Abstract
The development of respiratory drive for vocalization was studied by observing chest wall kinematics longitudinally in 4 typically developing children from the age of 9 to 48 months. Measurements of the relative contribution of rib cage and abdominal movement during vocalization (i.e., babbling and true words) and rest breathing were obtained every 3 months using respiratory plethysmography (Respitrace™). Extending earlier findings in 15-month-olds, 2 methods of analysis of rib cage and abdominal movement were used: (a) a dynamic index of the strength of coupling between the rib cage and abdomen, and (b) a classification scheme describing the moment-by-moment changes in each of the 2 components (C. A. Moore, T. J. Caulfield, & J. R. Green, 2001). The developmental course of relative chest wall kinematics differed between vocalization and rest breathing. The coupling of rib cage and abdomen during vocalization weakened significantly with development, whereas it remained consistently strong for rest breathing throughout the observed period. The developmental changes in frequency of occurrence of relative moment-by-moment changes varied across movement type. The results support previous findings that speech breathing is distinct from rest breathing based on the relative contributions of the rib cage and abdomen. Longitudinal changes are likely responsive to anatomic development, including changes to rib cage shape and compliance.
Keywords: normal respiration, breathing, children, speech, motor development
Key considerations in the development of speech motor control include biomechanical changes (i.e., growth and maturation), coordinative support afforded by extant nonspeech behaviors, and the form of the most appropriate developmental model (e.g., a linear, stage, or other nonmonotonic model). The respiratory system is an attractive system for evaluating these considerations, as respiratory drive is essential to vocalization, undergoes known biomechanical changes, and has been shown to exhibit different actions for speech and nonspeech behaviors in toddlers (Moore, Caulfield, & Green, 2001). The present investigation was designed to capitalize on these features during development of speech motor control by children from 9 to 48 months of age. This longitudinal investigation is an extension of an earlier study of 15-month-olds by Moore et al.
Investigations of the development of oral articulatory kinematics have previously elaborated our understanding of the coordinative relationships among supraglottal speech structures. Recent investigations of the development of coordination of lip and jaw kinematics have revealed the emergence of predictable and distinct coordinative relationships from the age of 1 year through 6 years. For example, speech production in young children was characterized by the predominance and relative stability of jaw movement. Further development of speech motor control was characterized by increasing independence of upper and lower lip movement, and increased integration of lip movement into bilabial closure (Green, Moore, Higashikawa, & Steeve, 2000; Green, Moore, & Reilly, 2002).
Physiologic investigations of speech development suggest that the underlying coordinative organization for speech versus nonspeech tasks is distinct, both for adults (Moore, 1993; Wohlert & Goffman, 1994) and young children (Moore et al., 2001; Moore & Ruark, 1996; Ruark & Moore, 1997). Moore et al. observed distinct patterns of rib cage and abdominal movement for vocalization and rest breathing in typically developing 15-month-old children. However, it is unclear whether this speech/nonspeech distinction in toddlers was part of a developmental trend, phase, or stage. For example, the onset and persistence of this distinction is unknown and is unaddressed by developmental models. Explication of the maturation of respiratory kinematics may reveal the influence of structural changes in the lungs and muskuloskeletal systems, contrasting them to ever-increasing task demands associated with speech motor control. Whereas maturational effects are expected to be manifest as gradual changes to the relationship of the chest wall components, motor control adjustment influences might be inferred from more abrupt changes (Thelen, 1993).
A sharp distinction between speech and rest breathing might be anticipated given the vastly different behavioral goals and behavioral complexity of rest breathing and breathing during vocalization: gas exchange with minimum energy expenditure during rest breathing (von Euler, 1982) versus moment-by-moment, linguistically meaningful modulations of vocal parameters (e.g., intensity, onset/offset timing, quality) during speech. Ventilation is mediated by the central nervous system, which generates rhythmic drive to the respiratory pump and coordinated discharge of respiratory motoneurons. This pattern is continuously, adaptively adjusted to maintain homeostasis across the full range of this system’s metabolic activities (Hlastala & Berger, 1996). Breathing for vocalization further requires adjustments for a wide range of parameters, including such considerations as assuring (i.e., by inspiratory volume and regulation of expiratory impedance) adequate and appropriate airflow over the predicted length of utterance (Kent, 1999). Respiratory demands are compounded during vocalization, as ventilatory requirements persist, even as the competing linguistic, phonatory, and articulatory demands vary with each syllabic unit. Moreover, syntactic rules will predominately determine the occurrence of inspirations during speech, as well as the depth of inspiration, which is highly related to the utterance’s length and the location of the inspiratory phase within the utterance (Winkworth, Davis, Adams, & Ellis, 1995). Speech breathing further requires rapid and varying inspiratory–expiratory frequency and volume adaptations to accommodate changes in speech rate, loudness, and vocal fundamental frequency (von Euler, 1982).
Physiologic differences between speech and non-speech behaviors in adults have been uniformly observed across studies evaluating respiratory behavior in adults (e.g., Estenne, Zocchi, Ward, & Macklem, 1990; Hixon, Goldman, & Mead, 1973; Hoit, Plassman, Lansing, & Hixon, 1988). Hoit and colleagues (1988) demonstrated higher amplitude abdominal electromyographic activity during speech breathing than rest breathing, suggesting greater abdominal activation during speech than rest. Observation of chest wall kinematics has revealed that rest breathing is characterized by the synchronous movement of the rib cage and abdomen (Hixon, 1973) and that speech breathing is characterized by occasional asynchronous (Hixon et al., 1973) and oppositional rib cage–abdominal movement (Hixon, 1973; Hodge & Rochet, 1989).
Studies of children have revealed similar distinctions across speech and nonspeech respiratory behaviors. Moore and colleagues (2001) described task-specific differences in toddlers, quantifying the relative contributions of rib cage and abdomen to respiration during vocalization. This investigation included a two-part analysis of relative respiratory kinematics in typically developing 15-month-old children. A correlational analysis of rib cage and abdominal movement, and classification of the relative direction of movement (e.g., coupled movement outward for inspiration) revealed significantly greater synchrony between rib cage and abdominal movement during rest breathing than during speech breathing. Oppositional movement of rib cage and abdomen was observed more frequently during speech breathing.
Although they did not specifically compare the underlying coordination of rest breathing versus vocalization, Boliek and colleagues conducted two longitudinal investigations that provided kinematic and volumetric data for a wide range of vocal behaviors (e.g., phoneme production, cries, and whimpers) of children from the age of 5 weeks to 1 year (Boliek, Hixon, Watson, & Morgan, 1996), and syllable and word productions of children from 1 to 3 years (Boliek, Hixon, Watson, & Morgan, 1997). These investigators found that chest wall kinematics were similar across vocalization types for males and females. Both intra- and interparticipant variability were relatively large. Older children exhibited proportionately smaller rib cage contributions to lung volume changes than younger children.
Though it seems clear that respiratory behaviors for rest and vocalization are distinct, especially in adults, the emergence of this distinction is undocumented. The context in which babbling and speech emerge is essential to an understanding of speech development and perhaps of the coordinative infrastructure of mature speech. A variety of developmental models may be appropriate to the acquisition of speech breathing. For example, are the distinct processes of speech and nonspeech breathing the consequence of a divergence of a common coordinative antecedent, or are these contrasting processes evident at the earliest emergence of babbling and speech? Are distinct stages evident in respiratory coordination across development, or is maturation better represented as a continuous process of refinement? Are changes in respiratory coordination coincident with anatomic changes or with the achievement of other milestones of motor development? Observation of the early stages of vocal and speech development can be applied to some of these persistent questions.
