Abstract
Background
US nationwide estimates indicate 50–80% of prisoners have a history of substance abuse or dependence. Tailoring substance abuse treatment to specific needs of incarcerated individuals could improve effectiveness of treating substance dependence and preventing drug abuse relapse. The purpose of the present study was to test the hypothesis that pre-treatment neural measures of a Go/NoGo task would predict which individuals would or would not complete a 12-week cognitive behavioral substance abuse treatment program.
Methods
Adult incarcerated participants (N=89; Females=55) who volunteered for substance abuse treatment performed a response inhibition (Go/NoGo) task while event-related potentials (ERP) were recorded. Stimulus- and response-locked ERPs were compared between individuals who completed (N=68; Females=45) and discontinued (N=21; Females=10) treatment.
Results
As predicted, stimulus-locked P2, response-locked error-related negativity (ERN/Ne), and response-locked error positivity (Pe), measured with windowed time-domain and principal component analysis, differed between groups. Using logistic regression and support-vector machine (i.e., pattern classifiers) models, P2 and Pe predicted treatment completion above and beyond other measures (i.e., N2, P300, ERN/Ne, age, sex, IQ, impulsivity, and self-reported depression, anxiety, motivation for change, and years of drug abuse).
Conclusions
We conclude individuals who discontinue treatment exhibited deficiencies in sensory gating, as indexed by smaller P2, error-monitoring, as indexed by smaller ERN/Ne, and adjusting response strategy post-error, as indexed by larger Pe. However, the combination of P2 and Pe reliably predicted 83.33% of individuals who discontinued treatment. These results may help in the development of individualized therapies, which could lead to more favorable, long-term outcomes.
Keywords: Drug Treatment, Event-Related Potentials, Principal Component Analysis, Response Inhibition, Response Errors, Support Vector Machine, Pattern Classifier
Substance abuse is a significant problem in US prisons with prevalence estimates ranging from 50–80% of inmates meeting DSM-IV-TR diagnostic criteria for a substance use disorder (SUD; (1, 2)). Depending on whether the correctional facility is a state or federal prison, as many as 40–49% (respectively) of inmates participate in some form of treatment for their SUD while incarcerated (3). The period shortly after release represents a time of significant risk for the return to substance use and accidental overdose death. For example, drug-related causes of death account for 76% of deaths within the first two weeks and 59% of deaths within the first three months following release from custody (4). Because more treatment has been consistently related to lower rates of relapse to substance use (5–9), and the stakes are high with an inmate population, the impetus to discover improved and novel predictors of treatment discontinuation are significant.
Recent investigations of event-related potentials (ERPs) in substance dependent individuals (10–13), some in treatment, provide compelling evidence for the investigation of the role of neurocognitive processing in inmate SUD treatment completion. One ERP component investigated is the P2, which is a positive deflection occurring 150–250 milliseconds (ms) following stimulus onset. The stimulus-locked P2 is believed to reflect early attentional processes including stimulus identification, comparison processes between predicted and actual representations, and early sensory gating (11, 14–16). Early sensory gating may impact subsequent cognitive functioning through the allocation of working memory and may explain some cognitive deficits exhibited by individuals with SUD. For example, reduced P2 amplitude has been found in cocaine-dependent individuals in an auditory paired-click paradigm and appeared to impact later memory and learning (11, 16). Additionally, long-term abstinence has been associated with some P2 amplitude recovery (11).
Stimulus-locked N2 and P300 ERP components have also been investigated in relation to treatment completion. In an investigation of the N2, which peaks around 275 ms post-stimulus onset in a Go/NoGo paradigm, reductions in amplitude were associated with improvements in externalizing tendencies after behavioral treatment (17). These findings were interpreted as executive functioning differences between those who did or did not improve with treatment. In studies of substance abuse treatment outcomes (i.e., whether an individual completes or discontinues treatment), reduced amplitude in stimulus-locked P300, which peaks between 250–500 ms post-stimulus onset, in visual and auditory oddball paradigms has been shown to be predictive of treatment discontinuation and relapse in community samples with SUDs (10, 12, 13). Here again, such findings indicate that measures of executive functioning may predict treatment outcomes; however, previous investigations have used stimulus-locked ERPs rather than response-locked ERPs. With response-locked ERPs, direct neural measurement of behavioral output is possible. Of particular interest, are response-locked ERP components elicited by an erroneous response to NoGo stimuli (False Alarms), the error-related negativity (ERN/Ne) and error positivity (Pe). The ERN/Ne is a negative deflection likely generated in the rostral cingulate zone, potentially within the caudal anterior cingulate cortex (cACC; (18–20)) and peaks between 50 and 100 ms after an incorrect response (21–24). The ERN/Ne is believed to be associated with cognitive detection of the response error (23, 25) or incorrect response tendencies (26). The Pe is a positive deflection generated from at least one source within the rostral ACC (rACC; (27)) and follows the ERN/Ne, peaking between 200 and 400 ms after an incorrect response. The Pe is believed to index further error processing, conscious evaluation of the error, response strategy adjustment and/or affective assessment of the error (23, 24, 28–31) and has been found to be negatively related with rACC activation (25). Successful error monitoring, as indexed by increased ERN/Ne amplitude, should lead to modulation of response strategies designed to reduce errors in the future, as indexed by reduced Pe amplitude.
Current Study
Given the previous associations of ERP indexed neurocognitive functioning with SUDs treatment completion, we investigated stimulus-locked and response-locked ERPs in a challenging Go/NoGo task. Specifically, we investigated NoGo stimulus-locked sensory processes via the P2 (11, 16, 32) and later cognitive control processes, including response inhibition, stimulus evaluation, and executive functioning via the N2 and P300 ERP components (33–35), and response-locked False Alarm cognitive control processes related to detection and evaluation of errors via the ERN/Ne and Pe ERP components (22–24) in a sample of treatment-seeking incarcerated individuals enrolled in a cognitive behavioral substance abuse treatment program. We hypothesized that participants who discontinued treatment before completing a full dose of therapy would exhibit, compared to participants who complete the full therapy, reduced P2 amplitude, increased N2, decreased P300, reduced ERN/Ne, and increased Pe amplitude(10–13, 36). Because classic time-domain measures of ERP components are inadequate at separating the inherently overlapping ERP components, we implement principal component analysis (PCA; (37)) which has been suggested to be optimal for ERP data analysis (38) and expect the PCA results to be more sensitive to predicting treatment outcomes than time-domain results.
