Abstract
Developmental coordination disorder (DCD) and attention deficit/hyperactivity disorder (ADHD) are prevalent childhood disorders that frequently co-occur. Evidence from neuroimaging research suggests that children with these disorders exhibit disruptions in motor circuitry, which could account for the high rate of co-occurrence. The primary objective of this study was to investigate the functional connections of the motor network in children with DCD and/or ADHD compared to typically developing controls, with the aim of identifying common neurophysiological substrates. Resting-state fMRI was performed on seven children with DCD, 21 with ADHD, 18 with DCD + ADHD and 23 controls. Resting-state connectivity of the primary motor cortex was compared between each group and controls, using age as a co-factor. Relative to controls, children with DCD and/or ADHD exhibited similar reductions in functional connectivity between the primary motor cortex and the bilateral inferior frontal gyri, right supramarginal gyrus, angular gyri, insular cortices, amygdala, putamen, and pallidum. In addition, children with DCD and/or ADHD exhibited different age-related patterns of connectivity, compared to controls. These findings suggest that children with DCD and/or ADHD exhibit disruptions in motor circuitry, which may contribute to problems with motor functioning and attention. Our results support the existence of common neurophysiological substrates underlying both motor and attention problems.
Keywords: Functional connectivity; Resting state fMRI, Motor networks; Developmental coordination disorder; Attention-deficit/hyperactivity disorder
Abbreviations: ADHD, attention deficit/hyperactivity disorder; DCD, developmental coordination disorder; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders (4th edition); DTI, diffusion tensor imaging; FC, functional connectivity; GLM general, linear model; fMRI, functional magnetic resonance imaging; ICA, independent component analysis; M1, primary motor cortex; PFC, prefrontal cortex; rs-fMRI, resting-state fMRI
Highlights
-
•
Functional connectivity of motor networks was examined in DCD and ADHD.
-
•
Disruptions in functional connectivity were found in children with DCD and/or ADHD
-
•
Co-occurring DCD and ADHD was associated with unique alterations in functional connectivity
-
•
Common neurophysiological substrates may underlie motor and attention problems
1. Introduction
Developmental coordination disorder (DCD) occurs in approximately 5–6% of children and is associated with impairments in fine and gross motor functions (American Psychiatric Association, 2000). Attention-deficit/hyperactivity disorder (ADHD), which affects approximately 5% of children, is characterized by age-inappropriate levels of inattention, hyperactivity and/or impulsivity (American Psychiatric Association, 2000). DCD and ADHD have been found to co-occur in up to 50% of affected children (Kadesjo and Gillberg, 1998; Pitcher et al., 2003) and have both been associated with neuropsychological deficits, academic difficulties and behavior problems that can lead to long-term issues in social and mental health (Able et al., 2007; Lingam et al., 2012). Early identification and intervention are therefore critical to improve outcomes.
Imaging studies suggest that disruptions in brain motor circuitry are associated with both ADHD and DCD. In children with ADHD, meta-analyses have consistently reported reduced volumes in the right hemisphere (Valera et al., 2007), including the right prefrontal cortex (PFC) (Valera et al., 2007), frontal white matter (Valera et al., 2007), right putamen/pallidus (Ellison-Wright et al., 2008; Frodl and Skokauskas, 2012; Nakao et al., 2011) and caudate (Frodl and Skokauskas, 2012; Nakao et al., 2011). Consistent with these findings, diffusion tensor imaging (DTI) studies of children with ADHD have reported reduced white matter integrity in the corticospinal tract (Hamilton et al., 2008) and in regions between the basal ganglia and PFC (de Zeeuw et al., 2012; Langevin et al., 2014). Functional magnetic resonance imaging (fMRI) studies have also implicated motor regions in children with ADHD (Booth et al., 2005; Durston et al., 2003; Durston et al., 2006; Mostofsky et al., 2006; Suskauer et al., 2008). Limited imaging research has been conducted on children with DCD; however, recent DTI studies have reported reduced white matter integrity within the corticospinal tract (Zwicker et al., 2012) and superior/posterior parietal regions of the corpus callosum and the left superior longitudinal fasciculus (Langevin et al., 2014). Functional MRI studies have implicated motor regions immediately overlying the corticospinal tract (Kashiwagi et al., 2009; Querne et al., 2008; Zwicker et al. 2010, Zwicker et al. 2011) and one fMRI study investigating functional connectivity (FC) (i.e., temporal synchrony between brain regions, which is an indicator of functional connection strength) during a Go/No Go task reported that children with DCD exhibit increased connectivity between the left middle frontal and inferior parietal cortices and reduced connectivity between the right striatum and parietal cortex (Querne et al., 2008). These findings suggest that the functional connections between the striatum and the parietal cortex, areas that integrate sensory information in motor responses, are altered in children with DCD.
Functional connectivity can also been examined when participants are not performing an explicit task during imaging, referred to as resting-state fMRI (rs-fMRI). Studies using rs-fMRI in children with ADHD have observed FC differences within frontostriatal circuits, attention circuits, and the default mode network (Cao et al., 2006; Cao et al., 2009; Tian et al., 2006; Zang et al., 2007). Findings of increased FC between the dorsal anterior cingulate cortex and the bilateral thalamus, cerebellum, insula and brainstem (Tian et al., 2006), as well as increased FC between the cerebellum and the right inferior frontal and left somatosensory cortices (Zang et al., 2007) support the involvement of motor pathways in ADHD. No studies, however, have specifically examined FC within the motor network. Furthermore, no rs-fMRI studies have investigated FC in the motor network of children with DCD or co-occurring DCD and ADHD.
In the present study, we used rs-fMRI to investigate brain regions that are functionally connected with the primary motor cortex (M1) in children with DCD and/or ADHD. The M1 was selected because motor circuitry converges upon M1 for movement execution and it is consistently identifiable on MR images because of its location and shape (Golestani and Goodyear, 2011; Yousry et al., 1995). We hypothesized that children with DCD and/or ADHD would exhibit altered FC between M1 and brain regions involved in motor functioning and sensorimotor processing compared to typically developing children. Regions exhibiting common FC alterations among children with DCD, ADHD or co-occurring DCD and ADHD compared to typically developing children would provide support for a common neurophysiological basis for these disorders.
2. Methods
2.1. Participants, recruitment details and assessments
Participants 8–17 years of age were recruited through advertisements posted in the local community, schools, and physicians' offices in Calgary, Alberta, Canada, and Alberta-based newsletters and websites devoted to learning disabilities, ADHD and developmental coordination disorder. These advertisements invited parents of children with motor or attention problems and parents of children who did not have motor or attention problems to contact the investigators regarding the study. Parents who responded were screened by telephone. Exclusion criteria included diagnosed metabolic or genetic condition, epilepsy or other seizure disorder, cerebral palsy, intellectual disability, autism spectrum disorder, fetal alcohol spectrum disorder, psychiatric disorder other than ADHD, prematurity (born at <36 weeks of gestation), and very low birth weight (<1500 g). Individuals with a previous diagnosis of DCD and/or ADHD and individuals who had not been diagnosed previously, but who met the above inclusion criteria were invited to participate in a detailed neuropsychological assessment. Based on their performance on standardized neuropsychological measures participants were classified as DCD, ADHD, DCD + ADHD or controls (Table 1). Those who scored less than the 16th percentile on the Movement Assessment Battery for Children — Second Edition (Henderson et al., 2007) and were reported by parents as exhibiting motor difficulties that interfered significantly with daily functioning on the Developmental Coordination Questionnaire (Wilson et al., 2000) were classified as DCD. Children were classified as ADHD if they met the diagnostic criteria on the Diagnostic Interview for Children and Adolescents-IV (Reich et al., 1997), or had a T score above the 95th percentile on the Conners' Parent Rating Scale — Revised (Conners et al., 1998) and were diagnosed by a physician as having ADHD based on DSM-IV criteria (American Psychiatric Association, 2000). Children meeting our research criteria for both DCD and ADHD were classified as DCD + ADHD. Children not meeting our research criteria for DCD, ADHD or DCD + ADHD were assigned to the typically developing control group.