Longitudinal changes in respiratory kinematics during rest and vocalization might be expected to reflect the well-documented rapid and dramatic developmental changes in anatomic structure. The structural characteristics of the young infant’s chest wall render his or her system surprisingly inefficient for respiration. For instance, the small zone of apposition limits the capacity of the diaphragm to expand the rib cage (Hershenson, 1992). Developmental changes that gradually facilitate respiration include decreasing rib cage compliance (Bryan & Wohl, 1986; Sharp, Druz, Balagot, Bandelin, & Danon, 1970) and gross changes in the shape of the rib cage (Openshaw, Edwards, & Helms, 1984). Additionally, changes in gravitational influences arising from the infant’s change to upright posture cause the ribs to assume a more diagonal orientation (Hershenson, 1992), which increases the capacity of the thoracic muscles to expand the rib cage (Hershenson, Colin, Wohl, & Stark, 1990).
These structural changes yield an increased rib cage contribution to breathing and a reduced reliance on abdominal activity; the child’s reliance on rib cage activity increases with maturation. The increased independence and greater potential contribution of the rib cage provide an additional mechanism by which the child can achieve fine control of respiration. This fine control of respiratory drive is essential to the development of the child’s full range of phonatory behaviors, including precise modulation of fundamental frequency and intensity, as well as utterance length and sentential phrasing. Investigations of suprasegmental stress production have consistently revealed controlled modulation of fundamental frequency and intensity throughout the first years of life (Kehoe, Stoel-Gammon, & Buder, 1995; Pollock, Brammer, & Hageman, 1993). The coordinative organization underlying these capacities is unknown. The present investigation was designed to evaluate development of speech breathing in children from the ages of 9 to 48 months. Rapidly changing anatomic and physiologic conditions, as well as developing speech capabilities, were expected to entail coordinative adjustments, which were investigated using respiratory plethysmography.
Method
Participants
Four children (3 girls, 1 boy) participated in this longitudinal study. Observations occurred at 3-month intervals (± 2 weeks) from the age of 9 months through 48 months, for a total of 14 sessions for each child. This age range was chosen to include speech development from babbling to multiple-word utterance production. Forty-three experimental sessions were completed; 13 sessions were not completed because of participant illness or unavailability. Participants had no known neurologic deficit. None of the 4 children ever exhibited hearing sensitivity less than normal limits; outer and middle ear function on the day of testing was assessed using otoscopy and tympanometric screenings whenever possible. As anticipated for toddlers, not all children tolerated otoscopy and tympanometry at each session without considerable effort. This effort and potential irritation of the child had to be balanced against each child’s temperament with respect to vocal output in the immediately ensuing experimental session. Parents reported achievement of gross motor, fine motor, cognitive, and speech and language milestones within normal limits.
Experimental Protocol
Rest breathing and vocalization breathing were transduced using a commercially available respiratory inductance plethysmograph (Respitrace™). This system transduces the circumferences of the rib cage and abdomen using two elasticized bands called “respibands.” The transducers were not calibrated, because of methodological limitations imposed by the children’s inability to comply with calibration task demands (e.g., production of vital capacity and isovolume maneuvers) and because only relative measures were used in the present analysis. The bands were placed on bare skin or over light clothing. The rib cage transducer was placed around the rib cage, under the arms and as high as possible. The abdominal transducer was centered vertically on the umbilicus and placed posteriorly so that it did not overlie the ribs, or did so minimally. The transducers did not overlap.
Each child sat upright in a highchair or at a play table to ensure that he or she did not apply external pressure to the Respitrace bands. Spontaneous and imitated sound productions were elicited using toys, books, and games, which were placed directly in front of the child to minimize extraneous reaching movement. An investigator seated next to the child provided a gloss of all sound productions, identified rest breathing periods, and noted occurrences of extraneous movement. The child’s caregiver, also seated next to the child, provided further assistance eliciting specific behaviors and identifying the child’s activities. Target behaviors were identified in the taped session and were subsequently parsed for further analysis. Artifacts resulting from postural changes and reaching movements were excluded from the analysis. Speech utterances were recorded over a period of approximately 20 min; each utterance was preceded by at least one cycle of rest breathing, although these cycles were not included in the kinematic analysis of the token. Inclusionary criteria required that each sample be free of acoustic artifact (e.g., audio signals free of simultaneous utterances by the experimenter or caregiver), movement artifact, crying, laughing, or chewing. Rest breathing data were obtained throughout the session and were identified as uninterrupted periods of rest breathing for at least 3 cycles.
Sampling Procedure
Samples were collected and categorized as rest breathing (uninterrupted periods of rest breathing at least 3 s in duration and including at least three contiguous cycles) or vocalization, which included babbling (including at least one consonant–vowel or vowel–consonant sequence) and true words. Utterances classified as true words included those productions that were clearly referential or explicit requests, or those recognized by the parent as a part of the child’s meaningful speech repertoire. Utterances that were nonreferential and nonrequests were classified as babbling.
Rest breathing samples ranged from 3 to 21 cycles and were analyzed as a single behavior. No rest breathing samples were included that immediately preceded or followed vocalization. Babbling and true word samples were identified using the audio channel, and the boundaries of the samples were demarcated by the acoustic onset and offset. Babbling and true word tokens did not include preceding or trailing rest breathing cycles. Multisyllabic and multiword utterances were treated as one token. Multiple sequential utterances, operationally defined as utterances separated by at least 300 ms, were digitized separately.
Signal Processing
Output signals from the transducers were low-pass filtered using analog filters with a cutoff of 30 Hz, and recorded continuously using an FM instrumentation recorder (frequency response: DC-1250 Hz; S/N ≥ 50 dB) for offline digitization. Vocalizations were recorded on a separate AM channel of the FM recorder using a wireless lapel microphone. Respitrace signals (two channels) were again filtered for anti-aliasing (flow-pass = 30 Hz) and digitized at 66.7 samples per second per channel. Audio signal processing included filtering (fhigh-pass = 350 Hz, flow-pass = 5000 Hz) and digitized at 25,600 samples per second. Following digitization, the audio waveforms were full-wave rectified and integrated to facilitate identification of speech onset and offset during the analysis.
Analysis
Because the respiratory signals were uncalibrated, only relative motions of the chest wall were analyzed (Moore et al., 2001). The two independent indices of respiratory kinematics were: (a) correlational analysis, which indicated the synchrony of changes in rib cage and abdominal dimensions, and (b) a classification scheme that described the moment-by-moment directional changes in each of the two components (i.e., both rib cage and abdomen expanding, both compressing, abdominal compression with rib cage expansion, abdominal expansion with rib cage compression).
Using a custom routine written for MATLAB (Version 11; The Mathworks, Inc., 1999), a simple correlation function using a 1-s sliding window was applied to the rib cage and abdominal signals. The window was advanced one point (15 ms) for each correlation computation, yielding a function (rmoving) comprising the coefficients derived from each iterative computation of the correlation. This method provided a basis for comparison with earlier investigations (e.g., Moore et al., 2001) and allowed for qualitative observation of changing chest wall kinematics within each token. The 1-s window width, selected empirically from a range of 100 ms to 2s, was judged to provide the most appropriate temporal resolution. Window sizes larger than 1 s were overly smoothed and insufficiently sensitive to detect speech intervals, which were all less than 1 s in duration. Smaller window sizes were not smooth enough, with the resultant function emphasizing very brief (e.g., less than 100 ms) or transient relationships between the two signals. Portions of the rmoving function associated with rest or speech were isolated during analysis using cursor placement (i.e., to identify acoustic onsets and offsets for speech production) and averaged, following Fisher’s Z transformation. This transform was required for the descriptive and inferential analyses. The utility of a moving correlation function has been described in detail by Moore et al. This function is limited in that it only quantifies the degree of covariation of these two channels. It does not code, for example, the direction of movement of either component. Because of this limitation, the classification analysis was implemented.