Method
Participants
Participants were 89 (55 females) treatment-seeking incarcerated individuals who identified their drug of choice as cocaine (N=32; 6 discontinued), methamphetamine (N=41; 10 discontinued), heroin (N=15; 4 discontinued), or poly-drug (N=1; 1 discontinued) recruited from two medium-security prisons in the state of New Mexico. The mean age of participants was 35.48 years (SD = 8.47) at the time of the baseline assessment, when electroencephalography (EEG) was collected, and the participants randomized into one of three 12-week manualized interventions. Because each treatment type was well represented (Addictions Counseling [AC], N=29; Relapse Prevention [RP], N=36; Substance Expectations Therapy [SET], N=22; two participants discontinued treatment before treatment group assignment) and the completion proportion of each group (completion group: AC, N=22; RP, N=28; SET, N=18; discontinuation group: AC, N=7; RP, N=8; SET, N=4; unassigned, N=2) were well represented, we collapsed across treatment types. Approximately 9% were left-hand dominant, 28% of the sample self-identified as White, 65% as Hispanic, 2% as Black/African American, 4% as American Indian, and 1% selected more than one category. Sixty-eight (45 females) participants completed the therapy protocol (i.e., at least nine sessions of the 12-session protocol (39)), and 21 (10 females) participants discontinued treatment before completing the therapy protocol, receiving eight or fewer sessions. Individuals who did not complete nine weeks of treatment for reasons other than voluntarily discontinuation (e.g., early release from prison or paroled, N=10, transferred to another facility N=3, transferred out of general population N=1, or enrolled in another drug treatment program N=8) were not included in the analyses.
Inclusion criteria
Participants included in the current study met the following inclusion criteria: (a) currently incarcerated, (b) cocaine, methamphetamine or heroine dependent at time of incarceration, (c) no history of head injury resulting in significant loss of consciousness, (d) no history of psychosis or first-degree relative with psychosis, (e) a sixth grade English reading level, and (f) an estimated IQ greater than 70.
Procedures and Ethical Considerations
Initial contact was made with potential study participants through announcements by research staff at the correctional facilities. Meetings were scheduled with interested participants and informed consent was obtained. Participants were informed of their right to discontinue participation at any point and that their participation was in no way associated with their status at the facility, their parole status, and there were no direct institutional benefits. Participants were paid at the rate of the hourly wage at the facility. All procedures were approved by the Human Research Review Committee at the research institution and correctional facilities where the study was conducted.
Trained researchers administered several questionnaires, including: the Psychopathy Checklist-Revised (PCL-R; (40)), Vocabulary and Matrix Reasoning subtests of the Wechsler Adult Intelligence Scale (41); self-report measures of anxiety (42), depression (43), motivation for change (44), and the Addiction Severity Index (ASI-X; (45)). The supplemental material includes further description and reliability analyses on these assessments. These measures did not differ between the treatment completion and treatment discontinuation groups, t’s<1.66 (Table S1).
EEG data were collected in a small room in an area separate from the general population housing provided by the facility. After placement of the electrodes, participants were seated in a comfortable chair 60 cm away from a computer monitor on which task stimuli were presented, and were instructed to refrain from excessive blinking or moving during data acquisition. Participants then performed a difficult, previously published Go/NoGo paradigm (19) containing a higher frequency of “Go” (84%, 412 trials) than “NoGo” stimuli (16%, 78 trials; see Supplemental material for additional information). EEG data were collected on a 64-channel BioSemi ActiveTwo amplifier in accordance with the 10–20 International System (46).
Event-Related Potential (ERP) Data Analysis
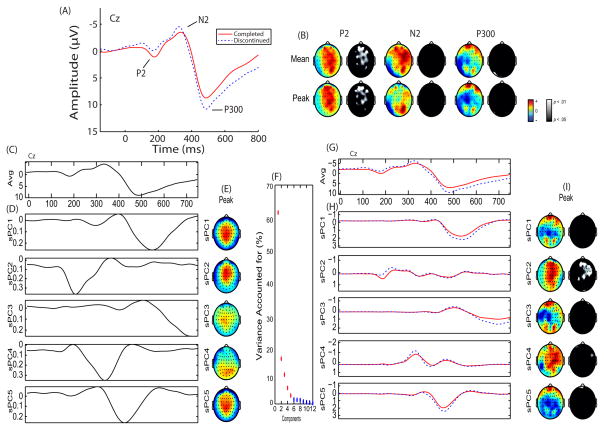
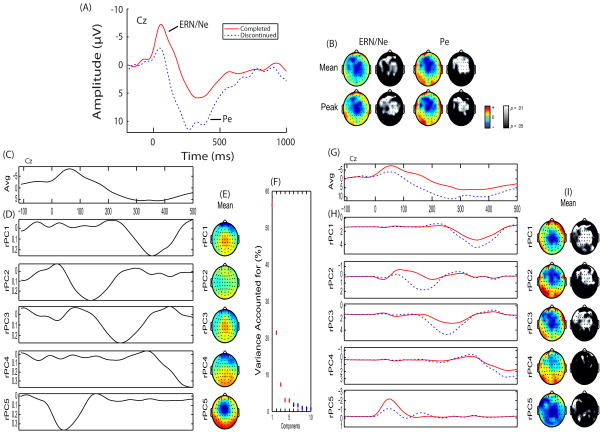
Standard preprocess steps and an independent components analysis eye-blink removal protocol (47) was performed. Classic time-domain stimulus-locked, defined relative to the onset of a NoGo stimulus, and response-locked components, defined relative to a False Alarm, were extracted (see Supplemental material for additional EEG data collection and analysis information). An additional data reduction method, principal component analysis (PCA; (37)), was also performed separately for stimulus- and response-locked data. A five-component stimulus-locked solution was extracted for correct trials (Figure 1) which accounted for 88.71% of the variance. A five-component response-locked solution was extracted for False Alarm trials (Figure 2) which accounted for 91.27% of the variance. To avoid confusion in referencing stimulus-locked and response-locked principal components, an ‘s’ or an ‘r’ will be included in the description (e.g., stimulus-locked principal component 1 and response-locked principal component 1 will be referred to as sPC1 and rPC1, respectively).
Figure 1.
