Abstract
The presence of maternal autoantibodies has been previously associated with pre-eclampsia, although the composition of the antibody repertoire in pre-eclampsia has not been well characterized. Given this, we applied bacterial display peptide libraries to identify peptides that preferentially react with plasma antibodies from patients with pre-eclampsia (n=15) versus healthy-outcome pregnancies (n=18). Screening using fluorescence activated cell sorting identified 38 peptides that preferentially bind to antibodies from individuals with pre-eclampsia. These pre-eclampsia specific peptides possessed similar motifs of RG/SG/−WWG/S, RWWG/S, or WGWGXXR/K distinct from the angiotensin II type 1 receptor epitope AFHYESQ. Seven library-isolated peptides and a cell-surface displayed type 1 receptor epitope were used to construct a diagnostic algorithm with a training set of 18 new pre-eclamptic and 22 healthy-outcome samples from geographically distinct cohorts. Cross-validation within the training group resulted in averaged areas underneath a receiver operating characteristic curve of 0.78 and 0.72 with and without the known receptor epitope, respectively. In a small validation set (pre-eclamptic = 12, healthy = 8), the algorithm consisting only of library-isolated peptides correctly classified 10 pre-eclamptic and 6 healthy, using a predefined cutoff that achieved 61% sensitivity (95%CI: 36–83%) at 95% specificity (95%CI: 77–100%) in training set (n=40) cross-validation. Our results indicate that antibodies with specificities other than anti-angiotensin II type 1 receptor are prevalent in pre-eclampsia patients and may be useful as diagnostic biomarkers.
Keywords: pre-eclampsia, diagnosis, peptide, bacterial display, antibody
Introduction
Pre-eclampsia (PE) is a serious disorder that affects 5–8% of pregnancies1 and causes 15–20% of maternal mortalities and morbidities in developed countries.2 Despite serious global impact, the primary method of PE diagnosis continues to rely on presentation of maternal symptoms, including hypertension and proteinuria, after 20 weeks of gestation. However, present diagnostic approaches are inadequate to identify patients likely to experience adverse outcomes since 10–15% of women who experience hemolysis, elevated liver enzymes, and low platelet levels (HELLP syndrome) and 20–25% who progress to eclampsia do not present with either hypertension or proteinuria.3 Consequently, there remains a need for non-invasive diagnostics that can accurately and reliably identify patients that develop PE and those at risk for adverse outcomes.
Diagnostic development efforts have focused on the identification of protein biomarkers with unique presentation in PE. Multiple proteins exhibit altered serum levels in PE and have been pursued as candidate biomarkers and/or therapeutic targets, including soluble VEGF receptor (sFlt-1) and placental growth factor (PlGF),4 soluble endoglin (sEng),5 placental protein 13,6 and angiotensin II type 1 receptor (AT1) autoantibodies (AT1-AAs).7 In particular, much effort has focused on evaluating the diagnostic utility of the ratio of the elevated sFlt-1 to lowered PlGF levels or the PlGF level alone.8 These biomarkers have yielded high diagnostic accuracy for detecting early-onset PE and predicting adverse outcomes.9 However, these biomarkers are less effective after 34 weeks gestation,8 the period where 90% of PE cases present.1 Finally, they do not enable accurate prediction of PE during the first trimester,10 since sFlt-1 levels only become significantly altered about five weeks before PE onset.4 Therefore, despite the utility of these biomarkers for early-onset PE detection, additional biomarkers are needed.
Circulating antibodies represent a rich source for additional biomarker discovery, and several observations link the immune system to PE pathogenesis. Most prominently, PE patients have been found to produce agonistic IgG AT1-AAs7 as early as 18 weeks gestation.11 Several in vivo and in vitro studies have demonstrated a potential pathological role for these antibodies. Injection of AT1-AAs or total IgG isolated from PE patients into pregnant mice induced the hallmark PE symptoms (hypertension, proteinuria, and increased sFlt-112 and fetal growth restriction13) while co-injection with an antibody blocking peptide epitope attenuated these effects. Interestingly, placental ischemia stimulated AT1-AAs similarly contribute to hypertension in an independent PE rat model.14 AT1-AAs increase complement protein C3 deposition in the placenta and kidney of pregnant mice,15 while mutations within complement system regulatory proteins appear to be a risk factor for PE.16 Complement activation has been further implicated in PE with increased C3 deposits in placental vessels from a transgenic PE rat model, and supernatant from PE placental explants stimulate C3 expression in rat vascular smooth muscle cells.17 Furthermore, isolated CD19+CD5+ B-cells are elevated in PE and produce AT1-AAs in culture upon addition of PE serum.18 At the same time, individuals with PE exhibit significantly reduced levels of CD4+CD25+ regulatory T cells,19 a finding consistent with increased autoantibody production.20 Collectively, these prior studies indicate that immunological alterations are a conserved feature in PE.
Despite a demonstrated role of AT1-AAs in the pathology of PE, their efficacy for PE diagnosis has not been established. Existing assays for AT1-AAs that rely upon cardiomyocyte beat rate7 or a luciferase reporter21 lack throughput and are unsuitable for point-of-care diagnostics. More importantly, AT1-AA prevalence varies significantly in different studies (70%22 to 95%21), and AT1-AAs are not specific to PE since they have been observed in individuals with HOP,22 chronic hypertension,23 and renal allograft rejection.24 Given these problems, we investigated whether additional PE specific antibodies exist that could serve as biomarker(s) for PE diagnosis and further implicate a pathophysiological role for an altered immune system. To simultaneously identify antibody biomarkers and peptide reagents for their detection, we screened bacterial display peptide libraries25 against antibodies enriched from the plasma of individuals with PE and HOPs. Our results demonstrate the existence of PE specific plasma antibodies, other than AT1-AAs, that may be useful for PE diagnosis.
