Abstract
Characterizing how the microenvironment, or niche, regulates stem cell activity is central to understanding stem cell biology and to developing strategies for therapeutic manipulation of stem cells1. Low oxygen tension (hypoxia) is commonly thought to be a shared niche characteristic in maintaining quiescence in multiple stem cell types2–4. However, support for the existence of a hypoxic niche has largely come from indirect evidence such as proteomic analysis5, expression of HIF-1 and related genes6, and staining with surrogate hypoxic markers (e.g. pimonidazole)6–8. Here we perform direct in vivo measurements of local oxygen tension (pO2) in the bone marrow (BM) of live mice. Using two-photon phosphorescence lifetime microscopy (2PLM), we determined the absolute pO2 of the BM to be quite low (<32 mmHg) despite very high vascular density. We further uncovered heterogeneities in local pO2, with the lowest pO2 (~9.9 mmHg, or 1.3%) found in deeper peri-sinusoidal regions. The endosteal region, by contrast, is less hypoxic as it is perfused with small arteries that are often positive for the marker nestin. These pO2 values change dramatically after radiation and chemotherapy, pointing to the role of stress in altering the stem cell metabolic microenvironment.
The precise location of a hematopoietic stem cell (HSC) niche within the BM remains elusive, with evidence supporting the existence of both a vascular niche9,10 and an endosteal niche11,12. As the BM is densely perfused13–16, including the endosteal region, we ask how such a highly vascularized tissue can harbor HSCs in a low oxygen microenvironment. The possibility of very steep pO2 gradients forming hypoxic zones at short distances away from the blood vessels is suggested by an in silico model17,18. However no direct measurement of local oxygen distribution within the BM has been reported.
Here we implemented 2PLM on a two-photon microscope designed specifically for live animal imaging19. The all-optical design (Extended Data Fig. 1) enabled non-contact pO2 measurement through the intact skull at precise locations within the BM with micrometer spatial resolution. Prior to imaging, a metalloporphyrin-based two-photon-enhanced phosphorescent probe PtP-C34320,21 was injected systemically. The emissive triplet state of the platinum porphyrin (PtP) is highly sensitive to local oxygen concentration20,21. Bimolecular collisions with dissolved oxygen shorten the probe’s triplet lifetime and quench phosphorescence22. Thus by measuring the phosphorescence decay time τ after an excitation pulse, the absolute pO2 value can be determined based on a pre-established τ vs. pO2 calibration curve (Extended Data Fig. 2). The metalloporphyrin in PtP-C343 is protected by a dendrimer with a polyethylene glycol (PEG) overcoat (Extended Data Fig. 3) to insure biocompatibility and to prevent unwanted interactions of the probe with bio-macromolecules20,23. To enhance the two-photon excitation cross section, several coumarin-343 (C343) moieties are grafted onto the dendrimer shell as two-photon “antennas” that funnel the captured energy to the metalloporphyrin by way of intramolecular energy transfer20,21.
The residual fluorescence of the coumarin units in the probe enabled visualization of the BM vasculature by conventional two-photon excited fluorescence, while the bone was visualized simultaneously by the collagen second harmonic generation (SHG) signal14. We confirmed that the BM contains high vascular density (Fig. 1a–c), with > 95% of voxels in the image stack located < 25 μm from the nearest blood vessel (Fig 1b–c). We then quantified BM pO2 at multiple intravascular locations using 2PLM and obtained values that ranged from 11.7 to 31.7 mmHg (1.5–4.2%) with a mean of 20.4 mmHg (2.7%). These values are significantly lower than the pO2 in the microvasculature of the brain24–26, the periosteum, and the cortical bone (Fig. 1d). In many instances we were able to follow individual blood vessels as they penetrated from the bone into the BM cavity (Fig. 1e). When traced along individual vessels, we detected steep drops in pO2, measured immediately before and after entrance into the BM cavity (Fig. 1e–f). The rapid depletion of oxygen along the direction of blood flow, where the blood transits from a region of low cellularity (cortical bone) to a region of high cellularity (BM), is reminiscent of the longitudinal pO2 gradient observed when blood vessels enter solid tumors with high metabolic demand27.
Figure 1. BM vascular density and oxygenation.