Stimulus-locked event-related potential (ERP) and Principal Component Analysis (PCA): (A) Representative ERP wavefore plotted at Cz for each group. Individuals who completed (solid red line) and discontinued (dashed blue line) substance abuse treatment are plotted. ERP components of interest (P2, N2, & P300) are identified. (B) Topographical difference (color) and statistical (black & white) maps are plotted for each component window highlighting individuals who discontinued treatment exhibited reduced P2 amplitude. (C) Grand average waveform plotted at Cz. (D) Principal components extracted accounting for 88.71% of the variance. (E) Topographical depiction of the peak spatial distribution for each principal component. (F) Scree plot of singular values which was used to determine a five-component solution. (G) Group average waveforms for individuals who completed (solid red line) and discontinued (dashed blue line) substance abuse treatment are plotted ad Cz. (H) Principal components plotted by group. (I) Topographical difference (color) and statistical (black & white) maps are plotted for each principal component highlighting individuals who discontinued treatment exhibited reduced sPC2 amplitude.
Figure 2.
Response-locked event-related potential (ERP) and Principal Component Analysis (PCA): (A) Representative ERP wavefore plotted at Cz for each group. Individuals who completed (solid red line) and discontinued (dashed blue line) substance abuse treatment are plotted. ERP components of interest (ERN/Ne & Pe) are identified. (B) Topographical difference (color) and statistical (black & white) maps are plotted for each component window highlighting individuals who discontinued treatment exhibited reduced ERN/Ne and increased Pe amplitude. (C) Grand average waveform plotted at Cz. (D) Principal components extracted accounting for 91.27% of the variance. (E) Topographical depiction of the mean spatial distribution for each principal component. (F) Scree plot of singular values which was used to determine a five-component solution. (G) Group average waveforms for individuals who completed (solid red line) and discontinued (dashed blue line) substance abuse treatment are plotted ad Cz. (H) Principal components plotted by group. (I) Topographical difference (color) and statistical (black & white) maps are plotted for each principal component highlighting individuals who discontinued treatment exhibited increased rPC1, rPC2, and rPC3 amplitudes.
Behavioral, ERP time-domain, and PCA measures were compared between the completion and discontinuation groups using repeated-measures analysis of variance (ANOVA). When significant violations of sphericity were detected, Greenhouse-Guisser corrected effects and epsilon (3) are reported, as recommended by Jennings and Wood (48, see also 49). Orthogonal linear and quadratic contrasts were calculated to clarify significant main effects and interactions. Logistic, stepwise regressions and classification using support vector machine (SVM) with two-nested leave-one-out validation (to avoid using the testing data in selecting and training the model; see Supplemental material) were carried out to predict treatment completion. Sequential backward feature selection (50) was used to identify which covariates were most useful in predicting treatment completion (see Supplemental material). Nine (Age, gender, IQ, contemplation and action measures of readiness for change, trait anxiety, depression, and both factors of the PCL-R) of the 12 were selected and used in logistic regression and SVM analyses predicting drug treatment completion. A subset of 20 electrode sites representing maximal time-domain component activation were selected (F3, F1, Fz, F2, F4, FC3, FC1, FCz, FC2, FC4, C3, C1, Cz, C2, C4, CP3, CP1, CPz, CP2, and CP4) and used in time-domain and PCA analyses below. Effects that did not reach statistical trend (p>.10) are not reported.
Results
Behavioral Results
Response times and frequency for False Alarms were analyzed. To test group differences, separate independent samples t-tests were conducted for response time and error rates to NoGo stimuli. Groups did not differ on response time (completion group M = 325 ms, SD = 45 ms; discontinuation group M = 332 ms, SD = 54 ms) or error rates (completion group: M = 16.00 trials, SD = 9.80 trials; discontinuation group: M = 17.62 trials, SD = 12.16 trials) to NoGo stimuli, t’s<1.
Event-Related Potentials
Average ERPs were computed for stimulus- and response-locked activations to NoGo stimuli for each group (completion and discontinued group; Figures S1 and S2). A subset of electrodes, defined above, was submitted to repeated-measures ANOVA. Coronal level of electrode location (electrode rows F, FC, C, and CP), lateral level of electrode location (electrode columns 3, 1, z, 2, and 4), and the interaction by group were also calculated. Main effects and interactions, which had a quadratic distribution with an uninteresting increase in activation over central and midline electrodes, were not of theoretical interest and are not reported here.
Group Differences
Participants in the discontinued group exhibited smaller P2 amplitude, smaller ERN/Ne amplitude, and greater Pe amplitude as measured in mean P2, F(1,86) = 4.85, p = .030, peak P2, F(1,86) = 6.95, p = .010, peak sPC1, F(1,86) = 4.59, p = .035, mean ERN/Ne, F(1,87) = 6.20, p = .015, peak ERN/Ne, F(1,87) = 10.02, p = .002, mean Pe, F(1,87) = 8.52, p = .004, peak Pe, F(1,87) = 4.10, p = .046, mean rPC2, F(1,87) = 7.11, p = .009, peak rPC2, F(1,87) = 3.13, p = .080, mean rPC3, F(1,87) = 7.10, p = .009, peak rPC3, F(1,87) = 4.80, p = .031, and mean rPC5, F(1,87) = 3.21, p = .077 (Figure 1; Figure 2; see Supplemental materials for complete ANOVA analyses)
Regression Analyses
Separate linear regressions, using the average of the subset of electrodes defined above, were computed predicting the peak P2 amplitude with the peak measure of the five principal component stimulus-locked solution and the mean ERN/Ne and mean Pe amplitude with the mean measure of the five principal components response-locked solution as predictive variables. Peak P2 was predicted by sPC1 (p<.001), mean ERN/Ne was predicted by rPC5 (p<.001), and mean Pe was predicted by rPC1, rPC3, and rPC4 (p’s<.040; Table S4). For simplicity, the principal components that carry group differences and explained time-domain components (peak sPC1, mean rPC3, and mean rPC5) were selected for subsequent analysis predicting treatment completion (see Supplemental materials for complete prediction models).
