Abstract
The capability model of frontal electroencephalographic (EEG) asymmetry suggests that brain activity during emotional challenge will be a more powerful indicator of predispositions toward psychopathology than activity observed at rest. EEG data were assessed during a resting baseline and a facial emotion task, wherein individuals with (n = 143) and without (n = 163) lifetime major depressive disorder (MDD) made approach (angry and happy) and withdrawal (afraid and sad) facial expressions. EEG asymmetry during emotional challenge was a more powerful indicator of MDD status than resting asymmetry for average, Cz, and linked mastoid references, results in support of the capability model. However, current-source-density (CSD) transformed asymmetry was indicative of lifetime MDD status under resting and task-elicited conditions. Findings suggest that CSD-transformed data may be more robust indicators of trait frontal EEG asymmetry.
In recent years, a considerable literature has examined the central roles motivational systems and associated brain mechanisms play in the emotional experience and expression of depressed individuals. Researchers have advanced the position that a behavioral activation system supports positive emotions, responds to rewarding stimuli, and leads to approach behavior and active avoidance, whereas a behavioral inhibition system underlies anxiety, responds to punishing stimuli, and leads to inhibition of action, passive avoidance, and heightened arousal (Cavanagh & Allen, 2009a, 2009b; Gray, 1982, 1987; Gray & McNaughton, 1996). It has been argued that individual differences in frontal brain asymmetry can be thought of a diathesis that biases one’s affective style, or tendency to engage in aspects of these motivational systems, and that these differences may influence an individual’s vulnerability to develop depression (Davidson, 1998a). A dispositional model of affective style asserts that individuals have a predisposition to respond with emotions linked to an approach system (reflected as relatively higher left than right frontal activity) or a withdrawal system (reflected as relatively higher right than left frontal activity) across many contexts (Davidson, 1992, 1998a), and resting electroencephalogram (EEG) research has provided some support for this model, demonstrating that relatively greater left frontal activity is linked to approach motivation, whereas relatively greater right frontal activity is linked to withdrawal motivation (e.g., Coan & Allen, 2003; Harmon-Jones & Allen, 1997; Sutton & Davidson, 1997).
Depressed individuals tend to display a pattern of relatively less left than right resting frontal activity (inferred by relatively more left than right alpha band activity; see Allen, Coan, & Nazarian, 2004a) thought to index reduced approach motivation and decreased sensitivity to reward (Davidson, Pizzagalli, Nitschke, & Putnam, 2002; Diego, Field, & Hernandez-Reif, 2001a). This pattern of resting EEG asymmetry distinguishes individuals who are currently depressed or euthymic with a past history of depression from never-depressed individuals (Allen, Urry, Hitt, & Coan, 2004b; Bruder, et al., 2005; Debener, et al., 2000; Diego et al., 2001a; Diego, Field, & Hernandez-Reif, 2001b; Gotlib, Ranganath, & Rosenfeld, 1998; Henriques & Davidson, 1990; Henriques & Davidson, 1991; Mathersul, Williams, Hopkinson, & Kemp, 2008; Miller, et al., 2002; Pössel, Lo, Fritz, & Seeman, 2008; Schaffer, Davidson, & Saron, 1983; Stewart, Bismark, Towers, Coan & Allen, 2010; Vuga, et al., 2006), suggesting that prefrontal brain asymmetry may tap a diathesis toward the development of depression (Allen, Urry, Hitt, & Coan, 2004b; Coan & Allen, 2003; Thibodeau, Jorgensen, & Kim, 2006).
However, some research has failed to confirm a link between left frontal EEG hypoactivity and depression (e.g., Bruder et al., 1997; Metzger et al., 2004; Nitschke, Heller, Palmieri, & Miller, 1999; Pizzagalli et al., 2002; Reid, Duke, & Allen, 1998). Inconsistent results may be due to clinical and/or methodological differences across laboratories, including comorbidity of depression and anxiety, sex differences in depression and/or EEG asymmetry, choice of EEG reference, uncontrolled experimental conditions, and the reliability and stability of EEG asymmetry within and across sessions (e.g., Allen et al., 2004a; Davidson, 1998b; Hagemann, 2004; Hagemann, Naumann, & Thayer, 2001; Hagemann, Naumann, Thayer, & Bartussek, 2002; Kline, Blackheart, & Joiner, 2002; Stewart, Bismark, et al., 2010). The dispositional model of asymmetry may be hampered by these as-yet-unresolved methodological limitations. Furthermore, the dispositional model of EEG asymmetry assumes that depressed individuals will react similarly across situations, but in fact particular contexts may exacerbate differences in regional brain activity between depressed and healthy individuals. Thus, examination of frontal brain activity during task manipulations, in addition to resting sessions, is important to test the limits of frontal EEG asymmetry as a marker of risk for depression.
There is some evidence that: (1) method variance involved with the measurement of frontal EEG asymmetry may be reduced and a better index of emotional response tendencies may be measured when brain activity is recorded under task manipulations rather than under resting conditions; and (2) EEG asymmetry linked to approach- and withdrawal-related state emotion tasks can replicate patterns of relationships between resting EEG asymmetry and approach and withdrawal motivation, but with larger effect sizes due to the elimination of uncontrolled variance. Coan, Allen, and McKnight (2006) proposed a capability model of individual differences in frontal EEG asymmetry, which asserts that frontal brain activity during an emotional challenge will be a more powerful detector of motivational differences than at rest, since it may reflect individuals’ capacity for emotion regulation in situations that demand it. This capability model was tested in healthy individuals using the Directed Facial Action (DFA) task, an emotional challenge paradigm that requires participants to move their facial muscles into certain configurations that represent approach-related emotions such as anger and happiness, and withdrawal-related emotions such as fear and sadness (Coan, Allen, and Harmon-Jones, 2001). In support of the capability model, Coan et al. (2006) demonstrated that: 1) the proportion of variance attributable to individual differences in the Coan et al. (2001) dataset was much lower at rest (16%) than during emotional challenges involving anger (72%), fear (88%), happiness (41%), and sadness (91%); 2) EEG asymmetry during approach- and withdrawal-related emotional challenges predicted positive and negative affect ratings just as well or better than brain activity at rest; and, 3) individual differences in EEG asymmetry were more resistant to variance attributable to choice of reference mode during emotional challenges than at rest. These results suggest that frontal EEG asymmetry in response to emotionally salient events may mitigate the contribution of uncontrolled variance and reference-specific variance plaguing the resting EEG literature.
The limited number of studies examining the relationship between EEG asymmetry and state emotion challenges in dysphoric populations has found that depressed individuals exhibit lower relative left frontal activity in response to approach- or withdrawal-related emotional challenges. Higher depression symptom scores have been linked to lower left frontal activity during an approach-related anger provocation paradigm (Harmon-Jones et al., 2002), and early onset-depressives exhibited lower left frontal activity than controls during an approach-related reward paradigm (Shankman, Klein, Tenke, & Bruder, 2007). Furthermore, a study that examined each hemisphere individually found results generally consistent with those reported above, as depressed individuals displayed higher right frontal activity but no differences in left frontal activity compared to control participants during a withdrawal-related challenge involving active listening to a sad narrative (Nitschke et al., 2004). Finally, recent work measuring EEG activity during the DFA task demonstrated that individuals with major depressive disorder (MDD) exhibited relatively less left than right frontal activity than controls across approach-related (anger, joy) and withdrawal-related (fear, sad) facial expressions (Stewart, Coan, Towers, & Allen, 2011). These studies, however, did not statistically test whether individual differences in activity during such emotional states are a more robust predictor of depression status than resting EEG activity.
