Abstract
The discovery of monoclonal antibodies (mAbs) that bind to a particular molecular target is now regarded a routine exercise. However, the successful development of mAbs that (1) express well, (2) elicit a desirable biological effect upon binding, and (3) remain soluble and display low viscosity at high concentrations is often far more challenging. Therefore, high throughput screening assays that assess self-association and aggregation early in the selection process are likely to yield mAbs with superior biophysical properties. Here, we report an improved version of affinity-capture self-interaction nanoparticle spectroscopy (AC-SINS) that is capable of screening large panels of antibodies for their propensity to self-associate. AC-SINS is based on concentrating mAbs from dilute solutions around gold nanoparticles pre-coated with polyclonal capture (e.g., anti-Fc) antibodies. Interactions between immobilized mAbs lead to reduced inter-particle distances and increased plasmon wavelengths (wavelengths of maximum absorbance), which can be readily measured by optical means. This method is attractive because it is compatible with dilute and unpurified mAb solutions that are typical during early antibody discovery. In addition, we have improved multiple aspects of this assay for increased throughput and reproducibility. A data set comprising over 400 mAbs suggests that our modified assay yields self-interaction measurements that are well-correlated with other lower throughput assays such as cross-interaction chromatography. We expect that the simplicity and throughput of our improved AC-SINS method will lead to improved selection of mAbs with excellent biophysical properties during early antibody discovery.
Keywords: nanoparticle, antibody developability, aggregation, self-interaction, self-association, high-throughput screening, cross-interaction
Introduction
The strong demand for monoclonal antibody (mAb) therapeutics has fueled rapid growth and maturation of antibody discovery platforms.1,2 In an effective antibody discovery program, both the biology and developability of the lead candidates must be carefully examined to ensure efficacy while minimizing downstream risks.3-6 In the past, the strongest focus has been placed on identifying biologically relevant, high affinity antibodies against selected targets. However, many discovery programs have ultimately failed due to poor antibody expression, low solubility and high viscosity, poor stability or high polyspecificity, the latter of which may lead to shorter serum half-life.7-12 These issues emphasize that developability is a critical determinant of the success of an antibody therapeutic program, and needs to be considered during early discovery.
Most developability problems arise from the intrinsic biophysical properties of an antibody, such as its conformational and colloidal stability. It is relatively simple to screen for candidate antibodies with high conformational (folding) stability using methods such as differential scanning calorimetry or fluorimetry.13 In contrast, it is more difficult to screen for antibodies with high colloidal stability (i.e., low self-association and high solubility). This is problematic because weak antibody self- and cross-interactions are often responsible for aggregation and polyreactivity, respectively.6,7,12,14-19 Nevertheless, numerous assays such as self-interaction chromatography (SIC)20-25 and cross-interaction chromatography (CIC)26-28 have been designed to identify these possibly troublesome antibodies early in the discovery program to avoid downstream issues. In these chromatography assays, increased retention of mAbs passing through a column conjugated with identical mAbs or a pool of polyclonal serum antibodies is indicative of attractive self- or cross-interactions, respectively. Antibodies that display attractive interactions typically have low solubility, but some antibodies with high solubility also show strong interaction with the column resin, which makes it difficult to measure their self- or cross-interactions.
Other methods for detecting weak antibody interactions have also been reported. For measuring non-specific cross-interactions, Hötzel et al.12 demonstrated that the propensity of mAbs to interact with baculovirus particles (BVPs) in an ELISA format is predictive of the antibody serum clearance rate in a variety of hosts. BVPs provide a large collection of representative surfaces that an antibody may encounter in the serum upon injection. Weak interaction with BVPs is often suggestive of polyspecificity of an antibody and thus faster clearance. A similar approach using soluble membrane proteins (SMPs)29 employed the speed of fluorescence-activated cell sorting (FACS) for polyspecificity screening during antibody selection or post-discovery characterization. Other approaches include direct detection of antibody self-interactions by surface plasmon resonance (SPR)30 or biolayer interferometry (BLI).31 These methods may be used to identify the mechanism of antibody aggregation by analysis of the interacting domains (e.g., Fab-Fab or Fab-Fc), which could be useful for reducing self-association via protein engineering.30,32-37
Nevertheless, there is still substantial need for improved screening assays capable of identifying mAbs with low propensity to self-associate during antibody discovery. To have the greatest effect, such assays should be compatible with the large number of antibody variants (hundreds to thousands) commonly selected during early antibody discovery, as well as the low concentrations (<0.1 mg/mL) and purities (unpurified cell supernatants) typical of this early stage. A promising assay, affinity-capture self-interaction nanoparticle spectroscopy (AC-SINS), that appears to address some of these remaining challenges has recently been reported.38-40 In this assay, gold nanoparticles (AuNPs) are first coated with polyclonal antibodies with specificity for human mAbs (e.g., goat anti-human), and then mAbs are captured by the conjugates from dilute solutions (1–100 μg/mL) without need for purification. Attractive self-interactions between adsorbed mAbs, which are amplified due to the polyvalency of the mAb conjugates, lead to reduced inter-particle separation distances. This can be detected by a change in color of the gold colloid solutions, which is simple to quantify via the change in the wavelength of maximum absorbance (plasmon wavelength) using a standard plate reader. Antibodies with tendency toward self-association lead to red-shifted plasmon wavelengths. Moreover, the plasmon wavelength can be evaluated as a function of the amount of immobilized mAb by coating gold particles with mixtures of capture (e.g., goat anti-human) and non-capture (e.g., goat non-specific) polyclonal antibodies for enhanced sensitivity, although this also leads to reduced throughput.
