Abstract
Background
Female runners have a high incidence of developing patellofemoral pain. Abnormal mechanics are thought to be an important contributing factor to patellofemoral pain. However, the contribution of abnormal trunk, hip, and foot mechanics to the development of patellofemoral pain within this cohort remains elusive. Therefore the aim of this study was to determine if significant differences during running exist in hip, trunk and foot kinematics between females with and without patellofemoral pain.
Methods
32 female runners (16 patellofemoral pain, 16 healthy control) participated in this study. All individuals underwent an instrumented gait analysis. Between-group comparisons were made for hip adduction, hip internal rotation, contra-lateral pelvic drop, contra-lateral trunk lean, rearfoot eversion, tibial internal rotation, as well as forefoot dorsiflexion and abduction
Findings
The patellofemoral pain group had significantly greater peak hip adduction and hip internal rotation. No differences in contra-lateral pelvic drop were found. A trend towards reduced contra-lateral trunk lean was found in the patellofemoral pain group. No significant differences were found in any of the rearfoot or forefoot variables but significantly greater shank internal rotation was found in the patellofemoral pain group.
Interpretation
We found greater hip adduction, hip internal rotation and shank internal rotation in female runners with patellofemoral pain. We also found less contra-lateral trunk lean in the patellofemoral pain group. This may be a potential compensatory mechanism for the poor hip Control seen. Rehabilitation programs that correct abnormal hip and shank kinematics are warranted in this population.
1 Introduction
The low cost and ease of access make running a popular form of exercise. Unfortunately, the positive health benefits associated with running are offset by a high incidence of musculoskeletal injuries (van Gent et al., 2007; van Mechelen, 1992). One of the most common injuries runners suffer from is patellofemoral pain (PFP) (Taunton et al., 2002). The symptoms of PFP often become chronic and may result in transient or permanent difficulty performing numerous tasks including running, stairs, and jumping. This can lead to reduced participation in running and other recreational activities (Blond and Hansen, 1998). Interestingly, females are twice as likely as males to develop PFP (Boling et al., 2009). Despite the high incidence of PFP in females, the mechanisms that contribute to this injury are still poorly understood.
A recent international PFP research retreat identified several factors believed to be related to the development of PFP (Davis and Powers, 2010). These included proximal factors related to the hip and pelvis, and distal factors related to the foot and ankle. Considerable debate remains as to the role of proximal and distal factors in the development of PFP in female runners. In addition, the consensus statement identified a need for research on proximal and distal factors to include the trunk and forefoot respectively, in order to better understand the etiology of PFP (Davis and Powers, 2010).
Currently, several conflicting studies exist concerning the role of proximal mechanics in the development of PFP. For example, greater hip internal rotation has been reported in females with PFP while performing a variety of tasks (Souza and Powers, 2009a). In contrast to this study, other authors have reported greater hip adduction but not greater hip internal rotation while running (Willson and Davis, 2008). However, neither study focused on habitual runners who specifically reported suffering from PFP during running (Souza and Powers, 2009a; Willson and Davis, 2008). A recent study that focused on runners with PFP reported that they had less hip adduction and no differences in hip internal rotation when compared to a healthy control group (Dierks et al., 2008). The inclusion of males and females may have influenced the results of this study as previous gender differences have been reported in running (Ferber et al., 2003). The varying cohorts and tasks studied in the literature to date have limited applicability to female runners and thus further research is required in that particular population.
Although poor control of the trunk has been hypothesized to contribute to PFP, few studies have examined this relationship (Powers, 2010). The collapsing of the trunk away from the stance leg may contribute to poor pelvic positioning and hip mechanics. This pattern is evident in females at risk for other injuries such as ACL injury (Zazulak et al., 2007). However, while greater contra-lateral pelvic drop has been reported in females with PFP (Willson and Davis, 2008) no study has yet examined the possible role of trunk lateral lean as a contributing mechanism to PFP.
