Abstract
We sought to address inconsistencies in the literature on amplitude of P3 brain potential response in offenders diagnosed with psychopathy. These inconsistencies contrast with the reliable finding of reduced P3 in relation to externalizing tendencies, which overlap with impulsive-antisocial features of psychopathy, as distinguished from the affective-interpersonal features. Employing a sample of incarcerated male offenders (N=154) who completed Hare’s (2003) Psychopathy Checklist-Revised along with a three-stimulus visual oddball task, we tested the hypothesis that impulsive-antisocial features of psychopathy would selectively exhibit an inverse relationship with P3 amplitude. Clear support for this hypothesis was obtained. Our findings clarify the discrepant findings regarding psychopathy and P3, and establish P3 as a neurophysiological point of contact between psychopathy and externalizing proneness from the broader psychopathology literature.
Keywords: psychopathy, externalizing, antisocial behavior, P3, EEG/ERP
Theoretical perspectives on antisocial behavior (e.g., Blair, 2005; Ishikawa & Raine, 2003; Kiehl, 2006) have emphasized abnormalities in frontal brain systems governing inhibitory control functions, and deviations in cortical response—including reduced amplitude of the P3 brain potential response—have consistently been reported in individuals with antisocial-externalizing conditions (Gao & Raine, 2009). However, findings from studies of P3 response in psychopathy have been mixed (Gao & Raine, 2009), with some investigative groups reporting negative associations (Kiehl, Bates, Laurens, Hare, & Liddle, 2006; Kiehl, Hare, Liddle, & McDonald, 1999; Kiehl, Smith, Hare, & Liddle, 2000), and others reporting null (Jutai, Hare, & Connolly, 1987) or in some cases positive associations (Raine & Venables, 1988). The current study sought to clarify the basis of these inconsistencies by considering psychopathy as a heterogeneous condition reflecting the confluence of multiple trait dispositions (cf. Skeem, Polaschek, Patrick, & Lilienfeld, 2011) as opposed to a unitary diagnostic entity. More specifically, the current study addressed notable gaps in the existing literature (Gao & Raine, 2009) by evaluating relations of distinguishable symptomatic components of psychopathy with P3 brain response amplitude in a visual oddball task that included incidental novel as well as task-relevant target stimuli. In particular, the current study sought to link the literature on psychopathy and P3 response with extensive existing work demonstrating reduced P3 amplitude in relation to disinhibitory problems and traits (cf. Iacono, Malone, McGue, 2003; Patrick et al., 2006, 2013).
Psychopathy and P3 Brain Potential Amplitude
Several studies have investigated relations between psychopathy and amplitude of the P3 (or P300)1 event-related potential (ERP) response by comparing groups of offenders classified as psychopathic or nonpsychopathic according to overall scores on Hare’s (2003) Psychopathy Checklist-Revised (PCL-R; for reviews, see Gao & Raine, 2009; Patrick, Venables, & Skeem 2012). Kiehl et al. (1999) reported that high psychopathic offenders exhibited smaller target P3 amplitude than nonpsychopaths over central and parietal recording sites during a classic visual P3 oddball task. In a follow-up study, Kiehl Smith, Hare, and Liddle (2000) examined psychopathy/P3 associations in the context of a visual Go/No-Go task (involving equally probable events, in contrast with the oddball paradigm) and reported smaller amplitude of P3 response to “Go” as compared to “No-Go” stimuli over anterior scalp sites in psychopathic (high PCL-R) offenders, whereas nonpsychopathic offenders exhibited the reverse pattern (i.e., No-Go < Go). More recently, Kiehl et al. (2006) examined P3 responses in high versus low PCL-R offenders using an auditory oddball task that included task-irrelevant novel stimuli along with task-relevant target-stimuli. Comparisons were reported for two separate high/low psychopathy samples, with psychopathic offenders exhibiting reduced P3 response to novel stimuli over lateral and midline scalp sites in both samples, and reduced P3 to target stimuli as well as novel stimuli over midline sites in one sample but not the other.
However, as previously noted, results from other studies of P3 response in PCL-R defined psychopathic offenders have been mixed. For example, Jutai et al. (1987) did not find reliable differences in P3 amplitude between psychopathic and nonpsychopathic offenders in an oddball task involving identification of speech sounds. Raine & Venables (1988) compared psychopathic and nonpsychopathic offenders (classified according to a median split of overall PCL-R scores) for amplitude of P3 response to target stimuli in a continuous visual-motor performance task. In contrast with findings from the above-noted studies conducted subsequently by Kiehl and colleagues (1999, 2000, 2006), these authors found evidence for enhanced P3 over parietal recording in psychopathic as compared to nonpsychopathic offenders.
An important point to consider in interpreting these findings is that studies on P3 response and psychopathy in offender samples to date have relied exclusively on overall psychopathy scores in analyses. There is accumulating evidence that psychopathy represents a configuration of separable trait dispositions as opposed to a unitary diagnostic syndrome (cf. Patrick & Bernat, 2009; Skeem et al., 2011). To explore whether distinct symptomatic facets of psychopathy might relate differentially to P3 response, Carlson, Thái, and McLaron (2009) examined P3 amplitude in relation to facets of psychopathy indexed by the self-report based Psychopathic Personality Inventory (PPI; Lilienfeld & Andrews, 1996) in an undergraduate participant sample. These authors reported a negative association between amplitude of target P3 over frontal recording sites in a visual oddball task and scores on the Self-Centered Impulsivity (or Impulsive Antisociality; Benning, Patrick, Salekin, & Leistico, 2005) factor of the PPI. By contrast, scores on the Fearless Dominance factor of the PPI (reflecting affective resilience and interpersonal dominance) were unrelated to P3 amplitude. However, while informative regarding the possibility that separable components of psychopathy may relate differentially to P3 response, the Carlson et al. (2009) study focused on an unselected, predominantly female student sample that would be expected to exhibit a limited range of psychopathic tendencies in comparison to incarcerated offenders.
Distinguishable Facets of PCL-R Psychopathy
Hare’s (2003) PCL-R has been the most widely used instrument for assessing psychopathy in research studies conducted in correctional and forensic settings. The PCL-R indexes psychopathy through 20 items scored on the basis of a diagnostic interview and review of institutional file records. Recent factor analytic work on the structure of the PCL-R item set has yielded evidence of a hierarchical structure in which two broad factors can be further parsed into four lower-order facets (Hare, 2003; Hare & Neumann, 2006). PCL-R Factor 1 reflects the core affective-interpersonal features of psychopathy whereas PCL-R Factor 2 reflects impulsive-antisocial tendencies.