Materials and Methods
Patient Samples
Whole blood samples were obtained from pregnant women as aliquots of samples taken for routine blood work during clinical assessments at the Santa Barbara Cottage Hospital (cohort 1). The study was approved by the Santa Barbara Cottage Hospital review board. To qualify as affected with PE, subjects fulfilled at least two of the following criteria: i) two documented blood pressures with readings greater than 140/90mm Hg at least 4 hours apart, with documented normal BPs in the first half of the pregnancy, ii) proteinuria as defined by ≥30 mg/dL on a spot urine check, ≥1+ dipstick reading, or ≥300 mg/24 hr iii) central nervous system (CNS) symptoms (visual disturbances or unremitting headaches), iv) epigastric pain associated with elevated liver enzymes unrelated to other abdominal pathology, v) or thrombocytopenia with platelet counts less than 100,000 U/mL. This ensured that PE samples met the American College for Obstetricians and Gynecologists (ACOG) criteria for mild or severe PE diagnosis. Pre-existing hypertension and lupus patients were excluded from cohort 1. Samples were divided into a discovery set (n=33) for initial peptide identification and a training set (n=20) for testing diagnostic ability of isolated peptides. Additional de-identified samples provided from University of Texas - Houston Medical School (cohort 2) were used in either the training set (n=20) or a validation set (n=20). These PE samples were diagnosed by clinical assessments based on the National High Blood Pressure Education Program Working Group Report.
This study did not distinguish between early or late onset PE and did not discriminate based on parity. Therefore, these cohorts represent a mix of presentation times and parities. All subjects provided informed consent, and samples were collected according to institutional guidelines. Blood samples for both cohorts were obtained near the time of delivery. In cohort 1, blood pressures were recorded at the time of presentation while cohort 2 recorded maximum blood pressure prior to delivery. Additionally, while cohort 1 mainly used the spot urine check, cohort 2 diagnosis used 24 hour analysis and the dipstick test (n=7).
Bacterial display and library screening
The AT1 epitope AFHYESQ was displayed on Escherichia coli MC106126 with flanking glycines as a fusion to the N-terminus of the eCPX scaffold27 along with a C-terminal peptide tag (P2x) that binds a fluorescent reporter (YPet-Mona) of scaffold expression.28 A 15-mer random peptide library displayed on the N-terminus of the eCPX scaffold was screened for peptides binding to PE specific antibodies. Library screening used antibody fractions in PBS (0.1% BSA) prepared by ammonium sulfate precipitation of patient plasma and depleted of E. coli binding antibodies. Magnetic selection enriched for peptides that bind pooled PE (n=9) Ig (5 µmol/L total concentration) labeled with Mini-Biotin-XX Labeling Kit (Invitrogen) while outcompeting an unlabeled pool of HOP (n=12) Ig (5 µmol/L total). To favor cross-reactivity, two pools of PE Ig with distinct fluorophores were prepared; group 1 (n=4) labeled with Alexa Fluor-488 (Invitrogen) (green) and group 2 (n=5) biotinylated (red) to enable detection with streptavidin-conjugated R-phycoerythrin (SA-PE) (Invitrogen). Cells were co-incubated with excess unlabeled HOP Ig and labeled PE Ig, and those cells exhibiting both red and green fluorescence were recovered by fluorescence activated cell sorting (FACS) (Figure 1A). Peptides with specificity for PE Ig were favored in separate sorts using a PE Ig pool (n=9) labeled with Alexa 488 and a biotinylated HOP Ig (n=12) pool. After recovering non-fluorescent cells that did not capture HOP Ig, cells exhibiting green not red fluorescence were collected after labeling with PE and HOP Ig pools followed by SA-PE (Figure 1B). Screening continued with a set of new PE and HOP (n=6 each) samples to enhance PE cross-reactivity and specificity.
Figure 1.
A two-color screening methodology isolated a pool of PE specific antibody-detecting peptides, enabling further characterization of individual peptides. A. After co-incubating a bacterial displayed peptide library with two distinctly labeled pools of PE (red or green fluorophore) and unlabeled pool of HOP antibodies enriched from plasma, cells expressing peptides that bind antibodies present in both PE groups were isolated. B. Subsequently, the library was co-incubated with a labeled HOP antibody pool (red) and a PE pool (green). Bacteria exhibiting only green fluorescence were isolated, ensuring disease specificity. C. Finally, sequence analysis of the enriched pool identified unique peptides for motif characterization and individual peptide PE and HOP binding activity (fluorescent intensity) was assessed.
Peptide sequence analysis and down-selection
Plasmid DNA from ~200 bacterial colonies was sequenced from the final sorting round, from which 83 unique sequences were identified using Geneious. Three additional peptides that demonstrated PE reactivity and specificity in an earlier screening round were incorporated into the motifs identified by inspection (Figure 1C). The binding activity, or fold fluorescence over a negative control (empty scaffold), of each library peptide and the AT1 epitope clone was measured in duplicate with discovery set PE and HOP (n=6 each) pools. After incubation with each Ig pool (1 µmol/L), cells were labeled using biotinylated anti-human IgA + IgG + IgM (Jackson ImmunoResearch), followed by SA-PE. On average, peptides exhibited 1.4-fold increased PE antibody binding activity over HOP. Therefore peptides demonstrating 50% higher PE reactivity over HOP (1.5-fold) were selected as the most specific and ranked according to the PE activity quotient defined as the PE binding activity multiplied by the ratio of PE activity to HOP (i.e. dynamic range).
Patient antibody reactivity assays
The AT1 epitope and seven downselected peptides were assayed in duplicate against biotinylated Ig (1 µmol/L) enriched from individual samples of a validation set. This set included 10 PE and 10 HOP from cohort 1 and 20 PE and 20 HOP from cohort 2. The mean cell fluorescence associated with each clone was divided by that of a negative control for each sample. While the class (PE or HOP) was known for cohort 1, cohort 2 samples’ classes were revealed only after testing peptide reactivity. To account for the varied peptide activity range, each clone’s binding activity was standardized by the average reactivity and standard deviation.
