Abstract
Dipeptidyl peptidase-4 inhibitors prevent the degradation of incretin hormones and reduce post-prandial hyperglycemia in patients with type 2 diabetes mellitus. Dipeptidyl peptidase-4 degrades other peptides with a penultimate proline or alanine, including bradykinin and substance P, which are also substrates of angiotensin-converting enzyme. During angiotensin-converting enzyme inhibition, substance P is inactivated primarily by dipeptidyl peptidase-4, while bradykinin is first inactivated by aminopeptidase P. This study tested the hypothesis that dipeptidyl peptidase-4 inhibition potentiates vasodilator and fibrinolytic responses to substance P when angiotensin-converting enzyme is inhibited. Twelve healthy subjects participated in this randomized, double-blinded, placebo-controlled crossover study. On each study day, subjects received sitagliptin 200 mg p.o. or placebo. Substance P and bradykinin were infused via brachial artery before and during intra-arterial enalaprilat. Sitagliptin and enalaprilat each reduced forearm vascular resistance and increased forearm blood flow without affecting mean arterial pressure, but there was no interactive effect of the inhibitors. Enalaprilat increased bradykinin-stimulated vasodilation and tissue plasminogen activator release; sitagliptin did not affect these responses to bradykinin. The vasodilator response to substance P was unaffected by sitagliptin and enalaprilat, however, substance P increased heart rate and vascular release of norepinephrine during combined angiotensin-converting enzyme and dipeptidyl peptidase-4 inhibition. In women, sitagliptin diminished tissue plasminogen activator release in response to substance P both alone and during enalaprilat. Substance P increases sympathetic activity during combined angiotensin-converting enzyme and dipeptidyl peptidase-4 inhibition.
Keywords: diabetes mellitus, norepinephrine, vasodilation, renin-angiotensin system, dipeptidyl peptidase-4, hypertension
Dipeptidyl peptidase-4 (DPP4) is a ubiquitously expressed cell surface protease that preferentially cleaves dipeptides from the amino terminus of peptides containing a penultimate alanine or proline. A soluble form of DPP4 also exists in plasma.1 The first selective DPP4 inhibitor, sitagliptin, was approved by the FDA in 2006 for the management of hyperglycemia in patients with type 2 diabetes mellitus (T2DM). DPP4 inhibition decreases the degradation of endogenous incretin hormones, including glucagon-like peptide 1 (GLP-1), and thereby augments nutrient-mediated insulin release, suppresses glucagon secretion, and slows gastric emptying.2
The widespread expression of DPP4 within the vasculature and immune system raises the possibility that DPP4 could affect vascular function.3 Among the vasoactive substrates cleaved by DPP4 are the angiotensin-converting enzyme (ACE) substrates bradykinin and substance P. ACE inactivates these peptides by cleaving them at the carboxy terminus. When ACE is inhibited, however, substance P is inactivated by DPP4,4,5 while bradykinin is inactivated primarily by aminopeptidase P before it is cleaved by DPP4.6 Substance P and bradykinin are potent vasodilators and enhance endothelial fibrinolytic function by stimulating the release of tissue plasminogen activator (tPA).7,8 Substance P released from primary afferent sensory nerve fibers also increases sympathetic activity.9–11
ACE inhibitors are widely prescribed to patients with T2DM in order to reduce cardiovascular risk.12 Thus, many patients are treated concurrently with an ACE inhibitor and DPP4 inhibitor. This study tested the hypothesis that DPP4 inhibition potentiates the vasodilator, fibrinolytic and sympathetic responses to substance P in the human forearm vasculature in the presence of ACE inhibition. Because bradykinin is inactivated by aminopeptidase P prior to cleavage by DPP4,6 we anticipated that ACE inhibition would potentiate the vasodilator response to bradykinin, but that DPP4 inhibition would not alter this effect.
Methods
Study Protocol
Twelve healthy, non-obese (BMI <30 kg/m2), non-smoking adults participated in a double-blind, randomized, placebo-controlled, cross-over study (Figure 1). (See online data supplement Table S1 for subject characteristics.) The study adhered to the principles of the Declaration of Helsinki and Title 45, U.S. Code of Federal Regulations, Part 46, Protection of Human Subjects following approval by the Vanderbilt University Institutional Review Board, and all subjects provided written informed consent prior to initiation of study procedures. Patients with a history of chronic illness, including diabetes, hypertension, cardiovascular disease, and chronic renal or hepatic insufficiency were excluded from participation. Medication use other than a multivitamin was prohibited at the time of study. Pregnancy was excluded in females of child-bearing age.
Figure 1.
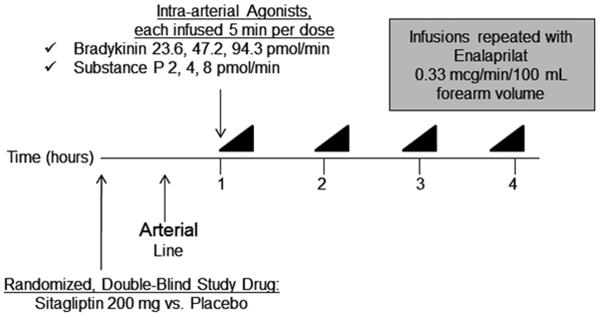
Study Protocol. Each intra-arterial peptide was given in a randomized fashion as 3 graded doses, lasting 5 minutes each. A 30-minute wash-out separated infusions. Forearm blood flow measurement, followed by arterial and venous sampling, was performed at baseline and at completion of each dose of peptide. A minimum of one week separated each study day.