Table 1.
Participant characteristics.
| Controls | ADHD | DCD | DCD + ADHD | |
|---|---|---|---|---|
| Age in years | 11.3 ± 2.8 | 12.5 ± 2.9 | 13.0 ± 2.5 | 11.5 ± 3.0 |
| N (females) | 23 (12) | 21 (1)a | 7 (2) | 18 (4) |
| Left-handed | 2 | 2 | 1 | 4 |
| WASI IQ | 113.0 ± 13.4 | 105.4 ± 11.8 | 107.0 ± 13.0 | 104.8 ± 15.8 |
| CPRSC-C score | 51.7 ± 9.5 | 72.5a,b ± 8.7 | 50.9 ± 3.9 | 72.2a,b ± 12.0 |
| CPRSC-H score | 51.6 ± 9.4 | 71.1a,b ± 14.1 | 49.7 ± 3.4 | 65.0a,b ± 13.8 |
| MABC-2 score | 10.1 ± 2.2 | 9.5 ± 1.6 | 5.1a,c ± 2.0 | 4.4a,c ± 2.2 |
WASI IQ = Wechsler Abbreviated Scale of Intelligence IQ; CPRSC-C = Conner's Parent Rating Scale Revised Children Cognitive Problems/Inattention; CPRSC-H = Conner's Parent Rating Scale Revised/Hyperactivity; MABC-2 = Movement Assessment Battery for Children — Second Edition.
CPRSC Scores were not available for one child in the ADHD group.
Children who were on stimulant treatment for ADHD were asked to refrain from taking their medication on the day of assessment. No children in the control and DCD groups were on stimulant medication, 11 of 21 the children in the ADHD group were on stimulant medication, and 9 of 18 children in the DCD + ADHD group were on stimulant medication. A significant difference was found between the control and ADHD groups for sex [χ (1, N = 44) = 9.69, p < 0.002]. This is consistent with research, which has reported higher prevalence of ADHD in males (Ramtekkar et al., 2010). No group differences were found for age, handedness or IQ (Table 1). This research was conducted in accordance with the code of ethics of the World Medical Association (Declaration of Helsinki) for experiments involving human subjects and was approved by the Conjoint Health Research Ethics Board of the University of Calgary. Written consent was obtained from parents/guardians, and verbal assent was obtained from the participants.
2.2. Image acquisition and analysis
Images were collected using a 3 Tesla GE MR scanner (Signa VH/i, GE Healthcare, Waukesha, WI) with an eight-channel phased-array radiofrequency head coil. Resting-state fMRI consisted of 5 min of a T2*-weighted gradient-recalled echo, echo planar imaging (EPI) sequence (TR/TE = 2000/30 ms, flip angle = 70°, matrix size 64 × 64, FOV = 220 mm × 220 mm, 4-mm slice thickness, 26 slices). Participants were instructed to look at a fixation cross at the center of a screen. T1-weighted images were obtained for anatomical registration of the fMRI data (multi-slice fast spoiled gradient echo; TR/TE = 200/2.5 ms, flip angle = 18°, matrix size = 128 × 128, FOV = 220 × 220 mm, 4-mm slice thickness, 40 slices).
Resting-state fMRI data were pre-processed prior to statistical analysis with the FMRIB Software Library (FSL, http://www.fmrib.ox.ac.uk/fsl). This included scalp and skull removal using the Brain Extraction Tool (BET) (Smith, 2002), motion correction using MCFLIRT (Jenkinson et al., 2002), interleaved slice timing correction, temporal high pass filtering (>0.01 Hz), spatial smoothing using a Gaussian kernel of 6 mm, and registration to the MNI standard template. T1-weighted images were segmented into grey matter, white matter and cerebrospinal fluid using FMRIB's Automated Segmentation Tool (FAST) (Zhang et al., 2001). MCFLIRT analysis revealed no group difference in head motion, M1 center of gravity, or degree of correlation among voxels within the mask. The center of gravity of the M1 mask and the degree of correlation among the voxels of the mask did not differ between groups. Thus, the M1 mask represented an equivalent region across all groups.
For each participant, a mask of left M1 was manually drawn on the anatomical image using the FSLView drawing tool, with the omega-shaped anatomical landmark of M1 as a guide (Yousry et al., 1995). The mask was registered to the native resting-state data space using FLIRT, and then reduced to a final volume of 100 contiguous voxels using the process of intervoxel cross-correlation, which identifies the region with the greatest homogeneity in terms of temporal synchrony (Golestani and Goodyear, 2011). The center of gravity and the degree of cross-correlation within the mask were each tested between subject groups using Student's t-tests, to determine if there was a group bias in this method of mask generation.
The average time series of all the voxels in the left M1 mask was generated from the preprocessed resting-state data, to act as the regressor of interest in a time-series analysis using the general linear model (GLM). This analysis generated a whole-brain voxel-by-voxel estimate of FC with M1. The average time series from the segmented white matter masks, CSF masks and six head motion parameters were used as nuisance regressors. Groups were compared using a mixed effects GLM model. FC maps were created for each participant group and for the differences between each group, using age as a co-factor. Average Z-statistic images for each group were generated for clusters of 25 or more voxels and a Z-score greater than 3.1, corresponding to a corrected cluster significance of p = 0.05 (Woolrich et al., 2004; Worsley, 2003). For differences between participant groups, Z-statistic images were generated for clusters of 75 or more voxels and a Z-score greater than 2.3 (p = 0.05). Brain regions within these Z-statistic images were anatomically identified by Brodmann's Area and the Harvard-Oxford Cortical and Subcortical Structural atlas (Lancaster et al., 2000; Lancaster et al., 2007).
3. Results
3.1. Resting-state fMRI
Each group exhibited resting-state connectivity of motor circuitry consistent with that reported by Deco and Corbetta (2011). Data analysis revealed that no differences in FC were associated with sex (i.e., males and females demonstrated no significant differences in FC patterns) or handedness; therefore, these variables were not included as co-factors in the group analysis, in order to maintain sufficient degrees of freedom. Brain regions exhibiting significant FC with the left M1 included the contralateral motor cortex, bilateral premotor cortices, somatosensory cortices and striatum. Fig. 1 shows brain regions that differed significantly between the control group and all diagnostic groups. Compared to the control group, the DCD group demonstrated decreased FC with M1 in the bilateral inferior frontal gyri, right frontal operculum cortex, right supramarginal gyrus, bilateral insular cortices and superior temporal gyri (Table 2). Subcortical structures exhibiting decreased FC included the bilateral caudate and the right nucleus accumbens, pallidum and putamen. No regions exhibited increased FC with M1. In the ADHD group, decreased FC with M1 was observed in the bilateral frontal eye fields, bilateral inferior frontal gyri, left middle frontal gyrus, right anterior cingulate cortex and frontopolar cortex. More posteriorly, decreased FC was observed in the bilateral supramarginal gyri, right auditory cortex and bilateral insular cortices. Subcortical structures that exhibited reduced connectivity with M1 in children with ADHD included the left amygdala, bilateral putamen, globus pallidus and brainstem. No regions exhibited greater FC with M1. Relative to controls, the DCD + ADHD group exhibited lower FC in the right motor cortex, left supramarginal gyrus, bilateral postcentral gyri, left putamen, left pallidum and left amygdala. Regions exhibiting greater FC with M1 included the left frontopolar cortex and lingual gyrus.
