Abstract
OBJECTIVES
Recent studies using histology alone in select patients have suggested that Helicobacter pylori-negative gastritis may be common. The objective of this study was to investigate the prevalence of H. pylori among individuals with histologic gastritis.
METHODS
Subjects between 40 and 80 years underwent elective esophagogastroduodenoscopy at a VA Medical Center. Gastric biopsies were mapped from seven prespecified sites (two antrum, four corpus, and one cardia) and graded by two gastrointestinal pathologists, using the Updated Sydney System. H. pylori-negative required four criteria: negative triple staining at all seven gastric sites, negative H. pylori culture, negative IgG H. pylori serology, and no previous treatment for H. pylori. Data regarding tobacco smoking, alcohol drinking, nonsteroidal anti-inflammatory drug, and proton pump inhibitor (PPI) use were obtained by questionnaire.
RESULTS
Of the 491 individuals enrolled, 40.7% (200) had gastritis of at least grade 2 in at least one biopsy site or grade 1 in at least two sites. Forty-one (20.5%) had H. pylori-negative gastritis; most (30 or 73.2%) had chronic gastritis, five (12.2%) had active gastritis, and six (14.6%) had both. H. pylori-negative gastritis was approximately equally distributed in the antrum, corpus, and both antrum and corpus. Past and current PPI use was more frequent in H. pylori-negative vs. H. pylori-positive gastritis (68.2% and 53.8%; P = 0.06).
CONCLUSIONS
We used multiple methods to define non-H. pylori gastritis and found it in 21% of patients with histologic gastritis. While PPI use is a potential risk factor, the cause or implications of this entity are not known.
INTRODUCTION
Gastritis is typically defined based on histologic examination of gastric mucosal biopsies. The discovery that Helicobacter pylori (H. pylori) infection was etiologically associated with peptic ulcer and gastric cancer has resulted in the frequent performance of gastric mucosal biopsies. The evaluating pathologist is expected to comment on whether gastritis was present and, if so, to provide additional details, especially in relation to H. pylori infection, to assist the clinician in patient management.
Worldwide the most common cause of gastritis is H. pylori infection. Treatment of an H. pylori infection results in the rapid disappearance of polymorphonuclear infiltration, followed by a reduction in the chronic inflammatory infiltrate, with gradual normalization of the mucosa (1). However, mucosal atrophy and metaplastic changes resolve slowly if at all (2). Of interest, a recent study of patients in clinical trials with erosive esophagitis reported that between 75 and 90% of H. pylori-negative subjects had gastritis (3). Similarly, 56–69% of H. pylori-negative subjects enrolled in clinical trials with functional dyspepsia or nonerosive gastroesophageal reflux were reported to have gastritis (3,4). In those studies, two biopsy specimens were taken from the gastric antrum and body (i.e., four biopsy samples per subject), and Warthin-Starry silver staining was used to examine for H. pylori infection. Gastric-body biopsies were obtained from the middle of the greater curvature of the gastric body, whereas antral biopsies were obtained from the lesser curvature. The unexpected results of these studies led us to question whether the concept that most stomachs could be characterized as either normal or H. pylori-infected (active or past infection) might be too simple. However, those two studies were relatively limited by their biopsy protocols, the reliance of histology alone (i.e., the lack of adjustment for previous H. pylori treatment or potential confounders such as smoking, alcohol, nonsteroidal anti-inflammatory drug (NSAID), or proton pump inhibitor (PPI) use) (5). To investigate the prevalence and risk factors associated with H. pylori-negative gastritis, we performed gastric mapping to identify gastritis in a large number of patients undergoing endoscopy. We also used histology, culture, serology, and past treatment history to identify H. pylori.
METHODS
Study population and design
We performed a cross-sectional study at the Michael E. DeBakey Veterans Affairs Medical Center in Houston, TX. Between 15 February 2008 and 18 February 2009, we invited all consecutive eligible patients who were scheduled for an elective esophagastroduodenoscopy (EGD) to participate. We also invited a group of randomly selected eligible patients who were scheduled for a primary care clinic visit at the same hospital to undergo an EGD at the same time as their screening colonoscopy.
Recruitment and eligibility criteria
The electronic medical records of all veterans with a scheduled EGD were screened to determine study eligibility, based on the following criteria: (i) age between 40 and 80 years; (ii) no previous esophageal surgery or surgical resection of the esophagus or stomach; (iii) no previous cancer of the esophagus; (iv) no active lung, liver, colon, breast, or stomach cancer; (v) no current use of anticoagulants; (vi) no significant liver disease, indicated by platelet count below 70,000, ascites, or known gastroesophageal varices; and (vii) no history of major stroke or mental condition that would limit ability to answer questions.
