Abstract
Objectives
White matter lesions (WMLs) are associated with depressive symptoms in older adults. However, it is not clear whether different symptom dimensions of depression have distinct associations with WMLs. We assessed the longitudinal relationships of the Center for Epidemiologic Studies Depression Scale (CES-D) total score and subscale scores with WML volume in the Baltimore Longitudinal Study of Aging (BLSA).
Design
Prospective observational design with examination of WML volume and depressive symptoms at 1–2 year intervals for up to 9 years.
Setting
Neuroimaging substudy of the BLSA.
Participants
116 dementia-free participants (mean age = 68.78 ± 7.68).
Measurements
At each visit, depressive symptoms were measured with the CES-D and WML volumes were quantified from structural magnetic resonance imaging scans.
Results
Higher CES-D full scale scores were associated with greater WML volume and with a faster rate of volume increases over time in women, especially at older ages. Higher depressed mood and somatic symptoms subscale scores were associated with greater increases in WML volume over time at older ages. In men, depressed mood and somatic symptoms were associated with larger WML volume at baseline.
Conclusions
Findings confirm an association between WMLs and depressive symptoms and suggest that depressed mood and somatic symptoms may be stronger predictors of depression-related brain changes than lack of well-being. Age and sex may moderate the relationships between depressive symptoms and WMLs. Understanding particular symptom dimensions of depressive symptoms has implications for treatment and may lead to targeted interventions and more precise knowledge of mechanisms underlying depression.
Keywords: depression, WML, symptom dimensions, aging, MRI
Objective
A large body of research has documented brain changes in late-life depression that are greater than would be expected due to age (1–4). For example, older depressed adults show disproportionate volume reduction, altered blood flow at rest, and functional changes in response to cognitive challenge in a network of frontolimbic brain regions. White matter changes are also common in late-life depression and are often observed as white matter hyperintensities (WMH), bright spots observable on T2-weighted magnetic resonance imaging (MRI) scans that are thought to reflect vascular and/or demyelinating brain disease (5,6). Studies using both subjective ratings of WMH severity and quantitative measurement of white matter lesion (WML) volume have documented greater severity of white matter changes in older depressed adults compared to elderly controls (7–13). Moreover, there is evidence that the severity of WMLs is greater in late onset versus early onset geriatric depression (6). Longitudinal studies are limited, but there is some evidence that older depressed patients with greater severity of subjectively rated WMHs have a more severe and persistent course of depression over 5 years than those with less extensive WMHs (14). Furthermore, longitudinal studies using volumetric segmentation of white matter signal abnormalities found that larger WML volume at baseline predicted a greater risk for depression during 4-year follow-up (15) and that WML changes predicted depressive symptom changes over one year, even after controlling for baseline depressive symptoms, quality of life and worsening disability (16).
Additional longitudinal studies are needed to clarify the relationship between WMLs and depressive symptoms over time. In light of recent findings that different symptom dimensions of depression (e.g., affective, somatic and interpersonal symptoms) have distinct associations with cognitive functioning, brain structure and function, and clinical outcomes (17–19), research that investigates the relationship of symptom dimensions of depression with WMLs is also needed. Moreover, correlates of depression, including health outcomes, cognition, quality of life, and cerebral blood flow, differ by sex, (20–22), emphasizing the need to clarify whether relationships between depressive symptoms and WMLs differ in men and women. The current study addressed these issues using data from the Baltimore Longitudinal Study of Aging (BLSA). Previous work in the BLSA showed that symptoms of depressed mood predicted greater severity of WMH ratings over time in men but not in women, while total depressive symptoms did not predict subsequent WMH changes in either sex (23). The present study expanded this research by using quantitative measures of WMLs with more time points and a larger sample, and including addition symptom dimensions of depression.
Based on previous evidence, we hypothesized that overall depressive symptoms would be associated with progression of WMLs over time. Previous work has shown that depressed mood and somatic symptoms have particularly salient associations with cognitive and brain alterations (17, 18, 24). For example, one study showed that the CES-D depressed affect and somatic complaints subscales were associated with cognitive performance, while lack of positive affect and ‘interpersonal difficulties were not (17), and another study reported that psychomotor retardation was associated with decreased cerebral blood flow to frontal regions while other symptoms of depression were not associated with blood flow (24). Therefore, we hypothesized that somatic symptoms and depressed mood would have stronger associations with WMLs compared to symptoms of lack of well-being. Based on evidence that men are more vulnerable to the negative effects of depressive syndromes than women (22, 25, 26), we also hypothesized that the association between depressive symptoms and WMLs would be stronger in men than in women.
Methods
Participants
Participants were drawn from the BLSA neuroimaging substudy (27). This study has been ongoing since 1994 and was designed to investigate associations between age-related cognitive and brain changes. Participants received annual neuroimaging, cognitive, and clinical evaluations, and at the time of data analysis had been followed for up to 9 years. Exclusionary criteria for enrollment into the neuroimaging study included central nervous system disease (epilepsy, stroke, bipolar illness, previous diagnosis of dementia), severe pulmonary disease, severe cardiovascular disease (myocardial infarction, coronary artery disease requiring angioplasty or bypass surgery), and metastatic cancer.