In addition to analysis of the magnitude of each coefficient on the correlation function (i.e., indicating the synchrony of rib cage and abdominal displacement), each rmoving point was categorized according to the slope of each Respitrace signal at that moment (i.e., indicating the relative direction of movement). The slope of each signal, categorized as upward, downward, or flat, was determined for the 200 ms window centered on the rmoving point and was moved through each signal in 16.7 ms increments. This window width was determined empirically to optimize the sensitivity of the slope measure.
Classifications of relative slopes included: (a) coupled inspiration (CI)—both waveforms positive-going (i.e., expanding); (b) coupled expiration (CE)—both waveforms negative-going (i.e., compressing); (c) oppositional movement of the rib cage and the abdomen, with the rib cage compressing and the abdomen expanding (AE); (d) oppositional movement, with the abdomen compressing and the rib cage expanding (RCE); and (e) unspecified (UN)—the average slope of either signal was not significantly different from zero (i.e., one or both of the signals was flat, most commonly occurring at the peaks and troughs of the waveforms). Because respiratory airflow was not obtained, no inference regarding airflow direction was possible and the oppositional component (i.e., rib cage or abdominal paradoxing) could not be specified. UN points were defined operationally as those occurrences for which the slope of the 200 ms analysis window was less than 30.3% of the standard deviation of the first derivative of the entire signal for that channel. The inclusion of the UN category was necessary to avoid false indications of paradoxical movement, which would arise when the slopes of each signal were near zero and slight differences or asynchronies could yield opposite slope directions.
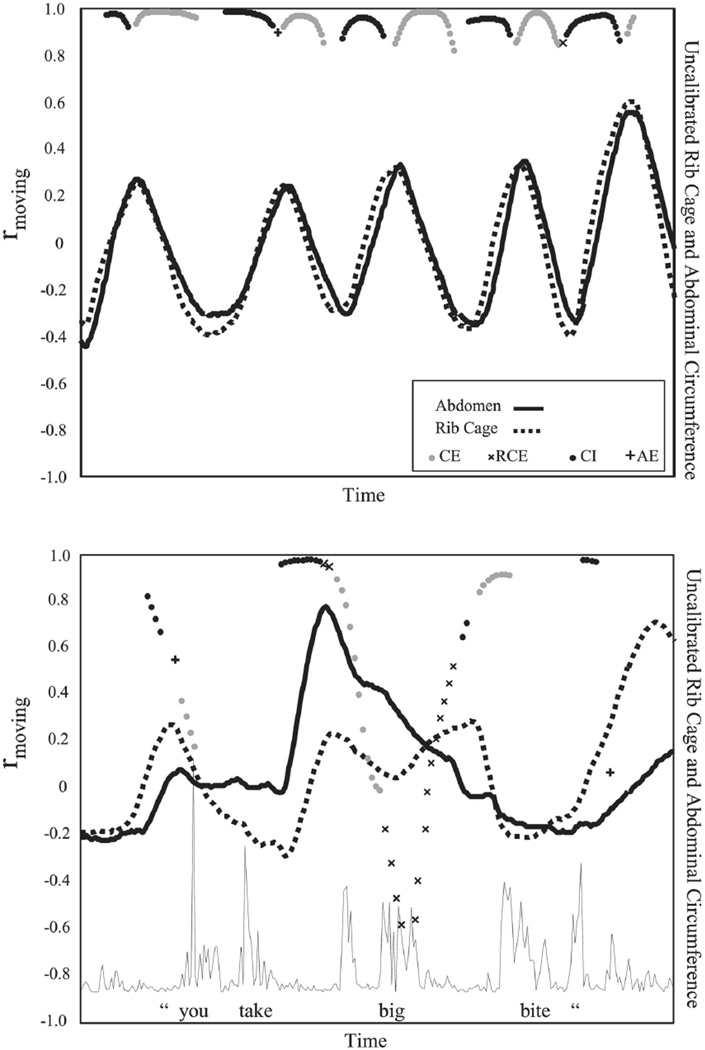
Figure 1 provides an example of the Respitrace signals and analysis of rmoving and kinematic types for a period of rest breathing and production of an utterance. The example includes a 24-month-old child’s rib cage and abdominal movement and correlations during rest (upper panel) and vocalization of “you take big bite” (lower panel). Although this figure includes rest breathing prior to and following the speech utterance, tokens were parsed at the acoustic signal onset and offset, so these rest breathing portions were not used in the analysis of this vocalization token. Tightly coupled, synchronous movement of the rib cage and abdomen (CI, plotted with a dark dot; CE, plotted with a light dot) characterized rest breathing; a sharp drop in the rmoving values suggested weak rib cage–abdominal coupling and was characteristic of breathing during vocalization. Whereas few oppositional movements (RCE, plotted with an x; AE, plotted with a +) were present in the rest breathing sample, frequent oppositional movements were apparent in the vocalization sample. Further analyses were conducted using the average of all of the rmoving values within a token. In addition, all kinematic category values were combined to calculate the percentage of occurrence for each type. Therefore, each utterance yielded five kinematic categories and one value for rmoving. For example, the rmoving value for the rest breathing panel in Figure 1 was .97. The occurrence of the kinematic types for the rest breathing panel was 38.6% CI, 39% CE, <1% AE, <1% RCE, and 22.4% UN, though this latter type is not indicated on the figure.
Figure 1.
Twenty-four-month-old child’s rib cage and abdominal movement, and corresponding rmoving and classification of moment-to-moment relationship during rest (upper panel) and vocalization of “you take big bite” (lower panel). The solid line (—) indicates movement of the abdomen, and the dashed line (- - -) indicates movement of the rib cage. The rmoving value is identified with a dark dot (CI = coupled inspiration), a light dot (CE = coupled expiration), an “x” (RCE = rib cage expanding), and a “+” (AE = abdomen expanding). For clarity, points in the unspecified (UN) classification are not plotted. Upward on the y-axes indicates increasing circumference.
The coupling strength and relative movement of the rib cage and abdomen for rest and vocalization breathing were compared using these measures. Quantitative differences among behaviors were tested by statistical analysis of rmoving and the proportionate occurrences of kinematic categories (i.e., %CI, %CE, %AE, %RCE, and %UN). Participant effects were statistically analyzed with analysis of variance (ANOVA). The use of nonparametric statistical analyses was necessary to test differences in averaged rmoving values and the proportionate occurrences of kinematic categories during speech and nonspeech behaviors because the data did not meet the assumption of normality and/or equal variance. These tests included the Mann–Whitney rank sum test to compare two groups and the Kruskal–Wallis one-way ANOVA on ranks to compare more than two groups. Post hoc analyses were conducted using Dunn’s method, which evaluates differences revealed by an ANOVA on ranks when the sample sizes of experimental groups are different.
Results
The present methods were designed to reveal differential developmental changes in respiratory kinematics for vocalization and nonspeech breathing, and to evaluate how developmental changes in kinematics might occur with respect to the relative movements of the ribcage and abdomen. Specifically, these analyses addressed the empirical questions of whether differences could be observed across types of vocalization, across tasks (i.e., vocalization vs. rest breathing), across participants, and over development. These differences were evaluated for each of the six measured variables (rmoving, frequency of occurrence of CI, CE, AE, RCE, and UN).
