Abstract
Dysregulation of fatty acid oxidation (FAO) is recognized as important in the pathophysiology of obesity and insulin resistance (IR). However, demonstrating FAO defects in vivo in humans has entailed complex and invasive methodologies. Recently, the identification of genetic blocks in FAO has been vastly simplified by using tandem mass spectrometry (MS/MS) of dried bloodspots to specify acylcarnitine (AcylCN) alterations characteristic for each disorder. This technology has recently been applied to examine FAO alterations in human and animal models of obesity and type 2 diabetes mellitus (T2DM). This study focused on characterizing AcylCN profiles in human plasma from individuals with obesity and T2DM during fasting and insulin-stimulated conditions. Following an overnight fast, plasma was obtained from lean (n = 12), obese nondiabetic (n = 14), and T2DM (n = 10) participants and analyzed for AcylCN using MS/MS. Plasma samples were also obtained at the end of a 4-h insulin-stimulated euglycemic clamp. In obesity and T2DM, long-chain AcylCNs were similarly significantly increased in the fasted state; free-CN levels were also elevated. Additionally, T2DM subjects of comparable BMI had increased short- and medium-chain AcylCNs, both saturated and hydroxy, as well as increased C4-dicarboxylcarnitine (C4DC–CN) that correlated with an index of poor glycemic control (HbA1c; r = 0.74; P < 0.0001). Insulin infusion reduced all species of plasma AcylCN but this reduction was blunted in T2DM. Plasma long-chain AcylCN species are increased in obesity and T2DM, suggesting that more fatty acids can enter mitochondria. In T2DM, many shorter species accumulate, suggesting that they have a generalized complex oxidation defect.
Introduction
Obesity and type 2 diabetes mellitus (T2DM) are characterized by lipid excess, with elevations of plasma nonesterified fatty acids (NEFAs) and excess lipid deposited in liver and skeletal muscle, which is associated with insulin resistance (IR) (1,2). This dysregulation of fatty acid and glucose metabolism induced by elevated lipids in β-cells, liver, and skeletal muscle has been termed lipotoxicity (3). The molecular pathogenesis of lipotoxicity is not understood, although increased levels of lipid metabolites have been shown to inhibit insulin signaling (1). This view assumes that lipid accumulation occurs as a result of excess NEFA availability, reduced NEFA oxidation or both. Rates of fatty acid oxidation (FAO) are elevated in obesity and T2DM under insulin-stimulated conditions (4) but reduced during fasting. The proposed mechanism underlying this reduction is malonyl-CoA inhibition of carnitine palmitoyltransferase-1 (CPT1)–mediated entry of NEFA into mitochondria (5). Reduced mitochondrial oxidative capacity is associated with skeletal muscle IR and might also reduce FAO capacity (6). The hypothesis that FAO is impaired in obesity and T2DM has been technically challenging to address in clinical investigation.
Profiling of blood acylcarnitine (AcylCN) by tandem mass spectrometry (MS/MS) is a proven technique for rapid and economical determination of genetic defects in FAO and in the electron transport chain (ETC) (7,8). The AcylCN analysis in newborn screening has resulted in the identification of several mild mutations that, although not associated with catastrophic disease, will theoretically reduce the efficiency of the subject’s stress response. Thus, we hypothesized that this technique might reveal the site of mild derangements in FAO or ETC activity in obesity and T2DM.
Recently, AcylCN profiling was used to identify FAO dysregulation in animal models of obesity, diabetes, and IR (9). These findings suggest that dysregulation of FAO in obesity and IR may entail mitochondrial oversupply of FA and increased incomplete FAO rather than blocked entry of fatty acids. Incomplete FAO with IR has previously been demonstrated in several tissues, including human skeletal muscle (10). Furthermore, the AcylCN profiles in these recent studies suggest inefficient coordination across the processes of β-oxidation, the tricarboxylic acid cycle and the ETC. In support of this hypothesis, impaired FAO and accumulation of intramuscular triglyceride correlates with abnormalities in the ETC and in the redox state (low NAD+/NADH ratios) within the mitochondrial matrix (11,12).
This study was undertaken to examine the utility of plasma AcylCN profiling as a minimally invasive probe to identify subtle defects in FAO in obese and T2DM participants compared to lean, healthy volunteers under fasting conditions and during insulin-stimulated euglycemia.
Methods and Procedures
Research participants
Participants were 30–55 years of age and separated into three groups. The first comprised lean, sedentary individuals with BMI <26 kg/m2 without metabolic syndrome or parental history of diabetes and with a normal oral glucose tolerance test. The second was obese (BMI 30–39 kg/m2) but not diabetic; most were glucose intolerant (n = 11; 2-h oral glucose tolerance test blood glucose = 140–200 mg/dl). The third consisted of obese (BMI 30–39 kg/m2) individuals who also have T2DM. Patients on insulin or incretin-mimetics were excluded from participation. Oral antidiabetic agents were held the night before the study and resumed on the next day after the clamp was concluded. Laboratory criteria for eligibility were as follows: normal urinary sediment, hematocrit, serum creatinine, thyrotropin, alkaline phosphatase, with aspartate and alanine aminotransferase <2.5× the upper limit of reference range. All participants gave written informed consent, per the protocol approved by the University of Pittsburgh institutional review board.
