Abstract
Ovarian hormone loss increases reactive oxidative species (ROS), endothelial dysfunction and cardiovascular disease. Since perivascular adipose tissue (PVAT) regulates endothelial function, we hypothesized that ROS in PVAT mediate adverse microvascular effects of ovarian hormone deficiency. Rats were ovariectomized (OVX) or sham-operated (SHAM) and given vehicle or tempol for 6 weeks. Mesenteric resistance arterioles from OVX compared to SHAM rats had dysfunctional responses to acetylcholine (ACh) including: decreased ACh induced endothelium-dependent relaxation; (50±6 vs 72±2%) and endothelium-dependent relaxation factor (EDRF; 17±4 vs 37±2%) and increased endothelium-dependent contracting factor (EDCF; 27±5 vs 9±3%). OVX rat mesenteric arterioles had increased contractions to the thromboxane/prostanoid receptor (TP-R) agonist U-46,619 (58±3 vs 40±5%) and increased of ROS (tempo-9-AC fluorescence) with U-46,619 (0.65±0.17 vs 0.14±0.06 units) or ACh (0.49±0.09 vs 0.09±0.05 units) and increased p22phox protein expression (0.89±0.05 vs 0.18±0.04 units) while nitric oxide (NO) activity (DAF-FM fluorescence) with ACh was reduced (0.39±0.1 vs 0.70±0.10 units). No differences were found in endothelium-dependent hyperpolarizing factor (EDHF) or contractile responses to phenylephrine (PE). PVAT enhanced ACh induced relaxation, EDRF and NO only in SHAM rats. Tempol prevented OVX-induced endothelial dysfunction and restored the enhancing effects of PVAT on ACh induced relaxation, EDRF and NO in OVX rat vessels but both tempol and PVAT were required to normalize the enhanced U-46,619 contractions after OVX. In conclusion, ovariectomy redirects endothelial responses from relaxation to contraction by reducing vascular NO, augmenting TP-R signaling and attenuating the vasodilatory effects of PVAT, all of which were dependent on ROS.
Keywords: reactive oxygen species, nitric oxide, endothelial dysfunction, ovariectomy, thromboxane prostanoid receptor
Introduction
Ovarian hormone loss in women is associated with increased vascular reactive oxygen species (ROS), reduced endothelial nitric oxide synthase (NOS) expression and NO, increased endothelial dysfunction1, 2 and risk of cardiovascular disease (CVD).2 Similar findings have been reported in ovariectomized (OVX) animal models.3, 4
Endothelial dysfunction entails both impaired relaxation and an endothelium dependent contracting factor (EDCF) generated by ROS.5 The EDCF is a prostaglandin (PG) or thromboxane (Tx) product of cyclooxygenase (COX) and/or thromboxane A2 (TxA2) synthase that vasoconstricts vascular smooth muscle cells (VSMCs) by activating thromboxane-prostanoid receptors (TP-Rs).5 OVX leads to a COX-dependent EDCF response in rat mesenteric arteries6 and in pig coronary arteries.7 Furthermore, endothelial dysfunction in cutaneous blood vessels of postmenopausal women was mediated by COX-2.8 However, the effects of OVX on ROS and TP-R signaling in microvessels remains poorly understood.
Obesity is an independent CVD risk factor9 whose incidence is increased in women with ovarian hormone loss,10 or animal models of ovarian hormone deficiency.11 Perivascular adipose tissue (PVAT) improves vascular function12 through generation of NO,13 hydrogen perioxide (H2O2).12 hydrogen sulphide14 and/or poorly characterized adipokine pathways.14, 15 ROS inhibit these protective effects of PVAT in hypertensive models15 and contribute to endothelial dysfunction in animal models16 and in patients with obesity and the metabolic syndrome.17
We tested the hypothesis that OVX impairs PVAT-induced microvascular NO-dependent relaxation and enhances endothelium-dependent contractions through ROS and enhanced TP-R signaling. We reduced ROS using tempol which is a redox cycling nitroxide18 and activated TP-Rs with the stable agonist U-46,619.19 These experiments are clinically significant because restoration of endothelial function and NO, and abrogation of EDCF and enhanced TP-R signaling, could attenuate the enhanced CVD risk after ovarian hormone deficiency but it is unclear if the microvessels and/or the surrounding PVAT should be the preferred therapeutic target.