Here, we report a modified form of AC-SINS with improved throughput and reproducibility for antibody developability screening during early stage discovery. With modified sample preparation procedures, assay precision is significantly improved to allow differentiation between antibodies with high and low self-association using only a single concentration of immobilized mAb. Good correlation between CIC, FACS-based SMP assay and our modified AC-SINS assay was observed for over 400 different antibodies. This modified procedure allows for self-interaction screening of thousands of antibodies within a single day with minimal material consumption.
Results
Figure 1A summarizes the procedure for performing AC-SINS. Citrate-stabilized gold nanoparticles (20 nm) are coated with polyclonal goat anti-human Fc antibodies, concentrated, and then incubated with mAbs of interest. For associative mAbs, the conjugates cluster and cause an increase in the plasmon wavelength. In contrast, non-associative antibodies do not cause clustering of conjugates or shifting of the plasmon wavelength.38 While the original AC-SINS assay is relatively straightforward, reproducibility is a concern if one were to use a single concentration, as would be required for high throughput implementation. When 11 data points are used for each test mAb formulation condition, the general trend of the resulting curve can be used for accurate qualitative assessment even when one or two points may be outliers. However, when using a single data point, robustness and reproducibility of each point becomes critical and the modifications described here directly address this concern.
Figure 1. (A) Schematic of the modified AC-SINS assay. (B) Images of the work flow for the AC-SINS assay.
To improve assay robustness and precision, we modified the particle-concentrating step prior to the addition of testing mAbs. In the original pelleting step, it appears that particle clusters can form and do not fully dissociate upon resuspension. Therefore, we developed an alternative particle concentrating method prior to addition of mAbs (Fig. 1B). In this procedure, particles coated with polyclonal goat antibodies (2 mM sodium acetate, pH 5.2) are filtered through PVDF membranes (0.22 µm). The coated particles fail to pass through the filter despite their small size (~40 nm) relative to the filter pore size (~220 nm). However, addition of a small amount of PBS (1/10 the original volume of the conjugates) elutes most of the particles from the membrane to form a concentrated colloid solution.
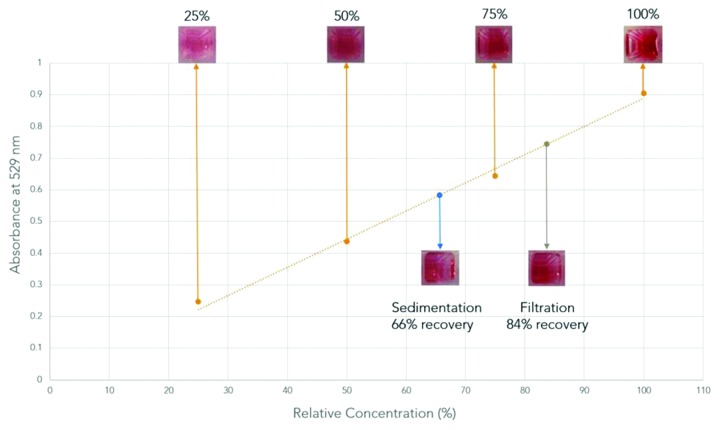
To evaluate the fractional particle recovery after filtration, we quantified the particle concentration by measuring the absorbance at 529 nm (Fig. 2). The maximum absorbances of concentrated particles after filtration or sedimentation were measured and their concentrations were calculated from the standard curve. The filtration method resulted in 84% recovery, while the sedimentation method results in a lower (66%) recovery. This data suggests that the filtration followed by elution is superior to the original method in terms of particle recovery and prevents the formation of particle clusters.
Figure 2. Standard curve of the absorbance intensity (529 nm) for gold nanoparticles coated with capture antibodies that is used for calculating the fractional particle recovery after concentration via sedimentation or filtration.