(a) Intravital maximum intensity image (~75 μm thick) of mouse calvarial BM showing blood vessels (red, Qtracker 655 vascular dye) and bone (blue, collagen SHG). (b) Corresponding single plane of the 3D intravital imaging stack showing blood vessels (red), bone SHG (blue), and the 3D Euclidean distance measurement (EDM, green) to the nearest blood vessel wall for each extravascular pixel in the BM. (c) Histogram of all EDMs from the full 3D imaging stack. (d) BM pO2 is significantly lower compared to pO2 in periosteal and cortical bone vessels. Each point represents a pO2 measurement in a separate blood vessel or interstitial position (n = 8, 21, 55, and 40 vessels/locations for periosteum, cortical bone, BM intravascular, and BM extravascular, respectively, from 8 mice). The mean (black line) and ± standard deviation (shaded box) for each data set is shown. (e) Maximum intensity projection image montage of a blood vessel entering the BM from the bone. Bone (blue) and blood vessels (yellow) are delineated with SHG and RhodamineB-dextran/PtP-C343 fluorescence, respectively. The two arrows point to locations of pO2 measurements just before and after the vessel enters the BM. (f) Drop in pO2 when tracking along individual vessels upon entry into the BM (n = 8 vessels from 4 mice). The mean (black line) is shown for each data set. Scale bars ~100 μm.
Because of the relatively high permeability of the BM vasculature, the injected probe (diameter ~3–4 nm)20 diffused out of the blood vessels and accumulated in sufficient concentrations within minutes (Extended Data Fig. 4) to allow pO2 measurements in the interstitial space. In accordance with the mathematical models17,18, we found a steep lateral pO2 gradient away from the blood vessels, with a mean extravascular pO2 of 13.3 mmHg (1.8%) and a range of 4.8 to 21.1 mmHg (0.6–2.8%) (Fig. 1d).
Our measured pO2 in the BM (~20.4 mmHg intravascular, ~13.3 mmHg extravascular) agreed well with a previously reported value of ~18 mmHg obtained using an oxygen electrode7. That measurement, however, lacked spatial resolution, and the insertion of the needle electrode most likely damaged the microvasculature so that only an averaged reading of intravascular and extravascular pO2 was recorded. Nevertheless, taken together these results support the notion that the BM as a whole is a hypoxic tissue despite its high vascularity.
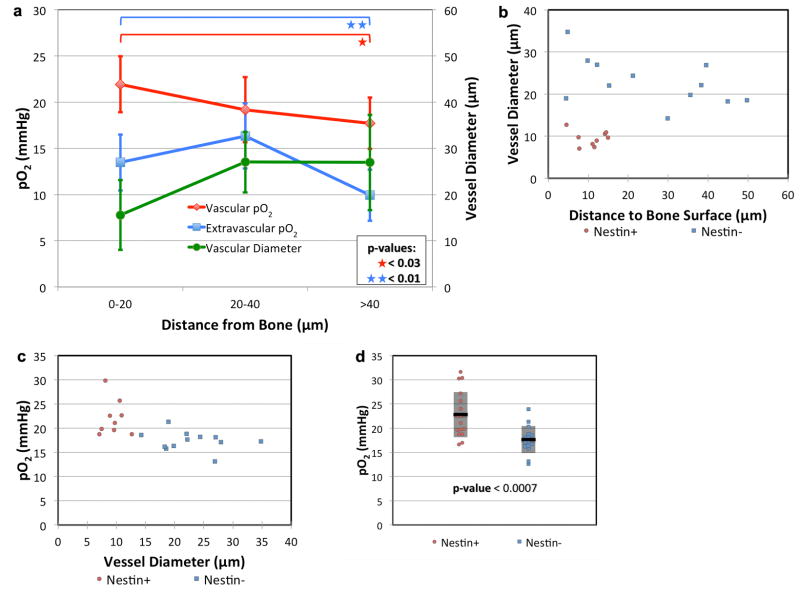
To provide a finer grained view of oxygen distribution within the BM, we noted that the vasculature is heterogeneous, with smaller vessels located closer to the endosteal surface (Fig. 2a). 2PLM of blood vessels with diameters < 15 μm shows higher pO2 (22.7 mmHg, 3.0%) than blood vessels with diameters > 15 μm (19.5 mmHg, 2.6%, p < 0.03). Accordingly, when we analyzed the pO2 values at different distances from the endosteum, we found the lowest pO2 to be in the regions > 40 μm from the bone, with values of 17.7 mmHg (2.4%) in the vessels and 9.9 mmHg (1.3%) outside the vessels (Fig. 2a). In the endosteal region (0–20 μm zone) where most of the smaller vessels are located, the pO2 readings were slightly higher, with values of 21.9 mmHg (2.9%, p < 0.03) in the vessels and 13.5 mmHg (1.8%, p < 0.01) outside the vessels (Fig. 2a). These measurements uncovered a moderate pO2 gradient with distance from the endosteum, but the direction of the gradient was unexpected. Instead of the endosteal zone being the most hypoxic, we found that the pO2 decreased with increasing distance away from the endosteum toward the more hypoxic sinusoidal region.