Methods
Animal preparation and protocols
Female Sprague Dawley rats (200–220 gm; Taconics Lab, Germantown, NY) maintained on tap water and standard chow (Na+ 0.3g · 100g−1) under conditions approved by the Institutional Animal Care and Use Committee of Georgetown University underwent ovariectomy (OVX) or sham surgery (SHAM)20 and received oral tempol (2 mmol·l−1) or vehicle in the drinking water for 6 weeks.5 After euthanasia by exsanguination, the second branch mesenteric resistance arterioles were isolated with the surrounding PVAT intact (PVAT+) or removed (PVAT−) and mounted in a four chamber myograph or snap frozen and stored at −80°C for analysis of p22phox protein.21
Vascular ACh-induced endothelial responses, NO, ROS activities and contractions
The media and lumen cross-sectional areas of mesenteric arterioles were measured.22 For the first series, mesenteric arterioles were preconstricted with 10−5 mol·l−1 norepinephrine (NE) to study: ACh induced relaxation (endothelium dependent relaxation, EDR); endothelium dependent relaxation factor (EDRF; change in EDR after 10−5 mol·l−1-L-NG-nitroarginine methylester, L-NAME); endothelium dependent hyperpolarizing factor (EDHF; change in EDRF after 10−6 mol·l−1 apamin (AP) and 10−5 mol·l−1 charabdotoxin (Chx) to block small and medium conductance calcium activated potassium channels); and endothelium independent responses (EIR; relaxation to sodium nitroprusside, SNP). To study ACh-induced nitric oxide (NO), arterioles were loaded with 5×10−5 mol·l−1 4-amino-5-methylamino-2',7'-difluorofluorescein diacetate (DAFFM-DA). The change of fluorescence (ΔF1/F0) with 10−5 mol·l−1 ACh quantitated vascular NO.5
For the second series, endothelium dependent contraction factor (EDCF) was measured in arterioles under spontaneous tone with relaxation pathways blocked by L-NAME + AP + Chx and contracted with ACh. Other vessels prepared similarly, were loaded with tempo-9-AC and the change of fluorescence with 10−4 mol·l−1 ACh determined to quantitate vascular ROS.5
For the third series, vessels were contracted with phenylephrine (PE) or U-46,619. ROS generation with 10−6 mol·l−1 U-46,619 was quantitated by tempo-9-AC fluorescence.
Expression of p22phox
The protein expression of the NADPH oxidase regulatory subunit p22phox in lysates of mesenteric arterioles was measured by Western blot21 and normalized to β-actin.
Statistics
Data are expressed as mean±SEM calculated from 6 rats per group. The concentration-response relationships were analyzed by two-way, repeated measures ANOVA to assess the effects of OVX, PVAT and the interaction (effects of PVAT on responses to OVX) followed, where appropriate, by Bonferroni tests. Separate analyses were undertaken in the groups given tempol. Significance was defined as P < 0.05.
Results
Body weight (BWt), vascular structure and p22phox protein expression
OVX increased BWt by 16% whereas tempol reduced it by a similar degree (Table 1). OVX almost doubled the MRA media area without changing the lumen area resulting in a doubling of the ratio of media to lumen (M/L). The p22phox protein expression was increased by 400% in OVX rat vessels (Fig.1). These effects of OVX were prevented by tempol.
Table 1.
Effect of ovariectomy and tempol on body weight and mesenteric arteriole (MRA) structure and p22phox expression
| Variable | Sham | ovx | Sham+Tempol | OVX + tempol | By ANOVA, effect of: |
||
|---|---|---|---|---|---|---|---|
| OVX | Tempol | Interaction | |||||
| Body weight (g) | 251±11 | 287±9 | 219±5 | 246±6 | P<0.001 | P<0.001 | NS |
| MRA media area (M; μm2) | 48±5 | 91±7 | 51±5 | 48±6 | P<0.01 | P<0.01 | P<0.01 |
| MRA lumen area (L; μm2) | 187±12 | 170±6 | 178±14 | 193±5 | NS | NS | NS |
| MRA M/L ratio (cross section area, μm2/μm2) | 1.54±0.29 | 3.43±0.38* | 1.53±0.24 | 1.63±0.19 | P<0.05 | P<0.05 | P<0.05 |
| MRA p22phox expression (relative to β- actin) | 0.18±0.04 | 0.89±0.05** | 0.28±0.11 | 0.26±0.14 | P<0.01 | P<0.05 | P<0.001 |
Mean ± SEM value (n=6 per group). Compared to SHAM:
P<0.05.
P<0.01
Fig. 1.
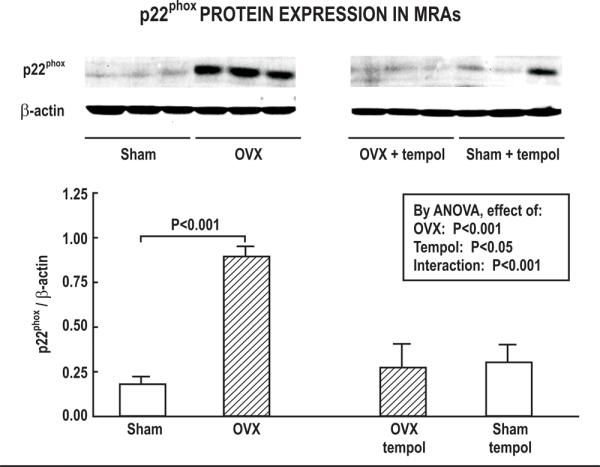
Effect of ovariectomy and tempol on p22phox protein expression. Mean± SEM values normalized to β-actin in mesenteric arterioles from SHAM (open bar) or OVX (hatched bar) rats after 6 weeks of vehicle or tempol treatment (n=6 per group). Top panel, Representative Western blot used to quantitate p22phox expression.