Each subject was studied on two days at least one week apart. Subjects were assigned to treatment order (sitagliptin or matching placebo) using a block randomization algorithm provided by the study biostatistician. Randomization was stratified by race and gender. On each study day, subjects reported to the Vanderbilt Clinical Research Center (CRC) in the morning after an overnight fast. All subjects were studied in the supine position in a temperature-controlled room. Subjects were given oral study drug (sitagliptin 200 mg or matching placebo) and an arterial line was placed in the brachial artery of their non-dominant arm with an adjacent peripheral intravenous line.
Baseline forearm blood flow (FBF) measurements and blood sampling were obtained 60 minutes following ingestion of study drug and at least 30 minutes after insertion of arterial catheter. (See online supplement for information regarding study procedures and medications.) Subjects then received sequential intra-arterial infusions of substance P and bradykinin in random order. Each peptide was infused in 3 graded doses for 5 minutes each. FBF was assessed during the last 2 minutes of each dose, and arterial and venous blood samples were then drawn simultaneously. A 30-minute washout separated each infusion. This sequence was then repeated during ACE inhibition by intra-arterial enalaprilat. On the second study day, the protocol was repeated using the opposite study drug (sitagliptin or matching placebo). Blood pressure and heart rate were continuously monitored throughout each study day.
Statistical Analysis
Data are presented as mean ± standard deviation, unless otherwise noted. Potential carry-over and period effects were tested by comparing the measures of FBF obtained prior to each infusion. Mixed-effect models were used to analyze the data with a random subject effect and with fixed effects of treatment (enalaprilat and/or sitagliptin), vasodilator dose, as well as their interaction. Interaction terms were removed from the final model when the p-value from the corresponding chunk test was greater than 0.2. For inferences of interest, a 2-sided P value <0.05 was considered significant. Statistical analyses were performed using IBM SPSS software v. 21.0, GraphPad Prism 5 and R 2.15.0 (www.r-project.org).
Results
Effect of Treatment on DPP4 Activity and Baseline Hemodynamic Parameters
DPP4 inhibition with sitagliptin significantly decreased DPP4 activity compared to placebo (p=0.003), while DPP4 antigen was unchanged (Table 1). ACE inhibition significantly decreased ACE activity both in the presence (p=0.008) or absence of DPP4 inhibitor (p=0.01). Neither DPP4 inhibition nor ACE inhibition, alone or in combination, significantly affected baseline mean arterial pressure (MAP) or heart rate at baseline. ACE inhibition significantly decreased baseline forearm vascular resistance (FVR) (p=0.04), as did DPP4 inhibition (p=0.01) (Table 1). Similarly, ACE inhibition (p=0.04) and DPP4 inhibition (p=0.03) each increased FBF. DPP4 inhibition did not alter the effect of ACE inhibition on FVR or FBF at baseline.
Table 1.
Baseline Parameters
| Placebo (n=12) |
DPP4 inhibition (n=11) |
|||
|---|---|---|---|---|
| Variable | Vehicle | ACE inhibition | Vehicle | ACE inhibition |
| DPP4 Activity (U/L) | 24.3±7.0 | 4.8±3.0* | ||
| DPP4 Antigen (ng/mL) | 571.5±187.0 | 561.7±116.4 | ||
| ACE Act (U/L) | 37.4±7.6 | 8.1±3.3† | 37.0±7.2 | 11.0±8.6‡ |
| MAP (mm Hg) | 86.4±6.2 | 84.2± 5.2 | 85.1±5.4 | 82.8±4.1 |
| Heart rate (bpm) | 61.5±9.7 | 62.4±8.3 | 62.9±10.0 | 65.0±10.0 |
| FVR (mmHg/ml/min/100mL) | 37.7±12.4 | 30.4± 10.0† | 30.6±8.5† | 27.1±7.3 |
| FBF (ml/min/100mL) | 2.6±0.8 | 3.2±1.1† | 3.1±0.9† | 3.4±1.0 |
Results are presented as mean± standard deviation
MAP, mean arterial pressure; DPP4, dipeptidyl peptidase-4; ACE, angiotensin-converting enzyme; bpm, beats per minute; FVR, forearm vascular resistance; FBF, forearm blood flow
p<0.05 versus placebo,
p<0.05 versus placebo/vehicle,
p<0.05 versus DPP4 inhibition/vehicle
Influence of DPP4 and ACE Inhibition on Forearm Blood Flow, Heart Rate and Norepinephrine Release
Vasodilator response is presented as FBF, as local intra-arterial infusion of bradykinin or substance P did not affect MAP in any treatment group. Intra-arterial bradykinin increased FBF in a dose-dependent manner (p<0.001), and ACE inhibition potentiated this effect (p<0.001) (Figure 2). Treatment with DPP4 inhibition did not affect the vasodilator response to bradykinin. ACE inhibition significantly increased venous bradykinin concentrations (p<0.001) and decreased the metabolite bradykinin (1–5) (p<0.001); DPP4 inhibition did not affect bradykinin concentrations. (Data not shown.) Intra-arterial substance P increased FBF in a dose-dependent manner (p<0.001), however, neither ACE inhibition nor DPP4 inhibition affected the vasodilator response to substance P.
Figure 2.
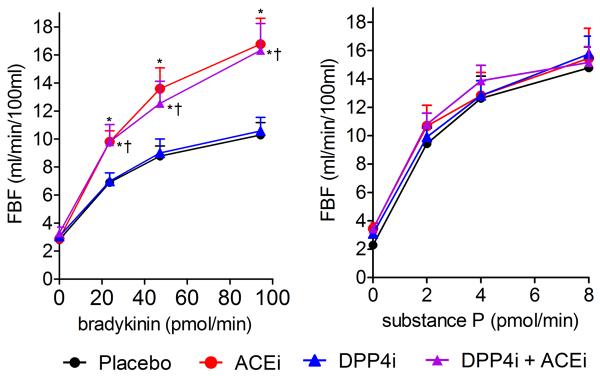
Effect of treatment on forearm blood flow (FBF) response to intra-arterial bradykinin, with and without intra-arterial enalaprilat, and to substance P, with and without intra-arterial enalaprilat (n=12). Data presented as mean ± standard error of the mean. Quadratic model-based p-values presented in the text. *p<0.05 versus placebo at same peptide dose, †p<0.05 versus dipeptidyl peptidase-4 inhibition (DPP4i) at same peptide dose, ACEi indicates angiotensin-converting enzyme inhibition.
