Abstract
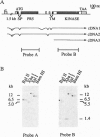
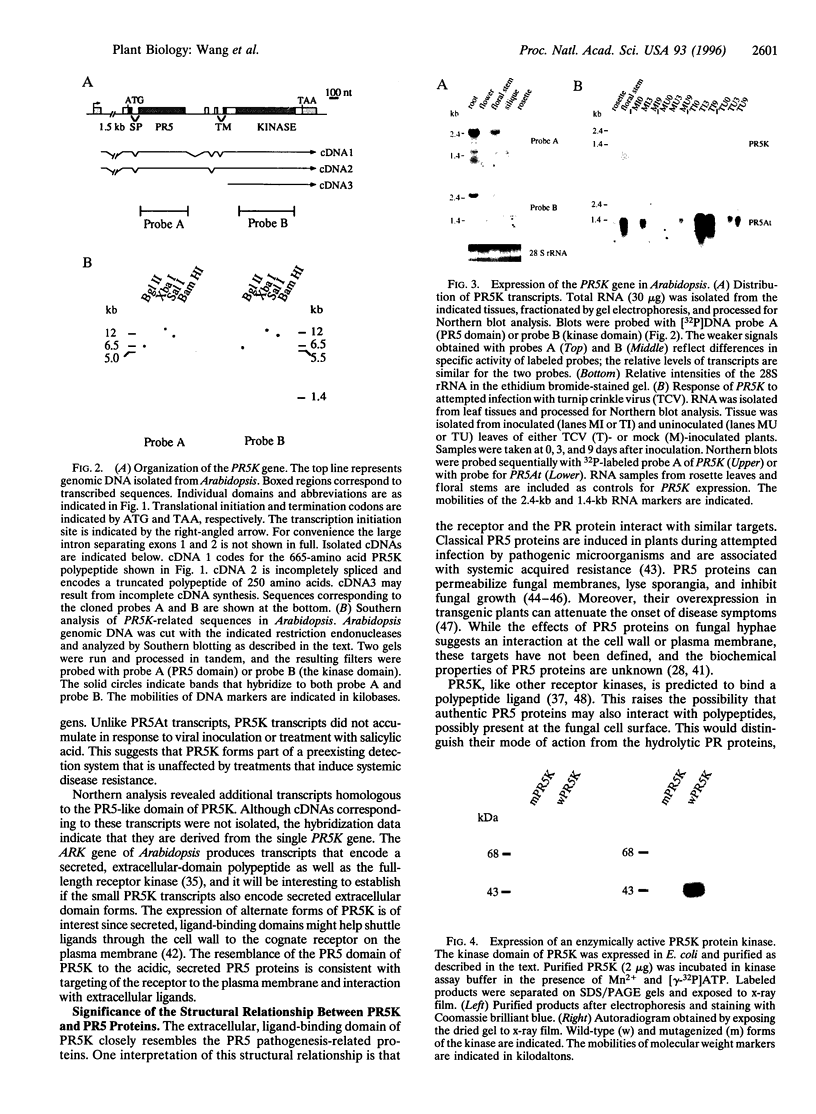
We have isolated an Arabidopsis thaliana gene that codes for a receptor related to antifungal pathogenesis-related (PR) proteins. The PR5K gene codes for a predicted 665-amino acid polypeptide that comprises an extracellular domain related to the PR5 proteins, a central transmembrane-spanning domain, and an intracellular protein-serine/threonine kinase. The extracellular domain of PR5K (PR5-like receptor kinase) is most highly related to acidic PR5 proteins that accumulate in the extracellular spaces of plants challenged with pathogenic microorganisms. The kinase domain of PR5K is related to a family of protein-serine/threonine kinases that are involved in the expression of self-incompatibility and disease resistance. PR5K transcripts accumulate at low levels in all tissues examined, although particularly high levels are present in roots and inflorescence stems. Treatments that induce authentic PR5 proteins had no effect on the level of PR5K transcripts, suggesting that the receptor forms part of a preexisting surveillance system. When the kinase domain of PR5K was expressed in Escherichia coli, the resulting polypeptide underwent autophosphorylation, consistent with its predicted enzyme activity. These results are consistent with PR5K encoding a functional receptor kinase. Moreover, the structural similarity between the extracellular domain of PR5K and the antimicrobial PR5- proteins suggests a possible interaction with common or related microbial targets.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chang C., Schaller G. E., Patterson S. E., Kwok S. F., Meyerowitz E. M., Bleecker A. B. The TMK1 gene from Arabidopsis codes for a protein with structural and biochemical characteristics of a receptor protein kinase. Plant Cell. 1992 Oct;4(10):1263–1271. doi: 10.1105/tpc.4.10.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J., Raikhel N. V. Short peptide domains target proteins to plant vacuoles. Cell. 1992 Feb 21;68(4):613–616. doi: 10.1016/0092-8674(92)90134-x. [DOI] [PubMed] [Google Scholar]
- Daum G., Eisenmann-Tappe I., Fries H. W., Troppmair J., Rapp U. R. The ins and outs of Raf kinases. Trends Biochem Sci. 1994 Nov;19(11):474–480. doi: 10.1016/0968-0004(94)90133-3. [DOI] [PubMed] [Google Scholar]
- De Lorenzo G., Cervone F., Bellincampi D., Caprari C., Clark A. J., Desiderio A., Devoto A., Forrest R., Leckie F., Nuss L. Polygalacturonase, PGIP and oligogalacturonides in cell-cell communication. Biochem Soc Trans. 1994 May;22(2):394–397. doi: 10.1042/bst0220394. [DOI] [PubMed] [Google Scholar]
- Dietrich A., Mayer J. E., Hahlbrock K. Fungal elicitor triggers rapid, transient, and specific protein phosphorylation in parsley cell suspension cultures. J Biol Chem. 1990 Apr 15;265(11):6360–6368. [PubMed] [Google Scholar]
- Dzelzkalns V. A., Nasrallah J. B., Nasrallah M. E. Cell-cell communication in plants: self-incompatibility in flower development. Dev Biol. 1992 Sep;153(1):70–82. doi: 10.1016/0012-1606(92)90092-u. [DOI] [PubMed] [Google Scholar]