Of the 153 participants enrolled in the neuroimaging substudy at the time of data analysis for whom WML volumes had been quantified, 149 had at least one Center for Epidemiologic Studies Depression Scale (CES-D) measurement. Two participants with severe cerebrovascular disease (e.g., subarachnoid hemorrhage, intracerebral hemorrhage, occlusion and stenosis of cerebral or precerebral arteries, and stroke) at the first neuroimaging visit were excluded. Participants who developed these conditions during the course of the study were included up until the time of diagnosis. Given the evidence that depression may be a prodrome or risk factor for mild cognitive impairment (MCI) and dementia (28,29), we excluded participants diagnosed with Alzheimer’s disease, other dementias, and mild cognitive impairment (MCI) not associated with depression (N = 31) at any point during the study. Diagnoses of dementia and MCI were determined at consensus conferences using Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised criteria for dementia and the National Institute of Neurological and Communication Disorders-Alzheimer’s Disease and Related Disorders Association criteria for Alzheimer’s disease, as described previously (30).
116 participants were included in the final sample. Participants were on average 68.78 years old (SD=7.68) at baseline and were followed for up to nine years (M= 6.02 SD=2.61). Twelve participants reported a history of depression; no other major psychiatric illness was present in the sample based on self report and review of available medical records. Participant demographic information is presented in Table 1. The study was approved by the local Institutional Review Boards and the National Institute on Aging Intramural Research Program, and all subjects gave written informed consent at each visit.
Table 1.
Sample characteristics
| Women (N = 49) | Men (N = 67) | Total (N = 116) | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| Baseline age (years) | 67.50 | 8.26 | 69.35 | 7.09 | 68.78 | 7.68 |
| Number of visits | 5.53 | 1.79 | 5.14 | 2.04 | 5.29 | 1.94 |
| Interval (years) | 6.33 | 2.33 | 5.84 | 2.78 | 6.02 | 2.61 |
| CES-D score | 5.20 | 5.24 | 4.98 | 5.46 | 5.08 | 5.36 |
| Depressed mood subscale | 1.13 | 2.01 | 0.92 | 1.88 | 1.01 | 1.94 |
| Somatic complaints subscale | 2.78 | 2.72 | 2.84 | 2.70 | 2.81 | 2.71 |
| Lack of well-being subscale | 1.15 | 1.97 | 1.06 | 1.76 | 1.09 | 1.85 |
| Interpersonal problems subscale | .15 | .54 | .16 | .57 | .15 | .56 |
| WML volume (mm3) | 1,339.00 | 1,648.43 | 1,264.49 | 2,194.59 | 1,320.27 | 1,970.83 |
Note: The interpersonal problems subscale was not included in the statistical analyses due to the restricted range of scores.
Depressive Symptomatology
At each visit, participants completed the CES-D. The CES-D is a widely used 20-item self-report scale that measures depressive symptom severity and frequency over the past week (31). The CES-D has adequate validity in older community-dwelling adults and is frequently used in longitudinal studies (32). Factor analytic studies have identified four subscales in the CES-D: depressed mood, somatic symptoms, lack of well-being, and interpersonal problems (31). Items in each subscale are summarized in Table 2. CES-D scores range from 0 to 60, with higher scorers indicating greater depressive symptom severity.
Table 2.
CES-D items by subscale
| Depressed Mood |
| I felt that I could not shake off the blues… |
| I felt depressed. |
| I thought my life had been a failure. |
| I felt fearful. |
| I felt lonely. |
| I had crying spells. |
| I felt sad. |
| Somatic Symptoms |
| I was bothered by things that usually don’t bother me. |
| I did not feel like eating… |
| I had trouble keeping my mind on what I was doing. |
| …everything I did was an effort. |
| My sleep was restless. |
| I talked less than usual. |
| I could not get “going.” |
| Lack of Well-being |
| I felt I was just as good as other people. |
| I felt hopeful about the future. |
| I was happy. |
| I enjoyed life. |
| Interpersonal Problems |
| People were unfriendly. |
| I felt that people dislike me. |
Since scores on the CES-D were skewed in our sample, analysis of total depressive symptoms was based on score quartiles (cutoffs = 0–1; 2–3; 4–6; 7+). Due to the restricted range of scores on the subscales, analysis of the depressed mood (0–1 vs. 2+), somatic symptoms (0–4 vs. 5+), and lack of well-being (0–1 vs. 2+) subscales were based on a binary variable comparing the upper quartile of scores to the lower three quartiles. The interpersonal problems subscale was not included in the analyses because of the restricted range of this subscale, which comprises only 2 items (see Table 2).
Image Acquisition and White Matter Lesion Quantitation
MRI scans were performed at each annual visit; acquisition procedures are detailed elsewhere (27). Briefly, a GE Signa 1.5 Tesla scanner was used to acquire oblique axial interleaved spin echo proton density and T2-weighted images (repetition time (TR) = 3000, echo time (TE) = 34/100, field of view (FOV) = 24 cm, matrix = 256×192, number of excitations (NEX) = 0.5, 5-mm slice thickness; oriented parallel to the anterior–posterior intercommissural line), as well as axial T1-weighted three-dimensional spoiled gradient refocused images (TR = 35 ms, TE = 5 ms, flip angle = 45degree, NEX = 1, voxel dimensionsof 0.94 × 0.94 × 1.5 mm slice thickness). A computer-assisted white matter segmentation method, based on local features extracted from T1-weighted, T2-weighted and PD sequences, was used for volumetric assessment of WML using a support vector machine classifier. (FLAIR imaging was added to the protocol subsequently, but was not available during this timeframe, which spanned 1994–2005.) The same scanner was used at each visit. This procedure has been previously detailed (33,34).
Statistical Analysis
We performed a series of mixed-effects regression analyses in SAS using the PROC MIXED procedure to analyze the longitudinal relationship between depressive symptoms and WML volume. This method produces information on the unique effects of each predictor, including fixed and random effects, adjusting for all other terms in the model. Mixed-effects models account for correlations among repeated measurements on the same participant, are unaffected by unequal numbers of visits among participants, and account for differences in the interval between visits. Thus, mixed-effects analyses are the preferred method of examining data with repeated outcome measurements obtained at non-uniform intervals, such as the data used in the present study (35).