Bradykinin did not affect heart rate either in the presence or absence of DPP4 and ACE inhibition (Figure 3). Substance P increased heart rate during ACE inhibition (from 61.2±8.8 to 65.7±6.8 beats per minute (bpm) at the maximum dose of substance P, p=0.01) and during combined ACE and DPP4 inhibition (from 61.2±8.8 to 68.2±12.1 bpm at the maximum dose of substance P, p=0.03). Substance P-stimulated heart rate was also significantly higher during combined ACE and DPP4 inhibition than during DPP4 inhibition alone (68.2±12.1 vs. 63.5±11.3 bpm, p=0.045).
Figure 3.
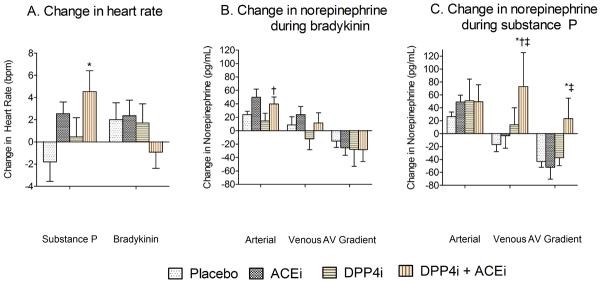
Effect of the maximal dose of substance P and bradykinin on heart rate (A) and norepinephrine release (B, C) after treatment with placebo, angiotensin-converting enzyme inhibitor (ACEi) alone, dipeptidyl peptidase-4 inhibitor (DPP4i) alone, or the combination (n=12). Data presented as mean ± standard error of the mean. P values from Wilcoxon signed rank test and separate means models presented in the text. * p<0.05 versus placebo, †p<0.05 versus DPP4i alone, ‡p<0.05 versus ACEi alone
At the highest dose of bradykinin, the arterial-venous (AV) norepinephrine gradient was similar during ACE, DPP4 and combined inhibition. Substance P did not significantly affect venous norepinephrine concentrations during ACE or DPP4 inhibition alone as compared to placebo. In contrast, during combined ACE inhibition and DPP4 inhibition, substance P increased venous norepinephrine (from 212.5±60.7 during placebo to 331.9±228.5 pg/mL at the highest dose during combined inhibition, p=0.02) to a greater extent than arterial norepinephrine (206.6±42.5 to 277.9±126.0 pg/mL, p=0.04). As a result, substance P increased the AV gradient of norepinephrine in the setting of combined DPP4 and ACE inhibition, as compared to treatment with placebo (p=0.05), ACE inhibition alone (p=0.007), or DPP4 inhibition alone (p=0.04). Similarly, net norepinephrine release in response to substance P was significantly higher following treatment with combined DPP4 and ACE inhibition as compared to treatment with placebo (estimated difference 418.8 pg/min/100mL, 95% CI 93.9 to 743.8, p=0.01), ACE inhibition alone (estimated difference 534.1 pg/min/100mL, 95% CI 209.1 to 859.1, p=0.001), or DPP4 inhibition alone (estimated difference 447.2 pg/min/100mL, 95% CI 123.0 to 771.5, p=0.007). There was no effect of substance P on arterial or venous tryptase concentrations. (Data not shown.)
Effect of DPP4 Inhibition on tPA Release in Response to Intra-arterial Bradykinin and Substance P
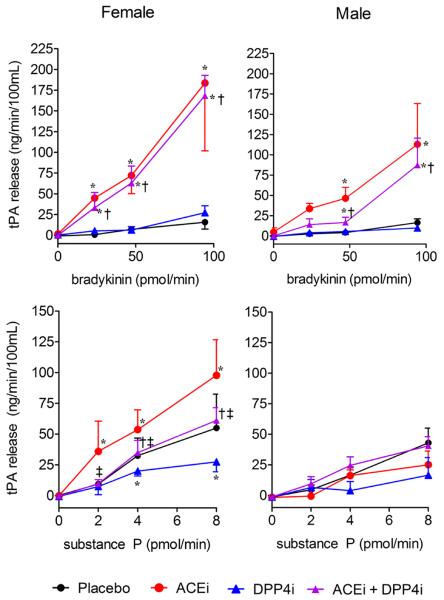
During placebo, bradykinin increased net tPA release (p<0.001) in a dose-dependent manner (Figure 4). ACE inhibition potentiated bradykinin-stimulated tPA release compared to placebo, in the absence of DPP4 inhibition (at the maximum bradykinin dose p<0.001 both males and females) and presence of DPP4 inhibition (at maximum bradykinin dose p<0.001 both males and females). As we have observed previously, the effect of ACE inhibition on bradykinin-stimulated tPA release was more pronounced in females.13 DPP4 inhibition did not influence bradykinin-stimulated tPA release.
Figure 4.
Effect of treatment on net tissue plasminogen activator (tPA) release in response to intra-arterial bradykinin, with and without intra-arterial enalaprilat, and substance P, with and without intra-arterial enalaprilat (n=7 females, n=5 males). Data presented as mean ± standard error of the mean. Linear model-based p-values presented in the text. * p<0.05 versus placebo at same peptide dose, †p<0.05 versus dipeptidyl peptidase-4 inhibition (DPP4i) at same peptide dose, ‡p<0.05 versus angiotensin-converting enzyme inhibition (ACEi) at same peptide dose. Bradykinin and Substance P increased tPA release in a dose-dependent manner (see text for p-values).