A total of 1644 respiratory samples were obtained from 4 children, each observed longitudinally across the ages of 9 to 48 months. Of the samples obtained, 188 were rest breathing and 1,456 were vocalization breathing samples (including 85 samples of babbling and 1,371 samples of true words). Babbling tokens occurred at the ages of 9 to 18 months. No true words were acquired at the 9-month visits, and true word data were captured from only 1 child at 12 months. Table 1 summarizes the data set, including the number of children participating at each age.
Table 1.
Composition of data set (no. of participants present and total types of tokens produced at each age).
| Months | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Task | Total | 9 | 12 | 15 | 18 | 21 | 24 | 27 | 30 | 33 | 36 | 39 | 42 | 45 | 48 |
| No. participants | 2 | 2 | 4 | 4 | 3 | 4 | 2 | 3 | 3 | 4 | 4 | 3 | 4 | 1 | |
| Rest breathing | 188 | 6 | 10 | 12 | 18 | 11 | 28 | 6 | 10 | 11 | 26 | 13 | 10 | 24 | 3 |
| Babbling | 85 | 22 | 34 | 16 | 13 | — | — | — | — | — | — | — | — | — | — |
| True words | 1371 | — | 9 | 69 | 77 | 90 | 239 | 38 | 109 | 96 | 168 | 155 | 118 | 173 | 30 |
Prior to computing the rmoving function and moment-by-moment classifications, preliminary statistical analyses were used to evaluate potential task differences across subsets (i.e., types of vocalization, individual participants) of the data set. These statistical results were used to assess the validity of combining babbling and true words into a single “vocalization” category. In addition, because of the small number of participants observed, a statistical evaluation of participant effects was used to evaluate whether any single participant disproportionately affected the results of the group analysis. Average rmoving functions and frequency of occurrence of kinematic types were collapsed across repetitions within each participant and each behavior type (i.e., true words, babbling, rest) prior to these statistical procedures.
Types of Vocalizations
Previous investigators have not found significant differences in respiratory measures among types of vocalization by young children (Boliek et al., 1996, 1997; Moore et al., 2001). Accordingly, comparisons among rest breathing, babbling, and true words were completed to evaluate the effect of combining babbling and true word samples into a single task category including all types of vocalization. The underlying assumption of this analysis was that if the two vocalization tokens (babbling and true words) did not differ from one another, yet did differ from rest tokens, the tokens could be reasonably considered as a single category in addressing the primary questions of this investigation. A one-way ANOVA was used to compare rest, babbling, and true word samples at the ages for which babbling occurred (9–18 months). Kruskal–Wallis one-way ANOVA on ranks was applied to rmoving and to the frequency of occurrence of each of the five kinematic types. Dunn’s method revealed significant pairwise differences (p < .05) only between rest breathing and babbling and between rest breathing and true words for rmoving, CI, RCE, and UN. For the frequency of AE occurrences, a significant difference was found only between babbling and rest breathing (p < .05). No significant differences were obtained between babbling and true words for any of the six measures. The frequency of occurrence of CE was not significantly different among the three tasks. Because no comparison of babbling and true word production was found to be statistically significant, these two task types were combined into a single category of vocalization for subsequent analyses.
Participant Effects
Because of the relatively small number of participants observed, an analysis was conducted to evaluate the effect and variability attributable to each participant. A Kruskal–Wallis one-way ANOVA on ranks was used to compare each participant’s averaged rmoving and frequency of kinematic types. There were no significant differences among participants for any of the measures, with the exception of CE, H(3) = 7.845, p = .049, which did reveal an effect for individual participants. The use of Dunn’s method for post hoc analysis did not reveal significant differences between any participant pairs. These findings suggested that no individual participant contributed disproportionately to the group effects observed. Accordingly, subsequent analyses were completed only for group data.
Comparison of Rest Breathing and Vocalization
One of the primary questions in the present investigation was whether there were coordinative differences in the movement of the ribcage and abdomen during rest breathing and tasks involving vocalization. A Mann–Whitney rank sum test was used to compare the averaged rmoving and kinematic category tallies. The results of these tests are shown in Table 2. A task analysis was used to determine whether the chest wall kinematics of rest breathing and vocalization differed when averaged across the age range of 9–48 months. Significant task differences were found for all measures (p < .001), suggesting that even young children demonstrate different chest wall kinematics while rest breathing versus vocalizing. Further analyses were used to reveal the developmental and task-specific nature of the differences in respiratory kinematics.
Table 2.
Mann–Whitney rank sum test comparing rmoving and relative frequency of kinematic type values for rest breathing versus vocalization.
| Measure | T |
|---|---|
| rmoving | 2794* |
| Coupled expiration (CE) | 2496* |
| Coupled inspiration (CI) | 2795* |
| Unspecified (UN) | 962* |
| Oppositional movement with rib cage expanding (RCE) | 952* |
| Oppositional movement with abdomen expanding (AE) | 1017* |
p < .001.
Developmental Changes
Of particular interest in this longitudinal investigation was how measures of respiratory kinematics change with development. Developmental effects were evaluated using linear regression, which revealed developmental rates as well as task-specific differences in developmental change. For example, a significant correlation between rmoving and participants’ age was taken to indicate a developmental change in chest wall kinematics. The lack of a significant correlation coefficient (i.e., not significantly different from zero) suggested that the parameter did not change with development. Regression results also permitted comparisons of developmental changes across tasks (i.e., rest breathing vs. combined vocalization tasks).
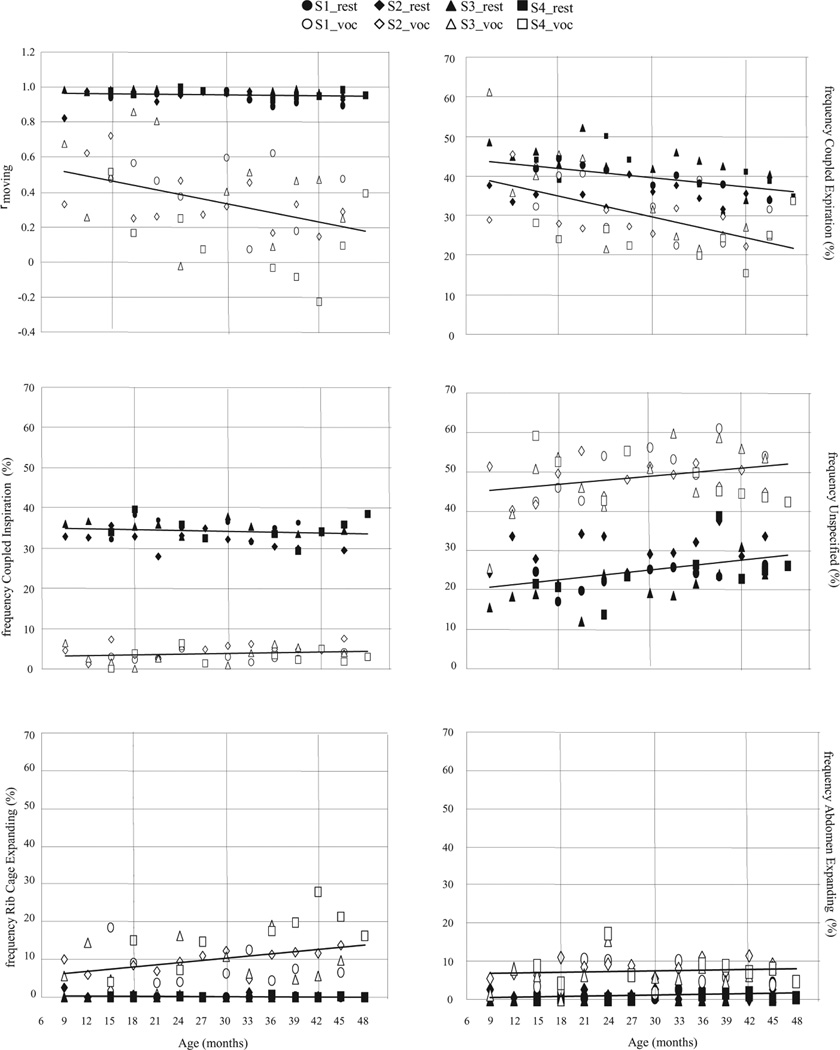
Table 3 provides the Pearson product–moment correlation coefficient, as well as the linear regression slope and intercept and the statistical probability of each linear regression being nonzero. Averages for rmoving and kinematic type were determined for each child at each age (i.e., an average across tokens such as those seen in Figure 1). Figure 2 illustrates these averages for each participant at each age and the linear regression line computed for each task type (rest breathing and vocalization) across all participants and ages. These six panels depict the developmental changes in ribcage–abdominal coupling and their relative movement during vocalization and rest breathing. Results of the regression analysis (see Table 3), as shown in Figure 2, revealed distinct developmental courses for rest breathing and vocalization. In general, these developmental changes can be described as being much more notable for vocalization. Coupling of ribcage and abdomen (rmoving), for example, remained extremely rigid for rest breathing throughout the developmental period studied (i.e., the average correlation coefficient was near 1.0 and did not change significantly with age; p = .46), whereas coupling declined significantly (p < .01) with development of vocalization. CE decreased significantly with age for both tasks (p < .01). The frequency of CI occurrences did not change with maturity for either task. The frequency of UN occurrences increased significantly with age for rest breathing only (p < .05). The frequency of oppositional movement with the RCE was relatively stable for rest breathing across ages, whereas a significant relationship between frequency of oppositional movement and age was observed during vocalization (p < .05). Occurrences of AE increased significantly only for rest breathing (p < .05).