Figure 2. Variation in BM pO2 according to vessel diameter and distance from the endosteal surface.
(a) Average vessel diameter (green line, n = 38 vessels from 4 mice) and intravascular (red line, n = 38 vessels from 4 mice) and extravascular (blue line, n = 39 locations from 3 mice) pO2 are plotted as a function of the distance from the bone surface to the measurement location. Error bars are the ± standard deviations. (b) The diameter of nestin+ (red circles, n = 9 vessels from 3 mice) and nestin- vessels (blue squares, n = 12 vessels from 3 mice) are plotted as a function of the distance from the endosteal surface. (c) The pO2 of the same nestin+ and nestin- vessels are plotted as a function of vessel diameter. (d) Nestin+ (red circles, n = 17 vessels from 7 mice) and nestin- (blue squares, n = 16 vessels from 5 mice) vessel pO2. The mean (black line) and ± standard deviation (shaded box) for each data set is shown.
As there are currently no HSC-specific markers for visualizing the endogenous stem cells under homeostatic conditions, we used the nestin-GFP mouse to help identify the locations where HSCs reside. Nestin is expressed by a subset of BM mesenchymal stem cells (MSCs) that are involved in HSC maintenance and trafficking9, and perivascular nestin-GFP bright cells define a quiescent HSC niche16. We reasoned that if hypoxia regulates quiescence5, then nestin-GFP cells should be a landmark for hypoxic regions in the BM. In nestin-GFP mice, we confirmed that nestin+ cells are predominantly perivascular, and transplanted HSCs home close to nestin+ vessels (Extended Data Fig. 5)9. Nestin+ vessels are anatomically distinct from nestin- vessels, being located closer to the bone surface (10.9 μm vs. 25.5 μm, p < 0.02) and much smaller in diameter (9.5 μm vs. 22.9 μm, p < 2×10−6) (Fig. 2b). Contrary to our expectation, we found the pO2 to be significantly higher in nestin+ compared to nestin- vessels (22.8 mmHg vs. 17.6 mmHg, p < 0.0007) (Fig. 2c–d).
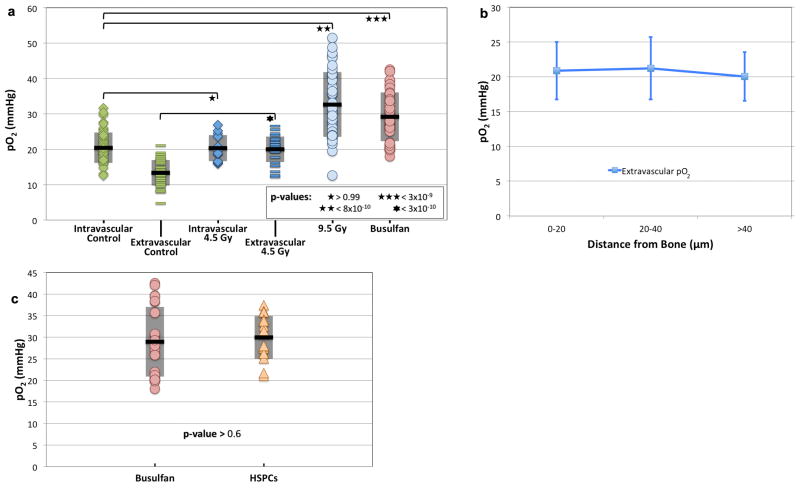
Next we examined whether transplanted hematopoietic stem/progenitor cells (HSPCs) home to BM niches with distinct pO2 levels. Prior to transplantation, recipient animals were given cytoreductive conditioning with either radiation or chemotherapy (busulfan, 35 mg/kg). Remarkably, both treatments resulted in a substantial elevation in the overall BM pO2 (Fig. 3a). The rise in pO2 was striking considering that the vasculature was severely damaged after both radiation and chemotherapy (Extended Data Fig. 6a–c). Moreover, while the intravascular pO2 values were essentially unchanged after sub-lethal irradiation (4.5 Gy), the gradient between intravascular and extravascular pO2 disappeared, with the extravascular pO2 rising to the same level as the intravascular pO2 (Fig. 3a). The “negative” gradient of decreasing pO2 with increasing distance away from the endosteum also disappeared (Fig. 3b). In the case of lethal irradiation and busulfan treatment, the extensive vascular damage caused such widespread dye leakage that the boundary between intravascular and extravascular spaces could no longer be delineated.