Effects of OVX and PVAT on endothelial relaxation and generation of NO
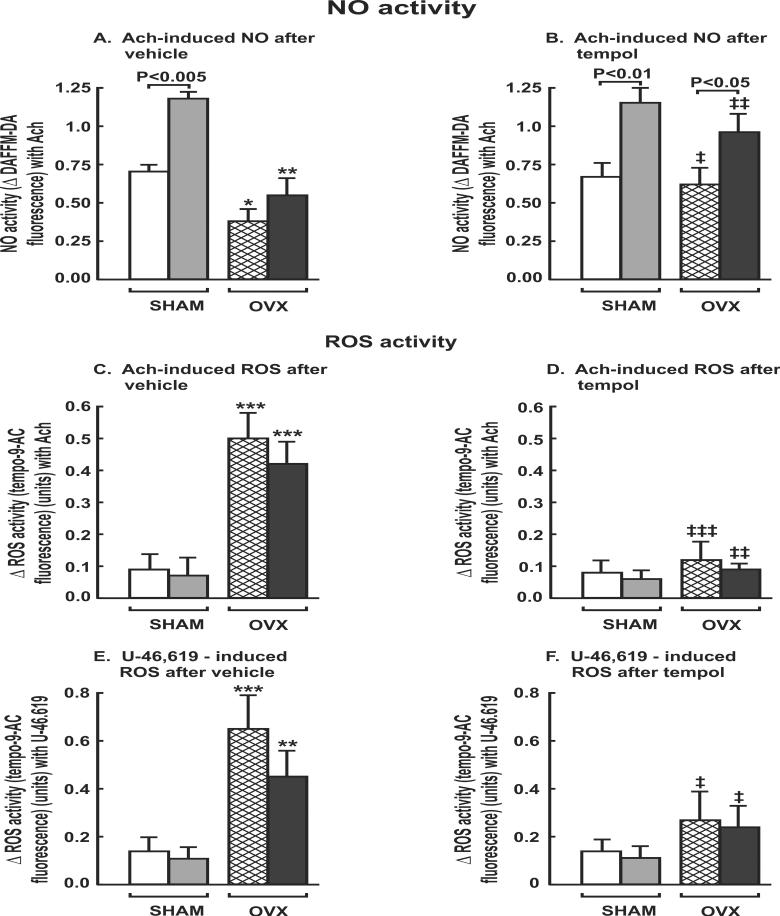
The maximum ACh- induced relaxation and EDRF responses in preconstricted mesenteric arterioles from OVX rats were reduced by 30 ±3% and 53±3% (P<0.05), respectively (Fig. 2A, 2B) but the EDHF and EIR responses were maintained (Table 2-I). Vascular NO activity with ACh was reduced in mesenteric arterioles from OVX rats by ≈2-fold (P<0.001; Table 2-I; Figure 3A).
Fig. 2.
Effect of perivascular adipose tissue and ovariectomy on ACh induced relaxation (ACh), EDRF and EDCF responses after 6 weeks of vehicle or tempol administration. Mean± SEM (n=6 per group) values for ACh induced relaxation (A&D), EDRF (B&E), and EDCF (C&F) in mesenteric arterioles with PVAT (solid symbol) or without PVAT (open symbol) from SHAM (circles) or OVX (squares) rats after 6 weeks of vehicle (A–C) or tempol (D–F). Comparing PVAT+ vs – in same treatment groups: *P<0.05; **, P<0.01. Comparing SHAM vs OVX in same treatment groups: ‡, P<0.01; ‡‡‡, P<0.005.
Table 2.
Effect of ovariectomy and PVAT on maximum vascular reactivity, NO and ROS in mesenteric artenoles.
| I. After vehicle | |||||||
|---|---|---|---|---|---|---|---|
| SHAM: |
OVX: |
By ANOVA, effect of: |
|||||
| Responses | PVAT− | PVAT+ | PVAT− | PVAT+ | OVX | PVAT | Interaction |
| ACh (%) | 71.9±2.1 | 89.5±4.4† | 50.1±6.2** | 56.7±6.4** | P<0.01 | P<0.05 | P<0.05 |
| EDRF (%) | 37.1±3.5 | 52.0±3.3††† | 17.0±1.3*** | 19.8±5.6*** | P<0.05 | P<0.001 | P<0.01 |
| EDHF (%) | 27.9±5.2 | 31.2±2.4 | 25.3±4.9 | 29.3±2.8 | NS | NS | NS |
| SNP (%) | 96.3±2.1 | 98.1±4.1 | 93.6±2.8 | 91.6±3.8 | NS | NS | NS |
| EDCF (%) | 8.7±2.9 | 7.5±2.7 | 26.7±2.9** | 17.4±2.7**† | P<0.001 | P<0.05 | NS |
| PE (%) | 67.0±5.3 | 55.7±8.1 | 65.6±6.1 | 55.9±9.3 | NS | NS | NS |
| U46,619(%) | 39.6±5.7 | 10.0±2.4††† | 57.5±3.4* | 35.4±8.5**††† | P<0.05 | P<0.001 | NS |
| ACh-NO (Δunit) | 0.69±0.07 | 1.17±0.07††† | 0.39±0.08* | 0.55±0.11*††† | P<0.001 | P<0.001 | P<0.01 |
| EDCF-ROS(Δunit) | 0.09±0.05 | 0.07±0.06 | 0.49±0.09*** | 0.42±0.07*** | P<0.001 | NS | NS |
| U46,619-ROS(Δunit) | 0.14±0.06 | 0.11±0.05 | 0.65±0.17*** | 0.45±0.14*† | P<0.01 | P<0.05 | P<0.05 |
| II. After tempol | |||||||
|---|---|---|---|---|---|---|---|
| SHAM: |
OVX: |
By ANOVA, effect of: |
|||||
| Responses | PVAT− | PVAT+ | PVAT− | PVAT+ | OVX | PVAT | Interaction |
| ACh (%) | 71.1±2.6 | 86.5±2.1† | 72.5±1.8 | 87.0±2.3† | NS | P<0.001 | NS |
| EDRF (%) | 31.3±4.2 | 51.2±4.2†† | 30.7±4.0 | 51.4±4.5†† | NS | P<0.001 | NS |
| EDHF (%) | 33.6±1.6 | 27.4±2.5 | 36.1±2.4 | 30.1±2.5 | NS | NS | NS |
| EDCF (%) | 6.7±2.1 | 5.1±1.8 | 11.5±3.1 | 9.4±1.8 | NS | NS | NS |
| PE (%) | 66.5±4.9 | 47.9±6.9 | 76.9±7.3 | 61.2±6.8 | NS | NS | NS |
| U-46,619 (%) | 37.9±3.9 | 8.9±3.6†† | 51.9±5.1† | 16.8±3.8††† | P<0.05 | P<0.01 | NS |