Table 3.
Pearson product–moment correlations and linear regression equations for respiratory kinematics of rest breathing and vocalization across the ages of 9 to 48 months.
| Variable and task |
r |
m (slope) |
b (intercepts) |
p |
|---|---|---|---|---|
| rmoving | ||||
| Rest | –.116 | –.0003 | .966 | .457 |
| Vocalization | –.416 | –.009 | .597 | <.01** |
| % CE | ||||
| Rest | –.436 | –.200 | 45.4 | <.01** |
| Vocalization | –.560 | –.436 | 42.6 | <.01** |
| % CI | ||||
| Rest | –.153 | –.034 | 35.1 | .327 |
| Vocalization | –.181 | –.030 | 2.87 | .246 |
| % RCE | ||||
| Rest | –.185 | –.007 | .451 | .235 |
| Vocalization | .367 | .193 | 4.60 | <.05* |
| % AE | ||||
| Rest | .328 | .030 | .068 | <.05* |
| Vocalization | .116 | .034 | 6.22 | .458 |
| % UN | ||||
| Rest | .384 | .203 | 19.0 | <.05* |
| Vocalization | .284 | .169 | 44.0 | .064 |
Significant regression value at p < .05.
Significant regression value at p < .01.
Figure 2.
Developmental changes from 9 to 48 months in amount of rib cage–abdominal coupling (rmoving) and percentage of occurrence of type of relative movement for vocalization and rest breathing. The linear regression line demonstrates the tendency of the behavior to become more prevalent or less prevalent, or to lack change with development.
To summarize these results, relatively large differences were observed with respect to the developmental course for rest breathing and breathing for vocalization. Developmental changes in the respiratory kinematics of rest breathing were generally characterized by consistently rigid coupling among ribcage and abdominal movements. A slight decrease in the proportion of CE occurrences during rest breathing appeared with development, concomitant with a comparable increase in the relative occurrence of UN specified kinematic events (i.e., periods during which one or both kinematic signals have slopes near zero).
Breathing during vocalization, in contrast, exhibited a marked decrease in ribcage–abdominal coupling, which was characterized by decreasing relative occurrence of CE and increasing RCE (presumably indicating ribcage paradoxing, as vocalization was always observed to occur with expiratory airflow). Thus, rest breathing appears to be characterized by consistent unitary behavior of the ribcage and abdomen throughout this developmental period, whereas development of speech vocalization appears to be characterized by decreasing coupling across the chest wall, including an increasing frequency of ribcage paradoxing. An exception to the consistent coupling during rest breathing was the significant increase of AE (abdominal expansion with oppositional compression of the rib cage) during rest breathing. However, these occurrences contributed less than 10% of the observed kinematic events on average.
The correlation coefficient did not remain constant across development, even for rest breathing. Of particular interest were brief decreases in the rmoving function, which were observed most often at the end of each inspiratory or expiratory phase, when the chest wall components asynchronously reversed direction. These brief periods of asynchrony probably reflected the individual biomechanical characteristics of the rib cage and abdomen as active muscular influences were momentarily minimized, inhibited, and reversed.
Discussion
The results of this investigation demonstrated task-specific relative respiratory kinematics for rest breathing and vocalization in children during the age range of 9–48 months. Rest breathing and vocalization breathing were clearly distinguished by the observed correlations between rib cage and abdominal movement during development. This distinction revealed increasing independence (i.e., weaker coupling) of the chest wall components during vocalization, compared to very high coupling during rest breathing. Additional evidence of increasing abdominal and rib cage independence was found in the increasing proportion of ribcage expansion during vocalization (i.e., the frequency of occurrence of RE increased approximately 7% over 3 years) and in the decrease in CE of rib cage and abdominal components during vocalization (i.e., the frequency of occurrence of CE decreased at a rate of approximately 15% over 3 years).
The present results also showed that these differences increased with age. Like earlier investigations of nonspeech motor behaviors, which revealed early synchrony of motor structures and the decoupling of the structures with development (Forssberg, 1985; Hofsten, 1989), the present findings revealed that maturation of breathing for vocalization was characterized by increasing independence of the chest wall components (i.e., decoupling of rib cage and abdomen). The results of linear regression analyses also suggested that changes in respiratory kinematics with development were gradual rather than abrupt, which would be anticipated if coordinative changes were consistent with a stage model of development (Kent, 1999; Stathopolous & Sapienza, 1993). This gradual change in respiratory kinematics may implicate parallel developmental changes in chest wall anatomy and biomechanics as the likely contributors to the observed increase in ribcage and abdominal independence. Greater detail with respect to the nature of these developmental changes was gleaned from closer inspection of the individual measures.
Specific Findings
Kinematic Types
Developmental changes in the moment-to-moment relationship between the two chest wall components provided several indications of changes in respiratory kinematics with the acquisition of speech. The strikingly higher frequency of occurrence of CI tokens during rest breathing than vocalization breathing was consistent across ages. This finding was not surprising, as inspiratory vocalizations were never observed, and only occasional, very brief inspirations (e.g., <300 ms) were noted during vocalization. These occasional inspiratory periods were seen in the productions of longer utterances by children at the later ages, as exemplified in Figure 1. Like rest breathing, these very brief inspiratory moments demonstrated very high coupling. Most importantly, as observed by the parallel, flat slopes of the regression lines for CI in Figure 2, this difference persisted throughout the developmental period observed, with no significant change in the magnitude of the difference.