With respect to distal factors, excessive foot pronation has often been implicated with PFP. The potential role of foot mechanics in PFP is highlighted by studies demonstrating the efficacy of foot orthotics in the treatment of the condition (Collins et al., 2008; Eng and Pierrynowski, 1993; Johnston and Gross, 2004; Sutlive et al., 2004). However, the scientific evidence for the link between abnormal pronation during gait and PFP remains inconclusive. A recent review of literature identified only one study comparing foot kinematics during running in PFP patients and controls (Barton et al., 2009). This study reported no group differences in the magnitude or timing of peak rearfoot eversion, but a small reduction in excursion was found during the first 10% of stance in PFP patients (Duffey et al., 2000). However, a more recent study identified no differences between PFP and controls (Dierks et al., 2011) Therefore, there is a clear need for future studies investigating the role of foot kinematics in the etiology of PFP.
Presently, the majority of published studies concerning the foot kinematics of PFP patients have quantified either rearfoot (Callaghan and Baltzopoulos, 1994; Duffey et al., 2000; Levinger and Gilleard, 2005; Levinger and Gilleard, 2007) or whole foot motion (Powers et al., 2002). There is evidence that significant motion occurs at the joints distal to the rearfoot. Indeed, forefoot kinematics has successfully been used to discriminate between those with and without pathology (Ness et al., 2008; Woodburn et al., 2004). Moreover, a recent study demonstrated that PFP patients who responded positively to an orthotic intervention had significantly greater rearfoot eversion and a trend towards greater forefoot dorsiflexion and abduction during walking, compared to patients who did not respond to the treatment (Barton et al., 2010). To the authors’ knowledge there have been no cross-sectional studies examining whether both rearfoot and forefoot kinematics differ in PFP patients during running.
In summary, the role of proximal and distal mechanics in the development of PFP in female runners remains inconclusive. Additionally, poor trunk mechanics and excessive forefoot motion have been implicated as contributing factors to PFP but this relationship has yet to be formally examined. Therefore, the purpose of this study is to determine if significant differences exist in trunk, hip, and foot mechanics between female runners with and without PFP. We hypothesized that female runners with PFP would exhibit greater peak hip adduction and internal rotation, contra-lateral pelvic drop, and contra-lateral trunk lean. In terms of distal factors we also 3hypothesized that PFP would demonstrate greater peak shank internal rotation, rearfoot eversion, and forefoot dorsiflexion and abduction during running.
2 Methods
Female runners between the ages of 18–45 were recruited from a sample of convenience through local races and posted flyers. The institutional review board approved the study and before inclusion into the study all subjects provided written informed consent. To be initially considered for the study, subjects had to report running at least 16 km/week. Females with PFP also had to report insidious onset anterior knee pain while running for at least the past two months. In addition, subjects could not have had any previous reconstructive surgery to the knee and had to report being free from any other lower extremity or lower back injury for the past 6 months.
Potential subjects with PFP were evaluated by a licensed physical therapist or athletic trainer to determine if they qualified for the study. Subjects were included in the PFP group if they reported pain of at least 3 out of 10 on the verbal analog scale during running. During the evaluation the patient had to report pain either during retropatellar/peripatellar palpation or during the patellar compression test. Also the examiner ruled out possible ligamentous, tendon and internal derangement during the evaluation. Subjects from the PFP group were also excluded if they had any previous history of patellar dislocations, instability, or any other condition that may influence their gait. Subjects in the control group were excluded if they reported any of the above conditions. A total of thirty-two subjects (16 PFP, 16 control) participated in this study.
Subjects that qualified for the study then underwent an instrumented gait analysis. Briefly, markers were placed bilaterally on the acromion process, iliac crest, L5S1, anterior superior iliac spines, and greater trochanter, Markers were also placed unilaterally on the medial and lateral femoral condyles, head of fibula, tibial tuberosity, medial and lateral malleolus, superior and inferior calcaneus, lateral calcaneus, 1st and 5th metatarsal heads and bases. Rigid shells containing a cluster of four tracking markers were also secured to the posterior aspect of the thigh and shank. Good reliability with placing these markers has previously been reported (Noehren et al., 2010). All participants wore custom laboratory shoes (Xccelerator, Nike Inc.), which contained cut out windows allowing the placement of the forefoot and foot markers directly on the skin of the foot. A standing calibration trial was then collected. After this trial a hip motion trial was performed for the purposes of establishing the hip joint center (Schwartz and 4Ozumalski, 2005). The subjects then walked on the instrumented treadmill (Bertec, Columbus, Ohio) until they felt comfortable and ready to run. They then ran for 3 min at their self-selected pace to warm up. The treadmill speed was gradually increased to 3.3 m/s where the subject then ran for an additional 2 min and 5 consecutive footfalls were recorded. A standardized running speed was selected to be consistent with the literature thus enabling a comparison of our data with other studies (Souza and Powers, 2009a; Willson and Davis, 2008). The three dimensional marker trajectories were recorded at 300 Hz with a 15-camera motion analysis system (Motion Analysis Corp, Santa Rosa, USA). Force data was collected at 1200 Hz and heel strike and toe off were determined when the vertical ground reaction force was greater or less than 30 N respectively.