The mean of 20 electrodes defined above was computed for each stimulus- and response-locked PCA measure and was used, in addition to the nine covariates, in logistic, stepwise regressions predicting treatment completion. Three models were calculated: 1) stimulus-locked PCA measure plus the nine covariates (Table S3); 2) response-locked PCA measures plus the covariates (Table S3) 3) stimulus- and response-locked PCA measures plus the covariates (Table 1). No group differences were found between these predictive measures (Table S1). PCA regression analyses of models 1, 2, and 3 produced significant prediction models, χ2(1) = 7.50, p = .006, χ2(1) = 5.18, p = .023, χ2(2) = 12.04, p =.002, respectively. Although these three overall models were significant and classified individuals with 81.7%, 81.7%, and 78.3% accuracy, respectively, peak sPC1 (model 1: p = .025; model 3: p = .035) and mean rPC3 (model 2: p = .031; model 3: p = .041) were unique predictors of treatment completion. However, none adequately predicted drug treatment discontinuation (model 1: 8.30%; model 2: 16.70%; model 3: 25.00%, respectively).
Table 1.
Principal Component Analyses used in Predicting Treatment Completion
| Predictors | B | SE B | Wald | Exp(B) | Sig | |
|---|---|---|---|---|---|---|
| Step 1 | sPC1 peak | 2.980 | 1.328 | 5.034 | 19.697 | .025 |
| rPC3 mean | 4.689 | .030 | ||||
| rPC5 mean | 2.066 | .151 | ||||
| Age | 0.006 | .938 | ||||
| Gender | 0.735 | .391 | ||||
| IQ | 0.888 | .346 | ||||
| Contemplation | 0.033 | .856 | ||||
| Action | 0.020 | .888 | ||||
| Trait Anxiety | 0.025 | .875 | ||||
| BDI Total | 0.046 | .831 | ||||
| PCL-R-F1 | 0.033 | .856 | ||||
| PCL-R-F2 | 0.255 | .614 | ||||
| Step 2 | sPC1 peak | 3.186 | 1.508 | 4.467 | 24.200 | .035 |
| rPC3 mean | −2.092 | 1.022 | 4.190 | 0.123 | .041 | |
| rPC5 mean | 1.400 | .237 | ||||
| Age | 0.414 | .520 | ||||
| Gender | 0.538 | .463 | ||||
| IQ | 1.453 | .228 | ||||
| Contemplation | 0.438 | .508 | ||||
| Action | 0.016 | .900 | ||||
| Trait Anxiety | 0.011 | .917 | ||||
| BDI Total | 0.097 | .755 | ||||
| PCL-R-F1 | 0.021 | .885 | ||||
| PCL-R-F2 | 0.002 | .961 |
Note. Step 1: -2 Log likelihood = 52.546, Cox & Snell R2 = .12; Nagelkerke R2 = .19; Model χ2 = 7.503, p = .006, df = 1. Classification of the completed group: 100 % (48 of 48); the discontinued group: 8.3 % (1 of 12); overall 81.7 % (49 of 60). Only the sPC1 mean measure was included in the first step of this regression. Step 2: -2 Log likelihood = 48.008, Cox & Snell R2 = .18; Nagelkerke R2 = .29; Model χ2 = 12.041, p = .002, df = 2. Classification of the completed group: 91.7 % (44 of 48); the discontinued group: 25.0 % (3 of 12); overall 78.3 % (47 of 60). Both sPC1 peak and rPC3 mean measures were included in this significant model. Assessments: Intelligence quotient (IQ) was calculated from the Wechsler Adult Intelligence Scale III (WAIS-III); Contemplation and Action are summary scores of subscales from the University of Rhode Island Change Assessment (URICA); Trait Anxiety is a summary score from the State-Trait Anxiety Inventory (STAI); Beck’s Depression is the total score from Beck’s Depression Inventory (BDI-II); PCL-R-F1 and PCL-R-F2 are the Factor 1 and Factor 2 summary scores from the Psychopath Checklist – Revised (PCL-R).
Classification with Support Vector Machine
Five SVM classification models to predict treatment completion will be discussed here (see supplemental material for additional SVM models). Three simple models containing only the nine covariates, only the time-domain measures (i.e, peak P2, mean ERN/Ne, and mean Pe), or only the PCA measures (i.e., peak sPC1, mean rPC3, and rPC5) were included. Two additional models combined either the time-domain or PCA measures with the covariates in a predictive model (Table 2). The model with only the covariates correctly classified individuals at 66.10% but by adding the time-domain measures (72.88%) and PCA measures (69.49%) the overall models increased in accuracy. The simple time-domain (74.58%) was more accurate than the model with only covariates and the simple PCA (66.10%) model. Of particular interest is how well each model identified who would discontinue treatment. The first SVM model, including only the covariates, predicted only 41.67% of those who discontinued treatment. The best model (simple PCA) accurately predicted discontinuation at a rate of 83.33%. The other three models predicted discontinuation reasonably well (simple time-domain: 75.00%; time-domain with covariates: 66.67%; PCA with covariates: 75.00%). All of these models were more successful at predicting treatment discontinuation than the logistic regressions reported above with the simple PCA model as the most predictive model.
Table 2.
Support Vector Machine Models Predicting Treatment Completion
| Stimulus-Locked | |||||
|---|---|---|---|---|---|
| Covariates | Time-Domain Measures | PCA Measures | Covariates with TD Measures | Covariates with PCA Measures | |
| Overall Classification Rate | 66.10% | 72.88% | 79.66% | 59.32% | 72.88% |
| Specificity | 72.34% | 72.34% | 78.72% | 55.32% | 78.72% |
| Sensitivity | 41.67% | 75.00% | 83.33% | 75.00% | 50.00% |
| Positive Predictive Value | 27.78% | 40.91% | 50.00% | 30.00% | 37.50% |
| Negative Predictive Value | 82.93% | 91.89% | 94.87% | 89.66% | 86.05% |
| Response-Locked | |||||
|---|---|---|---|---|---|
| Covariates | Time-Domain Measures | PCA Measures | Covariates with TD Measures | Covariates with PCA Measures | |
| Overall Classification Rate | 66.10% | 67.80% | 71.19% | 81.36% | 81.36% |
| Specificity | 72.34% | 65.96% | 78.72% | 89.36% | 85.11% |
| Sensitivity | 41.67% | 75.00% | 75.00% | 50.00% | 66.67% |
| Positive Predictive Value | 27.78% | 36.00% | 39.13% | 54.55% | 53.33% |
| Negative Predictive Value | 82.93% | 91.18% | 91.67% | 87.50% | 90.91% |
| Combination | |||||
|---|---|---|---|---|---|
| Covariates | Time-Domain Measures | PCA Measures | Covariates with TD Measures | Covariates with PCA Measures | |
| Overall Classification Rate | 66.10% | 74.58% | 66.10% | 72.88% | 69.49% |
| Specificity | 72.34% | 74.47% | 61.70% | 74.47% | 68.09% |
| Sensitivity | 41.67% | 75.00% | 83.33% | 66.67% | 75.00% |
| Positive Predictive Value | 27.78% | 42.86% | 35.71% | 40.00% | 37.50% |
| Negative Predictive Value | 82.93% | 92.11% | 93.55% | 89.74% | 91.43% |
Note. Five support vector machine (SVM) models predicting drug treatment completion were computed for each stimulus-locked (time-domain: peak P2; principal component analysis [PCA]: peak sPC1), response-locked (time-domain: mean ERN/Ne & mean Pe; PCA: mean rPC3 & rPC5), and a combination of stimulus- and response-locked (computed separately for time-domain and PCA measures) ERP data: All of the covariates of identified with feature selection were included; Covariates with time-domain for stimulus- and response-locked measures; Covariates with PCA for stimulus- and response-locked measures. Specificity is the measure of how well the model identified who will complete drug treatment and sensitivity is the measure of how well the model identified who will discontinue drug treatment. Positive predictive value represents the ratio of individuals who discontinued treatment to combined individuals identified correctly and incorrectly to be in the discontinued group. Negative predictive value represents the ratio of individuals who completed treatment to combined individuals identified correctly and incorrectly to be in the completion group.