Figure 3. BM pO2 after cytoreductive therapy and at sites of HSPC homing.
(a) BM pO2 two days after sublethal (4.5 Gy, n = 13 vessels and 29 extravascular locations from 5 mice) or lethal (9.5 Gy, n = 40 locations from 2 mice) gamma irradiation, or after busulfan (35 mg/kg, n = 40 locations from 6 mice) conditioning. The untreated control pO2 from Figure 1d is plotted for comparison. (b) Extravascular pO2 measurements are plotted as a function of distance to the bone surface from the measurement location 2 days after 4.5 Gy irradiation (n = 44 locations from 4 mice). (c) Comparison of pO2 at sites of HSPC homing to the overall BM pO2 in busulfan treated recipients (n = 17 HSPCs from 6 mice). No statistically significant difference was observed. For (a) and (c), the mean (black line) and ± standard deviation (shaded box) for each data set is shown.
We performed HSPC transplantation (~105 lineage-cKit+Sca1+ (LKS) cells per recipient) 24 hours after busulfan treatment and measured the pO2 around each LKS cell that homed to the calvarial BM 18–24 hours later. We obtained pO2 values that span nearly the entire range of the (elevated) BM pO2 in the post-chemotherapy setting (Fig. 3c), indicating that HSPCs did not seek out specific niches defined by low pO2 as sites for preferential homing.
These observations compelled us to reexamine blood flow in the BM28. Specifically we asked whether the differences in pO2 observed in nestin+ vs. nestin- vessels and in steady-state vs. radiation/chemotherapy treated animals could be accounted for, at least in part, by the blood flow profiles. We injected CFSE-labeled red blood cells (RBCs) and increased our imaging speed to 120 frames per second (scanning ¼ of the frame size) to enable tracking of individual RBC flow direction and speed. Interestingly, the highest RBC flow velocities are observed in nestin+ vessels (> 2 mm/sec, compared to 0.2–1 mm/sec in sinusoidal vessels). The vascular network map shows that the nestin+ vessels are always upstream of and drain into sinusoidal vessels (Fig. 4a), suggesting that nestin+ vessels have arterial characteristics. In vivo immunostaining29 with fluorescent anti-Sca-1 antibody confirms they are arteries15(Extended Data Fig. 7a).
Figure 4. BM vascular mapping and the effects of cellularity on local pO2 after cytoreductive conditioning.
(a) Vascular connectivity map of a nestin-GFP mouse reveals that nestin+ vessels are upstream of and drain into sinusoids. Arrows indicating blood flow direction, determined by tracking the movement of labeled RBCs with video-rate imaging, are superimposed on a maximum intensity projection image of BM vasculature (red = vascular dye, green = nestin-GFP/RBCs, blue = SHG bone signal). A solid green line indicates a vessel with an adjacent nestin+ cell and a dashed white line indicates a nestin- vessel. Scale bar ~100 μm. (b) In vivo image of the bone marrow of a universal DsRed recipient 5 days after transplantation with 25 million total marrow cells from a universal GFP donor. Red = DsRed, Green = GFP, Blue = SHG from bone. Scale bar ~100 μm. (c) Ki-67 staining of green (donor) and red (host) BM cells by FACS on day 2 (n = 3 mice) and day 5 (n = 2 mice) after transplantation. Each symbol denotes a different mouse. Scale bars (day 2) are the ± standard deviation of the mean. (d) The in vivo pO2 values in regions with large clusters of donor cells (n = 19 locations from 4 mice) compared to small donor clusters/single cells (n = 16 locations from 4 mice) and host clusters/single cells (n = 40 locations from 5 mice). The mean (black line) and ± standard deviation (shaded box) for each data set is shown.
Flow imaging with labeled RBCs also helps to overcome the dye leakage problem after radiation/chemotherapy and enables us to delineate the functional blood vessels by analyzing the tracks of the flowing RBCs in the video sequence. We found a surprisingly robust flow 2–6 days after lethal irradiation (Extended Data Fig. 7b and Extended Data Video 1), a time when the BM cellularity is declining precipitously (Extended Data Fig. 8). A combination of ample blood supply and reduced oxygen consumption (reduced BM cellularity) can explain the elevation of the pO2 after radiation and chemotherapy. The reduced BM cellularity can also explain why the gradient between intra- and extra-vascular pO2 disappears after the myelosuppressive conditioning.