We first examined the association of depressive symptoms as a whole with WML volume. CES-D score quartiles, sex, interval, interval2, and baseline age were independent variables in this model, while WML volume was the dependent variable. Baseline age captures cross sectional age differences, while interval represents the number of years since baseline testing and captures longitudinal changes. Interval2 was included to capture nonlinear longitudinal changes. All variables were continuous except sex (women, men) and CES-D score (quartiles 1 through 4). All two- and three-way interactions were included in the model. Independent variables and their interactions were modeled as fixed effects, while intercept and interval were modeled as random effects. A backward elimination procedure was utilized in which all main effects remained in the model while non-significant interaction terms (p > 0.05) were eliminated from the model in stages to reach the final solution (35).
To examine the association of symptom dimensions of depression with longitudinal change in WML, we performed separate mixed models using scores on the depressed mood, somatic symptoms, and lack of well-being subscales (binary variables: upper quartile vs. lower 3 quartiles), rather than the total score, as independent variables. Otherwise the models were identical to that described above.
Results
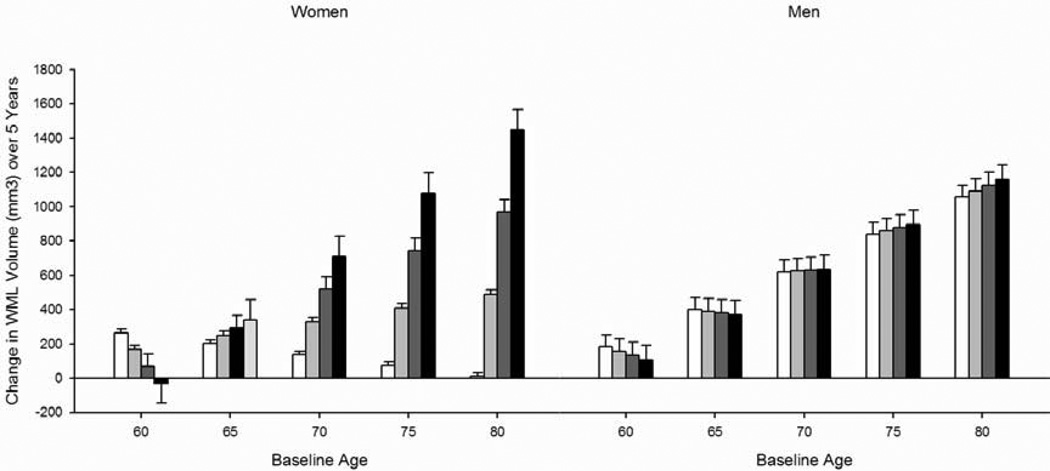
As we hypothesized, both cross-sectional and longitudinal relationships between depressive symptoms and WMLs were observed. Analysis of the total CES-D score revealed a four-way interaction between baseline age, interval, CES-D score, and sex [F(1, 473) = 4.73, p = 0.030] (Figure 1; Table 3). Baseline age is represented as a categorical variable in the figures for ease of display, but analyses were performed with age as a continuous variable. To better understand this four-way interaction, we performed a post hoc analysis stratified by sex. In women, higher CES-D scores were associated with larger WML volumes and a faster rate of increase in WMLs over time [CES-D F(1, 210) = 4.73, p = 0.031; interval × CES-D F(1, 210) = 24.48, p = 0.000], specifically at older baseline ages [baseline age × CES-D F(1, 210) = 4.96, p = 0.027; age × interval × CES-D F(1, 210) = 26.93, p = 0.000]. The total CES-D score was not associated with WML volumes in men.
Figure 1.
Change in white matter lesion volume over 5 years as a function of the Center for Epidemiologic Studies Depression Scale (CES-D) total score quartiles. Bars represent difference scores computed from estimated values at baseline and in year 5. Note: Age groupings are for visual representation; age was analyzed as continuous measures in all statistical analyses.
Table 3.
Regression coefficients in the final mixed effects regression model with the total CES-D score
| df | b | SE | |
|---|---|---|---|
| Baseline age* | 112 | 86.120 | 39.486 |
| Interval | 473 | 1172.250 | 617.770 |
| Interval2* | 473 | −109.060 | 48.438 |
| CES-D* | 473 | 1390.530 | 577.080 |
| Sex | 112 | −135.130 | 3664.420 |
| Baseline age × interval | 473 | −17.913 | 9.133 |
| Baseline age × interval2** | 473 | 1.8761 | 0.708 |
| Baseline age × CES-D* | 473 | −20.886 | 8.270 |
| Baseline age × sex | 112 | 1.733 | 53.101 |
| Interval × CES-D*** | 473 | −472.220 | 140.550 |
| Interval × sex* | 473 | −1947.990 | 818.460 |
| Interval2 × sex* | 473 | 158.330 | 69.896 |
| CES-D × sex | 473 | −1420.010 | 786.700 |
| Baseline age × interval × CES-D*** | 473 | 7.221 | 2.073 |
| Baseline age × interval × sex* | 473 | 30.626 | 12.045 |
| Baseline age × interval2 × sex* | 473 | −2.511 | 1.017 |
| Baseline age × CES-D × sex | 473 | 21.807 | 11.375 |
| Interval × CES-D × sex* | 473 | 419.570 | 201.380 |
| Baseline age × interval × CES-D × sex* | 473 | −6.475 | 2.977 |
Note: CES-D = Center for Epidemiologic Studies Depression Scale. Quartiles of CES-D scores were used in the statistical analyses.
