Abstract
Background
Parkinson’s Disease (PD) is a chronic progressive neurologic disorder, which affects approximately one million men and women in the U.S. alone. PD represents a heterogeneous disorder with common clinical manifestations and for the most part common neuropathological findings.
Objective
This short article reviews the role of the ubiquitin E3 ligase in sporadic PD.
Methods
The role of parkin in sporadic PD was reviewed by querying PubMed
Results
Parkin is inactivated in sporadic PD via S-nitrosylation, oxidative and dopaminergic stress, and phosphorylation by the stress activated kinase, c-Abl leading to the accumulation of AIMP2 and PARIS (ZNF746).
Conclusion
Strategies aimed at maintaining parkin in a catalytically active state or interfering with toxicity of AIMP2 and PARIS (ZNF746) offer new therapeutic opportunities.
Keywords: c-Abl, PARIS, ZNF746, AIMP2, S-nitrosylation, neurodegeneration
Mutations in parkin, an E3 ubiquitin ligase are the most common cause of autosomal recessive Parkinson’s disease (PD) [1,2]. Parkin has been proposed to regulate a variety of processes including receptor trafficking and mitochondrial quality control. Mutations include deletions, insertions and point mutations that for the most part lead to a loss of parkin’s catalytic activity [3–5]. In addition to mutations impairing parkin’s function, its enrichment with cysteines makes it prone to oxidative and nitrosative attack. There is a loss of parkin function due to S-nitrosylation, oxidative and dopaminergic stress, and phosphorylation by the stress activated kinase, c-Abl in the more common sporadic form of PD [6–13].
Different ubiquitin lysine linkages enable parkin to function as a multifunctional E3 ligase. Parkin regulates receptor trafficking and cell signaling via monoubiquitination of parkin substrates [5]. Inclusion body formation and autophagy are regulated by parkin through polyubiquitination via lysine 63 or 29 linkages [14,15]. Polyubiquitinated parkin substrates via lysine 48 linkages are degraded by the ubiquitin proteosome system. Loss of parkin function in PD would be expected to interfere with parkin E3 ligase functions and lead to defects in the ubiquitin proteasome system clearance of lysine 48 substrates [16]. Thus, parkin substrates should accumulate in situations where parkin is inactivated such as in patients with parkin mutations, sporadic PD, parkin knockout mice, and following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) intoxication in mice [9]. Parkin substrates that are elevated in all four conditions are strong candidates for parkin mediated polyubiquitination via lysine 48 linkages and subsequent ubiquitin proteosome degradation [9,17–19]. At least 4 independent groups and 6 different labs that have shown that parkin is inactivated in sporadic PD [6,7,9–13,17,20]. Our laboratory has focused its attention on two potential pathophysiologic substrates of parkin that meet the latter criteria.
Aminoacyl-tRNA synthetase complex interacting multifunctional protein-2 (AIMP2), also known as JTV-1 or P38, is a parkin substrate that is present in Lewy body inclusions of PD substantia nigra [18,21]. AIMP2 is a strong candidate as a pathogenic parkin substrate since AIMP2 levels are elevated in the ventral midbrain in parkin KO mice and post-mortem brain from patients with parkin mutations or sporadic PD [7,9,18]. AIMP2 also accumulates in the MPTP model of PD consistent with the notion that parkin is inactivated following MPTP intoxication [9].
PARIS (ZNF746) is another strong pathogenic parkin substrate since it accumulates in familial PD with parkin mutations, sporadic PD, parkin knockout mice and MPTP intoxicated mice [19]. Under pathologic conditions, where parkin is inactivated in PD, PARIS levels accumulate leading to mitochondrial dysfunction through down regulation of PGC-1α resulting in the loss of dopamine (DA) neurons. PARIS upregulation is required for the loss of DA neurons since conditional knockout of parkin in adult animals leads to progressive loss of DA neurons that is PARIS dependent. Moreover, overexpression of PARIS leads to the selective loss of DA neurons in the substantia nigra, which is reversed by either parkin or PGC-1α co-expression [19]. These findings are recapitulated in sporadic PD.