Fig. 1.
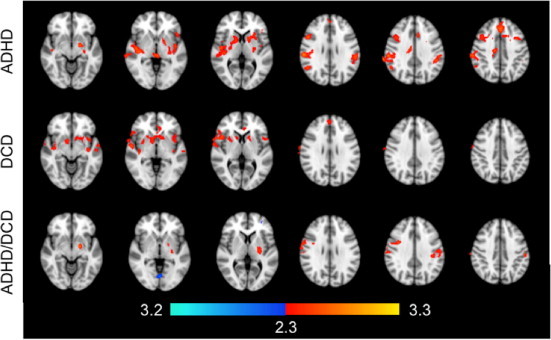
Regions exhibiting greater (red) and lower (blue) functional connectivity with left M1 in controls compared to children in the ADHD (top), DCD (middle), and DCD + ADHD (bottom) groups. Colors indicate statistical significance, expressed as Z-scores.
Table 2.
Regions exhibiting altered functional connectivity with left primary motor cortex in children with DCD, ADHD and DCD + ADHD.
| Comparison | Brain region | Z score | x | y | z | BA |
|---|---|---|---|---|---|---|
| DCD < controls | ||||||
| R anterior superior temporal gyrus/planum polare | 3.16 | 58 | −4 | −2 | 6, 22 | |
| R frontal operculum cortex | 3.0 | 46 | 16 | 4 | 13 | |
| R inferior frontal gyrus/precentral gyrus | 2.93 | 54 | 12 | 2 | 44, 45 | |
| R caudate | 2.88 | 8 | 10 | 0 | – | |
| R nucleus sccumbens | 2.85 | 10 | 8 | −6 | – | |
| L insular cortex | 2.79 | −40 | 8 | −6 | 13 | |
| L superior temporal gyrus (anterior) | 2.79 | −56 | −14 | −6 | −21 | |
| L inferior frontal gyrus/precentral | 2.76 | −54 | 10 | 2 | 13, 44 | |
| R insular cortex | 2.71 | 38 | 0 | −6 | 13 | |
| L anterior cingulate gyrus | 2.71 | −4 | 40 | −4 | 24 | |
| R pallidum | 2.66 | 14 | 4 | −4 | – | |
| L caudate (all) | 2.66 | −14 | 14 | 2 | – | |
| Paracingulate gyrus/superior frontal gyrus | 2.56 | 0 | 48 | 28 | 9 | |
| R putamen (ventral) | 2.53 | 28 | 8 | 2 | – | |
| ADHD < controls | R parietal operculum | 2.53 | 52 | −32 | 22 | 13 |
| R parietal operculum cortex/supramarginal gyrus | 3.69 | 60 | −26 | 28 | 40 | |
| R auditory cortex/insular cortex | 3.58 | 46 | −10 | 0 | 13 | |
| Frontal eye fields | 3.29 | 0 | 30 | 40 | 8 | |
| L pallidum | 3.28 | −18 | −4 | −8 | – | |
| R inferior frontal gyrus | 3.26 | 48 | 14 | 30 | 9 | |
| L angular/supramarginal gyri | 3.26 | −54 | −52 | 22 | 22, 39, 40 | |
| L supramarginal gyrus | 3.21 | −48 | −40 | 34 | 40 | |
| Brainstem | 3.17 | −2 | −28 | 2 | – | |
| L amygdala | 2.97 | −20 | −6 | −10 | – | |
| L insular cortex | 2.96 | −42 | −6 | −2 | 13 | |
| L inferior frontal gyrus | 2.89 | −44 | 32 | 2 | 45, 47 | |
| R anterior cingulate gyrus | 2.88 | −8 | 18 | 34 | 24, 32 | |
| L putamen | 2.71 | −28 | −12 | 2 | – | |
| R putamen, pallidum | 2.67 | 20 | 4 | 6 | – | |
| L middle frontal gyrus | 2.66 | −38 | 10 | 40 | 6 | |
| DCD + ADHD < controls | L pallidum | 3.55 | −18 | −6 | −8 | – |
| R postcentral gyrus | 3.49 | 64 | −8 | 26 | 4 | |
| R motor cortex | 3.32 | 62 | 2 | 26 | 6 | |
| L supramarginal gyrus (anterior) | 2.96 | −56 | −26 | 38 | 2, 40 | |
| L putamen/pallidum (dorsal) | 2.85 | −26 | −14 | 6 | – | |
| L amygdala | 2.79 | −20 | −8 | −12 | – | |
| L postcentral gyrus | 2.71 | −56 | −16 | 34 | 3 | |
| DCD + ADHD > controls | Lingual gyrus | 3.46 | 0 | −72 | −2 | – |
| L lingual gyrus | 3.41 | −4 | −84 | −16 | – | |
| L frontopolar cortex | 3.22 | −26 | 64 | 8 | 10 |
BA = Brodmann's Area, L = left, R = right. Coordinates (x, y, and z) given in mm of MNI template space.
Direct comparison of the clinical groups revealed greater FC with M1 in the left postcentral gyrus and left superior frontal gyrus in children with DCD compared to ADHD (Table 3). The ADHD group exhibited greater FC compared to the DCD group in the right caudate, middle frontal gyrus, left superior temporal gyrus and bilateral inferior frontal gyri. Compared to the DCD + ADHD group, children with ADHD exhibited greater FC between M1 and the left postcentral gyrus. Children with DCD + ADHD evidenced greater FC between M1 and the bilateral precuneus cortices and anterior cingulate gyri, the left premotor cortex and postcentral gyrus, and the right parietal operculum cortex and angular gyrus, compared to children with ADHD. Compared to the DCD group, children with DCD + ADHD exhibited greater FC between M1 and the bilateral caudate nuclei and anterior superior temporal gyri, the left premotor cortex, postcentral gyri and frontopolar cortex, and the right inferior frontal gyrus and parietal operculum cortex.
Table 3.
Regional difference in functional connectivity with left primary motor cortex between the DCD, ADHD and DCD + ADHD groups.
| Comparison | Brain region | Z score | x | y | z | BA |
|---|---|---|---|---|---|---|
| DCD > ADHD | L postcentral gyrus | 3.70 | −32 | −30 | 54 | 3 |
| L superior frontal gyrus | 2.67 | −2 | 44 | 40 | 8 | |
| ADHD > DCD | R caudate | 3.33 | 8 | 12 | −2 | – |
| R middle frontal gyrus | 3.01 | 52 | 44 | 4 | 10 | |
| L superior temporal gyrus | 2.98 | −56 | −2 | −10 | 22 | |
| R inferior frontal gyrus | 2.79 | 48 | 20 | 16 | 44 | |
| L inferior frontal gyrus | 2.72 | −56 | 10 | 2 | 44 | |
| ADHD > DCD + ADHD | L postcentral gyrus | 2.98 | −58 | −14 | 34 | 3 |
| DCD + ADHD > ADHD | L premotor cortex | 3.91 | −28 | −12 | 70 | 6 |
| L anterior cingulate cortex | 3.12 | −12 | 20 | 36 | 32 | |
| R parietal operculum cortex | 2.99 | 54 | −24 | 24 | 40 | |
| L postcentral gyrus | 2.94 | −28 | −30 | 56 | 3 | |
| R precuneus cortex | 2.73 | 18 | −72 | 40 | 31 | |
| R anterior cingulate cortex | 2.66 | 4 | 32 | 28 | 32 | |
| L precuneus cortex | 2.60 | −10 | −72 | 30 | 31 | |
| R angular gyrus | 2.59 | 54 | −52 | 32 | 40 | |
| DCD + ADHD > DCD | R caudate | 3.27 | 8 | 12 | −2 | – |
| L premotor cortex | 3.25 | −26 | −12 | 70 | 6 | |
| R inferior frontal gyrus | 3.10 | 52 | 12 | 4 | 44 | |
| L postcentral gyrus | 3.09 | −50 | −16 | 58 | 3 | |
| L anterior superior temporal gyrus | 3.02 | −58 | 0 | −8 | 22 | |
| L frontopolar cortex | 2.92 | −28 | 62 | 8 | 10 | |
| R anterior superior temporal gyrus | 2.86 | 58 | −4 | −2 | 22 | |
| R parietal operculum cortex | 2.75 | 54 | −22 | 20 | 40 | |
| L caudate | 2.62 | −8 | 14 | 0 | – |
BA = Brodmann's Area, L = left, R = right. Coordinates (x, y, and z) given in mm of MNI template space.
