 cm. For the medial pterygoid electrode pairs, each individual wire was inserted vertically using an extraoral approach to the inferior portion of the muscle, approximately 2 cm medially from the angle of the mandible. Interelectrode distance within each pair was approximately 1–2 cm at the electrode tips. A 30-gauge hypodermic needle was used to carry each Teflon-insulated, .001″-diameter, stainless steel, hooked-wire electrode into the muscle to a depth of 1–2 cm, at which point the needle was immediately withdrawn, leaving the wire electrode in place. Hooked-wire electrode pairs were placed similarly in ABD using an extraoral vertical approach approximately 1 cm posterior to the internal surface of the mental symphysis and 1 cm lateral to the midsagittal plane. Surface electrode pairs over ABD were separated by about 2 cm and were located over the main mass of the muscle as determined by palpation of the muscle and reference to the mental symphysis. Pre- and postexperimental verification of electrode placement and recording fields were accomplished by observation of the EMG patterns associated with behavioral tasks (e.g., incisal bite, molar bite, jaw depression against an opposing force), and that the recordings indicated high activation in rhythm with chewing.
Each EMG signal was amplified (Grass P511), filtered (passband: 3–1000 Hz), and full-wave rectified prior to recording. All EMG, mandibular-position, and acoustic signals were recorded simultaneously using an 8-track FM instrumentation recorder (Hewlett-Packard 3968A) configured to yield essentially flat frequency response from DC to 1250 Hz (S/N 35 dB). All analyses were completed later from these recordings.
Transduction of Mandible Position
The position of the subject’s mandible was transduced in the horizontal and frontal planes during speech production and voluntary oscillation, but not during chewing, using a dual-beam strain gauge cantilever system (adapted from Barlow, Cole, & Abbs, 1983). The movement transducer was coupled to the subject using a thin stainless steel wire fixed to the subject’s lower central incisors with a customized, lightweight dental appliance. The appliance had no significant effect on EMG patterns during speech production and voluntary oscillation of the jaw; however, earlier results have shown that the presence of the transducer significantly alters the patterns of activity during mastication (Moore, Smith, & Ringel, 1988).
Because we were limited to only seven data channels (and one audio channel), two of which were dedicated to mandibular position signals, only four EMG sites could be recorded simultaneously during any given experimental run. Subjects A and B were able to repeat the entire protocol to acquire data during the same tasks to represent all possible combinations of muscle pairs. Although this method was less than ideal, we have observed these measures to be quite robust within subjects (the average difference between correlation coefficients, r, for repeated runs observing homologous pairs within Subjects A and B was only .06), thereby reducing the problems inherent in nonsimultaneous observations and unbalanced sampling. Furthermore, all ipsilateral configurations were compared to the independent data set provided by Moore, Smith, & Ringel (1988). To accommodate the potential imbalance in individual subject effects, nonuniform sampling effects, and the large number of empty cells, a very conservative statistical treatment, including collapsing across compared pairs and experimental subtasks, was adopted as described below.
Experimental Protocol
A variety of tasks representing three different behaviors (i.e. chewing, voluntary continuous oscillation of the jaw, and speech production) was performed by each subject. This range of tasks was selected to include a broad representation of mandibular movements, including tasks that varied in task objectives such as rate and range of motion, force generation, and lateral displacement.
Tasks included three major task types, each with three or four subtasks:
Three mastication conditions—molar chewing of a chewy candy (a Tootsie Roll) on the right side and on the left side, and molar chewing of a breadstick on the preferred side.
Three voluntary oscillation conditions—slow, moderate, and fast (approximately 1, 3, and 5 Hz) elevation and depression of the mandible approximately sinusoidally.
Three speaking conditions—reading of the Hanley Passage, a passage designed to elicit frequent and high-amplitude mandibular displacement (Zimmermann & Hanley, 1983), at normal and fast rates, and rapid and spontaneous speech.
Each subtask was performed for a duration of at least 40 seconds, from which selected samples were drawn.
Signal Processing and Analysis of EMG Signals
Selected periods of activity for each condition were digitized for analysis as described below. Samples were selected to obtain the longest possible interval that met specific selection criteria. For mastication, samples were obtained from chewing periods after the initial chew cycles and before the final swallowing stage. These type II, or reduction series, chewing cycles are characterized by a smooth opening movement, followed by a fast closing movement to the working side, and a slow closing movement, or power stroke, during which the mandible moves back toward midline in a grinding motion (Luschei & Goodwin, 1974). The sampled intervals ranged from 10 seconds to more than one minute. Samples of voluntary oscillatory movements of the jaw were taken from periods during which the movement pattem was judged to be regular in amplitude and periodic. Sampled interval lengths ranged from 15 to 90 seconds. Samples obtained during speech production included the entire reading passage, for which the duration varied with reading rate, but ranged from 15 to 25 seconds.
Analysis of EMG signals followed the methods described earlier by Moore, Smith, & Ringel (1988) using a Digital Equipment Corporation PDP 11/23+ computer system using custom software. The recorded EMG and mandibular position signals were digitized with anti-aliasing lowpass filtering at 250 Hz (digital sampling rate: 680 samples/sec per channel; 12-bit A/D amplitude resolution; ±2.5V; 0.1% accuracy). Each EMG record was filtered digitally using a linear-phase, lowpass filter with a cutoff frequency of 40 Hz. This last filtering step yielded the activation “envelope” for each EMG site, an indicator of overall muscle activation level, and reduced the variability caused by high-frequency variations in the EMG interference patterns. Finally, these data were logarithmically transformed so that the coordinative relationships of reciprocally active muscle pairs yielded linear X-Y functions, rather than the typical curvilinear relation of the function y = 1/X (i.e., these data, which were expected to yield an inverse function, were linearized by logarithmic transform). Coactivated pairs were affected minimally by this logarithmic transformation.