We also tested other assay parameters (ratio of capture to non-capture antibodies and incubation time) to optimize throughput, sensitivity and robustness using six mAbs and one control sample (PBS buffer) as shown in Figure 3A. These mAbs were selected to represent antibodies with different developability issues. Adalimumab41 and mAb2 are well-behaved, highly soluble antibodies (soluble at >100 mg/mL in PBS). On the other hand, mAb1, mAb3, and CNTO60710,11 are poorly behaved antibodies with low solubility. Moreover, mAb4 is a highly viscous antibody that displays gel-like behavior at high concentrations (>50 mg/mL). Using these antibodies, we varied the amount of immobilized capture antibody by co-adsorbing non-capture antibodies at different ratios. As capture capacity increases, the difference between the plasmon wavelengths of the sample and control (Δλmax) generally increases, especially for the poorly behaved antibodies. The difference between the plasmon wavelengths for the positive and negative controls is most significant for the 60, 80, and 100% capture antibody concentrations, and after an incubation time of 2 h. Given our goal of increasing throughput of AC-SINS by reducing the number of different capture concentrations, we identified 80% capture antibody as the optimal condition that maximized sensitivity without causing non-specific interactions for well-behaved antibodies such as mAb2. The 80% capture antibody concentration is modestly more sensitive than the 60% condition at differentiating between well- and poorly-behaved antibodies. We expanded our studies to a total of 439 different antibodies using both 60 and 80% capture antibody concentrations, and compared the corresponding Δλmax values (Fig. 3B). More data points are observed under the y = x line, confirming our observation that 80% capture antibody is a more sensitive condition to detect mAb self-interactions.
Figure 3. (A) Plasmon wavelength shifts vs. incubation time for different capture (anti-Fc) antibody concentrations.
Figure 3B-C.(B) Comparison of plasmon wavelength shifts for 439 antibodies for 60% and 80% capture antibody concentrations. (C) AC-SINS assay precision for different analysts performing the experiments on multiple days.
Using these optimized assay procedures, we next evaluated assay robustness with a combination of different analysts on multiple days for 22 selected mAbs (Fig. 3C). Slightly larger standard deviations are observed for poor developability antibodies. However, assay precision is sufficient to reliably differentiate between good or poor developability antibodies. Using the same set of antibodies, we also found that these conjugates are stable in the coating solution at 4 °C for more than 2 wk (data not shown). The conjugates prepared from multiple batches also give similar results, further confirming the robustness of this assay.
To further validate AC-SINS predictions of antibody solution behavior, we evaluated the self-association characteristics of 32 human and humanized antibodies currently in late-stage clinical trials or FDA-approved drugs in the clinic (Fig. 4). Most of these clinical antibodies have Δλmax values less than 5 nm, confirming their good developability. However, there are a few clinical antibodies, such as ganitumab, tremelimumab and vesencumab that show Δλmax values greater than 10 nm, suggesting that these antibodies strongly self-associate in PBS buffer at pH 7.4.
Figure 4. Maximum plasmon wavelength shift for 32 different clinical antibodies in PBS.
We also compared AC-SINS results with CIC (Fig. 5A) for 447 antibodies. For the comparison of results obtained by AC-SINS and CIC, we used threshold values of 5 nm (AC-SINS) and 10 min (CIC) that divide the plot into four quadrants. These thresholds were chosen by an analysis of ROC curves.29,42 Most of the mAbs fall within quadrants I and III (~86% or 385 mAbs), confirming poor (quadrant I) and good (quadrant III) developability antibodies. In quadrant II, 39 self-interacting antibodies (9%) are identified as potentially problematic by AC-SINS, but not by CIC. In quadrant IV, 23 antibodies (5%) that interact non-specifically are identified by CIC, but not by the AC-SINS assay. We selected these CIC-positive mAbs and tested the AC-SINS method using alternative capture antibodies, including goat anti-human Fab and goat anti-human heavy plus light chain to control the presentation orientation of mAbs on the particle surface (data not shown). Nevertheless, these conjugates also displayed Δλmax values less than 5 nm, suggesting that the mAbs display low self-association.
Figure 5. (A) Comparison of assay results for AC-SINS and Cross-Interaction Chromatography (CIC). Assay cutoff values of 5 nm (AC-SINS) and 10 min (CIC) are used. (B) Comparison of assay results for AC-SINS and a FACS assay that uses a soluble membrane protein (SMP) reagent. Assay cutoff values of 5 nm (AC-SINS) and 200 fluorescence units (SMP) are used. (C) Comparison of assay results for CIC and SMP. Red squares represent clinical antibodies, orange squares represent ganitumab, tremelimumab or vesencumab.
Finally, we compared our AC-SINS results with those obtained from a recently reported FACS assay that employs a polyspecific reagent29 (PSR; Fig. 5B). Using a cutoff value of 200 fluorescent units, we observe a similar trend as observed in Figure 5A. A total of 346 antibodies (~78%) are in quadrants III (well-behaved in both assays) and I (poorly-behaved in both assays). In quadrant II, 11 self-interaction antibodies (~2%) are identified only by AC-SINS, but not by the FACS assay. In quadrant IV, 88 polyreactive antibodies (~20%) are detected only by FACS, but not by the AC-SINS assay. A similar comparison between CIC and PSR (SMP) assay is shown in Figure 5C. There is a 77% positive correlation between these two assays. 22% poly reactivity is detected only by PSR and 1% is detected only by CIC.