indicates significance at p <.05
indicates significance at p <.01
indicates significance at p <.001
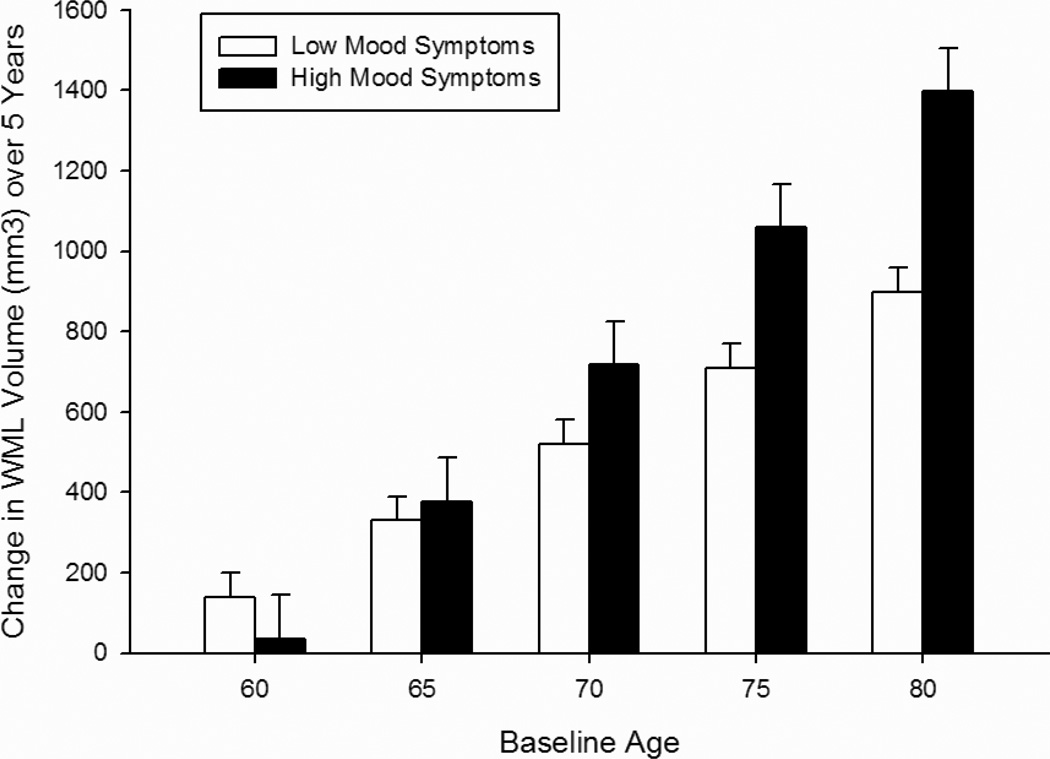
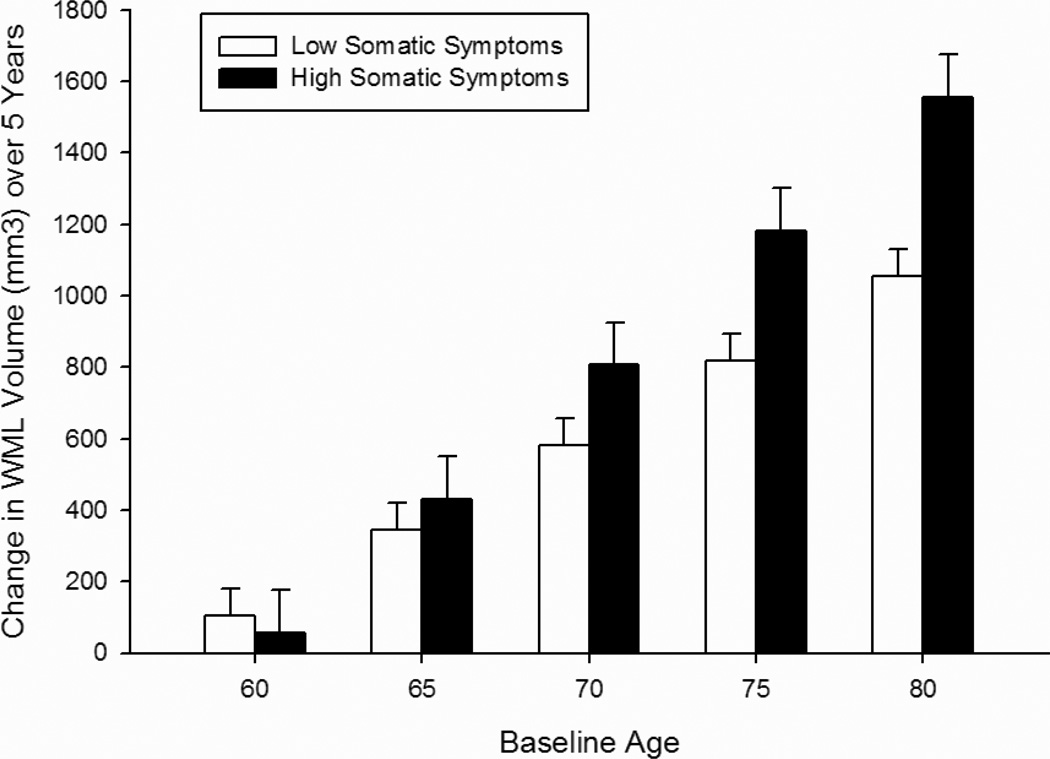
Analysis of the CES-D symptom subscales revealed that higher scores on both the depressed mood (Table 4; Figure 2) and somatic symptoms (Table 5; Figure 3) subscales were associated with a faster rate of increase in WML volume over time at older baseline ages in the total sample [baseline age × interval × mood symptoms F(1, 475) = 3.90, p = 0.049; baseline age × interval × somatic symptoms F(1, 478) = 4.02, p = 0.045]. In women, higher somatic symptoms were associated with a faster rate of increase in WML volume over time independent of baseline age [interval × somatic symptoms × sex F(1, 478) = 7.50, p = 0.006]. In men, WML volume was associated with higher mood and somatic symptoms at baseline [mood symptoms × sex F(1, 475) = 5.28, p = 0.022, baseline age × mood symptoms × sex F(1, 475) = 5.00, p = 0.026; somatic symptoms × sex F(1, 478) = 5.05, p = 0.025, baseline age × somatic symptoms × sex F(1, 478) = 7.14, p = 0.008]. Symptoms of lack of well-being were not associated with WML volumes (Table 6).