Visual 3D software (C-motion, Germantown, MD, USA) was used to filter the data, identify the functional hip joint center and calculate the joint angles. The marker trajectory data was filtered at 8 Hz and force data at 35 Hz using a 4th order Butterworth low pass filter. Joint angles were calculated as the distal segment relative to the proximal segment with an x-y-z (medio-lateral, antero-posterior, vertical) Cardan angle sequence. Trunk and pelvis angles were calculated relative to the lab coordinate system. A detailed description of the segment coordinate definitions is provided as supplementary online document (Appendix A). Forefoot and rearfoot kinematics were calculated using and adapted Oxford foot model, which has demonstrated good reliability (Carson et al, 2001; Pohl et al., 2007). Segment angles were not normalized to the standing calibration trial. Custom Labview code (National Instruments; Austin, TX) was used to extract the peak joint angle from the first 75% of each stance phase. Each subject’s joint angular values were calculated as the average of the five trials. Descriptive statistics (means and standard deviations) were then calculated for each group and an independent samples t-test was used to detect between-group differences. Statistical analysis was performed with SPSS version 18.0 (IBM SPSS, Chicago, IL). The effect size of each variable was also calculated. Standard definitions were used with a small effect equal to 0.020–0.50, a medium being between 0.50 and 0.80 and a large effect considered 0.80 or greater (Portney and Watkins, 1993). The proximal variable of interest included peak contra-lateral trunk lean, peak contra-lateral pelvic drop, peak hiup adduction and peak hip internal rotation. The distal variables of interest included peak shank internal rotation, peak rearfoot eversion, peak forefoot dorsiflexion, and peak forefoot abduction.
3 Results
The groups were well matched in height, weight and age (Table 1). However, there was a significant difference in running mileage (p= 0.018), with control subjects running on average 12 km/week more than the PFP subjects. Significantly greater peak hip adduction (p= 0.046), and hip internal rotation (p= 0.002) were found in the PFP group with a mean difference of 2.2 and 4.6° respectively (Table 2, Figs. 1A, B, and 2). However, no significant difference was found in peak contra-lateral pelvic drop (p= 0.304), (Fig. 1C). There was a non-significant trend towards differences in peak contra-lateral trunk lean (p= 0.071) (Table 2 and Fig. 1D) with the PFP group actually having lower values. All hip and trunk variables were associated with moderate to large effect sizes (Table 2).
Table 1.
Mean (standard deviation) subject demographics of the PFP and control groups.
| Patellofemoral Pain (PFP)
|
Control
|
|
|---|---|---|
| Height (m) | 1.64 (0.05) | 1.65 (0.07) |
| Mass (kg) | 57.4 (4.6) | 58.7 (6.5) |
| Age (years) | 27 (6) | 25 (4) |
| Weekly distance (km) | 23 (10) | 35 (16) |
Table 2.
Peak variables of interest expressed as mean (standard deviation). Hip adduction, hip internal rotation, and forefoot dorsiflexion are positive, whereas contra-lateral trunk, pelvic drop, rearfoot eversion, forefoot abduction, and shank internal rotation are negative.
| PFP
|
Control
|
p value
|
Effect size
|
|
|---|---|---|---|---|
| Hip adduction | 20.0° (3.5) | 17.8° (2.6) | 0.046 | 0.74 |
| Hip internal rotation | 9.8° (4.2) | 5.2° (3.3) | 0.002 | 1.26 |
| Lateral trunk lean | 5.0° (1.3) | 3.5° (3.0) | 0.071 | 1.74 |
| Pelvis contra-drop | −8.0° (2.7) | −6.6° (2.1) | 0.132 | 0.60 |
| Rearfoot eversion | −11.2° (4.0) | −9.4° (5.3) | 0.27 | 0.39 |
| Forefoot dorsiflex | 7.2° (2.5) | 7.5° (2.5) | 0.66 | 0.12 |
| Forefoot abduction | −12.5° (4.0) | −10.8° (3.2) | 0.16 | 0.49 |
| Shank internal rot | −10.0° (5.3) | −6.5° (3.0) | 0.03 | 0.83 |
Fig. 1.
