Abstract
The anti-ErbB2 antibody trastuzumab has shown significant clinical benefits in ErbB2-overexpressing breast and gastric cancer, but resistance to the drug is common. Here, we investigated the antitumor activity of the combination of trastuzumab and the SRC inhibitor saracatinib in ErbB2-overexpressing trastuzumab-resistant gastric cancer. The ErbB2-overexpressing human gastric cancer cell line NCI-N87 was treated with trastuzumab to obtain the trastuzumab-resistant cell line NCI-N87R. The NCI-N87R cell line showed a marked increase in SRC activity and ErbB signaling compared with the NCI-N87 cell line. Our data demonstrated that trastuzumab plus saracatinib was much more potent than either agent alone in reducing the phosphorylation of ErbB3 and AKT in both NCI-N87 and NCI-N87R gastric cancer cell lines. Trastuzumab and saracatinib synergistically inhibited the in vitro growth of NCI-N87 and NCI-N87R cell lines. Further data showed that combination therapy of trastuzumab with saracatinib resulted in a significant benefit over either agent alone in both NCI-N87 and NCI-N87R xenograft models, suggesting its potential use for treating ErbB2-overexpressing gastric cancer.
Keywords: gastric cancer, ErbB2, SRC, trastuzumab, saracatinib
Introduction
Overexpression of human epidermal growth factor receptor-2 (HER2 or ErbB2), a member of the ErbB family of receptor tyrosine kinases (RTKs), is found in many solid tumors.1,2 Trastuzumab, a humanized monoclonal antibody (mAb) directed against ErbB2, inhibits ErbB2-mediated signaling, induces antibody-dependent cellular cytotoxicity, and prevents cleavage of the extracellular domain of ErbB2.3,4 Trastuzumab was approved by the US Food and Drug Administration (FDA) for clinical use for patients with ErbB2-overexpressing metastatic breast cancer in 1998, and for ErbB2-positive metastatic gastric and gastro-esophageal junction cancer in 2010. Despite the effectiveness of trastuzumab, the majority of trastuzumab-responsive patients develop resistance within one year of treatment initiation.5,6 Thus, there is an urgent need to overcome trastuzumab resistance.
The nonreceptor tyrosine kinase c-SRC (SRC) is overexpressed in a wide range of tumors.7 The aberrant activation of SRC regulates multiple functions during tumor progression, such as apoptosis, proliferation, cell adhesion, cell migration and invasion, angiogenesis, and metastasis.8 SRC interacts with multiple RTKs and facilitates RTK-mediated signaling.9-12 The combination of SRC inhibitors and trastuzumab has been demonstrated to be effective in suppressing the in vitro and in vivo growth of trastuzumab-resistant breast cancer cell lines.13 In gastric cancer, SRC activity has been found to be increased.14,15 A recent study has shown that inhibition of SRC activity suppresses the in vitro and in vivo growth of gastric cancer cell lines.16 However, the antitumor activity of trastuzumab plus SRC inhibitor in gastric cancer has not yet been reported. In this study, we investigated the antitumor effect of trastuzumab in combination with the SRC inhibitor saracatinib on both trastuzumab-sensitive and -resistant gastric cancer cell lines, and we examined its mechanism of action.