To further establish the link between cellularity and oxygen consumption, we infused 25×106 total marrow cells from GFP donor mice into lethally irradiated DsRed recipients (Fig. 4b). We verified by FACS on day 2 and 5 that most of the proliferating (Ki-67+) cells are in the donor fraction (Fig. 4c). Then, by in vivo imaging, we showed that the bone marrow contained heterogeneous patches of donor (GFP+) and recipient (DsRed+) cells, with corresponding differences in local pO2 (Fig. 4b). Lower pO2 was associated with large clusters of green cells, consistent with the notion that the proliferating cells consume oxygen more avidly (Fig. 4d).
In summary, direct measurements of the absolute pO2 in the BM has revealed a unique hypoxic landscape organized by its dense vascularity (oxygen supply) and high cellularity (consumption). The balance between supply and consumption can be altered by stress such as radiation and chemotherapy. The local topography is further defined by the positioning of different types of blood vessels within the BM (Extended Data Fig. 9). In particular, HSCs near nestin+ arteries and those near sinusoidal vessels will experience different metabolic milieus, highlighting the need to further examine the role of distinct vascular niches in HSC regulation.
Methods
Two-photon pO2 microscope
The microscope is composed of two excitation arms: a video-rate laser-scanning two-photon imaging arm and a point-detection two-photon phosphorescence lifetime sensing arm (Extended Data Fig. 1). Output from a 80 MHz femtosecond laser source (MaiTai, Newport, Mountain View, CA) tuned to 840 or 913 nm is split into s- and p-polarizations by a polarizing beamsplitter (PBS1). The s-polarized beam passes through PBS1 and enters the imaging arm whereas the p-polarized beam is deflected 90° into the point detection arm. The power for each arm can be adjusted by rotating a half-wave plate in front of PBS1. These polarized beams are later recombined by a second polarizing beamsplitter (PBS2) immediately before entering the objective.
For the point-detection arm, the beam is swept across a slit aperture (NT38-560, Edmund Optics) using a galvanometric scanner (6220H, Cambridge Technology) and imaged onto the sample to form a ~3.5 μm scan line. Such one-dimensional scanning simultaneously served two purposes. First, it generated an excitation gate with adjustable pulse duration (15–40 μsec), determined by the scanning speed across the slit aperture. Secondly, it served to reduce the triplet state saturation effects that may accompany stationary excitation30, thus helping to prevent degradation of the point spread function in the axial dimension. After application of the excitation gate, the arrival time of individual phosphorescence photons was recorded using a custom photon counting circuit. The histogram of the photon arrival times was then analyzed to obtain the triplet state lifetime. We found that the standard deviation of the pO2 measurement was typically under 4 mmHg and inversely proportional to the signal-to-noise ratio (SNR, data not shown).
For the imaging arm, we use a similar scan engine as described previously and combine the beam with the point-detection arm using PBS219. The beam is then focused by a 60× 1.2 NA water immersion, infinity corrected, near-infrared coated objective (UPLSAPO 60XW, Olympus America Inc.) with a working distance of 280 μm.
Description and Calibration of PtP-C343
The two-photon antenna, coumarin 343, within PtP-C343 (Extended Data Fig. 3) has a two-photon absorption cross section (σ2) of ~28 GM at 840 nm and a fluorescent quantum yield ϕfl) of ~0.9020. This means that the two-photon action cross-section (η = σ2ϕfl) of PtP-C343 is ~25.2 GM. The FRET efficiency (φFRET) of C343 and PtP is ~0.6820. The phosphorescence lifetime of PtP-C343 in the absence of oxygen (τ0) is ~60 μs20 with a dynamic range well suited for the physiological range of pO2. For calibration, we recorded lifetime measurements in a pH 7.4 buffered solution of PtP-C343 at 38° C in vitro over a range of pO2 recorded independently by an oxygen electrode (OX-500, Unisense A/S, Extended Data Fig. 2). Our results match a published curve for the same batch of dye (Extended Data Fig. 2).
Animal Preparation
All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Massachusetts General Hospital. Male wild-type C57BL/6J (Jackson Laboratory) and C57BL/6 Nes-GFP mice < 6 months of age were used for all in vivo PtP-C343 experiments31. In a typical experiment, anesthesia was slowly induced in mice via inhalation of a mixture of 1.35–2% isoflurane and O2. To maintain anesthesia the mixture was reduced to 1.25% isoflurane with 98.75% O2. This protocol minimizes the effect of anesthesia on tissue pO232. Calvarial bone was exposed through a U-incision in the scalp to create a skin flap and 2% methocellulose gel was placed on the scalp for refractive index matching.