Discussion
The current study tested the hypothesis that stimulus-locked (P2, N2, & P300) and response-locked (ERN/Ne, & Pe) ERP components elicited during a Go/NoGo task would differentiate between incarcerated participants who did or did not discontinued drug treatment before completing a full dose of therapy. As predicted, those who discontinued treatment exhibited decreased P2, less negative ERN/Ne, and increased Pe amplitude. Somewhat surprisingly, however, no significant differences were found in stimulus-locked P300 amplitude between those who completed or discontinued treatment. Both time-domain and principal component analysis (PCA) ERP measures were analyzed and group differences were best isolated with PCA. Using PCA, logistic regression, and support vector machine (SVM; i.e., pattern classifier), PCA measures of P2 and Pe were identified as unique predictors of treatment completion. These two ERP components are measures of sensory gating (P2) and post-error response strategy modulation (Pe) suggesting individuals who discontinue treatment exhibit deficiencies in neural correlates of these cognitive processes relative to individuals who complete treatment. The two groups did not differ in behavioral response time, error rates, or other variables used as predictors. Most importantly, the SVM models outperformed the logistic regression models in correctly classifying individuals who would discontinue treatment with the best model accurately classifying 83.33% of individuals who discontinued (Table 2).
The P2 is thought to index early sensory gating and the ability of individuals to filter extraneous information in the allocation of attention (11, 16, 28, 29, 51, 52) and the Pe is thought to index conscious evaluation of errors, response strategy adjustment, and/or affective assessment of the error (28, 29, 52). It is possible that in our sample, those who discontinued treatment exhibit deficiencies in neural correlates of early sensory gating which may be linked to reduced working memory stores and could lead to deficiencies in subsequent cognitive processes associated response inhibition. Reduced P2 amplitudes have previously been associated with cocaine dependence and amplitudes become comparable with healthy controls with abstinence (11) suggesting the P2 to be a potential biomarker of treatment success. Also, in our sample, those who discontinued treatment exhibit deficiencies in neural correlates of conscious evaluation of errors and response strategy adjustments, as indexed by the Pe.
It is possible that the deficiencies in working memory are related to difficulty in processing information participants received in treatment and evaluating the consequences of their substance use and the benefits of treatment. These deficiencies could thereby interfere with their ability to evaluate consequences of substance use, benefits of treatment, and how their choices are related to their future. Conscious awareness of errors has been shown to modulate the Pe (23, 29, 30), though our groups did not differ on behavioral measures but differed on Pe amplitude suggesting no group differences in error awareness but differences on this neural-correlate of post-error response adjustment. Pe amplitude has been both negatively and positively correlated with ACC activation (25) and reduced ACC activation was related with poor future outcomes (53). The data presented here clearly identify post-error response adjustments as a specific cognitive function to target when developing new substance abuse treatments though future functional magnetic resonance imaging studies are necessary to identify the specific direction of neural activation. In using stimulus- and response-locked measures we found both time-domain and PCA were effective in isolating underlying cognitive functioning most predictive of treatment completion. Furthermore, PCA measures were more predictive of treatment outcomes than time-domain measures and PCA analyses delineated the underlying neurocognitive processes that might otherwise be indistinguishable with traditional time-domain measurements.
By using PCA, we identified the underlying cognitive processes related specifically to P2 and Pe, which were separable from those related to other ERP components. Distinguishing between P2, N2, and P300 helps to isolate the specific cognitive function the P2 component is thought to reflect, specifically sensory gating (11, 16). Similarly, disentangling the ERN/Ne and Pe is important when interpreting the previously delineated underlying neural generators (25, 27) and thus the associated cognitive processes that differentiate the treatment completion and discontinued groups. By isolating unique cognitive functions, we have, to some degree, eliminated potential confounds of overlapping stimulus- and response-locked components which have been shown to potentially interact with one another (54). Therefore, we identified neurocognitive measures of sensory gating, error monitoring, and post-error response adjustment to be deficient in individuals who discontinue substance abuse treatment. Only the measure of sensory gating and post-error response adjustment predicted treatment outcomes, however, and were incrementally more predictive than questionnaire measures.
Limitations
Limitations of this study should be considered in evaluating the generalizability of these findings. The first limitation is the BDI-II and the URICA had only acceptable reliability, which may have affected the ability of these scales to significantly predict who would discontinue treatment in our study. In addition, the measures were not available for a few participants and this, too, may have affected the ability of the scales to significantly predict treatment completion. A third limitation was that we did not have evidence of the participants’ ability to engage in sensory gating and post-error response adjustment. Additional research is recommended to examine the influence of the P2 and Pe finding on, for example, participants’ engagement in release disposition planning, examining the completeness of such plans, as well as participants’ abilities to remain abstinent from substance use post-release. Such research would emphasize the importance of the influence of neurocognitive processing on outcomes for these individuals.