A more complex interpretation is required for the task differences and developmental changes seen for the CE classification. The consistently lower proportion of CE occurrences during vocalization and the increasing number of UN specified occurrences was consistent with the notion that chest wall components exhibited greater independence with development of speech. The UN specified classification served two purposes: one as a conservative means of eliminating the spurious classification of very low slopes (essentially flat traces) as one of the four primary types (i.e., CI, CE, RE, AE), but another as a method for identifying periods during which only one of the two chest wall components was changing. Commonly observed patterns classified as UN included both the brief, static posturing of both the rib cage and abdomen and the persistent static posturing of the abdomen against rib cage compression. Inspection of the rmoving function was used to clarify the nature of these different types of UN intervals. For example, a higher occurrence of UN intervals during vocalization than rest was anticipated based on earlier observations of abdominal fixation and rib cage compression during speech production (Hixon et al., 1973). This respiratory posture is illustrated in two instances in the lower panel of Figure 1, during “you take” and during “bite”. The coincident dramatic drop in the value of rmoving confirms that these intervals of UN specified kinematics involved uncoupling of the chest wall components.
Rest breathing exhibited the contrasting type of UN specified kinematic relationship; consistently high values for rmoving indicated that both chest wall components remained relatively unchanged for longer periods of time with development. This pattern was also anticipated, as the slower respiratory rates that occur with development entail longer periods at the extremes of each cycle (i.e., the peaks and troughs of the kinematic traces), giving rise to a higher frequency of occurrence of UN specified, though highly correlated, kinematic events in the present quantitative method. Comparison of these two variables across development supported the suggestion that breathing for vocalization is qualitatively distinct from that of rest.
Individual Participant Data
Although statistical analysis did not reveal a significant difference among the observations of individual participants, several observations in the development of respiratory kinematics across these 4 participants were noteworthy. Whereas all 4 participants demonstrated lower averaged rmoving values at 48 months than at the earliest age of data collection (9 or 12 months), the participants did not consistently decrease in rmoving from month to month, indicating highly individual rates and patterns of development and variability. These changes deserve more intensive observation using finer grained sampling, greater morphometric detail, and larger participant pools. For example, for the occurrences of UN, Participant 4’s profile was opposite that of the group finding. This participant showed a decrease of UN occurrences during vocalization, unlike the remaining participants, who exhibited increased percentages of occurrences with development.
General Interpretation
The two primary findings of this investigation were task differences of respiratory kinematics and clear changes in respiratory kinematics with speech development during this period. These findings can be interpreted with respect to developmental trends of increasing independence of structures, anatomical/biomechanical changes, and models of speech development. The results expand the findings of Moore et al. (2001) to reveal not only task-specific differences between respiratory kinematics in childhood, but also differences in their developmental profiles with maturation. These findings also support previous investigations across speech structures that have consistently revealed differences in the underlying coordinative organization for speech versus nonspeech tasks for both adults (Moore, 1993; Wohlert & Goffman, 1994) and young children (Moore et al., 2001; Moore & Ruark, 1996; Ruark & Moore, 1997).
Developmental Relationships Among Orofacial and Respiratory Speech Structures
The task differences observed are consistent with increasing degrees of freedom among respiratory structures and are congruent with our understanding of a range of early skilled movement, including locomotion (Forssberg, 1985; Thelen & Cooke, 1987; Thelen & Fisher, 1982), reaching (Gatev, 1972; Hadders-Algra, Van Eykern, Klip-Van den Nieuwendijk, & Prechtl, 1992; Hofsten, 1989; Konczak & Dichgans, 1997), and bilabial speech production (Green et al., 2000), each of which has been characterized by the limited independence of functional components (Provins, 1997). These investigations of motor behaviors have further revealed increasing independence of the structures during development. The development of locomotion, reaching, and grasping are each characterized by a tightly constrained and uniform pattern of joint movement in infancy, progressing to a dissociated pattern in the adult (Forssberg, 1985; Hofsten, 1989). With respect to speech development, Green and colleagues (2000) speculated that the control of speech articulation follows a general-to-specific course, with increasing independence of the articulators across development. In this investigation of the development of labiomandibular coordination, these researchers found an early predominance of jaw control and increasing independence of the upper and lower lips with development.
Similar to these earlier findings in development of speech and nonspeech oral motor behaviors, the present findings indicated that the functional relationship between the rib cage and abdomen changed across development, with increasing independence and decoupling of the chest wall structures during vocalization. The increasing independence of the chest wall components for vocalization with development is consistent with the idea that breathing for vocalization exploits the multiple degrees of freedom available to the chest wall, whereas rest breathing does not. Unified movement of the rib cage and abdomen overwhelmingly characterized rest breathing (i.e., CI and CE). Vocalization breathing incorporated various independent movements of the components, which was consistent with earlier findings that children (Boliek et al., 1997) and adults (Hixon et al., 1973) use multiple degrees of freedom to produce respiratory drive for vocalization. This independent movement became increasingly apparent during vocalization with development and may have reflected an increase in coordinative plasticity (Green et al., 2000) complementary to the child’s diminishing need to reduce coordinative complexity by constraining the motor system’s degrees of freedom.
The Influence of Anatomic and Physiologic Changes
The present findings were especially interpretable with respect to well-known anatomic changes. These changes include decreasing compliance of the rib cage (Papastamelos, Panitch, England, & Allen, 1995; Sharp et al., 1970) and changes in the gross shape and orientation of the rib cage (Openshaw et al., 1984). The relationship of the rib cage and abdomen during rest breathing in infancy underlies significant biomechanical changes in the chest wall with development. One consequence of these changes is the gradual decrease in rib cage paradoxing during inspiration, which typifies breathing in early infancy (Gaultier, Praud, Canet, Delaperche, & D’Allest, 1987). This inspiratory paradoxing, characterized by the inward movement of the rib cage with concurrent expansion of the abdomen and contraction of the diaphragm, is widely understood to be the result of high rib cage compliance.
Unlike inspiratory paradoxing arising from biomechanical factors, oppositional movement during expiratory vocalization can be alternatively interpreted as reflecting the increasing independence of the chest wall components with development. In the present investigation, maturation of vocalization breathing exhibited an increase in oppositional movement with the RCE. This oppositional movement could be assumed to represent rib cage paradoxing, given expiratory flow for vocalization and abdominal compression. Though these changes in respiratory kinematics may represent passive changes resulting from biomechanical development, it may be that this coordinative organization provides a level of fine motor control that is best attained by modulation of abdominal compression against a passively expanding, increasingly stiff rib cage. Future investigations will be necessary to isolate these effects during vocal development.
Developmental changes in rib cage biomechanics may further explain the observed increase in rib cage contribution and the increased independence of the chest wall components during respiration. The role of the rib cage increases for rest breathing (Hershenson et al., 1990) and for speech (Hixon, 1982; Hoit, Hixon, Altman, & Morgan, 1989; Stathopoulos & Sapienza, 1993). The contribution of rib cage movement to tidal breathing during quiet sleep significantly increases through the first year of life, probably as a consequence of changes in rib cage shape, compliance, and deformability (Hershenson et al., 1990). Investigators have further demonstrated that children ages 4–14 years (Hoit, Hixon, Watson, & Morgan, 1990) and 7–16 years, (Stathopoulos & Sapienza, 1993), as well as adults (Hixon, 1982; Hoit et al., 1990; Stathopoulos & Sapienza, 1993), primarily use rib cage movement to produce lung volume changes for speech. The predominate contribution of rib cage motion to lung volume changes is consistent with the fact that rib cage motion is generally more efficient at producing lung volume exchange than abdominal motion. This greater efficiency is attributable to the greater surface area of the lung adjacent to the rib cage than the diaphragm (Solomon & Charron, 1998).