Results
Characterization of trastuzumab-resistant NCI-N87 cell line
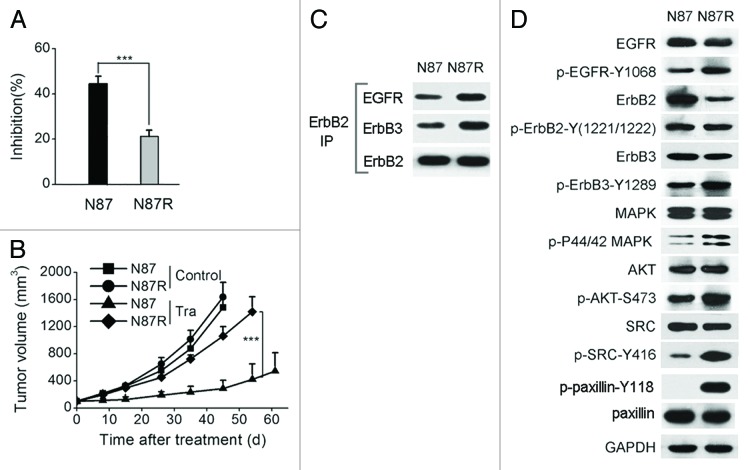
We treated the NCI-N87 cell line with 10 μg/ml of trastuzumab for nine months to obtain trastuzumab-resistant sub-line NCI-N87R. NCI-N87R was significantly more resistant to trastuzumab treatment than the parental cell line both in vitro and in vivo (Fig. 1A and B). We found that the amount of EGFR-ErbB2 and ErbB2-ErbB3 heterodimers was enhanced in the NCI-N87R cell line compared with the parental cell line (Fig. 1C). NCI-N87R cells also showed an increase in EGFR and ErbB3 phosphorylation (Fig. 1D). The phosphorylation of AKT and MAPK was also markedly enhanced in the NCI-N87R cell line (Fig. 1D). Moreover, NCI-N87R cells showed increased phosphorylation of SRC at Tyr416 and of the SRC downstream target paxillin at Tyr118 (Fig. 1D). These results suggest that SRC activation and increased ErbB signaling may be acquired resistance mechanisms.
Figure 1. Characterization of trastuzumab-resistant gastric cancer cell line NCI-N87R. (A) MTS assay evaluating cell proliferation of NCI-N87 and NCI-N87R cell lines upon treatment with trastuzumab (10 μg/ml). Error bars, SD ***P < 0.0001. (B) Tumor volume of NCI-N87 and NCI-N87R xenografts after treatment with control IgG, trastuzumab, saracatinib, or trastuzumab plus saracatinib. Data are shown as means ± SEM ***P < 0.0001, Mann-Whitney test. (C) Co-immunoprecipitation assay detecting ErbB2/EGFR and ErbB2/ErbB3 heterodimerization in the NCI-N87 and NCI-N87R cell lines. (D) Immunoblots comparing major cell signaling changes between NCI-N87 and NCI-N87R cell lines.
Trastuzumab and saracatinib synergistically inhibit the growth of both trastuzumab-sensitive and trastuzumab-resistant gastric cancer cell lines
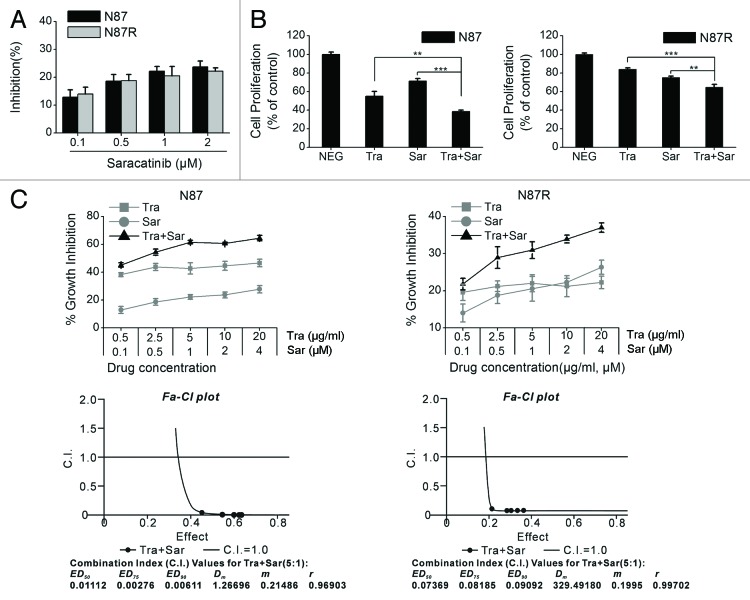
We examined the inhibitory effects of saracatinib on NCI-N87 and NCI-N87R cell lines. The results showed that saracatinib suppressed the in vitro proliferation of these two cell lines in a dose-dependent manner (Fig. 2A). Remarkably, the antiproliferative activity of saracatinib was similar in trastuzumab-sensitive and trastuzumab-resistant gastric cancer cell lines (Fig. 2A). Next, we evaluated and compared the ability of saracatinib and trastuzumab, either alone or in combination, to inhibit the in vitro growth of NCI-N87 and NCI-N87R cell lines. As shown in Figure 2B, saracatinib plus