Substance P increased net tPA release (p<0.001) in a dose-dependent manner during placebo (Figure 4 and online data supplement Table S2). In women, DPP4 inhibition attenuated net tPA release in response to substance P as compared to placebo (p=0.02 at maximum substance P dose). ACE inhibition potentiated substance P-stimulated tPA release in females but not males (at maximum substance P dose p<0.001 in females); this effect was attenuated by DPP4 inhibition (at maximum substance P dose p=0.001 in females), such that the effect of ACE and DPP4 inhibition combined was no longer significant compared to placebo.
Safety
One female experienced angioedema of the instrumented forearm following infusion of bradykinin and substance P on the sitagliptin treatment day. An upper extremity ultrasound did not show a deep vein thrombosis nor arterial thrombosis and the swelling resolved within 72 hours without intervention. Other adverse events included transient lightheadedness and orthostasis which resolved with increased oral fluid intake (n=3) and one episode of nephrolithiasis within two weeks of completion of the first study day (n=1). There were no instances of hypoglycemia. One subject did not complete the second study day due to inability to obtain adequate arterial access; the data presented includes this subject. All other subjects completed both study days.
Discussion
This study tested the hypothesis that DPP4 inhibition potentiates the vascular effects of substance P during ACE inhibition. We found no effect of combined DPP4 and ACE inhibition on substance P-mediated vasodilation; however, during concurrent DPP4 and ACE inhibition, intra-arterial administration of substance P stimulated the sympathetic nervous system. In addition, DPP4 inhibition diminished substance P-induced tPA release in women.
This study extends the findings of three prior studies examining the interactive effect of ACE inhibition and DPP4 inhibition on blood pressure. Marney et al. reported that the DPP4 inhibitor sitagliptin reduced blood pressure in subjects with the metabolic syndrome when given alone for five days. When given in combination with an ACE inhibitor, however, sitagliptin attenuated the hypotensive response to ACE inhibition and caused an increase in heart rate and circulating norepinephrine concentrations.14 Boschmann et al. reported a significant increase in post-prandial venous norepinephrine after a seven-day treatment with the DPP4 inhibitor vildagliptin in patients with T2DM.15 The authors did not comment on concurrent ACE inhibitor use and hypothesized that the increased sympathetic activity may be a result of GLP-1 receptor activation in the central nervous system, as has been demonstrated in animal models.16,17 Jackson et al. similarly reported that DPP4 inhibition increased blood pressure in spontaneously hypertensive rats only if they had been pretreated with an ACE inhibitor, and this effect was prevented by ganglionic blockade. The investigators postulated that decreased degradation of the vasoconstrictor neuropeptide Y by DPP4 may have contributed to increased blood pressure.18 The current study suggests, however, that activation of the sympathetic nervous system in the setting of combined ACE and DPP4 inhibition is in part mediated by substance P.
Substance P is a tachykinin neuropeptide which is released from afferent sensory nerve terminals and binds with highest affinity to the neurokinin-1 (NK1) receptor.19 Nerve fibers containing substance P localize to the vasculature.20–22 Substance P-mediated vasodilation in the human forearm is dependent upon activation of the NK1 receptor.23 Afferent sensory nerve stimulation or direct application of substance P results in depolarization of sympathetic neurons via the NK1 receptor.10,11,24 Substance P also stimulates release of catecholamines from the adrenal medulla in animal models10,25 and activates mast cells leading to increased formation of angiotensin II via mast cell-derived chymases.26 During concurrent DPP4 and ACE inhibition, substance P could increase heart rate and norepinephrine directly by stimulating sympathetic ganglia or indirectly by causing systemic vasodilation and reflex activation of the sympathetic nervous system. Two lines of evidence suggest that substance P facilitated local release of norepinephrine when ACE and DPP4 were inhibited. First, intra-arterial substance P increased the AV gradient during combined ACE and DPP4 inhibition, indicating local release. Second, intra-arterial infusion of substance P did not lower blood pressure even when both DPP4 and ACE were inhibited. On the other hand, the increase in heart rate observed during the highest dose of substance P during combined ACE and DPP4 inhibition suggests some degree of systemic sympathetic activation.
Increased sympathetic activation may also have contributed to the apparent decrease in substance P-stimulated tPA release in women during DPP4 inhibition. Net tPA release depends on both the AV gradient and blood flow and reflects the capacity of the vascular endothelium to release stored tPA. Norepinephrine, like substance P, stimulates tPA release from the vascular endothelium.27,28 Activation of the sympathetic nervous system during DPP4 inhibition may have caused systemic release of endothelial tPA, as evidenced by an increase in arterial tPA levels. Substance P infusion also stimulated local endothelial tPA release as supported by increased venous tPA levels. Stored endothelial tPA pools may have been depleted secondary to concurrent systemic tPA release mediated by the sympathetic nervous system and local tPA release mediated by substance P infusion, thus resulting in an apparent decrease in net tPA release.
