Abstract
Background
The number needed to treat ratio is an effective method for measuring accuracy in melanoma detection. Dermoscopy reduces the number of false positives and subsequently unnecessary excisions. In vivo confocal microscopy is a non-invasive technique which allows the examination of the skin with cellular resolution.
Objectives
To assess the impact of RCM analysis on the number of equivocal lesions, assumed to be melanocytic, excised for every melanoma.
Methods
Consecutive patients (n=343) presenting with doubtful lesions, were considered for enrolment. The lesions were analysed by dermoscopy and RCM and histopathological assessment was considered the reference standard. The main outcome was the number needed to treat, calculated as the proportion of equivocal lesions, excised for every melanoma.
Results
Dermoscopy alone obtained a hypothetical NNT of 3.73, the combination of dermoscopy and RCM identified 264 equivocal lesions that qualified for excision, 92 of which were confirmed to be a melanoma; resulting in a NNT of 2.87; whereas the analysis of RCM images classified as melanoma 103 lesions with a consequent NNT of 1.12; the difference in the reduction of this ratio was statistically significant (p< 0.0001) between the three groups. There was no significant improvement in sensitivity when comparing the combination of dermoscopy and RCM and RCM alone (94.56% vs. 97.82%; p = 0.043). However, the differences between specificities were statistically significant (p <0.000001), favouring RCM alone.
Conclusion
The addition of RCM analysis to dermoscopy reduces unnecessary excisions with a high diagnostic accuracy and could be a means for reducing the economic impact associated with the management of skin cancer.
INTRODUCTION
Concerns about the increasing incidence of melanoma in white populations have focused on the well documented association between early excision and reduction of mortality1–3. In line with this effort, dermoscopy has been shown to improve the diagnostic accuracy for melanoma in comparison with the unaided-eye examination in four meta-analyses performed on studies conducted in both clinical and experimental settings 4–7. One effective method for measuring the accuracy in melanoma detection is the number needed to treat (NNT), calculated as the number of pigmented lesions excised to detect a melanoma8. Although this value depends on the prevalence of the disease and varies according to physician and lesion-related variables 8–10, it has been proposed to be an useful indicator of the efficient use of healthcare resources11.
The addition of dermoscopy to melanoma screening has been associated with a reduction in the false-positive detection rate and a subsequent decrease of unnecessary excisions, showing a clinically relevant effect in terms of lesion management 12–20. The reported NNT between non-dermoscopy users ranged from about 10 to 15 14,19 and dramatically improves to at least 4 14,19 and as high as 2.4 for properly trained dermatologists with access to digital dermoscopy 20.
In vivo reflectance mode confocal microscopy (RCM) is a non-invasive technique which allows the examination of the epidermis and papillary dermis at cellular resolution. 21,22 Several studies have evaluated the diagnostic accuracy of RCM for equivocal melanocytic lesions, concluding that the use of this novel technique provides a significant improvement in melanoma detection 23–29, even in small, featureless or amelanotic melanoma.30–33
Our aim in this study was to assess the impact of RCM analysis on the number of dermoscopically equivocal pigmented lesions excised for every melanoma, in a clinical setting.
MATERIALS AND METHODS
Study Population
Consecutive patients presenting at the Melanoma Unit of Hospital Clinic in Barcelona with dermoscopically equivocal pigmented lesions, assumed to be melanocytic, were considered for enrolment.
Data collection
The previously published dermoscopic criteria for diagnosing melanoma and the criteria for changes in digital follow up 34 were used to establish the eligibility of lesions 35–37. Data regarding age, gender, anatomic location, melanoma risk factors and dermoscopic diagnosis was collected before the RCM examination and histopathological analyses were performed.
Instruments and Procedures
All the lesions were imaged with a digital camera (Canon PowerShot G10, Canon, Tokyo, Japan) and a high resolution dermatoscope (DermLite Photo, 3GEN, LLC Dana Point, CA, USA). Before biopsy, in vivo confocal microscopy was performed with a commercially available reflectance confocal microscope (Vivascope 1500; Lucid Inc., Henrietta, NY, USA), which uses a near-infrared laser at 830 nm wavelength with a maximum power of 35mW. The image acquisition method was published elsewhere 22 and we established a protocol including serial optical sections obtained at the stratum corneum, stratum granulosum and/or stratum spinosum, dermoepidermal junction (DEJ) and papillary dermis.