Metabolic assessments
Glycemic response to a 75-g oral glucose challenge was determined after an overnight fast. Body composition was assessed using dual-energy X-ray absorptiometry (Lunar Prodigy; GE Healthcare, Madison, WI). Participants were admitted to the University of Pittsburgh General Clinical Research Center. Following a standardized dinner (7 kcal/kg body weight; 50% carbohydrates, 20% protein, and 30% fat) and an overnight fast (12–14 h), participants underwent a 4-h euglycemic–hyperinsulinemic (40 mU/m2/min) clamp with variable intravenous glucose infusion (13). Insulin sensitivity was calculated as the systemic glucose disposal rate during the steady-state phase of the clamp. A plasma sample was obtained before and another during the last 20 min of the clamp for determination of NEFA (enzymatic method), insulin (radioimmunoassay), glucose (glucose-oxidase method), and AcylCN concentrations. Indirect calorimetry (DeltaTrac, Anaheim, CA) was performed 30 min before and during the last 30 min of insulin infusion to determine the respiratory quotient (RQ).
AcylCN profiling
Archived plasma specimens (−80 °C) were thawed and 25 µl were spotted onto S&S Grade 903 filter paper and dried at room temperature. Sample preparation and analyses of AcylCN profiles by MS/MS of butyl derivatives were conducted as previously described (7;see Supplementary Methods And Procedures online). Up to 46 AcylCN species were quantified by calculating the concentrations of metabolites relative to the deuterated internal standard (eight different masses; Cambridge isotopes, Andover, MA) closest in mass. Each of the AcylCN species presented in the tables and figures was detected in each plasma sample, except for C14–OH– (not detected in 0 basal, 10 clamp) and C16–OH–CN (2 and 6). In the analyses, undetectable AcylCNs were given a value of 0. A listing of all AcylCNs detected in most basal samples is provided in Supplementary Table S1 online.
Statistics
The concentrations of AcylCN species were compared using the General Linear Model analysis of variance (ANOVA; SAS Release 9.1.3 for Windows; SAS Institute, Cary, NC) using the full model including group (lean, obese, and T2DM) and insulin treatment (basal and insulin clamp). Data are presented as means ± s.d. Post hoc tests for significant differences (P < 0.05) in group means used a Tukey multiple comparison adjustment. In addition, levels of specific AcylCN species were correlated with metabolic parameters.
Results
Participants and clinical measures
Clinical characteristics are shown in Table 1. The proportion of males was similar in lean and obese (25 and 21%, respectively), but higher in the T2DM group (50%). Measures of adiposity were similar in the obese and T2DM groups, but significantly greater than in the lean group. Fasting plasma NEFA were similar in obese and T2DM and higher than in lean participants. The obese and T2DM groups had higher fasting RQ, suggesting (not significant) decreased reliance on lipid oxidation.
Table 1.
Participant characteristics and metabolic measures
| Parameter | Leana | Obese | T2DM |
|---|---|---|---|
| n (males/females) | 12 (3/9) | 14 (3/11) | 10 (5/5) |
| Age | 47 ± 7 | 43 ± 7 | 45 ± 10 |
| BMI | 23.9 ± 1.8 | 34.3 ± 3.1* | 34.2 ± 2.5* |
| Body weight, kg | 65.7 ± 7.5 | 93.8 ± 10.3* | 100.5 ± 12.8* |
| Fat free mass, kg | 43.4 ± 8.6 | 50.2 ± 9.3 | 60.0 ± 13.1* |
| Body fat, kg | 21.2 ± 4.3 | 41.3 ± 5.7* | 37.6 ± 8.1* |
| Waist, cm | 81.8 ± 5.7 | 108.4 ± 12.1* | 108.9 ± 7.6* |
| Baseline | |||
| Fasting NEFA,µmol/l | 311 ± 54 | 554 ± 121* | 550 ± 125* |
| Fasting RQ | 0.78 ± 0.03 | 0.80 ± 0.03 | 0.80 ± 0.02 |
| Fasting glucose, mg/dl | 90.3 ± 6.4 | 95.5 ± 5.0 | 161.4 ± 47.8*,** |
| Glucose, mg/dl, 120min | 105.9 ± 32.8 | 152.1 ± 27.3* | 258.8 ± 50.5*,** |
| HbA1c, % | 5.46 ± 0.37 | 5.35 ± 0.32 | 7.86 ± 1.63*,** |
| Insulin clamp | |||
| NEFA,µmol/l | 24 ± 13 | 71 ± 38* | 162 ± 79*,** |
| Insulin, µU/ml | 57.9 ± 10.2 | 88.1 ± 162* | 86.0 ± 23.1* |
| Glucose, mg/dl | 92.4 ± 3.6 | 91.7 ± 4.6 | 94.6 ± 8.1 |
| Glucose disposal (mg/min kg/FFM) | 12.04 ± 3.04 | 6.12 ± 1.93* | 2.54 ± 2.75*,** |
| RQ | 0.92 ± 0.05 | 0.90 ± 0.02 | 0.85 ± 0.03*,** |
FFM, free fat mass; NEFA, nonesterified fatty acid; RQ, respiratory quotient; T2DM, type 2 diabetes mellitus.
Means ± s.d.
Significantly (P < 0.05) different from lean.
Significantly (P < 0.05) different from obese.