| ACh-NO (Δunit) | 0.67±0.09 | 1.14±0.10†† | 0.62±0.11 | 0.96±0.12† | NS | P<0.01 | NS |
| EDCF-ROS(Δunit) | 0.08±0.04 | 0.06±0.03 | 0.12±0.06 | 0.09±0.02 | NS | NS | NS |
| U-46,619-ROS(Δunit) | 0.13±0.07 | 0.10±0.06 | 0.27±0.12 | 0.24±0.11 | NS | NS | NS |
Fig. 3.
Effects of perivascular adipose tissue and ovariectomy on NO and ROS generation in mesenteric resistant arterioles with 10−4 mol.l−1 of acetylcholine or 10−6 mol.l−1 of U-46,619. Comparing SHAM vs OVX in same treatment groups: *, P<0.05; **, P<0.01; ***, P<0.005. Comparing tempol vs vehicle in same treatment groups: ‡, P<0.05; ‡ ‡, P<0.01; ‡ ‡ ‡, P<0.005.
The presence of PVAT around mesenteric arterioles from SHAM rats increased their ACh induced relaxation and EDRF response by 20±4% and 29±3%, respectively (P<0.05; Fig. 2A, 2B) and increased their vascular NO activity with ACh by 41±4% (P<0.001; fig.3A) without changing their EDHF or EIR responses (table 2-I). However, these effects were lost in OVX rat vessels.
EDCF contractions were increased by 200% in vessels from OVX rats (Fig.2C), accompanied by a 500% (P< 0.001) increase in vascular ROS activity (Table 2-I; Fig.3C). PVAT reduced the EDCF responses in OVX rats (P<0.01) without reducing the associated vascular ROS generation.
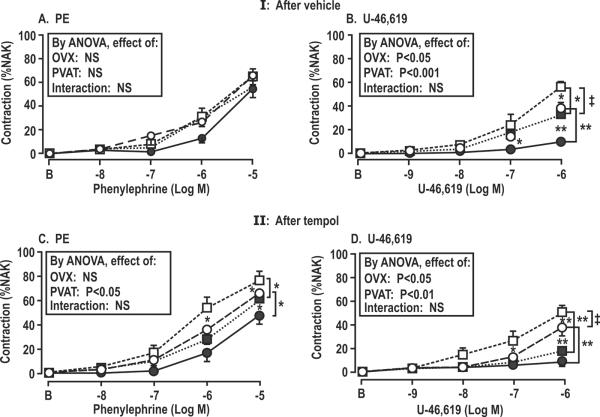
Contractions with PE were unchanged in mesenteric arterioles from OVX rats but contractions with U-46,619 were increased by 45% (P<0.05; Table 2-I; Fig. 4B) accompanied by a 350% increase in vascular ROS (P< 0.001; Fig.3E). PVAT moderated the contractions with U-46,619 in both SHAM and OVX rat vessels and moderated the accompanying ROS generation in vessels from OVX rats. Nevertheless, both the vascular contractions and the ROS generation with U-46,619 in OVX rat vessels with PVAT remained >3 fold higher than in vessels from SHAM rats despite the moderating effects of PVAT (Table 2-I).
Fig.4.
Effect of perivascular adipose tissue and ovariectomy on contractions to phenylephrine and U-46,619 in mesenteric arterioles with PVAT (solid symbols) or without PVAT (open symbols) from SHAM (circles) or OVX (squares) rats after 6 weeks of vehicle or tempol. Comparing PVAT+ vs – in same treatment groups: *P<0.05; **, P<0.01. Comparing SHAM vs OVX in same treatment groups: ‡, P<0.05.
Effects of tempol on endothelial function, contractility and generation of NO and ROS
Tempol did not affect the vascular responses or NO generation of vessels from SHAM rats. However, it improved these responses in OVX rat vessels and prevented the enhanced EDCF responses (Table 2-II; Fig.2F) and ROS generation (Table 2-II; Fig.3D). However, although tempol prevented the enhanced vascular ROS generation with U-46,619 in OVX rat vessels without PVAT, the enhanced contractions to U-46,619 persisted.