The previously described specific RCM criteria for melanoma including four diagnostic features were followed to assess all the images39. The presence of two protective criteria in the basal layer with a score of −1 were considered: 1) edged papillae and 2) presence of typical cells in the basal layer; and the presence of 2 risk criteria with a score of 1 were also considered: 1) presence of round pagetoid cells in upper layers of the epidermis and presence of the nucleated cells found within the dermal papillae. A threshold score greater than −1 was used to obtain a diagnosis of melanoma.
The dermoscopy and RCM diagnosis were made prospectively and therefore, blinded to pathological outcome, but not to clinical information such as age and anatomic location. All the images were interpreted independently by one of the three dermatologists with expertise in RCM (C.C; S.P; J.M). Histopathological assessment was considered the reference standard for diagnosing melanoma40 and was performed by certified dermatopathologists, blinded to the result of RCM examination, in order to avoid review bias 41.
Outcomes
The primary outcome was the NNT, calculated as the proportion of dermoscopically and RCM equivocal pigmented lesions, assumed to be melanocytic, excised for every melanoma. Secondary outcomes included sensitivity, specificity, positive predictive value and negative predictive value of each technique for diagnosing melanoma. According to the method used to decide the excision, the lesions were categorized into three groups. The first group included the lesions intended for excision based on dermoscopy alone; the second group contained the lesions for which dermoscopy and RCM were used to decide excision and the third group was comprised of the lesions for excision based on the analysis of the RCM images. All excised lesions considered to be a melanoma by means of dermoscopy or RCM and confirmed by histopathology were defined as true positives (TP) whereas the true negatives (TN) were the lesions assumed to be non melanoma and afterwards diagnosed as non melanoma (by histopathology). False negatives (FN) included all the melanomas excised with a diagnosis of non melanoma; and false positives (FP) were defined as lesions with a preoperative diagnosis of melanoma not confirmed by histopathology. All the patients with not excised lesions and those with excised lesions were scheduled for a strict follow up including at least 2 visits within a year.
Statistical Analysis
Statistical analyses were performed using SPSS 16.0 (SPSS, Chicago, IL) software. The NNT was calculated for all excised lesions and then adjusted for a range of clinical variables (patient sex, age groups and anatomic site); if distributed differently they were included in multivariate analysis using logistic regression model. In order to compare the two diagnostic tests for the paired sample of the study, a matched sample table was constructed and the raw data in this table was divided according to the final diagnosis as “melanoma” and “non-melanoma”. For these two groups, contingency tables were created, with the two examination techniques being referenced against each other. Sensitivity, specificity, positive and negative predictive values were estimated for each method and compared using Mac Nemar test for proportions.
RESULTS
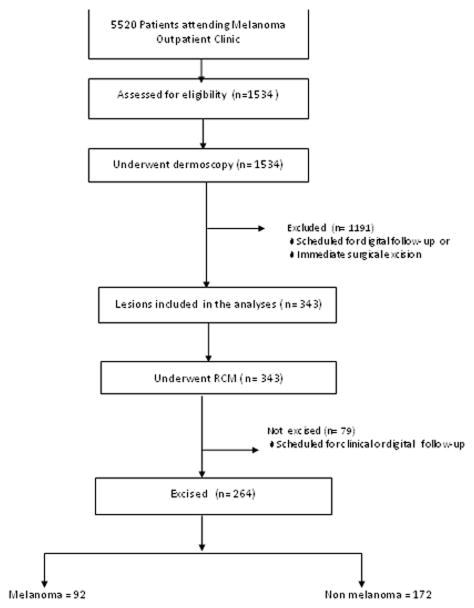
We prospectively assessed data from patients at the Melanoma Unit in Hospital Clinic of Barcelona between 1st June 2011 and 30th May 2012. From a target population of 5520 patients, we found an estimated 1534 lesions to be eligible; 1191 lesions were scheduled for digital follow-up or immediate surgical excision, leaving 343 lesions that qualified for this study. Of these lesions, 264 were finally excised (Fig. 1). The reason why patients were scheduled for digital follow up was the lack of a worrisome change in lesions already included in the digital follow program. The demographic characteristics of the study population, the melanoma characteristics, and the diagnosis of the non melanoma pathology are presented in Table 1. Patients were scheduled to undergo RCM before histopathological analysis, both of which were performed on the same day.