Table 4.
Regression coefficients in the final mixed effects regression model with the CES-D depressed mood subscale
| df | B | SE | |
|---|---|---|---|
| Baseline age | 112 | 40.753 | 38.451 |
| Interval | 475 | 411.250 | 571.950 |
| Interval2* | 475 | −95.788 | 48.359 |
| Depressed mood | 475 | 588.000 | 1173.300 |
| Sex* | 112 | −7785.300 | 3579.370 |
| Baseline age × interval | 475 | −6.276 | 8.447 |
| Baseline age × interval2* | 475 | 1.687 | 0.708 |
| Baseline age × depressed mood | 475 | −10.047 | 16.717 |
| Baseline age × sex* | 112 | 114.410 | 51.878 |
| Interval × depressed mood | 475 | −492.830 | 260.760 |
| Interval × sex | 475 | −715.650 | 649.300 |
| Interval2 × sex | 475 | 134.290 | 70.058 |
| Depressed mood × sex* | 475 | 3478.360 | 1514.380 |
| Baseline age × interval × depressed mood* | 475 | 7.533 | 3.813 |
| Baseline age × interval × sex | 475 | 11.702 | 9.441 |
| Baseline age × interval2 × sex* | 475 | −2.178 | 1.021 |
| Baseline age × depressed mood × sex* | 475 | −49.114 | 21.972 |
Note: CES-D = Center for Epidemiologic Studies Depression Scale. Statistical analyses compared the upper quartile of depressed mood subscale scores to the lower three quartiles.
indicates significance at p <.05.
Figure 2.
Change in white matter lesion volume over 5 years as a function of the Depressed Mood Subscale of the Center for Epidemiologic Studies Depression Scale (CES-D). Bars represent difference scores computed from estimated values at baseline and in year 5. Low Mood Symptoms = lower 3 quartiles of scores (0–1); High Mood Symptoms = upper quartile of scores (2+). Note: Age groupings are for visual representation; age was analyzed as continuous measures in all statistical analyses.
Table 5.
Regression coefficients in the final mixed effects regression model with the CES-D somatic symptoms subscale
| df | B | SE | |
|---|---|---|---|
| Baseline age* | 112 | 79.020 | 36.033 |
| Interval | 478 | −382.710 | 360.680 |
| Interval2** | 478 | 9.775 | 3.328 |
| Somatic symptoms** | 478 | 3465.760 | 1072.100 |
| Sex | 112 | −2243.270 | 3184.090 |
| Baseline age × interval | 478 | 5.004 | 5.240 |
| Baseline age × somatic symptoms*** | 478 | −56.385 | 15.673 |
| Baseline age × sex | 112 | 27.485 | 46.046 |
| Interval × somatic symptoms | 478 | −372.670 | 235.720 |
| Interval × sex | 478 | 144.380 | 76.565 |
| Somatic symptoms × sex* | 478 | −2837.440 | 1263.180 |
| Baseline age × interval × somatic symptoms* | 478 | 6.882 | 3.431 |
| Baseline age × somatic symptoms × sex** | 478 | 49.204 | 18.408 |
| Interval × somatic symptoms × sex** | 478 | −132.710 | 48.476 |
Note: CES-D = Center for Epidemiologic Studies Depression Scale. Statistical analyses compared the upper quartile of somatic symptoms subscale scores to the lower three quartiles.
indicates significance at p <.05.
indicates significance at p <.01.
indicates significance at p <.001.
Figure 3.
Change in white matter lesion volume over 5 years as a function of the Somatic Symptoms Subscale of the Center for Epidemiologic Studies Depression Scale (CES-D). Bars represent difference scores computed from estimated values at baseline and in year 5. Low Somatic Symptoms = lower 3 quartiles of scores (0–4); High Somatic Symptoms = upper quartile of scores (5+). Note: Age groupings are for visual representation; age was analyzed as continuous measures in all statistical analyses.
Table 6.
Regression coefficients in the final mixed effects regression model with the CES-D lack of well-being subscale
| df | b | SE | |
|---|---|---|---|
| Baseline age | 112 | 26.710 | 32.477 |
| Interval | 480 | −298.980 | 454.060 |
| Interval2 | 480 | −81.235 | 47.917 |
| Lack of well-being | 480 | 39.961 | 59.401 |
| Sex | 112 | −4097.300 | 3140.110 |
| Baseline age × interval | 480 | 4.723 | 6.632 |
| Baseline age × interval2* | 480 | 1.448 | 0.700 |
| Baseline age × sex | 112 | 62.812 | 45.473 |
| Interval × sex | 480 | −609.850 | 643.940 |
| Interval2 × sex | 480 | 128.980 | 69.084 |
| Baseline age × interval × sex | 480 | 9.913 | 9.353 |
| Baseline age × interval2 × sex* | 480 | −2.066 | 1.005 |
Note: CES-D = Center for Epidemiologic Studies Depression Scale. Statistical analyses compared the upper quartile of lack of well-being subscale scores to the lower three quartiles.
indicates significance at p <.05.