3.2. Connectivity association with age
Within group effects were investigated for age. Between group differences were not examined due to the limited sample size. In controls, increasing age was associated with greater FC between M1 and the bilateral precentral and postcentral gyri as well as the right superior frontal gyrus (Fig. 2, Table 4). Lower FC was observed in the bilateral occipital poles, left lingual gyrus, and right anterior cingulate cortex. In the DCD group, older age was associated with greater FC between M1 and the left frontal pole, left precuneus cortex and right paracingulate gyrus, and lower FC between M1 and the left occipital lobe, right motor cortex, left supplementary motor area and thalamus. In the ADHD group, age was positively associated with greater FC between M1 and a number of brain regions including the dorsolateral prefrontal cortices, paracingulate gyrus, right superior and middle temporal gyri, right insular cortex, lateral occipital cortices and precuneus. Older age was associated with lower FC between M1 and the angular gyri, left precentral and middle frontal gyri, right anterior cingulate cortex, and left superior lateral occipital cortex. In the DCD + ADHD group, increasing age was not associated with greater FC in any brain regions. Increasing age, however, was associated with lower FC between M1 and the right angular gyrus, lateral occipital cortex, and right superior and middle temporal gyri.
Fig. 2.
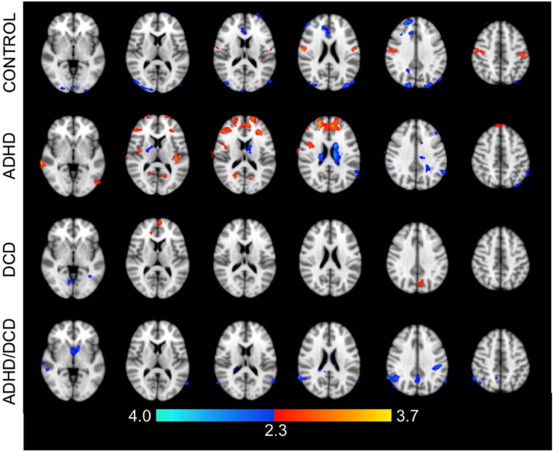
Regions exhibiting a significant association between age and functional connectivity with the left M1. Colors indicate statistical significance, expressed as Z-scores; red indicates a positive association with age; blue indicates a negative association with age.
Table 4.
Regions exhibiting a dependence of functional connectivity on age.
| Brain region | Z score | x | y | z | BA | |
|---|---|---|---|---|---|---|
| Increase with age (controls) | R precentral and postcentral gyrus (entire) | 4.24 | 58 | −8 | 24 | 4 |
| R superior frontal gyrus | 4.14 | 28 | 28 | 58 | 6 | |
| L precentral gyrus | 4.08 | −34 | −6 | 68 | 6 | |
| L postcentral gyrus (entire) | 3.41 | −46 | −22 | 46 | 1, 2 | |
| R precentral gyrus | 3.36 | 40 | −2 | 64 | 6 | |
| Decrease with age (controls) | L lingual gyrus | 3.74 | −6 | −84 | −10 | – |
| R lateral occipital cortex/occipital pole | 3.72 | 18 | −88 | 36 | 19 | |
| L lateral occipital cortex (superior)/occipital pole | 3.63 | −42 | −84 | 30 | 19 | |
| R anterior cingulate gyrus | 3.59 | 6 | 22 | 22 | 24 | |
| R frontal pole | 3.56 | 20 | 50 | 34 | 9 | |
| Increase with age (DCD) | L frontopolar cortex | 3.19 | −4 | 62 | 10 | 10 |
| L precuneus cortex | 2.85 | −8 | −70 | 36 | 7 | |
| R paracingulate gyrus | 2.69 | 12 | 48 | 2 | 32 | |
| Decrease with age (DCD) | L occipital fusiform gyrus | 3.28 | −24 | −74 | −14 | 19 |
| L occipital pole/lingual gyrus/occipital fusiform gyrus | 3.2 | −20 | −92 | −16 | 18 | |
| R precentral gyrus | 3.00 | 22 | −22 | 58 | 4 | |
| L supplementary motor area | 2.96 | −10 | −4 | 50 | 6 | |
| L intracalcarine cortex | 2.77 | −4 | −88 | 4 | 18 | |
| R thalamus | 2.73 | 4 | −18 | 4 | – | |
| Increase with age (ADHD) | R superior frontal gyrus/DLPFC | 4.17 | 20 | 52 | 24 | 9 |
| Paracingulate gyrus | 3.95 | −8 | 48 | 22 | 32 | |
| R superior and middle temporal gyrus, posterior | 3.94 | 70 | −26 | 2 | 21, 22 | |
| L Heschl's gyrus | 3.88 | −38 | −24 | 8 | 13 | |
| L DLPFC | 3.85 | −18 | 62 | 20 | 9, 10 | |
| Brainstem | 3.78 | 0 | −10 | −12 | – | |
| R inferior temporal gyrus (temporooccipital)/lateral occipital cortex, inferior | 3.68 | 56 | −54 | −12 | 20 | |
| L lateral occipital cortex, inferior | 3.63 | −40 | −64 | 4 | 37 | |
| L temporal occipital fusiform cortex | 3.49 | −30 | −46 | −16 | 37 | |
| R middle frontal gyrus | 3.42 | 48 | 36 | 18 | 46 | |
| R supracalcarine/precuneous cortices | 3.24 | 24 | −64 | 16 | 30, 31 | |
| R insular cortex | 3.19 | 40 | −4 | 12 | 13 | |
| L supracalcarine/precuneous/intracalcarine cortices | 3.03 | −16 | −64 | 12 | 30 | |
| Decrease with age (ADHD) | L angular gyrus/supramarginal gyrus | 3.76 | −58 | −54 | 26 | 40 |
| R anterior cingulate gyrus/paracingulate gyrus | 3.45 | 8 | 12 | 38 | 32 | |
| L superior lateral occipital cortex | 3.29 | −32 | −76 | 52 | 7 | |
| L precentral gyrus | 3.11 | −10 | −14 | 68 | 6 | |
| L middle frontal gyrus | 2.82 | −38 | 32 | 42 | 8 | |
| R supramarginal gyrus (posterior)/angular gyrus | 2.81 | 62 | −44 | 32 | 40 | |
| Decrease with age (DCD + ADHD) | R angular gyrus/lateral occipital cortex (superior) | 5.16 | 66 | −52 | 30 | 39, 40 |
| R planum polare/superior temporal gyrus/middle temporal gyrus (posterior) | 3.96 | 52 | −12 | −12 | 13, 21, 22 |
Coordinates (x, y, and z) are given in mm of MNI template space; BA = Brodmann's Area.