Figure 1.
Flow diagram illustrating the design of the study and main outcomes. n = number of lesions
Table 1.
Demographic characteristics
| Total | ||
|---|---|---|
|
| ||
| Sex | ||
| Male | 136 | |
| Female | 128 | |
|
| ||
| Age, and Median | 54,5 (31–78) | |
|
| ||
| Anatomic location | ||
| Head and neck | 73 | |
| Trunk | 135 | |
| Limbs | 49 | |
| Acral | 7 | |
|
| ||
| Melanoma Patients: | 92 | |
| Breslow thickness median (IQ 25–75) mm | 0.5 (0–1.3) | |
| < 1mm | 86 | |
| ≥ 1mm | 6 | |
| Phototype | ||
| I–II | 42 | |
| III–V | 50 | |
| CDKN2A Mutation | ||
| Carriers | 4 | |
| Wild Type | 20 | |
| Test not performed | 68 | |
|
| ||
| Non melanoma Patients: | 172 | |
| Nevi | 107 | |
| BCC | 12 | |
| Others* | 53 | |
Includes seborrheic keratoses, pigmented actinic keratoses.
Following the use of dermoscopy, 343 of the lesions classified as equivocal would eventually be excised. After the addition of RCM, 77 % (264 of 343) of lesions were judged as suggestive of malignancy according to the criteria followed in the study, and therefore, excised. The 79 lesions without criteria of malignancy upon RCM examination were scheduled for clinical or digital follow up. The consequent reduction of 23 % (79 of 343) of lesions selected for excisional biopsy following RCM was statistically significant compared to the percentage selected by means of dermoscopy alone (p < 0.0001).
In table 2, the effect of RCM on the NNT melanoma is shown. In the first group dermoscopy alone identified 343 equivocal pigmented lesions that qualified for excision, resulting in a hypothetical NNT of 3.73, if all these 343 lesions would had been excised; this is illustrated in Fig. 2, which shows a false positive of lentigo maligna, diagnosed by dermoscopy presenting clear features of solar lentigo under RCM examination. The second group contained the 264 lesions where excision was decided by both dermoscopy and RCM criteria, the resulting NNT was 2.87. In the third group, the analysis of the RCM images classified 103 lesions as melanoma, with a consequent hypothetical NNT of 1.12 if only the lesions selected by this method had been excised. The reduction in this ratio was statistically significant between the two methods of assessment (p< 0.0001). Based on the diagnosis made by the expert using dermoscopy, a total of six false negatives were found; all of them were diagnosed by pathology as in situ melanomas. Two false negatives were encountered for RCM, in both cases the lesions were considered as atypical nevi in the prospective evaluation, and after being excised, both of them were classified as in situ melanomas. Table 3 presents the statistical measures calculated for each method.
Table 2.
NNT according to the method used
| Lesions intended for excision | NNT | |
|---|---|---|
| Dermoscopy | 343 | 3.73 |
| Dermoscopy & RCM | 264 | 2.87 |
| RCM | 103 | 1.12 |
| Excised lesions (n=264) | Confirmed Melanoma by Histopathology (n=92) |
Figure 2.