More such work is needed to better understand the P2 and Pe amplitude cut-off that is most predictive of treatment completion. Specifically, the logistic regression models predicting which participants would complete treatment at a high rate but did not predict who would discontinue treatment at a similarly high rate (i.e., high sensitivity but low specificity). This highlights that though our sample is quite large (N=89), our groups were not of equal size therefore additional reports with a larger number of individuals who discontinue treatment are necessary to better understand the phenomenon reported here. However, the best SVM model was successful in identifying who would not complete treatment (83.33%) suggesting, with the correct data analytical strategy (i.e., resampling), a larger and more equal sample is not necessary for accurate neuroprediction. Additionally, after individuals discontinued treatment, we had no further ability to ask them research questions and were unable to obtain details as to why they discontinued treatment. Future explorations of this topic should investigate specific reasons for withdrawal (e.g., incompatibility with the treatment). Such information would benefit the development of treatment techniques targeting most frequent reasons for withdrawal.
Another potential limitation is no P300 differences were identified between our completed and discontinued groups; unlike previous explorations of treatment outcomes using ERPs. This could be because previous examples of reduced stimulus-locked P300 amplitudes predictive of treatment discontinuation have all used oddball paradigms (10, 12, 13, 36, 55) where the P300 component elicited is thought to reflect stimulus evaluation and executive functioning (e.g., contextual updating; (56)). NoGo stimulus-locked P300 is thought to reflect stimulus evaluation and response inhibition (35, 57, 58) and amplitude reductions suggest impairments in executive control, insufficient input from the prefrontal cortex (PFC), and increased impulsivity (59, 60). Although stimulus-locked P300s elicited during oddball and Go/NoGo paradigms have similar temporal and spatial characteristics, they are most likely neural markers of separable, distinct cognitive functions.
Conclusion and Implications for Treatment
The findings of the present study also have significant implications for treatment retention of incarcerated individuals seeking substance abuse treatment. The research on treatment retention and outcomes in individuals with histories of criminal justice involvement has shown that more treatment received is associated with better outcomes (61). These improved outcomes include reduced substance use and improved physical health and psychosocial functioning (62, 63), but also reduced recidivism (64, 65). The identification of neurocognitive processes that predict treatment discontinuation in incarcerated individuals represents a significant advance over the predominant measures of client treatment motivation and social desirability which are notoriously poor in predicting who will stay in treatment and who will discontinue (66). We demonstrated that a relatively simple neuroimaging measure coupled with logistic regression and pattern classifiers accurately predicted substance abuse treatment completion. Reliable predictors of treatment retention allow for the development of enhancements to therapies designed specifically to target those at greatest risk for treatment discontinuation. For example, despite its nascence, there is some evidence suggesting that working memory training may improve concurrent cognitive processing (67–69). Future studies could investigate working memory training as an enhancement to substance abuse treatment in those at greatest risk for early treatment discontinuation, to determine if it improves processing of treatment-related information, thereby improving treatment retention.
Supplementary Material
Acknowledgments
This research was supported by a grant from the National Institute on Drug Abuse (1 R01 DA020870-01) awarded to Kent A. Kiehl. Brandi C. Fink is supported by a National Institute on Alcohol Abuse and Alcoholism Institutional Research Training Grant (1 T32 AA018108-01A1; McCrady, PI).
Footnotes
Financial Disclosures
Dr. Clark reported having received honoraria from Boys Town National Research Hospital, consulting fees from George Mason University, and payment for work as Editor of Elsevier. Dr. Steele, Dr. Fink, Mr. Maurer, Mr. Arabshirani, Dr. Wilber, Dr. Jaffe, Ms. Sidz, Dr. Pearlson, Dr. Calhoun, and Dr. Keihl reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.US Department of Health and Human Services NIoDA. Treating offenders with drug problems: Integrating public health and public safety. 2011 Retrieved from National Institute on Drug Abuse website: http://www.drugabuse.gov/sites/default/files/drugs_crime.pdf.
- 2.(CASA) NCoAaSAaCU. Behind bars II: Substance abuse and America’s prison population. 2010. [Google Scholar]
- 3.Mumola CJ, Karberg JC. Drug use and dependence, state and federal prisoners, 2004. 2006. NCJ 213530. [Google Scholar]
- 4.Merrall EL, Kariminia A, Binswanger IA, Hobbs MS, Farrell M, Marsden J, et al. Meta-analysis of drug-related deaths soon after release from prison. Addiction. 2010;105:1545–1554. doi: 10.1111/j.1360-0443.2010.02990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gossop M, Marsden J, Stewart D, Kidd T. The National Treatment Outcome Research Study (NTORS): 4–5 year follow-up results. Addiction. 2002;98:291–303. doi: 10.1046/j.1360-0443.2003.00296.x. [DOI] [PubMed] [Google Scholar]
- 6.Hubbard RL, Craddock SG, Anderson J. Overview of 5-year followup outcomes in the drug abuse treatment outcome studies (DATOS) Journal of Substance Abuse Treatment. 2003;25:125–134. doi: 10.1016/s0740-5472(03)00130-2. [DOI] [PubMed] [Google Scholar]
- 7.Mattson ME, Del Boca FK, Carroll KM, Cooney NL, DiClemente CC, Donovan D, et al. Compliance with treatment and follow-up protocols in Project MATCH: Predictors and relationship to outcome. Alcoholism: Clinical and Experimental Research. 1998;22:1328–1339. [PubMed] [Google Scholar]
- 8.Sayre SL, Schmitz JM, Stotts AL, Averill PM, Rhoades HM, Grabowski JJ. Determining predictors of attrition in an outpatient substance abuse program. Am J Drug Alcohol Abuse. 2002;28:55–72. doi: 10.1081/ada-120001281. [DOI] [PubMed] [Google Scholar]