4. Discussion
This study is the first to report that children with motor and/or attention problems exhibit altered FC with M1 within the motor network as well as with brain regions involved in cognitive control of movement and sensorimotor processing.
4.1. Resting-state motor connectivity of children with DCD
The alterations in FC we observed in children with DCD are consistent with commonly reported deficits in motor execution, motor control, motor planning and sensorimotor processing. Reduced FC between M1 and the caudate, putamen, and globus pallidus in children with DCD suggests disrupted connectivity between regions associated with motor execution and motor control. Similarly, reductions in connectivity between M1 and the inferior frontal gyrus is consistent with disruptions in fine motor control, inhibition and integration of sensory input into action reported previously (Liakakis et al., 2011). Consistent with decreased insular cortex involvement during trail-tracing in children with DCD (Zwicker et al., 2010), we observed reduced FC between M1 and the posterior insular cortex, which could also be related to disruptions in sensorimotor processing and motor output (Cauda et al., 2012). Cognitive control regions related to working memory and motor planning (i.e., prefrontal cortex) also exhibited altered FC in children with DCD. As a result, motor execution regions may receive degraded input from areas responsible for motor planning, organization and regulation (Christoff and Gabrieli, 2000).
4.2. Resting-state motor connectivity of children with ADHD
Reduced FC in the striatum, putamen and other subcortical structures in children with ADHD is consistent with observations of previous imaging studies (Cao et al., 2009; Castellanos et al., 1996; Durston et al., 2003; Suskauer et al., 2008). Lower connectivity between M1 and the frontal eye fields, areas associated with visual attention (Schall, 2004), and the left postcentral gyrus, a key component of the neural network involved in working memory (du Boisgueheneuc et al., 2006), could be linked to deficits in visual attention and working memory observed in children with ADHD (Martinussen et al., 2005; Swanson et al., 1991). Decreased FC in the inferior frontal gyri could play a part in the motor inhibition difficulties associated with ADHD (Cao et al., 2006; Zang et al., 2007), which is further supported by previous task-related fMRI studies (Booth et al., 2005; Durston et al., 2006; Liakakis et al., 2011). Previous imaging research has reported reduced grey matter volume of the left insular cortex in children with ADHD (Brieber et al., 2007). Further, increased cortical thickness (Duerden et al., 2012) and increased activity during an fMRI-based attention–reorientation task (Konrad et al., 2006) have been reported in the right insular cortex. Given the multiple functions performed by the insular cortices, it is not surprising that abnormal connectivity between the M1 and the insular cortices are associated with attention difficulties.
4.3. Resting-state differences in single versus co-occurring disorders
Compared to typically developing controls, children with co-occurring DCD and ADHD evidenced lower FC between M1 and the somatosensory cortices, left supramarginal gyrus, striatum and amygdala, suggesting poor integration between sensorimotor input, motor execution and movement regulation. In contrast, compared to children with only DCD or ADHD, the DCD + ADHD group exhibited greater FC between M1 and brain regions involved in motor control (bilateral caudate, left premotor cortex, right inferior frontal gyrus), speech processing and prosody (bilateral anterior superior temporal gyri), sensorimotor processing (left postcentral gyrus, right parietal operculum cortex, bilateral precuneus cortices, angular gyri) and attention and error detection (bilateral anterior cingulate cortices) compared to children with only DCD or ADHD. These findings, though unexpected, may suggest that some connections between M1 and processing areas may be erroneous, such that greater involvement of these regions is needed to successfully plan and execute movement in children with co-occurring disorders (Zwicker et al., 2010). More research is needed to examine the influence of erroneous connectivity between brain areas on motor output.
4.4. Common neurophysiological substrates
Our findings support the hypotheses that common neurophysiological substrates may underlie both motor and attention problems. We found that a number of brain regions (i.e., bilateral inferior frontal gyri, the right supramarginal gyrus, angular gyri, insular cortices, amygdala, putamen and pallidum) exhibited similar FC alterations in children with DCD and/or ADHD, which may represent putative targets for future study and potential biomarkers for DCD and ADHD.
The angular gyri act as multimodal integration centers active during tasks such as reading, comprehension, spatial cognition and attention (Vannini et al., 2004). Imaging research has implicated these regions in both DCD (Kashiwagi et al., 2009; Querne et al., 2008; Zwicker et al. 2010, Zwicker et al. 2011) and ADHD (Dickstein et al., 2006). The angular gyri are also part of the fronto-parietal network, which is responsible for integrating internal and external information for response generation (Binkofski et al., 1999). In individuals affected by ADHD, fMRI tasks involving motor inhibition have been found to result in decreased activity within this network (Dickstein et al., 2006). Our observation of lower FC between M1 and the angular gyri in the DCD and ADHD groups compared to the controls suggests that the information provided by this region to M1 may be degraded, which in turn could impact motor performance. The putamen and pallidum, key regulators of movement and motor learning (Kandel et al., 2000), exhibited lower FC with M1 in all diagnostic groups. Reduced FC between this region and M1 could contribute to the movement difficulties associated with DCD and ADHD, as striatal structures receive information from regions throughout the brain and directly interact with M1.
The supramarginal gyri (secondary somatosensory cortices) are responsible for integrating tactile or pain-based stimuli with higher order functions such as attention (Chen et al., 2008). Decreased FC with M1 was observed in the right supramarginal gyrus (secondary somatosensory cortex) in the DCD and ADHD groups, and in the left supramarginal gyrus in the DCD + ADHD group. Other sensorimotor regions also displayed decreased FC with M1. In the DCD and ADHD groups, decreased FC was observed with the insular and secondary somatosensory cortices. In contrast, in the DCD + ADHD group decreased FC was observed with the primary somatosensory cortices. Further, the posterior insular cortices exhibited lower FC in the DCD and ADHD groups; however, a similar decrease in FC was not observed in the DCD + ADHD group. These findings suggest that alterations in FC between primary motor cortex and sensory networks differ between children with isolated DCD or ADHD and children with co-occurring DCD + ADHD. The functional consequences of these differences need to be investigated further; however, they could be associated with differences in severity of the sensory processing dysfunction (Crawford and Dewey, 2008).
4.6. Connectivity association with age
Volumetric MRI studies of children with ADHD have reported decreased volume and delayed cortical thickness as age progresses, particularly in regions associated with motor control (Castellanos et al., 2002; Shaw et al., 2007; Shaw et al., 2012). In our study, typically developing children exhibited increased FC between M1 and the bilateral motor and sensorimotor cortices as age increased; a finding not observed in children with DCD and/or ADHD. This is a novel finding and may provide the basis for a subjective biomarker of motor and attention problems in future studies. Our findings of increasing functional connectivity with age between M1 and frontal and parietal regions involved in executive functions, memory and visuospatial imagery in children with DCD are consistent with the research that has reported symptom remission in adolescence in children with milder symptoms of motor impairments (Cantell et al., 2003). In the ADHD group, increasing FC with age between M1 and regions of the frontal cortex is consistent with a delay in the development of regions associated with inhibition, impulsivity, and attention. Increased FC between M1 and frontal cognitive areas such as the dorsolateral prefrontal cortex, could be associated with the remission of symptoms such as hyperactivity, impulsivity and inattention, in children with ADHD as they mature (Biederman et al., 2000). Finally, in the DCD + ADHD group, the absence of increased FC with age between M1 and any brain structures suggests that brain development in children with co-occurring attention and motor disorders could be disrupted to a greater extent than that of children who present with only one disorder.