Pairwise zero-lag cross-correlation coefficients were computed for each of the 15 pairs possible among the six EMG recording sites for each sampled interval (e.g., one 30-sec sample of chewing on the left side by 1 subject). This statistic has been demonstrated to be a reliable indicator of coordinative relationships among muscle pairs (Cooper & Folkins, 1985; Loeb, Pratt, Chanaud, & Richmond, 1986; McLean, Goldsmith, & Cerf, 1984; Moore, Smith, & Ringel, 1988). The decision to evaluate the results of only the zero-lag result, rather than the entire cross-correlation function was based on several factors. A preliminary analysis of the lag for peaks in the cross-correlation function for synergist pairs during chewing revealed that most peaks were within 10 degrees (about 30 msec) of zero-lag with the cross-correlation function being fairly wide near zero-lag. This expected finding of small phase shifts among synergists has been demonstrated to be associated with differences of working and nonworking side muscles (Luschei & Goodwin, 1974). For antagonist pairs, most cross-correlation maxima were within 20 degrees of 180 degrees (i.e., within about 60 msec of being exactly out of phase), and minima were within 10 degrees of zero-lag. For speech production, maxima were usually within 60 msec of zero-lag for both antagonist and synergist pairs. Cross-correlation functions for speech showed only singular peaks near zero lag. The results for the oscillation condition were much less predictable and did not appear to vary systematically with oscillation rate. Thus, zero-lag correlation coefficients were accepted as good indicators of the degree of coactivation of synergists, and as reasonable indicators of reciprocal activation relationships for the conditions studied.
An additional motivating factor for considering only zero-lag coefficients was the theoretical orientation underlying the methodology of this investigation. In seeking to quantify the degree of shared neural drive to mandibular muscles, we assumed that simultaneous EMG signals might best reflect common inputs to the motorneuron pools. Of course, the rhythmic output of a CPG or other mechanisms of neuromuscular coupling may specify cyclic phase relationships among synergists as well as antagonists, but these timing and amplitude relationships are beyond the scope of the present investigation. Rather, we suggest that if, for example, the motorneurons for right and left masseter were driven by a common neural source (e.g., premotor neuron burst generators proximal to the trigeminal motor nucleus), small fluctuations in output levels will be reflected in the resultant simultaneous EMG output of each. To the extent that these signals are coordinated by more distant mechanisms (e.g., bilaterally mediated by separate inputs) the proportion of shared output within and across muscle pairs will decrease and will be reflected by the zero-lag cross-correlation coefficient. Furthermore, while correlated activity over time might reasonably be evaluated for strictly cyclic activities such as chewing and voluntary oscillation, phase relationships are much more difficult to assess for speech production during which periodicity may or may not be detectable.
Reliability
Overall reliability of the analysis procedures was assessed by repeating completely (i.e., starting with redigitization of the data from the FM tape recording) the analysis of 72 EMG pairs (i.e., six electrode sites for four different behaviors) taken from three different experimental runs. Samples of chewing, oscillation, and speech were included in this reanalysis. Although the same events were sampled from the taped experiment, reliability sampling was completed without reference to the earlier periods sampled. Thus it is possible that the second samples partially overlapped the originally digitized samples (usually less than 50% of the recorded period of activity was digitized and analyzed), but generally these samples provided a further evaluation of these measures across different samples of the same behaviors. Furthermore, this second analysis used completely revamped algorithms and hardware (reliability was evaluated on a PC-based platform running custom routines for MATLAB, a commercially available software package), and was completed by a second research assistant. Results of this reanalysis revealed a correlation between the Fisher’s-Z transformations of the original and the repeated correlation coefficients of 0.931 (r2 = 0.866). The mean difference between correlation coefficients was .038 (SD = .136; range = .20 to −.69). This very high level of reliability further demonstrated both the robust nature of the coordinative relations described and of this specific descriptive technique.
An additional control condition was completed in order to evaluate the magnitude of random variability arising from electronic and neurophysiologic noise and electrode placement. In this condition, duplicate simultaneous recordings were made from individual muscles during several chewing tasks in order to observe within-task, within-muscle variability. Four pairs of surface electrodes were used, two pairs on the right masseter and two pairs on the left, in an adult female who met the inclusion criteria stated earlier. Each of the two electrode pairs on each muscle was placed to span the length of the muscle with an interelectrode distance of about 3–4 cm. The electrode pairs were spaced about 1 cm from each other in the anterior-posterior dimension, such that the pairs had very similar recording fields over the main mass of the muscle. Completion of the complete correlational analysis revealed that zero-lag correlation coefficients ranged from .92 to .98 for these same-muscle pairs. This level of agreement suggests that the large recording fields employed satisfactorily sample the overall muscle activity, which may then be validly compared with the activation levels of other muscles. Furthermore, this condition defines the empirical limit of coefficients describing correlated activity.
Statistical Treatment
The specific objective of this experiment was to compare relative coactivity among jaw muscles along a continuum of behaviors across subjects. This comparison was facilitated by combining results across subjects and across groups of muscles (rather than within specific muscle pairs), which were defined by innervation and biomechanical relations. This collapsing of specific EMG recording sites into muscle groups served to focus on comparisons of organizational interest and further reduced the effects of sampling different muscle pairs in different subjects. These muscle groups included (a) homologous pairs (i.e., right masseter with left masseter, right medial pterygoid with left medial pterygoid, and right digastric with left digastric), (b) ipsilateral synergists (ipsilateral pairs of masseter with medial pterygoid), (c) contralateral synergists (contralateral pairs of masseter with medial pterygoid pairs), (d) ipsilateral antagonists (ipsilateral pairs of digastric with masseter and medial pterygoid), and (e) contralateral antagonists (contralateral pairs of digastric with masseter or medial pterygoid). The correlation coefficients of these five muscle groups were compared across the three behavioral tasks (i.e., chewing, voluntary oscillation, and speech) and across the subtasks within each task group (e.g., slow, moderate, and fast rates of oscillation).