The mouse then received an intravenous injection of 100–180 μl of 1.7 mM PtP-C343 suspended in 0.9% phosphate buffered saline (1x PBS, Invitrogen) and 40–60 μl of 10 mg/ml 70 kDa RhodamineB-Dextran (Life Technologies Corporation). We used relatively high amounts of PtP-C343 in order to accomplish a sufficient concentration outside of the blood vessels for interstitial measurement of pO2. The mouse was placed on a heated stage with the skull positioned under the objective as described previously29. An approximately 4×6 mm area of the calvarium encompassing most of the parasagittal BM cavities within the left and right frontal bones was scanned in each imaging session and appropriate locations were selected for pO2 measurements. In agreement with previous in vivo studies24–26, no apparent toxicity or phototoxicity was observed using the PtP-C343 probe. After imaging, the scalp was closed with 6-0 sutures and the mouse allowed to wake up. For terminal experiments, the mice were either sacrificed by dislodgement of the spinal cord or by euthanasia with CO2.
When analyzing vessel size as a function of distance to the endosteal surface, we noticed that vessels 20–40 μm and > 40 μm away from the bone surface were generally larger than the 0–20 μm zone with average diameters of 27.0 and 26.9 μm, respectively (p < 0.002 and 0.06, respectively, Fig. 2a). The region > 40 μm away from the bone surface constituted a dense network of sinusoidal vessels, which were more irregular in shape compared to vessels near the bone surface (Fig. 2a).
For cytoreductive conditioning, mice were conditioned on Day 0 with either irradiation (4.5 Gy or 9.5 Gy) or chemotherapy (busulfan, 35 mg/kg) and imaged on Day 2. For HSPC transplantation, ~105 FACS sorted HSPCs (lin-/c-kit+/Sca1+) were stained with Cell-Tracker Green (Life Technologies Corporation) and injected intravenously on Day 1.
For whole BM transplantation, four-month old male C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) or male actin-DsRed knock-in mice (Cg-Tg(CAG-DsRedMSTNagy/J, Jackson Laboratory, Bar Harbor, ME) were lethally irradiated with a single dose of 9.5 Gy gamma irradiation from a Cs137 source (Gammacel Exactor, Nordion, Ottawa, CA) 4 hours before rescue with 25×106 whole bone marrow cells from a donor actin-GFP knock-in mouse (C57BL/6-Tg(CAG-EGFP)131Osb/LeySopJ, Jackson Laboratory, Bar Harbor, ME).
On the fifth day following irradiation and transplantation, the actin-DsRed recipient mice were imaged and pO2 measurements acquired in the BM. C57BL/6J mice were sacrificed, femurs and tibias extracted, and the marrow prepared for analysis of cycling cells. RBCs were lysed on ice using Red Blood Cell Lysing Buffer (Sigma Chemical Co., St. Louis, MO). The remaining bone marrow cell fraction was counted, fixed, permeabilized and stained with PE-conjugated Ki-67 according to the manufacturer’s recommendations (BD Pharmingen/Biosciences, San Diego, CA). Analysis of cycling cells was performed on a SORP FACSAria II instrument (BD Biosciences, San Diego, CA).
For vascular mapping, we extracted blood from a donor C57BL/6 mouse, isolated the RBCs, labeled 7×106 RBCs with CFDA-SE (Life Technologies Corporation) @ 15 μM for 12 min @ 37° C (labeling concentration ~20×106 RBC/mL, in PBS without Ca + 0.2% bovine serum albumin + 0.2 g/L glucose), and washed 2x. We then suspended the RBCs in 200 μL of 400 nMQtracker 655 (Life Technologies Corporation) vascular label-PBS solution or 200 μL of 3 mg/ml 70 kDa RhodamineB-Dextran vascular label-PBS solution and intravenously injected the solution into the donor Nes-GFP mice immediately prior to imaging.
For in vivo anti-Sca-1 staining, we first demonstrated the in vivo specificity of the anti-Sca-1 antibody. We intravenously injected 20 μg of Trustainfcx (BioLegend catalog #B164564) into a C57BL/6J mouse to block non-specific Fc binding. Two hours later, we intravenously injected ~15 μg of PE-Cy7 anti-Sca-1 antibody (BD Biosciences catalog #108114) and ~15 μg of PerCP-Cy5.5 Rat IgG2a isotype control antibody (eBioscience catalog #45-4321-80). We then imaged the BM 24 hours later and found that anti-Sca-1 antibody had high specificity compared to the isotype control (data not shown). We then repeated the same protocol in a Nes-GFP mouse.