The Two-Process theory of psychopathy (Patrick & Bernat, 2009; see also Fowles & Dindo, 2009) posits separate neurobiologically-based mechanisms contribute to differing features of the disorder: 1) a weakness in defensive motivational reactivity to aversive (i.e., threat or distress) cues, related more so to the core-affective interpersonal-features, and 2) impairments in anterior-executive systems of the brain, related more to the impulsive-antisocial (externalizing) features. In support of the view that psychopathy is non-unitary and that separable dimensions underlie the construct, PCL-R Factor 1 and Factor 2 exhibit notable divergent relations with external criterion variables of various types. While PCL-R Factor 1 appears to be associated with adaptive tendencies of some kinds (e.g., social efficacy; reduced susceptibility to internalizing problems) as well as with deviant proclivities (e.g., narcissism, danger-seeking, low empathy; Benning et al., 2005), Factor 2 is associated more predominantly with maladaptive tendencies including disinhibitory proneness (Venables & Patrick, 2012), anger and hostility (Hicks & Patrick, 2006), suicidality (Verona, Patrick, & Joiner, 2001), persistent criminal deviance (Hare, 2003; Harpur et al., 1989; Verona et al., 2001), and substance-related problems (Taylor & Lang, 2006).
Externalizing Proneness: Associations with P3 Response Amplitude and Psychopathy
Whereas associations between psychopathy and P3 amplitude in offender samples have been mixed, studies with community samples have consistently reported reduced amplitude of P3 response in individuals broadly characterized as exhibiting disinhibitory problems and traits (i.e., externalizing proneness). Iacono and colleagues (2003) hypothesized that P3 amplitude reduction indexes the genetically transmitted vulnerability toward a spectrum of disinhibitory expressions encompassing impulsive-irresponsible behavior, aggression, antisocial deviance, and substance abuse/dependence. Overlap among problems of this type is thought to reflect a highly heritable trait vulnerability (Iacono et al., 2003; see also Krueger, Hicks, Patrick, Carlson, Iacono, & McGue, 2002), which appears to be reliably indexed by reduced amplitude of P3 brain potential response (Iacono et al., 2003). As direct evidence for this, Patrick, Bernat, Malone, Iacono, Krueger, & McGue (2006) reported that variance in common among antisocial and substance use disorders accounted for findings of reduced P3 response in relation to individual disorders of these types, and Hicks et al. (2007) demonstrated the relationship between P3 and externalizing proneness to be attributable mainly to genetic influence. Follow-up work with twins (Yancey, Venables, Hicks & Patrick, 2013) has replicated the findings of a robust association between externalizing proneness and P3 and a genetic basis to this association using a brief self-report index of disinhibitory tendencies consisting of items from a comprehensive inventory of externalizing tendencies (Krueger, Markon, Patrick, Benning, & Kramer, 2007).
Additionally, other work has presented evidence that P3 amplitude deficits in high externalizing individuals reflect dysfunction in anterior brain regions (Nelson, Patrick, & Bernat, 2011). Findings along these lines dovetail with theory and research pointing to deficits in frontal (‘executive’) systems in antisocial-externalizing individuals (Blair, 2005; Ishikawa & Raine, 2003; Kiehl, 2006; Morgan & Lilienfeld, 2000). In turn, this work suggests that reductions in P3 response among individuals high in impulsive-antisocial features of psychopathy might occur with greater robustness over more anterior as compared to posterior recording sites (cf. Carlson et al., 2011).
A notable facet of externalizing proneness in theoretical accounts and measurement operationalizations entails boredom susceptibility and need for stimulation (cf. Depue & Collins, 1999; Krueger et al., 2007). However, no study to date has evaluated whether reduced P3 amplitude in high externalizing individuals might be attributable to fatigue or reduced task engagement over the course of experimental testing. Given that externalizing proneness (operationalized as the variance in common among antisocial behavior, substance abuse, and disinhibitory personality traits; cf. Krueger et al., 2002) has been shown to overlap substantially with Factor 2 of the PCL-R (Patrick, Hicks, Krueger, & Lang 2005), we hypothesized that P3 amplitude reductions in criminal psychopathy would be specifically related to the impulsive-antisocial (Factor 2) features of PCL-R psychopathy and evaluated whether this effect might reflect greater-than-expected diminishment of phasic brain response across trials of testing.
Current Study Aims and Hypotheses
With the foregoing background in mind, the current study was conducted to clarify the basis of mixed findings in the existing literature on psychopathy and P3 brain response amplitude. Our study is the first to test for differential relations of separable diagnostic facets of PCL-R psychopathy with P3 amplitude in an offender sample. Specifically, the affective-interpersonal and impulsive-antisocial features of psychopathy were assessed using the PCL-R in a sample consisting of incarcerated prisoners, and their relative contributions to prediction of P3 responses in a three-stimulus visual oddball task were evaluated. By testing for differential relations of distinct facets of psychopathy with P3 amplitude, we sought to connect the psychopathy/P3 literature to considerable data from the general psychopathology literature indicating that P3 amplitude reduction indexes a heritable propensity toward impulsive-antisocial behavior (Hicks et al., 2007; Iacono et al., 2003; Krueger et al., 2002; Yancey et al., 2013). In line with suggestions and notable gaps in the literature highlighted by Gao and Raine (2009), we hypothesized (1) that reduced amplitude of P3 response to target and novel task stimuli would be related selectively to impulsive-antisocial (externalizing) features of psychopathy, while unrelated to affective-interpersonal features, and (2) that P3 reductions would be maximal at anterior scalp recording sites. Hypotheses were based on scalp-topography effects reported in studies of P3 and externalizing proneness in non-incarcerated samples (Carlson et al., 2009; Nelson et al., 2011) and data indicating frontal brain dysfunction in impulsive-antisocial individuals (Davidson, Putnam, & Larson, 2000; Gao & Raine, 2009; Morgan & Lilienfeld, 2000). In addition, we evaluated the possibility that reduced P3 amplitude in high impulsive-antisocial offenders may reflect greater-than-expected diminishment of brain response over the course of testing—perhaps due to progressive disengagement from the task—by evaluating effects for P3 across the first versus second halves of the experimental procedure.
Method
Participants
Study participants were 184 male prisoners from a medium-security state correctional facility in Minnesota who met the following inclusionary criteria: no current major mental disorder (i.e., schizophrenia, Bipolar I) as determined from questions pertaining to mental health history on a brief pre-test questionnaire and information contained in prison file records; competency in English; lack of visual or hearing impairments; and non-imminent release date. Thirty participants were excluded from analyses due to excessive artifact in the EEG recordings (n = 24), discontinuation of participation (n = 1), or technical problems with the EEG collection (n = 5), resulting in 154 participants for the reported analyses. The mean age of participants was 32.1 years (SD = 7.8, range = 21–54). The racial composition was as follows: Caucasian, 61.9%; African American, 12.9%; Hispanic, 7.1%; Native American, 3.2%; Asian, 1.9%; mixed race, 4.5%; other, 5.8%. All participants provided informed written consent prior to study participation and received a payment of $30 deposited into their institutional account. Procedures for the study were reviewed and approved by the Institutional Review Board of the University of Minnesota and the Research Review Committee of the Minnesota Department of Corrections.