Recent work suggests that the non-tyrosine receptor kinase, c-Abl, accounts for the inactivation of parkin via oxidative stress by phosphorylation of tyrosine 143 in sporadic PD [7,9]. This post-translational modification of parkin provides a unique opportunity to modify the phosphorylation status of parkin and maintain it in a catalytically active state by interfering with activation of c-Abl.
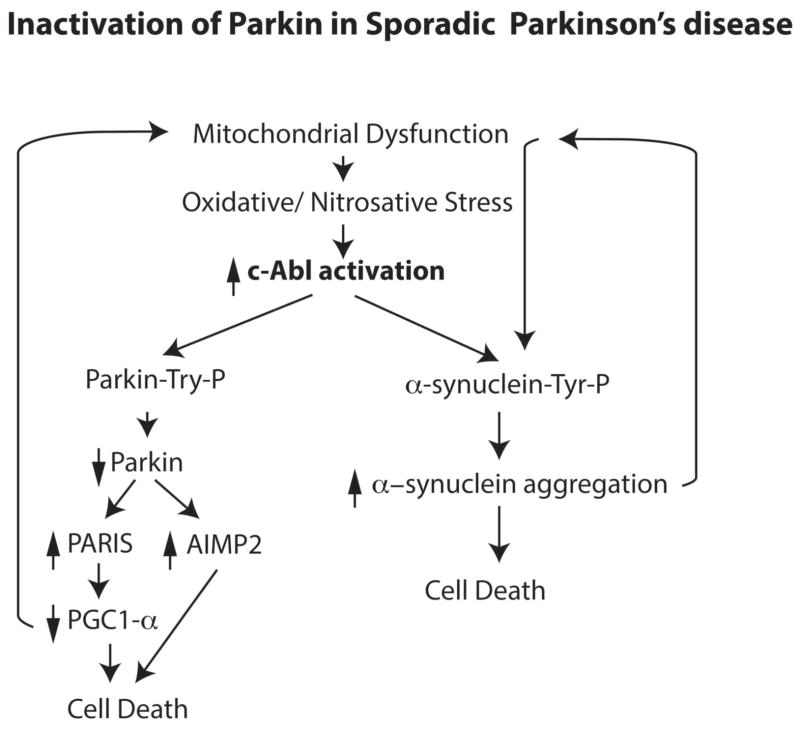
We propose a model in which c-Abl is activated due to mitochondrial dysfunction and/or oxidative stress leading to tyrosine phosphorylation of parkin and its subsequent inactivation followed by the accumulation of parkin substrates (Figure 1). In a parallel pathway, α-synuclein is tyrosine phosphorylated leading to its aggregation and subsequent toxicity [22]. Since aggregated α-synuclein can lead to mitochondrial dysfunction it creates a feed forward cycle. In this model, parkin is not required for α-synuclein toxicity consistent with our observations that the absence of parkin does not exacerbate α-synuclein induced toxicity in the A53T α-synuclein transgenic model [23]. However, preventing parkin’s tyrosine phosphorylation could enhance its protective function against α-synuclein toxicity by maintaining its cytoprotective function consistent with observations that overexpression of parkin protects against the toxic effects of α-synuclein induced toxicity [24]. Our central hypothesis is that stress-induced activation of the non-receptor tyrosine kinase, c-Abl contributes to the pathogenesis of sporadic PD via tyrosine phosphorylation of parkin and α-synuclein. We acknowledge that there are likely to be other mechanisms and these additional mechanisms will need to be evaluated in future studies to determine their relative contributions to DA neuron degeneration due to parkin inactivation.
Figure 1.
