Abstract
The use of antibodies in therapy and diagnosis has undergone an unprecedented expansion during the past two decades. This is due in part to innovations in antibody engineering that now offer opportunities for the production of “second generation” antibodies with multiple specificities or altered valencies. The targeting of individual components of the human epidermal growth factor receptor (HER)3-PI3K signaling axis, including the preferred heterodimerization partner HER2, is known to have limited anti-tumor effects. The efficacy of antibodies or small molecule tyrosine kinase inhibitors (TKIs) in targeting this axis is further reduced by the presence of the HER3 ligand, heregulin. To address these shortcomings, we performed a comparative analysis of two distinct approaches toward reducing the proliferation and signaling in HER2 overexpressing tumor cells in the presence of heregulin. These strategies both involve the use of engineered antibodies in combination with the epidermal growth factor receptor (EGFR)/HER2 specific TKI, lapatinib. In the first approach, we generated a bispecific anti-HER2/HER3 antibody that, in the presence of lapatinib, is designed to sequester HER3 into inactive HER2-HER3 dimers that restrain HER3 interactions with other possible dimerization partners. The second approach involves the use of a tetravalent anti-HER3 antibody with the goal of inducing efficient HER3 internalization and degradation. In combination with lapatinib, we demonstrate that although the multivalent HER3 antibody is more effective than its bivalent counterpart in reducing heregulin-mediated signaling and growth, the bispecific HER2/HER3 antibody has increased inhibitory activity. Collectively, these observations provide support for the therapeutic use of bispecifics in combination with TKIs to recruit HER3 into complexes that are functionally inert.
Keywords: bispecific antibody, receptor internalization, HER3, HER2, antibody engineering
Introduction
Multiple cancers are driven by aberrant signaling through the human epidermal growth factor receptor (HER), also known as ErbB, family members. Recent studies have indicated that HER2-HER3 heterodimers can play a central role in tumorigenesis.1-5 HER3 is a preferred dimerization partner for HER2, which has no known ligand and is constitutively active.5-7 Although HER3 has very low intrinsic kinase activity, there are six phosphorylation-dependent binding sites for PI3K on the cytosolic tail of this receptor.8 Consequently, HER2-HER3 heterodimers are the most effective activators identified to date of the PI3K/Akt pathway through both ligand-independent and ligand-dependent signaling.5,9 Ligand-dependent activation of HER3 involves the binding of heregulin or other ligands to induce a conformational switch in the dimerization arm, driving heterodimer formation with kinase competent partners such as HER2 or EGFR.5,6
Consistent with HER3 as a driver of tumorigenesis, loss of HER3 expression in breast cancer cells results in reductions in both PI3K/Akt signaling and proliferation.1,2 Further, modeling studies demonstrate that HER3 represents a central node in PI3K/Akt signaling.4 In conjunction with the limited efficacy of solely targeting HER2 with monotherapies such as trastuzumab,10-12 these observations have motivated the development of therapeutics targeting HER3 or HER2-HER3 heterodimers.3,13-15 Recent data indicate that the targeting of this axis with antibodies is less effective in the presence of heregulin,14 which is expressed in either autocrine or paracrine fashion in many tumor types.16-18 Thus, there is a need for the generation of improved therapeutics directed toward ligand-dependent activation of HER3. The current study involves a comparison of two distinct approaches using bispecific HER2/HER3 specific antibodies or multivalent HER3-specific antibodies to target HER3 in the presence of the HER3 ligand, heregulin.
As an alternative to targeting HER family members with antibodies, the use of small molecule inhibitors of the tyrosine kinase activity of EGFR or HER2, or of the downstream kinase, PI3K, has attracted much interest.19-25 However, these inhibitors can lead to tumor escape due to upregulation of compensatory signaling pathways and complex cross-regulatory networks involving negative feedback loops. For example, the delivery of lapatinib, a tyrosine kinase inhibitor (TKI) that targets both EGFR and HER2, results in upregulation of HER2 and HER3 expression and PI3K/Akt signaling.26-29 Lapatinib resistance pathways can also include activating mutations of PI3K,30 and the inhibitory effects of lapatinib can be dampened by the presence of the HER3 ligand, heregulin.14,31-33 This suggests that lapatinib in combination with antibodies or bispecifics that bind to HER3 might provide an effective route for therapy, particularly for tumors involving autocrine or paracrine heregulin loops that can result in limited efficacy of monotherapies.
Related to the limited therapeutic efficacy of TKIs such as lapatinib that inhibit EGFR and HER2 activation, HER3 can associate with other activating receptors such as cMET and insulin-like growth factor type I receptor I/insulin receptor substrate-1.5,7,34,35 A possible strategy to extinguish HER3-PI3K signaling would therefore be to inhibit multiple potential HER3 partners. This provides the impetus for directly targeting HER3, which would avoid the complexity of identifying and globally inhibiting all HER3 partners. Here, we have taken the approach of investigating the ability of a bispecific anti-HER2/HER3 antibody to drive HER3 into HER2-HER3 heterodimers that, through combination treatment with lapatinib, are “kinase-dead”. Locking HER3 into such inactive complexes is expected to sequester this receptor from interactions with other signaling competent partners and, as such, have anti-tumor effects.
A second route toward extinguishing HER3 signaling is to develop strategies to induce the efficient internalization and degradation of this receptor. In support of this approach, several studies have demonstrated that reduction of HER3 expression has anti-proliferative effects.1,2 HER3 is constitutively internalized into the endolysosomal pathway36 and it is well documented that receptor crosslinking leads to downregulation in multiple different cellular systems. We therefore reasoned that a multivalent (tetrameric) anti-HER3 antibody would induce more efficient HER3 internalization or degradation relative to its bivalent counterpart, thereby enhancing clearance from the cell surface. The efficacy of this approach has been compared with that of recruiting HER3 into kinase-inactivated HER2-HER3 heterodimers. These comparative studies have also been extended to microscopy analyses of the trafficking behavior of the different antibodies within cells, which relates to both drug delivery for antibody-drug conjugates and Fc-mediated cytotoxicity.37,38
Our study has led to several observations that are of relevance to targeting HER3. First, we show that in the presence of heregulin, combination treatment with antibodies and lapatinib is necessary to achieve inhibition of signaling and growth. Under these conditions, a tetravalent HER3-specific antibody induces increased degradation of HER3 and has more potent anti-tumor effects relative to its bivalent counterpart. Importantly, when used in combination with lapatinib, the bispecific anti-HER2/HER3 antibody is a more effective inhibitor of heregulin-driven signaling and growth compared with anti-HER3 antibodies, tetravalent anti-HER3 antibodies or mixtures of individual antibodies specific for HER2 and HER3. Our observations are consistent with a model in which the bispecific antibody recruits HER3 into HER2-HER3 heterodimers that are inactive in the presence of lapatinib. Collectively, these studies provide support for the combined use of multimeric anti-growth factor receptor antibodies with small molecule TKIs for the therapy of cancer.
Results
Antibodies specific for HER2 and HER3 have differential effects on signaling and proliferation
The anti-HER3 antibody, Ab6 (MM-1213), which competes with heregulin for binding to HER3, was generated in our laboratory as a biosimilar and used throughout these studies. To investigate whether a tetravalent anti-HER3 antibody is more effective than its bivalent counterpart in inhibiting cell growth and proliferation, we fused the Ab6 single chain variable fragment (scFv) to the CH3 domains of Ab6 via a Gly-Ser-Ser linker (Ab6tet). In addition, a bispecific trastuzumab (anti-HER2)-Ab6 antibody (TAb6) and a bispecific pertuzumab (anti-HER2)-Ab6 antibody (PAb6) were generated by fusing scFv fragments corresponding to Ab6 to the CH3 domains of trastuzumab and pertuzumab, respectively, using an analogous design (Fig. 1). All antibodies were expressed in transfected Chinese hamster ovary (CHO) cells. The expression yields of the bispecific antibodies were 4.5 mg/L, 2.5 mg/L, and 8 mg/L for Ab6tet, PAb6 and TAb6, respectively. Size exclusion and serum stability analyses of the bispecific proteins are presented in the Supplementary Materials (Fig. S1 and S2). For comparative purposes, size exclusion studies of Ab6, trastuzumab and pertuzumab are shown (Fig. S1). Ab6 and the bispecific antibodies also bound to immobilized, recombinant Fc-HER2 (TAb6, PAb6) or HER3 (Ab6, Ab6tet, TAb6, PAb6) in surface plasmon resonance experiments (BIAcore; data not shown). The in vivo half-lives (β-phase) of Ab6tet and TAb6 were also determined in BALB/c SCID mice and were 228 ± 14 (n = 4 mice) and 215 ± 11 (n = 5 mice) hours, respectively.