Contrary to our hypothesis, ACE inhibition and DPP4 inhibition did not affect the vasodilator response to intra-arterial substance P. This is consistent with prior studies by Labinjoh et al.29 and Benjamin and Webb4 that found no effect of acute or chronic ACE inhibition on substance P-stimulated arterial vasodilation, but conflicts with studies demonstrating the hydrolysis of substance P by ACE and DPP4.30,31 One explanation is that substance P-mediated vasodilation may primarily result from the rapid release of histamine or other mediators from mast cells, and neither ACE inhibition nor DPP4 inhibition decreases the degradation of histamine.32 We found no effect, however, of substance P on tryptase concentration, a marker of mast cell activation. Alternatively, enhanced sympathetic activation by substance P during combined ACE and DPP4 inhibition may result in norepinephrine-mediated vasoconstriction, thereby attenuating substance P-induced vasodilation. Finally, we may not have appreciated an effect of combined ACE and DPP4 inhibition on substance P induced-vasodilation due to tachyphylaxis, as has been previously observed during continuous infusion of substance P.33,34
As previously described, one female experienced edema of the instrumented forearm after receiving sitagliptin and subsequent intra-arterial infusions of bradykinin, substance P, and enalaprilat. Newby et al. noted patchy skin edema, beginning at the level of the elbow and extending distally, in select healthy male subjects receiving intra-arterial infusion of substance P at 16 pmol/min and in all subjects receiving 32 pmol/min of substance P.35 The maximum dose of substance P used in our protocol was 8 pmol/min, however, suggesting that the combination of ACE and DPP4 potentiated the effects of substance P on vascular permeability.36,37
As noted earlier, this study provides mechanistic insight to prior studies examining the interactive effect of ACE and DPP4 inhibition on blood pressure. When given alone, DPP4 inhibitors have been reported to decrease blood pressure and to have conflicting effects on endothelial function. For example, two groups separately reported that sitagliptin38 or vildagliptin39 therapy reduced blood pressure. In animal models and in vitro, DPP4 inhibition lowers blood pressure and protects endothelial function, an effect that has been attributed to both GLP-1-dependent and -independent increases in nitric oxide bioavailability.40–42 In patients with T2DM, DPP4 inhibition with vildagliptin potentiates endothelium-dependent vasodilation in response to acetylcholine.43 In contrast, Ayaori et al. observed in two independent clinical trials that sitagliptin or alogliptin attenuate endothelial function as evaluated by flow-mediated vasodilation in patients with T2DM.44 We propose that interpretation of these disparate data regarding the vascular effects of DPP4 inhibitors in clinical trials may require knowledge of concurrent ACE inhibitor treatment.
Two recent placebo-controlled clinical trials, EXAMINE45 and SAVOR-TIMI 53,46 demonstrated that neither alogliptin nor saxagliptin affected the rate of cardiovascular events in patients with T2DM who were at high cardiovascular risk. The rate of hospitalization for heart failure, however, was increased with saxagliptin.46 Augmented sympathetic activity has long been implicated in the pathophysiology of heart failure.47,48 Over 50% of individuals taking saxagliptin were also prescribed an ACE inhibitor throughout the SAVOR-TIMI 53 trial.46 The contribution of a substance P-mediated increase in sympathetic activity to the development of heart failure in patients receiving concurrent DPP4 and ACE inhibition merits further study.
A few study limitations warrant mention. We studied the effect of acute DPP4 inhibition on the vasodilatory response to locally administered substance P and bradykinin with and without local ACE inhibition in the human forearm in order to isolate the vascular responses to these specific peptide substrates and how these responses were altered by DPP4 inhibition and dual inhibition. We used 200 mg of sitagliptin because this dose inhibits DPP4 inhibition to the same extent as the clinically approved dose of 100 mg but within a shorter time period.49 We studied vascular function in healthy subjects in order to avoid the confounding effects of other medications and disease states on the endothelium. We also studied healthy individuals in the fasting state in order to control for the fluctuations in other vasoactive hormones, including GLP-1 and insulin, and to control for medications and diseases which may affect endothelial function. Substance P levels are both reported to be low in diabetic patients with coronary artery disease50 and high in obese individuals with diabetes.51 We did not measure substance P concentrations. Future studies using an NK1 receptor antagonist, aprepitant, are needed to investigate the specific contribution of endogenous substance P to changes in adrenergic tone during dual inhibition. Studies of prolonged ACE and DPP4 inhibition in individuals with T2DM will also be necessary to confirm the clinical relevance of these findings.
Perspective
Diabetes is associated with increased risk of heart attack and stroke. While many anti-diabetes therapies reduce hyperglycemia, most do not reduce cardiovascular risk and some even increase risk. DPP4 inhibitor therapy may influence cardiovascular risk and blood pressure by affecting the degradation of vasoactive peptides, including substance P. Our findings indicate that substance P increases sympathetic activity in the presence of combined ACE and DPP4 inhibition. Elevated sympathetic activity and heart rate could contribute to the recently reported increased rate of hospitalization for heart failure in patients taking the DPP4 inhibitor saxagliptin in the SAVOR-TIMI 53 trial.46 Over half of the patients participating in this trial were also prescribed an ACE inhibitor. Further studies will be needed to determine if decreased degradation of substance P during chronic concurrent ACE and DPP4 inhibition is associated with increased risk of heart failure. In the interim, physicians prescribing simultaneous DPP4 inhibitor and ACE inhibitor therapies in patients with T2DM should monitor for this undesirable, interactive hemodynamic effect.
Supplementary Material
Novelty and Significance.
What is New?
Decreased degradation of substance P during concurrent angiotensin converting enzyme (ACE) and dipeptidyl peptidase-4 (DPP4) inhibition increases sympathetic activity.
DPP4 inhibition in women decreases tissue plasminogen activator (tPA) release in response to substance P.
What is Relevant?
ACE inhibitors are widely prescribed to patients with type 2 diabetes mellitus in order to reduce cardiovascular risk and DPP4 inhibitors are prescribed in order to control post-prandial hyperglycemia.
Increased sympathetic activity contributes to the development of insulin resistance, vascular dysfunction, hypertension and heart failure.
Reduced fibrinolytic capacity is associated with thrombotic events.
