Abstract
Many farmers in water-scarce regions of developing countries use wastewater to irrigate vegetables and other agricultural crops, a practice that may expand with climate change. There is a number of health risks associated with wastewater irrigation for human food crops, particularly with surface irrigation techniques common in the developing world. The World Health Organization recommends using quantitative microbial risk assessment (QMRA) to determine if the irrigation scheme meets health standards. However, only a few vegetables have been studied for wastewater risk and little information is known about the disease burden of wastewater-irrigated vegetable consumption in China. To bridge this knowledge gap, an experiment was conducted to determine volume of water left on Asian vegetables and lettuce after irrigation. One hundred samples each of Chinese chard (Brassica rapa var. chinensis), Chinese broccoli (Brassica oleracea var. alboglabra), Chinese flowering cabbage (Brassica rapa var. parachinensis), and lettuce (Lactuca sativa) were harvested after overhead sprinkler irrigation. Chinese broccoli and flowering cabbage were found to capture the most water and lettuce the least. QMRAs were then constructed to estimate rotavirus disease burden from consumption of wastewater-irrigated Asian vegetables in Beijing. Results indicate that estimated risks from these reuse scenarios exceed WHO guideline thresholds for acceptable disease burden for wastewater use, signifying that reduction of pathogen concentration or stricter risk management is necessary for safe reuse. Considering the widespread practice of wastewater irrigation for food production, particularly in developing countries, incorporation of water retention factors in QMRAs can reduce uncertainty regarding health risks for consumers worldwide.
Keywords: Agriculture, irrigation, wastewater, public health, China
1. INTRODUCTION
Agricultural irrigation with wastewater is a common practice in many arid regions of the world, particularly of vegetable crops.(1) One of the major concerns associated with wastewater irrigation is the human health risks from the pathogens it carries. Microorganisms such as viruses, bacteria, and protozoa are common in urban wastewater and can cause gastroenteritis, giardiasis, campylobacteriosis, and other diseases through a waterborne or food borne route.(2) The World Health Organization (WHO) guidelines for the safe use of wastewater in agriculture require stringent assessment of pathogenic hazards, generally through epidemiological study or a probabilistic modeling technique called quantitative microbial risk assessment (QMRA) to determine the health risks from wastewater irrigation.(3) While epidemiological studies can determine spatial and temporal exposure factors to incidence of disease in a population,(1) such experimental work is difficult to carry out due to lack of opportunity or finances. Sample size is often restricted by logistics and cost, and a compromise is always struck between the power of the statistical significance and financial capability.(4)
The QMRA approach has thus been widely used for microbiological and human health assessments. This four-step process involves (i) hazard identification, (ii) exposure assessment, (iii) dose-response modeling, and (iv) risk characterization.(5, 6) Briefly, hazard identification constitutes determining the pathogen of concern, exposure assessment covers defining the exposure pathway and determining the pathogen concentration the consumer encounters, dose-response modeling frames the probability of infection as a function of pathogen dose, and finally, risk characterisation brings together the exposure and dose-response models to estimate probability of infection or illness.(1) This methodology is a powerful tool for estimating order-of-magnitude risk and has been successfully applied to a variety of pathogens and exposure scenarios.(7-9)
The difficulty with such QMRA models is the high input of information required. Among the knowledge gaps is the lack of information on pathogen exposure on vegetables from irrigation. Many studies have estimated this pathogen load through estimation of irrigation water retention on vegetables and the conservative assumption that all pathogens in the wastewater will attach to the plant surface.(7, 10, 9, 11, 12) There have been attempts to quantify the pathogen directly on the plant surface after wastewater irrigation to estimate microbial risk,(13, 14) but this method will allow only for modeling a particular set of conditions that led to that pathogen load on the vegetable. Utilizing water retention parameters can allow for more flexible modeling of multiple scenarios where the pathogen source changes.
Measurements of irrigation water retention on crops have only been done for lettuce, broccoli, cucumber, and cabbage,(15, 10) however, thus restricting the possibilities of modeling irrigation risks for other agricultural crops. Furthermore, the study of water retention on lettuce and cucumber was conducted under unrealistic irrigation conditions where the vegetables were submerged in water and then weighed.(15) While lettuce, broccoli, cucumber, and cabbage are grown world-wide, these are not the only vegetables consumed, particularly in Asia. Farmers in Asia were responsible for harvesting 73.5% of the world’s vegetable harvest by area in 2011.(16) Asia also accounts for the majority of the world’s wastewater irrigation. China is the dominant practitioner of wastewater irrigation, with at least 1.3 million ha of land under cultivation with raw or poorly-treated wastewater—an order of magnitude greater area than the rest of the world combined!(17) Considering that China is the world’s most populated country, there may be millions of people exposed to health risks from wastewater irrigation. Despite such persuasive figures, to date there have not been any studies on the microbial risks attendant with wastewater irrigation of vegetables in China, let al.one risks from local varieties such as Asian vegetables.
Asian vegetables are also increasing in prominence in the Western diet. For example, in Australia, production of Asian vegetables has increased from 37,000 tons to over 47,000 tons from 2005-2008.(18) Nevertheless, QMRAs for wastewater irrigation in the West have thus far been concerned with conventional vegetables, such as lettuce and cabbage.(7, 19, 15, 9, 10, 20) This may be because such vegetables still dominate the overall market in Western countries, but sound public health protection measures demand consideration of sub-populations, such as ethnic communities that consume other vegetables. Even if Asian vegetable consumption in Western countries is considered low compared to other vegetables, there is still a significant number of Asian countries that consume these varieties and with 60% of the world’s population in Asia,(21) it is necessary to take steps to estimating health risks for these communities. Additionally, Asian vegetables are structurally dissimilar to typical leafy vegetables in the West and may in consequence capture a markedly different volume of contaminated water and thus confer a different level of risk.