Esophagastroduodenoscopy
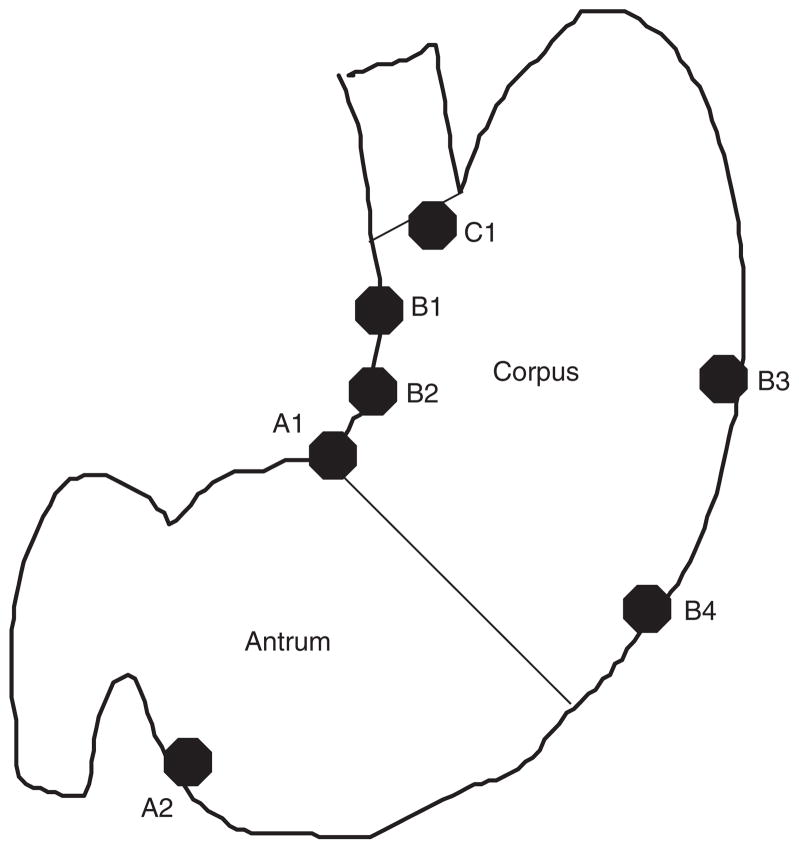
All study participants underwent an EGD with systematic recording of specific findings. Gastric mapping was performed by taking seven mucosal biopsy samples from the antrum, corpus, incisura angularis, lesser curvature, greater curvature, and cardia with biopsies separately identified (Figure 1). This protocol follows the recommendations of the International Workshop on Histopathology of Gastritis (6). We recorded the presence of gastric erosions. Live-case orientation sessions were conducted with the endoscopists involved in the study to demonstrate landmarks and biopsy sites.
Figure 1.
Biopsy sites for gastric mapping used in the study population.
Histopathological examination
All biopsy specimens were embedded in paraffin, oriented on edge, sectioned in 5-μ sections, and stained with: (i) a triple stain consisting of hematoxylin and eosin, alcian blue at pH 2.5; and in case of negative staining for H. pylori (ii) a modified silver stain; and (iii) alcian blue-Periodic acid Schiff stain. Biopsies were examined and graded by 2 gastrointestinal pathologists (M.R. and G.V.), who were blinded to the other H. pylori tests. Features of gastritis, gastric atrophy, and intestinal metaplasia were identified and graded according to the standardized Updated Sydney System (Table 1) (6). Equivocal cases were also examined using immunostaining for H. pylori. In addition, the study pathologist systematically recorded PPI effect defined as jagged protrusion of hyperplasic oxyntic cells into dilated glandular lumens.