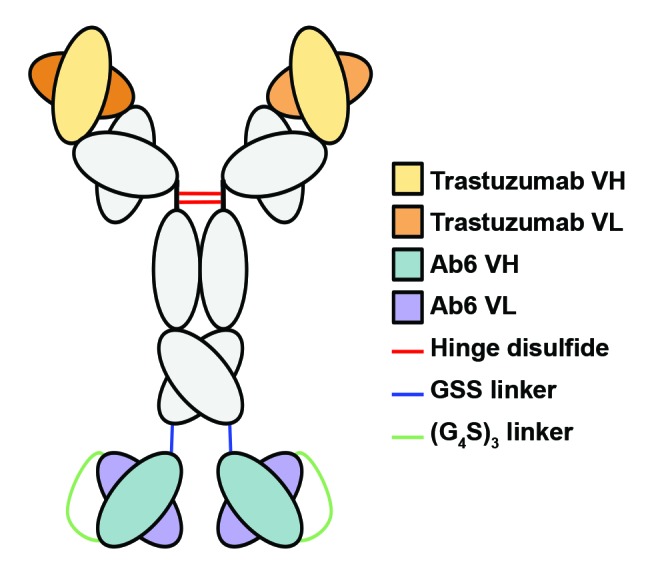
Figure 1. Schematic representation of the bispecific antibody (TAb6) comprising trastuzumab and a scFv derived from the anti-HER3 antibody, Ab6, used in the current study.
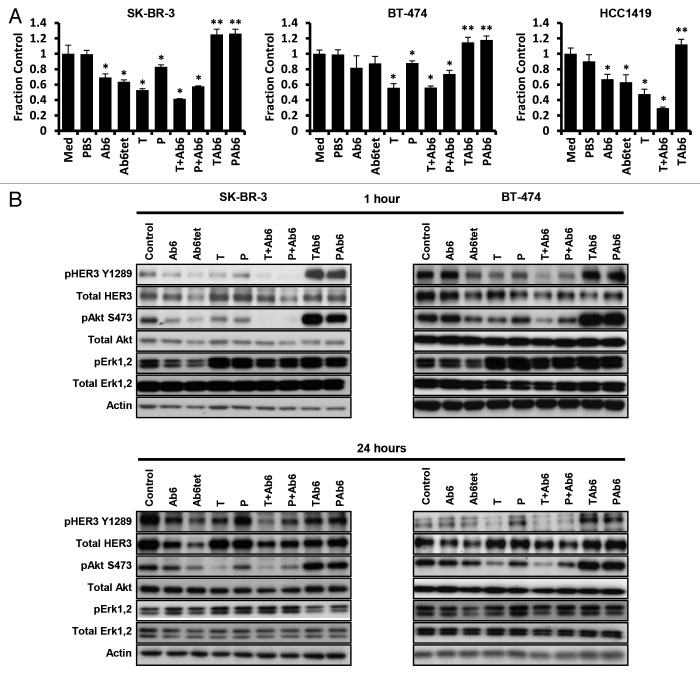
We initially assessed the effect of targeting HER3 and HER2/HER3 with several different antibody formats in the absence of the HER3 ligand, heregulin. For comparative purposes we also included the anti-HER2 antibodies, trastuzumab and pertuzumab, which bind to domain IV and the dimerization arm (domain II), respectively, of HER2.39,40 The anti-HER3 antibody, Ab6, and its tetrameric form, Ab6tet, reduced proliferation in the HER2-overexpressing cancer cell lines, SK-BR-3 and HCC1419 (Fig. 2A). Although Ab6 and Ab6tet treatment resulted in a trend toward reduced proliferation in BT-474 cells, the effects were not significant. By contrast with SK-BR-3 and HCC1419 cells, BT-474 cells express a gain of function variant of PI3K41,42 which could account for the reduced efficacy of anti-HER3 antibodies. Consistent with the observations of others,43 trastuzumab has higher anti-proliferative activity on ligand-independent growth of SK-BR-3 and BT-474 cells relative to pertuzumab (Fig. 2A). A mixture of pertuzumab and Ab6 is also less effective than trastuzumab and Ab6.
Figure 2. Effects of antibodies specific for HER2 and/or HER3 on HER2-overexpressing breast cancer cells. (A) Cells were incubated with 50 nM anti-HER3 (Ab6), tetrameric anti-HER3 (Ab6tet), trastuzumab (T), pertuzumab (P), trastuzumab or pertuzumab and Ab6 (T + Ab6 or P + Ab6), bispecific trastuzumab with anti-HER3 Ab6 scFv (TAb6), or bispecific pertuzumab with anti-HER3 Ab6 scFv (PAb6) for 5 d. Proliferative responses were assessed using the MTS reagent and were normalized against the proliferation of cells incubated in medium (Med) only. Data shown are means of triplicates ± standard deviation. The symbols * and ** indicate significantly lower or higher proliferative responses, respectively, between cells treated with antibody and PBS vehicle (Student t-test; P < 0.05). (B) SK-BR-3 or BT-474 cells were treated with anti-HER2/HER3 antibodies (50 nM) for 1 or 24 h, and cell lysates analyzed by immunoblotting. Data shown are representative of at least two independent experiments.
Although combinations of anti-HER2 and anti-HER3 antibodies (Ab6 combined with trastuzumab or pertuzumab) had anti-proliferative activities, exposure of cells to the bispecific, TAb6, comprising trastuzumab plus Ab6, resulted in increased proliferation (Fig. 2A). Further, the bispecific antibody comprising Ab6 and pertuzumab (PAb6) induced proliferation (Fig. 2A). The effects of both PAb6 and TAb6, which bind to different sites of HER2,39,40 indicate that proximity of HER2 and HER3 is sufficient for proliferative signaling, rather than a need for the receptors to dimerize in a specific configuration. This proximity model is also consistent with the observation that exposure of cells to a mixture of trastuzumab or pertuzumab and Ab6, which would not be expected to drive formation of HER2-HER3 heterodimers, results in reduced proliferation (Fig. 2A).
Analyses of the effects of the antibodies on the phosphorylation of HER3, Akt and Erk demonstrated that the anti-proliferative effects are associated with decreased pAkt levels in SK-BR-3 and BT-474 cells (Fig. 2B). Although pErk levels were lower following treatment of cells for one hour with Ab6, Ab6tet, or Ab6 combined with anti-HER2 antibodies than for cells treated with trastuzumab, pertuzumab, TAb6 or PAb6, these differences were not sustained at the 24 h time point (Fig. 2B). Exposure of SK-BR-3 or BT-474 cells to TAb6 or PAb6 resulted in increased pAkt (S473) levels that persisted for at least 24 h, consistent with the pro-proliferative effects of these bispecific antibodies. By contrast, the levels of pAkt at 24 h were decreased in cells treated with any of the other antibodies or antibody combinations (Fig. 2B). Pertuzumab as a single agent, or in combination with Ab6, was less effective than trastuzumab (with or without Ab6) in reducing pAkt (S473) phosphorylation, which is congruent with the lower anti-proliferative activity of this antibody (Fig. 2A).
Earlier studies have shown that relief of feedback inhibition of the FoxO1/3a transcription factors can lead to upregulation of multiple receptor tyrosine kinases such as HER3 and insulin-like growth factor-1 receptor (IGF-1R) and subsequent reactivation of Akt.27,44,45 However, the possibility of pAkt reactivation occurring in the current study following treatment of cells with the bispecific antibodies, TAb6 or PAb6, can be excluded by the relatively rapid kinetics of pAkt induction observed (1 h, Fig. 2B). To further support this, we observed phosphorylation of Akt following 15 min of exposure of SK-BR-3 and BT-474 cells to the bispecific antibodies (Fig. S3).
Total HER3 levels in the cells following treatment with anti-HER3 antibodies were also analyzed. In general, HER3 levels were reduced by treatment with anti-HER3 antibodies, Ab6 and Ab6tet, whereas exposure of cells to the bispecific antibodies, TAb6 and PAb6, resulted in less HER3 degradation (P < 0.05; Fig. 2B; Fig. S4). Reduced HER3 degradation following TAb6 or PAb6 treatment is consistent with the inhibitory effects of HER2 expression on the internalization of ligand-activated EGFR or HER3.46,47The increased HER3 degradation induced by Ab6tet relative to Ab6 was more marked for SK-BR-3 than BT-474 cells, although in both cases the differences were statistically significant (P < 0.05; Fig. 2B).
Microscopy analyses were used to further investigate the intracellular trafficking pathways taken by Ab6, Ab6tet and TAb6 (Fig. 3). These studies demonstrate that Ab6tet is internalized into EEA-1 positive early endosomes more rapidly than Ab6, and enters these compartments within 5 min of treatment. Following 15 min of treatment, both Ab6 and Ab6tet are internalized into early endosomes, although the levels of Ab6 remaining on the plasma membrane are greater than for Ab6tet (Fig. 3). By contrast, the majority of TAb6 is present on the plasma membrane following 5–60 min of treatment (Fig. 3; Fig. S5). Within 60 min of treatment, Ab6 and Ab6tet are present in LAMP-1+ lysosomes (Fig. S5). Multivalent antibody binding to HER3 therefore enhances the rate of HER3 internalization into the endolysosomal pathway, consistent with the increased degradation of HER3 in the presence of Ab6tet relative to Ab6.