The purpose of this study is two-fold. Firstly, we determine water retention volumes for three commonly grown Asian vegetables, and thus provide useful exposure information for Asian vegetable QMRAs constructed for any setting in the future. A detailed comparison of the water retention methodology to published data by Hamilton et al. (10) and Shuval et al. (15) is provided in the discussion. Secondly, we use QMRA to estimate rotavirus disease burdens associated with wastewater irrigation of Asian vegetables as a case study. Our focus is on diarrheal disease for children because diarrheal disease accounts for 74% of global deaths from diseases transmitted via wastewater and 90% of those deaths can be attributed to this vulnerable group.(3) Rotavirus is chosen as the pathogen of concern because the gastroenteritis it causes is largely a pediatric condition.(22) China is used as the case study for the modeling, not least because it hosts one fifth of the world’s population and likely a considerable portion of the world’s consumers of Asian vegetables, but also because the consumption behavior of these vegetables has been studied there. More specifically, Beijing is used as the illustrative city from a wastewater quality perspective, partly because of availability of data on rotavirus concentration in wastewater, but also because we believe it is a reasonable sanitation representative of a large Chinese city and because rotavirus has indeed been documented to be the overwhelmingly dominant viral cause of diarrheal disease in children in Beijing.(23-25)
2. METHODS
2.1 Vegetable Selection
Leafy Asian vegetables are commonly found all across Asia and consequently the same variety can be referred to by multiple names in different languages. Chinese chard (Brassica rapa var. chinensis) is a leafy vegetable with smooth dark green blades forming a cluster. There are many variations to its name and spelling, including the English names Chinese cabbage and Chinese mustard as well as Chinese names bok choy, pak choi, bai cai or qing cai (Fig 1A).(26) The Chinese flowering cabbage (Brassica rapa var. parachinensis), choy sum or cai xin in Chinese, is another common variety of Brassica rapa with more slender stems and yellow flowers (Fig 1B).(26) Chinese broccoli (Brassica oleracea var. alboglabra), known as gai lan or kai lan in Chinese, is another leafy vegetable with thick green leaves and thick stems (Fig 1C).(26) It belongs to the same species as broccoli, but in a different varietal group, alboglabra. Since these three leafy vegetables are all commonly grown and consumed in Asia and around the world,(27, 28) they were chosen to be modeled for this study and will be referred to as bok choy, choy sum, and gai lan respectively.
Figure 1.
Photos of a) bok choy, b) choy sum, c) gai lan, and d) green oak lettuce from Butler Market Garden.(31)
In addition to the leafy Asian vegetables, this study also focuses on lettuce (Lactuca sativa). Although water retention by iceberg lettuce has been measured before, the experiment involved the unrealistic and worst-case scenario of complete submersion in water as well as a small sample size of 12.(15) This quantification of water retention is likely contributing to overestimation of pathogen exposure on lettuce, thus it is important to refine the measurement with a larger sample size and more realistic irrigation conditions. In addition, while iceburg lettuce is popular in the US, it is structurally dissimilar to the other common types of lettuce such as leaf or butterhead lettuce due to its densely packed heart.(29) To account for cultivar variation, green oak lettuce was selected for this study. Green oak lettuce belongs to the butterhead lettuce group, a popular type of lettuce widely grown around the world with loose folds and large ruffled leaves.(30)
2.2 Volume Capture
The volumes of water caught by Chinese chard, Chinese flowering cabbage, Chinese broccoli, and green oak lettuce were determined from field experiments at Butler Market Gardens, a commercial market garden on the outskirts of Melbourne, Victoria, Australia (38°24 S, 144°53 E). All trials were conducted on 28 January 2013. All the vegetables were harvested immediately after a scheduled irrigation of 30 minutes, the normal irrigation duration. Crops were planted in beds that comprised three staggered rows, with plants in each row spaced 30 cm apart. Standard fixed-set overhead sprinklers were positioned between every two beds at 10-m intervals. A systematic sampling protocol was used, whereby every other plant in a 10 m by 10 m area around the sprinkler was harvested for a total of 100 plants per crop type. Plastic bags were carefully placed over the plant and gathered at the bottom where the soil met the bag before cutting off the plant at its base with a harvest knife.
All samples were transported to a laboratory, where they were weighed before and after being dried with a lettuce spinner and paper towel to remove surface water. Bok choy, gai lan, and choy sum had their leaves separated to ensure that no water was trapped. Likewise, lettuce was cut into small sections prior to being spun. Probability density functions (PDF) were fitted to the volume data using @Risk software (Palisade Corporation, Newfield, New York).
2.3 Quantitative microbial risk assessment model
2.3.1 Hazard assessment and exposure model
Probabilistic QMRAs were constructed using the experimental exposure factors to simulate the human health risks to children from year-round consumption of each of the vegetables after overhead irrigation with secondary treated wastewater in Beijing, China. Rotavirus is the leading cause of severe diarrhea in infants and young children, particularly in developing countries, where rotavirus vaccine is still not widely administered.(32, 22) The Lanzhou lamb rotavirus (LLR) vaccine has been in use in China since 2000, but the vaccination rate is low. A study of LLR vaccine effectiveness in Guangzhou, China found only 14.4% of its 6737 study participants had valid doses of the vaccine.(33) Rotavirus still remains the most common agent for acute diarrhea for children in Beijing.(23-25)
The rotavirus dose the consumer is exposed to on the kth day (λk; virus ingested/person day) was defined as
| (1) |
where C is the concentration of rotaviruses in the wastewater (viral unit/L), V is the volume of irrigation water caught by the crop from overhead spray irrigation (mL/g), M is the daily consumption of the vegetable per capita per person (g/person day), k is the in-field virus kinetic decay constant (day−1), W is the reduction in virus concentration through post-harvest vegetable washing (log10 unit), and t is the withholding time between the last wastewater irrigation event and harvest/consumption (days). Fit parameters for all probability distributions are shown in Table I.
Table I.
Distributions and fit parameters used in the models. AIC = Akaike information criterion.
| Model parameter | Unit | Distribution type (values)a ~ Medianb |
References and Fit Statistics |
|---|---|---|---|
|
C = rotavirus concentration in secondary treated wastewater at Beijing wastewater treatment plants |
viral unit/L | Mixture (A, B) with 60% recovery efficiency ~ 0.02 A = Lognormal (−2.53, 1.33) B = Lognormal (−13.69, 4.45) |
(34-36) |
|
V = volume of water captured by vegetable |
mL/g | ||
| Bok choy | Lognormal (−3.79, 0.37) ~ 0.02 |
Chi Sq = 12.8600 AIC = −668.2724 |
|
| Choy sum | Lognormal (−3.08, 0.48) ~ 0.05 |
Chi Sq = 10.7917 AIC = −457.7444 |
|
| Gai lan | Lognormal3 (−2.99, 0.45, 0.005) ~ 0.06 |
Chi sq = 10.2222 AIC = −461.97 |
|
| Lettuce | Lognormal3 (−4.57, 0.50, 0.006) ~ 0.02 |
Chi Sq = 9.0309 AIC = −739.0825 |
|
|
M = per capita consumption of vegetable in China |
g/person day |
||
| Bok choy | Lognormal (3.03, 2.12) ~ 4.59 |
(37) | |
| Choy sum | Lognormal (2.87, 1.99) ~ 16.25 |
(37) | |
| Gai lan | Lognormal (3.69, 0.58) ~ 39.33 |
(37) | |
| Lettuce | Lognormal (4.35, 1.27) ~ 76.64 |
(37) | |
|
k = in-field virus kinetic decay constant |
days−1 | Normal (1.07, 0.07) – truncated at zero ~ 1.07 |
(7, 38) |
| t = withholding period | days | Uniform (0, 2) ~ 0.97 | |
|
W = reduction of virus concentration by post-harvest vegetable washing |
log10 unit | PERT (0.1, 1, 2) ~ 1.01 | (39-41, 9) |
| Rotavirus dose-response parameters |
Hypergeometric Beta Poisson α = 0.167, β = 0.191 |
(42) | |
|
I = proportion of rotavirus-infected persons in developing countries who become ill |
proportion | 0.90 | (32) |
|
D = rotavirus disease burden for China |
DALY per case |
Uniform (5.79 × 10−2, 2.13 × 10−1) ~ 0.11 |
(43, 44) |
| SF = susceptibility fraction | proportion | 0.059 | (45, 21) |
Distribution types and values: Lognormal(μ, σ), where population parameters and are calculated as follows: and , where is the sample mean and s is the sample standard deviation; Lognormal3(μ, σ, location), where location is a shift parameter; Normal(mean, standard deviation); PERT(minimum, mode, maximum); and Uniform(minimum, maximum).