Image quantification
Using the axial-stacks, the distance of each pO2 measurement to the nearest bone surface (endosteum) was computed as described previously14. Briefly, the shortest distance to the endosteum was determined in three dimensions using the Pythagorean Theorem. The vessel diameter was measured using the Rhodamine B dextran signal. This measurement was only performed in the x-y plane and is the diameter of the lumen of the vessel. Both the distance and vessel diameter measurements were performed in ImageJ v. 1.47p. For display purposes, the brightness and contrast of Figure 1a–b, 1e, 4a–b, Extended Data Figures 4, 6, and 7, and Extended Data Video 1 were adjusted. All image analysis was performed on raw images. In Figure 1a, the maximum intensity projection image displayed only includes ~23 μm of depth in the blue channel. We did not include the full thickness for the blue channel because the bone SHG signal covers over the majority of the image at the most shallow depths.
For distance measurements to blood vessels, 3D stacks were re-sliced using Fiji (Image JA v. 1.45b) into the vertical planes (Y-Z, X-Z) and digitally rotated to remove the tilt of the skull relative to the imaging plane. Subsequently, for each R/G/B channel, the grayscale intensity profile along the Z direction was measured and fitted into an empirical exponential decay curve, the inverse of which was then multiplied to each stack image to equalize the image intensity as a function of depth. Next, the 3D stack was converted into binary images and manually verified for structural accuracy. Applying the Exact Euclidean Distance Transform (EEDT) function on the binarized red (blood vessel) channel stack returned the 3D distance of each non-vascular pixel to the nearest blood vessel wall as a 32-bit grayscale image stack. Pixels outside the bone marrow space were excluded using binarized blue (bone) channel stack. A histogram of EEDT distances hence described the distribution of distances from each pixel in the intra-marrow, non-vascular space to its nearest blood vessel.
Signal-to-noise ratio and statistical analysis
The signal-to-noise ratio (SNR) of the pO2 measurements was calculated using the following formula:
where n is the number of photon counts and nBD is the background count determined using the background value described above. nBD is the combined signal from dark counts and background from stray light with the majority arising from dark counts. Phosphorescent measurements with a SNR below 20 were excluded from this study.
Sample size required for the experiments was estimated based upon results of preliminary data. All p-values were determined using a two-tailed, unequal variance Student’s T Test except for Fig. 1f and 4c where a two-tailed, paired Students’s T Test and a one-tailed, paired Student’s T Test were used, respectively. For cases where multiple statistical tests were performed on the same data set, statistical significance was determined using the Bonferroni correction. No blinding or randomization was performed for any of the experiments.
Acknowledgments
We thank Dr. Sava Sakadzic for helpful discussion on setting up the 2PLM experiment. This work was supported by the US National Institutes of Health grant HL097748, EB017274 (to CPL), HL097794, HL096372 and EB014703 (to DTS).
Footnotes
Author Contributions
J.A.S. designed and built the microscope, designed experiments, conducted research, collected and analyzed data and wrote the manuscript; F.F. designed experiments, conducted research, collected and analyzed data, and wrote the manuscript; E.R. synthesized the PtP-C343 oxygen probe; A.K. helped conduct research and collected and analyzed data; J.W., J.M.R., W.Z., L.J.M., R.T., and R.Y. helped conduct research; C.A. and D.C. helped build the microscope; S.A.V. synthesized the PtP-C343 oxygen probe and wrote the manuscript; D.T.S. designed experiments and wrote the manuscript; C.P.L. designed experiments, sponsored the project, and wrote the manuscript.
Author Information
The authors declare no competing financial interests. Reprints and permissions information is available at www.nature.com/reprints.