Discussion
AC-SINS uses gold nanoparticles to locally cluster antibodies from dilute antibody solutions to achieve high local concentrations (>60 mg/mL) for detecting antibody self-interactions.30 This assay offers a practical solution to developability screening for early-stage antibody discovery during which a large number of candidates are typically available in relatively low quantities. The ability of AC-SINS to analyze complex samples such as unpurified antibodies due to the use of capture antibodies is also a strength of the assay. In the original form of this assay, several different capture antibody concentrations were used for each mAb to evaluate antibody self-association. While this extensive data set for each antibody is valuable for robustly differentiating between mAbs with different levels of self-association, it is less suitable for developability screening during early antibody discovery because of the reduced throughput. Therefore, we adapted AC-SINS into a single-point high-throughput assay for developability assessment. This required optimization of assay reproducibility and robustness because only one data point is used for self-interaction analysis.
To improve assay robustness, we made multiple changes to the sample preparation procedure for AC-SINS. We find that using sedimentation to concentrate the coated nanoparticles can result in particle clusters that are difficult to re-suspend and ultimately reduce reproducibility. Our initial attempts to remove these large particle clusters via filtration (0.22 µm filters) failed due to retention of the conjugates. This phenomenon is inconsistent with the size of the conjugates (~40 nm). The goat anti human Fc antibody coating solution (without particles) can easily pass through these filters with good recovery, ruling out the possibility that the conjugate are caught by the filter because of coating antibody and membrane interactions. We hypothesized that repulsive electrostatic interactions between the conjugates and membranes, which are magnified at the low ionic strength (2 mM sodium acetate) and pH (5.2), led to exclusion of the conjugates from the relatively large membrane pores. A similar membrane exclusion phenomenon has been observed during protein ultrafiltration.43 Indeed, we found that increasing solution pH to pH 7 led to the conjugates passing through the membranes. Presumably the increase in pH reduces the antibody net charge, which in turn reduces repulsive electrostatic interactions with the membrane. We exploited the retention of antibody conjugates at low pH and elution at neutral pH to concentrate the conjugates without sedimentation, which led to improved assay reproducibility.
Our use of two different plates for the adsorption of mAbs (plate 1) and measurement of plasmon wavelengths (plate 2) was also a modification of the original AC-SINS protocol. We noticed that (in some cases) particles adhered to the hydrophobic polystyrene plates and this in turn led to reduced reproducibility. Assay robustness was improved by performing the mAb adsorption in a polypropylene plate (plate 1) and then transferring the conjugates to a polystyrene plate (plate 2) just prior to measurement of plasmon wavelengths. It was not possible to use polypropylene plates in both steps due to the opaque nature of such plates. Briefly centrifuging the polystyrene plate (plate 2) to remove air bubbles and level the menisci also improved assay precision. Finally, increasing the sample volume from 50 to 100 μL in 384-well plates increases the optical path length during plate reading and enhanced signal-to-noise ratios.
We also found that the sensitivity of AC-SINS generally increases with increasing fraction of capture antibody. Nevertheless, 100% capture antibody appeared to induce self-association even for a well behaved antibody (mAb 2) at long incubation times (4 h). Thus, we chose 80% capture antibody concentration as the final assay condition to balance sensitivity and robustness. When adopting this assay in house, there is no need for further screening on this parameter and the 80% condition can be used directly. With these modified sample preparation procedures, we performed assay validation with a combination of different analysts on multiple days. When using Δλmax < 5 nm as a criterion for low self-association (good developability) antibodies, most of the mAbs can be easily categorized. The standard deviations for self-interacting antibodies are relatively large (average of ~4 nm) but this does not significantly affect their developability assignment. We used Δλmax < 5 nm as a fairly stringent cutoff to select only well-behaved mAbs for downstream development. This cutoff value can be adjusted based on the required stringency during antibody discovery.
When applying this single point self-interaction assay to 32 clinical antibodies, most of the mAbs showed small plasmon shifts (Δλmax < 5 nm), confirming their good developability. However, a few antibodies show significant self-interaction. We used a relatively harsh screening buffer (PBS) and a relatively high pH (7.4) compared with typical antibody formulation conditions (pH < 7). Usually, minimal formulation screening is needed if an antibody shows low self-interaction in this buffer. Self-interaction of an antibody depends on its pI, as well as its formulation, such as solution pH, ionic strength, and addition of detergent or sugar. We tested a few commonly used formulation conditions, other than just PBS, with pH ranging from 5.5‒6.5, with or without addition of histidine, sorbitol, trehalose and Tween 20 (data not shown). The results suggest that AC-SINS assay is compatible with all these conditions and is able to detect mAb self-interaction conditions with good sensitivity.
Notably, the three antibodies with the highest self-association (ganitumab,44 tremelimumab45 and vesencumab46) in PBS have been (to the best of our knowledge) administered only in IV formulations that are likely to reduce problems linked to self-association due to the dilute concentrations. In general, the demand for non-association antibodies is more intense when clinical indication or competitive pressures make it a priority to develop high-concentration formulations required for subcutaneous administration. Nevertheless, it is desirable to aim at identifying antibodies with the best biophysical properties from the beginning of the discovery process.