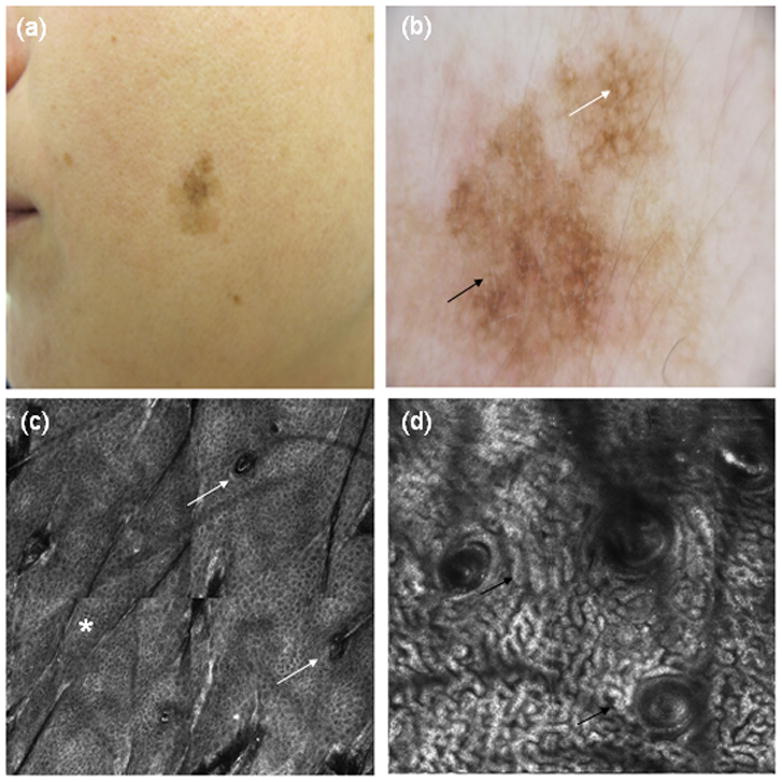
(a) Clinical picture and (b) dermoscopy showing a large asymmetric lesion with atypical pseudonetwork pattern, perifollicular pigmentation (white arrow) and some grey dots (black arrow). (c) RCM mosaic 1000×1000 μm showing a preserved honeycomb pattern in epidermis (*) without involvement of follicular openings (white arrows). (d) Cord-like structures (black arrows) in a cerebriform distribution without atypical cells, very suggestive of solar lentigo under RCM.
Table 3.
Sensitivity, specificity, positive predictive value and negative predictive value depending on the method used
| Dermoscopy n=264 | CI 95% | RCM n=264 | CI 95% | |
|---|---|---|---|---|
| Sensitivity % | 94.56 | (87.19 – 97.98) | 97.82 | (91.62 – 99.62) |
| Specificity % | 26.74 | (87.19 – 97.98) | 92.44 | (87.15 – 95.74) |
| Positive Predictive Value | 40.84 | (34.23 – 47.78) | 87.37 | (79.03 – 92.84) |
| Negative Predictive Value | 90.19 | (77.81 – 96.33) | 98.75 | (95.11 – 99.78) |
n = excised lesions
As shown in Table 4, when comparing dermoscopy diagnosis and RCM diagnosis in preselected lesions by dermoscopy, against histopathology, the reference standard, there was no significant improvement in sensitivity (94.56% vs 97.82%; p = 0.043) for the diagnosis of melanoma. However, the differences between specificities for the two methods were statistically significant (p <0.000001). After analysing the Breslow thickness and the pathology of melanoma type, neither the sensitivity nor the specificity varied according to each examination technique. After one year of follow up, all the patients included completed at least 2 visits and no additional melanoma was diagnosed.
Table 4.
Matched sample table for melanoma (a) and non-melanoma (b) groups as verified by histopathology (reference standard)
| (a) Comparison of sensitivities of the two methods
| |||||
|---|---|---|---|---|---|
| RCM in preselected lesions by dermoscopy | Mac Nemar Test CI 95% | ||||
| + | − | ||||
| Dermoscopy | + | 84 | 2 | 86 | 0.6 – 14.8 |
| − | 6 | 0 | 6 | p = 0.043 | |
|
|
|||||
| Total melanoma | 92 | ||||
| (b) Comparison of specificities of the two methods
| |||||
|---|---|---|---|---|---|
| RCM in preselected lesions by dermoscopy | Mac Nemar Test CI 95% | ||||
| + | − | ||||
| Dermoscopy | + | 11* | 117 | 128 | 14.4 – 26.3 |
| - | 2 | 42 | 44 | p< 0.000001 | |
|
|
|||||
| Total non-melanoma | 172 | ||||
False positives of RCM included Spitz Nevi (3) and histologically atypical nevi (8)
DISCUSSION
The major outcome of our study is the significant reduction on the NNT as a result of the addition of RCM to dermoscopy in real clinical practice. Since the performance of the examination technique has a direct effect on the NNT14,18,19, this low ratio (1.12) could be explained by the high diagnostic accuracy of RCM in melanoma detection. The hypothetical NNT we have calculated for dermoscopy (3.73) is in line with those of previous publications evaluating NNT for dermoscopy and digital dermoscopy 12–20. Although several studies on the diagnostic accuracy of RCM have been published, not many evaluated the impact of RCM in a clinical setting. Only two studies compared the performance of RCM with dermoscopy 23,29 and only the latter assessed the additive value of RCM in the