Statistical Analysis
A subset of the 60 samples (20 cohort 1 and 20 randomly selected cohort 2 samples) was used to train a classification algorithm followed by testing with a validation set (20 remaining cohort 2 samples) to verify peptide panel diagnostic accuracy. To reduce over-fitting to the training set, an Adaptive Boosting (AdaBoost) classification algorithm, which applies the algorithm successively to reweighted versions of the training set,29 was implemented through the ‘ada’ package in R30 using a four-split tree. Diagnostic algorithms were generated with and without the AT1 epitope included in the peptide panel. Three trials of ten-fold cross-validation across a combined set from both cohorts (n = 40) yielded each sample’s averaged probability for PE classification, which was used to perform receiver operating characteristic (ROC) curve analysis with the pROC R package and assess algorithm accuracy. Additional algorithms were trained using cohort 1 or 2 separately with or without the AT1 epitope. Subsequently, the six algorithms’ performance was assessed by generating ROC curves and determining the PE, HOP, and overall classification accuracy at a 0.5 probability cutoff in the final validation set.
Separately, ROC analysis was performed with Prism 4 software (GraphPad Software, Inc.) for each library-isolated peptide across the entire set of 60 validation samples based upon binding activity. ROC analysis of the AT1 epitope was performed on the same 60 samples for comparison to library-isolated peptides followed by analysis across the entire set of 45 PE and 48 HOP samples described in this study. Excluding Figures 3 and 4, data are presented as mean ± SEM. Statistical significance using a Student’s t-test or Mann-Whitney U-test and Spearman correlation was assessed with Prism 4 software. For all analyses, p < 0.05 was considered significant.
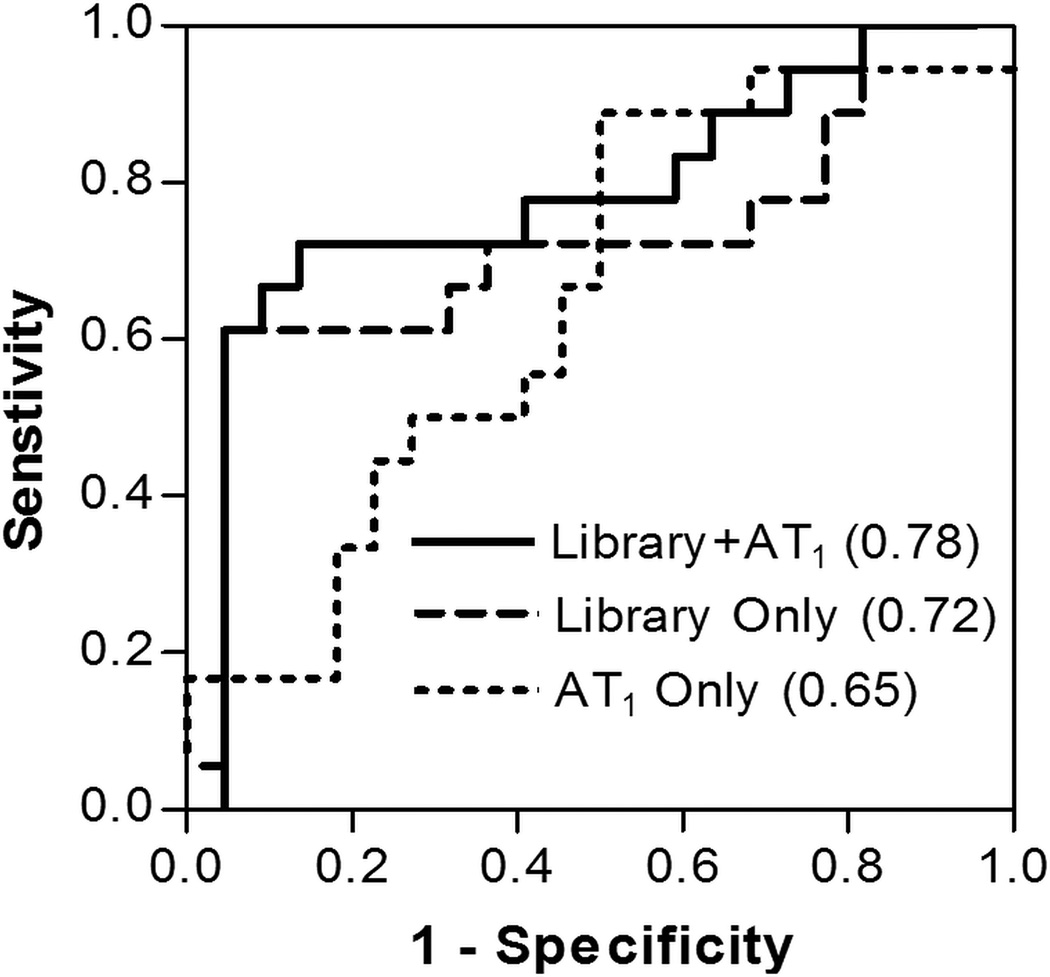
Figure 3.
Receiver operating characteristic (ROC) curves are shown for algorithm predictions using library peptides with and without the AT1 epitope and the AT1 epitope alone across the combined training sample set (n=40). The respective areas underneath the ROC curves (AUC) are also indicated.
Figure 4.