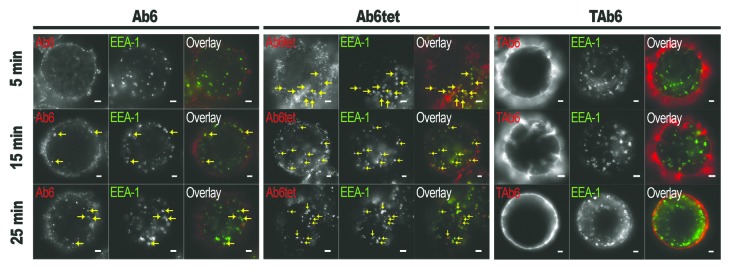
Figure 3. Ab6tet internalizes into SK-BR-3 cells more rapidly than Ab6. Cells were pulsed with 50 nM anti-HER3 (Ab6), tetrameric anti-HER3 (Ab6tet) or bispecific trastuzumab with anti-HER3 Ab6 scFv (TAb6) for 5 min at 37οC, chased for 0, 10 or 20 min, fixed, permeabilized and stained with anti-EEA-1 antibody. The combined pulse plus chase times are indicated on the left margin. Anti-HER3 or HER2/HER3 antibodies were detected with Alexa 555-labeled secondary antibody (pseudocolored red in overlay) and anti-EEA-1 antibody with Alexa 647-labeled secondary antibody (pseudocolored green in overlay). Yellow arrows in the images for Ab6tet-treated cells indicate examples of internalized Ab6tet that is associated with EEA-1 positive endosomes. Scale bars = 2 μm.
Targeting HER2/HER3 with antibodies is ineffective in the presence of heregulin
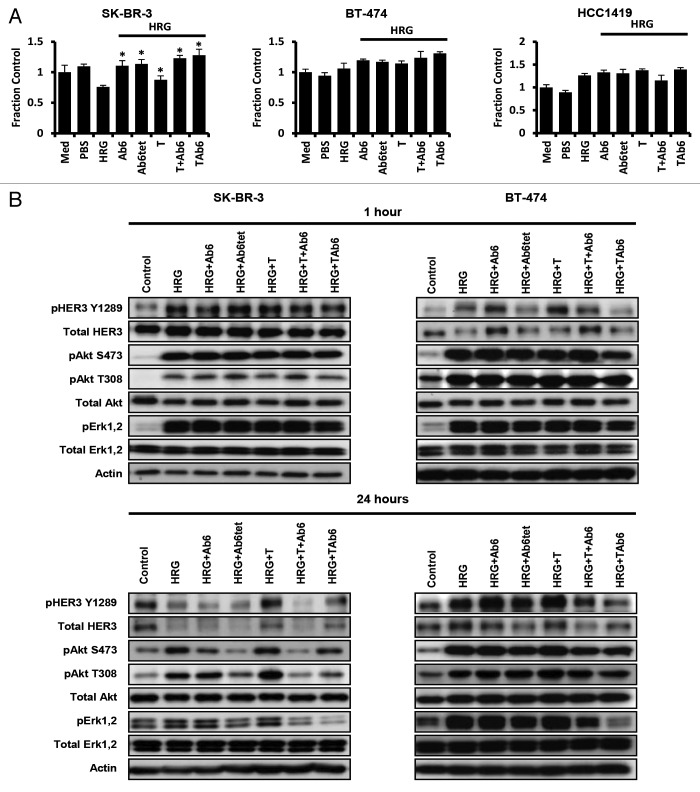
Heregulin is frequently present in tumors due to autocrine or paracrine production,16-18 motivating an investigation of the effects of the antibodies on tumor cell growth in the presence of this HER3 ligand. HCC1419 cells showed increased proliferation in the presence of heregulin, whereas reduced proliferation was observed for SK-BR-3 cells (Fig. 4A). Although BT-474 cells showed a trend toward heregulin-stimulated proliferation, differences between vehicle- and heregulin-treated cells were not always significant. Reduced proliferation of SK-BR-3 cells in response to heregulin has been described previously.48 Heregulin exposure ablated the inhibitory effects of Ab6, Ab6tet, trastuzumab or trastuzumab plus Ab6 on ligand-independent proliferation of BT-474 or HCC1419 cells (Fig. 2A and 4A). Slightly higher proliferation of HCC1419 cells was observed following TAb6 relative to trastuzumab plus Ab6 treatment, but differences for these two treatments were not significant for SK-BR-3 and BT-474 cells.
Figure 4. Antibodies specific for HER2 and/or HER3 have reduced efficacy in inhibiting proliferation and PI3K/Akt signaling in the presence of heregulin. (A) Cells were incubated with heregulin (HRG, 6.25 nM) and 50 nM anti-HER3 (Ab6), tetrameric anti-HER3 (Ab6tet), trastuzumab (T), trastuzumab and Ab6 (T + Ab6) or bispecific trastuzumab with anti-HER3 Ab6 scFv (TAb6) for 5 d. Proliferative responses were assessed using the MTS reagent and were normalized against the proliferation of cells incubated in medium (Med) only. Data shown are means of triplicates ± standard deviation. * indicates statistically significant differences between proliferative responses for cells treated with antibody in the presence of heregulin and cells treated with heregulin only (Student t-test; P < 0.05). (B) SK-BR-3 or BT-474 cells were treated with anti-HER2/HER3 antibodies (50 nM) in the presence of 6.25 nM heregulin for 1 or 24 h, and cell lysates analyzed by immunoblotting. Data shown are representative of at least two independent experiments.
The reduced efficacy of the antibodies in the presence of heregulin was accompanied by either no change (SK-BR-3 cells with trastuzumab and TAb6 24 h following treatment and BT-474 cells with all treatments), or a reduction (SK-BR-3 cells with Ab6, Ab6tet, or a mixture of trastuzumab plus Ab6 at 24 h) in pAkt levels (Fig. 4B). Collectively, the data indicate that antibody targeting of HER2 and HER3 has limited efficacy in the presence of intratumoral HER3 ligands.
Lapatinib combined with antibodies specific for HER2/HER3 overcomes heregulin-mediated resistance
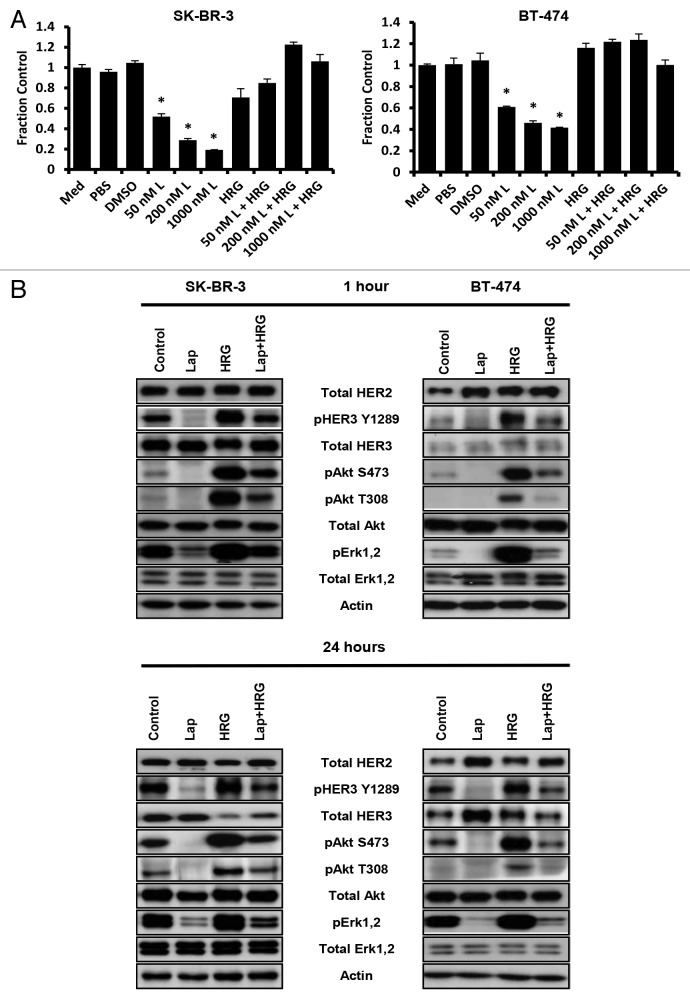
The induction of proliferative signaling by HER2-HER3 dimerization “forced” by TAb6 treatment suggested that the combination of this bispecific antibody with the small molecule inhibitor of HER2 (and EGFR) kinase activity, lapatinib, might stabilize HER2-HER3 heterodimers in an inactive state. Further, we hypothesized that such complexes would preclude the interaction of HER3 with signaling competent partners. Lapatinib treatment is known to result in upregulation of HER2 and HER3 levels on the plasma membrane,26-29 and we confirmed these observations under the conditions of our assay (except that HER2 was not upregulated on BT-474 cells; Fig. S6). Treatment of SK-BR-3 and BT-474 cells with lapatinib at doses ranging from 50 nM‒1 μM resulted in inhibition of proliferation (Fig. 5A). However, this growth inhibition was ablated by exposure of cells to heregulin (Fig. 5A). Although lapatinib treatment alone resulted in potent inhibition of HER3, Akt and Erk phosphorylation, this was partially reversed by incubation of cells with lapatinib plus heregulin (Fig. 5B).