The obtained correlation coefficients were transformed using Fisher’s-Z transformation. These transformed coefficients were normalized for each grouping using the mean and standard deviation (for each subtask or, in a second modeling procedure, for each task) according to the function ZFisher = Z−Z/sdz. Initial analyses included evaluation of a full model (unbalanced repeated-measures model with structured covariance matrices, BMDP Statistical Software, 1990) to test for effects and interactions of the five muscle groups, the three primary task types, and the subtasks. A reduced second model tested for effects and interactions of only the muscle groups and tasks. Post hoc analyses of differences among pairs of coefficients within tasks and within muscle groups were completed using a Newman-Keuls procedure.
Results
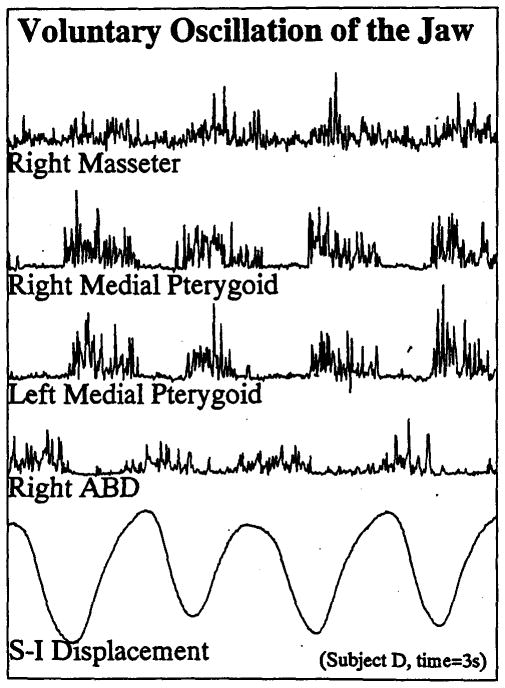
All subjects were able to complete the protocol easily, although incomplete runs resulted from the loss of individual electrode recordings, usually from a hooked-wire electrode dislodging from the muscle. A typical example of some of the EMG recordings and position signals is shown in Figure 1. This figure illustrates the activation pattern obtained during 3 seconds of voluntary oscillation of the jaw at about 1 cycle per second by Subject D (for display purposes only a short period of the 30-sec run is shown). The zero-lag correlation coefficients obtained for the paired comparisons in this entire run were .64 for right and left medial pterygoid, .48 for right masseter and right medial pterygoid, .39 for right masseter and left medial pterygoid, and −.25, −.31, and −.29 for right ABD with right masseter, right medial pterygoid, and left medial pterygoid, respectively. The final data set, shown in Table 1, was composed of 342 correlation coefficients computed across these seven runs, three major tasks (divided into three subtasks each), and six EMG sites. The ipsilateral data in this data can be compared directly with the results obtained from an earlier data set presented in Moore, Smith, & Ringel (1988).
FIGURE 1.
Integrated, full-wave rectified EMG and the vertical Jaw position trace obtained from Subject D during voluntary oscillation of the aw at a moderate rate. Moderately rigid coupling between the homologous pair (left and right medial pterygold) contrasts with the weaker coupling among synergists (each of the medial pterygold recordings with right masseter) and the reciprocal coupling among the antagonistic pairs (medial pterygold or masseter with digastric).
TABLE 1.
Result, of pairwise correlational analysis.
| Task | Subject | Homologous pairs
|
Ipsllateral synergists
|
Contralateral synergists
|
Antagonistic pairs
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | Pt | ABD | RM x RMPI | LM x LMPt | RM x LMPt | LM x RMPt | RM x D | LM x D | RMPt x D | LMPt x D | ||
| Chew Right | A1 | 0.65 | 0.16 | 0.88 | 0.31 | 0.06 | 0.63 | |||||
| A2 | 0.73 | 0.26 | −0.15 | −0.15 | ||||||||
| B1 | 0.76 | 0.14 | 0.19 | 0.45 | 0.49 | 0.13 | ||||||
| B2 | 0.80 | 0.83 | −0.32 | −0.27 | ||||||||
| B3 | 0.05 | 0.88 | 0.11 | −0.11 | ||||||||
| C | 0.71 | 0.88 | 0.64 | −0.15 | −0.13 | −0.02 | ||||||
| D | 0.47 | 0.68 | 0.28 | −0.05 | −0.04 | −0.07 | ||||||
| E | 0.49 | 0.73 | 0.43 | −0.12 | −0.19 | −0.06 | ||||||
| F | 0.37 | 0.74 | 0.55 | −0.10 | −0.08 | −0.08 | ||||||
| Chew Left | A1 | 0.68 | 0.22 | 0.69 | 0.41 | 0.25 | 0.43 | |||||
| A2 | 0.73 | 0.26 | −0.15 | −0.15 | ||||||||
| B1 | 0.68 | 0.22 | 0.34 | 0.40 | 0.59 | 0.51 | ||||||
| B2 | 0.62 | 0.79 | −0.02 | −0.21 | ||||||||
| B3 | 0.17 | 0.86 | −0.29 | 0.07 | ||||||||
| D | 0.36 | 0.53 | 0.53 | −0.14 | −0.16 | −0.16 | ||||||