To address the question of whether task-elicited EEG asymmetry, compared to resting EEG asymmetry, would be more a more sensitive indicator of a lifetime history of depression, an emotional challenge task involving facial expressions was used. A facial expression task was selected since state manipulations involving facial expressions provide some of the most robust changes in EEG asymmetry in healthy participants (e.g., Coan et al., 2001; Davidson, Ekman, Saron, Senulis, & Friesen, 1990; Ekman & Davidson, 1993; Fox & Davidson, 1988).
The present study examined frontal EEG asymmetry in individuals with and without a lifetime history of depression under rest and approach- and withdrawal-related emotional challenge conditions (the DFA task) in order to examine three hypotheses. First, it was predicted that individuals with a lifetime history of depression will display lower relative left frontal activity than never-depressed individuals across all conditions (approach, withdrawal, and rest), consistent with the available state and trait EEG asymmetry literature. Second, in a statistical test of the capability model of individual differences in frontal EEG asymmetry (Coan et al., 2006), it was predicted that frontal alpha asymmetry during the approach and withdrawal conditions of the DFA task will demonstrate larger differences between depressed and non-depressed individuals than frontal alpha asymmetry at rest. Third, despite shared variance between EEG and electromyographic (EMG) activity, it is predicted that to the extent that EMG is present, EMG asymmetry will not account for the overall pattern of EEG asymmetry differences between depressed and non-depressed groups, replicating prior research (Coan et al., 2001). This hypothesis is motivated from the fact that EMG activity due to facial muscle movements is prominent during the DFA task (Coan et al., 2001) and could contaminate patterns of EEG alpha asymmetry (although research indicates that alpha band activity is less susceptible to contamination than other bands such as gamma; see Shackman et al., 2009).
Method
Participants
A total of 306 participants (95 male, 73% Caucasian; also reported in Stewart, Bismark et al., 2010, Stewart, Towers, Coan, and Allen, 2010, and Stewart et al., 2011) with an age range of 17 to 34 years (M = 19.1, SE = 0.1) were enrolled in the study from a possible pool of over 10,000 individuals on the basis of their scores on the Beck Depression Inventory (BDI; Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) completed during pre-testing in a large introductory psychology course or online after learning about the study from a flier or referral source. Individuals participated in a phone screening session administered by a post-bachelors project manager to screen for preliminary inclusion and exclusion criteria. To be eligible, individuals were required to be strongly right-handed (a score greater than 35 on the 39 point scale of Chapman & Chapman, 1987) and to report no history of: head injury with loss of consciousness greater than 10 minutes, concussion, epilepsy, electroshock therapy, use of current psychotropic medications, and active suicidal potential necessitating immediate treatment (although participation in current psychotherapy was allowed). Those passing this brief phone screen were invited for an intake interview, administered by a trained graduate clinical rater. Individuals were enrolled in the study if the Structured Clinical Interview for DSM-IV (SCID, First, Spitzer, Gibbon, & Williams, 1997) indicated that they did not meet criteria for any DSM-IV Axis I disorder other than lifetime MDD and comorbid current dysthymia. Participants were selected to include currently non-depressed (MDD-, n = 163) individuals, individuals endorsing a current major depressive episode (n = 62), and also those with a history of depression but no current major depressive episode (n = 81); these latter two groups were considered jointly to be lifetime history positive for major depression (MDD+, n = 143).
Procedure and Task Parameters
The DFA task and two resting EEG sessions were completed each visit, on 4 separate days with no fewer than 24 hours between visits, and with all 4 visits completed within a 14 day period (such that the fourth day is not more than 14 days after the first day)1. Participants were seated in a sound-attenuated room, separate from the experimenter. Resting EEG was recorded for eight one-minute baselines, in blocks including periods of eyes-open (O) and eyes-closed (C), in one of two counterbalanced orders (OCCOCOOC or COOCOCCO) for 8 minutes per block.
The DFA task (see Coan et al., 2001 and Levenson, Ekman, and Friesen, 1990) was performed by participants in between the first and second blocks of resting EEG recording on each day of EEG assessment. Facial movements described below are numbered according to the Facial Action Coding System (FACS; Ekman & Friesen, 1978). Individual facial movements are referred to as action units (AU) in FACS. Four facial expressions were performed, representing the following emotions: anger (AUs 4 + 5 + 7 + 23/24), fear (AUs 1 + 2 + 4 + 5 + 15 + 20), happiness (AUs 6 + 12 + 25), and sadness (AUs 1 + 6 + 15 + 17). Facial expressions were each held for 1 minute, during which time EEG was recorded. The experimenter communicated with participants via microphone regarding how to make each facial movement, and participants’ faces were closely observed via video monitor to ensure that each facial movement was performed correctly. Participants had no visual feedback, and auditory feedback consisted of describing the intended facial movement again (e.g., “raise your upper eyelid”). Two FACS-trained (but not FACS-certified) observers rated each participants’ facial expression performance on a 7-point scale (1 = no target facial movements achieved; 7 = target facial movements prototypic).2 Mean levels of task quality across raters and days were: anger M =3.9, SE = .03; fear M = 4.5, SE = .04; happiness M = 4.3, SE = .03; sadness M = 3.8, SE = .03. Intraclass correlation coefficients (ICCs) between the two independent raters across participants and days ranged from .71 to .78. Experimenters did not interrupt recording for each face but instead ensured that the subject had the facial muscles in place before starting EEG recording. Immediately following each 1-minute facial expression sequence, participants were asked while making that particular face, how angry, afraid, happy, or sad they felt on a scale of 1 to 7 (1 = no experience at all; 7 = intense experience).
EEG Data Collection and Reduction
All EEG data were collected using a 64-channel NeuroScan Synamps2 amplifier (Charlotte, NC) and acquisition system, utilizing the international 10–20 system for electrode placement. Two electrooculogram (EOG) channels (vertical: superior and inferior orbit of the left eye; lateral: outer canthi) were collected for ocular artifact rejection of resting EEG data. All impedances were kept under 10K Ohms. Data were collected using 1000 Hz sampling rate, amplified 2816 times, and filtered with a 200Hz low pass filter prior to digitization. EEG data were acquired with an online reference site immediately posterior to Cz and subsequently re-referenced offline to four reference modes: the average of all EEG leads (AVG), Cz, averaged (“linked”) mastoids (LM), and to the reference-free current source density transformation (CSD; using algorithms from Kayser & Tenke, 2006, and based on the spherical spline approach summarized by Perrin, Pernier, Bertrand, and Echallier, 1989, 1990; although CSD is technically reference-free, it will be referred to as a reference mode to streamline description of analyses and results).