trastuzumab exhibited a significantly greater antiproliferative activity against NCI-N87 cells than either agent alone. Similar results were obtained with NCI-N87R cells (Fig. 2B). To further investigate whether the combination of saracatinib and trastuzumab is synergistic, we treated NCI-N87 and NCI-N87R cell lines with various clinically relevant concentration ranges of saracatinib and trastuzumab. Data were analyzed using the method of Chou and Talalay to establish drug C.I. values. Synergy is defined as C.I. values of < 1.0, antagonism as C.I. values > 1.0, and additivity as CI values equal to 1.0. Our results showed that saracatinib and trastuzumab synergistically inhibited the proliferation of both NCI-N87 and NCI-N87R cell lines (Fig. 2C).
Figure 2. The in vitro antitumor activity of trastuzumab plus saracatinib in NCI-N87 or NCI-N87R cell lines. (A) MTS assay comparing cell proliferation of the NCI-N87 and NCI-N87R cell lines upon trastuzumab treatment. Error bars, SD (B) MTS assay comparing the effects of control IgG (10 μg/ml), trastuzumab (10 μg/ml), saracatinib (1 μM), and trastuzumab (10 μg/ml) plus saracatinib (1 μM) on gastric cancer cell proliferation. Results are shown as percentage of control cell proliferation. Error bars, SD **P < 0.001, **P < 0.0001. (C) Trastuzumab and saracatinib synergistically inhibit the in vitro growth of NCI-N87 and NCI-N87R cell lines. Combination index (CI) values were calculated using the Chou-Talalay method. Drug synergy, addition, and antagonism are defined by C.I. values less than 1.0, equal to 1.0, or greater than 1.0, respectively.
Trastuzumab plus saracatinib potently inhibits ErbB2 signaling in both trastuzumab-sensitive and -resistant gastric cancer cell lines
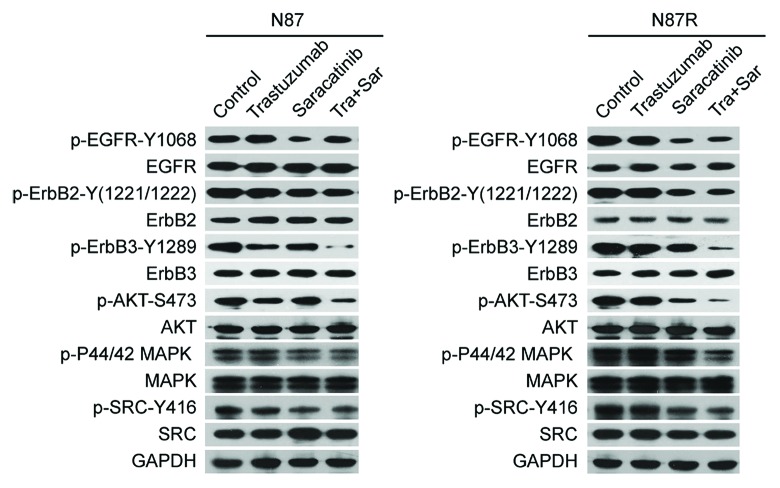
We examined the inhibitory effects of saracatinib, trastuzumab, or saracatinib plus trastuzumab on ErbB signaling pathways in NCI-N87 and NCI-N87R cell lines. As shown in Figure 3, trastuzumab treatment caused a decrease in ErbB3 and AKT phosphorylation in the NCI-N87 cell line, but not in the NCI-N87R cell line. We found that saracatinib inhibited the phosphorylation of SRC, EGFR, ErbB2, ErbB3, AKT and MAPK in both cell lines (Fig. 3). Remarkably, the addition of trastuzumab to saracatinib further reduced the phosphorylation of ErbB3 and AKT in both trastuzumab-sensitive and -resistant gastric cancer cell lines (Fig. 3).