Measurement of Psychopathy
The Psychopathy Checklist Revised (PCL-R; Hare, 2003) was developed to assess criminal psychopathy in forensic settings. Its 20 items are scored on the basis of data from a semi-structured interview in conjunction with information from collateral sources (i.e., institutional file records). Current analyses focused on scores for the two broad factors of the PCL-R (Factor 1 = affective-interpersonal features; Factor 2 = impulsive-antisocial features), and scores on lower-order facets comprising subsets of items from Factor 1 (Interpersonal = items reflecting social guile and manipulativeness; Affective = items reflecting callous-unemotionality) and Factor 2 (Impulsive-Irresponsible Lifestyle = items reflecting impulsive-irresponsible tendencies; Antisocial Behavior = items reflecting delinquent and aggressive behavior). Interrater reliabilities for PCL-R scores in the subset of study participants (49%) evaluated by independent diagnosticians were as follows: total scores, .88; Factor 1, .81; Factor 2, .85; Interpersonal facet, .80; Affective facet, .75; Impulsive-Irresponsible facet, .74; Antisocial facet, .82.
Experimental Task Stimuli
The current study employed a 3-stimulus oddball paradigm consisting of a modified version of the ‘rotated-heads’ visual oddball task designed by Begleiter, Porjesz, Bihari, and Kissin (1984), in which photographic pictorial stimuli were included as the third (novel) stimulus category (see Venables, Patrick, Hall, & Bernat, 2011 for more detail). Simple oval shapes served as the frequent non-target (standard) stimuli, which appeared 70% of the time (i.e., on 168 of the 240 task trials). The target stimuli, which called for participants to respond, were schematic heads, each consisting of the same oval shape accompanied by a stylized nose and ear (15% of all stimuli presented [36 trials]). For each target trial, the task was to press the left or right button on a button-box, with either the left or right hand respectively, to indicate whether the ear was on the left or right side of the head. The novel stimuli appeared on 15% of task trials (n = 36) and consisted of pleasant, neutral, and unpleasant pictures from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2008).2,3 IAPS pictures were used as novel stimuli to permit examination of the novelty-P3 response elicited by emotional and non-emotional cues, which are likely to be more representative of meaningful unexpected stimuli encountered in real-life environments, relative to abstract geometric shapes or auditory tones. Participants were required to achieve 85% accuracy on the task during a practice session (including only target and standard stimuli) before commencing the test procedure.
Stimulus Delivery and Recording Procedure
During the experiment, participants viewed the stimuli on a 21” computer monitor while seated in a comfortable recliner, and made responses using a serial response box. The monitor was situated 1 m from participants’ eyes, and the response box was positioned on their laps. All task stimuli (ovals, target heads, and novel pictures) were displayed within a rectangular frame, filled with dark gray, which appeared against a black background. Stimuli were presented for 100ms each, with a variable intertrial interval between 4 and 5 s, resulting in an average time of approximately 18 minutes to complete the task. Picture presentation was counterbalanced across participants using 12 different stimulus orders, in which the presentation of oval, target head, and novel stimuli was randomized with constraints (i.e., no more than four oval stimuli appeared consecutively, no two target types appeared consecutively, and no two novels appeared consecutively). A second computer coordinated data acquisition with Neuroscan amplifiers and software (Neuroscan, Inc.).
Physiological Measurement and Data Reduction
EEG activity was recorded from 53 scalp sites (AF3, AF4, AFz, C1, C2, C3, C4, C5, C6, CP1, CP2, CP3, CP4, CPz, Cz, F1, F2, F3, F4, F5, F6, F7, F8, FC1, FC2, FC3, FC4, FCz, FP1, FP2, FPz, FT7, FT8, Fz, O1, O2, Oz, P1, P2, P3, P4, P5, P6, P7, P8, PO3, PO4, POz, Pz, T7, T8, TP7, TP8) using Neuroscan Quik-Caps with sintered Ag-AgCl electrodes. Electrodes were positioned above and below the left eye to monitor vertical electrooculogram (VEOG) activity. All electrode impedances were kept below 10K Ohms. EEG signals were digitized on-line at 1000 Hz during data collection with an analog band pass filter of .05–200 Hz. Data were referenced on-line to electrode site Cz during data collection, and re-referenced off-line to the average of left and right mastoid electrodes for purposes of processing and analysis. Data epochs from −1000 ms to 2000 ms were extracted from the continuous EEG recordings using Neuroscan EDIT software (version 4.3, Neuroscan Inc.), and corrected for eye movements using the algorithm developed by Semlitsch, Anderer, Schuster, and Presslich (1986), as implemented within the EDIT software.
The epoched and eye-blink corrected EEG data were then imported to Matlab (Mathworks, Inc.) for subsequent data processing, including downsampling to 128 Hz using the Matlab resample command, which applies a low pass anti-aliasing filter before downsampling. Trials in which activity exceeded a range of +/− 75 μV, relative to a 500 ms baseline, were excluded from further processing. Trial epochs were then averaged within the two main stimulus conditions of interest (target heads, novel pictures). To evaluate the time-course of P3 effects, data were averaged separately (within stimulus conditions) for trials occurring during the first and second halves of the experimental task. Using the grand average across participants to guide window selection, target P3 amplitude and the shorter latency P3 for novel picture stimuli were defined, respectively, as the maximum peak values occurring between 250–602 and 250–555 ms.
Data Analyses
Statistical analyses focused on brain response at nine electrode sites (F3, Fz, F4, C3, Cz, C4, P3, Pz, P4; cf. Carlson et al., 2011), with data collapsed across lateral and mid-line sites to simplify reporting of frontality effects of major interest. Each analysis included P3 amplitude scores, square-root transformed to reduce positive skewness, as the dependent measure, and Stimulus Type (target, novel), Frontality (frontal, central, posterior), and Block (first, second half) as discrete within-subjects variables. Effects for the three-level Frontality variable were decomposed through use of planned orthogonal contrasts (frontal/central vs. parietal, and frontal vs. central) that highlighted the comparison of more anterior scalp sites with posterior sites.
Following an initial three-way repeated measures ANOVA evaluating effects for the three within-subjects variables across participants as a whole, mixed general linear models, analogous to multivariate regression analyses, were conducted in which continuous psychopathy scores were included as between-subjects variables along with the discrete within-subjects variables and their interactions as predictors of P3 response amplitude. One analysis included scores on the two PCL-R factors (Factor 1, affective-interpersonal; Factor 2, impulsive-antisocial) as between-subject variables to evaluate their distinctive contributions to prediction of P3 amplitude (i.e., contribution of Factor 2 while controlling for scores on Factor 1, and vice-versa). Follow-up analyses utilized scores on one or the other facet of PCL-R Factor 2 (Impulsive-Irresponsible, Antisocial) along with scores on Factor 1 as between-subjects factors in order to further clarify the source of the predicted Factor 2/P3 amplitude association (controlling for Factor 1). In cases where effects for PCL-R score variables on P3 response emerged as significant, depictions of high versus low quartile groups are presented to illustrate the nature of the effects.
Tests of omnibus effects involving the 3-level Frontality variable were Greenhouse-Geisser adjusted, with reported p values reflecting the adjusted statistical values. Partial eta square (ηp2) values are reported as an index of effect size.
Results
Overall Sample Effects
Results for the initial three-way repeated measures ANOVA focusing on effects for within-subject condition factors in the overall study sample were largely consistent with those from prior work using the same experimental task in non-incarcerated participants (Venables et al., 2011), and in turn, with the broad literature on P3 (Polich, 2007).4 The one notable exception was a Stimulus Type x Block interaction, F(1,153) = 145.7, p < .001, ηp2 = .49, indicating greater amplitude of P3 response on average during the second half of the task versus the first for novel stimuli specifically (t = −4.9, p < .001). By contrast, target stimuli exhibited an expected pattern of response habituation, with P3 amplitude smaller in the second half as compared to the first (t = 9.02, p < .001).