management of melanocytic skin lesions29. The former study prospectively evaluated the diagnostic accuracy of RCM and dermoscopy examining melanocytic lesions, and did not find any significant difference between the sensitivities and specificities of the two techniques. In contrast to these results, we found higher specificity when using RCM, but these results could be influenced by the design of the study. The fact that 79 lesions following RCM examination were not excised, impaired our specificity and sensitivity analysis, but our study design prioritized the real impact of RCM in the clinical arena. The two melanomas misdiagnosed by the expert evaluating RCM were in situ melanomas classified prospectively as nevi with atypical RCM features. When we review these two lesions retrospectively applying the RCM second step score for melanoma, the score was 0, and melanoma should be suspected (with this method only negative values were not associated with melanoma). The first lesion was an achromic papule showing arborizing vessels under dermoscopy and the second was a melanocytic lesion with globular pattern (Fig. 3). Interestingly, both cases were correctly suspected by clinical and dermoscopical judgement. The first case was a patient diagnosed with Xeroderma Pigmentosum who had seven previous melanomas, and the second patient presented a lesion suspected to be melanoma by means of observed changes in sequential dermoscopy imaging. Both techniques together did not miss any melanoma, and all the lesions classified as benign by RCM were scheduled for clinical or digital follow up. One year after the end of the study, no additional melanoma was diagnosed. The design of our study did not allow us to clarify whether RCM alone would have reduced the NNT as low as it did, as dermoscopy was the first step used to select the lesions to be subjected to RCM examination. We believe that the role of RCM is not to replace but to complement dermoscopy.
Figure 3.
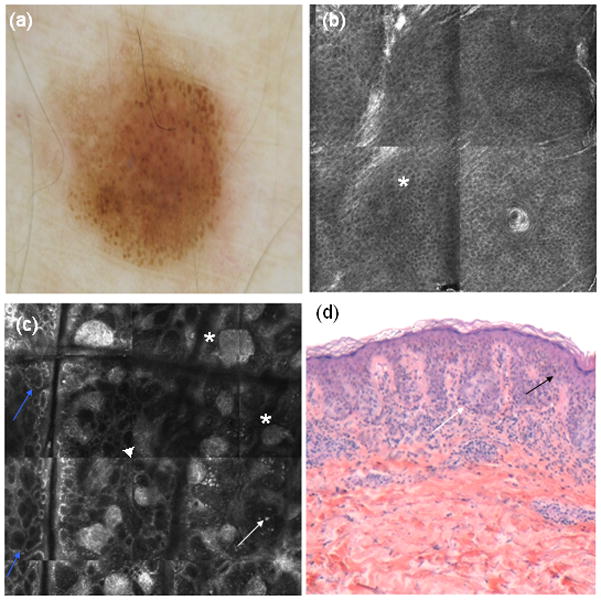
(a) Dermoscopy showing irregular globular pattern (b) RCM mosaic 1000×1000 μm showing a preserved honeycomb pattern in epidermis (*). (c) RCM mosaic at dermoepidermal junction showing irregular nests (*), with irregular edged papillae (blue arrows), junctional thickenings (▲) and some atypical hyperreflective cells within the dermal papillae (white arrows). (d) Histopathology (haematoxylin and eosin 20X) showing atypical cells (black arrow) and nests (white arrow) from a in situ melanoma.
There is one study 29 published using a set of images and simulating the conditions of a clinical setting, ours are the first data evaluating the impact of RCM on the NNT ratio in a clinical setting. In conclusion, the use of RCM in lesions preselected by dermoscopy or digital follow-up reduces unnecessary excisions with a high diagnostic accuracy and could be a means for reducing the economic impact associated with the management of skin cancer by reducing the excision of benign lesions.
What’s already known about this topic?
Dermoscopy enhances the diagnostic accuracy for melanoma and dramatically decreases the NNT ratio when used by properly trained dermatologists. RCM is a novel technique that provides a significant improvement in melanoma detection.
What does this study add?