Median values calculated after 10,000 iterations
The Mixture distribution for rotavirus concentration in secondary treated wastewater (C; viral unit/L) was obtained from two studies that monitored virus concentrations at six different urban wastewater treatment plants across Beijing.(34, 35) Although these studies by no means represent a comprehensive survey of wastewater quality in Beijing, there are few datasets for municipal wastewater quality in China, thus this is likely as complete a picture for wastewater given the lack of data available. Both studies used similar methodology: SiO2 and AlCl3 amendments added to water samples for concentration of viral particles, use of Qiagen RNA extraction kits, and amplification of rotavirus through reverse-transcription PCR with primers designed to target the VP7 gene of group A rotaviruses.(34, 35) This coupled with the fact that all the wastewater treatment plants in the studies used activated sludge processes with a sedimentation step justifies the combination of the datasets for this model. Concentrations determined by both studies were for rotavirus genomes,(34, 35) so it was assumed that the infectious rotavirus concentrations were 1/1000 of the rotavirus genomes.(46) Virus concentration was assumed to be Lognormally distributed.(47) Recovery efficiency was not reported in either study, so recovery efficiency of 60% from an analogous study that examined poliovirus concentration in wastewater in Switzerland using the same molecular extraction methods was used.(36) It was assumed that recovery efficiencies for poliovirus and rotavirus would be similar. This recovery efficiency was applied to the sample means () and standard deviations (s) of rotavirus concentrations from the Beijing studies before calculating μ and σ for two separate Lognormal distributions. Random samples from both distributions (n = 10,000) were used to create an overall Mixture distribution for C. Values were randomly drawn with replacement from this distribution for λk.
Daily consumption data were obtained from the China Health and Nutrition Survey.(37) Daily per capita consumption values were obtained for vegetable codes 45107, 45112, 45209, and 45315, which correspond to bok choy, choy sum, gai lan, and lettuce respectively.(48) Using the reported means and standard deviations, and following the assumption that vegetable consumption would be Lognormally distributed, distributions were created for daily vegetable consumption in China (M; g/person day).
The log10-reduction in virus concentration from vegetable washing has been investigated in several studies.(39-41) The findings from these studies indicate that viral reduction from washing produce with tap water is between 0.1 to 2 log10 units, with a mean reduction of 1 ± 0.2 log10 unit. Following the approach of Barker et al.,(9) this was used as the most likely value in the PERT distribution for W (Table I).
The constant of first-order virus decay model was assumed to be Normally distributed, following experiments on Bacteroides fragilis bacteriophage B40-8 on lettuce.(7, 38) Postharvest decay of virus was considered negligible (49) and therefore was not included in the model, following the methods of other QMRA studies.(9, 7, 10)
2.3.2 Dose-response
The probability of infection for the kth day (pinf; person−1 day−1) was calculated using the hypergeometric Beta-Poisson dose-response model, given as
| (2) |
where 1F1() is the Kummer confluent hypergeometric function. Values for fit parameters α and β (0.167, 0.191) were determined by Teunis and Havelaar (42) using data from a rotavirus infectivity study involving adult volunteers.(50)
The conditional probability of illness after infection (pill; person−1 day−1) was calculated as
| (3) |
where I is the proportion of rotavirus-infected persons who become ill. The point estimate for this proportion in developing countries was taken from Havelaar and Melse (32) and is based on studies of infected children. These daily probabilities were used as inputs for the annual probability of illness or infection calculation for the jth simulation of the model (P; person−1 year−1), which is given as
| (4) |
where pk is the illness or infection probability for the kth iteration of 365 daily exposure events in the jth of 1,000 simulations, and where events are assumed to be independent. It was assumed that wastewater irrigation of vegetables and consumption of those vegetables occurred year-round; therefore, the number of exposure events was set to 365. Variability in vegetable consumption by the Chinese population was accounted for in the empirical distribution for the parameter M (Table I). (37)
Annual disease burden was calculated using the Disability Adjusted Life-year (DALY) metric, expressed as the number of years lost due to illness, disability or premature death. The annual disease burden (A; DALY per person per year [pppy]) for rotavirus illness was estimated as
| (5) |
where P is the annual probability of illness, D is the rotavirus disease burden (DALYs per case of rotavirus illness), and SF is the proportion of population susceptible to the disease. Rotavirus disease burden for children under age of 5 for China and middle income countries in Asia were investigated by several studies and a Uniform distribution for disease burden was constructed from the values reported in the studies.(43, 44) The susceptibility fraction was determined based on findings by Pott et al.,(45) which indicated that the immune response to rotavirus was age-dependent and persons ages 5 and over show greater expression of the innate Toll-like immune receptor for viral dsRNA, meaning those under 5 have less immunity. Although infants from 6-24 months show the highest rotavirus infection rates,(24, 51, 23) children up to age 5 show vulnerability to rotavirus,(52, 22) so this latter age group was modeled as a conservative estimate of risk. The proportion of the Chinese population in 2013 for children from ages 0-4 is 5.9%,(21) which was used for the parameter SF.