When comparing results obtained using AC-SINS and CIC, a positive correlation is observed for most (86%) of the tested mAbs. However, there are some antibodies identified only by AC-SINS or CIC. This is likely due to the inherent difference between the molecular interactions measured by these two assays. AC-SINS detects self-interactions while CIC detects non-specific interactions between mAbs and polyclonal IgGs. For associative mAbs identified by AC-SINS but not by CIC, it may be that specific self-interactions are not well-represented by polyclonal IgGs. Conversely, problematic mAbs identified only by CIC but not AC-SINS may be due to unique non-specific interactions involving polyclonal antibodies that are absent in such mAbs. Another issue may be that mAbs interact non-specifically with the column resin, but do not display strong self-association (as indicated by AC-SINS). Our control experiments, however, confirmed that this is not the case for most mAbs (20 out of 23).
The AC-SINS results also correlated with those from a FACS-based assay that detects interactions between mAbs and solubilized membrane proteins. Most of the mAbs (~78%) display positive correlation between these two assays. Interestingly, a minor population (~20%) is only identified by the FACS assay, which is likely due to the complex nature of the membrane protein reagent that provides a broad coverage of non-specific interactions. These mAbs non-specifically interact with the membrane protein reagent but display low self-association. An even smaller population of mAbs (2%) is positive by the AC-SINS assay, but negative by the FACS assay. These antibodies may possess specific self-interactions that are not represented by the soluble membrane protein reagent.
When comparing the assay results between CIC and SMP, it is obvious that the SMP assay is more sensitive to detect polyreactivity. These two assays are both designed to capture poly-reactivity using a complex mixture of poly-clonal human serum IgGs or soluble membrane proteins. A more diversified reagent and/or surface is included in SMP to allow capture of more types of weak cross interactions.
In conclusion, we adapted AC-SINS into a robust, high-throughput, single point self-interaction screening assay that is compatible with the work flow of early stage antibody discovery. The adapted AC-SINS assay allows developability screening of thousands of antibodies in a single day with minimal material consumption and cost.
Materials and Methods
Citrate-stabilized 20 nm gold nanoparticles (15705) were purchased from Ted Pella Inc. Poly(ethylene glycol) methyl ether thiol (2000 Da, 729140) was from Sigma-Aldrich. Goat anti-human IgG, Fcγ fragment specific (109-005-098), goat non-specific antibody (005-000-003), goat anti-human IgG F(ab')2 fragment Specific (109-005-006) and goat anti-human IgG (H+L) (109-005-088) were obtained from Jackson ImmunoResearch Laboratories, Inc. Zeba Spin desalting columns (87766, 87768, and 87770) were from Thermo Fisher Scientific. 0.22 µm PVDF Millex-GV Filters (SLGVX13NK) were from EMD Millipore. Costar 384-Well Polystyrene Plates (12-565-506) were obtained from Fisher Scientific. 96-well polypropylene shallow-well plates (5042-1385) were obtained from Agilent Technologies, Inc. All the test antibodies were discovered in Adimab and expressed in yeast, unless otherwise stated. The clinical antibodies were made recombinantly in HEK293 using published v-region sequence in an IgG1 format. A Spectramax M2e model plate reader from Molecular Devices was used in this study.
AC-SINS
Polyclonal goat anti-human IgG Fc antibodies (capture) and goat non-specific antibodies (non-capture) are buffer exchanged into 20 mM NaAc (pH 4.3), followed by a concentration normalization to 0.4 mg/mL. A 4:1 volume ratio mix of capture:non-capture IgG solution is prepared for 80% capture capacity coating solution. A 9:1 volume ratio is used to mix gold nanoparticle solution with coating solution. After room temperature incubation for 1 h, thiolated PEG (final concentration 0.1 μM) is used to block empty sites on the AuNP. These coated and blocked particles are stable in the coating solution at 4 °C for up to 2 wk. The particle solution is then passed through a 0.22 μm PVDF membrane (Millex-GV, 13 mm, Millipore). The particles are retained on top of the membrane and the flow through solution is clear. PBS at 1/10 of the starting volume is used to elute the particles into the collection tube. Ten μL of the 10X concentrated coated particles is incubated with 100 μL of test antibody solution (50 μg/mL or above in PBS) at room temperature for 2 h in a polypropylene plate, then 100 μL of the resulting solution is transferred into a polystyrene UV transparent plate, followed by brief centrifugation to bring the solution menisci to the same level. Absorbance data are collected from 510 to 570 nm at in increment of 2 nm. Raw absorbance data are exported into an Excel file, followed by data processing using a macro. The macro first identifies the wavelength of maximum absorbance in the raw data, then stores the 20 data points around that wavelength in an array. Each point is averaged with the points directly before and after it to reduce error from noise. Using the Linest function in Excel, a second-order polynomial is fit to this set of data. The coefficients are used to calculate the wavelength where the slope is equal to zero and the macro then determines whether or not this point is a maximum or minimum. In the case of a maximum, the calculated wavelength is returned, unless it is greater than 560nm, in which case the macro returns “Max > 560.”