- Felix G., Grosskopf D. G., Regenass M., Boller T. Rapid changes of protein phosphorylation are involved in transduction of the elicitor signal in plant cells. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8831–8834. doi: 10.1073/pnas.88.19.8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix G., Regenass M., Spanu P., Boller T. The protein phosphatase inhibitor calyculin A mimics elicitor action in plant cells and induces rapid hyperphosphorylation of specific proteins as revealed by pulse labeling with [33P]phosphate. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):952–956. doi: 10.1073/pnas.91.3.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goring D. R., Glavin T. L., Schafer U., Rothstein S. J. An S receptor kinase gene in self-compatible Brassica napus has a 1-bp deletion. Plant Cell. 1993 May;5(5):531–539. doi: 10.1105/tpc.5.5.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goring D. R., Rothstein S. J. The S-locus receptor kinase gene in a self-incompatible Brassica napus line encodes a functional serine/threonine kinase. Plant Cell. 1992 Oct;4(10):1273–1281. doi: 10.1105/tpc.4.10.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Heldin C. H. Dimerization of cell surface receptors in signal transduction. Cell. 1995 Jan 27;80(2):213–223. doi: 10.1016/0092-8674(95)90404-2. [DOI] [PubMed] [Google Scholar]
- Jones D. A., Thomas C. M., Hammond-Kosack K. E., Balint-Kurti P. J., Jones J. D. Isolation of the tomato Cf-9 gene for resistance to Cladosporium fulvum by transposon tagging. Science. 1994 Nov 4;266(5186):789–793. doi: 10.1126/science.7973631. [DOI] [PubMed] [Google Scholar]
- Kieber J. J., Rothenberg M., Roman G., Feldmann K. A., Ecker J. R. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell. 1993 Feb 12;72(3):427–441. doi: 10.1016/0092-8674(93)90119-b. [DOI] [PubMed] [Google Scholar]
- Kohorn B. D., Lane S., Smith T. A. An Arabidopsis serine/threonine kinase homologue with an epidermal growth factor repeat selected in yeast for its specificity for a thylakoid membrane protein. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10989–10992. doi: 10.1073/pnas.89.22.10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb C. J., Lawton M. A., Dron M., Dixon R. A. Signals and transduction mechanisms for activation of plant defenses against microbial attack. Cell. 1989 Jan 27;56(2):215–224. doi: 10.1016/0092-8674(89)90894-5. [DOI] [PubMed] [Google Scholar]
- Levine A., Tenhaken R., Dixon R., Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994 Nov 18;79(4):583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Liu D., Raghothama K. G., Hasegawa P. M., Bressan R. A. Osmotin overexpression in potato delays development of disease symptoms. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1888–1892. doi: 10.1073/pnas.91.5.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. B., Brommonschenkel S. H., Chunwongse J., Frary A., Ganal M. W., Spivey R., Wu T., Earle E. D., Tanksley S. D. Map-based cloning of a protein kinase gene conferring disease resistance in tomato. Science. 1993 Nov 26;262(5138):1432–1436. doi: 10.1126/science.7902614. [DOI] [PubMed] [Google Scholar]
- Mu J. H., Lee H. S., Kao T. H. Characterization of a pollen-expressed receptor-like kinase gene of Petunia inflata and the activity of its encoded kinase. Plant Cell. 1994 May;6(5):709–721. doi: 10.1105/tpc.6.5.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah J. B., Nasrallah M. E. Pollen[mdash]Stigma Signaling in the Sporophytic Self-Incompatibility Response. Plant Cell. 1993 Oct;5(10):1325–1335. doi: 10.1105/tpc.5.10.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett R. A., Grabowski P. J., Konarska M. M., Seiler S., Sharp P. A. Splicing of messenger RNA precursors. Annu Rev Biochem. 