References
- 1.Lymperi S, Ferraro F, Scadden DT. The HSC niche concept has turned 31. Annals of the New York Academy of Sciences. 2010;1192:12–18. doi: 10.1111/j.1749-6632.2009.05223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suda T, Takubo K, Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell. 2011 doi: 10.1016/j.stem.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010 doi: 10.1016/j.stem.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Lee KE, Simon MC. From stem cells to cancer stem cells: HIF takes the stage. Current opinion in cell biology. 2012 doi: 10.1016/j.ceb.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Unwin RD. Quantitative proteomics reveals posttranslational control as a regulatory factor in primary hematopoietic stem cells. Blood. 2006;107:4687–4694. doi: 10.1182/blood-2005-12-4995. [DOI] [PubMed] [Google Scholar]
- 6.Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E. Regulation of the HIF-1α level is essential for hematopoietic stem cells. Cell Stem Cell. 2010 doi: 10.1016/j.stem.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 7.Ceradini DJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 8.Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci USA. 2007;104:5431–5436. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Méndez-Ferrer S, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiel MJ, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 11.Calvi LM, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 12.Wang LDL, Wagers AJA. Dynamic niches in the origination and differentiation of haematopoietic stem cells. Nat Rev Mol Cell Biol. 2011;12:643–655. doi: 10.1038/nrm3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lichtman MAM. The ultrastructure of the hemopoietic environment of the marrow: a review. Exp Hematol. 1981;9:391–410. [PubMed] [Google Scholar]
- 14.Celso, Lo C, et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature. 2009;457:92–96. doi: 10.1038/nature07434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nombela-Arrieta C, et al. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nat Cell Biol. 2013;15:533–543. doi: 10.1038/ncb2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kunisaki Y, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502:637–643. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chow DC, Wenning LA, Miller WM, Papoutsakis ET. Modeling pO 2 Distributions in the Bone Marrow Hematopoietic Compartment. I. Krogh’s Model. Biophys J. 2001 doi: 10.1016/S0006-3495(01)75732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chow DC, Wenning LA, Miller WM, Papoutsakis ET. Modeling pO 2 Distributions in the Bone Marrow Hematopoietic Compartment. II. Modified Kroghian Models. Biophys J. 2001 doi: 10.1016/S0006-3495(01)75733-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veilleux I, Spencer JA, Biss DP, Cote D, Lin CP. In Vivo Cell Tracking With Video Rate Multimodality Laser Scanning Microscopy. Journal of Selected Topics in Quantum Electronics, IEEE. 2008;14:10–18. [Google Scholar]
- 20.Lebedev AY, Troxler T, Vinogradov SA. Design of Metalloporphyrin-Based Dendritic Nanoprobes for Two-Photon Microscopy of Oxygen. J Porphyr Phthalocyanines. 2008;12:1261–1269. doi: 10.1142/S1088424608000649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finikova OS, et al. Oxygen Microscopy by Two-Photon-Excited Phosphorescence. Chem Phys Chem. 2008;9:1673–1679. doi: 10.1002/cphc.200800296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vanderkooi JMJ, Maniara GG, Green TJT, Wilson DFD. An optical method for measurement of dioxygen concentration based upon quenching of phosphorescence. J Biol Chem. 1987;262:5476–5482. [PubMed] [Google Scholar]
- 23.Lebedev AY, et al. Dendritic phosphorescent probes for oxygen imaging in biological systems. ACS Appl Mater Interfaces. 2009;1:1292–1304. doi: 10.1021/am9001698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakadzić S, et al. Two-photon high-resolution measurement of partial pressure of oxygen in cerebral vasculature and tissue. Nat Methods. 2010;7:755–759. doi: 10.1038/nmeth.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lecoq J, et al. Simultaneous two-photon imaging of oxygen and blood flow in deep cerebral vessels. Nat Med. 2011;17:893–898. doi: 10.1038/nm.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kazmi SMS, et al. Three-dimensional mapping of oxygen tension in cortical arterioles before and after occlusion. Biomed Opt Express. 2013;4:1061–1073. doi: 10.1364/BOE.4.001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dewhirst MWM, et al. Quantification of longitudinal tissue pO2 gradients in window chamber tumours: impact on tumour hypoxia. Br J Cancer. 1999;79:1717–1722. doi: 10.1038/sj.bjc.6690273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazo IB, et al. Total body irradiation causes profound changes in endothelial traffic molecules for hematopoietic progenitor cell recruitment to bone marrow. Blood. 2002;99:4182–4191. doi: 10.1182/blood.v99.11.4182. [DOI] [PubMed] [Google Scholar]
- 29.Sipkins DA, et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature. 2005;435:969–973. doi: 10.1038/nature03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinks LE, et al. Two-photon microscopy of oxygen: polymersomes as probe carrier vehicles. J Phys Chem B. 2010;114:14373–14382. doi: 10.1021/jp100353v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mignone JL, Kukekov V, Chiang AS, Steindler D, Enikolopov G. Neural stem and progenitor cells in nestin-GFP transgenic mice. J Comp Neurol. 2004;469:311–324. doi: 10.1002/cne.10964. [DOI] [PubMed] [Google Scholar]
- 32.Esipova TV, et al. Two new ‘protected’ oxyphors for biological oximetry: properties and application in tumor imaging. Anal Chem. 2011;83:8756–8765. doi: 10.1021/ac2022234. [DOI] [PMC free article] [PubMed] [Google Scholar]