Abstract
The anemia of inflammation, commonly observed in patients with chronic infections, malignancy, trauma, and inflammatory disorders, is a well-known clinical entity. Until recently, we understood little about its pathogenesis. It now appears that the inflammatory cytokine IL-6 induces production of hepcidin, an iron-regulatory hormone that may be responsible for most or all of the features of this disorder.
In 1932, Locke et al. made the important observation that infection was associated with hypoferremia (low serum iron), providing a partial explanation for the common finding of anemia in patients with chronic infections (1). Cartwright and Wintrobe went on to show that the anemia associated with infection was indistinguishable from the anemia of inflammation, and established that hypoferremia resulted from reticuloendothelial sequestration of iron and interruption of intestinal iron absorption (2, 3). Cartwright and Lee recognized that similar findings could be induced in mice by exposure to bacterial endotoxin (4). Others correlated the anemia of inflammation with elaboration of inflammatory cytokines, and ascribed changes in iron metabolism to the effects of these cytokines (5). Cytokines have been shown to modulate the expression of iron transport and storage proteins (6), but it was not clear that these changes accounted for the abnormalities of iron homeostasis observed in the anemia of inflammation.
The roles of hepcidin and IL-6
Over the past two years, a variety of experiments have converged to establish a role for hepcidin, a liver-derived peptide regulator of iron homeostasis, as a key mediator of hypoferremia in inflammation (7–9). In an elegant report in this issue of the JCI, Nemeth, Rivera, and colleagues have elucidated an important link between inflammatory cytokines and hepcidin (10). Using both mice and humans as experimental models, they have shown that IL-6 acts directly on hepatocytes to stimulate hepcidin production. Hepcidin, in turn, acts as a negative regulator of intestinal iron absorption and macrophage iron release.
In previous work Nemeth, Rivera, and colleagues showed that IL-6 induced hepcidin expression in hepatic cells (9). Here, they have replicated this effect using conditioned medium from endotoxin-treated macrophages and shown that a neutralizing antibody against IL-6 blocked hepcidin induction (10). Other inflammatory cytokines did not stimulate hepcidin production; in fact, TNF-α inhibited it.
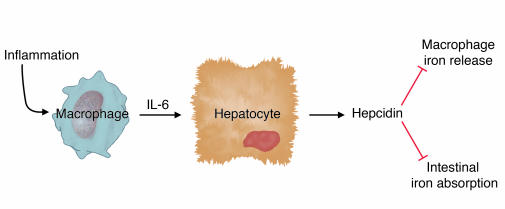
Cytokines have all sorts of effects on cultured cells, and it was important to show that IL-6 induction of hepcidin occurred in vivo and triggered hypoferremia, as predicted. First, Nemeth, Rivera, et al. used turpentine injection to cause inflammatory abscesses in wild-type and IL-6 knockout mice and analyzed the responses (10). Wild-type mice had increased hepcidin expression and a substantial decrease in serum iron levels. In contrast, IL-6 knockout mice had no increase in hepcidin expression and no decrease in serum iron. A complementary experiment carried out in human volunteers showed that IL-6 infusion stimulated urinary hepcidin excretion within 2 hours and induced hypoferremia. Taken together, these data provide strong support for the conclusions that IL-6 is a primary inducer of hepcidin expression and that increased hepcidin expression results in hypoferremia (Figure 1). This is gratifyingly consistent with clinical observations that hypoferremia occurs very quickly after the onset of inflammation.
Figure 1.
Regulation of hepcidin production in inflammation. Inflammation leads to macrophage elaboration of IL-6, which acts on hepatocytes to induce hepcidin production. Hepcidin inhibits macrophage iron release and intestinal iron absorption, leading to hypoferremia.
Earlier studies had shown that rodents with induced iron overload also had increased hepcidin expression (11, 12), presumably to try to compensate for iron excess. However, the signal to increase hepcidin expression was unknown. Here, Nemeth, Rivera, and colleagues have shown that IL-6 is not involved in the regulation of hepcidin in response to iron (10). Furthermore, their data suggest that hepcidin levels are not simply responding to increased iron stores. In human volunteers, urinary hepcidin levels were boosted soon after a single dose of oral iron, which should have no significant effect on iron stores. Perhaps the serum iron level, known to increase transiently after iron ingestion, might itself be the signal to induce hepcidin expression. Alternatively, the signal might relate to the degree of iron saturation of serum transferrin.
However, if transferrin iron saturation modulates hepcidin expression, other signals can clearly override its effects. The IL-6–mediated inflammatory induction of hepcidin does not appear to be offset by the hypoferremia it causes, at least in the short term. And mice with thalassemia intermedia (which presumably have elevated serum iron) have decreased hepcidin expression (13), as occurs in other mouse models with increased erythroid iron demand (7, 8).