Tempol administration OVX rats without PVAT restored EIR and EDRF and attenuated EDCF responses (Table 2-II; Fig.2D–F). However, the enhanced contractions to U-46,619 were not significantly moderated by tempol (Fig.4D), despite prevention of the enhanced generation of ROS. This indicated that there was a ROS-independent vascular effect of OVX to enhance U-46,619 contractions.
PVAT reduced the contractions to U-46,619 in OVX rat vessels by 40% (Table 2-I; Fig. 4B) but after tempol, PVAT reduced the contractions by 70% (Table 2-II; Fig.4D). The U-46,619 contractions in vessels from OVX rats given tempol was 17±4% with PVAT which was similar to the value of 10±2% in vessels with PVAT from SHAM rats (P=NS). Thus tempol +PVAT normalized the enhanced contraction to U-46,619 in vessels from OVX rats. Again, the effects of PVAT to moderate U-46,619 contraction after tempol occurred independent of ROS which was effectively suppressed by tempol in vessels without PVAT. Thus, neither PVAT, nor tempol alone were sufficient to prevent enhanced contractions to U-46,619, but the contractions were normalized by their combination. This suggests that OVX enhanced contractions to U-46,619 by independent effects of enhanced ROS and diminished blunting by PVAT. Thus, a primary effect of tempol on OVX rat vessels was to restore normal signaling from PVAT which blunted the response to U-46,619 in the adjacent blood vessels.
Discussion
This study confirms previous findings that OVX or ovarian hormone deficiency lead to endothelial dysfunction and enhanced vascular oxidative stress.3, 4, 23–25 The main new findings are that defects in endothelium dependent relaxation in OVX rat vessels were specific for EDRF since they did not impact EDHF and were accompanied by reduced NO generation. OVX rat vessels had an enhanced expression of p22phox which is a critical regulatory component of NADPH oxidase.21 Indeed, these vessels developed enhanced ACh-and U-46,619-induced ROS generation and contractions.
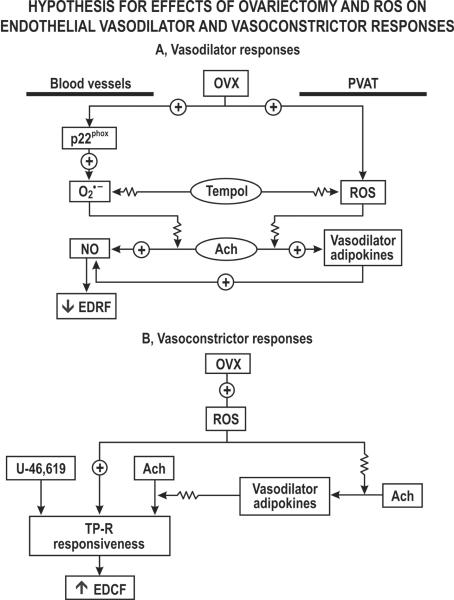
The main new findings related to PVAT signaling are summarized in fig.5. The presence of PVAT surrounding the mesenteric arterioles from SHAM rats enhanced their EDRF responses and NO generation and diminished their contractions to U-46,619 substantially without modifying the modest vascular ROS generation or contractions to PE. In contrast, PVAT surrounding OVX rat vessels failed to enhance EDRF responses or to restore NO generation. Furthermore, PVAT was less effective in moderating the contractions to U-46,619 after OVX and failed to prevent the associated ROS generation.
Fig. 5.
Flow diagram outlining pathways intrinsic to blood vessels or that originate in PVAT that may modulate vasodilator and vasoconstrictor pathways. OVX, ovariectomy; PVAT. perivasvular adipose tissue; ROS, reactive oxygen species; TP-R, thromboxane prostanoid receptor; ACh, acetylcholine.
As in prior studies,5, 18, 19 tempol did not affect relaxation or contraction responses or NO generation of SHAM vessels, implying that ROS have little effect on normal vascular function. The main new findings related to tempol in OVX rat vessels are that it prevented all of the endothelial dysfunction and diminished NO generation, and prevented the augmented EDCF contractions and ACh-induced ROS generation. Moreover, tempol given to OVX rats restored the effects of PVAT to enhance endothelial function. In contrast, tempol failed to prevent the enhanced contractions to U-46,619 despite preventing the excessive ROS generation.26 In fact, a combination of PVAT and tempol was required to reduce U-46,619 contractions in OVX rat vessels to SHAM levels.
In contrast, incubation of canine coronary arteries with bradykinin released an adipokine that reduced vascular ROS, yet inhibited vasodilation. Another apparently paradoxical effect of tempol was to reduce bradykinin-induced relaxations. There are important differences in the functions of PVAT between vessels and experimental circumstances.26 After OVX, tempol enhanced both the intrinsic relaxation to ACh (in vessels without PVAT) and the relaxations mediated by PVAT, while reducing ROS generation consistent with many other reports that tempol moderated ROS and contractility and enhanced relaxation during oxidative stress.18
Of interest was the finding that OVX increased weight gain similar to the menopause.10 However, tempol reduced the weight gain in both OVX and SHAM rats. Tempol given to fat-fed mice also prevented weight gain27 which was attributed to alteration in the microbiome and signaling via intestinal farnesoid x in receptors.