Associations of Psychopathy Factors with P3 Response Amplitude
The initial ANOVA, in which continuous scores on PCL-R Factors 1 and 2 were entered as predictors of P3 amplitude, did not reveal a main effect of Factor 1, F(1,151) < 1, or Factor 2, F(1,151) = 1.54, p > .21. However, a significant Factor 2 x Block interaction was evident, F(1,151) = 8.26, p = .005, ηp2 = .052—reflecting a greater negative relationship between Factor 2 and P3 amplitude in the second half of the task (zero-order r = −.15, partial r controlling for Factor 1 = −.17) as compared to the first half (zero-order and partial rs = −.02 and −.02, respectively). In addition, and consistent with hypotheses, a trend level Factor 2 x Frontality interaction was evident, F(1,151) = 3.36, p = .059, ηp2 = .022, reflecting a more robust negative association between Factor 2 and P3 amplitude at frontal sites (zero-order/partial rs = −.15/−.16) compared to central and parietal sites (rs = −.09/−.09 and .01/−.01).5 No corresponding effects were evident for Factor 1 (all ps > .10).
Associations of PCL-R Impulsive-Irresponsible and Antisocial Facets with P3 Response Amplitude
To further clarify the basis of the observed relationship between PCL-R Factor 2 scores and P3 amplitude, we undertook additional analyses evaluating the contributions of distinguishable Impulsive-Irresponsible and Antisocial Behavior facets of Factor 2 (cf. Hare, 2003; Hare & Neumann, 2006) to this relationship. Two separate repeated measures ANOVAs were performed in which scores on one or the other of these facets were included along with scores on PCL-R Factor 1 as between-subjects factors, and Stimulus Type (novel, target), Frontality (frontal, central, parietal), and Block (first, second half) were included as within-subjects factors. The analysis for Antisocial facet scores revealed a trend-level main effect for this facet in predicting P3 amplitude, F(1,151) = 3.7, p = .056, ηp2 = .024, along with a significant Antisocial facet x Frontality interaction, contrast F(1,151) = 3.98, p = .048, ηp2 = .026, reflecting more pronounced reduction in P3 amplitude as a function of higher Antisocial scores at anterior (frontal/central; zero-order r and partial r controlling for Factor 1 = −.18 and −.19, ps = .025/.021) relative to posterior (parietal) scalp sites (rs = −.04 and −.05, respectively, ps = .58 and .56. Figure 1 illustrates this finding in terms of waveform plots for novel and target stimuli at midline recording sites (Fz, Cz, Pz); it can be seen that participants scoring high as compared to low on the PCL-R Antisocial facet (i.e., upper- versus lower-quartile) exhibited reduced amplitude of P3 response for both novel and target stimuli, particularly at combined frontal/central recording sites. Thus, the hypothesis that Factor 2 related reductions in P3 amplitude would be especially evident at anterior recording sites was borne out for the Antisocial facet of Factor 2 in particular, reflecting the aggressive, criminal deviance features of PCL-R psychopathy.
Figure 1.
Average ERP waveforms for novel picture stimuli (left plots) and target ‘head’ stimuli (right plots) at electrode sites Fz, Cz, and Pz (upper, middle, and lower plots, respectively). High and Low PCL-R Antisocial groups consist of participants within the study sample falling within the upper and lower quartiles, respectively, of scores on the Antisocial Behavior facet of the Psychopathy Checklist-Revised (Hare, 2003).
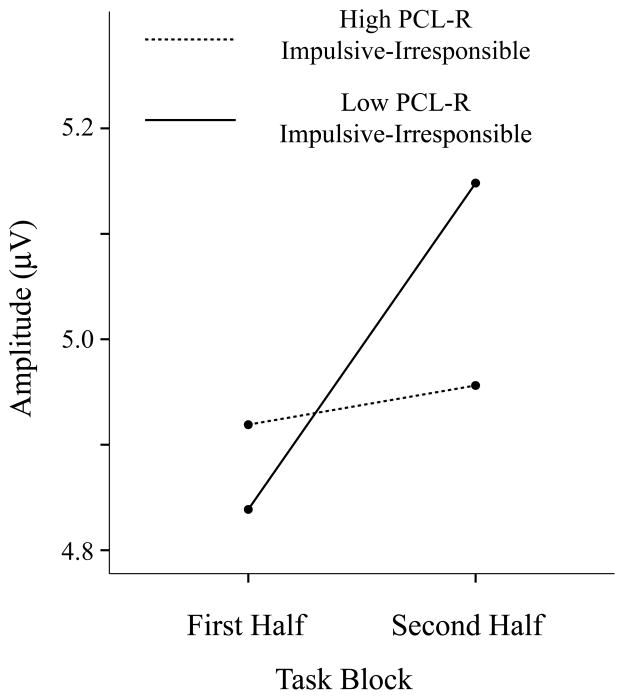
An interesting and complementary pattern of results emerged from the corresponding ANOVA for the Impulsive-Irresponsible facet of the PCL-R. While no main effect on P3 was evident for scores on this facet, F(1,151) < 1, p > .92, a significant Impulsive-Irresponsible x Stimulus Type x Block interaction was found, F(1,151) = 4.78, p = .03, ηp2 = .031. Decomposition by stimulus type revealed a significant Impulsive-Irresponsible x Block interaction for novel picture stimuli specifically, F(1,151) = 11.92, p < .001, ηp2 = .073 (F for targets < 1). Regarding the basis of this interaction, zero-order and partial (controlling for Factor 1) correlations computed separately for the two blocks revealed a shift in the direction of relationship between scores on this psychopathy facet and P3 response to novel stimuli from Block 1 to Block 2, rs = .12/.11 and −.06/−.09, respectively (Steiger’s Z for the difference of zero-order rs = −2.06, p = .04, and for partial rs = −2.28, p = .02). As an illustration of this shift, Figure 2 depicts mean amplitude of P3 response to novel stimuli across first and second halves of the task for groups scoring high versus low (upper vs. lower quartiles) on the Impulsive-Irresponsible facet of the PCL-R. It can be seen that high impulsive-irresponsible participants showed markedly less amplification of P3 response to novel stimuli across blocks compared to low impulsive-irresponsible participants.6 Taken together, these results indicate a moderating effect of high levels of this psychopathy facet on brain reactivity to incidental novel stimuli over the course of repeated occurrences.
Figure 2.
Mean novel P3 amplitude elicited during the first and second half of the task. High and Low PCL-R Impulsive-Irresponsible groups consist of participants within the study sample falling within the upper and lower quartiles, respectively, of scores on the Impulsive-Irresponsible Lifestyle facet of the Psychopathy Checklist-Revised (Hare, 2003).