| E | 0.41 | 0.35 | 0.32 | −0.09 | −0.05 | −0.20 | ||||||
| F | 0.62 | 0.73 | 0.69 | −0.15 | −0.11 | −0.14 | ||||||
| Chew Breadstick | D | 0.36 | 0.57 | 0.30 | −0.01 | 0.02 | 0.02 | |||||
| E | 0.54 | 0.72 | 0.45 | −0.12 | −0.11 | −0.11 | ||||||
| F | 0.16 | 0.52 | 0.29 | −0.07 | −0.02 | −0.09 | ||||||
| Oscillation, Slow | A1 | 0.66 | 0.08 | 0.19 | −0.08 | −0.20 | 0.17 | |||||
| A2 | 0.85 | 0.13 | −0.02 | −0.02 | ||||||||
| D | 0.04 | 0.09 | −0.03 | −0.05 | −0.11 | 0.01 | ||||||
| E | 0.00 | −0.02 | 0.00 | 0.00 | −0.09 | −0.03 | ||||||
| Oscillation, Moderate | A1 | 0.79 | 0.20 | 0.41 | −0.18 | −0.29 | 0.39 | |||||
| A2 | 0.90 | 0.04 | 0.08 | 0.04 | ||||||||
| B1 | 0.33 | 0.03 | −0.05 | 0.02 | 0.05 | −0.05 | ||||||
| B2 | 0.35 | 0.85 | 0.38 | 0.38 | ||||||||
| B3 | 0.12 | 0.88 | 0.01 | 0.08 | ||||||||
| D | 0.64 | 0.48 | 0.39 | −0.25 | −0.31 | −0.29 | ||||||
| E | 0.74 | 0.72 | 0.65 | −0.06 | −0.11 | −0.11 | ||||||
| Oscillation, Fast | A1 | 0.91 | 0.37 | 0.63 | −0.08 | −0.25 | 0.64 | |||||
| A2 | 0.90 | 0.18 | −0.30 | −0.34 | ||||||||
| B1 | 0.78 | 0.22 | 0.78 | 0.19 | 0.19 | 0.83 | ||||||
| B2 | 0.81 | 0.87 | −0.36 | −0.43 | ||||||||
| B3 | 0.25 | 0.88 | 0.01 | −0.08 | ||||||||
| C | 0.72 | 0.66 | 0.56 | −0.15 | −0.33 | −0.23 | ||||||
| D | 0.77 | 0.66 | 0.66 | −0.12 | −0.21 | −0.19 | ||||||
| E | 0.76 | 0.82 | 0.73 | −0.09 | −0.10 | −0.06 | ||||||
| F | 0.08 | 0.03 | 0.20 | −0.12 | −0.09 | 0.20 | ||||||
| Passage, Normal | A1 | 0.53 | 0.06 | 0.40 | 0.15 | 0.09 | 0.33 | |||||
| A2 | 0.81 | 0.06 | 0.06 | 0.11 | ||||||||
| B1 | 0.67 | 0.05 | 0.21 | 0.26 | 0.29 | 0.35 | ||||||
| B2 | 0.61 | 0.61 | 0.10 | 0.07 | ||||||||
| B3 | 0.09 | 0.63 | −0.04 | 0.00 | ||||||||
| D | 0.11 | 0.02 | 0.05 | −0.07 | 0.27 | 0.15 | ||||||
| F | 0.11 | 0.07 | 0.08 | 0.10 | 0.11 | 0.21 | ||||||
| Passage, Fast | A1 | 0.64 | 0.05 | 0.38 | 0.14 | 0.07 | 0.37 | |||||
| A2 | 0.78 | 0.18 | 0.00 | 0.36 | ||||||||
| B1 | 0.62 | 0.12 | 0.34 | 0.19 | 0.23 | 0.53 | ||||||
| B2 | 0.63 | 0.69 | 0.08 | 0.23 | ||||||||
| B3 | 0.17 | 0.68 | −0.06 | −0.09 | ||||||||
| D | 0.12 | 0.03 | 0.03 | −0.01 | 0.22 | 0.11 | ||||||
| E | 0.10 | 0.06 | 0.02 | −0.01 | 0.03 | 0.13 | ||||||
| F | 0.08 | 0.04 | 0.10 | 0.07 | 0.11 | 0.08 | ||||||
| Normal Speech | E | 0.16 | 0.03 | 0.04 | 0.00 | −0.02 | 0.16 | |||||
| F | −0.02 | 0.05 | 0.03 | 0.07 | 0.14 | 0.01 | ||||||
Note: R = right; L = left; M = masseter; MPt = medial pterygoid; D = anterior belly of the digastric.
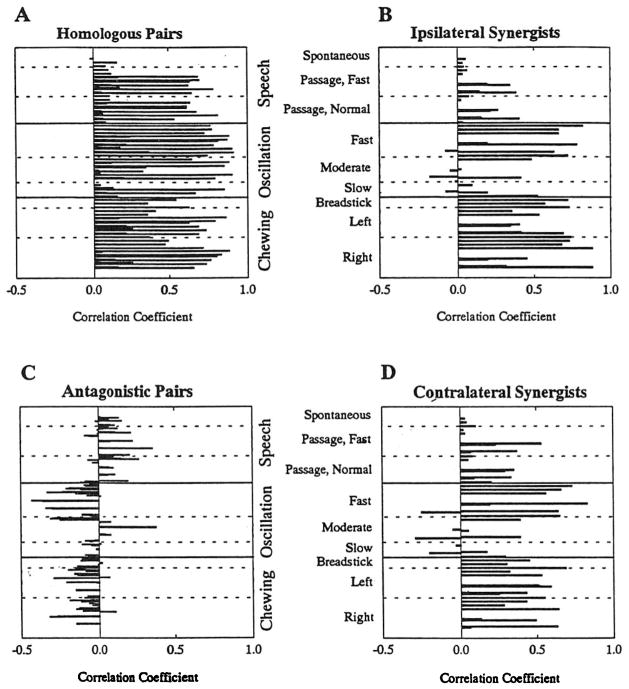
The complete data set is graphically presented in Figure 2. It is apparent from this figure alone that the patterns of muscle activation varied across the four muscle groupings. Inspection of Figure 2A, B, and D suggest that homologous and synergistic muscle pairs usually yielded highly positive correlation coefficients. The data in Figure 2C, however, compose a pattern that changes from task to task, with speech production exhibiting positive coefficients and chewing and oscillation exhibiting negative coefficients. This pattern of variation is consistent with the previous findings by Moore, Smith, & Ringel (1988), which demonstrated consistent coactivation of antagonistic muscles during normal speech production. The present investigation was designed to go further analytically to determine whether there are differences in levels of coactivation within muscle groups (e.g., among chewing, oscillation, and speech for homologous muscle pairs) and across muscle groups (e.g., among all muscle groups for speech production), as represented by the four panels of Figure 2.
FIGURE 2.
Distributions of correlation coefficients across tasks and muscle groupings as shown In Table 1.