Figure 3. Trastuzumab in combination with saracatinib inhibits ErbB2 signaling in both NCI-N87 and NCI-N87R gastric cancer cell lines. Immunoblots were used to determine the ability of control IgG (10 μg/ml), trastuzumab (10 μg/ml), saracatinib (1 μM), and trastuzumab (10 μg/ml) plus saracatinib (1 μM) to inhibit the phosphorylation of EGFR, ErbB2, ErbB3, AKT, MAPK and SRC in NCI-N87 or NCI-N87R gastric cancer cell lines.
Trastuzumab plus saracatinib suppresses the in vivo growth of both trastuzumab-sensitive and -resistant gastric cancer xenografts
The therapeutic efficacy of trastuzumab, saracatinib, and trastuzumab plus saracatinib was examined in nude mice bearing established NCI-N87 and NCI-N87R xenograft tumors. Trastuzumab suppressed tumor growth much better than saracatinib in the NCI-N87 xenograft model (Fig. 4). Both trastuzumab and saracatinib had a modest inhibitory effect on NCI-N87R tumor growth (Fig. 4). Combinatorial treatment with trastuzumab and saracatinib resulted in a significant benefit over either agent alone in both NCI-N87 and NCI-N87R xenograft models (Fig. 4).

Figure 4. Trastuzumab plus saracatinib combinatorial treatment inhibits the in vivo growth of both NCI-N87 and NCI-N87R gastric cancer cell lines. Tumor volume of NCI-N87 or NCI-N87R xenografts after treatment with control IgG (10 mg/kg), trastuzumab (10 mg/kg), saracatinib (25 mg/kg), or trastuzumab (10 mg/kg) plus saracatinib (25 mg/kg). Data are shown as means ± SEM ***P < 0.0001, Mann-Whitney test.
Discussion
The mechanism of trastuzumab resistance is not yet fully elucidated. A recent study has indicated that SRC activation induces trastuzumab resistance by facilitating EGFR, ErbB2 and ErbB3 activation in breast cancer cells.13 In this study, we treated the gastric cancer cell line NCI-N87 with trastuzumab for nine months to obtain the trastuzumab-resistant sub-line NCI-N87R. The NCI-N87R cell line showed a marked increase in SRC activity and ErbB signaling compared with the NCI-N87 cell line. Therefore, it can be concluded that SRC activation may contribute to acquired resistance to trastuzumab in NCI-N87R cell line. Moreover, our data showed that trastuzumab plus saracatinib was less potent in NCI-N87R cells than in parental NCI-N87 cells (Fig. 2B). This suggests that SRC activation may not be the only cause for the resistance of NCI-N87R cells to trastuzumab. Blockade of trastuzumab binding by MUC4, a membrane-associated mucin, has also been implicated as a mechanism of trastuzumab resistance.17 Here, we determined the expression of MUC4 in NCI-N87 and NCI-N87R cell lines. The results showed that NCI-N87R cells expressed a much higher level of MUC4 than did NCI-N87 cells (Fig. S1). These data suggest that overexpression of MUC4 may also contribute to trastuzumab resistance in NCI-N87R cell line.