Discussion
In contrast with consistent findings of reduced amplitude of oddball P3 response in antisocial-externalizing conditions (e.g., Iacono et al., 2003; Patrick et al., 2006; 2013), studies examining relations between psychopathy and P3 response have yielded more mixed results. Working from conclusions advanced by Gao and Raine (2009), and evidence for contrasting correlates of psychopathy subcomponents with a range of criterion variables (including externalizing proneness; Patrick et al., 2005), we hypothesized that scores on the impulsive-antisocial and affective-interpersonal factors of PCL-R psychopathy would show differential relations with P3 response. The current study tested this hypothesis, for the first time, in a sample of male incarcerated offenders.
In line with prediction, a reduction in P3 amplitude was evident in the current study as a function of higher scores on the impulsive-antisocial (Factor 2) component of the PCL-R. By contrast, P3 amplitude was unrelated to scores on the affective-interpersonal (Factor 1) component. In general, this effect occurred more strongly during the second half of task trials as compared to the first and over anterior (fronto-central) recording sites as compared to posterior (parietal) sites. This latter result is consistent with theoretical perspectives that have emphasized the role of abnormalities in frontal brain systems governing cognitive and affective regulatory processes in aggressive antisocial behavior (e.g., Blair, 2005; Davidson, Putnam, & Larson, 2000; Ishikawa & Raine, 2003; Kiehl, 2006), previous studies documenting anterior P3 reductions in relation to externalizing tendencies (Nelson et al., 2011), and psychopathy in community participants (Carlson et al., 2011).
Indeed, we found evidence that high scores on the Antisocial Behavior facet of PCL-R Factor 2 in particular were predictive of reduced P3 amplitude over anterior recording sites. By contrast, the Impulsive-Irresponsible facet of Factor 2, which reflects boredom proneness, lack of planfulness, and unreliability, contributed more to the finding that the Factor 2/P3 relationship (for novel stimuli in particular) was stronger in the second half of the task than the first. This is notable given that amplitude of P3 response to novel stimuli in the sample as a whole was greater during the second half of the task as compared to the first. The novel stimuli used in the present study consisted of differing IAPS pictures presented without repetition, such that each individual picture stimulus occurred as a distinct novel event. This contrasts with many prior studies of the novelty P3 response, in which rare non-target stimuli of one only type occurs repeatedly throughout the task (e.g., a single geometric shape, or auditory tone of a specific frequency; Friedman, Cycowicz, & Gaeta, 2001). This unique aspect of the current procedure may account for the observed increase in amplitude of the novelty P3 during the second as compared to the first half of the task for participants as a whole (except for those with high scores on Factor 2 of the PCL-R).
Implications for Understanding of Brain Processes in Psychopathy
Results from the present study indicate that P3 amplitude reductions in criminal psychopathy are selectively related to impulsive-antisocial tendencies and largely unrelated to affective-interpersonal features. This core finding is consistent with the two-process model of psychopathy (Fowles & Dindo, 2009; Patrick & Bernat, 2009), which posits that impairments in neural indicators of cognitive function should be related to the features of psychopathy that overlap most with externalizing tendencies. In turn, the current work suggests that mixed results from prior studies of P3 amplitude in psychopathy (Gao & Raine, 2009) are attributable, at least in part, to contrasting relations for differing symptomatic facets. In line with the current results, a study by Carlson et al. (2009) found P3 amplitude in young non-offender (student) participants to be reduced as a function of scores on the impulsive-antisocial factor of the self-report based PPI, but unrelated to scores on the PPI’s affective-interpersonal (Fearless Dominance) factor. However, the current study was the first to examine P3 amplitude in relation to distinct components of psychopathy as measured by the PCL-R in a correctional sample comparable in age to offender groups examined in prior published work (e.g., Jutai et al., 1987, Kiehl et al., 1999; 2000; 2006; Raine & Venables, 1988).
In addition to the finding of an inverse relationship between scores on PCL-R Factor 2 as a whole and P3 amplitude across task stimuli, interaction effects were evident, indicating greater reduction of P3 at anterior as compared to posterior recording sites, and a more pronounced reduction in the second half of the task as compared to the first. Follow-up analyses sought to clarify the basis of these interactions by examining effects for separable facets of Factor 2. One interesting result emerging from analyses in which the Impulsive-Irresponsible and Antisocial Behavior facets of Factor 2 were examined separately as predictors of P3 response was that the Antisocial facet accounted for the general reduction in P3 response to target and novel stimuli across task trials for, over anterior scalp sites in particular. This result suggests that, within the current offender sample, a history of antisocial behaviors reflecting criminality and violence/aggression was predictive of anterior brain deficits as reflected by reduced P3 amplitude. In turn, this finding is consistent with theory and research pointing to disruption of frontal brain circuitry as a basis for impairments in affective and behavioral control in antisocial-aggressive individuals (e.g., Blair, 2005; Davidson et al., 2000; Ishikawa & Raine, 2003; Kiehl, 2006).
A second interesting effect to emerge from these follow-up analyses was the finding of a moderating effect of the Impulsive-Irresponsible facet of the PCL-R on amplitude of P3 amplitude to novel picture stimuli specifically across trial blocks: Whereas the sample as a whole exhibited an increase in P3 response to novel stimuli from the first task block to the second, participants scoring high on this PCL-R facet exhibited no such increase, instead showing a significant decrease in response from Block 1 to Block 2. This result indicates that it was the Impulsive-Irresponsible facet in particular that accounted for the finding of a stronger negative relationship between PCL-R Factor 2 and P3 amplitude in the second half of the task as compared to the first.
What might account for the differing observed relations of the antisocial-aggressive and impulsive-irresponsible facets of psychopathy with P3 amplitude as a function of recording sites (anterior versus posterior) and task blocks (second versus first)? Theoretical accounts of P3 amplitude reduction in disinhibitory (externalizing) psychopathology (e.g., Iacono et al., 2003) posit impulsive-irresponsible tendencies common to antisocial and substance use disorders as the source of this effect. However, studies of this kind have relied primarily on community samples exhibiting a more restricted range of impulsive and antisocial tendencies. In correctional samples where disinhibitory tendencies are common and generally more severe than in the population at large, aggressive-antisocial behavior may be indicative of greater proneness to externalizing problems than tendencies towards boredom proneness, impulsivity, and irresponsible behavior. That is, from the standpoint of item response theory (e.g., Muthén, 1996), antisocial tendencies may discriminate at higher levels of an underlying externalizing liability dimension than impulsive-irresponsible tendencies per se, particularly within an offender sample. Consistent with this possibility, prior work has demonstrated reduced amplitude of P3 response in particular for offenders with histories of violent crime as opposed to only nonviolent crime (Bernat, Hall, Steffen, & Patrick, 2007). From this perspective, aggressive-antisocial tendencies reflected in the Antisocial facet of the PCL-R and reduced P3 may index more severe aggressive/violent tendencies with incarcerated offenders more effectively than tendencies associated with the Impulsive-Irresponsible facet. Relevant to this, previous work has shown the Antisocial facet to be positively correlated with assault charges and previous violent offending (Hall et al., 2004) and callous-aggressive forms of externalizing proneness (Venables & Patrick, 2012).