Fasting AcylCN alterations common to both obese and T2DM subjects
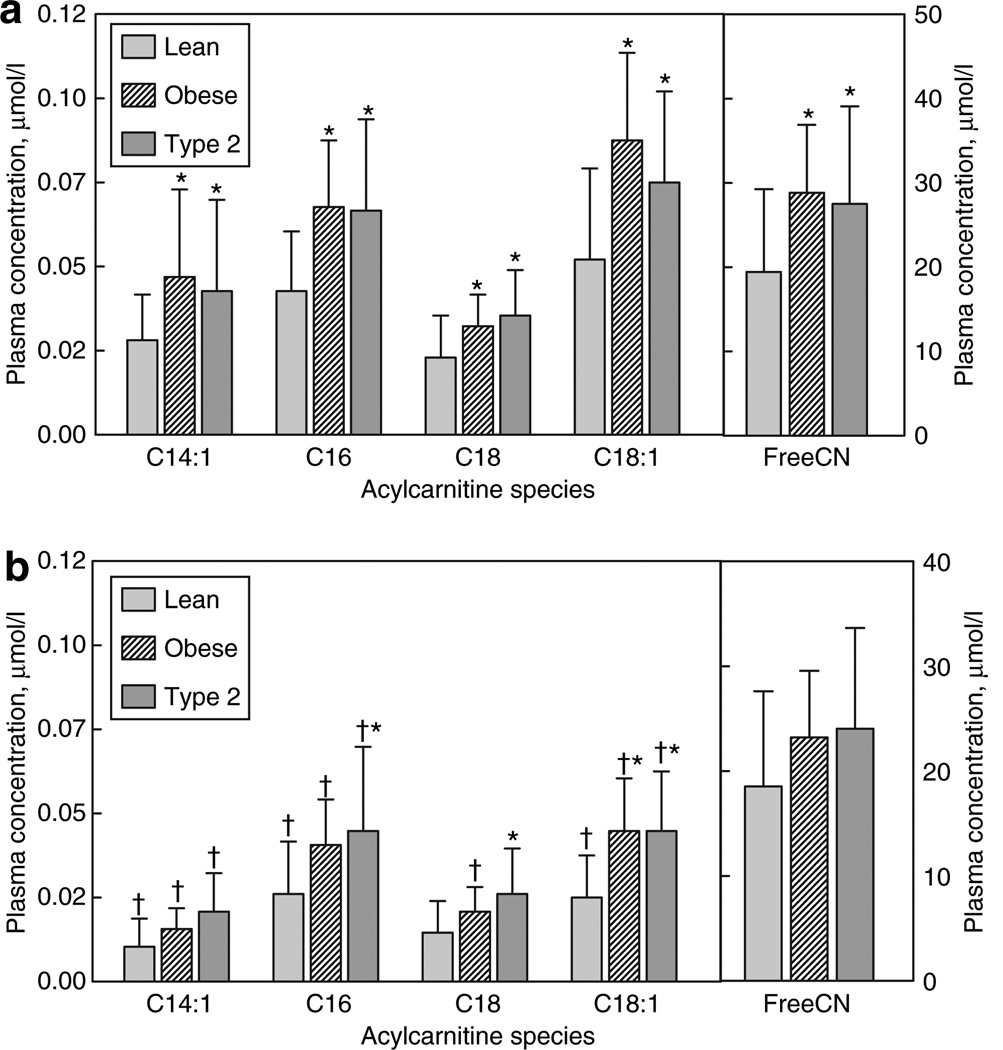
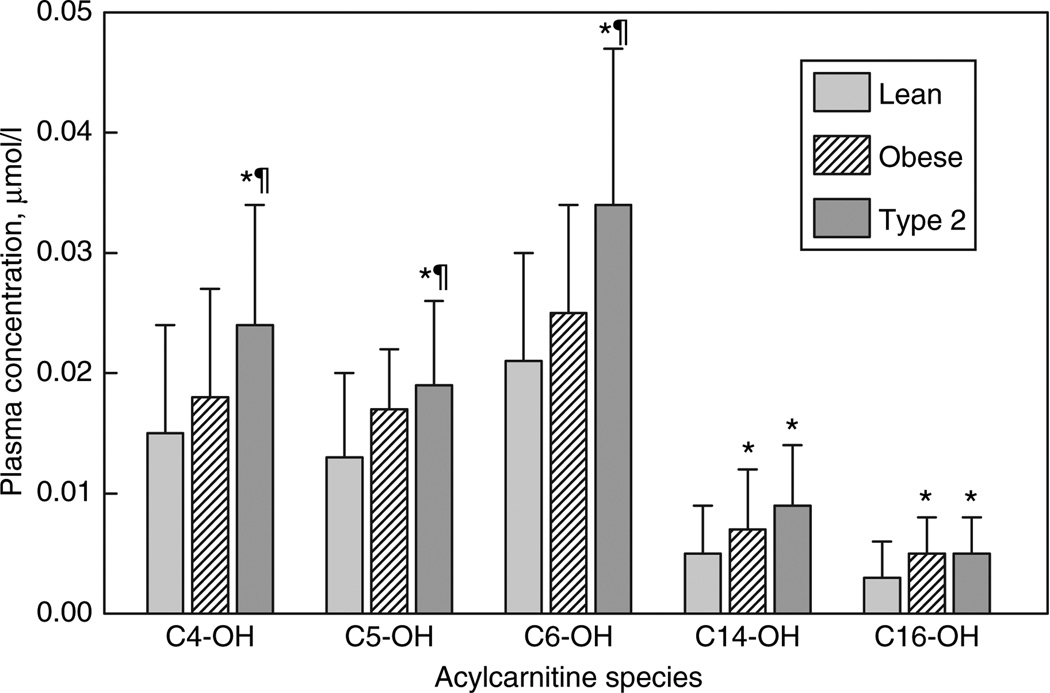
Individual long-chain AcylCN species, both saturated and monounsaturated were similarly increased in both obese and T2DM relative to lean participants (Figure 1a). In addition, C14–OH– and C16–OH–CN were equally increased in both groups (Figure 2). The plasma free-CN level was similarly increased in both groups relative to lean participants (Figure 1a).