After acquisition, each data file was visually inspected to remove epochs with movement and signal discontinuities. Data reduction was implemented using custom scripts in Matlab (release 2007b, The Mathworks Inc., Natick, MA) and an artifact rejection algorithm rejected segments with large fast deviations in amplitude in any channel (e.g., DC shifts and spikes) that may have been missed by human inspection. As per convention, a blink rejection algorithm rejected any data segments in the resting EEG data where ocular activity exceeded +/- 75 microvolts in the vertical EOG channel. However, since state emotion EEG data consisted of only one minute per facial expression, blink removal was not performed because it would have resulted in too few trials for analysis. To demonstrate that control of EOG artifacts should not introduce differences between resting and state emotion EEG asymmetry, ICCs computed between blink-retained versus blink-rejected resting frontal EEG data for 40 randomly selected participants in the present sample were excellent (ranging from .91 to .99 for AVG and LM reference modes across the four frontal channel pairs). These results replicate research demonstrating that retaining or rejecting blinks appears to have a negligible effect on EEG asymmetry in the alpha band (Hagemann & Naumann, 2001). Therefore, blinks were rejected for all resting data, as per convention, but not for any DFA task-related data.
Resting data were collected in one-minute EEG blocks, as were data during each DFA expression. Each one-minute block was then epoched into 117 2.048 epochs, overlapping by 1.5 seconds to compensate for the minimal weight applied to the end of the epoch by the use of the Hamming window function. Following windowing, a Fast Fourier Transform (FFT) was applied to all artifact-free epochs. For each state emotion facial expression and all eight minutes of each resting session, total alpha power (8–13 Hz) and EMG power (70–90 Hz) were then extracted from the power spectrum. An asymmetry score was then calculated for total alpha power by subtracting the natural log transformed scores (i.e., ln[Right] – ln[Left]) for each homologous left and right pair (FP1 & FP2, AF3 & AF4, F7 & F8, F5 & F6, F3 & F4, F1 & F2, FT7 & FT8, FC5 & FC6, FC3 & FC4, FC1 & FC2, T7 & T8, C7 & C6, C3 & C4, C1 & C2, TP7 & TP8, CP5 & CP6, CP3 & CP4, CP1 & CP2, P7 & P8, P5 & P6, P3 & P4, P1 & P2, PO7 & PO8, PO5 & PO6, PO3 & PO4, O1 & O2). Higher asymmetry score values are commonly believed to reflect relatively greater left activity (i.e., relatively greater right alpha; cf. Allen et al., 2004a). Although asymmetry scores were computed for all homologous channel pairs, analyses for the present study were performed on a specific subset of those pairs (frontal: F2-F1, F4-F3, F6-F5, F8-F7) that correspond to regions commonly studied throughout the asymmetry literature (F4-F3 and F8-F7: see review by Coan & Allen, 2004) as well as channels that neighbor these pairs, potentially providing better resolution of the lateral and medial extent of depression-related frontal asymmetry.
Results
Topography of Alpha Power and Asymmetry by Condition
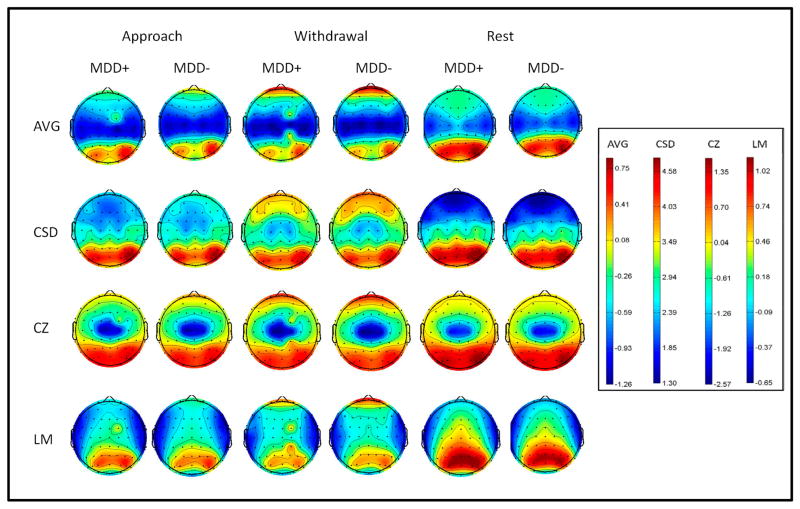
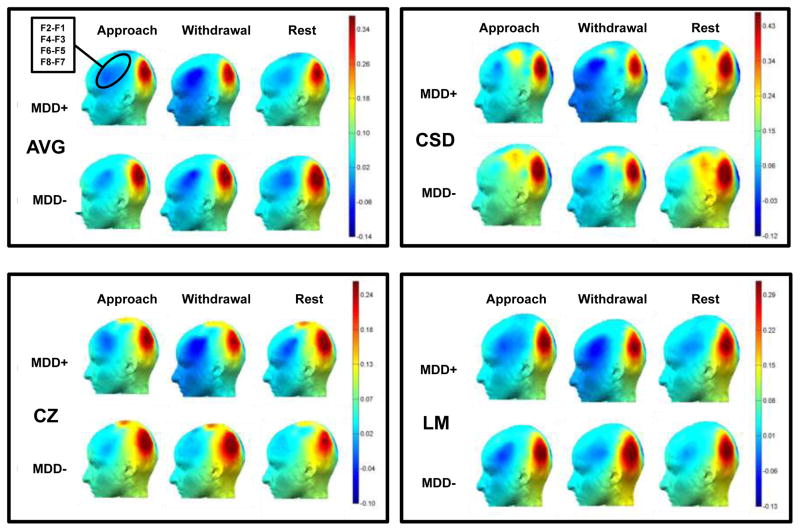
Figures 1 and 2 illustrate log-transformed total alpha power and alpha asymmetry score topographies, respectively, as a function of lifetime MDD status, condition, and channel location. Specifically, Figure 1 demonstrates that across reference modes, alpha power was greatest at posterior sites, replicating prior research (e.g., Debener et al., 2000), and frontal alpha power appeared strongest for the withdrawal condition. In addition, Figure 2 shows that group differences in asymmetry appeared to be more robust for the withdrawal condition than approach and rest conditions across references.
Figure 1.
Figure 2.