Regarding the observed effect for the Impulsive-Irresponsible facet on novel stimulus P3 response across task blocks, one possibility is that this effect was attributable to greater fatigue and/or decreased effort across task trials for individuals high on this psychopathy facet. However, follow-up analyses showed that this effect was due more so to increased response across blocks for individuals scoring low on this facet than to decreased response for individuals scoring high. Additionally, the fact that this effect was evident for incidental novel stimuli but not task-relevant target stimuli indicates that high impulsive-irresponsible individuals processed targets similarly across halves of the task. An alternative possibility is that this block-related effect reflected a processing difference of more direct functional relevance to disinhibitory problems—for example, reduced prioritization of attention to anomalous stimulus events over the course of repeated occurrences, perhaps related to tendencies toward boredom proneness and need for stimulation/excitement. However, this and other interpretations are necessarily tentative, and it will be important to further evaluate the nature and basis of effects for this facet of psychopathy on P3 response through additional systematic research.
Results from the present study link research on P3 response amplitude in criminal psychopathy to the nomological network of externalizing psychopathology, which posits P3 amplitude reduction to be an indicator of a heritable propensity toward disinhibitory problems and traits. In turn, our effort to connect findings for P3 and psychopathy to the broader literature on externalizing psychopathology is in line with initiatives directed at reconceptualizing mental disorder conditions in neuroscientific terms. In particular, the Research Domain Criteria (RDoC) initiative launched by the National Institute of Mental Health (Insel et al., 2010; Cuthbert & Kozak, 2013) seeks to incorporate concepts and methods of neuroscience into psychopathology classification through a focus on transdiagnostic “process” constructs that can be studied across multiple levels of analysis (i.e., from genetic-cellular through neural circuitry and physiology to observable behavior). Along this line, the current work serves as a link between the impulsive-antisocial component of criminal psychopathy and the construct-network (Patrick et al., 2013) of externalizing proneness (or disinhibition), which includes P3 amplitude as a neurobiological indicator.
Given prior work demonstrating diminished P3 amplitude in individuals diagnosed with depression and schizophrenia (Blackwood, Whalley, Christie, Blackburn, St. Clair, & McInnes, 1987; Ford, 1999), a key question concerns the specificity of P3 amplitude as an indicator of impulsive-externalizing tendencies per se. In the case of depression, Blackwood et al. (1987) reported that P3 normalized in in depressed subjects following treatment, indicating that reduced amplitude of response reflects the acute phase of depressive illness. By contrast, evidence does exist for reduced P3 as an indicator of liability for schizophrenia (Ford, 1999), but the association in this case is evident more for auditory than visual tasks such as that used in the current study (Jeon & Polich, 2003) and in other work on externalizing problems (Nelson et al., 2011; Patrick et al., 2006; Polich, Pollock, & Bloom, 1994). The implication is that the mechanisms underlying P300 reduction in schizophrenia and externalizing psychopathology are not identical. Further research will be needed to clarify the distinctiveness versus overlap in effects for these two forms of psychopathology.
Limitations and Future Directions
Some limitations of the present study are noteworthy and highlight important directions for future research. One limitation concerns the participant sample, consisting of incarcerated male offenders, which may limit the generalizability of the current findings. Notably, the previously mentioned study by Carlson and colleagues (2009) reported parallel results for younger female student participants assessed for facets of psychopathy using an alternative self-report based inventory (the PPI)—suggesting that current study effects may well extend to other samples and measures. Nonetheless, further research is needed evaluate whether impulsive-antisocial features of psychopathy evidence similar relations with P3 brain response in community participants or less severe offender samples (e.g., individuals on probation for comparatively minor offenses).
Another potential limitation concerns the approach used to partition sources of variance in psychopathy scores in the prediction of P3 amplitude. Specifically, analytic models included both Factor 1 and Factor 2 scores as predictors of P3 amplitude. This approach was used because scores on the two factors of the PCL-R (and its lower-order facets) are moderately intercorrelated, such that their overlap can conceal differential relations with physiological indicators and other criterion variables (cf. Hicks & Patrick, 2006). However, some concerns have been raised in the literature about reliance on correlational approaches to control for overlap among predictor variables (Lynam, Hoyle, & Newman, 2006), and thus it will be important in future research (following the example of Carlson et al., 2009) to evaluate differential predictive relations for facets of psychopathy operationalized as more independent of one another (cf. Patrick, Fowles, & Krueger, 2009; Skeem et al., 2011).
A further potential limitation of the current study concerns the reliability of P3 amplitude quantification in analyses utilizing subsets of trials, such as those focusing on effects for the first versus second half of the task, which utilized averages based on only 16–17 trials. While lower-level aggregation could potentially limit the reliability of P3 scores, we were able to detect effects in accordance with study hypotheses. Nonetheless, we encourage future work directed at systematically evaluating the quantity of trials needed to obtain stable indices of P3 response amplitude, particularly in the context of its use as an individual-difference indicator.
An additional limitation of the current study entails its use of P3 brain response as the sole indicator of neurocognitive function. Existing research and theory on the P3 event-related potential suggests that it reflects multiple cognitive processes, including attention and updating of working memory (Polich, 2007), arising from multiple coordinated sources within the brain. Consequently, P3 response lacks specificity with regard to processes or mechanisms that might be deficient in impulsive-antisocial individuals. Additional research will be needed to further elucidate the nature of processing deficits associated with reduced P3 amplitude in impulsive-antisocial individuals—for example, studies employing experimental tasks designed to index specific cognitive processes with clearer brain referents (cf. Carter & Barch, 2007), and incorporating alternative methods for assessing brain activity such as functional neuroimaging. Future work, consistent with the RDoC initiative, could expand upon the construct-network of externalizing proneness by evaluating coherence among performance on cognitive (effortful) control paradigms, P3 amplitude, and assessments (self-report and diagnostic) of impulsive-antisocial traits and problems (cf. Patrick et al., 2013).
Acknowledgments
We are grateful to the Minnesota Department of Corrections and the residents and staff of the Minnesota Correctional Facility at Lino Lakes, in particular chief psychologist Colette Morse, for their support of this work. We also thank Jason Hall, Justin Jobelius, and Elizabeth Reich for their assistance with interviewing and data collection, and Edward Bernat for his assistance with data reduction.
The work was supported by grants MH65137, MH072850, and MH089727 from the National Institute of Mental Health.
Footnotes
We use the term P3 to refer to a family of ERP components (cf. Polich, 2007) including the P3 response to attended target stimuli in a frequent/infrequent “oddball” task (aka “P300,” or “P3b”), and the P3 response to unexpected novel events (aka “novelty P3,” or “P3a”).
The 36 novel pictures, listed by their IAPS identification numbers, were as follows: erotic - 2030, 4320, 4666, 4669, 4672, 4770; action - 5626, 5629, 8034, 8200, 8340, 8490; threat - 1525, 6250, 6300, 6370, 6510, 6930; victim - 6315, 6540, 8485, 9050, 9250, 9600; neutral - 2480, 2870, 2890, 5390, 7004, 7010, 7020, 7090, 7100, 7491, 7595, 9700. Novel picture stimuli were interspersed evenly and randomly among other stimuli (nontarget ovals, target heads) in the task series.