In my opinion, this report from Nemeth, Rivera, et al. (10) leaves little room for doubt about the importance of hepcidin in the pathogenesis of anemia of inflammation. This was challenged by a recent report that concluded that elevated serum hepcidin levels were not useful in the diagnosis of the anemia of inflammation (14). However, that study did not provide data to support the authors’ contention that they had developed a sensitive, specific test for serum hepcidin. Furthermore, as they also pointed out, it was not clear that serum measurements were as useful as urinary hepcidin measurements. Hepcidin gene expression seems to be exquisitely sensitive to regulation, and the circulating peptide is small enough to be quantitatively filtered by the kidneys. Urine samples probably provide a better indication of recent hepcidin expression than individual serum samples.
A possible treatment?
If inflammatory induction of hepcidin causes hypoferremia, it is logical to predict that inhibition of hepcidin expression or activity would ameliorate the anemia of inflammation. Would that be advantageous? Perhaps, particularly in noninfectious inflammatory disorders. We know that patients (15) and mice (16) lacking hepcidin have increased intestinal iron absorption and increased serum iron, but this is unlikely to be harmful in the short term. However, there may be more cause for concern in patients with infections or malignancy. Decreased serum iron is believed to contribute to host defense against invading pathogens and tumor cells (17), and hepcidin itself has antimicrobial properties of uncertain importance (18). If hepcidin antagonists become available, careful clinical trials will be required to define appropriate indications for their use.
Footnotes
See the related article beginning on page 1271.
Conflict of interest: The author has declared that no conflict of interest exists.
References
- 1.Locke A, Main ER, Rosbach DO. The copper and non-hemoglobinous iron contents of the blood serum in disease. J. Clin. Invest. 1932;11:527–542. doi: 10.1172/JCI100432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cartwright GE, Wintrobe MM. The anemia of infection. XVII. A review. Adv. Intern. Med. 1952;5:165–226. [PubMed] [Google Scholar]
- 3.Cartwright GE. The anemia of chronic disorders. Semin. Hematol. 1966;3:351–375. [PubMed] [Google Scholar]
- 4.Cartwright GE, Lee GR. The anaemia of chronic disorders. Br. J. Haematol. 1971;21:147–152. doi: 10.1111/j.1365-2141.1971.tb03424.x. [DOI] [PubMed] [Google Scholar]
- 5.Means RT. Pathogenesis of the anemia of chronic disease: a cytokine-mediated anemia. Stem Cells. 1995;13:32–37. doi: 10.1002/stem.5530130105. [DOI] [PubMed] [Google Scholar]
- 6.Ludwiczek S, Aigner E, Theurl I, Weiss G. Cytokine-mediated regulation of iron transport in human monocytic cells. Blood. 2003;101:4148–4154. doi: 10.1182/blood-2002-08-2459. [DOI] [PubMed] [Google Scholar]
- 7.Weinstein DA, et al. Inappropriate expression of hepcidin is associated with iron refractory anemia: implications for the anemia of chronic disease. Blood. 2002;100:3776–3781. doi: 10.1182/blood-2002-04-1260. [DOI] [PubMed] [Google Scholar]
- 8.Nicolas G, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J. Clin. Invest. 2002;110:1037–1044. doi:10.1172/JCI200215686. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nemeth E, et al. Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood. 2003;101:2461–2463. doi: 10.1182/blood-2002-10-3235. [DOI] [PubMed] [Google Scholar]
- 10.Nemeth E, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J. Clin. Invest. 2004;113:1271–1276. doi:10.1172/JCI200420945. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pigeon C, et al. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J. Biol. Chem. 2001;276:7811–7819. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- 12.Muckenthaler M, et al. Regulatory defects in liver and intestine implicate abnormal hepcidin and Cybrd1 expression in mouse hemochromatosis. Nat. Genet. 2003;34:102–107. doi: 10.1038/ng1152. [DOI] [PubMed] [Google Scholar]
- 13.Adamsky K, et al. Decreased hepcidin mRNA expression in thalassemic mice. Br. J. Haematol. 2004;124:123–124. doi: 10.1046/j.1365-2141.2003.04734.x. [DOI] [PubMed] [Google Scholar]
- 14.Dallalio G, Fleury T, Means RT. Serum hepcidin in clinical specimens. Br. J. Haematol. 2003;122:996–1000. doi: 10.1046/j.1365-2141.2003.04516.x. [DOI] [PubMed] [Google Scholar]
- 15.Roetto A, et al. Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat. Genet. 2003;33:21–22. doi: 10.1038/ng1053. [DOI] [PubMed] [Google Scholar]
- 16.Nicolas G, et al. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc. Natl. Acad. Sci. U. S. A. 2001;98:8780–8785. doi: 10.1073/pnas.151179498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinberg ED. Iron, infection and neoplasia. Clin. Physiol. Biochem. 1986;4:50–60. [PubMed] [Google Scholar]
- 18.Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J. Biol. Chem. 2001;276:7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]