CIC
A CIC column was prepared by coupling ~30 mg of human polyclonal IgGs (Sigma I 4506) onto a HiTrap NHS-activated resin (GE Healthcare #17-0716-01) followed by passivation with ethanolamine. A “blank” column was prepared similarly except ethanolamine passivation performed with no prior IgG coupling. The columns were then connected to an HPLC and run at 0.1 mL per minutes using 1xPBS as the mobile phase until a flat baseline was reached. IgG or Fab at 0.5‒1 mg/mL in PBS or HBS were then injected (10 µL). Peak retention times on the column were monitored at 280 nm and compared with reference IgGs run on same column to determine level of sample IgG cross-interaction. Typical “non-interacting” IgGs eluted from the column under 10 min.
SMP preparation and binding assay
SMP preparation and binding assay is performed as described in details previously.29 In brief, soluble membrane proteins were extracted from mammalian cells, solubilized in detergent and biotinylated prior to FACS analysis.
Disclosure of Potential Conflicts of Interest
Liu Y, Caffry I, Wu J, Geng SB, Jain T, Sun T, Reid F, Cao Y, Estep P, Yu Y, Vásquez M, and Xu Y own company stock in Adimab. Tessier PM is an Adimab consultant.
Acknowledgments
All of the in-house antibodies used in this study were discovered by Adimab’s Antibody Discovery group, sequence confirmed by the Core and produced by the High Throughput Expression department. We appreciate the manuscript editing and critical review by Michael Ruse, Errik Anderson, Dane Wittrup, and Tillman Gerngross.
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/27431
References
- 1.Elvin JG, Couston RG, van der Walle CF. Therapeutic antibodies: market considerations, disease targets and bioprocessing. Int J Pharm. 2013;440:83–98. doi: 10.1016/j.ijpharm.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 2.TABS Therapeutic Antibody Database [Internet]. Available from: http://tabs.craic.com/
- 3.Drake AW, Papalia GA. Biophysical Considerations for Development of Antibody-Based Therapeutics. In: Tabrizi MA, Bornstein GG, Klakamp SL, editors. Development of Antibody-Based Therapeutics. New York, NY: Springer New York; 2012. page 95–139. [Google Scholar]
- 4.Yang X, Xu W, Dukleska S, Benchaar S, Mengisen S, Antochshuk V, Cheung J, Mann L, Babadjanova Z, Rowand J, et al. Developability studies before initiation of process development: improving manufacturability of monoclonal antibodies. MAbs. 2013;5:787–94. doi: 10.4161/mabs.25269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saxena V, Panicucci R, Joshi Y, Garad S. Developability assessment in pharmaceutical industry: An integrated group approach for selecting developable candidates. J Pharm Sci. 2009;98:1962–79. doi: 10.1002/jps.21592. [DOI] [PubMed] [Google Scholar]
- 6.Sathish H, Angell N, Lowe D, Shah A, Bishop S. Application of Biophysics to the Early Developability Assessment of Therapeutic Candidates and Its Application to Enhance Developability Properties. In: Narhi LO, editor. Biophysics for Therapeutic Protein Development. Springer New York; 2013. page 127–46. [Google Scholar]
- 7.Connolly BD, Petry C, Yadav S, Demeule B, Ciaccio N, Moore JMR, Shire SJ, Gokarn YR. Weak interactions govern the viscosity of concentrated antibody solutions: high-throughput analysis using the diffusion interaction parameter. Biophys J. 2012;103:69–78. doi: 10.1016/j.bpj.2012.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yadav S, Liu J, Shire SJ, Kalonia DS. Specific interactions in high concentration antibody solutions resulting in high viscosity. J Pharm Sci. 2010;99:1152–68. doi: 10.1002/jps.21898. [DOI] [PubMed] [Google Scholar]
- 9.Kanai S, Liu J, Patapoff TW, Shire SJ. Reversible self-association of a concentrated monoclonal antibody solution mediated by Fab-Fab interaction that impacts solution viscosity. J Pharm Sci. 2008;97:4219–27. doi: 10.1002/jps.21322. [DOI] [PubMed] [Google Scholar]
- 10.Bethea D, Wu S-J, Luo J, Hyun L, Lacy ER, Teplyakov A, Jacobs SA, O’Neil KT, Gilliland GL, Feng Y. Mechanisms of self-association of a human monoclonal antibody CNTO607. Protein Eng Des Sel. 2012;25:531–7. doi: 10.1093/protein/gzs047. [DOI] [PubMed] [Google Scholar]
- 11.Wu S-J, Luo J, O’Neil KT, Kang J, Lacy ER, Canziani G, Baker A, Huang M, Tang QM, Raju TS, et al. Structure-based engineering of a monoclonal antibody for improved solubility. Protein Eng Des Sel. 2010;23:643–51. doi: 10.1093/protein/gzq037. [DOI] [PubMed] [Google Scholar]