Mean normative valence and arousal ratings (Lang et al., 2008) for each picture content category were as follows: Erotic: valence (mean = 6.89, SD = 1.75); arousal (mean = 6.40, SD = 2.05). Action: valence (mean = 6.95, SD = 1.70); arousal (mean = 6.34, SD = 2.16). Threat: valence (mean = 3.08, SD = 1.79); arousal (mean = 6.37, SD = 2.25). Victim: valence (mean = 2.98, SD = 1.76); arousal (mean = 6.32, SD = 2.08). Neutral: valence (mean = 4.96, SD = 1.12); arousal (mean = 2.64, SD = 1.82).
A detailed description of basic experimental effects can be obtained from the authors upon request
We evaluated history of problematic substance use and head injury as potential mediators of the Factor 2/P3 association. Data for historic symptoms of drug and alcohol dependence (assessed through semi-structured interview, coded as present/absent, summed within drug and alcohol categories, and then aggregated to form a composite substance dependence variable) were available for a subset of 134 participants. Data for history of head injury (assessed via self-report on a medical screening form and coded as present/absent) were available for a subset of 151 participants. We evaluated whether these variables might account for Factor 2 associations with P3 by including scores for each as a covariate, in separate analyses. In each case, the Factor 2 x Block interaction remained significant, Fs(1, 130; 1, 147) = 6.26 and 8.42, respectively, ps = .014 and .004, ηp2s = .046 and .054, with the Frontality x Factor 2 interaction also remaining significant when substance abuse history was controlled for, F(2,130) = 8.41, p = .002, ηp 2 = .061, and trend-level when history of head injury was controlled for, F(2,147) = 2.93, p = .08, ηp 2 = .0. These supplemental analyses indicate that the inverse association between PCL-R Factor 2 and amplitude of P3 response to target and novel stimuli was not mediated by prior substance dependence or a history of head injury.
Results for the groupings in Figure 2 are provided purely for purposes of illustrating, in a simplified manner, the basis of the psychopathy by block interaction effect for continuous Impulsive-Irresponsible facet scores. However, to confirm that differential reactivity across blocks was evident for these groupings as it was for continuous facet scores, we performed tests of Block effects separately for the high versus low groups depicted in the Figure. Whereas low scorers showed an increase in amplitude of P3 response to novel stimuli from the first to the second half of the task, t(48) = 6.3, p <.001, reflecting the pattern observed in the sample as a whole, high scorers showed no such increase, t(50) < 1, p > .44. These results both confirm and clarify the interaction effect observed in the primary (continuous score) analysis.
This manuscript is based on work completed by the first author in partial fulfillment of the requirements for the degree of Master of Science at Florida State University, under the supervision of the second author
References
- Blair RJR. Applying a cognitive neuroscience perspective to the disorder of psychopathy. Development and Psychopathology. 2005;17:865–891. doi: 10.1017/S0954579405050418. [DOI] [PubMed] [Google Scholar]
- Benning SD, Patrick CJ, Salekin RT, Leistico AR. Convergent and discriminant validity of psychopathy factors assessed via self-report: A comparison of three instruments. Assessment. 2005;12:270–289. doi: 10.1177/1073191105277110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Bihari B, Kissin B. Event-related potentials in boys at risk for alcoholism. Science. 1984;225:1493–1496. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- Bernat EM, Hall JR, Steffen BV, Patrick CJ. Violent offending predicts P300 amplitude. International Journal of Psychophysiology. 2007;66:161–167. doi: 10.1016/j.ijpsycho.2007.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwood DH, Whalley LJ, Christie JE, Blackburn IM, St Clair DM, McInnes A. Changes in auditory P3 event-related potential in schizophrenia and depression. British Journal of Psychiatry. 1987;150:154–160. doi: 10.1192/bjp.150.2.154. [DOI] [PubMed] [Google Scholar]
- Carlson SR, Thái S, McLaron ME. Visual P3 amplitude and self-reported psychopathic personality traits: Frontal reduction is associated with self-centered impulsivity. Psychophysiology. 2009;46:100–113. doi: 10.1111/j.1469-8986.2008.00756.x. [DOI] [PubMed] [Google Scholar]
- Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment on cognition in schizophrenia: The CNTRICS initiative. Schizophrenia Bulletin. 2007;33:1131–1137. doi: 10.1093/schbul/sbm081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert NB, Kozak MJ. Constructing constructs for psychopathology: The NIMH research domain criteria. Journal of Abnormal Psychology. 2013;122:928–937. doi: 10.1037/a0034028. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation: a possible prelude to violence. Science. 2000;298:591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behavioral and Brain Sciences. 1999;22(3):491–517. doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- Fowles DC, Dindo L. Temperament and psychopathy: A dual-pathway model. Current Directions in Psychological Science. 2009;18:179–183. [Google Scholar]
- Ford JM. Schizophrenia: the broken P300 and beyond. Psychophysiology. 1999;36:667–682. [PubMed] [Google Scholar]
- Friedman D, Cycowicz YM, Gaeta H. The novelty P3: an event-related brain potential (ERP) sign of the brain’s evaluation of novelty. Neuroscience & Biobehavioral Reviews. 2001;25:355–373. doi: 10.1016/s0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- Gao Y, Raine A. P3 event-related potential impairments in antisocial and psychopathic individuals: A meta-analysis. Biological Psychology. 2009;82:199–210. doi: 10.1016/j.biopsycho.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Hall JR, Benning SD, Patrick CJ. Criterion-related validity of the three-factor model of psychopathy personality, behavior, and adaptive functioning. Assessment. 2004;11:4–16. doi: 10.1177/1073191103261466. [DOI] [PubMed] [Google Scholar]
- Hare RD. The Hare Psychopathy Checklist-Revised. 2. Toronto, Ontario, Canada: Multi-Health Systems; 2003. [Google Scholar]
- Hare RD, Neumann CS. The PCL-R assessment of psychopathy: Development, structural properties, and new directions. In: Patrick CJ, editor. Handbook of psychopathy. New York: The Guilford Press; 2006. pp. 58–88. [Google Scholar]
- Hicks BM, Bernat E, Malone SM, Iacono WG, Patrick CJ, Krueger RF, McGue M. Genes mediate the association between P3 amplitude and externalizing disorders. Psychophysiology. 2007;44:98–105. doi: 10.1111/j.1469-8986.2006.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Patrick CJ. Psychopathy and negative emotionality: Analyses of suppressor effects reveal distinct relations with emotional distress, fearfulness, and anger-hostility. Journal of Abnormal Psychology. 2006;115:276–287. doi: 10.1037/0021-843X.115.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpur TJ, Hare RD, Hakstian AR. Two factor conceptualization of psychopathy. Psychological Assessment. 1989;1:6–17. [Google Scholar]