To account for uncertainty and variability in the parameters, each model involved Monte Carlo simulation of 3,650,000 iterations of the daily probability calculations, which provided enough values for 10,000 simulations of the annual probability calculations. Each iteration involved drawing a set of values from the input parameter probability distributions (Table 1). The modeling was implemented through R version 3.0.1.(53) Confidence intervals for the predicted disease burdens were calculated using the percentile method.(54)
Sensitivity analysis is useful for investigating the influence of uncertainty and variation of input parameters on the output probability in QMRAs. It generally involves applying correlation or regression techniques to paired arrays of input and output variables, but this process was complicated here by the model structure. Since the annual risks P and A required 365 values from each of the input distribution rather than one, the sensitivity analyses addressed the uncertainty relationships between the input variables and pinf instead. Spearman rank order correlation was conducted after 10,000 iterations in R. Spearman’s correlation technique was preferred to linear regression because most of the relationships were non-linear. Significance testing for correlation between the paired samples through Spearman’s rho was also conducted to investigate whether the Spearman’s rank order coefficient was significantly different from zero.
3. RESULTS
3.1 Vegetable water retention
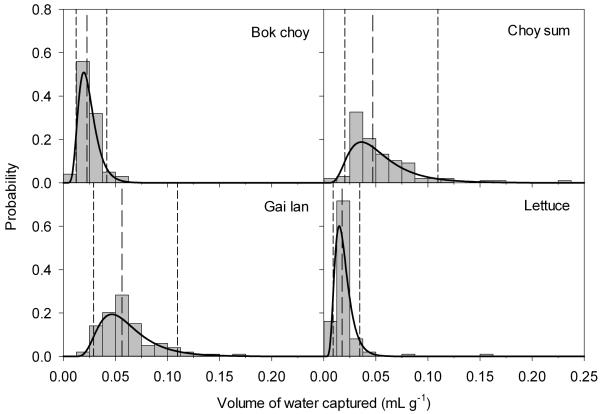
Overall, gai lan and choy sum captured the most water, with a median of 0.06 and 0.04 mL water/g vegetable respectively. Lettuce and bok choy captured the least amount of water at a median of 0.01 and 0.02 mL water/g vegetable respectively. Gai lan and choy sum also had the largest ranges, capturing up to 0.17 and 0.23 mL water/g vegetable respectively. The experimental volume data were successfully fitted with distributions in @Risk (Fig 2). Based on the goodness-of-fit statistics in @Risk, the fits for different distributions were ranked for the experimental data. Smaller goodness-of-fit statistics demonstrate better fits, and the Lognormal and Lognormal3 distributions gave the lowest goodness-of-fit statistics of all the distributions fitted by @Risk. This, coupled with the graphical comparison of the distributions with the experimental data (Fig 2), indicate that these distributions were satisfactory fits for irrigation water retention by these vegetables.
Figure 2.
Histograms of experimental water capture data for the four vegetables overlaid with the corresponding fitted models from @Risk. The y-axis represents probability mass and density for the histogram and the fitted model respectively. The short dashed lines represent the 5th and 95th percentiles of the fitted models and the long dashed lines represent the medians of the fitted models.
3.2 Microbial risk
Overall, choy sum posed the greatest risk of the four vegetables while the smallest risk was associated with consumption of wastewater-irrigated bok choy. The predicted median annual probability of infection ranged from 7.70 × 10−4 year−1 with bok choy to at 4.64 × 10−3 year−1 with choy sum, while gai lan and lettuce posed median risks of 2.67 × 10−3 year−1 and 2.64 × 10−3 year−1 respectively. Similarly, the median annual disease burden ranged from 5.38 × 10−6 DALY pppy with bok choy consumption to 3.25 × 10−5 DALY pppy with choy sum consumption, with gai lan and lettuce again in the middle with disease burdens of 1.89 × 10−5 DALY pppy and 1.87 × 10−5 DALY pppy respectively. The 90% confidence intervals for the DALYs of each vegetable are as follows: bok choy [7.78 × 10−6, 8.66 × 10−6], choy sum [4.50 × 10−5, 4.91 × 10−5], gai lan [2.02 × 10−5, 2.08 × 10−5], and lettuce [2.14 × 10−5, 2.24 × 10−5].
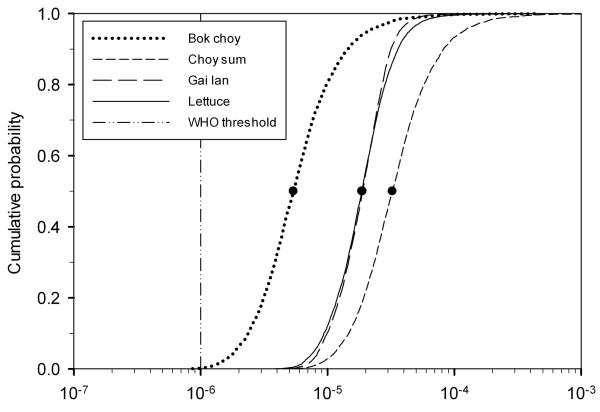
The cumulative probability curves for the annual disease burden show that all the scenarios were within the 10−6 to 10−4 DALY range (Fig 3), thus exceeding the WHO (3) threshold of ≤ 10−6 DALY pppy for acceptable level of risk from wastewater reuse by two to four orders of magnitudes. Bok choy posed the least risk and choy sum the greatest risk while lettuce and gai lan had similar risk profiles.
Figure 3.
Cumulative probability curves of annual rotavirus disease burden for children in Beijing consuming bok choy, choy sum, gai lan, and lettuce irrigated with secondary treated wastewater. The dashed line at 10−6 DALY pppy represents the WHO guideline threshold for acceptable level of annual disease burden and the solid black dots represent the median values in the cumulative probability curves.
Uncertainty in virus concentration had the most significant effects of the random variables on the uncertainty of the daily probability of infection, pinf (Table II). Variation in vegetable consumption had more of an effect for bok choy and choy sum than it did for lettuce and gai lan. The effect of t and washing reduction was similar across the different vegetable scenarios. Daily probability of infection was relatively insensitive to variations in the water retention by the vegetable or the kinetic decay constant in comparison to other parameters.
Table II.
Sensitivity analyses for daily probability of infection.
| Vegetable | C | V | M | k value | t | W |
|---|---|---|---|---|---|---|
| Bok choy | 0.751 *** | 0.090*** | 0.556 *** | −0.029** | −0.142*** | −0.208*** |
| Choy sum | 0.763 *** | 0.121*** | 0.504 *** | −0.008 | −0.166*** | −0.230*** |
| Gai lan | 0.904 *** | 0.119*** | 0.185*** | −0.041*** | −0.197*** | −0.271*** |
| Lettuce | 0.845 *** | 0.097*** | 0.385*** | −0.016 | −0.177*** | −0.239*** |
Values represent Spearman rank order correlation coefficients for input variables in relation to pinf and significance of the coefficients is marked by <0.05,
<0.01,
<0.001. Coefficients > 0.5 are bolded.