Summary
Substance P increases sympathetic activity during concurrent ACE and DPP4 inhibition in healthy subjects. DPP4 inhibition attenuates tPA release by substance P in healthy women. Further studies are needed to determine if simultaneous prescription of these common therapies in patients with type 2 diabetes mellitus contributes to the risk of heart failure.
Acknowledgements
We thank Stacy Gilbert RN, Delia Woods RN, and Carol Meisch RN for their recruitment of volunteers and nursing assistance. We thank Shouzuo Wei, Anthony Dematteo, Zuofei Wang, the Hormone Assay Core and the Clinical Research Center Core Laboratory for their technical assistance.
Clinical Trial Registration: NCT01413542
Sources of Funding This research was supported by NIH grants RO1HL079184 and HL060906, and in part by Vanderbilt CTSA grant UL1 TR000445 from NCATS/NIH (NJB). JKD was supported by the Vanderbilt Physician Scientist Development Award and NIH grant 5P30GM092386. FTB was supported by K23GM102676.
Footnotes
Disclosures None
Reference List
- 1.Zhong J, Rao X, Rajagopalan S. An emerging role of dipeptidyl peptidase 4 (DPP4) beyond glucose control: potential implications in cardiovascular disease. Atherosclerosis. 2013;226:305–314. doi: 10.1016/j.atherosclerosis.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 2.Herman GA, Stein PP, Thornberry NA, Wagner JA. Dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes: focus on sitagliptin. Clin Pharmacol Ther. 2007;81:761–767. doi: 10.1038/sj.clpt.6100167. [DOI] [PubMed] [Google Scholar]
- 3.Fadini GP, Avogaro A. Cardiovascular effects of DPP-4 inhibition: beyond GLP-1. Vascul Pharmacol. 2011;55:10–16. doi: 10.1016/j.vph.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin N, Webb DJ. The effect of local converting enzyme inhibition on the dilator response to substance P in the human forearm. Br J Clin Pharmacol. 1990;29:774–776. doi: 10.1111/j.1365-2125.1990.tb03702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmad S, Wang L, Ward PE. Dipeptidyl(amino)peptidase IV and aminopeptidase M metabolize circulating substance P in vivo. J Pharmacol Exp Ther. 1992;260:1257–1261. [PubMed] [Google Scholar]
- 6.Pesquero JB, Jubilut GN, Lindsey CJ, Paiva AC. Bradykinin metabolism pathway in the rat pulmonary circulation. J Hypertens. 1992;10:1471–1478. doi: 10.1097/00004872-199210120-00006. [DOI] [PubMed] [Google Scholar]
- 7.Brown NJ, Gainer JV, Stein CM, Vaughan DE. Bradykinin stimulates tissue plasminogen activator release in human vasculature. Hypertension. 1999;33:1431–1435. doi: 10.1161/01.hyp.33.6.1431. [DOI] [PubMed] [Google Scholar]
- 8.Oliver JJ, Webb DJ, Newby DE. Stimulated tissue plasminogen activator release as a marker of endothelial function in humans. Arterioscler Thromb Vasc Biol. 2005;25:2470–2479. doi: 10.1161/01.ATV.0000189309.05924.88. [DOI] [PubMed] [Google Scholar]
- 9.Dzurik MV, Diedrich A, Black B, Paranjape SY, Raj SR, Byrne DW, Robertson D. Endogenous substance P modulates human cardiovascular regulation at rest and during orthostatic load. J Appl Physiol. 2007;102:2092–2097. doi: 10.1152/japplphysiol.00969.2006. [DOI] [PubMed] [Google Scholar]
- 10.Hancock JC, Lindsay GW. Pressor and tachycardic responses to intravenous substance P in anesthetized rats. Peptides. 1995;16:1439–1445. doi: 10.1016/0196-9781(95)02049-7. [DOI] [PubMed] [Google Scholar]
- 11.Hancock JC, Lindsay GW. Enhanced ganglionic responses to substance P in spontaneously hypertensive rats. Peptides. 2000;21:535–541. doi: 10.1016/s0196-9781(00)00170-4. [DOI] [PubMed] [Google Scholar]
- 12.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 13.Pretorius M, Luther JM, Murphey LJ, Vaughan DE, Brown NJ. Angiotensin-converting enzyme inhibition increases basal vascular tissue plasminogen activator release in women but not in men. Arterioscler Thromb Vasc Biol. 2005;25:2435–2440. doi: 10.1161/01.ATV.0000186185.13977.94. [DOI] [PubMed] [Google Scholar]
- 14.Marney A, Kunchakarra S, Byrne L, Brown NJ. Interactive hemodynamic effects of dipeptidyl peptidase-IV inhibition and angiotensin-converting enzyme inhibition in humans. Hypertension. 2010;56:728–733. doi: 10.1161/HYPERTENSIONAHA.110.156554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boschmann M, Engeli S, Dobberstein K, Budziarek P, Strauss A, Boehnke J, Sweep FC, Luft FC, He Y, Foley JE, Jordan J. Dipeptidyl-peptidase-IV inhibition augments postprandial lipid mobilization and oxidation in type 2 diabetic patients. J Clin Endocrinol Metab. 2009;94:846–852. doi: 10.1210/jc.2008-1400. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, Hollenberg AN, Baggio L, Saper CB, Drucker DJ, Elmquist JK. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002;110:43–52. doi: 10.1172/JCI15595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Osaka T, Endo M, Yamakawa M, Inoue S. Energy expenditure by intravenous administration of glucagon-like peptide-1 mediated by the lower brainstem and sympathoadrenal system. Peptides. 2005;26:1623–1631. doi: 10.1016/j.peptides.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Jackson EK, Dubinion JH, Mi Z. Effects of dipeptidyl peptidase iv inhibition on arterial blood pressure. Clin Exp Pharmacol Physiol. 2008;35:29–34. doi: 10.1111/j.1440-1681.2007.04737.x. [DOI] [PubMed] [Google Scholar]