Table 1.
| Mucosa phenotype |
| (A=antrum; O=oxyntic; T=transitional) |
| Helicobacter pylori on triple staining |
| 0=Absent |
| 1=Isolated/difficult to find (low-grade) |
| 2=Easy to find (high-grade) |
| 3=Absent, but suspected |
| Neutrophils 0=within the normal range |
| 1=Sparse within lamina propria (LP) no glands abscess (low-grade) |
| 2=Several within LP + glands microabscess (high-grade) |
| Mononuclear cells |
| 0=Within the normal range |
| 1=Sparse within the LP (low-grade) |
| 2=Several within LP with follicles (high-grade) |
| Atrophy |
| (Metaplastic atrophy prevails on the non-metaplastic variant; for assessment) |
| 0=Absent |
| 1=Indefinite |
| 2.1=Atrophy (intestinal metaplasia positive) |
| (2.1.1=Mild; 2.1.2=moderate; 2.1.3=severe) |
| 2.2=Atrophy (intestinal metaplasia negative) |
| (2.2.1=Mild; 2.2.2=moderate; 2.2.3=severe) |
Serology
Serum samples from all study subjects were evaluated for IgG antibody to the high-molecular-weight, cell-associated proteins of H. pylori, using an enzyme-linked immunosorbent assay ELISA (HM-CAP, EPI, Westbury, NY). The test was scored positive when the optical density was >2. This test has a reported sensitivity of >97% and a specificity of >94% for adults (7).
Cultures
Biopsy specimens from all study subjects were placed in sterile vials containing cysteine medium with 20% glycerol and stored at −80°C until cultured. The frozen specimens were then thawed and the tissue was homogenized and inoculated onto two types of selective media; Brain Heart Infusion and H. pylori Special Peptone Agar plates with 7% horse blood. The plates were incubated at 37°C under microaerophilic conditions. The negative plates were reincubated and then read every 24h up to 14 days. Positive growth was transferred to a fresh, nonselective Brain Heart Infusion blood agar plate and then incubated for 48–72h. H. pylori was identified definitively when the oxidase, catalase, and urease reactions were positive with a compatible Gram-negative stain.
Survey questionnaire
All study participants completed a computer-assisted survey before the EGD that included questions on demographic features, use of several medications, previous treatment for H. pylori, tobacco-smoking status, and alcohol drinking. For this analysis, past and current PPI use was based on self-reported use of any of the following medications: omeprazole, lansoprazole, pantoprazole, rabeprazole, or esomeprazole. Histamine-2 receptor antagonist use was based on self-reported use of any of the following medications: cimetidine, ranitidine, nizatidine, or famotidine. Aspirin or NSAID use was defined based on self-reported current use of any of 31 named medications. Given some features of gastritis persist for some time after eradicating H. pylori, previous H. pylori treatment was ascertained to avoid misclassifying H. pylori-negative gastritis Alcohol drinking was defined based on answers to 28 questions about current and past drinking and was operationalized as the patient’s being a nondrinker, current drinker, or past drinker if the patient had quit drinking >1 year earlier. Smoking status was defined as being a current smoker by smoking at least 100 cigarettes, cigars, or pipes in a lifetime and being a current smoker on the date of the interview; being a nonsmoker, with a lifetime history of smoking < 100 cigarettes, cigars or pipes; or being a past smoker, who had quit smoking >1 year ago.
For cases negative for H. pylori (staining, culture, serology), the slides were re-examined for features of sarcoidosis, eosinophilic gastritis, lymphocytic gastritis, and autoimmune gastritis. We also examined the medical records and self-reported history of inflammatory bowel disease.
Analysis
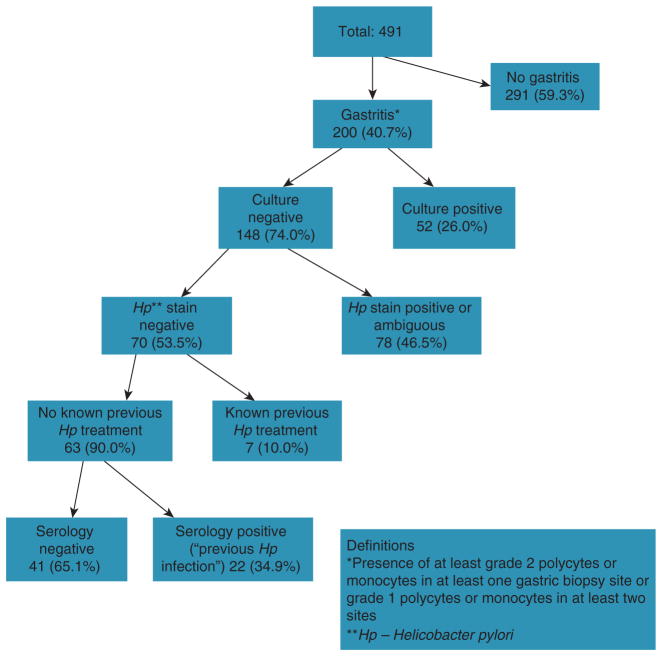
We defined gastritis by the presence of at least grade 2 neutrophils or mononuclear cells in at least one gastric biopsy site, or grade 1 neutrophils or mononuclear cells in at least two sites (Table 1; 8,9). In a sensitivity analysis, gastritis was defined as at least grade 2 neutrophils or mononuclear cells in at least two biopsy sites. To be considered H. pylori-negative, an individual had to fulfill all four of the following criteria: (i) a negative triple staining of gastric mucosa in all seven sites (hematoxylin and eosin, alcian blue at pH 2.5, and a modified silver stain); (ii) a negative H. pylori culture; (iii) a negative IgG H. pylori serology (HM-CAP ELISA); and (iv) no self-reported previous H. pylori treatment (Figure 2).