- 12.Hötzel I, Theil F-P, Bernstein LJ, Prabhu S, Deng R, Quintana L, Lutman J, Sibia R, Chan P, Bumbaca D, et al. A strategy for risk mitigation of antibodies with fast clearance. MAbs. 2012;4:753–60. doi: 10.4161/mabs.22189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He F, Hogan S, Latypov RF, Narhi LO, Razinkov VI. High throughput thermostability screening of monoclonal antibody formulations. J Pharm Sci. 2010;99:1707–20. doi: 10.1002/jps.21955. [DOI] [PubMed] [Google Scholar]
- 14.He F, Woods CE, Becker GW, Narhi LO, Razinkov VI. High-throughput assessment of thermal and colloidal stability parameters for monoclonal antibody formulations. J Pharm Sci. 2011;100:5126–41. doi: 10.1002/jps.22712. [DOI] [PubMed] [Google Scholar]
- 15.Esfandiary R, Hayes DB, Parupudi A, Casas-Finet J, Bai S, Samra HS, Shah AU, Sathish HA. A systematic multitechnique approach for detection and characterization of reversible self-association during formulation development of therapeutic antibodies. J Pharm Sci. 2013;102:3089–99. doi: 10.1002/jps.23654. [DOI] [PubMed] [Google Scholar]
- 16.Salinas BA, Sathish HA, Bishop SM, Harn N, Carpenter JF, Randolph TW. Understanding and modulating opalescence and viscosity in a monoclonal antibody formulation. J Pharm Sci. 2010;99:82–93. doi: 10.1002/jps.21797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sahin E, Grillo AO, Perkins MD, Roberts CJ. Comparative effects of pH and ionic strength on protein-protein interactions, unfolding, and aggregation for IgG1 antibodies. J Pharm Sci. 2010;99:4830–48. doi: 10.1002/jps.22198. [DOI] [PubMed] [Google Scholar]
- 18.Sahin E, Weiss WF, 4th, Kroetsch AM, King KR, Kessler RK, Das TK, Roberts CJ. Aggregation and pH-temperature phase behavior for aggregates of an IgG2 antibody. J Pharm Sci. 2012;101:1678–87. doi: 10.1002/jps.23056. [DOI] [PubMed] [Google Scholar]
- 19.Roberts CJ, Das TK, Sahin E. Predicting solution aggregation rates for therapeutic proteins: approaches and challenges. Int J Pharm. 2011;418:318–33. doi: 10.1016/j.ijpharm.2011.03.064. [DOI] [PubMed] [Google Scholar]
- 20.Ahamed T, Ottens M, van Dedem GWK, van der Wielen LAM. Design of self-interaction chromatography as an analytical tool for predicting protein phase behavior. J Chromatogr A. 2005;1089:111–24. doi: 10.1016/j.chroma.2005.06.065. [DOI] [PubMed] [Google Scholar]
- 21.Bengali AN, Tessier PM. Biospecific protein immobilization for rapid analysis of weak protein interactions using self-interaction nanoparticle spectroscopy. Biotechnol Bioeng. 2009;104:240–50. doi: 10.1002/bit.22392. [DOI] [PubMed] [Google Scholar]
- 22.García CD, Hadley DJ, Wilson WW, Henry CS. Measuring protein interactions by microchip self-interaction chromatography. Biotechnol Prog. 2003;19:1006–10. doi: 10.1021/bp025788z. [DOI] [PubMed] [Google Scholar]
- 23.Johnson DH, Parupudi A, Wilson WW, DeLucas LJ. High-throughput self-interaction chromatography: applications in protein formulation prediction. Pharm Res. 2009;26:296–305. doi: 10.1007/s11095-008-9737-6. [DOI] [PubMed] [Google Scholar]
- 24.Le Brun V, Friess W, Bassarab S, Mühlau S, Garidel P. A critical evaluation of self-interaction chromatography as a predictive tool for the assessment of protein-protein interactions in protein formulation development: a case study of a therapeutic monoclonal antibody. Eur J Pharm Biopharm. 2010;75:16–25. doi: 10.1016/j.ejpb.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Patro SY, Przybycien TM. Self-interaction chromatography: a tool for the study of protein-protein interactions in bioprocessing environments. Biotechnol Bioeng. 1996;52:193–203. doi: 10.1002/(SICI)1097-0290(19961020)52:2<193::AID-BIT2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 26.Jacobs SA, Wu S-J, Feng Y, Bethea D, O’Neil KT. Cross-interaction chromatography: a rapid method to identify highly soluble monoclonal antibody candidates. Pharm Res. 2010;27:65–71. doi: 10.1007/s11095-009-0007-z. [DOI] [PubMed] [Google Scholar]
- 27.Tessier PM, Sandler SI, Lenhoff AM. Direct measurement of protein osmotic second virial cross coefficients by cross-interaction chromatography. Protein Sci. 2004;13:1379–90. doi: 10.1110/ps.03419204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spencer S, Bethea D, Raju TS, Giles-Komar J, Feng Y. Solubility evaluation of murine hybridoma antibodies. MAbs. 2012;4:319–25. doi: 10.4161/mabs.19869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Y, Roach W, Sun T, Jain T, Prinz B, Yu T-Y, Torrey J, Thomas J, Bobrowicz P, Vásquez M, et al. Addressing polyspecificity of antibodies selected from an in vitro yeast presentation system: a FACS-based, high-throughput selection and analytical tool. Protein Eng Des Sel. 2013;26:663–70. doi: 10.1093/protein/gzt047. [DOI] [PubMed] [Google Scholar]