Conclusions
Our results confirm an association between WMLs and depressive symptoms over time in older adults. Consistent with our hypotheses, we found that depressed mood and somatic symptoms are more potent predictors of longitudinal changes in WML volume than are symptoms of lack of well-being. Contrary to our prediction, longitudinal associations between depressive symptoms and WML volumes were not stronger in men than in women, but we did observe cross-sectional relationships of WML volume with depressed mood and somatic symptoms in men, but not women.
A wealth of research has documented a relationship between depression or subthreshold depressive symptoms and WMLs in older adults (7,8,14,15), although there are some exceptions (36). This work has primarily been cross-sectional in nature, although there are longitudinal studies examining the relationship over time for up to 4 years that have shown that depression at baseline predicts longitudinal increases in WMLs (15). Our current finding that depressive symptoms predict longitudinal increases in WML volume over up to 9 years expands upon previous research by including more CES-D and WML measurements, and a quantitative measure of WML volume rather than WMH ratings that are commonly used. These differences may explain the discrepancy between the current findings and previous work in the BLSA, in which total depressive symptoms were not associated with WMH ratings (23).
The current findings also expand upon previous research by examining whether the relationship between depressive symptoms and WML volume differs for distinct symptom dimensions of depression. Depression is a heterogeneous disorder that comprises myriad symptoms, including sad mood, interpersonal problems, somatic complaints, cognitive symptoms, and anhedonia. Emerging research has documented distinct clinical outcomes, cognitive correlates and neural alterations associated with different symptom dimensions (17–19). Indeed, we previously found that only depressed mood symptoms predicted subsequent increases in WMH ratings in older men in the BLSA (23). In the current study, depressed mood and somatic symptoms were associated with increases in WML volume over time. This is consistent with previous research showing that the affective symptoms and somatic subscales of the Beck Depression Inventory were related to demyelinating lesions in the arcuate fasciculus of multiple sclerosis patients, while performance difficulties and cognitive distortions were not (37). While the association of somatic symptoms with WML may be confounded by the prevalence of somatic complaints in individuals with vascular disease, the strong association of depressed mood with WML suggests that depression is a risk factor for WML independent of physical symptoms.
We predicted that men would show stronger associations between depressive symptoms and WML than women. We did find cross-sectional relationships of higher depressed mood and somatic symptoms with larger WML volumes in men, but not women. However, our hypothesis was only partially supported, as the total CES-D score was associated with WML volumes in women, but not in men. Moreover, depressed mood and somatic symptoms were associated with increases in WML volumes over time in both men and women at older ages, but only in women were somatic symptoms associated with increasing WML volume independent of age. This is in contrast to our previous study (23), in which depressed mood predicted subsequent increases in WMH ratings in men, but not in women. The reasons for the discrepancy are unclear but may be related to methodological differences between the two studies. In the previous study, statistical models predicted WMH ratings at one visit from CES-D scores 1–2 years prior, thus capturing the long-term impact of depressive symptoms on white matter changes. In contrast, the current study focused on coincident changes in WML volumes and depressive symptoms over time. Additional research is needed to clarify sex differences in the relationships between white matter changes and depressive symptoms, including examining the possibility of a differential impact of sex on the short-term versus long-term association of depressive symptoms with WML volumes.
Both cross-sectional and longitudinal associations of depressive symptoms with WML in our study were greater at older ages. This is not unexpected considering previous findings that age and depression have an additive or synergistic effect on a number of outcomes, including cognitive functioning, brain structure, and brain function (38). Moreover, age, in the absence of depression, is a risk factor for vascular disease and greater severity of WMLs (39). These findings suggest that identification and treatment of both vascular disease and depression are particularly important at older ages.
It is possible that distinct trajectories of CES-D change over time are present in our sample, and that these longitudinal changes in CES-D would have differential associations with changes in WML volumes over time. Examination of patterns of CES-D change is beyond the scope of the present study. Future work will examine the relationship between WML change over time and trajectories of CES-D symptoms. Recent research has found that lesion location may be more important than volume in determining effects on mood. In particular, lesions in the dorsolateral prefrontal cortex, orbitofrontal cortex, anterior cingulate, uncinate fasciculus and superior longitudinal fasciculus have been associated with depression (36,40). We were not able to examine whether associations with depressive symptoms and WMLs were affected by lesion location since regional WML volumes were not available for the BLSA neuroimaging substudy at the time of data analysis. Future research will examine regional volumes as those data become available. The demographic characteristics of our sample is also a limitation, as the homogenous sample of primarily Caucasian participants may limit generalizability, particularly considering ethnic group differences in vascular disease risk. Nonetheless, in light of the strengths of the study, including the large sample size, long follow-up period, and use of WML volumetric procedures rather than WMH ratings, current findings are informative and contribute to the literature on vascular disease and late-life depressive disorders.
This research has important implications for treatment. Vascular disease and depression appear to have a bidirectional relationship (41). Considering that WML are associated with an increased risk of disability, cognitive impairment, dementia, and mortality, identifying and treating elderly individuals experiencing depressive symptoms is paramount. Our results suggest that depressed mood symptoms are particularly important warning signs for risk for WMLs in older adults, and for men, somatic symptoms are also an important predictor. Effective intervention strategies for these symptoms may slow the progression of white matter disease and subsequent negative outcomes.