4. DISCUSSION
Wastewater reuse for agricultural irrigation is becoming more prevalent around the world as rainfall variability and food needs increase, yet there is still a significant gap in the assessment of human health impacts for such scenarios. Given the long history of wastewater reuse for agricultural irrigation in China,(55) it is significant that this is the first presentation of water retention measurements for Asian vegetables as well as the first viral risk assessment for consumption of vegetables from wastewater irrigation in China. Gai lan and choy sum were found to capture the most irrigation water of all the leafy vegetables studied, but consumption of wastewater irrigated choy sum posed the greatest risk, while consumption of bok choy posed the lowest risk in these scenarios (Fig 3).
The volumes of water captured by the vegetables in this study are for the most part within the same order of magnitude as volumes for other vegetables. Hamilton et al. (10) conducted a similar water capture experiment for broccoli and cabbage and found that the mean volume captured by broccoli was 0.02 mL water/g vegetable while the mean volume for Savoy and Grand Slam cabbage cultivars was 0.04 mL water/g vegetable. Gai lan and choy sum likely captured more water per gram of vegetable due to the leaf structure and the surface area exposed to irrigation. In contrast, the mean volume captured by lettuce as found by Shuval et al. (15) was 0.11 mL water/g vegetable, an order of magnitude larger than the mean volume found for lettuce in this study. The difference in volumes may be due to the different cultivars used (iceberg versus green oak lettuce), but the experimental design may also be important. The Shuval et al. (15) study only had a sample size of 12, in contrast to the sample size of 100 used in this study, and the lettuces were submerged into a container of water, which likely exposed them to more water than in the overhead irrigation scenario studied here. It is important to note that more water captured by the plant does not necessarily indicate more pathogens will adhere to the plant surface. It was assumed in this study, as in others,(7, 15, 9) that all pathogens in the irrigation water would transfer to the plant, but in reality this may vary.
Although water retention parameters are necessary for modeling vegetables in these reuse scenarios, variation in consumption rates proved to have more influence on pinf than variation in water retention in this study (Table II). The bok choy scenarios likely produced the lowest risks because bok choy had the lowest consumption rate among the vegetables here. The vegetable consumption rate in different countries differs drastically; the mean daily per capita lettuce consumption in Australia is 21.81 g lettuce/person day (56) compared to a mean of 171.94 g lettuce/person day in China.(37) Thus, to construct a model that most accurately reflects the scenario at hand, it is important to use as much country or region-specific input data as possible.
The viral decay rate would also vary depending on the plant structure. While viral decay studies have been conducted on various crops,(57, 58) few k values have been derived.(7, 59) The k values used here are for lettuce since viral decay constants for Asian vegetables are not available. Variation in k can have significant bearing on the resultant risk prediction, as seen in this study (Table II) and by others,(10, 60) thus further research on crop-specific viral decay factors would be useful for increasing accuracy of risk estimations.
The dose-response model is a considerable source of uncertainty for this study. The data used to calibrate the rotavirus dose-response equations came from an infectivity trial involving adult volunteers.(50, 42) However, rotavirus primarily affects young children (22, 52, 45) and this model in particular focused on the risks to that age group. It is possible that the rotavirus dose for adults differs from the doses for children. Likely, lower doses induce infectivity in children faster than they do in adults, making the estimated disease burdens from this QMRA model underestimations of the actual risk to children. However, rotavirus infectivity data for children is not available, thus this dose-response relationship is the best estimate for children at this time.
Seasonal variation in the estimated risks would be an area for further research. For this model, further information about the seasonal variation in vegetable consumption would be needed. The China Health and Nutrition Survey is one of the most comprehensive studies of Chinese health and nutrition, but the consumption data was collected over a 3-day period,(37) thus it is impossible to estimate seasonal variation from this dataset. Based on the short vegetable growing season in north-eastern China where Beijing is located, vegetables such as bok choy, gai lan, and choy sum would only be available locally in the summer months,(61) indicating consumption may be higher in summer months than in winter months. However, rotavirus shedding and infection rates are higher in winter months from October – March than in summer months,(34, 35, 23-25) so the lower consumption may be counterbalanced by the higher rotavirus concentrations in the wastewater. More in-depth consumption data would be needed to determine which parameter has a stronger influence on the overall risk.
Since the QMRA models indicated that consumption of wastewater-irrigated vegetables in Beijing did not meet the WHO benchmark, reduction of disease burden will be necessary, which in turn may require higher pathogen reduction standards in the wastewater treatment process. Although there are several Chinese national standards pertaining to wastewater quality, such as The Reuse of Urban Recycling Water-Water Quality Standard for Industrial Uses (GB/T 19923-2005) and The Reuse of Urban Recycling Water-Quality of Farmland Irrigation Water (GB 20922-2007) (55) as well as the Guideline of Urban Sewage Treatment and Pollution Prevention and Control Technology (62), they present only threshold concentrations for E. coli and fecal coliforms, not viruses.(63, 64) While E. coli is often used as an index of fecal pollution to indicate the probability of pathogens, the bacterium does not well represent the presence of protozoa and viral pathogens.(65, 66) There are many factors that influence the correlation between indicator and pathogen species, including shedding patterns among host populations, time of year, carriage rates, environmental stressors, growth, and transport characteristics.(67, 65, 68) If standard pathogen concentrations are to be used effectively, there should be a move away from indicator species such as E. coli towards the pathogens of interest such as viruses. Virus threshold concentrations can aid wastewater treatment plants to better manage their facilities and reduce risk from wastewater reuse.