Figure 2.
Flowchart showing the presence, distribution, and causes of gastritis in the study population. Histopathology, serology, and culture were applied to all study subjects.
We compared the demographic and clinical features for patients with and without gastritis, and among those with gastritis we compared those with and without H. pylori infection. For the latter group, we also examined the proportions with antrum or corpus atrophy or intestinal metaplasia as well as overall atrophy (OLGA staging). χ2 tests were used for categorical variables and t-tests for continuous variables. We also conducted an adjusted analysis using multivariable logistic regression to examine the possible predictors of H. pylori-negative gastritis. To avoid overfitting the model, we adjusted each predictor of interest for age and race only. SAS (version 9.2, SAS Institute, Cary, NC, USA) was used to conduct the analyses.
The study was approved by the Institutional Review Board at Baylor College of Medicine and by Research and Development at the Michael E. DeBakey Veterans Affairs Medical Center, both in Houston, Texas.
RESULTS
The participation rates among patients who appeared in person to their endoscopy or clinic appointments were 78 and 50%, respectively. In the 12-month period described in the Methods, we included the first 491 participants who gave informed consent, completed the survey, and underwent endoscopy in this study (Figure 2); 458 (93.3%) were from those referred to endoscopy and 33 (7.7%) from patients recruited from primary care for colonoscopy and EGD. There were no significant differences in age, sex, or race distribution based on the source of enrollment. Of these study subjects, 200 (40.7%) were found to have histological gastritis, defined as at least grade 1 in at least two gastric sites (n=189) or at least grade 2 in at least one gastric site (n=11). The group of 159 subjects with H. pylori-positive gastritis were identified based on positive culture (n=52), positive triple staining (44 definite and 34 possible), positive serology (n=22), and previous self-reported H. pylori treatment (n=7). The remaining 41 individuals (20.5% of all subjects with gastritis and 8.4% of the total study population) had gastritis on histological examination but were negative for active or past H. pylori infection.
In general, subjects with any gastritis were more likely to be older, male, and Black; but only the higher proportion of Blacks was statistically significant compared with those without gastritis (Table 2). Subjects with H. pylori-positive gastritis were also slightly more likely to be Black (P=0.08) than subjects with H. pylori-negative gastritis.
Table 2.
Demographic features of 491 individuals with or without gastritis in the study population
| Gastritis | Non-gastritis | Total | |||
|---|---|---|---|---|---|
| Helicobacter pylori negativea | Helicobacter pylori positiveb | Total | |||
| Number of individuals | 41 | 159 | 200 | 291 | 491 |
| Endoscopy source | 38 (92.7%) | 148 (93.1%) | 186 (93.0%) | 272 (93.5%) | 458 (93.3%) |
| Men | 41 (100%) | 146 (91.8%) | 187 (93.5%) | 267 (91.8%) | 454 (92.5%) |
| Mean age (s.d.) | 62.8 (8.1) | 61.1 (7.3) | 61.5 (7.6) | 60.8 (7.8) | 61.1 (7.7) |
| Blacksc | 11 (26.8%) | 71 (44.7%) | 82 (41.0%) | 42 (14.4%) | 124 (25.3%) |
| Whites | 29 (70.7%) | 81 (50.9%) | 110 (55.0%) | 232 (79.7%) | 342 (69.7%) |
| Other | 1 (2.4%) | 7 (4.4%) | 8 (4.0%) | 17 (5.8%) | 25 (5.0%) |
| Mean body mass index (s.d.) | 29.2 (5.3) | 29.8 (5.7) | 29.7 (5.6) | 29.9 (6.2) | 29.8 (6.0) |
| Gastric erosions | 4 (9.8%) | 36 (22.6%) | 40 (13.8%) | 40 (13.8%) | 80 (16.3%) |
Negative on triple staining, culture, serology, and past treatment.