Figure 1. Long-chain- and free-acylcarnitine profile (mean ± s.d.).
(a) Fasting levels. (b) Levels during euglycemic clamp. *Within treatment (e.g., fasting or clamp) significantly different from lean. †Mean for insulin clamp significantly different from basal condition.
Figure 2. Fasting hydroxyacylcarnitine profiles (mean ± s.d.).
*Significantly different from lean. ¶Significantly different from obese.
AcylCN differences specific to T2DM participants
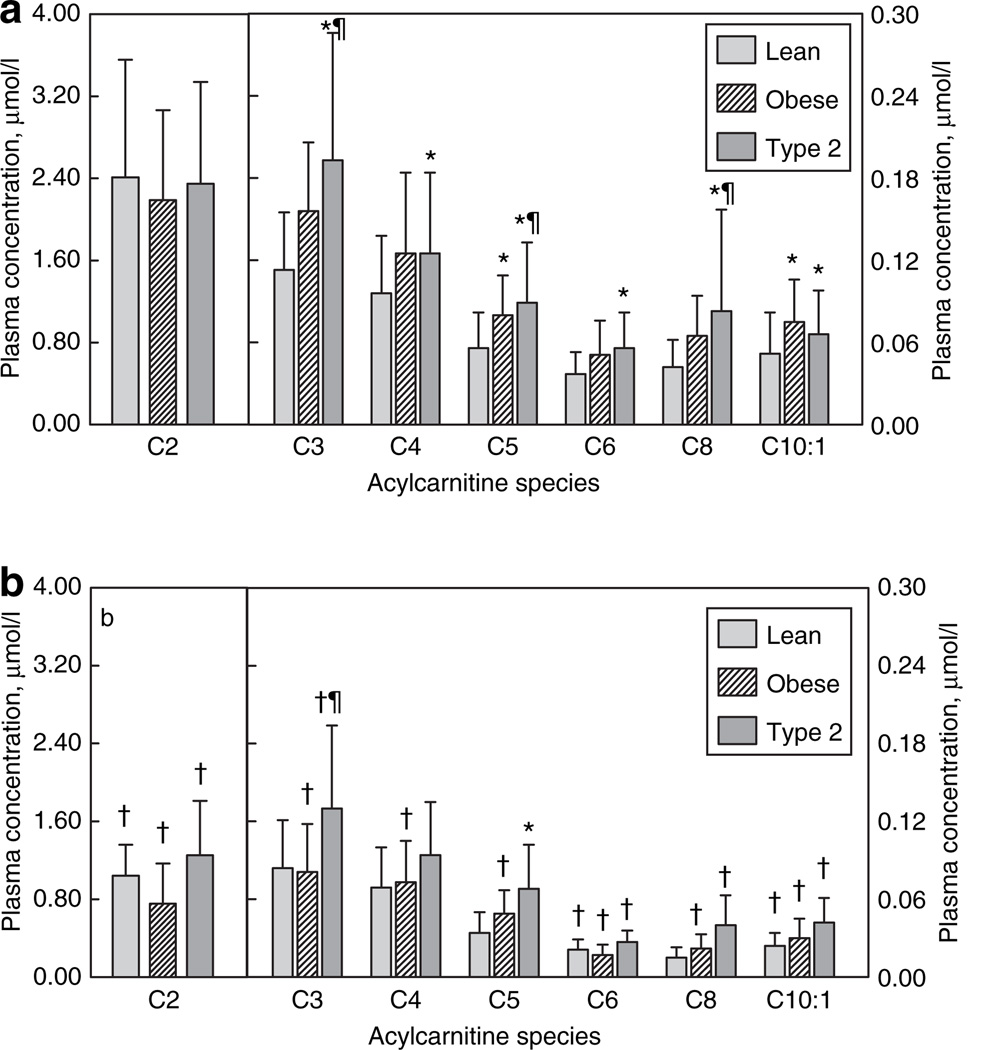
Relative to obese and lean, T2DM participants had significantly higher levels of several short- and medium-chain species, including the C3–, C5–, and C8–CN (Figure 3). Furthermore, T2DM but not obese had significant elevations of C4– and C6–CN relative to lean subjects, although the mean values for C4–CN were nearly identical in obese and T2DM. In contrast, the C2–CN (acetylcarnitine) concentration was similar in all three groups. T2DM participants also had elevated C4–OH–, C5–OH–, and C6–OH–CN levels (Figure 2) relative to both lean and obese. Finally, levels of C4-dicarboxylcarnitine (C4DC–CN) were significantly higher in T2DM participants (0.053 ± 0.034 µmol/l), compared to obese (0.027 ± 0.010 µmol/l; P < 0.0001) (despite similar BMI) or lean (0.025 ± 0.010 µmol/l; P < 0.002).
Figure 3. Short- and medium-chain acylcarnitine profiles (mean ± s.d.).
(a) Fasting levels. (b) Levels during euglycemic clamp. *Significantly different from lean. ¶Significantly different from obese. †Mean for insulin clamp significantly different from fasting condition.