Figure 5. Heregulin treatment reverses the anti-proliferative effects of lapatinib in HER2 overexpressing cell lines. (A) Cells were incubated with different concentrations of lapatinib (L) in the presence and absence of heregulin (HRG; 6.25 nM) for 5 d. Proliferative responses were assessed using the MTS reagent and were normalized against the proliferation of cells incubated in medium (Med) only. Data shown are means of triplicates ± standard deviation. * indicates statistically significant differences between proliferative responses for cells treated with lapatinib and DMSO vehicle (Student t-test; P < 0.001). (B) SK-BR-3 or BT-474 cells were treated with 1 μM lapatinib (Lap) in the presence and absence of heregulin (6.25 nM) for 1 or 24 h, and cell lysates analyzed by immunoblotting. Data shown are representative of at least two independent experiments.
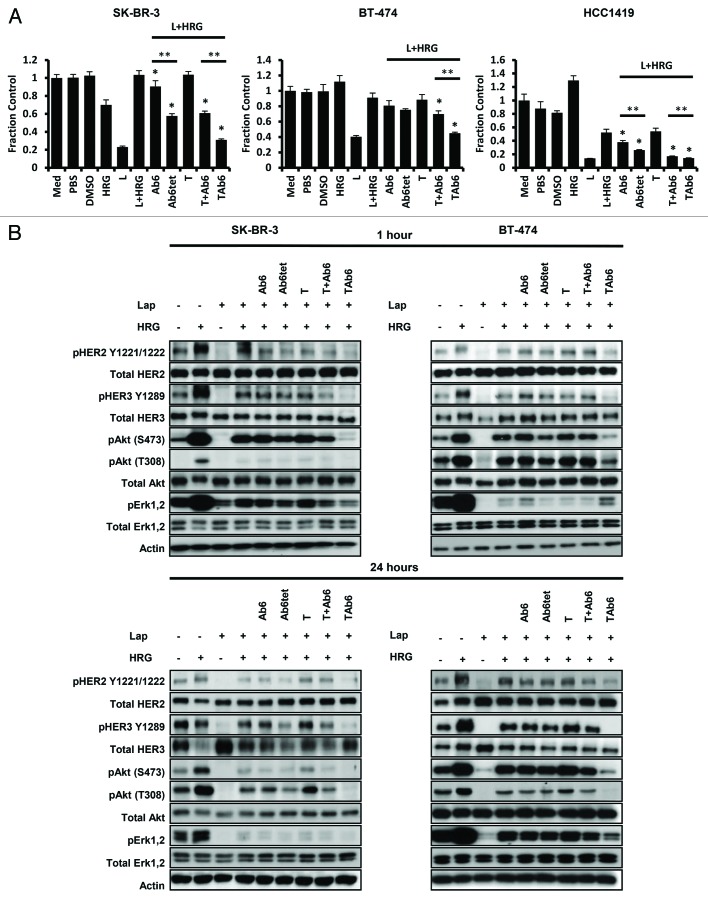
The observation that lapatinib alone has limited efficacy in blocking cell proliferation in the presence of heregulin prompted us to investigate the effect of combination treatment of heregulin-exposed SK-BR-3, BT-474 and HCC1419 cells with lapatinib and HER3-targeting antibodies. Addition of Ab6 or Ab6tet to lapatinib resulted in anti-proliferative effects compared with the effects of lapatinib alone (Fig. 6A). In addition, for SK-BR-3 and HCC1419 cells, Ab6tet was more effective than Ab6 (Fig. 6A). Consistent with the inability of trastuzumab to inhibit ligand-dependent HER2-HER3 signaling,43 this HER2-specific antibody did not reduce proliferation in the presence of heregulin and lapatinib. By contrast, of the antibodies/bispecifics tested, the anti-HER2/HER3 bispecific, TAb6, was the most potent of the antibodies tested in combination with lapatinib in reducing proliferation of heregulin-treated cells (Fig. 6A). Importantly, this bispecific also had increased anti-proliferative effects relative to a mixture of trastuzumab and Ab6 (Fig. 6A). Trastuzumab has superior activity over pertuzumab in inhibiting ligand-independent activity43 (Fig. 2), suggesting that TAb6 would more effectively target both ligand-dependent (heregulin) and ligand-independent signaling than PAb6. In combination with the lower expression yield of PAb6 relative to that of TAb6 (2.5 mg/L vs. 8 mg/L), we therefore focused on the use of TAb6 in the lapatinib/bispecific combination studies. In the absence of heregulin, none of the antibodies further reduced the anti-proliferative activity of lapatinib (Fig. S7).
Figure 6. The bispecific anti-HER2/HER3 antibody, TAb6, has the highest activity in reducing cell proliferation and PI3K/Akt signaling in the presence of heregulin and lapatinib. (A) Cells were incubated with 1 μM lapatinib (L) in the presence of heregulin (HRG; 6.25 nM) and treated with 50 nM anti-HER3 (Ab6), tetrameric anti-HER3 (Ab6tet), trastuzumab (T), trastuzumab and Ab6 (T + Ab6) or bispecific trastuzumab with anti-HER3 Ab6 scFv (TAb6) for 5 d. Proliferative responses were assessed using the MTS reagent and were normalized against the proliferation of cells incubated in medium (Med) only. Data shown are means of triplicates ± standard deviation. * indicates statistically significant differences between proliferative responses for cells treated with antibodies vs. vehicle in the presence of lapatinib and heregulin (Student t-test; P < 0.05). ** indicates statistically significant differences between proliferative responses for the pairwise comparison of the two indicated treatments (horizontal bars). (B) SK-BR-3 or BT-474 cells were treated with lapatinib (Lap), heregulin (6.25 nM) and anti-HER2/HER3 antibodies (50 nM) as indicated for 1 or 24 h, and cell lysates analyzed by immunoblotting. Data shown are representative of at least two independent experiments.
In the presence of lapatinib and heregulin, Ab6tet, trastuzumab plus Ab6 or TAb6 treatment resulted in lower levels of pHER3, pErk and pAkt (S473, T308) for SK-BR-3 and HCC1419 cells (Fig. 6B; Fig. S8). The improved activity of Ab6tet compared with Ab6 in SK-BR-3 and HCC1419 cells is consistent with the increased degradation of HER3 in the presence of the tetrameric antibody (P < 0.05; Fig. S8 and S9). TAb6 was the most potent antibody in reducing downstream signaling in SK-BR-3, BT-474 and HCC1419 cells, and was more inhibitory than mixtures of trastuzumab and Ab6 (Fig. 6B; Fig. S8). Further, TAb6 was the only antibody in combination with lapatinib that reduced heregulin-induced pAkt and pErk in BT-474 cells. Collectively, the data indicate that in the presence of lapatinib the bispecific antibody, TAb6, is the most potent inhibitor of ligand-induced activation.
Discussion
The limitations of targeting individual components of the HER3/PI3K/Akt signaling axis, including the activity of the preferred dimerization partner HER2, is well documented. Several factors contribute to the poor efficacy of this approach: first, antibodies specific for HER2 and HER3 have reduced activity in inhibiting proliferation and signaling in the presence of the HER3 ligand heregulin14,31,32 (this study), which is expressed in multiple tumor types.16-18 Second, the use of small molecule TKIs such as lapatinib or gefitinib results in compensatory HER2 and HER3 upregulation and signaling through either incomplete blockade of HER2 kinase activity or HER3 association with other signaling competent partners.5,7,26-29,34,35 Third, these compensatory effects are exacerbated by the presence of heregulin.14,31-33 Consequently, TKIs such as lapatinib are ineffective as single agents in inhibiting breast tumor cell proliferation, indicating a need for the development of combination therapies.14,26-29
In the current study, we analyzed the efficacy of two HER3-focused strategies directed toward reducing breast cancer cell signaling and proliferation. These approaches also have broader relevance to the targeting of other cell surface receptors, and involve the use of distinct antibody designs to recruit HER3 into kinase-inhibited HER2-HER3 complexes or induce HER3 degradation. We demonstrate that the recruitment of HER3 into lapatinib-inactivated HER2-HER3 complexes by a bispecific anti-HER2/HER3 antibody (TAb6, comprising trastuzumab and an anti-HER3 scFv) is efficacious in both ablating PI3K/Akt signaling and reducing tumor cell proliferation in the presence of heregulin. Importantly, and of direct relevance to therapy, the bispecific antibody is a more potent inhibitor of signaling and growth than either HER2- or HER3-specific antibodies alone or a mixture of antibodies of both specificities. In a second approach, we compared the effect on tumor cell signaling and growth of a multivalent anti-HER3 antibody (Ab6tet) that is designed to enhance HER3 degradation with its bivalent counterpart, Ab6. In the presence of heregulin and lapatinib, Ab6tet has higher activity. By comparison with bivalent Ab6, this multivalent construct induces more rapid HER3 internalization into early endosomes. By contrast, the bispecific TAb6 induces less HER3 degradation than either Ab6 or Ab6tet, and yet is more effective in reducing cell signaling and proliferation. This indicates that the internalization resistance or recycling behavior of HER246,47 has a dominant effect on HER3 in the forced HER2-HER3 heterodimers. Further, the internalization resistance is consistent with earlier studies in which endocytic uptake of EGFR or HER3 is reduced by dimerization with HER2.46,47 Importantly, our data indicate that the locking of HER3 into inactive HER2-HER3 complexes, even if they remain on the plasma membrane, is a more effective strategy for the ablation of heregulin-mediated signaling than induction of increased HER3 degradation.