The addition of RCM to dermoscopy has a significant impact on the number of dermoscopically equivocal pigmented lesions excised for every melanoma, reducing the excision of benign lesions.
Acknowledgments
Funding /Support: The research at the Melanoma Unit in Barcelona was partially funded by Grants 06/0265 and 09/01393 from Fondo de Investigaciones Sanitarias, Spain; by the CIBER de Enfermedades Raras of the Instituto de Salud Carlos III, Spain; by the AGAUR 2009 SGR 1337 of the Catalan Government, Spain; by the European Commission under the 6th Framework Programme, Contract nr: LSHC-CT-2006-018702 (GenoMEL) and by the National Cancer Institute (NCI) of the US National Institute of Health (NIH) (CA83115).
Footnotes
Conflict of interest: The authors state no conflict of interest.
References
- 1.Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27 :6199–206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balch CM, Soong S-J, Atkins MB, et al. An evidence-based staging system for cutaneous melanoma. CA Cancer J Clin. 2004;54:131–49. doi: 10.3322/canjclin.54.3.131. [DOI] [PubMed] [Google Scholar]
- 3.Balch CM, Soong SJ, Gershenwald JE, et al. Prognostic factors analysis of 17,600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol. 2001;19:3622–34. doi: 10.1200/JCO.2001.19.16.3622. [DOI] [PubMed] [Google Scholar]
- 4.Vestergaard ME, Macaskill P, Holt PE, Menzies SW. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669–76. doi: 10.1111/j.1365-2133.2008.08713.x. [DOI] [PubMed] [Google Scholar]
- 5.Kittler H, Pehamberger H, Wolff K, Binder M. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3:159–65. doi: 10.1016/s1470-2045(02)00679-4. [DOI] [PubMed] [Google Scholar]
- 6.Bafounta M, Beauchet A, Aegerter P, Saiag P. Is Dermoscopy (Epiluminescence Microscopy) Useful for the Diagnosis of Melanoma? Arch Dermatol. 2001;137 :1343–50. doi: 10.1001/archderm.137.10.1343. [DOI] [PubMed] [Google Scholar]
- 7.Mayer J. Systematic review of the diagnostic accuracy of dermatoscopy in detecting malignant melanoma. Med J Aust. 1997;167:206–10. doi: 10.5694/j.1326-5377.1997.tb138847.x. [DOI] [PubMed] [Google Scholar]
- 8.Baade PD, Youl PH, Janda M, et al. Factors associated with the number of lesions excised for each skin cancer. Arch Dermatol. 2008;144:1468–76. doi: 10.1001/archderm.144.11.1468. [DOI] [PubMed] [Google Scholar]
- 9.English DR, Del Mar C, Burton RC. Factors influencing the number needed to excise: excision rates of pigmented lesions by general practitioners. Med J Aust. 2004;180:16–9. doi: 10.5694/j.1326-5377.2004.tb05766.x. [DOI] [PubMed] [Google Scholar]
- 10.Hansen C, Wilkinson D, Hansen M, Argenziano G. How good are skin cancer clinics at melanoma detection? Number needed to treat variability across a national clinic group in Australia. J Am Acad Dermatol. 2009;61:599–604. doi: 10.1016/j.jaad.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 11.Sidhu S, Bodger O, Williams N, Roberts DL. The number of benign moles excised for each malignant melanoma: the number needed to treat. Clin Exp Dermatol. 2012;37 :6–9. doi: 10.1111/j.1365-2230.2011.04148.x. [DOI] [PubMed] [Google Scholar]
- 12.Carli P, de Giorgi V, Chiarugi A, et al. Addition of dermoscopy to conventional naked-eye examination in melanoma screening: a randomized study. J Am Acad Dermatol. 2004;50 :683–9. doi: 10.1016/j.jaad.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 13.Carli P, Mannone F, De Giorgi V, et al. The problem of false-positive diagnosis in melanoma screening: the impact of dermoscopy. Melanoma Res. 2003;13 :179–82. doi: 10.1097/00008390-200304000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Carli P, De Giorgi V, Crocetti E, et al. Improvement of malignant/benign ratio in excised melanocytic lesions in the “dermoscopy era”: a retrospective study 1997–2001. Br J Dermatol. 2004;150:687–92. doi: 10.1111/j.0007-0963.2004.05860.x. [DOI] [PubMed] [Google Scholar]