Although the study was not powered to specifically determine gender effects, when we examined AcylCN levels by gender, many of the species were significantly higher in men. Men also had poorer glucose control (HbA1c 5.7 ± 0.8% in women and 6.3 ± 1.5% in men, P < 0.05) and were more insulin resistant (glucose disposal, 9.03 ± 4.6 mg/min kg/free fat mass in women and 6.60 ± 4.1 mg/min kg/free fat mass in men, P < 0.05). However, the results for both men and women individually (data not shown) were similar to that for all participants presented in Figures 1–3. Furthermore, results for group differences presented in the figures were nearly identical when gender was included as a covariate in the ANOVA. Two notable exceptions were that the higher C3 and C5 observed in the T2DM subjects compared to obese subjects was driven almost entirely by the men (mean values were similar for obese and T2DM in women).
Plasma AcylCN during insulin infusion
Although plasma NEFA levels decreased in all groups during insulin infusion, levels in the T2DM remained higher compared to the lean or obese groups (Table 1). Systemic RQ rose similarly in lean and obese, indicating that they became less reliant on fat oxidation. In contrast, T2DM participants demonstrated a blunted rise in RQ, resulting in a significantly lower value, which indicates that the T2DM group oxidized relatively more fat during insulin infusion. In response to insulin, there was a significant decrease in every AcylCN species detected between carbon lengths of 2 and 18 (−43 ± 12%; most P < 0.0001).
Although there was an overall decrease in all AcylCN species, groups differed in their response to insulin infusion. Levels of C14:1–, C16–, and C18:1–CN decreased in all groups, whereas C18–CN decreased significantly only in the obese group (Figure 1b). Even during insulin infusion, levels of C16–, C18–, and C18:1–CN were significantly higher in T2DM compared to lean. Although there was a significant decrease in C14:1–CN in the T2DM subjects in response to insulin, the decrease (43%) was significantly less than (P < 0.05) that observed in the lean (61%) or obese (63%).
Most short- and medium-chain AcylCNs were decreased in each group in response to insulin (Figure 3b). However, the decrease in C8–, C10–, and C10:1–CN was significantly lower in the T2DM compared to lean and obese (39% vs. 58% and 61%; 42% vs. 67% and 71%; and 22% vs. 45% and 56%, respectively). Levels of C2–CN decreased more in obese (65%) compared to lean (50%) or T2DM (41%). Significant decreases were observed in most of the short-chain hydroxy-AcylCN species in each group in response to insulin. For the long-chain hydroxy-AcylCNs, no significant decreases were observed except for C14–OH–CN in the T2DM.
Correlations with physiological assessments
Correlation analysis was undertaken to identify associations between specific fasting plasma AcylCN levels and either adiposity, plasma NEFA levels, IR, or glycemic control (Table 2). Moderate correlations were observed between many AcylCN species and BMI; these associations were slightly stronger for waist circumference, particularly for C12:1–, C16–, and C18–CN. The correlations between the AcylCN species and both basal NEFA levels and insulin sensitivity were generally similar to those observed for BMI. The correlations between AcylCNs and basal glucose concentrations or levels of HbA1c were also moderate and no stronger than that observed for BMI or free fatty acid levels with one exception. The C4DC–CN species was strongly correlated with two measures of glycemic control (basal glucose, r = 0.74 and HbA1c, r = 0.72; P < 0.0001 for both). Thus, C4DC–CN is a strong candidate for a marker of “glucotoxicity”.
Table 2.
Correlations between basal acylcarnitine levels and metabolic parameters
| AcylCN | BMI | Waist | Basal NEFA |
Glucose disposal |
Basal glucose |
HbA1c |
|---|---|---|---|---|---|---|
| Free CN | 0.39 | 0.54 | 0.43 | −0.37 | ||
| C3 | 0.44 | 0.51 | −0.51 | 0.38 | 0.34 | |
| C4DC | 0.40 | −0.40 | 0.74 | 0.72 | ||
| C6 | 0.44 | 0.31 | 0.56 | −0.39 | ||
| C6–OH | 0.39 | 0.44 | 0.33 | −0.44 | 0.45 | 0.33 |
| C8 | 0.40 | 0.44 | 0.54 | −0.40 | 0.39 | 0.42 |
| C8:1 | 0.34 | 0.42 | 0.48 | −0.34 | 0.42 | |
| C10 | 0.40 | 0.46 | 0.45 | −0.36 | ||
| C10:1 | 0.34 | 0.50 | 0.33 | |||
| C12 | 0.56 | 0.44 | 0.40 | |||
| C12:1 | 0.38 | 0.56 | 0.40 | |||
| C16 | 0.42 | 0.59 | 0.53 | −0.39 | ||
| C18 | 0.37 | 0.56 | 0.53 | −0.41 |
All correlations are significant at P < 0.001 for r > 0.52, P < 0.01 for |r| > 0.40 and <0.52, P < 0.05 for |r| < 0.41.
AcylCN, acylcarnitine; C4-DC, C4-dicarboxylcarnitine; NEFA, nonesterified fatty acid.