The question arises as to why the anti-HER3 antibody, which competes for heregulin binding,3 is not as effective either alone or in combination with trastuzumab as the bispecific antibody, TAb6, in reducing HER3-PI3K signaling. In addition to locking HER3 into dimers, TAb6 would be expected to enhance the avidity of binding of Ab6 to HER3 through bridging of trastuzumab-HER2 complexes. The trastuzumab-HER2 interaction is of very high affinity (100 pM49), and this, combined with high HER2 expression levels on the cell surface, should increase avidity effects. Further, although Ab6tet is expected to bind with increased avidity over Ab6 through increased valency, the Ab6-HER3 interaction is of lower affinity (0.8 nM4). Thus, the improved efficacy of Ab6tet relative to Ab6 could be due to both increased degradation of HER3 and higher avidity binding, but the avidity enhancement resulting from multivalent binding would be lower than that for TAb6.
The use of bispecific antibodies of different formats is an active area in the development of cancer therapeutics.50 For example, strategies to target both HER2 and HER3 using several different bispecific antibody formats or mixtures of antibodies have been described.13-15 Of relevance to the use of bispecifics, however, we observe that in the absence of lapatinib (and heregulin), exposure of SK-BR-3, BT-474 or HCC1419 cells to bispecific antibodies comprising either trastuzumab or pertuzumab and the anti-HER3 scFv, Ab6 (TAb6 or PAb6, respectively), results in increased PI3K/Akt signaling and proliferation. The possibility that this activation is due to relief of feedback inhibition of the FoxO1/3a transcription factors, leading to upregulation of multiple receptor tyrosine kinases such as HER3 and IGF-1R,27,44,45 is excluded by the rapid kinetics of pAkt induction (15 min). The observation that bispecifics containing either trastuzumab (TAb6) or pertuzumab (PAb6) are both active in inducing HER3 phosphorylation and PI3K/Akt signaling suggests that proximity rather than a specific configuration of HER2 and HER3 is sufficient for HER3 transphosphorylation. This is consistent with our observation that bispecific HER2/HER3 specific antibodies can sequester HER3 into HER2-HER3 heterodimers that, if inhibited by HER2-specific TKIs, effectively silence HER3.
We also characterized the effect of using antibodies of different designs on intracellular trafficking. The increased internalization and trafficking to lysosomes induced by using a multivalent anti-HER3 antibody (Ab6tet) suggests that this approach could be used to enhance the activity of antibody-drug conjugates for which lysosomal delivery is required.38 By contrast, a high proportion of the bispecific, TAb6, persists on the plasma membrane where it is exposed for recognition by FcγRs or complement receptors, allowing antibody dependent cell-mediated cytotoxicity/phagocytosis (ADCC/ADCP) or complement-mediated cytotoxicity by appropriate effector cells.37
Earlier studies have demonstrated that dual targeting of HER2 and HER3, using either bispecific scFvs or mixtures of individual antibodies specific for HER2 and HER3, reduces tumor cell growth in the presence of lapatinib.14,15 Significantly, we demonstrate that TAb6 is more effective than mixtures of trastuzumab and Ab6. This is consistent with the concept that TAb6 anchors HER3 into lapatinib-inactivated HER2-HER3 dimers, thereby sequestering it from interactions with other kinase competent partners and enabling Ab6 to bind to HER3 with increased avidity. By analogy with our observations, the use of a bispecific scFv-human albumin fusion (MM-111) has been shown to have anti-tumor effects in the presence of heregulin that were increased by the addition of lapatinib,14 although the efficacy of MM-111 was not directly compared with that of anti-HER2 or anti-HER3 antibodies of the same specificities as those present in MM-111. Further, and by contrast with this earlier report, we observe lower levels of heregulin-induced proliferation and, for most conditions or cell lines, no significant anti-proliferative effects of monotherapy with lapatinib or trastuzumab in the presence of heregulin. These apparent discrepancies may be due to differences in assay conditions, such as the addition of heregulin following antibody treatment14 rather than simultaneous addition as used in the current study.
An important difference between the activity of TAb6 and MM-111 is that, in the absence of heregulin and lapatinib, MM-111 does not induce proliferation.14 The design of TAb6 and MM-111 differs in several respects: first, the scFvs specific for HER2 and HER3 in MM-111 are distinct from those in TAb6. Although both the anti-HER3 scFv (H351) and Ab6 compete with heregulin for binding to HER3, this does not exclude the possibility that the two antibodies recognize distinct epitopes. The HER2-specific scFv, B1D2, binds to an epitope that does not overlap with trastuzumab.14,52 Second, TAb6 has two anti-HER2 and two anti-HER3 Fabs/Fvs per molecule, whereas MM-111 has one scFv of each specificity. Tetravalency is expected to enhance the avidity of the interaction with HER2/HER3. Third, TAb6 has an antibody Fc region whereas the scFvs in MM-111 are linked to albumin, resulting in variations in the “span” distance of the Fv components of the two constructs. One or more of these factors could contribute to the different activities of MM-111 compared with TAb6. Significantly, the presence of an Fc region in TAb6 confers effector function activities such as ADCC. The surface retention of targeting antibodies such as TAb6 is expected to enhance ADCC and other cell-mediated cytotoxicity pathways, which in turn could enhance anti-tumor activity.37
Other multivalent antibody-based or designed ankyrin repeat proteins (DARPins) that target HER2 or HER3 have been recently described.53-55 Specifically, a tetravalent antibody (MM-141), with a similar design to TAb6 in the current study, that targets both HER3 and IGF-1R has been shown to have anti-proliferative effects both in vitro and in vivo.54 Analogously to our observations using TAb6 in combination with lapatinib, MM-141 is more effective in reducing tumor cell signaling and growth than mixtures of the individual antibodies of the corresponding specificities. However, MM-141 may not be as effective as TAb6 in targeting HER2-overexpressing tumors, given the importance of the HER2/HER3 signaling axis in such malignancies. In the context of targeting HER2, a divalent anti-HER2 DARPin has been optimized to “lock” HER2 into an inactive dimeric configuration on the cell surface.55 This DARPin-based construct represents a notable expansion to the possible types of molecular approaches for targeting HER2-addicted tumors.
Future experiments will be directed toward analyzing the anti-tumor activity of a combination of lapatinib and our lead antibody, TAb6, in tumor-bearing mice. Earlier studies using lapatinib with the bispecific anti-HER2/HER3 antibody, MM-111, indicated that weekly dosing of antibody with daily lapatinib was unexpectedly more effective than MM-111 delivery every three days with daily lapatinib.14 This indicates that the dosing regimen of lapatinib and TAb6, which has a longer in vivo persistence than MM-111,14 will require optimization.
Collectively, we compared the effects of targeting HER2, HER3 or both receptors with several different antibody formats on the proliferation and signaling of breast cancer cell lines. These studies demonstrate that in the presence of the TKI inhibitor, lapatinib, a bispecific anti-HER2/HER3 antibody has higher activity than individual (multivalent) antibodies specific for HER2 and HER3 in inhibiting heregulin-induced signaling through the PI3K/Akt pathway and cell proliferation. These observations suggest that the use of such bispecifics in combination therapy with lapatinib to sequester HER3 into inactive heterodimers may provide an effective pathway for the treatment of cancer.
Materials and Methods
Cell lines and reagents
The human breast cancer cell lines BT-474 and SK-BR-3 were obtained from the American Type Culture Collection (ATCC, catalog nos. HTB-20 and HTB-30, respectively) and cultured in Hybricare Medium (ATCC, catalog no. 46-X) and McCoy 5a (Gibco, catalog no. 12330–031/Hyclone, catalog no. SH30200.01), with 1% penicillin/streptomycin and 10% FCS, respectively. The human breast cancer cell line HCC1419 (a generous gift of Drs Adi Gazdar, John Minna, and Kenneth Huffman, Hamon Center for Therapeutic Oncology Research, University of Texas Southwestern Medical Center at Dallas, Dallas, Texas) was cultured in RPMI 1640 with 1% penicillin/streptomycin and 5% FCS.
For imaging experiments, cells were cultured in phenol-red free DMEM (Gibco, catalog no. 31053–028) supplemented with 1% penicillin/streptomycin, 1% L-Glutamine, 10 mM HEPES buffer, 1 mM sodium pyruvate, 100 nM MEM non-essential amino acids, 55 nM 2-mercaptoethanol and 10% FCS.
Polyclonal antibodies specific for phospho-Akt-T308 (catalog no. 9275S), Akt, phospho-Erk1,2 (catalog no. 9101S), Erk1,2 (catalog no. 9102S), phospho-HER2 Y1221/1222 (catalog no. 2249S), and monoclonal antibodies against phospho-Akt-S473 (D9E) (catalog no. 4060L), and phospho-HER3 Y1289 (D1B5) (catalog no. 2842) were obtained from Cell Signaling Technologies. Polyclonal anti-HER3 antibody (C-17) (catalog no. SC-285) was from Santa Cruz Biotechnology and monoclonal anti-c-erbB2 antibody (Ab-3 3B5) (catalog no. OP15L) was from Millipore. The monoclonal anti-actin antibody (Ab-5) (catalog no. 612656) was from BD Bioscience. Horseradish peroxidase-labeled goat anti-rabbit and anti-mouse IgG (H+L) (catalog nos. 111-035-003 and 115-035-003, respectively) were purchased from Jackson ImmunoResearch Laboratories. Lapatinib (catalog no. L-4804) was obtained from LC Laboratories and recombinant human heregulin-β1 (HRG-β1; EGF-like domain, catalog no. 100–03) was obtained from Peprotech.