Positive on at least one of triple staining, culture, or serology.
P < 0.0001 between gastritis and nongastritis, and 0.08 between H. pylori-negative and -positive gastritis.
Most of the gastritis in the H. pylori-negative group was chronic, whereas both acute and chronic were present in most cases in the H. pylori-positive group (Table 3). In the H. pylori-negative group, gastritis was also more likely to be present in isolated portions of the stomach than in the H. pylori-positive group. In the non-H. pylori group, acute or chronic gastritis was present in the antrum only in 15 (37%), in the corpus only in 13 (32%) and in both the antrum and corpus in 13 (32%), with none in the cardia alone (data not shown). This was different from subjects with H. pylori-positive acute or chronic gastritis, 70% of whom had both antrum and corpus distribution (n=111). There were also no significant differences in the proportions of patients with atrophy or intestinal metaplasia between patients with H. pylori-negative or -positive gastritis (Table 3). Although 17, 41.5% of patients with H. pylori-negative gastritis had atrophy recorded in antral or body biopsies, the overall atrophy as indicated by OLGA score was mild in most cases (15 of 19 had grade 1 or 2).
Table 3.
Type and distribution of gastritis in Helicobacter pylori-negative individuals
| Type/place of gastritis | Helicobacter pylori negative (n=41) % | Helicobacter pylori positive (n=159) % | P value |
|---|---|---|---|
| Acute antral | 8 (19.5) | 98 (61.6) | < 0.0001 |
| Chronic antral | 22 (53.7) | 130 (81.8) | 0.0002 |
| Acute body | 3 (7.3) | 102 (64.2) | < 0.0001 |
| Chronic body | 25 (61.0) | 125 (78.6) | 0.02 |
| Atrophy | 15 (36.6) | 55 (34.6) | 0.94 |
| Atrophy antral or body | 19 (46.3%) | 71 (44.6%) | 0.70 |
| Intestinal metaplasia antral or body | 17 (41.5%) | 63 (39.6%) | 0.83 |
| PPI effect | 22 (53.7%) | 86 (54.1%) | 0.96 |
PPI, proton pump inhibitor.
Patients with H. pylori-negative gastritis were more likely to have a history of past alcohol drinking and smoking rather than current drinking or smoking than patients with H. pylori-positive gastritis (Table 4). Both past and current PPI use was more commonly reported in the H. pylori-negative gastritis group, but these differences did not reach statistical significance (Table 4). Histological changes in the gastric mucosa suggestive of PPI use were recorded in 53.7 and 54.1% of H. pylori -negative and -positive gastritis, respectively. In addition, there were no significant differences in the proportions of subjects with self-reported histamine 2 receptor antagonists or aspirin/NSAID between the two gastritis groups.
Table 4.
Possible risk factors for non-H. pylori gastritis
| Helicobacter pylori negative, n=41 (%) | Helicobacter pylori positive, n=159 (%) | P value | |
|---|---|---|---|
| Alcohol | 0.05 | ||
| Current | 13 (31.7) | 81 (50.9) | |
| Past | 24 (58.5) | 58 (36.5) | |
| None | 4 (9.8) | 12 (7.5) | |
| Missing | 0 | 8 (5.0) | |
| Tobacco smoking | 0.04 | ||
| Current | 5 (12.2) | 51 (32.1) | |
| Past | 24 (58.5) | 57 (35.8) | |
| None | 8 (19.5) | 41 (25.8) | |
| Missing | 4 (9.8) | 10 (6.3) | |
| PPI | 0.14 | ||
| Current | 22 (53.7) | 73 (45.9) | |
| Past | 6 (14.6) | 12 (7.5) | |
| Current or past | 28 (68.2) | 85 (53.5) | |
| None | 8 (19.5) | 61 (38.3) | |
| Missing | 5 (12.2) | 13 (8.2) | |
| Histamine 2 receptor antagonists | |||
| Current or past | 7 (17.1) | 23 (14.5) | 0.75 |
| ASA/NSAIDs | |||
| Current use | 14 (34.1) | 65 (40.9) | 0.32 |
ASA, aspirin; NSAIDs, nonsteroidal anti-inflammatory drugs; PPI, proton pump inhibitor.