Library-isolated peptides and the AT1 epitope demonstrate cross-reactivity with PE with decreased HOP binding. A. Peptide reactivity with 30 samples each of PE and HOP in a dot-plot, where lines represent the mean of the population. Area underneath the ROC curve is also indicated for each peptide (AUC). B. A heat map shows cross-reactivity and application as a peptide panel. Statistical significance indicated for Mann-Whitney U-test (*), Student’s t-test (†) where applicable. * or † p < 0.05, ** or †† p < 0.01 Standardized Reactivity = (Sample Fluorescence − Average Fluorescence)/Standard Deviation of Fluorescence.
Results
Identification of peptides binding to plasma antibodies from PE patients
The 45 PE samples used in this study reflect the heterogeneity of PE presentation including early and late onset PE cases and atypical severe cases without proteinuria (Tables 1 and S1). To identify PE specific antibody-detecting peptide reagents within this diverse group, a bacterial display library was screened for peptides that bind antibodies present in multiple PE patients but not HOP subjects. This screening strategy utilized FACS to quantitatively measure the enrichment of PE antibody binding peptides from 7% to 87% (Figure S1 A,B) of the bacterial cell population and reduction of HOP antibody reactive peptides (Figure S1 C,D). From this enriched pool, DNA sequencing identified 83 unique peptides, enabling further characterization of each member alongside three peptides from an earlier screening round. None of the library-isolated peptides were similar to the known AT1 receptor epitope AFHYESQ, but several different motifs were identified (Table S2). Three motifs (a-1 to a-3) were similar, and four additional motifs were distinct. The library peptide consensus sequence motifs were not sufficiently strong to enable identification of antigens that elicited the antibodies. Roughly 48 candidate autoantigens were identified by rank ordering proteins with high similarity scores obtained using blastp and ScanProsite searches (Table S3); however, a much larger number of non-self proteins in the entire database also carried these motifs.
Table 1.
Clinical characteristics of patients
| Patient Characteristics |
Discovery Samples (Cohort 1) |
Training Samples (Cohort 1) |
Training Samples (Cohort 2) |
Validation Samples (Cohort 2) |
||||
|---|---|---|---|---|---|---|---|---|
| HOP (n=18) |
PE (n=15) |
HOP (n=10) |
PE (n=10) |
HOP (n=12) |
PE (n=8) |
HOP (n=8) |
PE (n=12) |
|
| Age, yr | 29.8 (1.6) | 29.9 (2.0) | 27.9 (2.7) | 30.1 (2.6) | 26.6 (1.7) | 25.1 (2.1) | 25.5 (2.2) | 26.5 (1.3) |
| GAD, wk | 38.0 (0.7) | 35.3 (0.8) | 38.9 (0.4) | 36.8 (0.9) | 39.1 (0.5) | 34.8 (1.2) | 39.6 (0.3) | 35.7 (0.6) |
| Highest SBP, mm Hg | 112.0 (3.3) | 164.7 (5.0)* | 114.4 (3.3) | 161.1 (6.8)* | 113.1 (3.4) | 156.3 (6.1)* | 124.1 (2.7) | 152.3 (5.4)* |
| Highest DBP, mm Hg | 65.4 (2.2) | 100.2 (2.2)* | 64.4 (1.9) | 101.7 (1.7)* | 66.5 (2.8) | 94.3 (4.2) | 73.9 (2.0) | 89.8 (3.9)† |
| Proteinuria, n (%) | 3 (17%) | 13 (87%)‡ | 0 (0%) | 8 (80%)‡ | 0 (0%) | 6 (75%) | 1 (13%) | 7 (58%) |
Data are given as the mean (SEM) unless otherwise indicated;
* and † indicate a significant difference (p < 0.01 and p < 0.05, respectively) compared to HOP using a Mann-Whitney U-test;
PE subjects without proteinuria met the ACOG criteria for severe PE. GAD, gestational age at delivery; SBP, systolic blood pressure; DBP, diastolic blood pressure
In total, 38/86 novel peptides and the AT1 epitope exhibited an average 1.5-fold increased PE over HOP binding activity, and these peptides were ranked according to their PE specific antibody binding activity (i.e., activity quotient) (Figure S2). The most PE specific and reactive peptides exhibited an activity quotient of at least six, and the most highly represented motifs amongst these peptides were determined (Figure 2). The greatest fraction (10/22) of peptides with a high PE activity quotient represented the a-1 motif (RG/SG/−WWG/S), which was also comprised of the greatest number of unique peptides. Individual peptides from these motifs did not exhibit reduced PE antibody binding after depletion with the AT1 epitope AFHYESQ (Figure S3), indicating that library-isolated peptides did not mimic the AT1 epitope.
Figure 2.
Consensus library peptides recognized by PE specific antibodies. Twenty-two peptides exhibited a high reactivity quotient (q > 6) and were grouped according to consensus families, indicated on the right. q=(PE Activity)2/HOP Activity.
Peptides demonstrate diagnostic ability on a validation set
To assess the diagnostic efficacy of these PE specific peptides, seven library-isolated peptides with PE activity quotients greater than six and the AT1 epitope were tested for reactivity against 30 new PE and 30 HOP patients. Together, the panel of library-isolated peptides performed well, achieving 100% accuracy within the cohort 1 validation set (Figure S4) while the AT1 epitope alone accurately classified 6/10 PE and 9/10 HOP in cohort 1. Cross-validation trials across the combined set of both cohorts (n=40) using library-isolated peptides with and without the AT1 epitope yielded averaged areas under the curve (AUC) of 0.78 and 0.72, respectively (Figure 3), achieving 61% (95%CI: 36–83%) sensitivity at 95% (95%CI: 77–100%) specificity (Table S4). Comparatively, the AT1 epitope alone demonstrated an AUC of 0.65 in this set of 40 samples and at 61% sensitivity exhibited 55% (95% CI: 32–76%) specificity. Next, the classification accuracy of algorithms trained using cohort 1, cohort 2, or the combined set with and without the AT1 epitope was assessed using an external validation set (20 remaining cohort 2 samples). The ROC curves of all six predictive algorithms revealed that utilization of samples from both cohorts in training resulted in the highest AUC, especially when including the AT1 epitope (0.83) (Figure S5). The algorithm trained using only the library peptides with the combined set attained a similar AUC (0.78), and both algorithms achieved comparable overall accuracy, 75% and 80%, respectively, at the prescribed cutoff. While the algorithm including the AT1 epitope yielded only one false positive, it misclassified two more PE samples than the algorithm using only library peptides (Table 2). Lastly, classification accuracy was not significantly different when analysis was restricted to cases of PE (n=19) identified strictly by new onset hypertension and proteinuria. The array algorithm excluding the AT1 epitope correctly detected 12 (63%) subjects of this PE subgroup, compared to 70% in the full, more heterogeneous group (n=30).