The SRC inhibitor saracatinib is well tolerated in Phase 1 and 2 clinical trials.18 A recent study demonstrated that saracatinib sensitized trastuzumab-resistant breast cancer cells to trastuzumab treatment in vitro and in vivo.13 Here, we showed that trastuzumab and saracatinib synergistically inhibited the in vitro growth of both trastuzumab-sensitive and -resistant gastric cancer cell lines. Further data showed trastuzumab in combination with saracatinib was significantly more effective than either agent alone in inhibiting tumor growth in both NCI-N87 and NCI-N87R xenograft models. Previous studies have indicated that PI3K-AKT pathway activity is directly linked to the proliferation of ErbB2-overexpressing cells and that inhibition of proliferation corresponds with the ability of trastuzumab to inhibit ErbB3-PI3K-AKT pathway.19 Consistent with this, our results also demonstrated that trastuzumab plus saracatinib was much more potent than either agent alone in reducing the phosphorylation of ErbB3 and AKT in both trastuzumab-sensitive and -resistant gastric cancer cell lines.
In conclusion, our data suggest that SRC activation and increased ErbB signaling may be acquired resistance mechanisms to trastuzumab in trastuzumab-resistant gastric cancer. Trastuzumab plus saracatinib was significantly more effective than either agent alone in inhibiting the in vitro and in vivo growth of both trastuzumab-sensitive and -resistant gastric cancer cell lines, suggesting that the combination therapy of trastuzumab and saracatinib may provide a new effective strategy for the treatment of ErbB2-overexpressing gastric cancer.
Materials and Methods
Cell lines and animals
The human gastric cancer cell line NCI-N87 was purchased from the American Type Culture Collection (ATCC). To obtain trastuzumab-resistant NCI-N87 cell line (NCI-N87R), NCI-N87 cells were treated with 10 μg/ml of trastuzumab for nine months. Five-week-old female BALB/c nude mice were obtained from the Shanghai Experimental Animal Center of Chinese Academy of Sciences (Shanghai, China). All animals were treated in accordance with guidelines of the Committee on Animals of the Second Military Medical University.
Cell proliferation assay
Trastuzumab and saracatinib were purchased from Roche and BioVision, respectively. Cells were incubated with increasing concentrations of trastuzumab, saracatinib, or trastuzumab and saracatinib in combination. Four days later, cell proliferation was determined by CellTiter 96 AQueous One Solution Cell Proliferation Assay (MTS Assay) kit (Promega). Percentage of inhibition of cell proliferation was calculated as [1-(treated cells ÷ untreated cells) × 100]. Combination index (CI) values were calculated using the Chou-Talalay method. Drug synergy, addition, and antagonism are defined by C.I. values less than 1.0, equal to 1.0, or greater than 1.0, respectively.
In vivo therapy study
NCI-N87 or NCI-N87R cells (5 × 106 per mouse) were inoculated subcutaneously into the right flank of female BALB/c nude mice. When tumor volumes reached an average of about 100 mm3, the mice were randomly divided into groups of 10 mice each. Mice were intravenously injected with control human IgG or trastuzumab (10 mg/kg) twice weekly. We treated mice with saracatinib (25 mg/kg) daily via oral gavage. Tumors were measured with digital calipers, and tumor volumes were calculated by the formula: volume = [length × (width)]2 ÷ 2.
Immunoprecipitation
We detected the ligand-independent ErbB2-containing heterodimers using the reversible chemical crosslinking procedure described by Junttila et al.,19 with minor modifications. Briefly, after washing twice with ice-cold HEPES-NaCl buffer (50 mM HEPES (pH 7.2), 150 mM NaCl), the cells were incubated with 2 mM DTSSP (Thermo Scientific) dissolved in HEPES-NaCl buffer for 1 h at 4 °C. The cells were then washed three times with ice-cold 25 mM Tris (pH 7.1), 150 mM NaCl and lysed in NP-40 lysis buffer supplemented with protease and phosphatase inhibitors. For co-immunoprecipitation experiments, we incubated the total cell lysate with an agarose-conjugated anti-ErbB2 monoclonal antibody (sc-7301 AC; Santa Cruz Biotechnology) overnight at 4 °C. The precipitated proteins were subjected to SDS-PAGE followed by western blot analysis with antibodies specific for EGFR (sc-03; Santa Cruz Biotechnology), ErbB2 (sc-7301; Santa Cruz Biotechnology) or ErbB3 (sc-285; Santa Cruz Biotechnology).