In summary, present findings confirm an association between depressive symptoms and WMLs. Moreover, this study expands upon previous findings by demonstrating that different symptom dimensions of depression have distinct associations with WMLs, with depressed mood predicting increased WMLs over time in both men and women and somatic symptoms predicting greater WML volume only in men. These findings have important implications for understanding the bidirectional relationship between depression and vascular disease and for targeting interventions in depressed elders. Future research should examine the causal relationship between symptoms dimensions of depression and WMLs.
Acknowledgements
Supported in part by the Intramural Research Program, National Institute on Aging, National Institutes of Health. The authors wish to thank Yang An, M.S. for assistance with statistical analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors have no potential conflicts of interest to be disclosed
Presented in part at the 41st annual meeting of the Society for Neuroscience, Washington D.C., November 12–16, 2011
References
- 1.Benjamin S, Steffens DC. Structural neuroimaging of geriatric depression. The Psychiatric clinics of North America. 2011;34:423–435. ix. doi: 10.1016/j.psc.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Disabato BM, Sheline YI. Biological basis of late life depression. Current psychiatry reports. 2012;14:273–279. doi: 10.1007/s11920-012-0279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naismith SL, Norrie LM, Mowszowski L, et al. The neurobiology of depression in later-life: clinical, neuropsychological, neuroimaging and pathophysiological features. Progress in neurobiology. 2012;98:99–143. doi: 10.1016/j.pneurobio.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Sexton CE, Mackay CE, Ebmeier KP. A systematic review and meta-analysis of magnetic resonance imaging studies in late-life depression. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2013;21:184–195. doi: 10.1016/j.jagp.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 5.Culang-Reinlieb ME, Johnert LC, Brickman AM, et al. MRI-defined vascular depression: a review of the construct. International journal of geriatric psychiatry. 2011;26:1101–1108. doi: 10.1002/gps.2668. [DOI] [PubMed] [Google Scholar]
- 6.Herrmann LL, Le Masurier M, Ebmeier KP. White matter hyperintensities in late life depression: a systematic review. J Neurol Neurosurg Psychiatry. 2008;79:619–624. doi: 10.1136/jnnp.2007.124651. [DOI] [PubMed] [Google Scholar]
- 7.Lavretsky H, Zheng L, Weiner MW, et al. The MRI brain correlates of depressed mood, anhedonia, apathy, and anergia in older adults with and without cognitive impairment or dementia. Int J Geriatr Psychiatry. 2008;23:1040–1050. doi: 10.1002/gps.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steffens DC, Pieper CF, Bosworth HB, et al. Biological and social predictors of long-term geriatric depression outcome. Int Psychogeriatr. 2005;17:41–56. doi: 10.1017/s1041610205000979. [DOI] [PubMed] [Google Scholar]
- 9.Firbank MJ, Lloyd AJ, Ferrier N, et al. A volumetric study of MRI signal hyperintensities in late-life depression. Am J Geriatr Psychiatry. 2004;12:606–612. doi: 10.1176/appi.ajgp.12.6.606. [DOI] [PubMed] [Google Scholar]
- 10.Gunning-Dixon FM, Hoptman MJ, Lim KO, et al. Macromolecular white matter abnormalities in geriatric depression: a magnetization transfer imaging study. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2008;16:255–262. doi: 10.1097/JGP.0b013e3181602a66. [DOI] [PubMed] [Google Scholar]
- 11.Lamar M, Charlton RA, Morris RG, et al. The impact of subcortical white matter disease on mood in euthymic older adults: a diffusion tensor imaging study. Am J Geriatr Psychiatry. 2010;18:634–642. doi: 10.1097/JGP.0b013e3181cabad1. [DOI] [PubMed] [Google Scholar]
- 12.O'Brien JT, Firbank MJ, Krishnan MS, et al. White matter hyperintensities rather than lacunar infarcts are associated with depressive symptoms in older people: the LADIS study. Am J Geriatr Psychiatry. 2006;14:834–841. doi: 10.1097/01.JGP.0000214558.63358.94. [DOI] [PubMed] [Google Scholar]
- 13.Wendell CR, Hosey MM, Lefkowitz DM, et al. Depressive symptoms are associated with subclinical cerebrovascular disease among healthy older women, not men. Am J Geriatr Psychiatry. 2010;18:940–947. doi: 10.1097/JGP.0b013e3181d57a2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heiden A, Kettenbach J, Fischer P, et al. White matter hyperintensities and chronicity of depression. J Psychiatr Res. 2005;39:285–293. doi: 10.1016/j.jpsychires.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Godin O, Dufouil C, Maillard P, et al. White matter lesions as a predictor of depression in the elderly: the 3C-Dijon study. Biol Psychiatry. 2008;63:663–669. doi: 10.1016/j.biopsych.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Teodorczuk A, O'Brien JT, Firbank MJ, et al. White matter changes and late-life depressive symptoms: longitudinal study. Br J Psychiatry. 2007;191:212–217. doi: 10.1192/bjp.bp.107.036756. [DOI] [PubMed] [Google Scholar]