Table 2.
Classification algorithm performance
| Training Set | PE (n/N) |
HOP (n/N) |
Overall (n/N) |
|---|---|---|---|
| Library Peptides and AT1 epitope | |||
| Cohort 1 | 4/12 (33%) | 7/8(88%) | 11/20 (55%) |
| Cohort 2 | 8/12 (67%) | 5/8 (63%) | 13/20 (65%) |
| Combined | 8/12 (67%) | 7/8 (88%) | 15/20 (75%) |
| Library Peptides | |||
| Cohort 1 | 7/12 (58%) | 5/8 (63%) | 12/20 (60%) |
| Cohort 2 | 5/12 (42%) | 7/8 (88%) | 12/20 (60%) |
| Combined | 10/12 (83%) | 6/8 (75%) | 16/20 (80%) |
In addition to evaluating overall diagnostic performance, the antibody-detecting peptide algorithm was assessed for adverse outcome detection utility. Due to the inclusion criteria used in this study, eight PE patients out of the 30 used in training and validation exhibited severe symptoms, such as CNS disturbances, thrombocytopenia, and elevated liver enzymes, without proteinuria. Importantly, the antibody-detecting peptide panel identified seven of these eight atypical PE patients. Furthermore, the peptide panel detected six out of seven nonproteinuric PE patients that delivered prior to 37 weeks gestation (n=18) (Table 3). This could help stratify patients requiring more timely delivery thereby complementing severe symptom detection. Overall, combining clinical criteria of high blood pressure and proteinuria along with the antibody-detecting peptide panel achieved the highest sensitivity for severe symptoms and early delivery
Table 3.
Association of antibody-detecting peptide panel reactivity with adverse outcomes.
| Detection method | Severe symptoms (n=16) |
Early delivery (<37 weeks) (n=18) |
|---|---|---|
| Proteinuria | 10 (63%) | 11 (61%) |
| Peptide panel (+AT1 epitope) | 12 (75%) | 12 (67%) |
| Peptide panel | 13 (81%) | 13 (72%) |
| Proteinuria + Peptide panel | 15 (94%) | 17 (94%) |
Severe symptoms include CNS disturbances, elevated liver enzymes, and/or thrombocytopenia. Proteinuria refers to using criteria for diagnosis of mild PE of hypertension and proteinuria
Statistical analysis of individual peptide performance
Individual peptides comprising the panel exhibited differing diagnostic efficacy. The AT1 epitope exhibited significantly (p < 0.05) higher PE antibody binding when evaluated across the entire sample set (45 PE and 48 HOP) (Figure S6). Here, the AT1 epitope detected binding antibodies in 78% of PE and 44% of HOP, resulting in an AUC of 0.66. However, binding of antibodies from PE subjects to the AT1 epitope was not significantly increased in the validation set composed of 60 samples. In contrast, five library-isolated peptides exhibited significantly (p < 0.05) higher reactivity with PE samples than with HOP samples (Figure 4A). Additionally, library-isolated peptides achieved comparable or higher AUCs than the AT1 epitope. Peptides cross-reacted with multiple PE patient Ig, and Ig from PE that reacted strongly with one peptide tended to bind multiple peptides (Figure 4B). Similarly, HOP antibodies that reacted with one peptide also tended to bind multiple peptides including the AT1 epitope. Nevertheless, the seven member panel exhibited stronger diagnostic efficacy than any individual peptide. Interestingly, peptide binding activity, especially peptide 36 (rs = −0.62), inversely correlated with PE patient platelet count in this set (Figure S7). Summing the standardized binding activity of the seven library-isolated peptides and AT1 epitope yielded the overall correlation (rs = −0.56) with platelet count. Analysis of other patient characteristics (i.e. BP or proteinuria) did not reveal strong correlations with peptide standardized reactivity.
Discussion
In this study, we present evidence that PE is associated with a distinctive signature of antibody binding specificities. This signature was represented in a PE antibody-detecting peptide panel composed of multiple epitope specificities. One such specificity corresponds to a 7-mer epitope of the AT1 receptor.7 The pathobiological significance of these AT1-AAs is now supported by multiple independent studies, demonstrating their ability to increase blood pressure, proteinuria, and sFlt-112 and complement deposition.15 Despite this increased complement deposition in the placenta and kidney of pregnant mice, blocking the AT1 receptor did not fully reduce deposition to HOP levels, leaving open the possibility that other antibodies could play a role in PE pathology. The pursuit for additional PE antibody markers has identified an association between PE and antibodies binding various autoantigens, including β1, β2 and α1 adrenoreceptors,31 cardiolipin,32 and prothrombin.33 However, the use of unbiased discovery approaches to characterize the complete antibody repertoire has not been described. Furthermore, the antigens responsible for eliciting these autoantibodies have not been determined, and their binding epitope specificities remain uncharacterized. Using an unbiased antibody repertoire analysis method, we observed a unique epitope reactivity pattern and used representative peptides to develop a PE antibody-detecting panel for diagnosis. In spite of the strong evidence for AT1-AAs in PE, we did not identify peptides with similarity to the AT1 epitope AFHYESQ. This result might be explained by an insufficient antibody affinity or titer, or the high frequency of AT1-AA in HOP subjects (used for subtraction) in this and previous studies11,22 and overall increased activity over non-pregnant normotensive subjects.34 Although the mechanism responsible for the PE antibody signature is unclear, the reduced prevalence of T-regulatory cells in PE19 may contribute to elevated autoantibody production. Additionally, the observation that several non-self proteins also carry these motifs raises the possibility that the antibodies may originally be responding to an environmental trigger as previously proposed for AT1-AAs.22 Regardless of the production mechanism, this study supports an altered immune response in PE pathophysiology by verifying the presence of PE specific antibodies in addition to AT1-AAs that could be useful for diagnosis.