Immunoblotting
Cells were treated with control IgG, trastuzumab, saracatinib, or trastuzumab plus saracatinib for 1 h at 37 °C. After washing, the cells were lysed in SDS lysis buffer and the cell lysates were subjected to SDS-PAGE and immunoblotted with antibodies against EGFR (2232; Cell Signaling), phospho-EGFR-Tyr1068 (2236; Cell Signaling), ErbB2 (2248; Cell Signaling), phospho-ErbB2-Tyr1221/1222 (2243; Cell Signaling), ErbB3 (4754; Cell Signaling), phospho-ErbB3-Tyr1289 (4791; Cell Signaling), AKT (9272; Cell Signaling), phospho-AKT-Ser473 (4060; Cell Signaling), p44/42 MAPK (9102; Cell Signaling), phospho-p44/42 MAPK-Thr202/Tyr204 (9106; Cell Signaling), SRC (2110, Cell Signaling), phospho-SRC-Y416(2101, Cell Signaling).
Real-time quantitative PCR
Total RNA was isolated using the TRIzol reagent (Invitrogen) and reverse transcribed with PrimeScript RT reagent kit (Takara). The real-time quantitative PCR was performed on a 7500 Fast Real-Time PCR System (Applied Biosystems) using the SYBR Premix Ex Tag kit (Takara). β-actin was used as an endogenous control to normalize expression levels. The primers used were as follows. β-actin: (F) 5′- AGC GAG CAT CCC CCA AAG TT -3′, (R) 5′-GGG CAC GAA GGC TCA TCA TT -3′; MUC4: (F)_5′-CGT TCT GGG ACG ATG CTG AC-3′, (R) 5′-GAT GGC TTG GTA GGT GTT GCT-3′.
Statistical analysis
Statistical analysis was performed by Student's unpaired t test to identify significant differences unless otherwise indicated. Differences were considered significant at P < 0.05.
Supplementary Material
Disclosure of Potential Conflicts of Interest Statement
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by National Natural Science Foundation of China, Ministry of Science and Technology of China (973 program projects), National Key project for New Drug Development and Manufacture, Shanghai Commission of Science and Technology, and Shanghai Leading Academic Discipline Project (B905).
Authors’ Contributions
Li B and Guo Y designed the study. Han S, Meng Y, Tong Q, Li G, Zhang X, Hu S, Zheng L, Tan W, Lu H, Chen Y, and Zhang G performed the experiments. Han S, Meng Y, Tong Q, Tan W, Li B, and Guo Y analyzed the data. Li B and Guo Y wrote the paper.