Thus, oxidative stress in microvessels from OVX rats redirected endothelial function from NO-dependent vasorelaxation to ROS-dependent vasoconstriction. About one half of these changes related to effects of ROS mediated by PVAT. The endothelial dysfunction is analogous to the effects on prolonged Ang II-infusion in rabbits19 and rats5 which also enhanced p22phox expression. EDCF requires vascular ROS generation after endothelial activation by shear stress,28 ACh,5, 19 Ang II19 or endothelin-1.29 Vascular ROS generate vasoconstrictor PGs and TxA2 from endothelial COX-1 or -2 and TxA2 synthase that activate TP-Rs on adjacent VSMCs to mediate EDCF responses.19 A second effect of vascular ROS is to enhance TP-R responsiveness of VSMCs19 by reducing the recycling of TP-Rs.30 The enhanced TP-R responsiveness of mesenteric arterioles from OVX rats confirms a prior study.6 However, an enhanced microvascular TP-R responsiveness is an incomplete explanation for the enhanced EDCF responses of mesenteric arterioles from OVX rats since tempol prevented the enhanced EDCF responses without fully correcting the enhanced responses to U-46,619. Apparently, by an ROS in PVAT change the release or responsiveness of an adipokine acting on TP-Rs or their signaling in adjacent VSMCs. The moderation of EDCF responses by PVAT in OVX rat vessels cannot be ascribed to upregulation of counterveiling endothelial vasodilation since this was blocked by L-NAME, apamin and charybdatoxin.
OVX rats had a similar increase in vascular ROS generation with TP-R activation as with ACh yet neither was prevented by PVAT alone and required co-administration of tempol. Thus, although PVAT enhanced ACh induced NO generation in OVX rat vessels, it did not restore EDRF responses or abrogate EDCF or U-46,619 contractions without first preventing ROS generation with tempol. This extends findings at the whole animal31 or cellular level32 that TP-R activation is a cause of oxidative stress, rather than just its consequence.
EDRF responses of normal rodent mesenteric arterioles depend on NO but not on PGs.19 The reduced EDRF responses and NO generation after OVX, and their restoration by tempol, implies a ROS-dependent bio-inactivation of NO or oxidation of the NOS cofactor, tetrahydrobiopterin.33 The maintenance of EDHF responses is consistent since EDHF does not normally depend on NO. PVAT enhanced EDRF responses and NO only in mesenteric arterioles from SHAM rats but an enhancing effect of PVAT in OVX rats was restored by tempol administration. This implies that ROS in PVAT surrounding OVX rat vessels impaired the adipokine signaling that normally enhanced NO generation and EDRF responses or that ROS in blood vessels prevented the signaling effects of the adipokine. Thus, ROS in PVAT were implicated in both enhancing the EDCF responses and reducing the EDRF/NO responses in vessels from OVX rats.
This study has some limitations. It was confined to mesenteric arterioles. However, their function parallels that of renal afferent arterioles and systemic vessels.5, 31 We did not test the effects of ovarian hormone replacement in OVX rats. However, prior studies have reported that concomitant 17β-estradiol replacement at the time of OVX prevents endothelial dysfunction and enhanced TP-R signaling.6 The adipokine(s) released from PVAT was not identified. However, these studies suggest it was not hydrogen sulfide, which activates vascular ATP-dependent K+ channels and leads to hyperpolarization of VSMCs since we did not detect any effect of PVAT on EDHF responses.
Perspective
TP-R signaling not only mediates EDCF responses but also contributes to hypertension, vascular remodeling, renal vasoconstriction, oxidative stress and platelet aggregation, as observed during angiotensin31 or Goldblatt renovascular hypertension.34, 35 Thus, enhanced TP-R signaling may contribute to the microvascular remodeling, endothelial dysfunction and oxidative stress observed in OVX rat vessels and also to the loss of protection from CVD observed in women with ovarian hormone deficiency. The finding that microvessels from OVX rats have defects in endothelial relaxation, an EDCF response and enhanced TP-R signaling that were mediated by adverse ROS-dependent signaling in the vessels and the surrounding PVAT suggests that full restoration of the beneficial signaling from PVAT would be a valuable therapeutic approach for reducing CVD risk in women with ovarian hormone deficiency.
Supplementary Material
Novelty and Significance.
What is New?
Microvascular endothelial dysfunction and reduced NO in this model of ovarian hormone deficiency derives from ROS and impaired relaxant signaling from PVAT. Correction of oxidative stress and presence of PVAT are both required to normalize the greatly enhanced TP-R responses.
What is Relevant?
Correction of vascular ROS alone is insufficient to correct microvascular dysfunction in full. Vascular TP-Rs and PVAT are novel targets to correct menopausal microvascular dysfunction.
Summary
Microvascular endothelial dysfunction and enhanced TP-R signaling after ovariectomy originates from interactive effects of oxidative stress in blood vessel and adverse adipokine signaling from PVAT.
Acknowledgements
We thank Miss Sarah Stalnaker for preparing the manuscript.
Sources of funding This work was supported by grants to DW from the National Kidney Foundation Capital Area and the Marriott Cardiovascular Research Fellowship Award, to KS from NIH (AG/HL-19291, AG-039779 & AG-16902) and to CSW and WJW from the NIDDK (DK-049870 and DK-036079) and the NHLBI (HL-68686) and to CSW by funds from the George E. Schreiner Chair of Nephrology and the Hypertension, Kidney and Vascular Research Center and to CW by a Chinese Government Scholarship Program.
Footnotes
Disclosures None.