4.7. Limitations and future research
Cortical development in children is influenced by factors, including environmental and social occurrences not covered by our exclusion criteria. Our seed based correlation approach is a highly reliable and allows for straightforward interpretation of the results; however, it is prone to spatial confounds and structural noise (motion, scanner artifacts), and may underrepresent the data due to the strict area selected. One future alternative approach would be independent component analysis (ICA), which is less prone to noise but is susceptible to statistical complexities (Cole et al., 2010). Our limited sample size and cross-sectional design limit the validity of the findings; however, the feasibility of the design provides a strong foundation from which to build future, longitudinal study paradigms, including investigation of DCD and ADHD subtypes.
FC alterations may reflect atypical brain development in children with DCD and/or ADHD and could explain the activation differences frequently observed in task-based fMRI studies (Booth et al., 2005; Durston et al., 2003; Durston et al., 2006; Kashiwagi et al., 2009; Mostofsky et al., 2006; Suskauer et al., 2008; Zwicker et al. 2010, Zwicker et al. 2011). Future research investigating the relationship between FC and task-based fMRI activity is warranted to shed light on how abnormal inter-regional communication may lead to the observed deficits.
Our findings of altered FC between M1 and the insular, somatosensory cortices, striatum, and inferior frontal gyri in children with DCD support the hypothesis that DCD is a disorder of sensorimotor processing (Wilson and McKenzie, 1998). Central problems in DCD are movement control and visuospatial processing, which rely on integrated information from visual, kinesthetic and vestibular systems (Wilson and McKenzie, 1998). The results of studies investigating kinesthetic and sensorimotor ability in children with ADHD have been inconsistent, possibly because of the confounding effect of unacknowledged motor problems (Piek and Dyck, 2004). Future research is needed to determine if children with only ADHD have overt problems with sensorimotor processing.
4.8. Conclusions
Compared to typically developing controls, children with DCD and/or ADHD exhibit FC alterations between M1 and brain regions involved in motor functioning and sensorimotor processing. Our findings support the hypothesis that common neurophysiological substrates underlie motor and attention problems. The decreased FC between the primary motor cortex, and the striatum and angular gyrus observed in all groups of children with motor and attention problems suggests that the these brain regions are common neurophysiological substrates underlying DCD and ADHD. Our results also indicated that children with co-occurring DCD and ADHD appear to have unique alterations in FC between primary motor cortex and sensory networks compared to children with DCD or ADHD alone; suggesting that co-occurrence of neurodevelopmental disorders may have a distinct impact on FC.
Funding
This study was supported by on operating grant from the Canadian Institutes of Health Research (grant number MOP-88588) to DD and BGG. KRM was the recipient of an Alberta Children's Hospital Research Institute Studentship through the CIHR Training Program in Genetics, Child Development and Health and a Katharina Zeigler Scholarship, University of Calgary. The funding agencies had no role in the design and conduct of the study, in the collection, analysis and interpretation of the data, or in the preparation, review or approval of the manuscript.
Acknowledgements
We thank Holly Johnston, University of Calgary, for her assistance in image acquisition and Nadia Barnieh, Ashley Marsh and Sally Powis-Campbell, University of Calgary, for their assistance with neurocognitive assessments. We also thank the children who participated in this study.
References
- Able S.L., Johnston J.A., Adler L.A., Swindle R.W. Functional and psychosocial impairment in adults with undiagnosed ADHD. Psychological Medicine. 2007;37:97–107. doi: 10.1017/S0033291706008713. 16938146 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. fourth edition, Text Revision. American Psychiatric Association; Washington, D.C.: 2000. [Google Scholar]
- Biederman J., Mick E., Faraone S.V. Age-dependent decline of symptoms of attention deficit hyperactivity disorder: impact of remission definition and symptom type. American Journal of Psychiatry. 2000;157:816–818. doi: 10.1176/appi.ajp.157.5.816. 10784477 [DOI] [PubMed] [Google Scholar]
- Binkofski F., Buccino G., Posse S., Seitz R.J., Rizzolatti G., Freund H. A fronto-parietal circuit for object manipulation in man: evidence from an fMRI-study. European Journal of Neuroscience. 1999;11:3276–3286. doi: 10.1046/j.1460-9568.1999.00753.x. 10510191 [DOI] [PubMed] [Google Scholar]
- Booth J.R., Burman D.D., Meyer J.R., Lei Z., Trommer B.L., Davenport N.D., Li W., Parrish T.B., Gitelman D.R., Mesulam M.M. Larger deficits in brain networks for response inhibition than for visual selective attention in attention deficit hyperactivity disorder (ADHD) Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2005;46:94–111. doi: 10.1111/j.1469-7610.2004.00337.x. 15660647 [DOI] [PubMed] [Google Scholar]
- Brieber S., Neufang S., Bruning N., Kamp-Becker I., Remschmidt H., Herpertz-Dahlmann B., Fink G.R., Konrad K. Structural brain abnormalities in adolescents with autism spectrum disorder and patients with attention deficit/hyperactivity disorder. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2007;48:1251–1258. doi: 10.1111/j.1469-7610.2007.01799.x. 18093031 [DOI] [PubMed] [Google Scholar]
- Cantell M.H., Smyth M.M., Ahonen T.P. Two distinct pathways for developmental coordination disorder: persistence and resolution. Human Movement Science. 2003;22:413–431. doi: 10.1016/j.humov.2003.09.002. 14624826 [DOI] [PubMed] [Google Scholar]
- Cao Q., Zang Y., Sun L., Sui M., Long X., Zou Q., Wang Y. Abnormal neural activity in children with attention deficit hyperactivity disorder: a resting-state functional magnetic resonance imaging study. Neuroreport. 2006;17:1033–1036. doi: 10.1097/01.wnr.0000224769.92454.5d. 16791098 [DOI] [PubMed] [Google Scholar]
- Cao X., Cao Q., Long X., Sun L., Sui M., Zhu C., Zuo X., Zang Y., Wang Y. Abnormal resting-state functional connectivity patterns of the putamen in medication-naive children with attention deficit hyperactivity disorder. Brain Research. 2009;1303:195–206. doi: 10.1016/j.brainres.2009.08.029. 19699190 [DOI] [PubMed] [Google Scholar]
- Castellanos F.X., Giedd J.N., Marsh W.L., Hamburger S.D., Vaituzis A.C., Dickstein D.P., Sarfatti S.E., Vauss Y.C., Snell J.W., Lange N., Kaysen D., Krain A.L., Ritchie G.F., Rajapakse J.C., Rapoport J.L. Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Archives of General Psychiatry. 1996;53:607–616. doi: 10.1001/archpsyc.1996.01830070053009. 8660127 [DOI] [PubMed] [Google Scholar]
- Castellanos F.X., Lee P.P., Sharp W., Jeffries N.O., Greenstein D.K., Clasen L.S., Blumenthal J.D., James R.S., Ebens C.L., Walter J.M., Zijdenbos A., Evans A.C., Giedd J.N., Rapoport J.L. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA: the Journal of the American Medical Association. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. 12365958 [DOI] [PubMed] [Google Scholar]