As is true for most developmental processes, multiple pressures probably influence the development of speech motor control. In this regard, it seems probable that in addition to anatomic changes, factors such as social, cognitive, and linguistic influences (e.g., lexical, phonetic, syntactic) affect articulatory and respiratory kinematics. The reduced linguistic complexity in children’s speech may not adequately tax the respiratory system. Positional and gravitational effects will vary for individuals of different body morphology (Boliek et al., 1997). Determination of these multiple effects will rely on multifactorial models of speech development, which might predict, for example, continuous growth effects or abrupt state changes paralleling linguistic stages. In this context, the current findings support a model of gradual change in respiratory kinematics associated with commensurate changes in chest wall anatomy. Future investigations may delineate these and other influences on speech motor development.
Another challenge with respect to any developmental data set is inferring the developmental sequence between the observed period and maturity, and the applicability of adult models to very young children (Kent, 1999; Stathopolous & Sapienza, 1993). Speech production differences between adults and children are qualitatively different, as is evident in the current results demonstrating rapidly changing use patterns for the components of the respiratory system. Previous investigations have also shown, for example, that children demonstrate greater relative rib cage excursions during speech breathing and use a higher percentage of vital capacity for speech utterances than adults (Hoit et al., 1990; Stathopoulos & Sapienza, 1993). Finally, speech motor development depends on the interaction of variable and nonlinear growth within and across systems (Kent, 1999). Howatt and DeMuth (1965), for example, described changes in thoracic dimensions as nonlinear, with differing rates of maturity for thoracic width, sternal length, and anterior–posterior diameter.
Preliminary data paralleling the present measures are available from a study by Steeve, Moore, Connaghan, and Reilly (2000). These investigators presented respiratory kinematic data obtained from 13 adults under very similar behavioral and analytic conditions, though the use of adult participants permitted the acquisition of calibrated data. Similar to the data obtained from the children, the adult data revealed task differences between rest breathing and speech breathing during a reading aloud task. The values for rmoving during rest expiration ranged from .39 to .75 and generally were greater than those during speech expiration, which ranged from 0 to .68. CE appeared to occur with greater frequency during rest breathing than speech breathing, whereas the occurrence of UN specified type occurred more frequently during speech than rest breathing. These results were consistent with the endpoints of the trends observed in the current results.
The suggestion that maturation is related to independence of the chest wall components was also supported by the adult data. In particular, the occurrence of UN specified and paradoxical patterns of movement indicated uncoupled movement of the rib cage and abdomen. Although seen only infrequently, rib cage (RCE) and abdominal (AE) paradoxing were observed only during expiration for speech breathing, not for rest breathing. Two of the 13 participants exhibited abdominal paradoxing (AE), although only to a maximum of 3% of the kinematic types. Rib cage paradoxing (RCE) was also observed in 5 of the 13 participants. Although RCE increased significantly in the children over the age range studied, this kinematic type does not appear to be a mature coordinative strategy, based on the preliminary adult data. The observation of oppositional rib cage movement is, however, consistent with the hypothesis that maturation entails greater independence of the chest wall components. The inconsistency of the increased frequency of RCE observed over 9–48 months and the limited presence of rib cage paradoxing (RCE) in adulthood further undermines the use of adult models to describe speech motor development. These inconsistencies are certain to emerge from the interaction of myriad asynchronous, nonlinear influences, which create at any point in development a unique array of motor control problems with complementarily unique behavioral solutions.
Future Research
Future investigations will address the problem of identifying, isolating, and tracking the operative influences throughout speech motor development generally, and respiratory motor control specifically. The present results have provided evidence of distinct coordinative relationships for chest wall components during vocalization and rest breathing. These differences appear to become greater with development and with the presumed increases in the task demands associated with speech production. Several questions emerge directly from the present results: Given their well-documented capacity to generate adult-like prosodic contours, how do very young children use respiratory systems to modulate vocal fundamental frequency and intensity? How do these developmental changes in function correlate with known anatomic changes in the respiratory and laryngeal systems? How does the respiratory motor control system accommodate the rapidly mounting task demands associated with longer utterances and more complex suprasegmental features? Can the interactions of these parallel maturational changes be isolated in individual children? The distinct developmental trajectories of anatomic changes and linguistic development provide the opportunity to isolate these effects. Investigations are currently underway to address these and other questions of speech development.
Acknowledgments
This work was supported by a research grant (R01 DC00822) from the National Institute on Deafness and Other Communication Disorders and by the University of Pittsburgh and the University of Washington, Seattle. Preliminary results of this investigation were presented at the annual convention of the American Speech-Language-Hearing Association in San Antonio, TX, November 1998.
Contributor Information
Kathryn P. Connaghan, University of Washington, Seattle
Christopher A. Moore, University of Washington, Seattle
Masahiko Higashakawa, Osaka Medical College, Osaka, Japan.
References
- 1.Boliek CA, Hixon TJ, Watson PJ, Morgan WJ. Vocalization and breathing during the first year of life. Journal of Voice. 1996;10:1–22. doi: 10.1016/s0892-1997(96)80015-4. [DOI] [PubMed] [Google Scholar]
- 2.Boliek CA, Hixon TJ, Watson PJ, Morgan WJ. Vocalization and breathing during the second and third years of life. Journal of Voice. 1997;11:373–390. doi: 10.1016/s0892-1997(97)80033-1. [DOI] [PubMed] [Google Scholar]
- 3.Bryan AC, Wohl MEB. Respiratory mechanics in children. In: Macklem PT, Mead J, editors. Handbook of physiology: Vol. 3. The respiratory system. Bethesda, MD: American Physiological Society; 1986. pp. 179–191. [Google Scholar]
- 4.Estenne M, Zocchi L, Ward M, Macklem PT. Chest wall motion and expiratory muscle use during phonation in normal humans. Journal of Applied Physiology. 1990;68:2075–2082. doi: 10.1152/jappl.1990.68.5.2075. [DOI] [PubMed] [Google Scholar]
- 5.von Euler C. Some aspects of speech breathing physiology. In: Grillner S, Lindblom B, Lubker J, Persson A, editors. Speech motor control. Oxford, UK: Pergamon Press; 1982. pp. 95–103. [Google Scholar]
- 6.Forssberg H. Ontogeny of human locomotor control: I. Infant stepping, supported locomotion and transition to independent locomotion. Experimental Brain Research. 1985;57:480–493. doi: 10.1007/BF00237835. [DOI] [PubMed] [Google Scholar]
- 7.Gatev V. Role of inhibition in the development of motor co-ordination in early childhood. Developmental Medicine and Child Neurology. 1972;14:336–341. doi: 10.1111/j.1469-8749.1972.tb02599.x. [DOI] [PubMed] [Google Scholar]