Discussion
There is growing evidence that subtle aberrations in lipid metabolism occur in obesity and T2DM. As advanced MS/MS technology for detection of altered AcylCN levels enables the detection of mild defects in FAO, we hypothesized that it would also detect FAO defects in obese participants with or without T2DM. Although our primary focus has been on skeletal muscle FAO, plasma AcylCN could also be derived from other tissues, such as liver and cardiac muscle. However, in a study in which elevated AcylCNs were observed in serum and skeletal muscle from insulin-resistant rats, there was no increase observed in liver, suggesting that the serum AcylCN originated mainly from the skeletal muscle (9). Our results show that both obese and T2DM subjects with similarly increased NEFAs and similar BMIs equally accumulated AcylCNs of both full-length fatty acids and of the first intermediates of β-oxidation. The T2DM subjects also accumulated a wide variety of later β-oxidation intermediates. When NEFA release was inhibited by short-term insulin clamp, plasma AcylCN values were reduced, with a significantly blunted response in the T2DM subjects, as would be expected if they are “metabolically inflexible” (4).
Interpretations were based on AcylCN profiles of patients with known genetic disorders of mitochondrial FAO (8). The AcylCN profile includes substrates of both fatty acid and amino acid β-oxidation. Even-chain AcylCNs containing up Integrative Physiology to 20 carbons accumulate in response to incomplete fatty acid β-oxidation. In contrast, amino acid catabolism produces oddchain- length species such as C3–CN and C5–CN, although C4–CN can be derived from either amino acid or fatty acid metabolism. Acetyl (C2)–CNs derive from carbohydrate catabolism and from the ultimate product of β-oxidation, acetyl- CoA. After an overnight fast, FAO is stimulated, increasing saturated AcylCN levels while branched-chain amino acid (BCAA) metabolism is spared, reducing C5 entry from leucine and isoleucine, resulting in reduced C5–CN levels.
As both obese and T2DM subjects had similarly elevated NEFAs during fasting, the next question is whether they efficiently process this flood of fatty acids. One locus of regulation is the limitation of entry into β-oxidation by CPT1, which transesterifies the activated long-chain acyl-CoA to its AcylCN before entry into mitochondria. This regulation limits C16–, C18–, and C18:1–CN levels, resulting in increased free-CN:C16–CN. However, both obese and T2DM groups had similarly elevated C16–, C18–, and C18:1–CN and free-CN, with the free-CN:C16–CN ratio decreased in obese participants (P < 0.05) with a similar trend in T2DM participants (P < 0.07) (Table 3). This finding is inconsistent with CPT1-based limitation of β-oxidation flux. Thus, in both obesity and T2DM, similar quantities of NEFAs are readily activated into AcylCN that can either pass into the mitochondria via carnitine AcylCN translocase or can exit the cell for other disposal. Inside, the AcylCN is de-esterified back to acyl-CoA and enters the β-oxidation spiral. We also observed increased levels of C14:1–CN and C16–OH–CN, species that arise from activity in the initial rounds of β-oxidation involving very-long-chain acyl-CoA dehydrogenase and longchain 3-hydroxyacyl-CoA dehydrogenase, respectively, yielding unaltered C14:1–CN:C16–CN and the C16–OH–CN:C16–CN ratios. This overall pattern is consistent with obese and T2DM subjects having similarly increased initial fluxes through β-oxidation in response to elevated NEFAs rather than having a CPT1 generated block, as had been suggested previously (5).
Table 3.
A cylcarnitine ratios
| Ratio | Lean | Obese | T2DM |
|---|---|---|---|
| Free/C16 | 657.4 ± 260.1 | 545.0 ± 186.1* | 556.6 ± 235.9 |
| C16–OH/C16 | 0.094 ± 0.09 | 0.078 ± 0.132 | 0.086 ± 0.051 |
| C8/C16 | 0.922 ± 0.573 | 0.818 ± 0.429 | 1.18 ± 0.316** |
| C14:1/C16 | 0.714 ± 0.317 | 0.694 ± 0.328 | 0.648 ± 0.272 |
| C4/C3 | 0.885 ± 0.285 | 0.921 ± 0.419 | 0.831 ± 0.315 |
| AcylCN/free | 0.249 ± 0.141 | 0.159 ± 0.077* | 0.178 ± 0.090 |
AcylCN, acylcarnitine; C4-DC, C4-dicarboxylcarnitine; T2DM, type 2 diabetes mellitus.
Significantly (P < 0.05) different from lean group.
Significantly (P < 0.05) different from obese group. (P < 0.07 for T2DM vs. lean).
With AcylCNs of ≤10 carbons, the pattern of AcylCN accumulation by groups diverged. Most straight-chain- and hydroxy-AcylCN products from these later steps of β-oxidation (medium- and short-chain processing) accumulated in a stepwise pattern from nonsignificant elevations in the obese subjects to significant elevations in the T2DM subjects (Figures 2 and 3), with several species significantly increased in T2DM relative to both the obese and lean subjects. In addition, the C8–CN:C16–CN ratio was significantly increased in the T2DM relative to the obese subjects, supporting this trend toward a more severe defect in the T2DM participants. The trends are consistent with a study of rat diabetic myocardium, which found that overall AcylCN levels increased with an accumulation of 3-OH–AcylCNs (14).
Thus, T2DM subjects display a second more global defect in their mitochondrial function that is characterized by an enhanced accumulation of intermediates such as the chainshortened straight-chain products of FAO (C4– to C14–CN) characteristic of a more generalized dysfunction in either FAO or at the interface of FAO and the ETC (15). In addition, they accumulate intermediates from substrates that would be expected to decrease in quantity rather than accumulating during fasting, such as the products of BCAA oxidation (C5–, C5–OH–, and C3–CN). As the BMI, waist circumference, and basal free fatty acid levels of the T2DM subjects were nearly identical to levels in the obese subjects, the more “abnormal” AcylCN patterns are not due to increased body fat or basal NEFA levels but most likely result from IR.