- Cauda F., Costa T., Torta D.M., Sacco K., D'Agata F., Duca S., Geminiani G., Fox P.T., Vercelli A. Meta-analytic clustering of the insular cortex: characterizing the meta-analytic connectivity of the insula when involved in active tasks. Neuroimage. 2012;62:343–355. doi: 10.1016/j.neuroimage.2012.04.012. 22521480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T.L., Babiloni C., Ferretti A., Perrucci M.G., Romani G.L., Rossini P.M., Tartaro A., Del Gratta C. Human secondary somatosensory cortex is involved in the processing of somatosensory rare stimuli: An fMRI study. Neuroimage. 2008;40:1765–1771. doi: 10.1016/j.neuroimage.2008.01.020. 18329293 [DOI] [PubMed] [Google Scholar]
- Christoff K., Gabrieli J. He frontopolar cortex and human cognition: evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology. 2000;28:168–186. [Google Scholar]
- Cole D.M., Smith S.M., Beckman C.F. Advances and pitfalls in the analysis and interpretation of resting state fMRI data. Frontiers in Systems Neuroscience. 2010;4:8. doi: 10.3389/fnsys.2010.00008. 20407579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners C.K., Sitarenios G., Parker J.D., Epstein J.N. The revised Conners' Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. Journal of Abnormal Child Psychology. 1998;26:257–268. doi: 10.1023/A:1022602400621. 9700518 [DOI] [PubMed] [Google Scholar]
- Crawford S.G., Dewey D. Co-occurring disorders: a possible key to visual perceptual deficits in children with developmental coordination disorder? Human Movement Science. 2008;27:154–168. doi: 10.1016/j.humov.2007.09.002. 18192047 [DOI] [PubMed] [Google Scholar]
- de Zeeuw P., Mandl R.C., Hulshoff Pol H.E., van Engeland H., Durston S. Decreased frontostriatal microstructural organization in attention deficit/hyperactivity disorder. Human Brain Mapping. 2012;33:1941–1951. doi: 10.1002/hbm.21335. 21826757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G., Corbetta M. The dynamical balance of the brain at rest. Neuroscientist: a Review Journal Bringing Neurobiology, Neurology and Psychiatry. 2011;17:107–123. doi: 10.1177/1073858409354384. 21196530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein S.G., Bannon K., Castellanos F.X., Milham M.P. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2006;47:1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. 17073984 [DOI] [PubMed] [Google Scholar]
- du Boisgueheneuc F.1., Levy R., Volle E., Seassau M., Duffau H., Kinkingnehun S., Samson Y., Zhang S., Dubois B. Functions of the left superior frontal gyrus in humans: a lesion study. Brain. 2006;129:3315–3328. doi: 10.1093/brain/awl244. Epub 2006 Sep 19. [DOI] [PubMed] [Google Scholar]
- Duerden E.G., Tannock R., Dockstader C. Altered cortical morphology in sensorimotor processing regions in adolescents and adults with attention-deficit/hyperactivity disorder. Brain Research. 2012;1445:82–91. doi: 10.1016/j.brainres.2012.01.034. 22325095 [DOI] [PubMed] [Google Scholar]
- Durston S., Mulder M., Casey B.J., Ziermans T., van Engeland H. Activation in ventral prefrontal cortex is sensitive to genetic vulnerability for attention-deficit hyperactivity disorder. Biological Psychiatry. 2006;60:1062–1070. doi: 10.1016/j.biopsych.2005.12.020. 16712804 [DOI] [PubMed] [Google Scholar]
- Durston S., Tottenham N.T., Thomas K.M., Davidson M.C., Eigsti I.M., Yang Y., Ulug A.M., Casey B.J. Differential patterns of striatal activation in young children with and without ADHD. Biological Psychiatry. 2003;53:871–878. doi: 10.1016/S0006-3223(02)01904-2. 12742674 [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I., Ellison-Wright Z., Bullmore E. Structural brain change in attention deficit hyperactivity disorder identified by meta-analysis. BMC Psychiatry. 2008;8:51. doi: 10.1186/1471-244X-8-51. 18590567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T., Skokauskas N. Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatrica Scandinavica. 2012;125:114–126. doi: 10.1111/j.1600-0447.2011.01786.x. 22118249 [DOI] [PubMed] [Google Scholar]
- Golestani A.M., Goodyear B.G. Regions of interest for resting-state fMRI analysis determined by inter-voxel cross-correlation. Neuroimage. 2011;56:246–251. doi: 10.1016/j.neuroimage.2011.02.038. 21338691 [DOI] [PubMed] [Google Scholar]
- Hamilton L.S., Levitt J.G., O'Neill J., Alger J.R., Luders E., Phillips O.R., Caplan R., Toga A.W., McCracken J., Narr K.L. Reduced white matter integrity in attention-deficit hyperactivity disorder. Neuroreport. 2008;19:1705–1708. doi: 10.1097/WNR.0b013e3283174415. 18841089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson S.E., Sudgen D.A., Barnett A.L. Movement Assessment Battery for Children. second edition. Psychological Corporation; London, UK: 2007. [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1006/nimg.2002.1132. 12377157 [DOI] [PubMed] [Google Scholar]
- Kadesjo B., Gillberg C. Attention deficits and clumsiness in Swedish 7-year-old children. Developmental Medicine and Child Neurology. 1998;40:796–804. doi: 10.1111/j.1469-8749.1998.tb12356.x. 9881675 [DOI] [PubMed] [Google Scholar]
- Kandel E.R., Schwartz J.H., Jessell T.M. The Principles of Neural Science. fourth edition. McGraw-Hill; New York: 2000. [Google Scholar]
- Kashiwagi M., Iwaki S., Narumi Y., Tamai H., Suzuki S. Parietal dysfunction in developmental coordination disorder: a functional MRI study. Neuroreport. 2009;20:1319–1324. doi: 10.1097/WNR.0b013e32832f4d87. 19730138 [DOI] [PubMed] [Google Scholar]
- Konrad K., Neufang S., Hanisch C., Fink G.R., Herpertz-Dahlmann B. Dysfunctional attentional networks in children with attention deficit/hyperactivity disorder: evidence from an event-related functional magnetic resonance imaging study. Biological Psychiatry. 2006;59:643–651. doi: 10.1016/j.biopsych.2005.08.013. 16197925 [DOI] [PubMed] [Google Scholar]
- Lancaster J.L., Tordesillas-Gutierrez D., Martinez M., Salinas F., Evans A., Zilles K., Mazziotta J.C., Fox P.T. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Human Brain Mapping. 2007;28:1194–1205. doi: 10.1002/hbm.20345. 17266101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster J.L., Woldorff M.G., Parsons L.M., Liotti M., Freitas C.S., Rainey L., Kochunov P.V., Nickerson D., Mikiten S.A., Fox P.T. Automated Talairach atlas labels for functional brain mapping. Human Brain Mapping. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. 10912591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin L., MacMaster F.P., Crawford S., Lebel C., Dewey D. Common white matter microstructure alterations in pediatric motor and attention disorders. The Journal of Pediatrics. 2014 doi: 10.1016/j.jpeds.2014.01.018. [published online ahead of print 25 Feb] [DOI] [PubMed] [Google Scholar]
- Liakakis G., Nickel J., Seitz R.J. Diversity of the inferior frontal gyrus — a meta-analysis of neuroimaging studies. Behavioural Brain Research. 2011;225:341–347. doi: 10.1016/j.bbr.2011.06.022. 21729721 [DOI] [PubMed] [Google Scholar]
- Lingam R., Jongmans M.J., Ellis M., Hunt L.P., Golding J., Emond A. Mental health difficulties in children with developmental coordination disorder. Pediatrics. 2012;129:e882–91. doi: 10.1542/peds.2011-1556. 22451706 [DOI] [PubMed] [Google Scholar]