Asymmetry Analyses involving DFA and Rest Data: Test of the Capability Model
Lifetime MDD status
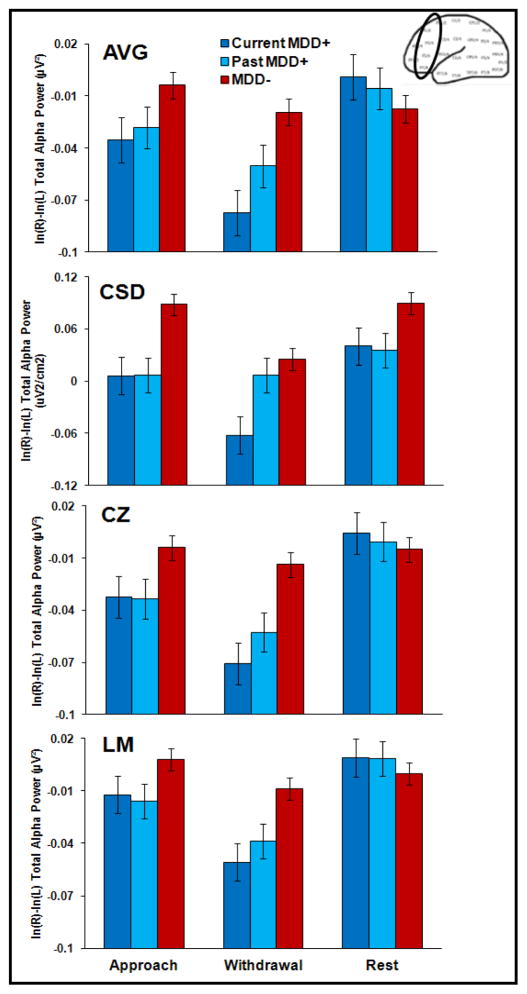
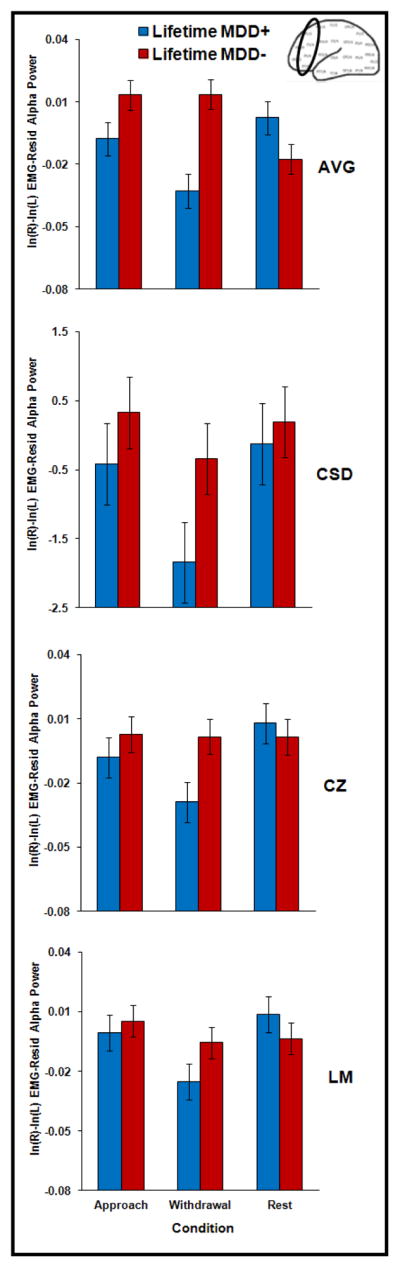
Although resting frontal EEG alpha asymmetry data from the present sample were reported in Stewart, Bismark, et al. (2010) and state frontal EEG asymmetry data were reported in Stewart et al. (2011), they are included here to test the capability model hypothesis that state EEG manipulations produce larger differences between depressed and non-depressed individuals than resting EEG. To examine the relationship between lifetime MDD status and frontal EEG asymmetry, full factorial mixed linear models (SAS 9.2) were run for each reference mode (AVG, CSD, Cz, and LM) separately with lifetime MDD status (past and/or current MDD = lifetime MDD+, never depressed = lifetime MDD−) and biological sex (male, female) as between-subjects variables and condition (approach faces, withdrawal faces, rest) and channel (F2-F1, F4-F3, F6-F5, F8-F7) as within-subjects variables. EEG asymmetry score based on total (8–13 Hz) alpha power was the dependent variable. An approach facial-expression DFA asymmetry score was computed by averaging across asymmetry during angry and happy faces for the four days of EEG recording, whereas a withdrawal facial-expression DFA score was calculated by averaging across asymmetry scores during fearful and sad faces for the four days. In addition, a resting EEG asymmetry score was created by averaging asymmetry scores across all eight resting sessions (2 sessions per day). The result of these calculations was a total of twelve asymmetry scores (one rest, approach, and withdrawal score for each of four reference modes) per participant at each homologous pair. The lifetime MDD by condition interaction was of interest in order to investigate the capability model of EEG asymmetry. Cohen’s d is reported to quantify effect size for significant differences between lifetime MDD+ and MDD− groups.
For all four reference modes, a main effect of lifetime MDD emerged (CSD: F(1, 302) = 29.0, p < .001, d = .62; AVG: F(1, 302) = 9.3, p < .01, d = .35; Cz: F(1, 302) = 13.1, p < .001, d = .42; LM: F(1, 302) = 11.9, p < .001, d = .40). For AVG, Cz, and LM, but not CSD, this main effect was qualified by a lifetime MDD by condition interaction (AVG: F(2, 603) = 6.5, p < .01; Cz: F(2, 603) = 6.4, p < .01; LM: F(2, 603) = 5.5, p < .01; CSD, p >.54), which indicated that approach and withdrawal conditions differentiated lifetime MDD+ and MDD− groups more robustly than the rest condition (see Figure 3). Specifically, the lifetime MDD+ group displayed relatively less left frontal activity than the lifetime MDD− group during the approach condition (AVG: p < .05 and d = .28; Cz: p < .05 and d = .29; LM: p < .05 and d = .29) and the withdrawal condition (AVG: p < .001 and d = .45; Cz: p < .001 and d = .50; LM: p < .01 and d = .46) but not the rest condition for Cz or LM (both p > .52). Although the groups differed during the rest condition for AVG (p < .01 and d = .12) the pattern of means was in the unpredicted direction, with the lifetime MDD+ group exhibiting higher relative left frontal activity than the lifetime MDD− group. Biological sex did not moderate lifetime MDD by condition results for any reference (all p > .60). In summary, findings for three out of four reference modes supported the capability model of EEG asymmetry, which asserts that state emotion challenges will be more powerful in detecting individual differences than resting sessions. In contrast, findings indicate that CSD–transformed frontal EEG alpha asymmetry at rest may be robust marker of risk for depression (see Stewart, Bismark, et al, 2010).
Figure 3.
Follow-up analysis: Role of current MDD status
To determine whether lifetime MDD findings were due to depression status, analogous mixed model analyses were performed for three groups: current MDD+ (n = 62), past MDD+ (n = 75), and MDD− (n = 163) (six lifetime MDD+ who met criteria for past MDD and current dysthymia were excluded from analysis). Figure 4 illustrates that a current MDD status by condition interaction emerged for AVG (F(4, 587) = 3.74, p < .05), Cz (F(4, 587) = 3.46, p < .05), and LM (F(4, 587) = 2.94, p < .05), wherein current and past MDD+ exhibited lower relative left frontal activity than MDD− for approach (AVG: d = .32/.25; Cz: d = .31/.32; LM: d = .25/.29) and withdrawal (AVG: d = .60/.31; Cz: d = .63/.41; LM: d = .52/.37) conditions but groups did not differ from each other during the rest condition. In contrast, results for CSD-transformed data indicated that a main effect of current MDD status emerged (F(2,294) = 15.87, p < .001), wherein current and past MDD+ displayed lower relative left frontal activity than MDD− (d = .45/.31) across approach, withdrawal, and rest conditions.