The insulin infusion studies further support the hypothesis that the T2DM participants differ in their FAO regulation. Overall, in response to insulin infusion, all AcylCN species decreased significantly. This finding verifies that plasma AcylCN species are responsive to short-term alterations in NEFA levels. However, the T2DM subjects had a blunted decrease in the levels of many AcylCNs, leading to an AcylCN pattern that did not normalize to the extent observed in the obese group. The higher NEFA levels and lower RQ seen in the T2DM participants during insulin infusion, suggest that the insulin clamp did not block release of free fatty acids or flux into β-oxidation to the degree found in either lean or obese participants. This finding is consistent with the hypothesis that T2DM participants exhibit metabolic inflexibility (4). Only one other group has reported decreased plasma AcylCNs (C10–, C12–, and C14:1–CN) with elevated insulin and that was in response to an oral glucose tolerance test (16).
One marker of abnormal FAO was specifically associated with T2DM. C4DC–CN levels in T2DM were nearly double than those observed in obese or lean participants. Furthermore, C4DC–CN correlated highly with fasting plasma glucose (r = 0.72) and with HbA1c (r = 0.74). Consequently, this AcylCN species may be a biomarker at the intersection of combined glucoand lipotoxicity (17,18). It should be noted that C4DC–CN as identified using this methodology represents the sum of both methylmalonylcarnitine and succinylcarnitine, which have the same molecular mass. Therefore, additional analyses will be required to specifically identify the affected species. However, whether the elevation is due to methylmalonylcarnitine and/or succinylcarnitine, this finding is consistent with our observation of increased C3–CN and suggests a potential defect in the utilization of succinyl-CoA in the tricarboxylic acid cycle in T2DM.
Our findings are consistent with recent reports in rodents and in cell culture showing that insulin-resistant skeletal muscle displays increased incomplete β-oxidation, resulting in increased AcylCN intermediates in serum and muscle (9,19,20). When rats were fed a high-fat diet, obesity and IR were induced and many of the same AcylCN species we observed in humans were increased in serum and skeletal muscle (9). Muscle AcylCNs were even more elevated in Zucker diabetic fatty rats, a model of severe IR where incomplete FAO has been experimentally confirmed (10). These rodent data indicate that IR in skeletal muscle is associated with excessive (at least in the fed state) yet incomplete β-oxidation (fed and fasted state) and impaired switching to carbohydrate substrate during the fasted-to-fed transition.
Two recent human studies comparing AcylCN patterns in lean and obese (21) or obese individuals with or without T2DM (22) observed some of the same patterns we observed in this study. The first study reported no increase in C2–CN and similar elevations of four of the AcylCN that we observed in obese participants (21). Higher C3– and C5–CNs and BCAAs were observed in obese individuals, and these metabolites correlated with degree of IR (21). In addition, they observed elevations of C6– and C8:1–CN. In contrast, another recent study found that women with T2DM had decreased C3–CN with increased C2–CN levels compared to obese women (22). However, consistent with our findings, they also observed that T2DM subjects accumulated medium-chain intermediates. Although we cannot explain these discrepant findings, the lean participants in the first study appeared to have impaired fasting glucose (100.5 mg/dl) and a higher percentage of males (70 vs. 57%, P = 0.09440) and the second study was limited to African-American women, who have been shown to have a lower FAO compared to the matched white women (23). Nonetheless, there is general agreement for the association of T2DM with accumulation of medium-chain fatty acid intermediates and for IR to be associated with excessive BCAA metabolism. However, these contradictory studies along with our finding that when results were examined by sex, nearly all of the increases in BCAA-derived CN metabolites (C3–CN and C5–CN) in T2DM relative to obese subjects occurred in male subjects suggest that BCAA metabolism may be regulated differently by race and sex or even by some other unexpected variable such as BCAA composition of the diet (21).
In summary, the circulating AcylCNs in obese and T2DM participants fall into two distinct patterns. First, the T2DM and obese participants have a similar accumulation of long-chain AcylCNs and AcylCN species that arise from activity in the initial rounds of β-oxidation, consistent with increased flux at entry into mitochondrial β-oxidation. Diabetic participants also displayed a secondary accumulation of many shorter chain AcylCNs that are suggestive of inefficient complete FAO or interactions between β-oxidation and ETC. They also showed an inability to efficiently switch from fat metabolism during the insulin clamp, as reflected in their inability to lower their AcylCNs as effectively as either the lean or obese subjects. Finally, the elevation in C4DC–CN in the T2DM participants is intriguing; its robust correlation with two indexes of glycemic control supports its association with elevated blood glucose. As such, it is possible that C4DC–CN may be a useful biomarker of gluco-and lipotoxicity in T2DM. Future studies will address whether the AcylCN profile can serve as a biomarker to assess the response of obese and T2DM participants to interventions.
Supplementary Material
Acknowledgments
This study was supported by the University of Pittsburgh General Clinical Research Center (5 M01RR00056), the Obesity and Nutrition Research Center (P30DK462) National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Grants DK49200-08 and DK78755, and the Pennsylvania Department of Health.
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/oby
Disclosure
The authors declared no conflict of interest.