- Iacono WJ, Malone SM, McGue M. Substance use disorders, externalizing psychopathology, and P300 event-related potential amplitude. International Journal of Psychophysiology. 2003;48:147–178. doi: 10.1016/s0167-8760(03)00052-7. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Wang P. Research domain criteria: Toward a new classification framework for research on mental disorders. The American Journal of Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Ishikawa SS, Raine A. Prefrontal deficits and antisocial behavior: A causal model. In: Lahey BB, Moffitt TE, Caspi A, editors. Causes of conduct disorder and juvenile delinquency. New York: Guilford; 2003. pp. 277–304. [Google Scholar]
- Jeon YW, Polich J. Meta-analysis of P300 and schizophrenia: Patients, paradigms, and practical implications. Psychophysiology. 2003;40:684–701. doi: 10.1111/1469-8986.00070. [DOI] [PubMed] [Google Scholar]
- Jutai JW, Hare RD, Connolly JF. Psychopathy and event-related potentials (ERPs) associated with attention to speech stimuli. Personality and Individual Differences. 1987;8:175–184. [Google Scholar]
- Kiehl KA. A cognitive neuroscience perspective on psychopathy: Evidence for paralimbic system dysfunction. Psychiatry Research. 2006;142:107–128. doi: 10.1016/j.psychres.2005.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl KA, Bates AT, Laurens KR, Hare RD, Liddle PF. Brain potentials implicate temporal lobe abnormalities in criminal psychopaths. Journal of Abnormal Psychology. 2006;115:443–453. doi: 10.1037/0021-843X.115.3.443. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Harem RD, Liddle PF, McDonald JJ. Reduced P300 responses in criminal psychopaths during a visual oddball task. Biological Psychiatry. 1999;45:1498–1507. doi: 10.1016/s0006-3223(98)00193-0. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, Liddle PF. An event-related potential investigation of response inhibition in schizophrenia and psychopathy. Biological Psychiatry. 2000;48:210–221. doi: 10.1016/s0006-3223(00)00834-9. [DOI] [PubMed] [Google Scholar]
- Krueger RF. The structure of common mental disorders. Archives of General Psychiatry. 1999;56:921–926. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson S, Iacono WG, McGue M. Etiological connections among substance dependence, antisocial behavior, and personality: Modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111:411–424. [PubMed] [Google Scholar]
- Krueger RF, Markon KE, Patrick CJ, Benning SD, Kramer MD. Linking antisocial behavior, substance use, and personality: an integrative quantitative model of the adult externalizing spectrum. Journal of abnormal psychology. 2007;116(4):645. doi: 10.1037/0021-843X.116.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-8. University of Florida; Gainesville, FL: 2008. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- Lilienfeld SO, Andrews BP. Development and preliminary validation of a self-report measure of psychopathic personality traits in noncriminal populations. Journal of Personality Assessment. 1996;66:488–524. doi: 10.1207/s15327752jpa6603_3. [DOI] [PubMed] [Google Scholar]
- Lynam DR, Hoyle RH, Newman JP. The perils of partialling: cautionary tales from aggression and psychopathy. Assessment. 2006;13:328–341. doi: 10.1177/1073191106290562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AB, Lilienfeld SO. A meta-analytic review of the relation between antisocial behavior and neuropsychological measures of executive function. Clinical psychology review. 2000;20(1):113–136. doi: 10.1016/s0272-7358(98)00096-8. [DOI] [PubMed] [Google Scholar]
- Muthén BO. Psychometric evaluation of diagnostic criteria: Application to a two-dimensional model of alcohol abuse and dependence. Drug and Alcohol Dependence. 1996;41:101–112. doi: 10.1016/0376-8716(96)01226-4. [DOI] [PubMed] [Google Scholar]
- Nelson LD, Patrick CJ, Bernat EM. Operationalizing proneness to externalizing psychopathology as a multivariate psychophysiological phenotype. Psychophysiology. 2011;48:64–72. doi: 10.1111/j.1469-8986.2010.01047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Bernat EM. Neurobiology of psychopathy: A two-process theory. In: Berntson GG, Cacioppo JT, editors. Handbook of neuroscience for the behavioral sciences. New York: John Wiley & Sons; 2009. pp. 1110–1131. [Google Scholar]
- Patrick CJ, Bernat EM, Malone SM, Iacono WG, Krueger RF, McGue M. P300 amplitude as an indicator of externalizing in adolescent males. Psychophysiology. 2006;43:84–92. doi: 10.1111/j.1469-8986.2006.00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Hicks BM, Krueger RF, Lang AR. Relations between psychopathy facets and exetrnalizing in a criminal offender sample. Journal of Personality Disorders. 2005;19:339–356. doi: 10.1521/pedi.2005.19.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CJ, Fowles DC, Krueger RF. Triarchic conceptualization of psychopathy: Developmental origins of disinhibition, boldness, and meanness. Development and Psychopathology. 2009;21:913–938. doi: 10.1017/S0954579409000492. [DOI] [PubMed] [Google Scholar]
- Patrick CJ, Venables NC, Skeem JL. Psychopathy and brain function: Empirical findings and legal implications. In: Häkkänen-Nyholm H, Nyholm J, editors. Psychopathy and law: A Practitioner’s Guide. New York: Wiley; 2012. pp. 39–78. [Google Scholar]
- Patrick CJ, Venables NC, Yancey JR, Hicks BM, Nelson LD, Kramer MD. A construct-network approach to bridging diagnostic and physiological domains: Application to assessment of externalizing psychopathology. Journal of Abnormal Psychology. 2013;122:902–916. doi: 10.1037/a0032807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J. Updating the P300: An integrative theory of P3a and P3b. Clinical Neurophysiology. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Pollock VE, Bloom FE. Meta-analysis of P300 amplitude from males at risk for alcoholism. Psychological Bulletin. 1994;115:55–73. doi: 10.1037/0033-2909.115.1.55. [DOI] [PubMed] [Google Scholar]
- Raine A, Venables PH. Enhanced P3 evoked potentials and longer P3 recovery times in psychopaths. Psychophysiology. 1988;25:30–38. doi: 10.1111/j.1469-8986.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction for ocular artifacts, applied to the P300. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- Skeem JL, Ploaschek DL, Patrick CJ, Lilienfeld SO. Psychopathic personality: Bridging the gap between scientific evidence and public policy. Psychological Science in the Public Interest. 2011;12(3):95–162. doi: 10.1177/1529100611426706. [DOI] [PubMed] [Google Scholar]
- Taylor J, Lang AR. Psychopathy and substance use disorder. In: Patrick CJ, editor. Handbook of psychopathy. New York: The Guilford Press; 2006. pp. 58–88. [Google Scholar]
- Verona E, Patrick CJ, Joiner TE. Psychopathy, antisocial personality, and suicide risk. Journal of Abnormal Psychology. 2001;110:462–470. doi: 10.1037//0021-843x.110.3.462. [DOI] [PubMed] [Google Scholar]
- Venables NC, Patrick CJ. Validity of the Externalizing Spectrum Inventory in a criminal offender sample: Relations with disinhibitory psychopathology, personality, and psychopathic features. Psychological Assessment. 2012;24:88–100. doi: 10.1037/a0024703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables NC, Patrick CJ, Hall JR, Bernat EM. Clarifying relations between dispositional aggression and brain potential response: Overlapping and distinct contributions of impulsivity and stress reactivity. Biological Psychology. 2011;86:279–288. doi: 10.1016/j.biopsycho.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey JR, Venables NC, Hicks BM, Patrick CJ. Evidence for a heritable brain basis to deviance-promoting deficits in self-control. Journal of Criminal Justice. 2013;41:309–317. doi: 10.1016/j.jcrimjus.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]