Figure 4.
EMG influence on EEG alpha asymmetry
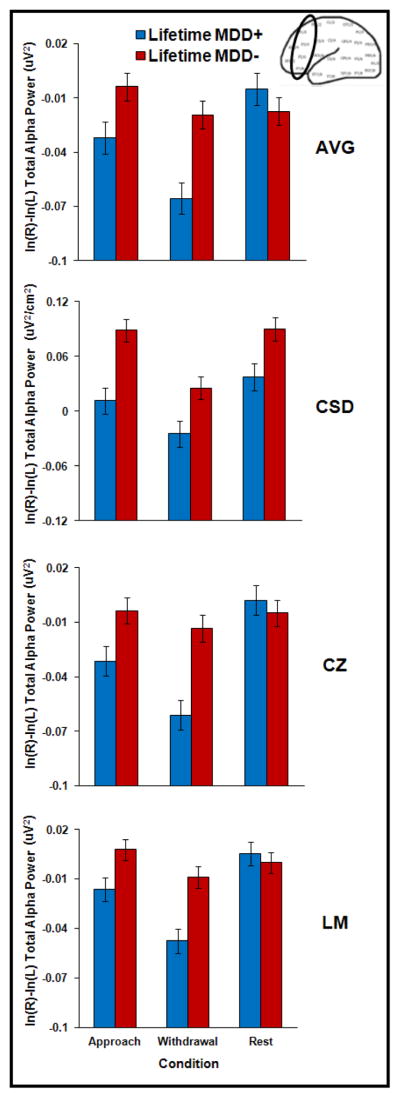
To examine whether EMG-related alpha asymmetry differences could account for the lifetime MDD by condition interaction reported above, asymmetry analyses were repeated for AVG, CSD, Cz, and LM, substituting EMG-residualized alpha asymmetry scores (e.g., McMenamin et al., 2009; Shackman et al., 2009b) as the dependent variable (F2-F1, F4-F3, F6-F5, and F8-F7).
For AVG, Cz, and LM modes, the lifetime MDD by condition interaction again emerged (AVG F(2, 603) = 10.5; Cz F(2, 603) = 12.0; LM F(2, 603) = 8.3; all p < .001), indicating that the withdrawal condition, but not the approach condition, differentiated lifetime MDD+ and MDD− groups more robustly than the rest condition (see Figure 5). Specifically, the lifetime MDD+ group displayed relatively less left frontal activity than the lifetime MDD− group during the withdrawal condition (AVG: p < .001 and d = .48; Cz: p < .001 and d = .56; LM: p < .001 and d = .49) but not the rest condition for LM (p > .15). Although the groups differed during the rest condition for AVG (p < .05 and d = .25) and Cz (p < .05 and d = .24), the pattern of means was again in the unpredicted direction, with the lifetime MDD+ group exhibiting higher relative left frontal activity than the lifetime MDD− group. The approach condition, although replicating the pattern of means evident in the main results (wherein the lifetime MDD+ group displayed relatively less left frontal activity than the MDD− group), became non-significant for all three references when accounting for possible EMG contributions to EEG asymmetry (AVG, Cz, and LME: .05 < p < .13). For the CSD mode, the lifetime MDD main effect that was significant in the main analysis was reduced to marginal significance once EMG was taken into account (F(1, 302) = 3.6, p < .07).
Figure 5.
Discussion
Alpha EEG Asymmetry, Depression, and Test of the Capability Model
The present study examined the relationship between asymmetries in frontal brain activity and depression during a resting state and an emotional challenge task involving approach and withdrawal-related facial expressions to statistically test the capability model of individual differences in frontal EEG asymmetry, which predicted that individual differences in EEG asymmetry (associated with depression status) would be stronger during emotional challenge than during a baseline rest session. In pursuit of this goal, three hypotheses were tested. First, it was predicted that individuals with a lifetime history of depression would display lower relative left frontal activity than never-depressed individuals across all conditions (approach, withdrawal, and rest), consistent with much of the state and trait EEG asymmetry literature. This prediction was confirmed in individuals with current and past depression for the CSD-transformed EEG asymmetry data. Although this hypothesis was also supported for approach and withdrawal state emotion conditions for AVG, Cz, and LM reference modes, depressed and never-depressed groups did not differ in frontal brain activity during the resting condition for these montages. The second hypothesis asserted that, in line with the capability model of individual differences, frontal EEG asymmetry during the approach and withdrawal conditions of the emotional challenge ask would show larger differences between depressed and never-depressed individuals than frontal EEG asymmetry at rest. This prediction was supported for AVG, Cz, and LM references, wherein lifetime MDD+ participants displayed significantly less relative left frontal activity than MDD− participants during approach and withdrawal facial expressions but not during resting sessions. In contrast, results for the CSD-transformed data indicated that EEG asymmetry during all three conditions – approach, withdrawal, and rest –significantly differed as a function of lifetime MDD status.
As explained in Stewart, Bismark, et al. (2010), resting EEG asymmetry derived from AVG, Cz, and LM references has produced an inconsistent pattern of findings, wherein null results between depressed and non-depressed individuals have been reported in several cases (e.g., Bruder et al., 1997; Metzger et al., 2004; Nitschke et al., 1999; Pizzagalli et al., 2002; Reid et al., 1998) and asymmetry results for depressed women have also been more robust than those for men when these montages are utilized (Stewart, Bismark, et al., 2010). In addition, researchers have shown that convergence of AVG, Cz, and LM montages is not particularly high for frontal EEG data collected at rest (Hagemann et al., 2001; Reid et al., 1998). Furthermore, unlike the CSD-transformed data, which is most likely to reflect predominantly frontal sources (Hagemann et al., 2001; Kayser & Tenke, 2006; Kayser & Tenke, 2012), these other references are thought to index both proximal (frontal) and distal (parietal and occipital) sources, so it is not surprising that discrepant findings emerged for resting data as a function of reference mode. CSD is advantageous as a reference-free algorithm that eliminates volume conduction contributions to EEG alpha power, and in contrast to conventional scalp EEG reference measures, results in unambiguous indices of current sources underlying EEG topography (Kayser & Tenke, 2012). Findings of the present study indicate that CSD-transformed EEG asymmetry may be a liability marker, identifying a vulnerability to develop depression, since its reduced relative left frontal activity characterizes depressed individuals independent of emotional state (during approach and withdrawal conditions as well as at rest, and independent of current levels of depression severity, see Stewart, Bismark, et al., 2010). In contrast to resting EEG results, which reflected some inconsistencies as a function of reference and gender differences, EEG findings from the emotional challenge task demonstrated consistent findings across all four reference modes, consistent with the idea that emotional challenges produce much more powerful asymmetry effects that overcome method variance.