Despite the demonstrated role of AT1-AAs in PE and potential for use in diagnosis and guiding therapy, their detection has proven exceptionally difficult. Current detection techniques rely upon complex biologic function assays,7,21 which are unlikely to be effective for point-of-care diagnosis. To address this problem, we developed a unique binding assay for AT1-AA detection using the 7-mer epitope displayed in a high avidity format on the bacterial cell-surface. One recent study demonstrated higher AT1-AA titers in PE patients (n=13) over HOPs (n=30) using the 27 amino acid second extracellular loop in an ELISA;34 however, here the cell-displayed 7-mer was sufficient to detect epitope specific antibodies in 78% of PE patients and 44% of HOP (45 PE, 48 HOP). Thus, AT1-AA detection can be performed with the 7-mer and does not require the entire loop. Assays based upon the minimal 7-mer may be important for developing therapeutics designed to block this antibody specificity12 and differentiate this from specificities present in other diseases such as renal allograft rejection.24 For comparison, a biologic function assay identified AT1-AAs in roughly 70% of PE patients and 20% of HOPs22 in addition to 62% of HOPs with abnormal perfusion.11 The percentage of HOP subjects in our study that experienced abnormal perfusion during pregnancy is not known, since the test is not part of routine practice. Regardless, these results demonstrate the utility of an epitope specific binding assay for AT1-AA detection in PE patients, for which researchers have previously relied upon complex biologic function assays.
The seven member PE antibody-detecting panel demonstrated a potential utility for detecting PE cases that is comparable to current protein biomarkers. The peptide panel exhibited 100% accuracy in the validation set from cohort 1 and maintained strong accuracy (80%) despite the inclusion of a second cohort from a distinct geographic location, which affects a population’s antibody repertoire.35 Despite this effect, the panel exhibited comparable diagnostic efficacy to biomarkers in clinical development, such as sFlt-1 and PlGF. Studies evaluating these markers have primarily focused on early onset PE where they show the strongest accuracy; however, the accuracy drops in late onset (≥34 weeks) PE.36 Commercially available diagnostic kits for the sFlt-1/PlGf ratio and PlGF alone exhibited 59% sensitivity at 100% specificity and 77% sensitivity at 95% specificity, respectively, across all gestational onsets.8 Similarly, this study did not discriminate between early and late onset and the antibody-detecting peptide panel achieved 61% sensitivity at 95% specificity within the larger cross-validated training set. It remains to be determined whether the PE specific peptide panel can perform similarly in larger cohorts. Nevertheless, our results indicate that a PE antibody-detecting peptide panel can effectively discriminate PE and HOP samples and demonstrate that PE patients possess a distinctive antibody repertoire signature.
This PE specific antibody signature was associated with an increased detection of adverse outcomes. A substantial percentage of patients that develop HELLP syndrome (10–15%) or eclampsia (20–25%) are not detected by the current clinical criteria of hypertension and proteinuria.3 Thus, there exists an unmet need to identify those at risk of developing these adverse outcomes. The antibody-detecting peptide panel outperformed (81%) clinical criteria (63%) in detecting severe PE patients (n=16), suggesting that this antibody signature is more strongly associated with severe PE. Furthermore, peptide binding activity inversely correlated with platelet levels. Continued investigation of this association may link a pathophysiological role for these antibodies to these severe symptoms.
Perspectives
Here, we demonstrated the existence of antibody biomarkers present in PE patients distinct from known AT1-AAs that achieve strong diagnostic accuracy (80%) for PE. Thus, our results provide supporting evidence for an altered immune response in PE. Identifying the antigen(s) mimicked by our library-isolated peptides may enable characterization of the antibody’s contribution to PE pathogenesis while elucidating potential therapeutic targets. Furthermore, use of the whole or partial antigen(s) mimicked by library peptides may further increase sensitivity/specificity of the assay. Additionally, the ability to detect PE early in pregnancy prior to clinical presentation would aid patient management. A time course study using the PE antibody-detecting peptides described here could identify when these antibodies first present to assess their potential utility in the early diagnosis of PE. Lastly, since this methodology does not require purified antibodies, screening can be conducted using unprocessed, diluted plasma to identify peptide diagnostic reagents that bind PE specific antibodies without the additional purification step.
Supplementary Material
Novelty and Significance.
What is New?
This study identified antibody specificities that occur in pregnant women with PE that are distinct from known AT1-AAs with utility for PE diagnosis.
A binding assay for AT1-AA detection was developed using bacterial cells that display on their surface the 7-mer AT1 epitope.
What is Relevant?
This peptide panel diagnostic assay demonstrated a high specificity (95%) at 61% sensitivity in the training set and maintained 80% accuracy in an independent validation set.
Further characterization may identify antigen(s) mimicked by these PE specific peptides potentially leading to novel therapeutic targets.
The improved throughput of the binding assay for AT1-AA detection will enable larger cohort studies to improve understanding of their prevalence in PE and HOP.