It is not imperative to have standard pathogen concentrations for reuse however. Risk from wastewater agricultural irrigation schemes depend on a multitude of factors such as irrigation withholding periods, frequency of public access during wastewater application, irrigation method, buffer zones, and level of food processing before consumption.(69) If risks from wastewater agricultural irrigation are to be managed, there should be a process for wastewater users to determine whether the risks associated with their reuse scheme are acceptable or not. Although there are several Chinese national standards regarding wastewater quality, there is to date no Chinese guideline for risk management around wastewater reuse. The risk management approach involves more pro-active identification and management of risk, rather than simply relying on post-treatment testing for managing reuse schemes. Consideration of irrigation withholding periods, buffer zones, and irrigation method through such risk management techniques can offer more flexibility and points of control. Furthermore, given the widespread practice of wastewater irrigation in China,(17) there is a need for better wastewater regulation that will protect consumers’ health and a risk management approach may be the best way to achieve this. The WHO guidelines for wastewater reuse provide a structure for building country-specific reuse guidelines (3) and various countries have incorporated the QMRA method or the ≤ 10−6 DALY pppy threshold into their guidelines.(69, 6)
The interpretation of the threshold should not be prescriptive, however. The ≤ 10−6 DALY pppy value corresponds to a tolerable excess lifetime risk of fatal cancer per person.(3) Using this value as a threshold for acceptable risk would only make sense if the actual incidence of cancer for the place in question was within the order of magnitude, but this is not the case in China. The estimated age-adjusted incidence of all cancers in all sexes across China in 2000 was 2.1 million cases.(70) Dividing this by the population of 1.26 billion in China in 2000,(21) the annual cancer incidence is 1.67 × 10−3 pppy. Aiming to reduce waterborne disease burden when the actual cancer incidence is three orders of magnitude higher will not only burden a significant cost upon a developing country, but also have little impact on overall disease burden. The WHO guidelines state that if the overall burden of disease from other exposure routes is very high, setting a less stringent level of acceptable risk, such as 10−5 or 10−4 DALY pppy may be more realistic for providing safe water quality.(71) As argued by Mara and Sleigh,(20) a 10−4 DALY pppy threshold is a more realistic margin of safety for wastewater reuse in comparison to actual disease incidences. In the scenarios investigated here, none of the irrigated vegetables would have been considered safe to consume under the ≤ 10−6 DALY pppy threshold, but all the vegetables except gai lan would have satisfied the less stringent 10−4 DALY pppy threshold (Fig 3).
QMRA can be a powerful tool for estimating health risks from wastewater reuse. Having the water retention factors for Asian vegetables can open up risk assessments of vegetable irrigation scenarios. These vegetables are widely consumed in Asia and given the large populations in these countries and the widespread use of wastewater irrigation,(1) it would be important to pursue risk assessments for these contexts. The reach of the Asian vegetable market extends well beyond Asia. The Asian vegetable market worth in Australia alone exceeds $204 million,(18) and more than 20,000 Asian American farmers in the US that are catering to the rapidly growing demand for Asian vegetables.(28) Considering the growing practice of wastewater reuse for agricultural irrigation, assessing the health risks from such reuse schemes involving Asian vegetables would aid in the development of better wastewater management policies and better protection of the health of farmers and consumers worldwide.
ACKNOWLEDGEMENTS
This research uses data from the China Health and Nutrition Survey (CHNS). We thank the National Institute of Nutrition and Food Safety, China Center for Disease Control and Prevention; the Carolina Population Center, University of North Carolina at Chapel Hill; the National Institutes of Health (NIH; R01-HD30880, DK056350, and R01-HD38700); and the Fogarty International Center, NIH, for financial support for the CHNS data collection and analysis files since 1989. We thank those parties, the China-Japan Friendship Hospital, and the Ministry of Health for support for CHNS 2009 and future surveys. We also thank Greg Rankin and Butler Market Gardens for their help in the Asian vegetable volume capture trial.
REFERENCES
- 1.Hamilton AJ, Stagnitti F, Xiong X, et al. Wastewater irrigation: The state of play. Vadose Zone Journal. 2007;6(4):823–40. [Google Scholar]
- 2.Toze S. Reuse of effluent water—benefits and risks. Agricultural Water Management. 2006;80(1–3):147–59. [Google Scholar]
- 3.WHO . Series Who guidelines for the safe use of wastewater, excreta and greywater. Volume ii: Wastewater use in agriculture. World Health Organisation; Geneva, Switzerland: 2006. Who guidelines for the safe use of wastewater, excreta and greywater. Volume ii: Wastewater use in agriculture. [Google Scholar]
- 4.Bonita R, Beaglehole R, Kjellstrom T. Basic epidemiology. 2nd edition World Health Organization; Geneva, Switzerland: 2006. [Google Scholar]
- 5.Haas C, Rose J, Gerba C. Quantitative microbial risk assessment. John Wiley & Sons; New York: 1999. [Google Scholar]
- 6.US EPA . Series EPA/600/r-12/618 guidelines for water reuse. U.S. Environmental Protection Agency, National Risk Management Research Laboratory, U.S. Agency for International Development; Washington, D.C.: 2012. EPA/600/r-12/618 guidelines for water reuse. [Google Scholar]
- 7.Petterson SR, Ashbolt NJ, Sharma A. Microbial risks from wastewater irrigation of salad crops: A screening-level risk assessment. Water Environment Research. 2001;73(6):667–72. doi: 10.2175/106143001x143402. [DOI] [PubMed] [Google Scholar]
- 8.Mara D, Sleigh A. Estimation of norovirus and ascaris infection risks to urban farmers in developing countries using wastewater for crop irrigation. Journal of Water and Health. 2010;8(3):572–6. doi: 10.2166/wh.2010.097. [DOI] [PubMed] [Google Scholar]