Recombinant antibodies
Clinical grade trastuzumab and pertuzumab were obtained from the UT Southwestern Pharmacy. For comparative purposes, trastuzumab and pertuzumab were also expressed in recombinant form using the same expression host as the other antibodies used in this study. Expression plasmids for the production of antibodies in stably transfected CHO cells were generated as follows: the genes encoding the heavy and light chain variable domains (VH and VL, respectively) of trastuzumab,49 pertuzumab,40 and Ab6 (US patent 20100266584A1; MM-1213) were synthesized commercially (Integrated DNA Technology, Genscript, or Thermo Fisher) and used to generate full-length human IgG1 and human kappa genes with the leader peptide (MGWSCIILFLVATATGVHS) from the anti-lysozyme antibody, Hulys10,56 using standard methods of molecular biology. The vectors pcDNA 3.3 TOPO and pOptiVEC-TOPO (OptiCHO Ab Express Kit, Life Technologies, catalog nos. K8300–01 and 12744017, respectively) were used for the expression of the light and heavy chain genes, respectively.
To generate expression constructs for bispecific, tetravalent antibodies, a similar design to that described previously was used.57 A linker sequence containing a unique XhoI site was inserted at the 3′ end of the heavy chain genes (trastuzumab and pertuzumab) using a designed oligonucleotide and the PCR. A scFv gene encoding the Ab6 scFv with codons encoding a (Gly4Ser)3 linker peptide between the VH (JH) and VL gene was generated using standard methods of molecular biology. XhoI sites and Gly-Ser-Ser codons to connect the CH3 domain to the VH gene, were appended to the 5′ and 3′ ends of the scFv gene using the PCR. This scFv gene was cloned into the XhoI sites at the 3′ ends of the trastuzumab and pertuzumab heavy chain genes to generate full-length heavy chains linked to the Ab6 scFv. Complete sequences of all expression plasmids are available upon request.
Transfections and expression of recombinant antibodies
Light chain expression constructs were transfected into CD/DG44 CHO cells (Life Technologies, catalog no. A11000-01) using electroporation. Desired clones were selected in CD/DG44 CHO medium (Life Technologies, catalog no. 12610010) containing 500 μg/ml geneticin without the HT supplement. The clone expressing the highest levels of light chain was identified by screening culture supernatants with ELISAs using goat anti-human kappa light chain antibody for detection (Sigma-Aldrich, catalog no. A7164). Heavy chain expression constructs were then transfected into their respective stably transfected light chain expressing CD/DG44 CHO clones via electroporation and selected with Opti-CHO Medium (Life Technologies, catalog no. 12681-011) containing 500 μg/ml geneticin. Supernatants of clones were screened by sandwich ELISA using goat anti-human IgG (Fab-specific, Sigma-Aldrich, catalog no. I5260) as capture antibody and goat anti-human IgG (Fc-specific) conjugated to horseradish peroxidase (Sigma-Aldrich, catalog no. A0170) as detection antibody. The clones expressing the highest levels of antibody were expanded and cultured in increasing concentrations of methotrexate (MTX, 50 nM‒4 μM), to induce gene amplification. Clones were further expanded in shake flasks (130 rpm) in 8% CO2 and antibody purified from culture supernatants using protein G-Sepharose (GE Healthcare, catalog no. 17-0618-05). Several antibodies were also scaled up and purified by BioXCell.
Surface plasmon resonance analyses
Surface plasmon resonance experiments were performed using a BIAcore 2000 and previously described methods.58,59 Recombinant Fc-HER2 (R&D Systems, catalog no. 1129-ER-050) and HER3 (Acro Biosystems, catalog no. ER3-H5223) were coupled to flow cells of CM5 sensor chips to densities of 758 and 467 RU, respectively. Antibodies at a concentration of 50 nM were injected in phosphate buffered saline (PBS) plus 0.01% Tween at a flow rate of 10 μl/min. At the end of an 800 s dissociation phase, bound antibody was stripped from the flow cells using 0.1 M glycine, 0.9 M NaCl, pH 3.8. Data were analyzed using BIAevaluation software.
Pharmacokinetics
The in vivo half-lives of 125I labeled Ab6tet and TAb6 were analyzed following intravenous or intraperitoneal delivery in BALB/c SCID mice (Jackson Laboratories, Bar Harbor, ME) using previously described methods and whole body counting.60
Proliferation assays
Cells were plated in 96-well plates at a density of 2,500 cells per well and incubated overnight. Cells were treated with lapatinib (1 μM), HRG-β1 (6.25 nM), anti-HER3 antibody Ab6 or the tetrameric form, Ab6tet, trastuzumab (anti-HER2), pertuzumab (anti-HER2), or the bispecific anti-HER2/ HER3 antibodies TAb6 and PAb6 as indicated in the figure legends. Antibodies were used at a concentration of 50 nM unless otherwise indicated. Dimethyl sulfoxide or PBS were used as vehicle controls for lapatinib or HRG-β1/antibodies, respectively. After 5 d of incubation in a 37 °C 5% CO2 incubator, cell proliferation was quantitated using CellTiter 96 AQueous One Solution Proliferation Assay kit (Promega, catalog no. G3580) according to the manufacturer’s instructions.
Immunoblotting
Cells cultured to near confluence in 6-well plates were treated with lapatinib, HRG-β1 or antibodies as for proliferation assays. Following 15 min, 1 h, or 24 h incubation, cells were lysed using RIPA buffer (50 mM Tris, 150 mM NaCl, 0.5% sodium deoxycholate, 1% Triton X-100, 0.1% sodium dodecyl sulfate, 2 μg/mL aprotinin, 2 μg/mL leupeptin, 1 μg/mL pepstatin A, and 0.4 mg/mL pefabloc SC PLUS). Lysates were centrifuged for 20 min at 14,600 rpm at 4 °C and supernatants were collected. Protein concentrations in each lysate were determined using the BCA protein assay reagent (Pierce, catalog no. 23223). Total lysates were fractionated by SDS-PAGE and transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, catalog no. IPVH00010) or nitrocellulose membranes (Bio-Rad, catalog no. 162-0145). Membranes were incubated with 5% bovine serum albumin in Tris-buffered saline with 0.1% Tween (TBST, pH 8.0) for 1 h following transfer to block non-specific binding sites. Blocked membranes were incubated with primary antibodies overnight at 4 °C with agitation. Prior to incubation with the secondary antibodies conjugated to horseradish peroxidase, blots were washed three times (10 min/wash) with TBST. Following incubation with secondary antibodies for 1 h and washing, bound secondary antibody was detected using chemiluminescent detection regent (GE Healthcare, catalog no. RPN 2209/AF).
Fluorescence microscopy
SK-BR-3 cells incubated overnight at a density of 50,000 cells per dish were treated with 50 nM Ab6, Ab6tet, or TAb6 for 5 or 15 min at 37 °C and then either immediately washed and fixed or chased in medium at 37 °C for 10, 20, or 45 min prior to washing and fixation. For fixation, cells were treated with 1.7% (w/v) paraformaldehyde for 10 min at 37 °C and then permeabilized with 0.05% (v/v) saponin for 10 min at room temperature in PBS. A pre-block with 4% BSA/PBS was performed prior to staining with 2 μg/mL of polyclonal rabbit anti-LAMP-1 antibody (Abcam, catalog no. AB24170) and 12.5 μg/mL monoclonal mouse anti-EEA-1 (clone 14) from BD Bioscience (catalog no. 610456) for 30 min at room temperature. After blocking for 30 min with goat serum (Sigma-Aldrich, catalog no. G6767), bound primary antibodies were detected by incubating cells with the following secondary antibody conjugates for 25 min at room temperature: Alexa 555-labeled goat anti-human IgG (H+L) (Life Technologies, catalog no. A21433), Alexa 647-labeled goat anti-mouse IgG (H+L) (Life Technologies, catalog no. A21236) and Alexa 488-labeled goat anti-rabbit IgG (H+L) (Life Technologies, catalog no. A11034). Cells were washed twice with PBS between each incubation step and were stored at 4 °C in 1% BSA/PBS.
Images were acquired using a Zeiss Axiovert 200M inverted fluorescence microscope with a 63X Plan Apochromat objective as described previously.61,62 Data was processed using custom written software in MatLab (MIAtool/LABSoft; http://www.wardoberlab.com/software/miatool/).
Statistical analyses
Statistical significance for differences in mean values of triplicate samples (immunoblots, ELISAs) was determined by Student t test. P values < 0.05 were considered to be significant.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
We thank Drs Adi Gazdar, John Minna and Kenneth Huffman (Hamon Center of Therapeutic Oncology Research, University of Texas Southwestern Medical Center at Dallas, Dallas, Texas) for generously providing HCC1419 cells. We would also like to thank Dr Sripad Ram for expert advice and assistance with microscopy experiments, and Dr Siva C Devanaboyina, Dr Sahar M Nodehi and J Bregg Reedy for help with expression plasmid generation and CHO cell transfections. This research was supported in part by grants from the Cancer Prevention and Research Institute of Texas (CPRIT; RP110069 and RP110070).