References
- 1.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. New Eng J Med. 340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 2.Wenger NK, Speroff L, Packard B. Cardiovascular health and disease in women. N Engl J Med. 329:247–256. doi: 10.1056/NEJM199307223290406. [DOI] [PubMed] [Google Scholar]
- 3.Lam KK, Lee YM, Hsiao G, Chen SY, Yen MH. Estrogen therapy replenishes vascular tetrahydrobiopterin and reduces oxidative stress in ovariectomized rats. Menopause. 13:294–302. doi: 10.1097/01.gme.0000182806.99137.5e. [DOI] [PubMed] [Google Scholar]
- 4.Wassmann S, Baumer AT, Strehlow K, van EM, Grohe C, Ahlbory K, Rosen R, Bohm M, Nickenig G. Endothelial dysfunction and oxidative stress during estrogen deficiency in spontaneously hypertensive rats. Circ. 103:435–441. doi: 10.1161/01.cir.103.3.435. [DOI] [PubMed] [Google Scholar]
- 5.Wang D, Luo Z, Wang X, Jose PA, Falck JR, Welch WJ, Aslam S, Teerlink T, Wilcox CS. Impaired endothelial function and microvascular asymmetrical dimethylarginine in angiotensin II-infused rats: effects of tempol. Hypertens. 56:950–955. doi: 10.1161/HYPERTENSIONAHA.110.157115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidge ST, Zhang Y. Estrogen replacement suppresses a prostaglandin H synthase-dependent vasoconstrictor in rat mesenteric arteries. Circ Res. 83:388–395. doi: 10.1161/01.res.83.4.388. [DOI] [PubMed] [Google Scholar]
- 7.Thompson LP, Weiner CP. Long-term estradiol replacement decreases contractility of guinea pig coronary arteries to the thromboxane mimetic U46619. Circ. 95:709–714. doi: 10.1161/01.cir.95.3.709. [DOI] [PubMed] [Google Scholar]
- 8.Calkin AC, Sudhir K, Honisett S, Williams MR, Dawood T, Komesaroff PA. Rapid potentiation of endothelium-dependent vasodilation by estradiol in postmenopausal women is mediated via cyclooxygenase 2. J Clin Endocrinol Metab. 87:5072–5075. doi: 10.1210/jc.2002-020057. [DOI] [PubMed] [Google Scholar]
- 9.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 10.Shi H, Clegg DJ. Sex differences in the regulation of body weight. Physiol Behav. 97:199–204. doi: 10.1016/j.physbeh.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown LM, Gent L, Davis K, Clegg DJ. Metabolic impact of sex hormones on obesity. Brain Res. 1350:77–85. doi: 10.1016/j.brainres.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao YJ, Lu C, Su LY, Sharma AM, Lee RM. Modulation of vascular function by perivascular adipose tissue: the role of endothelium and hydrogen peroxide. Br J Pharmacol. 151:323–331. doi: 10.1038/sj.bjp.0707228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee RM, Lu C, Su LY, Gao YJ. Endothelium-dependent relaxation factor released by perivascular adipose tissue. J Hypertens. 27:782–790. doi: 10.1097/HJH.0b013e328324ed86. [DOI] [PubMed] [Google Scholar]
- 14.Vacek TP, Gillespie W, Tyagi N, Vacek JC, Tyagi SC. Hydrogen sulfide protects against vascular remodeling from endothelial damage. Amino Acids. 39:1161–1169. doi: 10.1007/s00726-010-0550-2. [DOI] [PubMed] [Google Scholar]
- 15.Galvez-Prieto B, Dubrovska G, Cano MV, Delgado M, Aranguez I, Gonzalez MC, Ruiz-Gayo M, Gollasch M, Fernandez-Alfonso MS. A reduction in the amount and anticontractile effect of periadventitial mesenteric adipose tissue precedes hypertension development in spontaneously hypertensive rats. Hypertens Res. 31:1415–1423. doi: 10.1291/hypres.31.1415. [DOI] [PubMed] [Google Scholar]
- 16.Marchesi C, Ebrahimian T, Angulo O, Paradis P, Schiffrin EL. Endothelial nitric oxide synthase uncoupling and perivascular adipose oxidative stress and inflammation contribute to vascular dysfunction in a rodent model of metabolic syndrome. Hypertens. 54:1384–1392. doi: 10.1161/HYPERTENSIONAHA.109.138305. [DOI] [PubMed] [Google Scholar]
- 17.Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, Laing I, Yates AP, Pemberton PW, Malik RA, Heagerty AM. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circ. 119:1661–1670. doi: 10.1161/CIRCULATIONAHA.108.821181. [DOI] [PubMed] [Google Scholar]
- 18.Wilcox CS, Pearlman A. Chemistry and antihypertensive effects of tempol and other nitroxides. Pharm Rev. 60:418–469. doi: 10.1124/pr.108.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang D, Chabrashvili T, Wilcox CS. Enhanced contractility of renal afferent arterioles from angiotensin-infused rabbits: roles of oxidative stress, thromboxane-prostanoid receptors and endothelium. Circ Res. 94:1436–1442. doi: 10.1161/01.RES.0000129578.76799.75. [DOI] [PubMed] [Google Scholar]