Parkin is inactivated in sporadic PD. Mitochondrial dysfunction in sporadic PD leads to oxidative and nitrosative stress that directly inactivates parkin, but it also leads to the activation of c-Abl. Parkin is also inactivated by tyrosine phosphorylation of parkin. α-Synuclein may also be tyrosine phosphorylated by c-Abl leading to its aggregation and subsequent toxicity creating a feed-forward cycle of mitochondrial dysfunction followed by parkin inactivation. Inactivation of parkin leads to the loss of its ubiquitin E3 ligase activity and parkin substrates such as PARIS and AIMP2 accumulate leading to the death of dopamine neurons.
Future studies are needed to explore the relationship of PARIS and AIMP2 in dopaminergic cell death. What are the underlying mechanisms of PARIS and AIMP2 induced loss of dopaminergic neurons? It will be important to determine whether AIMP2 and PARIS or other mechanisms of parkin-induced DA neurodegeneration intersect in a common pathway or whether they are separate pathways. Interfering with these molecular mechanisms offer new therapeutic strategies to treat PD. Maintaining parkin in a catalytically active state through inhibition of c-Abl is an attractive avenue to pursue as inhibitors of c-Abl are in wide clinical use for the treatment of chronic myeloid leukemia. Repurposing of these agents could quickly be translated into a disease modifying therapy for Parkinson’s disease.
Acknowledgments
This work was supported by grants from the NIH NS38377 and the JPB Foundation. T.M.D. is the Leonard and Madlyn Abramson Professor in Neurodegenerative Diseases. The authors acknowledge the joint participation by the Adrienne Helis Malvin Medical Research Foundation and the Diana Helis Henry Medical Research Foundation through its direct engagement in the continuous active conduct of medical research in conjunction with The Johns Hopkins Hospital and the Johns Hopkins University School of Medicine and the Foundation’s Parkinson’s Disease Programs.
References
- 1.Abbas N, Lucking CB, Ricard S, et al. A wide variety of mutations in the parkin gene are responsible for autosomal recessive parkinsonism in Europe. French Parkinson's Disease Genetics Study Group and the European Consortium on Genetic Susceptibility in Parkinson's Disease. Hum Mol Genet. 1999;8:567–574. doi: 10.1093/hmg/8.4.567. [DOI] [PubMed] [Google Scholar]
- 2.Kitada T, Asakawa S, Hattori N, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 3.Corti O, Lesage S, Brice A. What genetics tells us about the causes and mechanisms of Parkinson's disease. Physiol Rev. 2011;91:1161–1218. doi: 10.1152/physrev.00022.2010. [DOI] [PubMed] [Google Scholar]
- 4.Dawson TM. Parkin and defective ubiquitination in Parkinson's disease. Journal of neural transmission Supplementum. 2006:209–213. doi: 10.1007/978-3-211-45295-0_32. [DOI] [PubMed] [Google Scholar]
- 5.Moore DJ. Parkin: a multifaceted ubiquitin ligase. Biochem Soc Trans. 2006;34:749–753. doi: 10.1042/BST0340749. [DOI] [PubMed] [Google Scholar]
- 6.Chung KK, Thomas B, Li X, et al. S-nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function. Science. 2004;304:1328–1331. doi: 10.1126/science.1093891. [DOI] [PubMed] [Google Scholar]
- 7.Imam SZ, Zhou Q, Yamamoto A, et al. Novel regulation of parkin function through c-Abl-mediated tyrosine phosphorylation: implications for Parkinson's disease. J Neurosci. 2011;31:157–163. doi: 10.1523/JNEUROSCI.1833-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim MY, Mauro S, Gevry N, Lis JT, Kraus WL. NAD+-dependent modulation of chromatin structure and transcription by nucleosome binding properties of PARP-1. Cell. 2004;119:803–814. doi: 10.1016/j.cell.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Ko HS, Lee Y, Shin JH, et al. Phosphorylation by the c-Abl protein tyrosine kinase inhibits parkin's ubiquitination and protective function. Proc Natl Acad Sci U S A. 2010;107:16691–16696. doi: 10.1073/pnas.1006083107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LaVoie MJ, Ostaszewski BL, Weihofen A, Schlossmacher MG, Selkoe DJ. Dopamine covalently modifies and functionally inactivates parkin. Nat Med. 2005;11:1214–1221. doi: 10.1038/nm1314. [DOI] [PubMed] [Google Scholar]