- Martinussen R., Hayden R., Hogg-Johnson S., Tannock R. A meta-analysis of working memory impairments in children with attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:377–384. doi: 10.1097/01.chi.0000153228.72591.73. 15782085 [DOI] [PubMed] [Google Scholar]
- Mostofsky S.H., Rimrodt S.L., Schafer J.G., Boyce A., Goldberg M.C., Pekar J.J., Denckla M.B. Atypical motor and sensory cortex activation in attention-deficit/hyperactivity disorder: a functional magnetic resonance imaging study of simple sequential finger tapping. Biological Psychiatry. 2006;59:48–56. doi: 10.1016/j.biopsych.2005.06.011. 16139806 [DOI] [PubMed] [Google Scholar]
- Nakao T., Radua J., Rubia K., Mataix-Cols D. Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. American Journal of Psychiatry. 2011;168:1154–1163. doi: 10.1176/appi.ajp.2011.11020281. 21865529 [DOI] [PubMed] [Google Scholar]
- Piek J.P., Dyck M.J. Sensory-motor deficits in children with developmental coordination disorder, attention deficit hyperactivity disorder and autistic disorder. Human Movement Science. 2004;23:475–488. doi: 10.1016/j.humov.2004.08.019. 15541530 [DOI] [PubMed] [Google Scholar]
- Pitcher T.M., Piek J.P., Hay D.A. Fine and gross motor ability in males with ADHD. Developmental Medicine and Child Neurology. 2003;45:525–535. doi: 10.1017/s0012162203000975. 12882531 [DOI] [PubMed] [Google Scholar]
- Querne L., Berquin P., Vernier-Hauvette M.P., Fall S., Deltour L., Meyer M.E., de Marco G. Dysfunction of the attentional brain network in children with developmental Coordination disorder: A fMRI study. Brain Research. 2008;1244:89–102. doi: 10.1016/j.brainres.2008.07.066. 18718456 [DOI] [PubMed] [Google Scholar]
- Ramtekkar U.P., Reiersen A.M., Todorov A.A., Todd R.D. Sex and age differences in attention-deficit/hyperactivity disorder symptoms and diagnoses: implications for DSM-V and ICD-11. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:217–228. doi: 10.1016/j.jaac.2009.11.011. 20410711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich W., Weltner Z., Herjanic B. The Diagnostic Interview for Children and Adolescents-IV. Multi-Health Systems; North Tonawanda, NY: 1997. [Google Scholar]
- Schall J.D. On the role of frontal eye field in guiding attention and saccades. Vision Research. 2004;44:1453–1467. doi: 10.1016/j.visres.2003.10.025. 15066404 [DOI] [PubMed] [Google Scholar]
- Shaw P., Eckstrand K., Sharp W., Blumenthal J., Lerch J.P., Greenstein D., Clasen L., Evans A., Giedd J., Rapoport J.L. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. 18024590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Malek M., Watson B., Sharp W., Evans A., Greenstein D. Development of cortical surface area and gyrification in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2012;72:191–197. doi: 10.1016/j.biopsych.2012.01.031. 22418014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. 12391568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suskauer S.J., Simmonds D.J., Caffo B.S., Denckla M.B., Pekar J.J., Mostofsky S.H. fMRI of intrasubject variability in ADHD: anomalous premotor activity with prefrontal compensation. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:1141–1150. doi: 10.1097/CHI.0b013e3181825b1f. 18724253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J.m., Posner M., Potkin S., Bonforte S., Youpa D., Fiore C., Cantwell D., Crinella F. Activating tasks for the study of visual–spatial attention in ADHD children: a cognitive anatomic approach. Journal of Child Neurology. 1991;6:S119–S127. doi: 10.1177/0883073891006001s12. 2002210 [DOI] [PubMed] [Google Scholar]
- Tian L., Jiang T., Wang Y., Zang Y., He Y., Liang M., Sui M., Cao Q., Hu S., Peng M., Zhuo Y. Altered resting-state functional connectivity patterns of anterior cingulate cortex in adolescents with attention deficit hyperactivity disorder. Neuroscience Letters. 2006;400:39–43. doi: 10.1016/j.neulet.2006.02.022. 16510242 [DOI] [PubMed] [Google Scholar]
- Valera E.M., Faraone S.V., Murray K.E., Seidman L.J. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2007;61:1361–1369. doi: 10.1016/j.biopsych.2006.06.011. 16950217 [DOI] [PubMed] [Google Scholar]
- Vannini P., Almkvist O., Franck A., Jonsson T., Volpe U., Kristoffersen Wiberg M., Wahlund L.O., Dierks T. Task demand modulations of visuospatial processing measured with functional magnetic resonance imaging. Neuroimage. 2004;21:58–68. doi: 10.1016/j.neuroimage.2003.09.033. 14741642 [DOI] [PubMed] [Google Scholar]
- Wilson B.N., Kaplan B.J., Crawford S.G., Campbell A., Dewey D. Reliability and validity of a parent questionnaire on childhood motor skills. American Journal of Occupational Therapy: Official Publication of the American Occupational Therapy Association. 2000;54:484–493. doi: 10.5014/ajot.54.5.484. 11006808 [DOI] [PubMed] [Google Scholar]
- Wilson P.H., McKenzie B.E. Information processing deficits associated with developmental coordination disorder: a meta-analysis of research findings. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 1998;39:829–840. doi: 10.1017/S002196309800276510.1111/1469-7610.00384. 9758192 [DOI] [PubMed] [Google Scholar]
- Woolrich M.W., Behrens T.E., Beckmann C.F., Jenkinson M., Smith S.M. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. 15050594 [DOI] [PubMed] [Google Scholar]
- Worsley K.J. Detecting activation in fMRI data. Statistical Methods in Medical Research. 2003;12:401–418. doi: 10.1191/0962280203sm340ra. 14599003 [DOI] [PubMed] [Google Scholar]
- Yousry T.A., Schmid U.D., Jassoy A.G., Schmidt D., Eisner W.E., Reulen H.J., Reiser M.F., Lissner J. Topography of the cortical motor hand area: prospective study with functional MR imaging and direct motor mapping at surgery. Radiology. 1995;195:23–29. doi: 10.1148/radiology.195.1.7892475. 7892475 [DOI] [PubMed] [Google Scholar]
- Zang Y.F., He Y., Zhu C.Z., Cao Q.J., Sui M.Q., Liang M., Tian L.X., Jiang T.Z., Wang Y.F. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain & Development. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. 16919409 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Brady M., Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation–maximization algorithm. IEEE Transactions on Medical Imaging. 2001;20:45–57. doi: 10.1109/42.906424. 11293691 [DOI] [PubMed] [Google Scholar]
- Zwicker J.G., Missiuna C., Harris S.R., Boyd L.A. Brain activation of children with developmental coordination disorder is different than peers. Pediatrics. 2010;126:e678–e686. doi: 10.1542/peds.2010-0059. 20713484 [DOI] [PubMed] [Google Scholar]
- Zwicker J.G., Missiuna C., Harris S.R., Boyd L.A. Brain activation associated with motor skill practice in children with developmental coordination disorder: an fMRI study. International Journal of Developmental Neuroscience: the Official Journal of the International Society for Developmental Neuroscience. 2011;29:145–152. doi: 10.1016/j.ijdevneu.2010.12.002. 21145385 [DOI] [PubMed] [Google Scholar]
- Zwicker J.G., Missiuna C., Harris S.R., Boyd L.A. Developmental coordination disorder: a pilot diffusion tensor imaging study. Pediatric Neurology. 2012;46:162–167. doi: 10.1016/j.pediatrneurol.2011.12.007. 22353291 [DOI] [PubMed] [Google Scholar]