- 17.Baune BT, Suslow T, Arolt V, et al. The relationship between psychological dimensions of depressive symptoms and cognitive functioning in the elderly - the MEMO-Study. J Psychiatr Res. 2007;41:247–254. doi: 10.1016/j.jpsychires.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Heinzel A, Grimm S, Beck J, et al. Segregated neural representation of psychological and somatic-vegetative symptoms in severe major depression. Neurosci Lett. 2009;456:49–53. doi: 10.1016/j.neulet.2009.03.097. [DOI] [PubMed] [Google Scholar]
- 19.John PDS, Montgomery PR. Do depressive symptoms predict mortality in older people? Aging & Mental Health. 2009;13:674–681. doi: 10.1080/13607860902774493. [DOI] [PubMed] [Google Scholar]
- 20.Beekman AT, Kriegsman DM, Deeg DJ, et al. The association of physical health and depressive symptoms in the older population: age and sex differences. Soc Psychiatry Psychiatr Epidemiol. 1995;30:32–38. doi: 10.1007/BF00784432. [DOI] [PubMed] [Google Scholar]
- 21.Lai CH. Major depressive disorder: gender differences in symptoms, life quality, and sexual function. J Clin Psychopharmacol. 2011;31:39–44. doi: 10.1097/JCP.0b013e318205a670. [DOI] [PubMed] [Google Scholar]
- 22.Dotson VM, Beason-Held L, Kraut MA, et al. Longitudinal study of chronic depressive symptoms and regional cerebral blood flow in older men and women. Int J Geriatr Psychiatry. 2009;24:809–819. doi: 10.1002/gps.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dotson VM, Zonderman AB, Kraut MA, et al. Temporal relationships between depressive symptoms and white matter hyperintensities in older men and women. International Journal of Geriatric Psychiatry. 2012 doi: 10.1002/gps.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Videbech P, Ravnkilde B, Pedersen TH, et al. The Danish PET/depression project: clinical symptoms and cerebral blood flow. A regions-of-interest analysis. Acta Psychiatr Scand. 2002;106:35–44. doi: 10.1034/j.1600-0447.2002.02245.x. [DOI] [PubMed] [Google Scholar]
- 25.Bay E, Sikorskii A, Saint-Arnault D. Sex differences in depressive symptoms and their correlates after mild-to-moderate traumatic brain injury. J Neurosci Nurs. 2009;41:298–309. doi: 10.1097/jnn.0b013e3181b6be81. quiz 310-291. [DOI] [PubMed] [Google Scholar]
- 26.Staley JK, Sanacora G, Tamagnan G, et al. Sex differences in diencephalon serotonin transporter availability in major depression. Biol Psychiatry. 2006;59:40–47. doi: 10.1016/j.biopsych.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Resnick SM, Goldszal AF, Davatzikos C, et al. One-year age changes in MRI brain volumes in older adults. Cereb Cortex. 2000;10:464–472. doi: 10.1093/cercor/10.5.464. [DOI] [PubMed] [Google Scholar]
- 28.Jorm AF. History of depression as a risk factor for dementia: an updated review. Aust N Z J Psychiatry. 2001;35:776–781. doi: 10.1046/j.1440-1614.2001.00967.x. [DOI] [PubMed] [Google Scholar]
- 29.Ownby RL, Crocco E, Acevedo A, et al. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch Gen Psychiatry. 2006;63:530–538. doi: 10.1001/archpsyc.63.5.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawas C, Gray S, Brookmeyer R, et al. Age-specific incidence rates of Alzheimer's disease: the Baltimore Longitudinal Study of Aging. Neurology. 2000;54:2072–2077. doi: 10.1212/wnl.54.11.2072. [DOI] [PubMed] [Google Scholar]
- 31.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 32.Haringsma R, Engels GI, Beekman AT, et al. The criterion validity of the Center for Epidemiological Studies Depression Scale (CES-D) in a sample of self-referred elders with depressive symptomatology. Int J Geriatr Psychiatry. 2004;19:558–563. doi: 10.1002/gps.1130. [DOI] [PubMed] [Google Scholar]
- 33.Zacharaki EI, Kanterakis S, Bryan RN, et al. Measuring brain lesion progression with a supervised tissue classification system. Med Image Comput Comput Assist Interv. 2008;11:620–627. doi: 10.1007/978-3-540-85988-8_74. [DOI] [PubMed] [Google Scholar]
- 34.Lao Z, Shen D, Liu D, et al. Computer-assisted segmentation of white matter lesions in 3D MR images using support vector machine. Acad Radiol. 2008;15:300–313. doi: 10.1016/j.acra.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morrell CH, Pearson JD, Brant LJ. Linear Transformations of Linear Mixed-Effects Models. Am Stat. 1997;51:338–343. [Google Scholar]
- 36.Dalby RB, Chakravarty MM, Ahdidan J, et al. Localization of white-matter lesions and effect of vascular risk factors in late-onset major depression. Psychol Med. 2010;40:1389–1399. doi: 10.1017/S0033291709991656. [DOI] [PubMed] [Google Scholar]
- 37.Pujol J, Bello J, Deus J, et al. Beck Depression Inventory factors related to demyelinating lesions of the left arcuate fasciculus region. Psychiatry Res. 2000;99:151–159. doi: 10.1016/s0925-4927(00)00061-5. [DOI] [PubMed] [Google Scholar]
- 38.Crocco EA, Castro K, Loewenstein DA. How late-life depression affects cognition: neural mechanisms. Curr Psychiatry Rep. 2010;12:34–38. doi: 10.1007/s11920-009-0081-2. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt R, Schmidt H, Haybaeck J, et al. Heterogeneity in age-related white matter changes. Acta Neuropathol. 2011;122:171–185. doi: 10.1007/s00401-011-0851-x. [DOI] [PubMed] [Google Scholar]
- 40.Alexopoulos GS. Frontostriatal and limbic dysfunction in late-life depression. Am J Geriatr Psychiatry. 2002;10:687–695. [PubMed] [Google Scholar]
- 41.Kales HC, Maixner DF, Mellow AM. Cerebrovascular disease and late-life depression. Am J Geriatr Psychiatry. 2005;13:88–98. doi: 10.1176/appi.ajgp.13.2.88. [DOI] [PubMed] [Google Scholar]