- 9.Barker S, O’Toole J, Sinclair M, et al. A probabilistic model in norovirus disease burden associated with greywater irrigation of lettuce in households in melbourne, Australia. Water Research. 2013;47(3):1421–32. doi: 10.1016/j.watres.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton AJ, Stagnitti F, Premier R, et al. Quantitative microbial risk assessment models for consumption of raw vegetables irrigated with reclaimed water. Applied and Environmental Microbiology. 2006;72(5):3284–90. doi: 10.1128/AEM.72.5.3284-3290.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seidu R, Heistad A, Amoah P, et al. Quantification of the health risk associated with wastewater reuse in accra, ghana: A contribution toward local guidelines. Journal of Water and Health. 2008;6(4):461–71. doi: 10.2166/wh.2008.118. [DOI] [PubMed] [Google Scholar]
- 12.Ayuso-Gabella N, Page D, Masciopinto C, et al. Quantifying the effect of managed aquifer recharge on the microbiological human health risks of irrigating crops with recycled water. Agricultural Water Management. 2011;99(1):93–102. [Google Scholar]
- 13.Bastos RKX, Bevilacqua PD, Silva CAB, et al. Wastewater irrigation of salad crops: Further evidence for the evaluation of the who guidelines. Water Science and Technology. 2008;57(8):1213–9. doi: 10.2166/wst.2008.244. [DOI] [PubMed] [Google Scholar]
- 14.Aiello R, Cirelli GL, Consoli S, et al. Risk assessment of treated municipal wastewater reuse in sicily. Water Science and Technology. 2013;67(1):89–98. doi: 10.2166/wst.2012.535. [DOI] [PubMed] [Google Scholar]
- 15.Shuval H, Lampert Y, Fattal B. Development of a risk assessment approach for evaluating wastewater reuse standards for agriculture. Water Science and Technology. 1997;35(11-12):15–20. [Google Scholar]
- 16.FAO Faostat 201212. 2012 Sep; [Google Scholar]
- 17.Jiménez B. Irrigation in developing countries using wastewater. International Review for Environmental Strategies. 2006;6(2):229–50. [Google Scholar]
- 18.RIRDC . Series Taking stock of the australian asian vegetables industry. Autsralian Rural Industries Research and Development Corporation and Horticulture Australia Limited; NWS Australia: 2011. Taking stock of the australian asian vegetables industry. [Google Scholar]
- 19.Stine SW, Song I, Choi CY, et al. Application of microbial risk assessment to the development of standards for enteric pathogens in water used to irrigate fresh produce. Journal of Food Protection. 2005;68(5):913–8. doi: 10.4315/0362-028x-68.5.913. [DOI] [PubMed] [Google Scholar]
- 20.Mara D, Sleigh A. Estimation of norovirus infection risks to consumers of wastewater-irrigated food crops eaten raw. Journal of Water and Health. 2010;8(1):39–43. doi: 10.2166/wh.2009.140. [DOI] [PubMed] [Google Scholar]
- 21.US Census Bureau . Series International data base. United States Census Bureau; Washington DC: 2013. International data base. [Google Scholar]
- 22.CDC Rotavirus 201307. 2013 Aug; [Google Scholar]
- 23.Fang ZY, Yang H, Zhang J, et al. Child rotavirus infection in association with acute gastroenteritis in two chinese sentinel hospitals. Pediatrics International. 2000;42(4):401–5. doi: 10.1046/j.1442-200x.2000.01249.x. [DOI] [PubMed] [Google Scholar]
- 24.Qiao HP, Nilsson M, Abreu ER, et al. Viral diarrhea in children in beijing, China. Journal of Medical Virology. 1999;57(4):390–6. [PubMed] [Google Scholar]
- 25.Liu CY, Grillner L, Jonsson K, et al. Identification of viral agents associated with diarrhea in young children during a winter season in beijing, China. Journal of Clinical Virology. 2006;35(1):69–72. doi: 10.1016/j.jcv.2005.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DPI Glossary of asian vegetables 200906. 2013 Aug; [Google Scholar]
- 27.Fernando N. Growing asian vegetables. Victoria Department of Primary Industries; Bendigo, VIC: [Google Scholar]
- 28.Wei Y. Asian vegetables entice growers. China Daily USA 2013. 2013 Mar 26; [Google Scholar]
- 29.Washington State University. Winter lettuce 201330. 2013 Aug; [Google Scholar]
- 30.Nolte K. Oak leaf lettuce. University of Arizona College of Agriculture and Life Sciences; University of Arizona: [Google Scholar]
- 31.Butler Market Gardens Butler market gardens 201328. 2013 Jul; [Google Scholar]
- 32.Havelaar AH, Melse J. Series Quantifying public health risk in the who guidelines for drinking-water quality: A burden of disease approach. World Health Organization (WHO), National Institute for Public Health and the Environment (RIVM); Bilthoven, Netherlands: 2003. Quantifying public health risk in the who guidelines for drinking-water quality: A burden of disease approach. [Google Scholar]
- 33.Fu CX, He Q, Xu JX, et al. Effectiveness of the lanzhou lamb rotavirus vaccine against gastroenteritis among children. Vaccine. 2012;31(1):154–8. doi: 10.1016/j.vaccine.2012.10.078. [DOI] [PubMed] [Google Scholar]
- 34.He XQ, Cheng L, Zhang DY, et al. One-year monthly survey of rotavirus, astrovirus and norovirus in three sewage treatment plants (stps) in beijing, China and associated health risk assessment. Water Science and Technology. 2011;64(6):1202. doi: 10.2166/wst.2011.080. [DOI] [PubMed] [Google Scholar]
- 35.Li D, Gu AZ, Zeng SY, et al. Monitoring and evaluation of infectious rotaviruses in various wastewater effluents and receiving waters revealed correlation and seasonal pattern of occurrences. Journal of Applied Microbiology. 2011;110(5):1129–37. doi: 10.1111/j.1365-2672.2011.04954.x. [DOI] [PubMed] [Google Scholar]
- 36.Zurbriggen S, Tobler K, Abril C, et al. Isolation of sabin-like polioviruses from wastewater in a country using inactivated polio vaccine. Applied and Environmental Microbiology. 2008;74(18):5608–14. doi: 10.1128/AEM.02764-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.CCDCP, UNCPC . Series China health and nutrition survey. National Institute of Nutrition and Food Safety, Chinese Center for Disease Control and Prevention, University of North Carolina Population Center; Chapel Hill, NC: 2006. China health and nutrition survey. [Google Scholar]
- 38.Petterson SR, Ashbolt NJ, Sharma A. Of: Microbial risks from wastewater irrigation of salad crops: A screening-level risk assessment. Water Environment Research. 2002;74(4):411. doi: 10.2175/106143002x140161. [DOI] [PubMed] [Google Scholar]
- 39.Gulati BR, Allwood PB, Hedberg CW, et al. Efficacy of commonly used disinfectants for the inactivation of calicivirus on strawberry, lettuce, and a food-contact surface. Journal of Food Protection. 2001;64(9):1430–4. doi: 10.4315/0362-028x-64.9.1430. [DOI] [PubMed] [Google Scholar]
- 40.Dawson DJ, Paish A, Staffell LM, et al. Survival of viruses on fresh produce, using ms2 as a surrogate for norovirus. Journal of Applied Microbiology. 2005;98(1):203–9. doi: 10.1111/j.1365-2672.2004.02439.x. [DOI] [PubMed] [Google Scholar]
- 41.Croci L, De Medici D, Scalfaro C, et al. The survival of hepatitis a virus in fresh produce. International Journal of Food Microbiology. 2002;73(1):29–34. doi: 10.1016/s0168-1605(01)00689-4. [DOI] [PubMed] [Google Scholar]