- 9.Simpson DD, Joe GW, Brown BS. Treatment retention and follow-up outcomes in the Drug Abuse Treatment Outcome Study (DATOS) Journal of Addictive Behaviors. 1997;11:294–307. [Google Scholar]
- 10.Anderson NE, Baldridge RM, Stanford MS. P3 amplitude predicts successful treatment program completion in substance dependent individuals. Substance Use and Misuse. 2011;46:669–677. doi: 10.3109/10826084.2010.528123. [DOI] [PubMed] [Google Scholar]
- 11.Boutros NN, Gooding D, Sundaresan K, Burroughs S, Johanson CE. Cocaine-dependence and cocaine-induced paranoia and mid-latency auditory evoked responses and sensory gating. Psychiatry Res. 2006;145:147–154. doi: 10.1016/j.psychres.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Bauer LO. Frontal P300 decrements, childhood conduct disorder, family history, and the prediction of relapse among abstinent cocaine abusers. Drug Alcohol Depend. 1997;44:1–10. doi: 10.1016/s0376-8716(96)01311-7. [DOI] [PubMed] [Google Scholar]
- 13.Bauer LO. CNS recovery from cocaine, cocaine and alcohol, or opioid dependence: A P300 study. Clinical Neurophysiology. 2001;112:1508–1515. doi: 10.1016/s1388-2457(01)00583-1. [DOI] [PubMed] [Google Scholar]
- 14.Luck SJ, Hillyard SA. Electrophysiological correlates of feature analysis during visual search. Psychophysiology. 1994;31:291–308. doi: 10.1111/j.1469-8986.1994.tb02218.x. [DOI] [PubMed] [Google Scholar]
- 15.Ferreira-Santos F, Silveira C, Almeida PR, Palha A, Barbosa F, Marques-Teixeira J. The auditory P200 is both increased and reduced in schizophrenia? A meta-analytic dissociation of the effect for standard and target stimuli in the oddball task. Clinical Neurophysiology. 2012;123:1300–1308. doi: 10.1016/j.clinph.2011.11.036. [DOI] [PubMed] [Google Scholar]
- 16.Lijffijt M, Lane SD, Meier SL, Boutros NN, Burroughs S, Steinberg JL, et al. P50, N100, and P200 sensory gating: Relationships with behavioral inhibition, attention, and working memory. Psychophysiology. 2009;46:1059–1068. doi: 10.1111/j.1469-8986.2009.00845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woltering S, Granic I, Lamm C, Lewis MD. Neural changes associated with treatment outcome in children with externalizing problems. Biol Psychiatry. 2011;70:873–879. doi: 10.1016/j.biopsych.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 18.Carter CS, Braver TS, Barch DM, Botvinick M, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- 19.Kiehl KA, Liddle PF, Hopfinger JB. Error processing and the rostral anterior cingulate: An event-related fMRI study. Psychophysiology. 2000;37:216–223. [PubMed] [Google Scholar]
- 20.Miltner WHR, Lemke U, Weiss T, Holroyd C, Scheffers MK, Coles MGH. Implementation of error-processing in the human anterior cingulate cortex: A source analysis of the magnetic equivalent of the error-related negativity. Biol Psychol. 2003;64:157–166. doi: 10.1016/s0301-0511(03)00107-8. [DOI] [PubMed] [Google Scholar]
- 21.Holroyd CB, Coles MGH. The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- 22.Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychol Sci. 1993;4:385–390. [Google Scholar]
- 23.Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography and clinical neurophysiology. 1991;78:447–455. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- 24.Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of errors in choice reaction tasks on the ERP under focused and divided attention. In: Brunia CHMAWKG, Kok A, editors. Psychophysiological brain research. Tilburg: Tilburg University Press; 1990. pp. 192–195. [Google Scholar]
- 25.Edwards BG, Calhoun VD, Kiehl KA. Joint ICA of ERP and fMRI during error-monitoring. Neuroimage. 2012;59:1896–1903. doi: 10.1016/j.neuroimage.2011.08.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carbonnell L, Falkenstein M. Does the error negativity reflect the degree of response conflict? Brain Res. 2006;1095:124–130. doi: 10.1016/j.brainres.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 27.van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- 28.Overbeek TJM, Nieuwenhuis S, Ridderinkhof KR. Dissociable components of error processing: On the functional significance of the Pe vis-à-vis the ERN/Ne. Journal of Psychophysiology. 2005;19:319–329. [Google Scholar]
- 29.Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GPH, Kok A. Error-related brain potentials are differentially related to awareness of response errors: Evidence from an antisaccade task. Psychophysiology. 2001;38:752–760. [PubMed] [Google Scholar]
- 30.Leuthold H, Sommer S. ERP correlates of error processing in spatial S-R compatibility tasks. Clinical Neurophysiology. 1999;110:342–357. doi: 10.1016/s1388-2457(98)00058-3. [DOI] [PubMed] [Google Scholar]
- 31.Endrass T, Reuter B, Kathmann N. ERP correlates of conscious error recognition: Aware and unaware errors in an antisaccade task. European Journal of Neuroscience. 2007;26:1714–1720. doi: 10.1111/j.1460-9568.2007.05785.x. [DOI] [PubMed] [Google Scholar]
- 32.Silva-Pereyra J, Bernal J, Rodriguez-Camacho M, Yanez G, Prieto-Corona B, Luviano L, et al. Poor reading skills may involve a failure to focus attention. Neuroreport. 2010;21:34–38. doi: 10.1097/WNR.0b013e328332c566. [DOI] [PubMed] [Google Scholar]
- 33.Huster RJ, Westerhausen R, Pantev C, Konrad C. The role of the cingulate cortex as neural generator of the N200 and P300 in a tactile response inhibition task. Hum Brain Mapp. 2010;31:1260–1271. doi: 10.1002/hbm.20933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Folstein JR, Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology. 2008;45:152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Polich J. Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campanella S, Petit G, Maurage P, Kornreich C, Verbanck P, Noel X. Chronic alcoholism: Insights from neurophysiology. Clinical Neurophysiology. 2009;39:191–207. doi: 10.1016/j.neucli.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Chapman RM, McCarry JW. EP component identification and measurment by principal component analysis. Brain Cogn. 1995;27:288–310. doi: 10.1006/brcg.1995.1024. [DOI] [PubMed] [Google Scholar]
- 38.Dien J, Khoe W, Mangun GR. Evaluation of PCA and ICA of simulated ERPs: Promax vs. Infomax rotations. Hum Brain Mapp. 2007;28:742–763. doi: 10.1002/hbm.20304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaffe AJ, Wilber CH. Substance Abuse Treatment Unit. Connecticut Mental Health Center, Yale University School of Medicine; 2001. A treatment manual for Substance Expectation Therapy (SET): Expectations, motivation, and personal meaning in the treatment of substance abuse. [Google Scholar]