- 11.Meng F, Yao D, Shi Y, et al. Oxidation of the cysteine-rich regions of parkin perturbs its E3 ligase activity and contributes to protein aggregation. Mol Neurodegener. 2011;6:34. doi: 10.1186/1750-1326-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C, Ko HS, Thomas B, et al. Stress-induced alterations in parkin solubility promote parkin aggregation and compromise parkin's protective function. Hum Mol Genet. 2005;14:3885–3897. doi: 10.1093/hmg/ddi413. [DOI] [PubMed] [Google Scholar]
- 13.Yao D, Gu Z, Nakamura T, et al. Nitrosative stress linked to sporadic Parkinson's disease: S-nitrosylation of parkin regulates its E3 ubiquitin ligase activity. Proc Natl Acad Sci U S A. 2004;101:10810–10814. doi: 10.1073/pnas.0404161101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geisler S, Holmstrom KM, Skujat D, et al. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 15.Olzmann JA, Chin LS. Parkin-mediated K63-linked polyubiquitination: a signal for targeting misfolded proteins to the aggresome-autophagy pathway. Autophagy. 2008;4:85–87. doi: 10.4161/auto.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cookson MR. Parkin's substrates and the pathways leading to neuronal damage. Neuromolecular medicine. 2003;3:1–13. doi: 10.1385/NMM:3:1:1. [DOI] [PubMed] [Google Scholar]
- 17.Ko HS, Kim SW, Sriram SR, Dawson VL, Dawson TM. Identification of far upstream element-binding protein-1 as an authentic Parkin substrate. J Biol Chem. 2006;281:16193–16196. doi: 10.1074/jbc.C600041200. [DOI] [PubMed] [Google Scholar]
- 18.Ko HS, von Coelln R, Sriram SR, et al. Accumulation of the authentic parkin substrate aminoacyl-tRNA synthetase cofactor, p38/JTV-1, leads to catecholaminergic cell death. J Neurosci. 2005;25:7968–7978. doi: 10.1523/JNEUROSCI.2172-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin JH, Ko HS, Kang H, et al. PARIS (ZNF746) Repression of PGC-1alpha Contributes to Neurodegeneration in Parkinson's Disease. Cell. 2011;144:689–702. doi: 10.1016/j.cell.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vandiver MS, Paul BD, Xu R, et al. Sulfhydration mediates neuroprotective actions of parkin. Nat Commun. 2013;4:1626. doi: 10.1038/ncomms2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corti O, Hampe C, Koutnikova H, et al. The p38 subunit of the aminoacyl-tRNA synthetase complex is a Parkin substrate: linking protein biosynthesis and neurodegeneration. Hum Mol Genet. 2003;12:1427–1437. doi: 10.1093/hmg/ddg159. [DOI] [PubMed] [Google Scholar]
- 22.Hebron ML, Lonskaya I, Moussa CE. Nilotinib reverses loss of dopamine neurons and improves motor behavior via autophagic degradation of alpha-synuclein in Parkinson's disease models. Hum Mol Genet. 2013 doi: 10.1093/hmg/ddt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Von Coelln R, Thomas B, Savitt JM, et al. Loss of locus coeruleus neurons and reduced startle in parkin null mice. Proc Natl Acad Sci U S A. 2004;101:10744–10749. doi: 10.1073/pnas.0401297101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrucelli L, O'Farrell C, Lockhart PJ, et al. Parkin protects against the toxicity associated with mutant alpha-synuclein: proteasome dysfunction selectively affects catecholaminergic neurons. Neuron. 2002;36:1007–1019. doi: 10.1016/s0896-6273(02)01125-x. [DOI] [PubMed] [Google Scholar]