1986;55:1119–1150. doi: 10.1146/annurev.bi.55.070186.005351. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard L., Arró M., Hoebeke J., Meeks-Wagner D. R., Van K. T. Immunological Evidence of Thaumatin-Like Proteins during Tobacco Floral Differentiation. Plant Physiol. 1992 Jan;98(1):337–342. doi: 10.1104/pp.98.1.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe J. L., Rivin C. J., Sessions R. A., Feldmann K. A., Zambryski P. C. The Tousled gene in A. thaliana encodes a protein kinase homolog that is required for leaf and flower development. Cell. 1993 Dec 3;75(5):939–950. doi: 10.1016/0092-8674(93)90537-z. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N. K., Nelson D. E., Kuhn D., Hasegawa P. M., Bressan R. A. Molecular Cloning of Osmotin and Regulation of Its Expression by ABA and Adaptation to Low Water Potential. Plant Physiol. 1989 Jul;90(3):1096–1101. doi: 10.1104/pp.90.3.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein J. C., Howlett B., Boyes D. C., Nasrallah M. E., Nasrallah J. B. Molecular cloning of a putative receptor protein kinase gene encoded at the self-incompatibility locus of Brassica oleracea. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8816–8820. doi: 10.1073/pnas.88.19.8816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobias C. M., Howlett B., Nasrallah J. B. An Arabidopsis thaliana Gene with Sequence Similarity to the S-Locus Receptor Kinase of Brassica oleracea: Sequence and Expression. Plant Physiol. 1992 May;99(1):284–290. doi: 10.1104/pp.99.1.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uknes S., Mauch-Mani B., Moyer M., Potter S., Williams S., Dincher S., Chandler D., Slusarenko A., Ward E., Ryals J. Acquired resistance in Arabidopsis. Plant Cell. 1992 Jun;4(6):645–656. doi: 10.1105/tpc.4.6.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Vigers A. J., Roberts W. K., Selitrennikoff C. P. A new family of plant antifungal proteins. Mol Plant Microbe Interact. 1991 Jul-Aug;4(4):315–323. doi: 10.1094/mpmi-4-315. [DOI] [PubMed] [Google Scholar]
- Walker J. C. Receptor-like protein kinase genes of Arabidopsis thaliana. Plant J. 1993 Mar;3(3):451–456. doi: 10.1111/j.1365-313x.1993.tb00164.x. [DOI] [PubMed] [Google Scholar]
- Walker J. C., Zhang R. Relationship of a putative receptor protein kinase from maize to the S-locus glycoproteins of Brassica. Nature. 1990 Jun 21;345(6277):743–746. doi: 10.1038/345743a0. [DOI] [PubMed] [Google Scholar]
- Woloshuk C. P., Meulenhoff J. S., Sela-Buurlage M., van den Elzen P. J., Cornelissen B. J. Pathogen-induced proteins with inhibitory activity toward Phytophthora infestans. Plant Cell. 1991 Jun;3(6):619–628. doi: 10.1105/tpc.3.6.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S., Lam E. Characterization of a DNA Topoisomerase II cDNA from Arabidopsis thaliana. Plant Physiol. 1994 Dec;106(4):1701–1702. doi: 10.1104/pp.106.4.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalpani N., Raskin I. Salicylic acid: a systemic signal in induced plant disease resistance. Trends Microbiol. 1993 Jun;1(3):88–92. doi: 10.1016/0966-842x(93)90113-6. [DOI] [PubMed] [Google Scholar]
- Zhang S. H., Lawton M. A., Hunter T., Lamb C. J. atpk1, a novel ribosomal protein kinase gene from Arabidopsis. I. Isolation, characterization, and expression. J Biol Chem. 1994 Jul 1;269(26):17586–17592. [PubMed] [Google Scholar]