Initial data reduction required transformation, using Fisher’s Z-transform, and normalization of all correlation coefficients as described in the previous section. Transformed coefficients were combined across subjects, within subtasks (e.g., slow, medium, and fast oscillation were combined into one task) and normalized to yield a final data set with the dimensions of five muscle groups by three tasks, each with three subtasks. Preliminary analysis focused on modeling the data set with respect to the effects associated with experimental tasks and the muscle groupings. This analysis yielded an indication of the strength of the overall effects associated with tasks, subtasks, and muscle relationships. Initial modeling of the full set of muscle groups, tasks, and subtasks yielded nonconvergent results for most comparisons. No main effects for subtasks (e.g., fast, moderate, and slow oscillation) were found. Accordingly, a reduced model was applied, which grouped the data within tasks (i.e., the data set was collapsed across subtasks).
The results of the reduced statistical model are shown in Table 2. This model yielded highly significant effects for the parameters of muscle group and task. Thus the data set used for post hoc analysis was confined to the dimensions of three tasks and five muscle groups. This reduction had the desirable effect of minimizing task differences that were caused simply by differences in activation level (i.e., lower levels of muscle activation yield poorer signal-to-noise (S/N) ratios, which lead to correlation coefficients that tend toward zero), and led to a reduction in the dimensions of the data set to three tasks and five muscle groups. If we had been unable to collapse across subtasks, comparisons of slow oscillation and chewing, for example, might demonstrate differences simply because of activation level differences rather than organizational differences, even though the overall patterns of activity could be quite similar.
TABLE 2.
Results of modeling correlated muscle activity across tasks and muscle groups.
| Test | Comparing | df | χ2 |
|---|---|---|---|
| Homologous muscles | across tasks | 2 | 22.99 |
| Ipsilateral synergists | across tasks | 2 | 27.93 |
| Contralateral synergists | across tasks | 2 | 10.35 |
| Ipsilateral antagonists | across tasks | 2 | 31.97 |
| Contralateral antagonists | across tasks | 2 | 19.67 |
| Chewing | across muscle groups | 4 | 165.86 |
| Voluntary oscillation | across muscle groups | 4 | 94.47 |
| Speech | across muscle groups | 4 | 18.76 |
Note. All results were significant at p < .001.
Accordingly, changes in EMG amplitude across tasks have not been factored into these calculations. Although correlational analyses are not sensitive to global amplitude variations within a record, higher levels of muscle activity give rise to improved S/N ratios, which in turn may, because of the reduced random noise process, yield higher correlation coefficients. As seen in figures presented by Moore, Smith, and Ringel (1988), these magnitude differences are predictably large, with EMG amplitudes during chewing being more than 10 times larger than those seen in other tasks. In the present analyses, signal rectification, integration, and lowpass filtering partially ameliorate this complication by extracting the amplitude envelope of the EMG signal. Most notably in terms of empirical findings, however, with respect to the present comparison of correlation coefficients within muscle groups across tasks, despite large difference in EMG amplitude, coefficients for homologous pairs during mastication were not shown to be statistically different from the other two tasks (see below).
The collapsed data set is shown in Table 3. This table summarizes the transformed and normalized mean correlation coefficients and standard deviations across tasks (collapsed across subtasks) and muscle groups used in post hoc analyses. The values shown in Table 3 are not directly comparable to those reported by Moore, Smith, and Ringel (1988), since the earlier study reported mean correlation coefficients and did not collapse data across muscle groups. Nevertheless, the magnitudes and directions of the differences among tasks are similar.
TABLE 3.
Means and standard deviations (in parentheses) of Fisher’s-Z transformed and normalized correlation coefficients for all muscle groups across tasks (shown graphically In Figure 3).
| Task | Homologous | Ipsllateral synergists | Contralateral synergists | Ipsilateral antagonists | Contralateral antagonists |
|---|---|---|---|---|---|
| Chewing | .656 (.420) | .515 (.182) | .340 (.059) | −.132 (.127) | −.112 (.146) |
| Oscillation | .804 (.520) | .289 (.267) | .252 (.059) | −.108 (.248) | −.112 (.195) |
| Speech | .428 (.434) | .107 (.112) | .120 (.057) | .112 (.132) | .113 (.061) |
Post hoc tests, using a Newman-Keuls procedure, revealed that the degree of coactivation among the jaw muscles, as indicated by correlation of EMG activity, varies significantly across tasks. The results of the post hoc analyses are summarized in Figure 3. This figure illustrates the relative coactivity within and among muscle groups and tasks. Our approach was to suggest that differences between pairs of average correlation coefficients represent differences in muscle activation synchrony, which may imply a change in coordinative strategy, especially in those cases where the sign of the coefficient changed. At one extreme are muscle groups that yield highly positive correlation coefficients, whose synchronous activity is tightly linked and might be viewed as exhibiting common neural drive. At the opposite end of this continuum are muscles that are asynchronously active, with correlation coefficients near zero, or reciprocally innervated, as indicated by negative coefficients. By observing these differences across and within tasks, the degree of shared synchronous activity and common neural control and task demands might be inferred.
FIGURE 3.
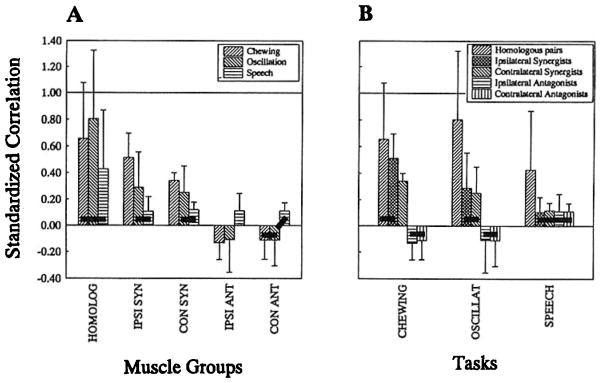
Normalized, Flsher’s-Z transformed coefficients collapsed across subjects and subtasks. Panel A plots these results by muscle group. Panel B Is a plot of the same results grouped by task. Connecting bars denote values that were not found to be statistically different In post hoc analyses.