- 40.Hare RD. Manual for the Hare Psychopathy Checklist-Revised. 2. Toronto, Canada: Multi-Health Systems; 2003. [Google Scholar]
- 41.Wechsler D. Wechsler adult intelligence scale. New York, NY: Psychological Corporation; 1997. [Google Scholar]
- 42.Spielberger CD. State-Trait Anciety Expression Inventiry-2 (STAXI-2) Lutz, FL: Psychological Assessment Resources, Inc; 2002. [Google Scholar]
- 43.Beck AT, Steer RA, Brown GK. Beck Depression Inventory-II (BDI-II) San Antonio, TX: Harcourt Assessment, Inc; 1996. [Google Scholar]
- 44.McConaughy EA, Prochaska JO, Velicer WG. Stages of change in psychotherapy: Measuremnt and sample profiles. Psychotherapy. 1983;20:368–375. [Google Scholar]
- 45.McLellan AT, Kushner HI, Metzger D, Peters R, Smith I, Grissom G, et al. The fifth edition of the Addiction Severity Index. Journal of Substance Abuse Treatment. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 46.Jasper HH. The ten-twenty electrode system of the International Federation. Electroencephalography and Clinical Neurophysiology. 1958;10:371–375. [PubMed] [Google Scholar]
- 47.Jung T-P, Makeig S, Westerfield M, Townsend J, Courchesne E, Sejnowski TJ. Removal of eye activity artifacts from visual event-related potentials in normal and clinical subjects. Clinical Neurophysiology. 2000;111:1745–1758. doi: 10.1016/s1388-2457(00)00386-2. [DOI] [PubMed] [Google Scholar]
- 48.Jennings JR, Wood CC. The e-Adjustment procedure for repeated-measures analyses of variance. Psychophysiology. 1976;13:277–278. doi: 10.1111/j.1469-8986.1976.tb00116.x. [DOI] [PubMed] [Google Scholar]
- 49.Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson RJ, et al. Guidlines for using human event-related potentials to study congnition: Recording standards and publication criteria. Psychophysiology. 2000;37:127–152. [PubMed] [Google Scholar]
- 50.Jain AK, Duin RPW, Mao JC. Statistical pattern recognition: A review. IEEE Transactions on Pattern Analysis and Machine Intelligence. 2000;22:4–37. [Google Scholar]
- 51.Kemp AH, Hopkinson PJ, Hermens DF, Rowe DL, Sumich AL, Clark CR, et al. Fronto-temporal alterations within the first 200 ms during an attentional task distinguish major depression, non-clinical participants with depressed mood and healthy controls: A potential biomarker? Hum Brain Mapp. 2009;30:602–614. doi: 10.1002/hbm.20528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Falkenstein M, Hoorman J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: A tutorial. Biol Psychol. 2000;51:87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- 53.Aharoni E, Vincent GM, Harenski CL, Calhoun VD, Sinnott-Armstrong W, Gazzaniga MS, et al. Neuro-prediction of future rearrest. Proc Natl Acad Sci U S A. 2013;110:6223–6228. doi: 10.1073/pnas.1219302110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hajcak G, Vidal F, Simons RF. Difficulties with easy tasks: ERN/Ne and stimulus component overlap. In: Ullsperger M, Falkenstein M, editors. Error, Conflicts, and the Brain: Current Opinions on Performance Monitoring. Leipzig: MPI of Cognitive Neuroscience; 2004. pp. 204–211. [Google Scholar]
- 55.Wan L, Baldridge RM, Colby AM, Stanford MS. Association of P3 amplitude to treatment completion in substance dependent individuals. Psychiatry Res. 2010;177:223–227. doi: 10.1016/j.psychres.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 56.Donchin E, Coles MG. Is the P300 a manifestation of context updating? Behavioral and Brain Sciences. 1988;11:357–427. [Google Scholar]
- 57.Fallgatter AJ, Strik WK. The NoGo anteriorization as a neurophysiological standard-index for cognitive response control. International Journal of Psychophysiology. 1999;32:233–238. doi: 10.1016/s0167-8760(99)00018-5. [DOI] [PubMed] [Google Scholar]
- 58.Salisbury DF, Griggs CB, Shenton ME, McCarley RW. The NoGo P300 ‘anteriorization’ effect and response inhibition. Clinical Neurophysiology. 2004;115:1550–1558. doi: 10.1016/j.clinph.2004.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kamarajan C, Porjesz B, Jones KA, Choi K, Chorlian DB, Padmanabhapillai A, et al. Alcoholism is a disinhibitory disorder: Neurophysiological evidence from a Go/No-Go task. Biol Psychol. 2005;69:353–373. doi: 10.1016/j.biopsycho.2004.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ruchsow M, Groen G, Kiefer M, Hermle L, Spitzer M, Falkenstein M. Impulsiveness and ERP components in a Go/Nogo task. J Neural Transm. 2008;115:909–915. doi: 10.1007/s00702-008-0042-7. [DOI] [PubMed] [Google Scholar]
- 61.Evans E, Jaffe A, Urada D, Anglin MD. Differential outcomes of court-supervised substance abuse treatment among California parolees and probationers. International journal of offender therapy and comparative criminology. 2012;56:539–556. doi: 10.1177/0306624X11404827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Landry MJ. Understanding drugs of abuse: The processes of addiction, treatment, and recovery. Amer Psychiatric Pub Incorporated; 1994. [Google Scholar]
- 63.McLellan AT, Woody GE, Metzger D, McKay J, Durell J, Alterman AI, et al. Evaluating the effectiveness of addiction treatments: Reasonable expectations, appropriate comparisons. The Milbank Quarterly. 1996;74:51–85. [PubMed] [Google Scholar]
- 64.Farabee D, Prendergast M, Anglin MD. The effectiveness of coerced treatment for drug-abusing offenders. Federal Probation. 1998;62:3–10. [Google Scholar]
- 65.Harrell A, Roman J. Reducing drug use and crime among offenders: The impact of graduated sanctions. Journal of Drug Issues. 2001;31:207–231. [Google Scholar]
- 66.Zemore SE. The effect of social desirability on reported motivation, substance use severity, and treatment attendance. Journal of Substance Abuse Treatment. 2012;42:400–412. doi: 10.1016/j.jsat.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ. Improving fluid intelligence with training on working memory. Proc Natl Acad Sci U S A. 2008;105:6829–6833. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morrison AB, Chein JM. Does working memory training work? The promise and challenges of enhancing cognition by training working memory. Psychon Bull Rev. 2011;18:46–60. doi: 10.3758/s13423-010-0034-0. [DOI] [PubMed] [Google Scholar]
- 69.Olesen PJ, Westerberg H, Klingberg T. Increased prefrontal and parietal activity after training of working memory. Nat Neurosci. 2004;7:75–79. doi: 10.1038/nn1165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.