Results of unadjusted χ2 analyses.
The medical records of the 41 individuals with H. pylori-negative gastritis were manually reviewed in detail by one investigator (D.Y.G.) to check for previous H. pylori diagnosis or treatment. Only one subject was found to have had previous H. pylori treatment that was not captured by the survey. One patient had lymphocytic gastritis, and another one had autoimmune gastritis.
In the age- and race-adjusted analyses conducted among the 200 patients with any gastritis, none of the lifestyle factors (alcohol or smoking) or medication use (PPI, histamine 2 receptor antagonists, or NSAID) were significantly associated with H. pylori-negative gastritis (Table 5). The risk estimates derived from the model were, however, in the same direction as those reported in the unadjusted analyses (Table 4).
Table 5.
Predictors of Helicobacter pylori-negative gastritis (41 cases among 200 with gastritis) examined with multivariate logistic-regression models
| Parameter estimate (standard error) | Odds ratio (95% confidence intervals) | |
|---|---|---|
| Current PPI | 0.52 (0.39) | 1.68 (0.78–3.66) |
| Past PPI | 0.86 (0.59) | 2.37 (0.74–7.57) |
| Current or past PPI | 0.64 (0.38) | 1.90 (0.90–3.99) |
| Current drinking | −0.89 (0.67) | 0.41 (0.11–1.52) |
| Former drinking | 0.29 (0.65) | 1.34 (0.38–4.77) |
| Current or former drinking | −0.28 (0.62) | 0.76 (0.22–2.54) |
| Current smoking | −0.22 (0.57) | 0.80 (0.26–2.44) |
| Past smoking | 0.72 (0.48) | 2.06 (0.80–5.31) |
| Current or past smoking | 0.37 (0.45) | 1.45 (0.60–3.52) |
PPI, proton pump inhibitor.
The estimates for each variable are derived from a separate model logistic regression adjusting for age and race only.
Lastly, the definition of gastritis used was robust in the sensitivity analysis. The number of subjects with gastritis changed very little with a definition of at least grade 2 neutrophils or mononuclear cells in at least two biopsy sites (n=192, all of whom were part of the 200 considered in the main analyses).
DISCUSSION
In this population, we found that H. pylori -negative gastritis, as confirmed by three different diagnostic methods and without previous H. pylori treatment, was relatively infrequent (8.4%). However, H. pylori-negative gastritis was found in about 21% of our patients with gastritis on histopathologic examination. Although differences were not statistically significant, patients with H. pylori-negative gastritis were more likely to use PPIs and to be past alcohol drinkers and tobacco smokers (rather than current ones) compared with patients with H. pylori-positive gastritis.
Previous studies on the prevalence of gastritis in H. pylori-negative individuals have shown that as many as 75–90% had evidence of gastritis (3,4). However, the patient population in these studies was part of clinical trials of PPIs for upper gastrointestinal diagnoses (erosive esophagitis, nonerosive reflux, and functional dyspepsia). The demographic composition was 33% women and 90% white (as compared with 7% women and 70% white in our study). Although the authors of one of these study report that the H. pylori-negative status remain unchanged in almost 7 years of follow-up 3, some patients who were characterized as H. pylori-negative in these studies might actually have been positive. First, only four biopsies were taken (two from the antrum and two from the corpus), whereas the new Sydney System requires at least five specimens, as well as a visual analog scale to compare and score the finding. Second, histopathologic review was done by only one pathologist. Finally, H. pylori diagnosis was made only on the basis of Warthin-Starry silver staining, potentially misclassifying H. pylori-positive individuals as H. pylori negative; and past history of H. pylori treatment was not accounted for. These biases were likely to underestimate H. pylori infection. Our definition of H. pylori negative was meant to be stringent and highly specific by using multiple methods of ascertainment. We acknowledge that in routine clinical practice where one method of diagnosis is usually used, the prevalence of H. pylori-negative gastritis would be higher.
Reasons for H. pylori-negative gastritis in our study are unknown but may include the following:
Previous H. pylori infection that was treated but not recollected by study participants or not captured by the VA medical records due to therapy being obtained at a non-VA facility. However, IgG serology used in this study has been shown to remain positive a long time after H. pylori eradication. In one study, 3.5 years after successful H. pylori treatment, 72% remained seropositive according to ELISA, and 62% remained positive according to rapid serum immunoassay (10). Therefore, previous infection was likely to have been detected on ELISA.