REFERENCES
- 1.Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and β-cell dysfunction. Eur J Clin Invest. 2002;32(Suppl: 3):14–23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 2.Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46:1579–1585. doi: 10.2337/diacare.46.10.1579. [DOI] [PubMed] [Google Scholar]
- 3.Unger RH. Lipotoxic diseases. Annu Rev Med. 2002;53:319–336. doi: 10.1146/annurev.med.53.082901.104057. [DOI] [PubMed] [Google Scholar]
- 4.Kelley DE. Skeletal muscle fat oxidation: timing and flexibility are everything. J Clin Invest. 2005;115:1699–1702. doi: 10.1172/JCI25758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGarry JD, Brown NF. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur J Biochem. 1997;244:1–14. doi: 10.1111/j.1432-1033.1997.00001.x. [DOI] [PubMed] [Google Scholar]
- 6.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 7.Chace DH, DiPerna JC, Kalas TA, Johnson RW, Naylor EW. Rapid diagnosis of methylmalonic and propionic acidemias: quantitative tandem mass spectrometric analysis of propionylcarnitine in filter-paper blood specimens obtained from newborns. Clin Chem. 2001;47:2040–2044. [PubMed] [Google Scholar]
- 8.Chace DH, Kalas TA, Naylor EW. Use of tandem mass spectrometry for multianalyte screening of dried blood specimens from newborns. Clin Chem. 2003;49:1797–1817. doi: 10.1373/clinchem.2003.022178. [DOI] [PubMed] [Google Scholar]
- 9.Koves TR, Ussher JR, Noland RC, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Veerkamp JH, Van Moerkerk HT, Glatz JF, Van Hinsbergh VW. Incomplete palmitate oxidation in cell-free systems of rat and human muscles. Biochim Biophys Acta. 1983;753:399–410. doi: 10.1016/0005-2760(83)90064-4. [DOI] [PubMed] [Google Scholar]
- 11.Watmough NJ, Bindoff LA, Birch-Machin MA, et al. Impaired mitochondrial β-oxidation in a patient with an abnormality of the respiratory chain. Studies in skeletal muscle mitochondria. J Clin Invest. 1990;85:177–184. doi: 10.1172/JCI114409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ritov VB, Menshikova EV, He J, et al. Deficiency of subsarcolemmal mitochondria in obesity and type 2 diabetes. Diabetes. 2005;54:8–14. doi: 10.2337/diabetes.54.1.8. [DOI] [PubMed] [Google Scholar]
- 13.Toledo FG, Menshikova EV, Ritov VB, et al. Effects of physical activity and weight loss on skeletal muscle mitochondria and relationship with glucose control in type 2 diabetes. Diabetes. 2007;56:2142–2147. doi: 10.2337/db07-0141. [DOI] [PubMed] [Google Scholar]
- 14.Su X, Han X, Mancuso DJ, Abendschein DR, Gross RW. Accumulation of long-chain acylcarnitine and 3-hydroxy acylcarnitine molecular species in diabetic myocardium: identification of alterations in mitochondrial fatty acid processing in diabetic myocardium by shotgun lipidomics. Biochemistry. 2005;44:5234–5245. doi: 10.1021/bi047773a. [DOI] [PubMed] [Google Scholar]
- 15.Sim KG, Carpenter K, Hammond J, Christodoulou J, Wilcken B. Acylcarnitine profiles in fibroblasts from patients with respiratory chain defects can resemble those from patients with mitochondrial fatty acid β-oxidation disorders. Metab Clin Exp. 2002;51:366–371. doi: 10.1053/meta.2002.30521. [DOI] [PubMed] [Google Scholar]
- 16.Zhao X, Peter A, Fritsche J, et al. Changes of the plasma metabolome during an oral glucose tolerance test: is there more than glucose to look at? Am J Physiol Endocrinol Metab. 2009;296:E384–E393. doi: 10.1152/ajpendo.90748.2008. [DOI] [PubMed] [Google Scholar]
- 17.Prentki M, Joly E, El-Assaad W, Roduit R. Malonyl-CoA signaling, lipid partitioning, and glucolipotoxicity: role in β-cell adaptation and failure in the etiology of diabetes. Diabetes. 2002;51(Suppl 3):S405–S413. doi: 10.2337/diabetes.51.2007.s405. [DOI] [PubMed] [Google Scholar]
- 18.Cnop M, Igoillo-Esteve M, Cunha DA, Ladrière L, Eizirik DL. An update on lipotoxic endoplasmic reticulum stress in pancreatic β-cells. Biochem Soc Trans. 2008;36:909–915. doi: 10.1042/BST0360909. [DOI] [PubMed] [Google Scholar]
- 19.An J, Muoio DM, Shiota M, et al. Hepatic expression of malonyl-CoA decarboxylase reverses muscle, liver and whole-animal insulin resistance. Nat Med. 2004;10:268–274. doi: 10.1038/nm995. [DOI] [PubMed] [Google Scholar]
- 20.Koves TR, Li P, An J, et al. Peroxisome proliferator-activated receptor-γ co-activator 1α-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem. 2005;280:33588–33598. doi: 10.1074/jbc.M507621200. [DOI] [PubMed] [Google Scholar]
- 21.Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams SH, Hoppel CL, Lok KH, et al. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid β-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr. 2009;139:1073–1081. doi: 10.3945/jn.108.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chitwood LF, Brown SP, Lundy MJ, Dupper MA. Metabolic propensity toward obesity in black vs white females: responses during rest, exercise and recovery. Int J Obes Relat Metab Disord. 1996;20:455–462. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.