Within-muscle-group comparisons (across tasks) are illustrated in Figure 3A. In this figure, dark horizontal bars connect average standardized coefficients that were not found to be significantly different. These comparisons of activation patterns of each muscle group across tasks revealed that the homologous pairs were coactive to a degree that was not significantly different across tasks. Both the ipsilateral and contralateral synergist groups, on the other hand, were most highly correlated during chewing, as compared to oscillation and speech production, which were not significantly different from each other. Correlations compared among ipsilateral antagonists were significantly different across all three tasks, being most negative for chewing, somewhat less negative for oscillation, and positive for speech. Finally, comparisons among contralateral antagonists revealed significant differences between speech and chewing only.
Within-task comparisons are illustrated in Figure 3B. These comparisons within tasks across all five muscle groups revealed that correlations among homologous pairs and ipsilateral synergists were not significantly different during chewing. Contralateral synergists had significantly lower correlation coefficients than homologous or ipsilateral synergist muscles groups, and significantly higher coefficients than those obtained from antagonistic muscle groups. Again, the activation patterns of the antagonistic groups were not significantly different from each other. These groupings did not hold for the other two tasks. During voluntary oscillation of the jaw, coupling of homologous muscle pairs significantly exceeded that of ipsilateral and contralateral synergists, which were not significantly different from each other. Conversely, coefficients for contralateral and ipsilateral antagonists were each significantly lower than those obtained for any of the synergistic or homologous groups. Finally, for speech production, coefficients obtained for homologous pairs exceeded those for any of the other muscle groups, the only case where this distinct grouping occurred. None of the other muscle groups demonstrated coactivation that was significantly different from any of the other groups.
Discussion
The present results suggest, in support of the earlier results of Moore, Smith, and Ringel (1988), that the coordinative organization of the mandibular system exploits a variety of muscle synergies in executing a range of speech and nonspeech tasks, presumably because of task-specific differences in demands (e.g., generation of occlusal force vs. achievement of vocal tract configurations) and coordinative complexity (e.g., interarticulator coordination versus rhythmic repetition). If we consider muscle coactivation to be one easily accessible indicator of one type of coordinative linkage among those muscles (i.e., in the range of timed, coupled neuromuscular events) and we assume further that consistently coactivated muscles are governed at some level by a common control signal, we can concisely describe some of the organizational patterns observed by noting groups of muscles that are synchronously active for specific behaviors.
Of course, it is probably true that timing of muscle activation can be varied continuously to generate timing relationships ranging from synchronous coactivation to reciprocity. Variable patterns of activity, dependent upon changing task demands, can arise from various mechanisms, including brainstem and corticomotoneuronal inputs (Fetz & Cheney, 1987). One model of synchrony among mandibular synergists for mastication depicts the coactivation of jaw-elevating motorneurons as arising from brain stem-level burst generators (Lund, 1991), although a cortical ablation study in macaques has shown that bilateral lesions of the lateral precentral cortex disrupt the normal chewing pattern with postoperative patterns being much smaller in amplitude and narrower in lateral displacement (Larson, Byrd, Garthwaite, & Luschei, 1980). In studies of limb movement in monkeys, Fetz and Cheney (1987) found that corticomotoneuronal cells can flexibly (i.e., dependent upon behavior) facilitate sets of coactivated limb agonist muscles and inhibit antagonists. The present results do not specifically address mechanisms of neuromuscular coupling, but may be interpreted as an indication of how (i.e., with what degree of plasticity) the mandibular system is variously configured to accomplish these specific tasks.
As a point of reference, the linkage among the homologous muscle pairs did not change significantly among all three tasks (see Figure 3A) despite large changes in activation levels, and the correlation coefficients obtained were comparable to those obtained when, as a control condition, two EMG signals from the same muscle were analyzed. Since these coefficients significantly exceeded those obtained for the other pairs in 11 of 12 possible comparisons (see Figure 3B), the coactivation of homologous pairs is seen as the limit of what is observed in correlated muscle synchrony. Even though asymmetries within homologous pairs characterize some behaviors, including mastication (Luschei & Goodwin, 1974), the overall strength and consistency of their coactivation support the notion that the activation of these muscles is tightly linked.
The application of this analytic approach to the data obtained for chewing illustrates the applicability of this analysis to known conditions. Coordinative linkages among these muscles for chewing emerge in three distinct functional groups (see Figure 3B): (a) homologous pairs and ipsilateral synergists (i.e., working and nonworking side jaw elevators), (b) contralateral synergists, and (c) antagonists. This resulting representation has the appeal of retaining homologous pairs as unitary motor components as well as reflecting the asymmetry of masticatory movements by the difference in phase of activity among contralateral synergists (Luschei & Goldberg, 1981; Luschei & Goodwin, 1974). Of course the distinct phases of aw elevation and depression yield a well-established pattern of reciprocity among antagonistic muscles, which accounts for the third functional grouping. The nearly equal (in relative amplitude) and opposite (in phase) activation of antagonists gave rise to negative correlation coefficients, which further characterize this relationship.
The findings for voluntary oscillation of the mandible were similar to those obtained for chewing, except that the coactivation of ipsilateral and contralateral synergists was quite low with respect to that of the homologous pairs. In fact, the coactivation among both ipsilateral and contralateral synergists was stronger for chewing than for either speech or voluntary oscillation (see Figure 3A). This result is somewhat difficult to interpret quantitatively because we did not complete a full cross-correlational analysis of these patterns. It is possible, for example, that brief, phase-delayed agonist and antagonist bursts, similar to those found for limb movement (Cooke & Brown, 1990; Feldman, 1980a; Ghez & Martin, 1982) underlie these results, although nothing like the typical triphasic pattern associated with limb movement has been observed in the mandible. Inspection of the individual records revealed that these differences in coactivation resulted primarily from overall recruitment of these muscles. That is, consistent with earlier results (Moore, Smith, & Ringel, 1988), oscillation tended to be accomplished by recruiting various subsets of the mandibular muscles by different subjects, as opposed to chewing, during which all subjects recruited all mandibular muscles. One consistency observed was that reciprocity was maintained by all subjects at all rates in this condition. This pattern of activation would be consistent with a coordinative organization consisting of the three bound homologous pairs, which may be coupled variously, including reciprocal inhibition of antagonists, to achieve this specific task.