The Relationship between Alpha EEG Activity and EMG Activity
The third and final prediction was that, despite shared variance between EEG and EMG activity, EMG (70–90 Hz) asymmetry would not eliminate the overall pattern of EEG asymmetry differences between depressed and never-depressed groups. Comparison of Figure 3 (alpha asymmetry findings) and Figure 5 (EMG-residualized alpha asymmetry findings) indicates that the overall pattern of mean differences between depressed and never-depressed participants persisted when EMG-related activity was taken into account, supporting this hypothesis. However, statistical analyses demonstrated that differences between MDD+ and MDD− groups largely remained for the withdrawal condition but not the approach condition when shared variance between EMG and the alpha band was removed. Overall, these findings show that brain asymmetry associated with muscle movements during happy and angry facial expressions may account for some portion of asymmetry in the alpha band during these expressions, and suggest that differences between lifetime MDD+ and MDD− groups for these faces are partially influenced by facial activity, consistent with assertions that alpha power asymmetries may be vulnerable to contributions from facial muscle activity (e.g., Davidson, 1998; Friedman & Thayer, 1991. Although Coan et al. (2001) did not find that EMG asymmetry accounted for EEG asymmetry during the DFA task in healthy participants, they could not determine whether EMG accounted for between-subjects differences as they did not enroll depressed subjects in that study.4 Moreover, robustness of findings for the withdrawal condition when shared EMG-alpha variance was removed may also be due to greater frontal non-EMG residualized alpha power evident in both MDD+ and MDD− participants during the withdrawal condition than approach and rest conditions (see Figure 1). Although group asymmetry differences across conditions for CSD-transformed data were reduced to marginal significance when shared EMG-alpha variance was removed, this finding is difficult to interpret because CSD may not accurately measure EMG activity. The CSD algorithm estimates radial current flow into and out of the skull from underlying neural tissue (Tenke & Kayser, 2005) and, as such, is not designed to provide a meaningful estimate of potentials that originate from muscles overlaying the skull.
Summary
Results from the present study largely support the capability model of individual differences in EEG asymmetry, which asserts that state emotion manipulations will be a more powerful indicator of individual differences (in this case, individual differences associated with depression status) than resting sessions, since emotion response systems (approach and withdrawal motivational tendencies, for example) are more likely to be activated. Depressed individuals showed relatively less left frontal activity than never-depressed individuals during approach- and withdrawal-related facial expressions, consistent with previous research on state emotion paradigms in dysphoric samples (e.g., Harmon-Jones et al., 2002; Nitschke et al., 2004; Shankman et al., 2007; Stewart et al., 2011). EEG asymmetry scores based on CSD-transformed data, however, were more consistent across task and resting data. Consistent with other findings (Velo et al., 2012, Stewart, Bismark et al., 2010), the CSD transform may be advantageous for examining stable trait estimates of frontal EEG asymmetry.
Acknowledgments
This research was supported in part by grants from the National Institutes of Health (R01–MH066902) and the National Alliance for Research on Schizophrenia and Depression (NARSAD) to John Allen. The authors wish to thank Eliza Fergerson, Jamie Velo, Dara Halpern, Andrew Bismark, Craig Santerre, Eynav Accortt, Amanda Brody, and Jay Hegde for assistance with subject recruitment, and myriad research assistants who helped to collect and review EEG data.
Footnotes
Of the 21 participants who did not complete their sessions within a 14-day period, 15 completed all sessions within 16 days, whereas the remaining 6 completed all sessions within 18–20 days. In addition, a total of 9 participants did not complete all 4 days of EEG sessions (n = 5 did not complete days 2, 3, and 4; n = 3 did not complete days 3 and 4; and n = 2 did not complete day 4); conditions were averaged over the days completed.
Several participants had only one face rater on a particular day (Day 1: n = 17, Day 2: n = 12, Day 3: n = 16; Day 4: n = 16), whereas on each of the four days, 3 participants had no face raters.
EEG data for faces with fewer than 40 useable epochs were excluded from data analysis per recommendations of Tower and Allen (2009). A total of 264 faces (5.8% of the DFA data) met this criterion. Mixed model analyses were able to accommodate these missing cells. To examine stability of frontal EEG asymmetry across all four days of recording, ICCs were computed using a one-way random effects model for each reference mode and frontal asymmetry score (F2-F1, F4-F3, F6-F5, F8-F7). Results indicated that ICCs were comparable in magnitude within condition across references (average ICC for asymmetry scores across frontal sites: approach AVG = 0.59, CSD = 0.62, Cz = 0.59, LM = 0.58; withdrawal AVG = 0.62, CSD = 0.59, Cz = 0.64, LM = 0.61; rest AVG = 0.74, CSD = 0.70, Cz = 0.70, LM = 0.64), suggesting that findings across conditions for CSD-transformed data were not due to differences as a function of reference mode stability across EEG recording sessions.
Although it could be argued that depressed participants may be more susceptible to show asymmetry differences from never-depressed participants for the withdrawal- than approach-related emotion induction given the larger effect sizes for group differences for the withdrawal than approach conditions, Stewart et al. (2011) determined that within the context of the DFA task alone, individuals with lifetime MDD subjectively felt more anger (Cohen’s d = .43), less happiness (d = .73) and more sadness (d = .58) across all facial expressions than individuals without lifetime MDD (all p < .001). Since both approach- and withdrawal-related types of emotional experience were heightened in the lifetime MDD group during the DFA task, not just negative affect specific to withdrawal motivation, it is not likely that depressives were more susceptible to the withdrawal than approach related emotion induction. Results of the present study are more consistent with the assertion that state inductions mainly eliminate unsystematic individual differences in state variance.