- 19.Saito R, Nonaka S, Konishi H, Takano Y, Shimohigashi Y, Matsumoto H, Ohno M, Kamiya H. Pharmacological properties of the tachykinin receptor subtype in the endothelial cell and vasodilation. Ann N Y Acad Sci. 1991;632:457–459. doi: 10.1111/j.1749-6632.1991.tb33155.x. [DOI] [PubMed] [Google Scholar]
- 20.Wharton J, Gulbenkian S, Mulderry PK, Ghatei MA, McGregor GP, Bloom SR, Polak JM. Capsaicin induces a depletion of calcitonin gene-related peptide (CGRP)-immunoreactive nerves in the cardiovascular system of the guinea pig and rat. J Auton Nerv Syst. 1986;16:289–309. doi: 10.1016/0165-1838(86)90035-4. [DOI] [PubMed] [Google Scholar]
- 21.Furness JB, Costa M, Papka RE, Della NG, Murphy R. Neuropeptides contained in peripheral cardiovascular nerves. Clin Exp Hypertens A. 1984;6:91–106. doi: 10.3109/10641968409062553. [DOI] [PubMed] [Google Scholar]
- 22.Fleming BP, Gibbins IL, Morris JL, Gannon BJ. Noradrenergic and peptidergic innervation of the extrinsic vessels and microcirculation of the rat cremaster muscle. Microvasc Res. 1989;38:255–268. doi: 10.1016/0026-2862(89)90004-6. [DOI] [PubMed] [Google Scholar]
- 23.Newby DE, Sciberras DG, Ferro CJ, Gertz BJ, Sommerville D, Majumdar A, Lowry RC, Webb DJ. Substance P-induced vasodilatation is mediated by the neurokinin type 1 receptor but does not contribute to basal vascular tone in man. Br J Clin Pharmacol. 1999;48:336–344. doi: 10.1046/j.1365-2125.1999.00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keeler JR, Charlton CG, Helke CJ. Cardiovascular effects of spinal cord substance P: studies with a stable receptor agonist. J Pharmacol Exp Ther. 1985;233:755–760. [PubMed] [Google Scholar]
- 25.Krause W, Michael N, Lubke C, Livett BG, Oehme P. Substance P and epibatidine-evoked catecholamine release from fractionated chromaffin cells. Eur J Pharmacol. 1997;328:249–254. doi: 10.1016/s0014-2999(97)83052-x. [DOI] [PubMed] [Google Scholar]
- 26.Reid AC, Brazin JA, Morrey C, Silver RB, Levi R. Targeting cardiac mast cells: pharmacological modulation of the local renin-angiotensin system. Curr Pharm Des. 2011;17:3744–3752. doi: 10.2174/138161211798357908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith D, Gilbert M, Owen WG. Tissue plasminogen activator release in vivo in response to vasoactive agents. Blood. 1985;66:835–839. [PubMed] [Google Scholar]
- 28.Newby DE, Wright RA, Labinjoh C, Ludlam CA, Fox KA, Boon NA, Webb DJ. Endothelial dysfunction, impaired endogenous fibrinolysis, and cigarette smoking: a mechanism for arterial thrombosis and myocardial infarction. Circulation. 1999;99:1411–1415. doi: 10.1161/01.cir.99.11.1411. [DOI] [PubMed] [Google Scholar]
- 29.Labinjoh C, Newby DE, Pellegrini MP, Johnston NR, Boon NA, Webb DJ. Potentiation of bradykinin-induced tissue plasminogen activator release by angiotensin-converting enzyme inhibition. J Am Coll Cardiol. 2001;38:1402–1408. doi: 10.1016/s0735-1097(01)01562-5. [DOI] [PubMed] [Google Scholar]
- 30.Yokosawa H, Endo S, Ohgaki Y, Maeyama J, Ishii S. Hydrolysis of substance P and its analogs by angiotensin-converting enzyme from rat lung. Characterization of endopeptidase activity of the enzyme. J Biochem. 1985;98:1293–1299. doi: 10.1093/oxfordjournals.jbchem.a135396. [DOI] [PubMed] [Google Scholar]
- 31.Wang LH, Ahmad S, Benter IF, Chow A, Mizutani S, Ward PE. Differential processing of substance P and neurokinin A by plasma dipeptidyl(amino)peptidase IV, aminopeptidase M and angiotensin converting enzyme. Peptides. 1991;12:1357–1364. doi: 10.1016/0196-9781(91)90220-j. [DOI] [PubMed] [Google Scholar]
- 32.Izumi H, Karita K. Axon reflex vasodilatation in human skin measured by a laser Doppler technique. Jpn J Physiol. 1991;41:693–702. doi: 10.2170/jjphysiol.41.693. [DOI] [PubMed] [Google Scholar]
- 33.Wong BJ, Tublitz NJ, Minson CT. Neurokinin-1 receptor desensitization to consecutive microdialysis infusions of substance P in human skin. J Physiol. 2005;568:1047–1056. doi: 10.1113/jphysiol.2005.095372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McEwan JR, Benjamin N, Larkin S, Fuller RW, Dollery CT, MacIntyre I. Vasodilatation by calcitonin gene-related peptide and by substance P: a comparison of their effects on resistance and capacitance vessels of human forearms. Circulation. 1988;77:1072–1080. doi: 10.1161/01.cir.77.5.1072. [DOI] [PubMed] [Google Scholar]