The finding for speech production was quite distinct from observations of chewing and voluntary oscillation. What was most clear in the results of this condition was the absence of reciprocal inhibition of antagonistic muscles, an essential element of models of mastication (Lund, 1991). Whereas the coactivation of homologous pairs remained rigid for speech production, the relationships among synergistic and antagonistic muscles changed quite dramatically. In fact, there was no significant difference in the activation patterns among nonhomologous muscle pairs, regardless of whether they were antagonistic. This linkage was actually a reversal for the antagonistic pairs in which a predominately reciprocal pattern for chewing was in contrast to the coactive pattern during speech production. Remarkably, there was no statistical distinction among the antagonist or synergist pairs. This representation of the coordinative organization for this system for speech emphasized the bilateral symmetry of homologous muscles and the weaker relationships among the remaining coordinative synergies, including the marked absence of reciprocity among antagonists. Finally, the tendency toward coactivation of antagonistic muscles suggested increased mechanical stiffness for the mandibular system during speech production, an adjustment that is well adapted to the rapid movements and reversals characteristic of speech. This tendency was further supported by the control condition analysis of the cross-correlation functions for 4 subjects. For speech production, peaks in the cross-correlation function were consistently close to zero-lag and sloped gently away to each side of the function (see Methods), suggesting that the reported zero-lag coefficients are reasonable representations of the coactivity and timing relationships among these antagonistic pairs. Again, because of the variety of potential mechanisms for implementing coupling of homologous pairs, it is not possible to speculate regarding the origin of these patterns. Nevertheless, the absence of reciprocal inhibition suggests an underlying organizational framework quite different from those of mastication or voluntary oscillation (Lund, 1991).
One potentially confounding effect of the present paradigm was the large differences in activation levels observed across tasks. The correlational techniques used are sensitive to signal-to-noise ratios, with poorer S/N ratios giving rise to lower coefficients. The impact of this effect was minimized in the present analyses by collapsing across subtasks, which invariably resulted in combining results from higher amplitude signals with those for lower amplitude signals (e.g., slow voluntary oscillation with rapid voluntary oscillation). Furthermore, for those cases in which homologous muscle pairs demonstrated large differences in activation level, no significant differences among correlation coefficients was observed. Thus, rather than obscuring the coordinative relationship characterizing a behavior, the generality of these coordinative organizations was found to extend across variations in levels of activity associated with the subtasks of each primary task.
It will be important for future investigations to evaluate this evidence of organizational plasticity in other systems such as the lips and the tongue, although the task promises to be much more challenging given the loosely restricted ranges of motion for those structures. The design of the present investigation exploited the bilateral articulation, the unitary structure, and the mechanical linkage of the mandible, as well as the familiarity of the activation patterns underlying mastication. In this limited context, orofacial coordination was shown to exhibit a few limited consistent patterns of organization, including rigid coactivation of homologous pairs and varying relationships among other muscles with changing task demands. Extension of the present descriptive framework to other systems will necessarily involve structures whose anatomical configurations permit more degrees of freedom of movement, and will involve behaviors that are less thoroughly documented. The complexity of these systems may preclude a simplistic categorization of the coordinative patterns observed; however, it seems quite likely that the biomechanics of systems such as the lips and tongue will reveal changing muscle synergies that, like those in the present investigation, may be taken to suggest distinct movement goals and coordinative strategies. Indeed, other orofacial systems, which are characterized by greater mechanical independence and more lateralized corticobulbar innervation, may manifest more coordinative freedom even within homologous muscle pairs. In contrast to the mandible, for example, the behavior of the tongue can be modeled as that of a muscular hydrostat, a system that would certainly entail very different motor control objectives from those discussed here.
It will also be important to extend this description to a wider range of behaviors and speakers. We would anticipate that, for normally developing speakers, this coordinative flexibility will emerge as a hallmark of orofacial systems, although the characteristics and specific objectives of most orofacial behaviors remain unknown. As a means of evaluating these systems in potentially simpler and more revealing states, those presumably more limited in their capacities and having less predictable coordinative organization, we are currently focusing our attention on quantification of the coordinative breakdown and compensatory movement associated with various dysarthrias, and on characterization of the developmental patterns of orofacial coordination.
Acknowledgments
The author wishes to acknowledge the efforts in support of this investigation by Margaret Denny, Janine Janosky, Erich Luschei, Angela Rahn, Jacki Ruark, and Anne Smith. Support was provided by a grant from the NIDCD (DC00822).