References
- Allen JJB, Coan JA, Nazarian M. Issues and assumptions on the road from raw signals to metrics of frontal EEG asymmetry in emotion. Biological Psychology. 2004a;67:183–218. doi: 10.1016/j.biopsycho.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Allen JJB, Urry HL, Hitt SK, Coan JA. The stability of resting frontal electroencephalographic asymmetry in depression. Psychophysiology. 2004b;41:269–280. doi: 10.1111/j.1469-8986.2003.00149.x. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. http://archpsyc.ama-assn.org/ [DOI] [PubMed] [Google Scholar]
- Bruder GE, Fong R, Tenke CE, Leite P, Towey JP, Stewart JE, Quitkin FM. Regional brain asymmetries in major depression with or without an anxiety disorder: A quantitative electroencephalographic study. Biological Psychiatry. 1997;41:939–948. doi: 10.1016/S0006-3223(96)00260-0. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Tenke CE, Warner V, Nomura Y, Grillon C, Hille J, Weissman MM. Electroencephalographic measures of regional hemispheric activity in offspring at risk for depressive disorders. Biological Psychiatry. 2005;57:328–335. doi: 10.1016/j.biopsych.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Cavanagh J, Allen JJB. Behavioral Activation System. In: Sander D, Scherer KR, editors. Oxford Companion to Affective Sciences. New York: Oxford University Press; 2009a. http://www.oup.com/us/ [Google Scholar]
- Cavanagh J, Allen JJB. Behavioral Inhibition System. In: Sander D, Scherer KR, editors. Oxford Companion to Affective Sciences. New York: Oxford University Press; 2009b. http://www.oup.com/us/ [Google Scholar]
- Chapman LJ, Chapman JP. The measurement of handedness. Brain and Cognition. 1987;6:175–183. doi: 10.1016/0278-2626(87)90118-7. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJB. The state and trait nature of frontal EEG asymmetry in emotion. In: Hugdahl K, Davidson RJ, editors. The Asymmetrical Brain. 2. MIT Press; Cambridge, MA: 2003. pp. 565–615. http://mitpress.mit.edu/ [Google Scholar]
- Coan JA, Allen JJB. Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology. 2004;67:7–49. doi: 10.1016/j.biopsycho.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJB, Harmon-Jones E. Voluntary facial expression and hemispheric asymmetry over the frontal cortex. Psychophysiology. 2001;38:912–925. doi: 10.1111/1469-8986.3860912. [DOI] [PubMed] [Google Scholar]
- Coan JA, Allen JJB, McKnight PE. A capability model of individual differences in frontal EEG asymmetry. Biological Psychology. 2006;72:198–207. doi: 10.1016/j.biopsycho.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Ekman P, Saron CD, Senulis JA, Friesen WV. Approach-withdrawal and cerebral asymmetry: Emotional expression and brain physiology I. Journal of Personality and Social Psychology. 1990;58:330–341. doi: 10.1037/0022-3514.58.2.330. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Emotion and affective style: Hemispheric substrates. Psychological Science. 1992;3:39–43. doi: 10.1111/j.1467-9280.1992.tb00254.x. [DOI] [Google Scholar]
- Davidson RJ. Affective style and affective disorders: Perspectives from affective neuroscience. Cognition and Emotion. 1998a;12:307–330. doi: 10.1080/026999398379628. [DOI] [Google Scholar]
- Davidson RJ. Anterior electrophysiological asymmetries, emotion, and depression: Conceptual and methodological conundrums. Psychophysiology. 1998b;35:607–614. doi: 10.1017/S0048577298000134. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: Perspectives from affective neuroscience. Annual Review of Psychology. 2002;53:545–74. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Schaffer CE, Saron C. Effects of lateralized presentations of faces on self-reports of emotion and EEG asymmetry in depressed and non-depressed subjects. Psychophysiology. 1985;22:353–364. doi: 10.1111/j.1469-8986.1985.tb01615.x. [DOI] [PubMed] [Google Scholar]
- Debener S, Beauducel A, Nessler D, Brocke B, Heilemann H, Kayser J. Is resting anterior EEG alpha asymmetry a trait marker for depression. Neuropsychobiology. 2000;41:31–37. doi: 10.1159/000026630. [DOI] [PubMed] [Google Scholar]
- Diego MA, Field T, Hernandez-Reif M. BIS/BAS scores are correlated with frontal EEG asymmetry in intrusive and withdrawn depressed mothers. Infant Mental Health Journal. 2001a;22:665–675. doi: 10.1002/imhj.1025. [DOI] [Google Scholar]
- Diego MA, Field T, Hernandez-Reif M. CES-D depression scores are correlated with frontal EEG alpha asymmetry. Depression and Anxiety. 2001b;13:32–37. doi: 10.1002/1520-6394(2001)13:1<32::AID-DA5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Ekman P, Davidson RJ. Voluntary smiling changes regional brain activity. Psychological Science. 1992;4:342–345. doi: 10.1111/j.1467-9280.1993.tb00576.x. [DOI] [Google Scholar]
- Ekman P, Friesen WV. The Facial Action Coding System (FACS): A Technique for the Measurement of Facial Action. Palo Alto, CA: Consulting Psychologists Press; 1978. http://isbndb.com/d/publisher/consulting_psychologists_press.html. [Google Scholar]
- First MG, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV Axis I disorder—clinical version, administration booklet. New York, NY: Biometrics Research Department; 1997. http://cpmcnet.columbia.edu/dept/scid/ [Google Scholar]
- Fox NA, Davidson RJ. Patterns of brain electrical activity during facial signs of emotion in 10 month-old infants. Developmental Psychology. 1988;24:230–246. doi: 10.1037/0012-1649.24.2.230. [DOI] [Google Scholar]
- Gaebel W, Wölwer W. Facial expression and emotional face recognition in schizophrenia and depression. European Archives of Psychiatry and Clinical Neuroscience. 1992;242:46–52. doi: 10.1007/BF02190342. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Ranganath C, Rosenfeld JP. Frontal EEG alpha asymmetry, depression, and cognitive functioning. Cognition & Emotion. 1998;12:449–478. doi: 10.1080/026999398379673. [DOI] [Google Scholar]
- Gray JA. The neuropsychology of anxiety: An inquiry into the functions of the septohippocampal system. Oxford, England: Oxford University Press; 1982. http://www.oup.com/us/ [Google Scholar]
- Gray JA. The neuropsychology of fear and stress. Cambridge, England: Cambridge University Press; 1987. http://www.cambridge.org/ [Google Scholar]
- Gray JA, McNaughton N. The neuropsychology of anxiety: Reprise. In: Hope DA, editor. Perspectives on anxiety, panic, and fear. Lincoln: Nebraska: University of Nebraska Press; 1996. pp. 61–134. http://www.nebraskapress.unl.edu/ [PubMed] [Google Scholar]
- Hagemann D. Individual differences in anterior EEG asymmetry: Methodological problems and solutions. Biological Psychology. 2004;67:157–182. doi: 10.1016/j.biopsycho.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Hagemann D, Naumann E. The effects on ocular artifacts on (lateralized) broadband power in the EEG. Clinical Neurophysiology. 2001;112:215–231. doi: 10.1016/S1388-2457(00)00541-1. [DOI] [PubMed] [Google Scholar]
- Hagemann D, Naumann E, Thayer JF. The quest for the EEG reference revisited: A glance from brain asymmetry research. Psychophysiology. 2001;38:847–857. doi: 10.1017/S0048577201001081. [DOI] [PubMed] [Google Scholar]