PPI use. In the study population, 68% of those with H. pylori-negative gastritis either were previous or current users of PPI, and 54% had histologic features of PPI use. Animal studies have suggested that long-term PPI use can lead to gastric inflammation, possibly through microbial overgrowth (11). Furthermore, in previous studies of H. pylori-negative gastritis, long-term lansoprazole treatment was associated with reductions in acute and chronic inflammation of the gastric body and antrum (3). It is also possible that PPI is a marker of GERD (gastroesophageal reflux disease), which is less likely to be present in Hp-negative subjects. This may also explain why Hp-gastritis was more common in the prior referenced studies that were enriched with GERD subjects.
Autoimmune gastritis, associated with serum antiparietal and anti-intrinsic factor antibodies and the cause of pernicious anemia, is characterized by a chronic atrophic gastritis limited to the mucosa of the corpus and fundus and by a marked diffuse atrophy of parietal and chief cells. While gastric atrophy was present in 36.6% of subjects with H. pylori-negative gastritis, the gastritis was limited to the corpus in only one-third of these subjects. Further detailed examination confirmed only one case of autoimmune gastritis. We did not measure anti-parietal antibodies in these patients.
Infection by organisms other than H. pylori (e.g., Mycobacterium avium-intracellulare, Herpes simplex, and Cytomegalovirus are a few examples of organisms that can invade the gastric mucosa and cause inflammation). However, these infections are extremely rare in patients who are not immunocompromised. We have not specifically evaluated for other infective causes of gastritis.
Chemical or reactive gastritis, often caused by bile reflux or NSAID use. Almost half of the 41 subjects with H. pylori-negative gastritis were either past or current NSAID users. However, NSAID intake usually causes lesions with only minimal inflammation and should be easily distinguishable on histopathology.
Causes such as collagenous gastritis, Crohn’s disease-associated gastritis, sarcoidosis, eosinophilic gastritis, lymphocytic gastritis are all rare and relatively easy to distinguish on histopathologic examination.
The clinical relevance and prognosis of H. pylori-negative gastritis is unknown and need to be further examined given the relatively high prevalence of this entity. For example, it is unclear if the risk of ulcer of cancer is increased, or if it predisposes to upper gastrointestinal symptoms. However, it is at least clear that in clinical practice, empirical treatment for H. pylori is not warranted in patients with gastritis without clear evidence for the presence of this infection.
This study has some limitations, including the characteristics of the study base population, mostly men with low socioeconomic status and high prevalence of H. pylori infection, PPI use and NSAID use, which could decrease the external validity of the study to nonveteran men or women. The small sample size may have resulted in lack of power to detect the differences in possible risk factors between H. pylori-positive and -negative groups. However, the internal validity is strengthened by the use of three different methods to diagnose H. pylori infection and the additional medical record review to confirm H. pylori-negative status, thus reducing the risk of misclassification of H. pylori status. Using self-reported past H. pylori treatment to define H. pylori as a cause of the gastritis may have misclassified few cases with H. pylori-negative gastritis that arose subsequent to H. pylori eradication. Furthermore, strict histopathological criteria were used for diagnosis; and all cases were reviewed by two gastrointestinal pathologists.
In conclusion, H. pylori-negative gastritis was present in ~21% of gastritides. Very few cases had an alternative known cause (autoimmune in only one person). Non-H. pylori gastritis was typically mild and more focal than H. pylori gastritis and tended to be chronic rather than chronic-active or active. PPI use is a potential risk factor for H. pylori-negative gastritis, but the causes and implications of this entity are unknown and are worthy of future studies.
Study Highlights.
WHAT IS CURRENT KNOWLEDGE?
Helicobacter pylori is often assumed to cause most or all of gastritis encountered in clinical practice.
Two studies that used suboptimal methodology for H. pylori detection reported extremely high rates of H. pylori-negative gastritis among patients with GERD (gastroesophageal reflux disease) and dyspepsia.
Neither the true prevalence nor the risk factors for H. pylori-negative gastritis is known.
This information is important both for clinical practice and for research of other gastritis etiological factors.
WHAT IS NEW HERE?
Using multiple concomitant methods (serology, histology of gastric mapping biopsies, culture, and ascertainment of past treatment), we used a highly stringent method of excluding H. pylori infection.
Approximately 21% of 200 patients with histological gastritis were H. pylori-negative.
There needs to be studies of etiological factors for gastritis.