- 42.Teunis PFM, Havelaar AH. The beta poisson dose-response model is not a single-hit model. Risk Analysis. 2000;20(4):513–20. doi: 10.1111/0272-4332.204048. [DOI] [PubMed] [Google Scholar]
- 43.Liu N, Yen C, Fang ZY, et al. Projected health impact and cost-effectiveness of rotavirus vaccination among children < 5 years of age in China. Vaccine. 2012;30(48):6940–5. doi: 10.1016/j.vaccine.2012.05.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Podewils LJ, Antil L, Hummelman E, et al. Projected cost-effectiveness of rotavirus vaccination for children in asia. Journal of Infectious Diseases. 2005;192:S133–S45. doi: 10.1086/431513. [DOI] [PubMed] [Google Scholar]
- 45.Pott J, Stockinger S, Torow N, et al. Age-dependent tlr3 expression of the intestinal epithelium contributes to rotavirus susceptibility. PLoS Pathogens. 2012;8(5):1–11. doi: 10.1371/journal.ppat.1002670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rutjes SA, Lodder WJ, van Leeuwen AD, et al. Detection of infectious rotavirus in naturally contaminated source waters for drinking water production. Journal of Applied Microbiology. 2009;107(1):97–105. doi: 10.1111/j.1365-2672.2009.04184.x. [DOI] [PubMed] [Google Scholar]
- 47.Tanaka H, Asano T, Schroeder ED, et al. Estimating the safety of wastewater reclamation and reuse using enteric virus monitoring data. Water Environment Research. 1998;70(1):39–51. [Google Scholar]
- 48.Yang Y, Wang G, Pan X. Vegetables and vegetable products. In: Yang Y, Wang G, Pan X, editors. translator and editor China food composition table. Peking University Medical Press; China: 2002. pp. 49–74. [Google Scholar]
- 49.Badawy AS, Gerba CP, Kelley LM. Survival of rotavirus sa-11 on vegetables. Food Microbiology. 1985;2(3):199–205. [Google Scholar]
- 50.Ward RL, Bernstein DI, Young EC, et al. Human rotavirus studies in volunteers - determination of infectious dose and serological response to infection. Journal of Infectious Diseases. 1986;154(5):871–80. doi: 10.1093/infdis/154.5.871. [DOI] [PubMed] [Google Scholar]
- 51.Orenstein EW, Fang ZY, Xu J, et al. The epidemiology and burden of rotavirus in China: A review of the literature from 1983 to 2005. Vaccine. 2007;25(3):406–13. doi: 10.1016/j.vaccine.2006.07.054. [DOI] [PubMed] [Google Scholar]
- 52.HPSC . Series Infectious intestinal disease: Public health and clinical guidance - rotavirus. Health Protection Surveillance Centre; Dublin, Ireland: 2012. Infectious intestinal disease: Public health and clinical guidance - rotavirus. [Google Scholar]
- 53.The R Foundation for Statistical Computing The r project for statistical computing. Series The r project for statistical computing. 2013 [Google Scholar]
- 54.Buckland ST. Monte-carlo confidence intervals. Biometrics. 1984;40(3):811–7. [Google Scholar]
- 55.Yi L, Jiao W, Chen X, et al. An overview of reclaimed water reuse in China. Journal of Environmental Sciences. 2011;23(10):1585–93. doi: 10.1016/s1001-0742(10)60627-4. [DOI] [PubMed] [Google Scholar]
- 56.ABS . Series 4804.0 - national nutrition survey: Foods eaten. Australian Bureau of Statistics; Australia. Canberra, Australia: 1995. 4804.0 - national nutrition survey: Foods eaten, Australia. [Google Scholar]
- 57.Larkin EP, Tierney JT, Sullivan R. Persistence of virus on sewage-irrigated vegetables. Journal of the Environmental Engineering Division-Asce. 1976;102(1):29–35. [Google Scholar]
- 58.Ward BK, Irving LG. Virus survival on vegetables spray-irrigated with wastewater. Water Research. 1987;21(1):57–63. [Google Scholar]
- 59.Stine SW, Song I, Choi CY, et al. Effect of relative humidity on preharvest survival of bacterial and viral pathogens on the surface of cantaloupe, lettuce, and bell peppers. Journal of Food Protection. 2005;68(7):1352–8. doi: 10.4315/0362-028x-68.7.1352. [DOI] [PubMed] [Google Scholar]
- 60.Barker-Reid F, Harper GA, Hamilton AJ. Affluent effluent: Growing vegetables with wastewater in melbourne, Australia - a wealthy but bone-dry city. Irrigation and Drainage Systems. 2010;24(1/2):79–94. [Google Scholar]
- 61.Lee SH. Vegetable crops growing in China. Scientia Horticulturae. 1982;17(3):201–9. [Google Scholar]
- 62.MEP . Series Guideline of urban sewage treatment and pollution prevention and control technology. Ministry of Environmental Protection; China: 2001. Guideline of urban sewage treatment and pollution prevention and control technology. [Google Scholar]
- 63.Chinese Government National Standards . Series Gb 5084-2005 standards for irrigation water quality. Chinese Government National Standards; China: 2006. Gb 5084-2005 standards for irrigation water quality. [Google Scholar]
- 64.He P, Phan L, Guowei G, et al. Reclaimed municipal wastewater - a potential water resource in China. Series Reclaimed municipal wastewater - a potential water resource in China. 2001;Vol. 43:51–8. [PubMed] [Google Scholar]
- 65.Wu J, Long SC, Das D, et al. Are microbial indicators and pathogens correlated? A statistical analysis of 40 years of research. Journal of Water and Health. 2011;9(2):265–78. doi: 10.2166/wh.2011.117. [DOI] [PubMed] [Google Scholar]
- 66.Payment P, Locas A. Pathogens in water: Value and limits of correlation with microbial indicators. Ground Water. 2011;49(1):4–11. doi: 10.1111/j.1745-6584.2010.00710.x. [DOI] [PubMed] [Google Scholar]
- 67.Feachem RG, Bradley DJ, Garelick H, et al. translator and editor Sanitation and disease: Health aspects of excreta and wastewater management. John Wiley & Sons; Chichester, UK: 1983. Elements and health risks of excreta and wastewater; pp. 3–22. [Google Scholar]
- 68.Ahmad F, Tourlousse DM, Stedtfeld RD, et al. Detection and occurence of indicator organisms and pathogens. Water Environment Research. 2009;81(10):959–80. doi: 10.2175/106143015X14338845155147. [DOI] [PubMed] [Google Scholar]
- 69.NRMMC, EPHC, AHMC Australian guidelines for water recycling: Managing health and environmental risks. In., Series Australian guidelines for water recycling: Managing health and environmental risks. Natural Resource Management Ministerial Council, Environmnet Protection and Heritage Council, and the Australian Health Ministers’ Conference; Canberra, Australia. 2006. [Google Scholar]
- 70.Yang L, Parkin DM, Ferlay J, et al. Estimates of cancer incidence in China for 2000 and projections for 2005. Cancer Epidemiology Biomarkers & Prevention. 2005;14(1):243–50. [PubMed] [Google Scholar]
- 71.WHO . Series Guidelines for drinking-water quality, third edition. Incorporating the first and second addenda - volume 1: Recommendations. World Health Organization; Geneva, Switzerland: 2008. Guidelines for drinking-water quality, third edition. Incorporating the first and second addenda - volume 1: Recommendations. [Google Scholar]