- 8.Gaultier C, Praud JP, Canet E, Delaperche MF, D’Allest AM. Paradoxical inward rib cage motion during rapid eye movement sleep in infants and young children. Journal of Developmental Physiology. 1987;9:391–397. [PubMed] [Google Scholar]
- 9.Green JR, Moore CA, Higashikawa M, Steeve RW. The physiologic development of speech motor control: Lip and jaw coordination. Journal of Speech, Language, and Hearing Research. 2000;43:239–255. doi: 10.1044/jslhr.4301.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green JR, Moore CA, Reilly KJ. The sequential development of jaw and lip control for speech. Journal of Speech, Language, and Hearing Research. 2002;45:66–79. doi: 10.1044/1092-4388(2002/005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadders-Algra M, Van Eykern LA, Klip-Van den Nieuwendijk AW, Prechtl HF. Developmental course of general movements in early infancy. II. EMG correlates. Early Human Development. 1992;28:231–251. doi: 10.1016/0378-3782(92)90170-l. [DOI] [PubMed] [Google Scholar]
- 12.Hershenson MB. The respiratory muscles and chest wall. In: Beckerman RC, Brouillette RT, Hunt CE, editors. Respiratory control disorders in infants and children. Baltimore: Williams & Wilkins; 1992. pp. 28–44. [Google Scholar]
- 13.Hershenson MB, Colin AA, Wohl MEB, Stark AR. Changes in the contribution of the rib cage to tidal breathing during infancy. American Review of Respiratory Disorders. 1990;141:922–925. doi: 10.1164/ajrccm/141.4_Pt_1.922. [DOI] [PubMed] [Google Scholar]
- 14.Hixon T. Respiratory function in speech. In: Minifie FD, Hixon TJ, Williams F, editors. Normal aspects of speech, hearing, and language. Englewood Cliffs, NJ: Prentice Hall; 1973. pp. 73–125. [Google Scholar]
- 15.Hixon T. Speech breathing kinematics and mechanism inferences therefrom. In: Grillner S, Lindblom B, Lubker J, Persson A, editors. Speech motor control. New York: Pergamon Press; 1982. pp. 75–93. [Google Scholar]
- 16.Hixon TJ, Goldman MD, Mead J. Kinematics of the chest wall during speech production: Volume displacements of the rib cage, abdomen, and lung. Journal of Speech and Hearing Research. 1973;16:78–115. doi: 10.1044/jshr.1601.78. [DOI] [PubMed] [Google Scholar]
- 17.Hlastala MP, Berger AJ. Physiology of respiration. New York: Oxford University Press; 1996. [Google Scholar]
- 18.Hodge MM, Rochet AP. Characteristics of speech breathing in young women. Journal of Speech and Hearing Research. 1989;32:466–480. doi: 10.1044/jshr.3203.466. [DOI] [PubMed] [Google Scholar]
- 19.Hofsten CV. Mastering reaching and grasping: The development of manual skills in infancy. In: Wallace SA, editor. Perspectives on the coordination of movement. New York: Elsevier Science; 1989. pp. 233–258. [Google Scholar]
- 20.Hoit JD, Hixon TJ, Altman M, Morgan W. Speech breathing in women. Journal of Speech and Hearing Research. 1989;32:353–365. doi: 10.1044/jshr.3202.353. [DOI] [PubMed] [Google Scholar]
- 21.Hoit JD, Hixon TJ, Watson PJ, Morgan WJ. Speech breathing in children and adolescents. Journal of Speech and Hearing Research. 1990;33:51–69. doi: 10.1044/jshr.3301.51. [DOI] [PubMed] [Google Scholar]
- 22.Hoit JD, Plassman BL, Lansing RW, Hixon TJ. Abdominal muscle activity during speech production. Journal of Applied Physiology. 1988;65:2656–2664. doi: 10.1152/jappl.1988.65.6.2656. [DOI] [PubMed] [Google Scholar]
- 23.Howatt WF, DeMuth GR. The growth of lung function: II. Configuration of the chest. Pediatrics. 1965;35:177–184. [PubMed] [Google Scholar]
- 24.Kehoe M, Stoel-Gammon C, Buder EH. Acoustic correlates of stress in young children’s speech. Journal of Speech and Hearing Research. 1995;38:338–350. doi: 10.1044/jshr.3802.338. [DOI] [PubMed] [Google Scholar]
- 25.Kent RD. Motor control: Neurophysiology and functional development. In: Caruso AJ, Strand EA, editors. Clinical management of motor speech disorders in children. New York: Thieme; 1999. pp. 29–71. [Google Scholar]
- 26.Konczak J, Dichgans J. The development toward stereotypic arm kinematics during reaching in the first 3 years of life. Experimental Brain Research. 1997;117:346–354. doi: 10.1007/s002210050228. [DOI] [PubMed] [Google Scholar]
- 27.The Mathworks, Inc. MATLAB (Version 11) Natick, MA: Author; 1999. [Computer software]. [Google Scholar]
- 28.Moore CA. Symmetry of mandibular muscle-activity as an index of coordinative strategy. Journal of Speech and Hearing Research. 1993;36:1145–1157. doi: 10.1044/jshr.3606.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore CA, Caulfield TJ, Green JR. Relative kinematics of the rib cage and abdomen during speech and nonspeech behaviors by 15-month-old children. Journal of Speech, Language, and Hearing Research. 2001;44:80–94. doi: 10.1044/1092-4388(2001/008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moore CA, Ruark JL. Does speech emerge from earlier appearing motor behaviors? Journal of Speech and Hearing Research. 1996;39:1034–1047. doi: 10.1044/jshr.3905.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Openshaw P, Edwards S, Helms P. Changes in rib cage geometry during childhood. Thorax. 1984;39:624–627. doi: 10.1136/thx.39.8.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papastamelos C, Panitch HB, England SE, Allen JL. Developmental changes in chest wall compliance in infancy and early childhood. Journal of Applied Physiology. 1995;78:179–184. doi: 10.1152/jappl.1995.78.1.179. [DOI] [PubMed] [Google Scholar]
- 33.Pollock KE, Brammer DM, Hageman CF. An acoustic analysis of young children’s productions of word stress. Journal of Phonetics. 1993;21:183–203. [Google Scholar]
- 34.Provins KA. The specificity of motor skill and manual asymmetry: A review of the evidence and its implications. Journal of Motor Behavior. 1997;29:183–192. doi: 10.1080/00222899709600832. [DOI] [PubMed] [Google Scholar]
- 35.Ruark JL, Moore CA. Coordination of lip muscle activity by two-year-old children during speech and nonspeech tasks. Journal of Speech, Language, and Hearing Research. 1997;40:1373–1385. doi: 10.1044/jslhr.4006.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharp JT, Druz WS, Balagot RC, Bandelin VR, Danon J. Total respiratory compliance in infants and children. Journal of Applied Physiology. 1970;29:775–779. doi: 10.1152/jappl.1970.29.6.775. [DOI] [PubMed] [Google Scholar]
- 37.Solomon N, Charron S. Speech breathing in able-bodied children and children with cerebral palsy: A review of the literature and implications for clinical intervention. American Journal of Speech-Language Pathology. 1998;7:61–78. [Google Scholar]
- 38.Stathopoulos ET, Sapienza CM. Respiratory and laryngeal measures of children during vocal intensity variation. Journal of the Acoustical Society of America. 1993;94:2531–2543. doi: 10.1121/1.407365. [DOI] [PubMed] [Google Scholar]
- 39.Steeve RW, Moore CA, Connaghan KP, Reilly KJ. Dynamic measures of chest wall kinematics; Presented at the Conference on Speech Motor Control; San Antonio, TX. 2000. Feb, [Google Scholar]
- 40.Thelen E. Timing and developmental dynamics in the acquisition of early motor skills. In: Turkewitz G, Devenny DA, editors. Developmental time and timing. Hillsdale, NJ: Erlbaum; 1993. pp. 85–104. [Google Scholar]
- 41.Thelen E, Cooke DW. Relationship between newborn stepping and later walking: A new interpretation. Developmental Medicine and Child Neurology. 1987;29:380–393. doi: 10.1111/j.1469-8749.1987.tb02492.x. [DOI] [PubMed] [Google Scholar]
- 42.Thelen E, Fisher DM. Newborn stepping: An explanation for a “disappearing” reflex. Developmental Psychology. 1982;18:760–775. [Google Scholar]
- 43.Winkworth AL, Davis PJ, Adams RD, Ellis E. Breathing patterns during spontaneous speech. Journal of Speech and Hearing Research. 1995;38:124–144. doi: 10.1044/jshr.3801.124. [DOI] [PubMed] [Google Scholar]
- 44.Wolhert A, Goffman L. Human perioral muscle activation patterns. Journal of Speech and Hearing Research. 1994;37:1032–1040. doi: 10.1044/jshr.3705.1032. [DOI] [PubMed] [Google Scholar]