Acknowledgments
The views expressed reflect those of the authors and not necessarily those of the National Cancer Institute, the National Institute of Diabetes and Digestive and Kidney Diseases, the Department of Veterans Affairs and/or Baylor College of Medicine. The NIDDK had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Financial support: This work was partly supported by the VA HSR&D Houston Center of Excellence (HFP90-020), Grant support: NCI R01 116845 and NIDDK K24-04-107.
Footnotes
Guarantor of the article: Hashem B. El-Serag, MD, MPH.
Specific author contributions: Funding, conception, design, analysis, interpretation of the results, manuscript writing, editing, and decision to publish: Hashem B. El-Serag; conception, data collection, editing manuscript, and decision to publish: Helena Nordensted; analysis, editing manuscript, and decision to publish: David Y. Graham, Massimo Rugge, Yasser Shaib, Maria E. Velez, Neena Abraham, and Bhupinderjit Anand; data collection, editing manuscript, and decision to publish: Jennifer R. Kramer, Stephanie Fitzgerald, and Abeer Alsarraj; analysis, editing manuscript, and decision to publish: Gordana Verstovsek; analysis, data collection, editing manuscript, and decision to publish: Rhonda Cole.
Potential competing interests: None.
References
- 1.Di Napoli A, Petrino R, Boero M, et al. Quantitative assessment of histological changes in chronic gastritis after eradication of Helicobacter pylori. J Clin Pathol. 1992;45:796–8. doi: 10.1136/jcp.45.9.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung JJ, Lin SR, Ching JY, et al. Atrophy and intestinal metaplasia one year after cure of H. pylori infection: a prospective, randomized study. Gastroenterol. 2000;119:7–14. doi: 10.1053/gast.2000.8550. [DOI] [PubMed] [Google Scholar]
- 3.Haber MM, Hunt B, Freston JW, et al. Changes of gastric histology in patients with erosive oesophagitis receiving long-term lansoprazole maintenance therapy. Aliment Pharmacol Ther. 2010;32:83–96. doi: 10.1111/j.1365-2036.2010.04310.x. [DOI] [PubMed] [Google Scholar]
- 4.Peura DA, Haber MM, Hunt B, et al. Helicobacter pylori-negative gastritis in erosive esophagitis, nonerosive reflux disease or functional dyspepsia patients. J Clin Gastroenterol. 2010;44:180–5. doi: 10.1097/MCG.0b013e3181ac9830. [DOI] [PubMed] [Google Scholar]
- 5.Merchant SH, VanderJagt T, Lathrop S, et al. Sporadic duodenal bulb gastrin-cell tumors: association with Helicobacter pylori gastritis and long-term use of proton pump inhibitors. Am J Surg Pathol. 2006;30:1581–7. doi: 10.1097/01.pas.0000213326.86992.98. [DOI] [PubMed] [Google Scholar]
- 6.Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney System. Am J Surg Pathol; International Workshop on the Histopathology of Gastritis; Houston. 1994; 1996. pp. 1161–81. [DOI] [PubMed] [Google Scholar]
- 7.van Der Ende A, van Der Hulst RW, Roorda P, et al. Evaluation of three commercial serological tests with different methodologies to assess Helicobacter pylori infection. J Clin Microbiol. 1999;37:4150–2. doi: 10.1128/jcm.37.12.4150-4152.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rugge M, de Boni M, Pennelli G, et al. Gastritis OLGA-staging and gastric cancer risk: a twelve-year clinico-pathological follow-up study. Aliment Pharmacol Ther. 2010;31:1104–11. doi: 10.1111/j.1365-2036.2010.04277.x. [DOI] [PubMed] [Google Scholar]
- 9.Rugge M, Correa P, Di Mario F, et al. OLGA staging for gastritis: a tutorial. Dig Liver Dis. 2008;40:650–8. doi: 10.1016/j.dld.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 10.Cutler AF, Prasad VM, Santogade P. Four-year trends in Helicobacter pylori IgG serology following successful eradication. Am J Med. 1998;105:18–20. doi: 10.1016/s0002-9343(98)00134-x. [DOI] [PubMed] [Google Scholar]
- 11.Zavros Y, Rieder G, Ferguson A, et al. Genetic or chemical hypochlorhydria is associated with inflammation that modulates parietal and G-cell populations in mice. Gastroenterol. 2002;122:119–33. doi: 10.1053/gast.2002.30298. [DOI] [PubMed] [Google Scholar]