Summary
PE specific antibody-detecting peptides isolated from a bacterial display library exhibited strong diagnostic efficacy within independent sample sets from distinct geographic locations. Using a unique AT1-AA detection assay the prevalence of AT1-AA in PE and HOP subjects was comparable to that observed in prior studies using more complex function based bioassays.
Acknowledgements
We thank Monika Foster for assisting with sample collection.
Source of Funding
This work was supported by the National Institutes of Health NIAID and NIDDK Grant R01 23628, National Institutes of Health Grant HD34130 and the Santa Barbara Cottage Hospital Grant 246.
Footnotes
Disclosures
None
References
- 1.Lain KY, Roberts JM. Contemporary concepts of the pathogenesis management of preeclampsia. JAMA. 2002;287:3183–3186. doi: 10.1001/jama.287.24.3183. [DOI] [PubMed] [Google Scholar]
- 2.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 3.Sibai BM. Biomarker for hypertension-preeclampsia: are we close yet? Am J Obstet Gynecol. 2007;197:1–2. doi: 10.1016/j.ajog.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 4.Levine RJ, Maynard SE, Qian C, Lim K, England L, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. New Engl J Med. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 5.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D'Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA. Soluble endoglin contributes to the pathogensis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 6.Cowans NJ, Stamatopoulou A, Khalil A, Spencer K. PP13 as a marker of pre-eclampsia: a two platform comparison study. Placenta. 2011;32:S37–S41. doi: 10.1016/j.placenta.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 7.Wallukat G, Homuth V, Fischer T, Lindschau C, Horstkamp B, Jupner A, Baur E, Nissen E, Vetter K, Neichel D, Dudenhausen JW, Haller H, Luft FC. Patients with preeclampsia develop agonistic autoantibodies against the angiotensin AT1 receptor. J Clin Invest. 1999;103:945–952. doi: 10.1172/JCI4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benton SJ, Hu Y, Xie F, Kupfer K, Lee S-W, Magee LA, von Dadelszen P. Angiogenic factors as diagnostic tests for preeclampsia: a performance comparison between two commercial immunoassays. Am J Obstet Gynecol. 2011;205:469.e1–469.e8. doi: 10.1016/j.ajog.2011.06.058. [DOI] [PubMed] [Google Scholar]
- 9.Rana S, Powe CE, Salahuddin S, Verlohren S, Perschel FH, Levine RJ, Lim K-H, Wenger JB, Thadhani R, Karumanchi SA. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation. 2012;125:911–919. doi: 10.1161/CIRCULATIONAHA.111.054361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneuer FJ, Nassar N, Guilbert C, Tasevski V, Ashton AW, Morris JM, Roberts CL. First trimester screening of serum soluble fms-like tyrosine kinase-1 and placental growth factor predicting hypertensive disorders of pregnancy. Preg Hyper: An Int J Women’s Card Health. 2013;3:215–221. doi: 10.1016/j.preghy.2013.04.119. [DOI] [PubMed] [Google Scholar]
- 11.Walther T, Wallukat G, Jank A, Bartel S, Schultheiss H, Faber R, Stepan H. Angiotensin II type 1 receptor agonistic antibodies reflect fundamental alterations in the uteroplacental vasculature. Hypertension. 2005;46:1275–1279. doi: 10.1161/01.HYP.0000190040.66563.04. [DOI] [PubMed] [Google Scholar]
- 12.Zhou CC, Zhang Y, Irani RA, Zhang H, Mi T, Popek EJ, Hicks MJ, Ramin SM, Kellem RE, Xia Y. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med. 2008;14:855–862. doi: 10.1038/nm.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irani RA, Zhang Y, Blackwell SC, Zhou CC, Ramin SM, Kellems RE, Xia Y. The detrimental role of angiotensin receptor agonistic autoantibodies in intrauterine growth restriction seen in preeclampsia. J Exp Med. 2009;206:2809–2822. doi: 10.1084/jem.20090872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LaMarca B, Wallukat G, Llinas M, Herse F, Dechend R, Granger JP. Autoantibodies to the angiotensin type I receptor in response to placental ischemia and tumor necrosis factor α in pregnant rats. Hypertension. 2008;52:1168–1172. doi: 10.1161/HYPERTENSIONAHA.108.120576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang W, Irani RA, Zhang Y, Ramin SM, Blackwell SC, Tao L, Kellems RE, Xia Y. Autoantibody-mediated complement C3a receptor activation contributes to the pathogenesis of preeclampsia. Hypertension. 2012;60:712–721. doi: 10.1161/HYPERTENSIONAHA.112.191817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salmon JE, Heuser C, Triebwasser M, Liszewski MK, Kavanagh D, Roumenina L, Branch DW, Goodship T, Fremeaux-Bacchi V, Atkinson JP. Mutations in the complement regulatory proteins predispose to preeclampsia: a genetic analysis of the PROMISSE cohort. PLoS Med. 2011;8:e1001013, 1–9. doi: 10.1371/journal.pmed.1001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hering L, Herse F, Verlohren S, Park J-K, Wellner M, Qadri F, Pijnenborg R, Staff AC, Huppertz B, Muller DN, Luft FC, Dechen R. Trophoblasts reduce the vascular smooth muscle cell proatherogenic response. Hypertension. 2008;51:554–559. doi: 10.1161/HYPERTENSIONAHA.107.102905. [DOI] [PubMed] [Google Scholar]