References
- Baragar FA, Osborn JW. A model relating patterns of human jaw movement to biomechanical constraints. Journal of Biomechanics. 1984;17:757–767. doi: 10.1016/0021-9290(84)90106-4. [DOI] [PubMed] [Google Scholar]
- Barlow SM, Cole KJ, Abbs JH. A new head-mounted lip-jaw movement transduction system for the study of motor speech disorders. Journal of Speech and Hearing Research. 1983;26:283–288. doi: 10.1044/jshr.2602.283. [DOI] [PubMed] [Google Scholar]
- Basmajian JV, Stecko GA. A new bipolar electrode for electromyography. Journal of Applied Physiology. 1962;17:849. [Google Scholar]
- Cooke JD, Brown SH. Movement-related phasic muscle activation. II. Generation and functional role of the triphasic pattern. Journal of Neurophysiology. 1990;63:465–472. doi: 10.1152/jn.1990.63.3.465. [DOI] [PubMed] [Google Scholar]
- Cooper DS, Folkins JW. Comparison of electromyographic signals from different electrode placements in the palatoglossus muscle. Journal of the Acoustical Society of America. 1985;78:1530–1540. doi: 10.1121/1.392788. [DOI] [PubMed] [Google Scholar]
- Delcomyn F. Neural basis of rhythmic behavior in animals. Science. 1980;210:492–498. doi: 10.1126/science.7423199. [DOI] [PubMed] [Google Scholar]
- Fel’dman AG. Superposition of motor programs–I. Rhythmic forearm movements in man. Neuroscience. 1980a;5:81–90. doi: 10.1016/0306-4522(80)90073-1. [DOI] [PubMed] [Google Scholar]
- Fel’dman AG. Superposition of motor programs–II. Rapid forearm flexion in man. Neuroscience. 1980b;5:91–95. doi: 10.1016/0306-4522(80)90074-3. [DOI] [PubMed] [Google Scholar]
- Fetz EE, Cheney PD. Functional relations between primate motor cortex cells and muscles: Fixed and flexible. Ciba Foundation Symposium. 1987;132:98–117. doi: 10.1002/9780470513545.ch7. [DOI] [PubMed] [Google Scholar]
- Gentil M, Gay T. Jaw muscle activity for speech and non-speech gestures. Proceedings of the 106th Meeting.Acoustical Society of America; 1983. [Google Scholar]
- Ghez C, Martin JH. The control of rapid limb movement in the cat: III. Agonist-antagonist coupling. Experimental Brain Research. 1982;45:115–125. doi: 10.1007/BF00235770. [DOI] [PubMed] [Google Scholar]
- Grillner S. Possible analogies in the control of innate motor acts and the production of sound in speech. In: Grillner S, Lindblom B, Lubker J, Persson A, editors. Speech motor control. New York: Pergamon Press; 1981. pp. 217–230. [Google Scholar]
- Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. Journal of Neurophysiology. 1965;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Larson CR, Byrd KE, Garthwalte CR, Luschel ES. Alterations in the pattern of mastication after ablations of the lateral precentral cortex in rhesus macaques. Experimental Neurology. 1980;70:638–651. doi: 10.1016/0014-4886(80)90189-2. [DOI] [PubMed] [Google Scholar]
- Loeb GE, Pratt CA, Chanaud CM, Richmond FJR. Cross-correlation of EMG reveals widespread synchronization of motor units during some slow movements in intact cats. Journal of Neuroscience Methods. 1986;17:207–208. doi: 10.1016/0165-0270(87)90119-1. [DOI] [PubMed] [Google Scholar]
- Lund JP. Mastication and its control by the brain stem. Critical Reviews in Oral Biology and Medicine. 1991;2:33–64. doi: 10.1177/10454411910020010401. [DOI] [PubMed] [Google Scholar]
- Luschel ES. Development of objective standards of non-speech oral strength and performance: An advocate’s views. In: Moore CA, Yorkston KM, Beukelman DR, editors. Dysarthria and apraxia of speech: Perspectives on management. Baltimore: Brookes; 1991. [Google Scholar]
- Luschel ES, Goldberg LJ. Neural mechanisms of mandibular control: Mastication and voluntary biting. In: Brooks VB, editor. Handbook of physiology—Section I: The nervous system, Volume II, Motor control. Bethesda, Maryland: American Physiological Society; 1981. pp. 1237–1274. [Google Scholar]
- Luschel ES, Goodwin G. Patterns of mandibular movement and jaw muscle activity during mastication in the monkey. Journal of Neurophysiology. 1974;37:954–966. doi: 10.1152/jn.1974.37.5.954. [DOI] [PubMed] [Google Scholar]
- McClean M, Goldsmith H, Cerf A. Lower-lip EMG and displacement during bilabial disfluencies in adult stutterers. Journal of Speech and Hearing Research. 1984;27:342–349. doi: 10.1044/jshr.2703.342. [DOI] [PubMed] [Google Scholar]
- McMillan AS, Hannam AG. Task-related behaviour of motor units in different regions of the human masseter muscle. Archives of Oral Biology. 1992;37:849–857. doi: 10.1016/0003-9969(92)90119-s. [DOI] [PubMed] [Google Scholar]
- Møller E. The chewing apparatus. An electromyographic study of the action of muscles of mastication and its correlation to facial morphology. Acta Physiologica Scandinavica. 1966;69(Supplement 280) [PubMed] [Google Scholar]
- Moore CA, Smith A, Ringel RL. Task-specific organization of activity in human jaw muscles. Journal of Speech and Hearing Research. 1988;31:670–680. doi: 10.1044/jshr.3104.670. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Kubo Y. Masticatory rhythm in intracellular potential of trigeminal motoneurons induced by stimulation of orbital cortex and amygdala in cats. Brain Research. 1978;148:504–509. doi: 10.1016/0006-8993(78)90738-2. [DOI] [PubMed] [Google Scholar]
- Nozaki S, Iriki A, Nakamura Y. Localization of central rhythm generator involved in cortically induced rhythmical masticatory jaw-opening movement in the guinea pig. Journal of Neurophysiology. 1986;55:806–825. doi: 10.1152/jn.1986.55.4.806. [DOI] [PubMed] [Google Scholar]
- Smith A. The control of orofacial movements in speech. Critical Reviews in Oral Biology and Medicine. 1992;3:233–267. doi: 10.1177/10454411920030030401. [DOI] [PubMed] [Google Scholar]
- Smith A, Denny M. High-frequency oscillations as indicators of neural control mechanisms in human respiration, mastication, and speech. Journal of Neurophysiology. 1990;63:745–758. doi: 10.1152/jn.1990.63.4.745. [DOI] [PubMed] [Google Scholar]
- Tal M. Neural basis for initiation of rhythmic digastric activity upon midbrain stimulation in the guinea pig. Brain Research. 1987;411:58–64. doi: 10.1016/0006-8993(87)90680-9. [DOI] [PubMed] [Google Scholar]
- Zimmermann GN, Hanley JM. A cinefluorographic investigation of repeated fluent productions of stutterers in an adaptation procedure. Journal of Speech and Hearing Research. 1983;26:35–42. doi: 10.1044/jshr.2601.35. [DOI] [PubMed] [Google Scholar]