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/27443
References
- 1.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–12. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 3.Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 4.Valabrega G, Montemurro F, Aglietta M. Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann Oncol. 2007;18:977–84. doi: 10.1093/annonc/mdl475. [DOI] [PubMed] [Google Scholar]
- 5.Nahta R, Esteva FJ. HER2 therapy: molecular mechanisms of trastuzumab resistance. Breast Cancer Res. 2006;8:215. doi: 10.1186/bcr1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nahta R, Yu D, Hung MC, Hortobagyi GN, Esteva FJ. Mechanisms of disease: understanding resistance to HER2-targeted therapy in human breast cancer. Nat Clin Pract Oncol. 2006;3:269–80. doi: 10.1038/ncponc0509. [DOI] [PubMed] [Google Scholar]
- 7.Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4:470–80. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- 8.Kim LC, Song L, Haura EB. Src kinases as therapeutic targets for cancer. Nat Rev Clin Oncol. 2009;6:587–95. doi: 10.1038/nrclinonc.2009.129. [DOI] [PubMed] [Google Scholar]
- 9.Bromann PA, Korkaya H, Courtneidge SA. The interplay between Src family kinases and receptor tyrosine kinases. Oncogene. 2004;23:7957–68. doi: 10.1038/sj.onc.1208079. [DOI] [PubMed] [Google Scholar]
- 10.Parsons JT, Parsons SJ. Src family protein tyrosine kinases: cooperating with growth factor and adhesion signaling pathways. Curr Opin Cell Biol. 1997;9:187–92. doi: 10.1016/S0955-0674(97)80062-2. [DOI] [PubMed] [Google Scholar]
- 11.Biscardi JS, Maa MC, Tice DA, Cox ME, Leu TH, Parsons SJ. c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J Biol Chem. 1999;274:8335–43. doi: 10.1074/jbc.274.12.8335. [DOI] [PubMed] [Google Scholar]
- 12.Belsches-Jablonski AP, Biscardi JS, Peavy DR, Tice DA, Romney DA, Parsons SJ. Src family kinases and HER2 interactions in human breast cancer cell growth and survival. Oncogene. 2001;20:1465–75. doi: 10.1038/sj.onc.1204205. [DOI] [PubMed] [Google Scholar]
- 13.Zhang S, Huang WC, Li P, Guo H, Poh SB, Brady SW, Xiong Y, Tseng LM, Li SH, Ding Z, et al. Combating trastuzumab resistance by targeting SRC, a common node downstream of multiple resistance pathways. Nat Med. 2011;17:461–9. doi: 10.1038/nm.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humar B, Fukuzawa R, Blair V, Dunbier A, More H, Charlton A, Yang HK, Kim WH, Reeve AE, Martin I, et al. Destabilized adhesion in the gastric proliferative zone and c-Src kinase activation mark the development of early diffuse gastric cancer. Cancer Res. 2007;67:2480–9. doi: 10.1158/0008-5472.CAN-06-3021. [DOI] [PubMed] [Google Scholar]
- 15.Okamoto W, Okamoto I, Yoshida T, Okamoto K, Takezawa K, Hatashita E, Yamada Y, Kuwata K, Arao T, Yanagihara K, et al. Identification of c-Src as a potential therapeutic target for gastric cancer and of MET activation as a cause of resistance to c-Src inhibition. Mol Cancer Ther. 2010;9:1188–97. doi: 10.1158/1535-7163.MCT-10-0002. [DOI] [PubMed] [Google Scholar]
- 16.Nam HJ, Im SA, Oh DY, Elvin P, Kim HP, Yoon YK, Min A, Song SH, Han SW, Kim TY, et al. Antitumor activity of saracatinib (AZD0530), a c-Src/Abl kinase inhibitor, alone or in combination with chemotherapeutic agents in gastric cancer. Mol Cancer Ther. 2013;12:16–26. doi: 10.1158/1535-7163.MCT-12-0109. [DOI] [PubMed] [Google Scholar]
- 17.Nagy P, Friedländer E, Tanner M, Kapanen AI, Carraway KL, Isola J, Jovin TM. Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a herceptin-resistant, MUC4-expressing breast cancer cell line. Cancer Res. 2005;65:473–82. [PubMed] [Google Scholar]
- 18.Aleshin A, Finn RS. SRC: a century of science brought to the clinic. Neoplasia. 2010;12:599–607. doi: 10.1593/neo.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Junttila TT, Akita RW, Parsons K, Fields C, Lewis Phillips GD, Friedman LS, Sampath D, Sliwkowski MX. Ligand-independent HER2/HER3/PI3K complex is disrupted by trastuzumab and is effectively inhibited by the PI3K inhibitor GDC-0941. Cancer Cell. 2009;15:429–40. doi: 10.1016/j.ccr.2009.03.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.