- 35.Newby DE, Sciberras DG, Mendel CM, Gertz BJ, Boon NA, Webb DJ. Intra-arterial substance P mediated vasodilatation in the human forearm: pharmacology, reproducibility and tolerability. Br J Clin Pharmacol. 1997;43:493–499. doi: 10.1046/j.1365-2125.1997.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cappugi P, Tsampau D, Lotti T. Substance P provokes cutaneous erythema and edema through a histamine-independent pathway. Int J Dermatol. 1992;31:206–209. doi: 10.1111/j.1365-4362.1992.tb03938.x. [DOI] [PubMed] [Google Scholar]
- 37.Smith CH, Barker JN, Morris RW, MacDonald DM, Lee TH. Neuropeptides induce rapid expression of endothelial cell adhesion molecules and elicit granulocytic infiltration in human skin. J Immunol. 1993;151:3274–3282. [PubMed] [Google Scholar]
- 38.Ogawa S, Ishiki M, Nako K, Okamura M, Senda M, Mori T, Ito S. Sitagliptin, a dipeptidyl peptidase-4 inhibitor, decreases systolic blood pressure in Japanese hypertensive patients with type 2 diabetes. Tohoku J Exp Med. 2011;223:133–135. doi: 10.1620/tjem.223.133. [DOI] [PubMed] [Google Scholar]
- 39.Bosi E, Camisasca RP, Collober C, Rochotte E, Garber AJ. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care. 2007;30:890–895. doi: 10.2337/dc06-1732. [DOI] [PubMed] [Google Scholar]
- 40.Shah Z, Pineda C, Kampfrath T, Maiseyeu A, Ying Z, Racoma I, Deiuliis J, Xu X, Sun Q, Moffatt-Bruce S, Villamena F, Rajagopalan S. Acute DPP-4 inhibition modulates vascular tone through GLP-1 independent pathways. Vascul Pharmacol. 2011;55:2–9. doi: 10.1016/j.vph.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mason RP, Jacob RF, Kubant R, Ciszewski A, Corbalan JJ, Malinski T. Dipeptidyl peptidase-4 inhibition with saxagliptin enhanced nitric oxide release and reduced blood pressure and sICAM-1 levels in hypertensive rats. J Cardiovasc Pharmacol. 2012;60:467–473. doi: 10.1097/FJC.0b013e31826be204. [DOI] [PubMed] [Google Scholar]
- 42.Liu L, Liu J, Wong WT, Tian XY, Lau CW, Wang YX, Xu G, Pu Y, Zhu Z, Xu A, Lam KS, Chen ZY, Ng CF, Yao X, Huang Y. Dipeptidyl peptidase 4 inhibitor sitagliptin protects endothelial function in hypertension through a glucagon-like peptide 1-dependent mechanism. Hypertension. 2012;60:833–841. doi: 10.1161/HYPERTENSIONAHA.112.195115. [DOI] [PubMed] [Google Scholar]
- 43.van Poppel PC, Netea MG, Smits P, Tack CJ. Vildagliptin improves endothelium-dependent vasodilatation in type 2 diabetes. Diabetes Care. 2011;34:2072–2077. doi: 10.2337/dc10-2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ayaori M, Iwakami N, Uto-Kondo H, Sato H, Sasaki M, Komatsu T, Iizuka M, Takiguchi S, Yakushiji E, Nakaya K, Yogo M, Ogura M, Takase B, Murakami T, Ikewaki K. Dipeptidyl peptidase-4 inhibitors attenuate endothelial function as evaluated by flow-mediated vasodilatation in type 2 diabetic patients. J Am Heart Assoc. 2013;2:e003277. doi: 10.1161/JAHA.112.003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.White WB, Cannon CP, Heller SR, Nissen SE, Bergenstal RM, Bakris GL, Perez AT, Fleck PR, Mehta CR, Kupfer S, Wilson C, Cushman WC, Zannad F. Alogliptin after Acute Coronary Syndrome in Patients with Type 2 Diabetes. N Engl J Med. 2013;369:1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 46.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I. Saxagliptin and Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus. N Engl J Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 47.Braunwald E, Chidsey CA, Pool PE, Sonnenblick EH, Ross J, Jr., Mason DT, Spann JF, Covell JW. Congestive heart failure. Biochemical and physiological considerations. Combined clinical staff conference at the National Institutes of Health. Ann Intern Med. 1966;64:904–941. doi: 10.7326/0003-4819-64-4-904. [DOI] [PubMed] [Google Scholar]
- 48.Chidsey CA, Braunwald E. Sympathetic activity and neurotransmitter depletion in congestive heart failure. Pharmacol Rev. 1966;18:685–700. [PubMed] [Google Scholar]
- 49.Bergman AJ, Stevens C, Zhou Y, Yi B, Laethem M, De SM, Snyder K, Hilliard D, Tanaka W, Zeng W, Tanen M, Wang AQ, Chen L, Winchell G, Davies MJ, Ramael S, Wagner JA, Herman GA. Pharmacokinetic and pharmacodynamic properties of multiple oral doses of sitagliptin, a dipeptidyl peptidase-IV inhibitor: a double-blind, randomized, placebo-controlled study in healthy male volunteers. Clin Ther. 2006;28:55–72. doi: 10.1016/j.clinthera.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 50.Wang LH, Zhou SX, Li RC, Zheng LR, Zhu JH, Hu SJ, Sun YL. Serum levels of calcitonin gene-related peptide and substance P are decreased in patients with diabetes mellitus and coronary artery disease. J Int Med Res. 2012;40:134–140. doi: 10.1177/147323001204000114. [DOI] [PubMed] [Google Scholar]
- 51.Fu J, Liu B, Liu P, Liu L, Li G, Wu B, Liu X. Substance P is associated with the development of obesity, chronic inflammation and type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2011;119:177–181. doi: 10.1055/s-0030-1261965. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.