- 15.van der Rhee JI, Bergman W, Kukutsch N. Impact of dermoscopy on the management of high-risk patients from melanoma families: a prospective study. Acta Derm Venereol. 2011;91 :428–31. doi: 10.2340/00015555-1100. [DOI] [PubMed] [Google Scholar]
- 16.van der Rhee JI, Bergman W, Kukutsch N. The impact of dermoscopy on the management of pigmented lesions in everyday clinical practice of general dermatologists: a prospective study. Br J Dermatol. 2010;162:563–7. doi: 10.1111/j.1365-2133.2009.09551.x. [DOI] [PubMed] [Google Scholar]
- 17.Argenziano G, Soyer HP, Chimenti S, et al. Impact of dermoscopy on the clinical management of pigmented skin lesions. Clin Dermatol. 2002;20 :200–2. doi: 10.1016/s0738-081x(02)00234-1. [DOI] [PubMed] [Google Scholar]
- 18.Argenziano G, Cerroni L, Zalaudek I, et al. Accuracy in melanoma detection: a 10-year multicenter survey. J Am Acad Dermatol. 2012;67:54–9. doi: 10.1016/j.jaad.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 19.Menzies SW, Emery J, Staples M, et al. Impact of dermoscopy and short-term sequential digital dermoscopy imaging for the management of pigmented lesions in primary care: a sequential intervention trial. Br J Dermatol. 2009;161:1270–7. doi: 10.1111/j.1365-2133.2009.09374.x. [DOI] [PubMed] [Google Scholar]
- 20.Tromme I, Sacré L, Hammouch F, et al. Availability of digital dermoscopy in daily practice dramatically reduces the number of excised melanocytic lesions: results from an observational study. Br J Dermatol. 2012;167:778–86. doi: 10.1111/j.1365-2133.2012.11042.x. [DOI] [PubMed] [Google Scholar]
- 21.Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol. 1995;104:946–52. doi: 10.1111/1523-1747.ep12606215. [DOI] [PubMed] [Google Scholar]
- 22.Rajadhyaksha M, González S, Zavislan JM, et al. In vivo confocal scanning laser microscopy of human skin II: advances in instrumentation and comparison with histology. J Invest Dermatol. 1999;113:293–303. doi: 10.1046/j.1523-1747.1999.00690.x. [DOI] [PubMed] [Google Scholar]
- 23.Langley RGB, Walsh N, Sutherland AE, et al. The diagnostic accuracy of in vivo confocal scanning laser microscopy compared to dermoscopy of benign and malignant melanocytic lesions: a prospective study. Dermatology. 2007;215:365–72. doi: 10.1159/000109087. [DOI] [PubMed] [Google Scholar]
- 24.Pellacani G, Guitera P, Longo C, et al. The impact of in vivo reflectance confocal microscopy for the diagnostic accuracy of melanoma and equivocal melanocytic lesions. J Invest Dermatol. 2007;127:2759–65. doi: 10.1038/sj.jid.5700993. [DOI] [PubMed] [Google Scholar]
- 25.Gerger A, Koller S, Weger W, et al. Sensitivity and specificity of confocal laser-scanning microscopy for in vivo diagnosis of malignant skin tumors. Cancer. 2006;107:193–200. doi: 10.1002/cncr.21910. [DOI] [PubMed] [Google Scholar]
- 26.Gerger A, Koller S, Kern T, et al. Diagnostic applicability of in vivo confocal laser scanning microscopy in melanocytic skin tumors. J Invest Dermatol. 2005;124:493–8. doi: 10.1111/j.0022-202X.2004.23569.x. [DOI] [PubMed] [Google Scholar]
- 27.Gerger A, Hofmann-Wellenhof R, Samonigg H, Smolle J. In vivo confocal laser scanning microscopy in the diagnosis of melanocytic skin tumours. Br J Dermatol. 2009;160:475–81. doi: 10.1111/j.1365-2133.2008.08995.x. [DOI] [PubMed] [Google Scholar]