- Hagemann D, Naumann E, Thayer JF, Bartussek D. Does resting electroencephalograph asymmetry reflect a trait?: An application of latent state-trait theory. Journal of Personality and Social Psychology. 2002;82:619–641. doi: 10.1037/0022-3514.82.4.619. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Allen JB. Behavioral activation sensitivity and resting frontal EEG asymmetry: Covariation of putative indicators related to risk of mood disorders. Journal of Abnormal Psychology. 1997;106:159–163. doi: 10.1037/0021-843X.106.1.159. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Abramson LY, Sigelman J, Bohlig A, Hogan ME, Harmon-Jones C. Proneness to hypomania/mania symptoms or to depression symptoms and asymmetrical frontal cortical responses to an anger-evoking event. Journal of Personality and Social Psychology. 2002;82:610–618. doi: 10.1037/0022-3514.82.4.610. [DOI] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Regional brain electrical asymmetries discriminate between previously depressed and healthy control subjects. Journal of Abnormal Psychology. 1990;99:22–31. doi: 10.1037/0021-843X.99.1.22. [DOI] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. Journal of Abnormal Psychology. 1991;100:535–545. doi: 10.1037/0021-843X.100.4.535. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Principal components analysis of Laplacian waveforms as a generic method for identifying ERP generator patterns: I. Evaluation with auditory oddball tasks. Clinical Neurophysiology. 2006;117:348–368. doi: 10.1016/j.clinph.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Kayser J, Tenke CE. Generator localization by current source density (CSD): Implications of volume conduction and field closure at intracranial and scalp resolutions. Clinical Neurophysiology. 2012;123:2328–2345. doi: 10.1016/j.clinph.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline JP, Blackhart GC, Joiner TE. Sex, lie scales, and electrode caps: An interpersonal context for defensiveness and anterior electroencephalographic asymmetry. Personality and Individual Differences. 2002;33:459–478. doi: 10.1016/S0191-8869(01)00167-2. [DOI] [Google Scholar]
- Levenson RW, Ekman P, Friesen WV. Voluntary facial action generates emotion-specific autonomic nervous system activity. Psychophysiology. 1990;27:363–384. doi: 10.1111/j.1469-8986.1990.tb02330.x. [DOI] [PubMed] [Google Scholar]
- Mathersul D, Williams LM, Hopkinson PJ, Kemp AH. Investigating models of affect: Relationships among EEG alpha asymmetry, depression, and anxiety. Emotion. 2008;8:560–572. doi: 10.1037/a0012811. [DOI] [PubMed] [Google Scholar]
- McMenamin BW, Shackman AJ, Maxwell JS, Greischar LL, Davidson RJ. Validation of regression-based myogenic correction techniques for scalp and source-localized EEG. Psychophysiology. 2009;46:578–592. doi: 10.1111/j.1469-8986.2009.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger LJ, Paige SR, Carson MA, Lasko NB, Paulus LA, Pittman RK, Orr SP. PTSD arousal and depression symptoms associated with increased right-sided parietal EEG asymmetry. Journal of Abnormal Psychology. 2004;113:324–329. doi: 10.1037/0021-843X.113.2.324. [DOI] [PubMed] [Google Scholar]
- Miller A, Fox NA, Cohn JF, Forbes EE, Sherrill JT, Kovacs M. Regional patterns of brain activity in adults with a history of childhood-onset depression: Gender differences and clinical variability. American Journal of Psychiatry. 2002;159:934–940. doi: 10.1176/appi.ajp.159.6.934. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Heller W, Palmieri PA, Miller GA. Contrasting patterns of brain activity in anxious apprehension and anxious arousal. Psychophysiology. 1999;36:628–637. doi: 10.1017/S0048577299972013. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Heller W, Etienne MA, Miller GA. Prefrontal cortex activity differentiates processes affecting memory in depression. Biological Psychology. 2004;67:125–143. doi: 10.1016/j.biopsycho.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalography and clinical Neurophysiology. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Corrigenda. Electroencephalography and clinical Neurophysiology. 1990;76:565–566. doi: 10.1016/0013-4694(90)90009-9. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Nitschke JB, Oakes TR, Hendrick AM, Horras KA, Larson CL, Davidson RJ. Brain electrical tomography in depression: The importance of symptom severity, anxiety, and melancholic features. Biological Psychiatry. 2002;52:73–85. doi: 10.1016/S0006-3223(02)01313-6. [DOI] [PubMed] [Google Scholar]
- Pollock VE, Schneider LS. Quantitative, waking EEG research on depression. Biological Psychiatry. 1990;27:757–780. doi: 10.1016/0006-3223(90)90591-O. [DOI] [PubMed] [Google Scholar]
- Pössel P, Lo H, Fritz A, Seeman S. A longitudinal study of cortical EEG activity in adolescents. Biological Psychology. 2008;78:173–178. doi: 10.1016/j.biopsycho.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Power MJ, Tarsia M. Basic and complex emotions in depression and anxiety. Clinical Psychology and Psychotherapy. 2007;14:19–31. doi: 10.1002/cpp.515. [DOI] [Google Scholar]
- Reid SA, Duke LM, Allen JJB. Resting frontal electroencephalographic asymmetry in depression: Inconsistencies suggest the need to identify mediating factors. Psychophysiology. 1998;35:389–404. doi: 10.1017/S0048577298970986. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Vaughan C. Emotion expression in depression: Emerging evidence for emotion context-insensitivity. In: Vingerhoets A, Nyklicek I, editors. Emotion regulation: Conceptual and clinical issues. New York: Springer Science and Business Media; 2008. pp. 125–139. http://www.springer.com/ [Google Scholar]
- Schaffer CE, Davidson RJ, Saron C. Frontal and parietal electroencephalogram asymmetry in depressed and nondepressed subjects. Biological Psychiatry. 1983;18:753–762. http://www.journals.elsevierhealth.com/periodicals/bps. [PubMed] [Google Scholar]
- Shackman AJ, McMenamin BW, Slagter HA, Maxwell JS, Greischar LL, Davidson RJ. Electromyogenic artifacts and electroencephalographic inferences. Brain Topography. 2009;22:7–12. doi: 10.1007/s10548-009-0079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman SA, Klein DN, Tenke CE, Bruder GE. Reward sensitivity in depression: A biobehavioral study. Journal of Abnormal Psychology. 2007;116:95–104. doi: 10.1037/0021-843X.116.1.95. [DOI] [PubMed] [Google Scholar]
- Stewart JL, Bismark AW, Towers DN, Coan JA, Allen JJB. Resting frontal EEG asymmetry as an endophenotype for depression risk: Sex-specific patterns of frontal brain asymmetry. Journal of Abnormal Psychology. 2010;119:502–512. doi: 10.1037/a0019196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, Coan JA, Towers DN, Allen JJB. Frontal EEG asymmetry during emotional challenge differentiates individuals with and without lifetime major depressive disorder. Journal of Affective Disorders. 2011;129:167–174. doi: 10.1016/j.jad.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, Towers DN, Coan JA, Allen JJB. The oft-neglected role of parietal EEG asymmetry and risk for major depressive disorder. Psychophysiology. 2010;48:82–95. doi: 10.1111/j.1469-8986.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton SK, Davidson RJ. Prefrontal brain asymmetry: A biological substrate of the behavioral approach and inhibition systems. Psychological Science. 1997;8:204–210. doi: 10.1111/j.1467-9280.1997.tb00413.x. [DOI] [Google Scholar]
- Velo JR, Stewart JL, Hasler BP, Towers DN, Allen JJ. Should it Matter When We Record? Time of Year and Time of Day as Factors Influencing Frontal EEG Asymmetry. Biological Psychology. 2012;91:283–291. doi: 10.1016/j.biopsycho.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuga M, Fox NA, Cohn JF, George CJ, Levenstein RM, Kovacs M. Long term stability of frontal electroencephalographic asymmetry in adults with a history of depression and controls. International Journal of Psychophysiology. 2006;59:107–115. doi: 10.1016/j.ijpsycho.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Thibodeau R, Jorgensen RS, Kim S. Depression, anxiety, and resting frontal EEG asymmetry: A meta-analytic review. Journal of Abnormal Psychology. 2006;115:715–729. doi: 10.1037/0021-843X.115.4.715. [DOI] [PubMed] [Google Scholar]