Author Contributions
Kang JC, Poovassery JS, Ober RJ, and Ward ES designed the research; Kang JC, Poovassery JS, Bansal P, You S, and Manjarres IM performed the research; Kang JC, Poovassery JS, You S, Manjarres IM, Ober RJ, and Ward ES analyzed the data; Kang JC, Poovassery JS, Ober RJ, and Ward ES wrote the paper.
Glossary
Abbreviations:
- TKI
tyrosine kinase inhibitor
- scFv
single chain variable fragment
- ADCC
antibody dependent cell-mediated cytotoxicity
- ADCP
antibody dependent cell-mediated phagocytosis
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/27658
References
- 1.Lee-Hoeflich ST, Crocker L, Yao E, Pham T, Munroe X, Hoeflich KP, Sliwkowski MX, Stern HM. A central role for HER3 in HER2-amplified breast cancer: implications for targeted therapy. Cancer Res. 2008;68:5878–87. doi: 10.1158/0008-5472.CAN-08-0380. [DOI] [PubMed] [Google Scholar]
- 2.Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF, 3rd, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci U S A. 2003;100:8933–8. doi: 10.1073/pnas.1537685100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schoeberl B, Faber AC, Li D, Liang MC, Crosby K, Onsum M, Burenkova O, Pace E, Walton Z, Nie L, et al. An ErbB3 antibody, MM-121, is active in cancers with ligand-dependent activation. Cancer Res. 2010;70:2485–94. doi: 10.1158/0008-5472.CAN-09-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schoeberl B, Pace EA, Fitzgerald JB, Harms BD, Xu L, Nie L, Linggi B, Kalra A, Paragas V, Bukhalid R, et al. Therapeutically targeting ErbB3: a key node in ligand-induced activation of the ErbB receptor-PI3K axis. Sci Signal. 2009;2:ra31. doi: 10.1126/scisignal.2000352. [DOI] [PubMed] [Google Scholar]
- 5.Baselga J, Swain SM. Novel anticancer targets: revisiting ERBB2 and discovering ERBB3. Nat Rev Cancer. 2009;9:463–75. doi: 10.1038/nrc2656. [DOI] [PubMed] [Google Scholar]
- 6.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 7.Tzahar E, Waterman H, Chen X, Levkowitz G, Karunagaran D, Lavi S, Ratzkin BJ, Yarden Y. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol. 1996;16:5276–87. doi: 10.1128/mcb.16.10.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hellyer NJ, Kim MS, Koland JG. Heregulin-dependent activation of phosphoinositide 3-kinase and Akt via the ErbB2/ErbB3 co-receptor. J Biol Chem. 2001;276:42153–61. doi: 10.1074/jbc.M102079200. [DOI] [PubMed] [Google Scholar]
- 9.Campbell MR, Amin D, Moasser MM. HER3 comes of age: new insights into its functions and role in signaling, tumor biology, and cancer therapy. Clin Cancer Res. 2010;16:1373–83. doi: 10.1158/1078-0432.CCR-09-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy CG, Modi S. HER2 breast cancer therapies: a review. Biologics. 2009;3:289–301. [PMC free article] [PubMed] [Google Scholar]
- 11.Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006;3:269–80. doi: 10.1038/ncponc0509. [DOI] [PubMed] [Google Scholar]
- 12.Hurvitz SA, Hu Y, O’Brien N, Finn RS. Current approaches and future directions in the treatment of HER2-positive breast cancer. Cancer Treat Rev. 2013;39:219–29. doi: 10.1016/j.ctrv.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson MK, Hodge KM, Horak E, Sundberg AL, Russeva M, Shaller CC, von Mehren M, Shchaveleva I, Simmons HH, Marks JD, et al. Targeting ErbB2 and ErbB3 with a bispecific single-chain Fv enhances targeting selectivity and induces a therapeutic effect in vitro. Br J Cancer. 2008;99:1415–25. doi: 10.1038/sj.bjc.6604700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDonagh CF, Huhalov A, Harms BD, Adams S, Paragas V, Oyama S, Zhang B, Luus L, Overland R, Nguyen S, et al. Antitumor activity of a novel bispecific antibody that targets the ErbB2/ErbB3 oncogenic unit and inhibits heregulin-induced activation of ErbB3. Mol Cancer Ther. 2012;11:582–93. doi: 10.1158/1535-7163.MCT-11-0820. [DOI] [PubMed] [Google Scholar]
- 15.Garrett JT, Sutton CR, Kuba MG, Cook RS, Arteaga CL. Dual blockade of HER2 in HER2-overexpressing tumor cells does not completely eliminate HER3 function. Clin Cancer Res. 2013;19:610–9. doi: 10.1158/1078-0432.CCR-12-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Q, Ahmed S, Loeb JA. Development of an autocrine neuregulin signaling loop with malignant transformation of human breast epithelial cells. Cancer Res. 2004;64:7078–85. doi: 10.1158/0008-5472.CAN-04-1152. [DOI] [PubMed] [Google Scholar]
- 17.Montero JC, Rodríguez-Barrueco R, Ocaña A, Díaz-Rodríguez E, Esparís-Ogando A, Pandiella A. Neuregulins and cancer. Clin Cancer Res. 2008;14:3237–41. doi: 10.1158/1078-0432.CCR-07-5133. [DOI] [PubMed] [Google Scholar]
- 18.Dunn M, Sinha P, Campbell R, Blackburn E, Levinson N, Rampaul R, Bates T, Humphreys S, Gullick WJ. Co-expression of neuregulins 1, 2, 3 and 4 in human breast cancer. J Pathol. 2004;203:672–80. doi: 10.1002/path.1561. [DOI] [PubMed] [Google Scholar]
- 19.Kaufman B, Trudeau M, Awada A, Blackwell K, Bachelot T, Salazar V, DeSilvio M, Westlund R, Zaks T, Spector N, et al. Lapatinib monotherapy in patients with HER2-overexpressing relapsed or refractory inflammatory breast cancer: final results and survival of the expanded HER2+ cohort in EGF103009, a phase II study. Lancet Oncol. 2009;10:581–8. doi: 10.1016/S1470-2045(09)70087-7. [DOI] [PubMed] [Google Scholar]
- 20.Gomez HL, Doval DC, Chavez MA, Ang PC, Aziz Z, Nag S, Ng C, Franco SX, Chow LW, Arbushites MC, et al. Efficacy and safety of lapatinib as first-line therapy for ErbB2-amplified locally advanced or metastatic breast cancer. J Clin Oncol. 2008;26:2999–3005. doi: 10.1200/JCO.2007.14.0590. [DOI] [PubMed] [Google Scholar]
- 21.Burstein HJ, Sun Y, Dirix LY, Jiang Z, Paridaens R, Tan AR, Awada A, Ranade A, Jiao S, Schwartz G, et al. Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol. 2010;28:1301–7. doi: 10.1200/JCO.2009.25.8707. [DOI] [PubMed] [Google Scholar]
- 22.Gradishar WJ. Emerging approaches for treating HER2-positive metastatic breast cancer beyond trastuzumab. Ann Oncol. 2013;24:2492–500. doi: 10.1093/annonc/mdt217. [DOI] [PubMed] [Google Scholar]
- 23.Baselga J, Albanell J, Ruiz A, Lluch A, Gascón P, Guillém V, González S, Sauleda S, Marimón I, Tabernero JM, et al. Phase II and tumor pharmacodynamic study of gefitinib in patients with advanced breast cancer. J Clin Oncol. 2005;23:5323–33. doi: 10.1200/JCO.2005.08.326. [DOI] [PubMed] [Google Scholar]
- 24.Folkes AJ, Ahmadi K, Alderton WK, Alix S, Baker SJ, Box G, Chuckowree IS, Clarke PA, Depledge P, Eccles SA, et al. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J Med Chem. 2008;51:5522–32. doi: 10.1021/jm800295d. [DOI] [PubMed] [Google Scholar]
- 25.Brachmann SM, Hofmann I, Schnell C, Fritsch C, Wee S, Lane H, Wang S, Garcia-Echeverria C, Maira SM. Specific apoptosis induction by the dual PI3K/mTor inhibitor NVP-BEZ235 in HER2 amplified and PIK3CA mutant breast cancer cells. Proc Natl Acad Sci U S A. 2009;106:22299–304. doi: 10.1073/pnas.0905152106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sergina NV, Rausch M, Wang D, Blair J, Hann B, Shokat KM, Moasser MM. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445:437–41. doi: 10.1038/nature05474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chakrabarty A, Sánchez V, Kuba MG, Rinehart C, Arteaga CL. Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors. Proc Natl Acad Sci U S A. 2012;109:2718–23. doi: 10.1073/pnas.1018001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scaltriti M, Verma C, Guzman M, Jimenez J, Parra JL, Pedersen K, Smith DJ, Landolfi S, Ramon y Cajal S, Arribas J, et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene. 2009;28:803–14. doi: 10.1038/onc.2008.432. [DOI] [PubMed] [Google Scholar]