- 18.Jensen F, Wallukat G, Herse F, Budner O, El-Mousleh T, Costa S, Dechend R, Zenclussen AC. CD19+CD5+ Cells as indicators of preeclampsia. Hypertension. 2012;59:861–868. doi: 10.1161/HYPERTENSIONAHA.111.188276. [DOI] [PubMed] [Google Scholar]
- 19.Darmochwal-Kolarz D, Saito S, Tabarkiewicz J, Kolarz B, Rolinski J, Leszczynska-Gorzelak B, Oleszczuk J. Apoptosis signaling is altered in CD4+CD25+FoxP3+ T regulatory lymphocytes in pre-eclampsia. Int J Mol Sci. 2012;13:6548–6560. doi: 10.3390/ijms13066548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakaguchi S, Sakaguchi N, Shimizu J, Yamazaki S, Sakihama T, Itoh M, Kuniyasu Y, Nomura T, Toda M, Takahashi T. Immunologic tolerance maintained by CD25+ CD4+ regulatory T cells: their common role in controlling autoimmunity, tumor immunity, and transplantation tolerance. Immunological Reviews. 2001;182:18–32. doi: 10.1034/j.1600-065x.2001.1820102.x. [DOI] [PubMed] [Google Scholar]
- 21.Siddiqui AH, Irani RA, Blackwell SC, Ramin SM, Kellems RE, Xia Y. Angiotensin receptor agonistic autoantibody is highly prevalent in preeclampsia. Hypertension. 2010;55:386–393. doi: 10.1161/HYPERTENSIONAHA.109.140061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herse F, Verlohren S, Wenzel K, Pape J, Muller DN, Modro S, Wallukat G, Luft FC, Redman CW, Dechend R. Prevalence of agonistic autoantibodies against the angiotensin II Type I receptor and soluble fms-like tyrosine kinase 1 in a gestational age-matched case study. Hypertension. 2009;53:393–398. doi: 10.1161/HYPERTENSIONAHA.108.124115. [DOI] [PubMed] [Google Scholar]
- 23.Liao Y-H, Wei Y-M, Wang M, Wang Z-H, Yuan H-T, Cheng L-X. Autoantibodies against AT1-receptor and alpha1-adrenergic receptor in patients with hypertension. Hypertension Res. 2002;25:641–646. doi: 10.1291/hypres.25.641. [DOI] [PubMed] [Google Scholar]
- 24.Dragun D, Muller DN, Brasen J-H, Fritsche L, Neminen-Kelha M, Dechend R, Kintscher U, Rudolph B, Hoebeke J, Eckert D, Mazak I, Plehm R, Schonemann C, Unger T, Budde K, Neumayer H-H, Luft FC, Wallukat G. Angiotensin II type 1-receptor activating antibodies in renal allograft rejection. NEJM. 2005;352:558–569. doi: 10.1056/NEJMoa035717. [DOI] [PubMed] [Google Scholar]
- 25.Hall SS, Daugherty PS. Quantitative specificity-based display library screening identifies determinants of antibody-epitope binding specificity. Prot Sci. 2009;18:1926–1934. doi: 10.1002/pro.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Casadaban MJ, Cohen SN. Analysis of gene control signals by DNA fusion and cloning in Escherchia coli. J Mol Bio. 1980;138:179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- 27.Rice JJ, Daugherty PS. Directed evolution of a biterminal bacterial display scaffold enhances display of diverse peptides. Prot Eng, Des Sel. 2009;21:435–442. doi: 10.1093/protein/gzn020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kenrick SA, Daugherty PS. Bacterial display enables efficient and quantitative peptide affinity maturation. Prot Eng Des Sel. 2010;23:9–17. doi: 10.1093/protein/gzp065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friedman J, Hastie T, Tibshirani R. Additive logistic regression: a statistical view of boosting. The Annals of Statistics. 2000;28:337–407. [Google Scholar]
- 30.Culp M, Johnson K, Michailidis G. ada: An R package for stochastic boosting. Journal of Statistical Software. 2006;17:1–27. [Google Scholar]
- 31.Ma G, Li Y, Zhang J, Liu H, Hou D, Zhu L, Zhang Z, Zhang L. Association between the presence of autoantibodies against adrenoreceptors and severe pre-eclampsia: a pilot study. PLoS ONE. 2013;8:e57983. doi: 10.1371/journal.pone.0057983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Do Prado AD, Piovesan DM, Staub HL, Horta BL. Association of anticardiolipin antibodies with preeclampsia: a systematic review and meta-analysis. Obstet Gynecol. 2010;116:1433–1443. doi: 10.1097/AOG.0b013e3181fe02ec. [DOI] [PubMed] [Google Scholar]
- 33.Marozio L, Curti A, Botta G, Canuto E, Salton L, Tavella AM, Benedetto C. Anti-Prothrombin Antibodies are associated with adverse pregnancy outcome. Am J Reprod Immunol. 2011;66:404–409. doi: 10.1111/j.1600-0897.2011.01031.x. [DOI] [PubMed] [Google Scholar]
- 34.Rossitto G, Regolisti G, Rossi E, Negro A, Nicoli D, Toniato A, Caroccia B, Seccia TM, Walther T, Rossi GP. Elevation of angiotensin-II type-1 receptor autoantibodies titer in primary aldosteronism as a result of aldosterone-producing adenoma. Hypertension. 2013;61:526–533. doi: 10.1161/HYPERTENSIONAHA.112.202945. [DOI] [PubMed] [Google Scholar]
- 35.Rabel PO, Planitzer CB, Farcet MR, Kreil TR. Tick-borne encephalitis virus-neutralizing antibodies in different immunoglobulin preparations. Clin Vaccine Immunol. 2012;19:623–625. doi: 10.1128/CVI.05705-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaiworapongsa T, Romero R, Korzeniewski SJ, Kusanovic JP, Soto E, Lam J, Dong Z, Than NG, Yeo L, Hernandez-Andrade E, Conde-Agudelo A, Hassan SS. Maternal plasma concentrations of angiogenic/antiangiogenic factors in the third trimester of pregnancy to identify the patient at risk for stillbirth at or near term and severe late preeclampsia. Am J Obstet Gynecol. 2013;208:287.e1–287.e15. doi: 10.1016/j.ajog.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.