- 28.Gerger A, Langsenlehner U, Richtig E, et al. In vivo confocal laser scanning microscopy of melanocytic skin tumours: diagnostic applicability using unselected tumour images. Br J Dermatol. 2008;158:329–33. doi: 10.1111/j.1365-2133.2007.08389.x. [DOI] [PubMed] [Google Scholar]
- 29.Guitera P, Pellacani G, Longo C, et al. In vivo reflectance confocal microscopy enhances secondary evaluation of melanocytic lesions. J Invest Dermatol. 2009;129:131–8. doi: 10.1038/jid.2008.193. [DOI] [PubMed] [Google Scholar]
- 30.Pupelli G, Longo C, Veneziano L, et al. Small diameter melanocytic lesions: morphological analysis by means of in vivo confocal microscopy. Br J Dermatol. 2013;168:1027–33. doi: 10.1111/bjd.12212. [DOI] [PubMed] [Google Scholar]
- 31.Maier T, Sattler EC, Braun-Falco M, et al. Reflectance confocal microscopy in the diagnosis of partially and completely amelanotic melanoma: report on seven cases. J Eur Acad Dermatol Venereol. 2013;27:e42–52. doi: 10.1111/j.1468-3083.2012.04465.x. [DOI] [PubMed] [Google Scholar]
- 32.Curchin C, Wurm E, Jagirdar K, et al. Dermoscopy, reflectance confocal microscopy and histopathology of an amelanotic melanoma from an individual heterozygous for MC1R and tyrosinase variant alleles. Australas J Dermatol. 2012;53:291–4. doi: 10.1111/j.1440-0960.2012.00882.x. [DOI] [PubMed] [Google Scholar]
- 33.Busam KJ, Hester K, Charles C, et al. Detection of clinically amelanotic malignant melanoma and assessment of its margins by in vivo confocal scanning laser microscopy. Arch Dermatol. 2001;137 :923–9. [PubMed] [Google Scholar]
- 34.Kittler H, Guitera P, Riedl E, et al. Identification of clinically featureless incipient melanoma using sequential dermoscopy imaging. Arch Dermatol. 2006;142:1113–9. doi: 10.1001/archderm.142.9.1113. [DOI] [PubMed] [Google Scholar]
- 35.Salerni G, Carrera C, Lovatto L, et al. Benefits of total body photography and digital dermatoscopy (“two-step method of digital follow-up”) in the early diagnosis of melanoma in patients at high risk for melanoma. J Am Acad Dermatol. 2012;67:e17–27. doi: 10.1016/j.jaad.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pehamberger H, Steiner A, Wolff K. In vivo epiluminescence microscopy of pigmented skin lesions. I. Pattern analysis of pigmented skin lesions. J Am Acad Dermatol. 1987;17:571–83. doi: 10.1016/s0190-9622(87)70239-4. [DOI] [PubMed] [Google Scholar]
- 37.Steiner A, Pehamberger H, Wolff K. In vivo epiluminescence microscopy of pigmented skin lesions. II. Diagnosis of small pigmented skin lesions and early detection of malignant melanoma. J Am Acad Dermatol. 1987;17:584–91. doi: 10.1016/s0190-9622(87)70240-0. [DOI] [PubMed] [Google Scholar]
- 38.Pehamberger H, Binder M, Steiner A, Wolff K. In vivo epiluminescence microscopy: improvement of early diagnosis of melanoma. J Invest Dermatol. 1993;100:356S–362S. doi: 10.1111/1523-1747.ep12470285. [DOI] [PubMed] [Google Scholar]
- 39.Segura S, Puig S, Carrera C, et al. Development of a two-step method for the diagnosis of melanoma by reflectance confocal microscopy. J Am Acad Dermatol. 2009;61:216–29. doi: 10.1016/j.jaad.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 40.Price NM, Rywlin AM, Ackerman AB. Histologic criteria for the diagnosis of superficial spreading malignant melanoma: formulated on the basis of proven metastatic lesions. Cancer. 1976;38:2434–41. doi: 10.1002/1097-0142(197612)38:6<2434::aid-cncr2820380631>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 41.Bossuyt PM, Reitsma JB, Bruns DE, et al. The STARD Statement for Reporting Studies of Diagnostic Accuracy: Explanation and Elaboration. Clin Chem. 2003;49:7–18. doi: 10.1373/49.1.7. [DOI] [PubMed] [Google Scholar]