- 20.Ji H, Zheng W, Menini S, Pesce C, Kim J, Wu X, Mulroney SE, Sandberg K. Female protection in progressive renal disease is associated with estradiol attenuation of superoxide production. Gend Med. 4:56–71. doi: 10.1016/s1550-8579(07)80009-x. [DOI] [PubMed] [Google Scholar]
- 21.Modlinger P, Chabrashvili T, Gill PS, Mendonca M, Harrison DG, Griendling KK, Li M, Raggio J, Wellstein A, Chen Y, Welch WJ, Wilcox CS. RNA silencing in vivo reveals role of p22phox in rat angiotensin slow pressor response. Hypertens. 47:238–244. doi: 10.1161/01.HYP.0000200023.02195.73. [DOI] [PubMed] [Google Scholar]
- 22.Heagerty AM, Aalkjaer C, Bund SJ, Korsgaard N, Mulvany MJ. Small artery structure in hypertension. Dual processes of remodeling and growth. Hypertens. 21:391–397. doi: 10.1161/01.hyp.21.4.391. [DOI] [PubMed] [Google Scholar]
- 23.Wong CM, Yao X, Au CL, Tsang SY, Fung KP, Laher I, Vanhoutte PM, Huang Y. Raloxifene prevents endothelial dysfunction in aging ovariectomized female rats. Vascul Pharmacol. 44:290–298. doi: 10.1016/j.vph.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Widder J, Pelzer T, von Poser-Klein C, Hu K, Jazbutyte V, Fritzemeier KH, Hegele-Hartung C, Neyses L, Bauersachs J. Improvement of endothelial dysfunction by selective estrogen receptor-alpha stimulation in ovariectomized SHR. Hypertens. 42:991–996. doi: 10.1161/01.HYP.0000098661.37637.89. [DOI] [PubMed] [Google Scholar]
- 25.Squadrito F, Altavilla D, Squadrito G, Saitta A, Cucinotta D, Minutoli L, Deodato B, Ferlito M, Campo GM, Bova A, Caputi AP. Genistein supplementation and estrogen replacement therapy improve endothelial dysfunction induced by ovariectomy in rats. Cardiovasc Res. 45:454–462. doi: 10.1016/s0008-6363(99)00359-4. [DOI] [PubMed] [Google Scholar]
- 26.Szasz T, Bomfim GF, Webb RC. The influence of perivascular adipose tissue on vascular homeostasis. Vasc Health Risk Manag. 9:105–116. doi: 10.2147/VHRM.S33760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samuni Y, Cook JA, Choudhuri R, DeGraff W, Sowers AL, Krishna MC, Mitchell JB. Inhibition of adipogenesis by Tempol in 3T3-L1 cells. Free Radic Biol Med. 49:667–673. doi: 10.1016/j.freeradbiomed.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang A, Sun D, Koller A. Shear stress-induced release of prostaglandinH2 in arterioles of hypertensive rats. Hypert. 35:925–930. doi: 10.1161/01.hyp.35.4.925. [DOI] [PubMed] [Google Scholar]
- 29.Wang D, Wang X, Welch WJ, Ji H, Sandberg K, Wu X, Jose P, Wilcox CS. Endothelin is a potent endothelium-derived contracting factor released by the renal afferent arterioles and mesenteric resistance vessels of mice with prolonged oxidative stress due to EC-SOD knockout. J Am Soc Nephrol. 2006;17:716A. Ref Type: Abstract. [Google Scholar]
- 30.Valentin F, Field M, Tippins JR. The mechanism of oxidative stress stabilization of the thromboxane receptor in COS-7 cells. J Biol Chem. 279:8316–8324. doi: 10.1074/jbc.M306761200. [DOI] [PubMed] [Google Scholar]
- 31.Kawada N, Dennehy K, Solis G, Modlinger P, Hamel R, Kawada JT, Aslam S, MOriyama T, Imai E, Welch WJ, Wilcox CS. TP receptors regulate renal hemodynamics during angiotensin II slow pressor response. Am J Physiol. 287:F753–F759. doi: 10.1152/ajprenal.00423.2003. [DOI] [PubMed] [Google Scholar]
- 32.Wilson SJ, Cavanagh CC, Lesher AM, Frey AJ, Russell SE, Smyth EM. Activation dependent stabilization of the human thromboxane receptor: Role of reactive oxygen species. J Lipid Res. 50:1047–1056. doi: 10.1194/jlr.M800447-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuzkaya N, Weissmann N, Harrison DG, Dikalov S. Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem. 278:22546–22554. doi: 10.1074/jbc.M302227200. [DOI] [PubMed] [Google Scholar]
- 34.Welch WJ, Patel K, Modlinger P, Mendonca M, Kawada N, Dennehy K, Aslam S, Wilcox CS. Roles of vasoconstrictor prostaglandins, COX-1 and -2, AT1, AT2 and TP receptors in a rat model of early 2K, 1C hypertension. Am J Physiol. 293:H2644–H2649. doi: 10.1152/ajpheart.00748.2007. [DOI] [PubMed] [Google Scholar]
- 35.Schildknecht S, van der LB, Weber K, Tiefenthaler K, Daiber A, Bachschmid MM. Endogenous peroxynitrite modulates PGHS-1-dependent thromboxane A2 formation and aggregation in human platelets. Free Radic Biol Med. 45:512–520. doi: 10.1016/j.freeradbiomed.2008.04.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.