- 30.Nishi H, Miyajima M, Wakiyama N, Kubota K, Hasegawa J, Uchiyama S, Fukui K. Fc domain mediated self-association of an IgG1 monoclonal antibody under a low ionic strength condition. J Biosci Bioeng. 2011;112:326–32. doi: 10.1016/j.jbiosc.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 31.Sun T, Reid F, Liu Y, Cao Y, Estep P, Nauman C, Xu Y. High throughput detection of antibody self-interaction by bio-layer interferometry. MAbs. 2013;5:5. doi: 10.4161/mabs.26186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perchiacca JM, Ladiwala ARA, Bhattacharya M, Tessier PM. Aggregation-resistant domain antibodies engineered with charged mutations near the edges of the complementarity-determining regions. Protein Eng Des Sel. 2012;25:591–601. doi: 10.1093/protein/gzs042. [DOI] [PubMed] [Google Scholar]
- 33.Perchiacca JM, Tessier PM. Engineering aggregation-resistant antibodies. Annu Rev Chem Biomol Eng. 2012;3:263–86. doi: 10.1146/annurev-chembioeng-062011-081052. [DOI] [PubMed] [Google Scholar]
- 34.Dudgeon K, Rouet R, Kokmeijer I, Schofield P, Stolp J, Langley D, Stock D, Christ D. General strategy for the generation of human antibody variable domains with increased aggregation resistance. Proc Natl Acad Sci U S A. 2012;109:10879–84. doi: 10.1073/pnas.1202866109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Singh SK, Kumar S. Potential aggregation-prone regions in complementarity-determining regions of antibodies and their contribution towards antigen recognition: a computational analysis. Pharm Res. 2010;27:1512–29. doi: 10.1007/s11095-010-0143-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pepinsky RB, Silvian L, Berkowitz SA, Farrington G, Lugovskoy A, Walus L, Eldredge J, Capili A, Mi S, Graff C, et al. Improving the solubility of anti-LINGO-1 monoclonal antibody Li33 by isotype switching and targeted mutagenesis. Protein Sci. 2010;19:954–66. doi: 10.1002/pro.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saluja A, Fesinmeyer RM, Hogan S, Brems DN, Gokarn YR. Diffusion and sedimentation interaction parameters for measuring the second virial coefficient and their utility as predictors of protein aggregation. Biophys J. 2010;99:2657–65. doi: 10.1016/j.bpj.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sule SV, Dickinson CD, Lu J, Chow C-K, Tessier PM. Rapid analysis of antibody self-association in complex mixtures using immunogold conjugates. Mol Pharm. 2013;10:1322–31. doi: 10.1021/mp300524x. [DOI] [PubMed] [Google Scholar]
- 39.Sule SV, Sukumar M, Weiss WF, 4th, Marcelino-Cruz AM, Sample T, Tessier PM. High-throughput analysis of concentration-dependent antibody self-association. Biophys J. 2011;101:1749–57. doi: 10.1016/j.bpj.2011.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tessier PM, Jinkoji J, Cheng Y-C, Prentice JL, Lenhoff AM. Self-interaction nanoparticle spectroscopy: a nanoparticle-based protein interaction assay. J Am Chem Soc. 2008;130:3106–12. doi: 10.1021/ja077624q. [DOI] [PubMed] [Google Scholar]
- 41.Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, Teoh LA, Fischkoff SA, Chartash EK. Adalimumab, a fully human anti-tumor necrosis factor α monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48:35–45. doi: 10.1002/art.10697. [DOI] [PubMed] [Google Scholar]
- 42.Fawcett T. An introduction to ROC analysis. Pattern Recognit Lett. 2006;27:861–74. doi: 10.1016/j.patrec.2005.10.010. [DOI] [Google Scholar]
- 43.Rohani MM, Zydney AL. Role of electrostatic interactions during protein ultrafiltration. Adv Colloid Interface Sci. 2010;160:40–8. doi: 10.1016/j.cis.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 44.Tolcher AW, Sarantopoulos J, Patnaik A, Papadopoulos K, Lin C-C, Rodon J, Murphy B, Roth B, McCaffery I, Gorski KS, et al. Phase I, pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin-like growth factor receptor 1. J Clin Oncol. 2009;27:5800–7. doi: 10.1200/JCO.2009.23.6745. [DOI] [PubMed] [Google Scholar]
- 45.NCT01843374—Randomized, Double-blind Study Comparing Tremelimumab to Placebo in Subjects With Unresectable Malignant Mesothelioma [Internet]. Available from: http://clinicaltrials.gov/show/NCT01843374
- 46.NCT00747734—A Study of MNRP1685A in Patients With Locally Advanced or Metastatic Solid Tumors [Internet]. Available from: http://clinicaltrials.gov/ct2/show/NCT00747734