- 29.Amin DN, Sergina N, Ahuja D, McMahon M, Blair JA, Wang D, Hann B, Koch KM, Shokat KM, Moasser MM. Resiliency and vulnerability in the HER2-HER3 tumorigenic driver. Sci Transl Med. 2010;2:ra7. doi: 10.1126/scitranslmed.3000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eichhorn PJ, Gili M, Scaltriti M, Serra V, Guzman M, Nijkamp W, Beijersbergen RL, Valero V, Seoane J, Bernards R, et al. Phosphatidylinositol 3-kinase hyperactivation results in lapatinib resistance that is reversed by the mTOR/phosphatidylinositol 3-kinase inhibitor NVP-BEZ235. Cancer Res. 2008;68:9221–30. doi: 10.1158/0008-5472.CAN-08-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E, Peng J, Lin E, Wang Y, Sosman J, et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature. 2012;487:505–9. doi: 10.1038/nature11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xia W, Petricoin EF, 3rd, Zhao S, Liu L, Osada T, Cheng Q, Wulfkuhle JD, Gwin WR, Yang X, Gallagher RI, et al. An heregulin-EGFR-HER3 autocrine signaling axis can mediate acquired lapatinib resistance in HER2+ breast cancer models. Breast Cancer Res. 2013;15:R85. doi: 10.1186/bcr3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato Y, Yashiro M, Takakura N. Heregulin induces resistance to lapatinib-mediated growth inhibition of HER2-amplified cancer cells. Cancer Sci. 2013;104:1618–25. doi: 10.1111/cas.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 35.Knowlden JM, Gee JM, Barrow D, Robertson JF, Ellis IO, Nicholson RI, Hutcheson IR. erbB3 recruitment of insulin receptor substrate 1 modulates insulin-like growth factor receptor signalling in oestrogen receptor-positive breast cancer cell lines. Breast Cancer Res. 2011;13:R93. doi: 10.1186/bcr3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sak MM, Breen K, Rønning SB, Pedersen NM, Bertelsen V, Stang E, Madshus IH. The oncoprotein ErbB3 is endocytosed in the absence of added ligand in a clathrin-dependent manner. Carcinogenesis. 2012;33:1031–9. doi: 10.1093/carcin/bgs128. [DOI] [PubMed] [Google Scholar]
- 37.Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol. 2010;10:301–16. doi: 10.1038/nri2761. [DOI] [PubMed] [Google Scholar]
- 38.Sievers EL, Senter PD. Antibody-drug conjugates in cancer therapy. Annu Rev Med. 2013;64:15–29. doi: 10.1146/annurev-med-050311-201823. [DOI] [PubMed] [Google Scholar]
- 39.Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW, Jr., Leahy DJ. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421:756–60. doi: 10.1038/nature01392. [DOI] [PubMed] [Google Scholar]
- 40.Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317–28. doi: 10.1016/S1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 41.Gymnopoulos M, Elsliger MA, Vogt PK. Rare cancer-specific mutations in PIK3CA show gain of function. Proc Natl Acad Sci U S A. 2007;104:5569–74. doi: 10.1073/pnas.0701005104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weigelt B, Warne PH, Downward J. PIK3CA mutation, but not PTEN loss of function, determines the sensitivity of breast cancer cells to mTOR inhibitory drugs. Oncogene. 2011;30:3222–33. doi: 10.1038/onc.2011.42. [DOI] [PubMed] [Google Scholar]
- 43.Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, Sampath D, Sliwkowski MX. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15:429–40. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 44.Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, Majumder PK, Baselga J, Rosen N. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell. 2011;19:58–71. doi: 10.1016/j.ccr.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Serra V, Scaltriti M, Prudkin L, Eichhorn PJ, Ibrahim YH, Chandarlapaty S, Markman B, Rodriguez O, Guzman M, Rodriguez S, et al. PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene. 2011;30:2547–57. doi: 10.1038/onc.2010.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haslekås C, Breen K, Pedersen KW, Johannessen LE, Stang E, Madshus IH. The inhibitory effect of ErbB2 on epidermal growth factor-induced formation of clathrin-coated pits correlates with retention of epidermal growth factor receptor-ErbB2 oligomeric complexes at the plasma membrane. Mol Biol Cell. 2005;16:5832–42. doi: 10.1091/mbc.E05-05-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sak MM, Szymanska M, Bertelsen V, Hasmann M, Madshus IH, Stang E. Pertuzumab counteracts the inhibitory effect of ErbB2 on degradation of ErbB3. Carcinogenesis. 2013;34:2031–8. doi: 10.1093/carcin/bgt173. [DOI] [PubMed] [Google Scholar]
- 48.Le XF, McWatters A, Wiener J, Wu JY, Mills GB, Bast RC., Jr. Anti-HER2 antibody and heregulin suppress growth of HER2-overexpressing human breast cancer cells through different mechanisms. Clin Cancer Res. 2000;6:260–70. [PubMed] [Google Scholar]
- 49.Carter P, Presta L, Gorman CM, Ridgway JB, Henner D, Wong WL, Rowland AM, Kotts C, Carver ME, Shepard HM. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci U S A. 1992;89:4285–9. doi: 10.1073/pnas.89.10.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reichert JM, Dhimolea E. The future of antibodies as cancer drugs. Drug Discov Today. 2012;17:954–63. doi: 10.1016/j.drudis.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 51.Horak E, Heitner T, Robinson MK, Simmons HH, Garrison J, Russeva M, Furmanova P, Lou J, Zhou Y, Yuan QA, et al. Isolation of scFvs to in vitro produced extracellular domains of EGFR family members. Cancer Biother Radiopharm. 2005;20:603–13. doi: 10.1089/cbr.2005.20.603. [DOI] [PubMed] [Google Scholar]
- 52.Schier R, Marks JD, Wolf EJ, Apell G, Wong C, McCartney JE, Bookman MA, Huston JS, Houston LL, Weiner LM, et al. In vitro and in vivo characterization of a human anti-c-erbB-2 single-chain Fv isolated from a filamentous phage antibody library. Immunotechnology. 1995;1:73–81. doi: 10.1016/1380-2933(95)00007-0. [DOI] [PubMed] [Google Scholar]
- 53.Xu L, Kohli N, Rennard R, Jiao Y, Razlog M, Zhang K, Baum J, Johnson B, Tang J, Schoeberl B, et al. Rapid optimization and prototyping for therapeutic antibody-like molecules. MAbs. 2013;5:237–54. doi: 10.4161/mabs.23363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fitzgerald JB, Johnson BW, Baum J, Adams S, Iadevaia S, Tang J, Rimkunas V, Xu L, Kohli N, Rennard R, et al. MM-141, an IGF-1R and ErbB3 directed bispecific antibody, overcomes network adaptations that limit activity of IGF-1R inhibitors. Mol Cancer Ther. 2013 doi: 10.1158/1535-7163.MCT-13-0255. [DOI] [PubMed] [Google Scholar]
- 55.Jost C, Schilling J, Tamaskovic R, Schwill M, Honegger A, Plückthun A. Structural basis for eliciting a cytotoxic effect in HER2-overexpressing cancer cells via binding to the extracellular domain of HER2. Structure. 2013;21:1979–91. doi: 10.1016/j.str.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 56.Foote J, Winter G. Antibody framework residues affecting the conformation of the hypervariable loops. J Mol Biol. 1992;224:487–99. doi: 10.1016/0022-2836(92)91010-M. [DOI] [PubMed] [Google Scholar]
- 57.Coloma MJ, Morrison SL. Design and production of novel tetravalent bispecific antibodies. Nat Biotechnol. 1997;15:159–63. doi: 10.1038/nbt0297-159. [DOI] [PubMed] [Google Scholar]
- 58.Zhou J, Mateos F, Ober RJ, Ward ES. Conferring the binding properties of the mouse MHC class I-related receptor, FcRn, onto the human ortholog by sequential rounds of site-directed mutagenesis. J Mol Biol. 2005;345:1071–81. doi: 10.1016/j.jmb.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 59.Vaccaro C, Zhou J, Ober RJ, Ward ES. Engineering the Fc region of immunoglobulin G to modulate in vivo antibody levels. Nat Biotechnol. 2005;23:1283–8. doi: 10.1038/nbt1143. [DOI] [PubMed] [Google Scholar]
- 60.Vaccaro C, Bawdon R, Wanjie S, Ober RJ, Ward ES. Divergent activities of an engineered antibody in murine and human systems have implications for therapeutic antibodies. Proc Natl Acad Sci U S A. 2006;103:18709–14. doi: 10.1073/pnas.0606304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ober RJ, Martinez C, Vaccaro C, Zhou J, Ward ES. Visualizing the site and dynamics of IgG salvage by the MHC class I-related receptor, FcRn. J Immunol. 2004;172:2021–9. doi: 10.4049/jimmunol.172.4.2021. [DOI] [PubMed] [Google Scholar]
- 62.Gan Z, Ram S, Vaccaro C, Ober RJ, Ward ES. Analyses of the recycling receptor, FcRn, in live cells reveal novel pathways for lysosomal delivery. Traffic. 2009;10:600–14. doi: 10.1111/j.1600-0854.2009.00887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.