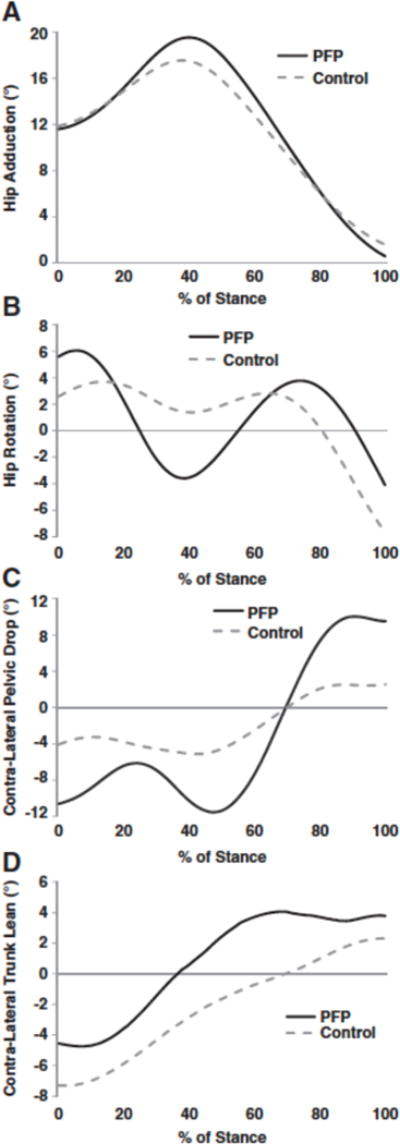
A) Hip adduction ensemble averages for the PFP group and the healthy control group. Hip adduction is positive. B) Hip internal rotation ensemble averages for the PFP group and the healthy control group. Hip internal rotation is positive. C) Contra-lateral pelvic drop ensemble averages for the PFP group and the healthy control group. Contra-lateral pelvic drop is negative. D) Contra-lateral trunk lean ensemble averages for the PFP group and the healthy control group. Contra-lateral lean is negative.
Fig. 2.
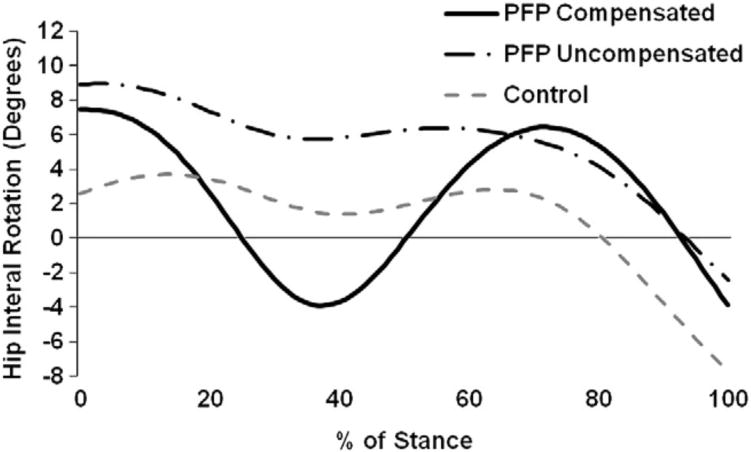
A comparison between hip internal rotation between PFP subjects who overly compensated vs. those who did not. Both subgroups of PFP subjects landed in greater hip internal rotation than the control group. Hip internal rotation is positive.
We found few differences in foot mechanics. Although there was no difference in peak rearfoot eversion (p= 0.27), forefoot dorsiflexion (p= 0.66), or forefoot abduction (p= 0.16), peak foot eversion and forefoot abduction were associated with a moderate effect size (Table 2, Fig. 3). We did find significantly greater peak shank internal rotation in the PFP group with an average difference of 4.5° between groups (p= 0.03).
Fig. 3.
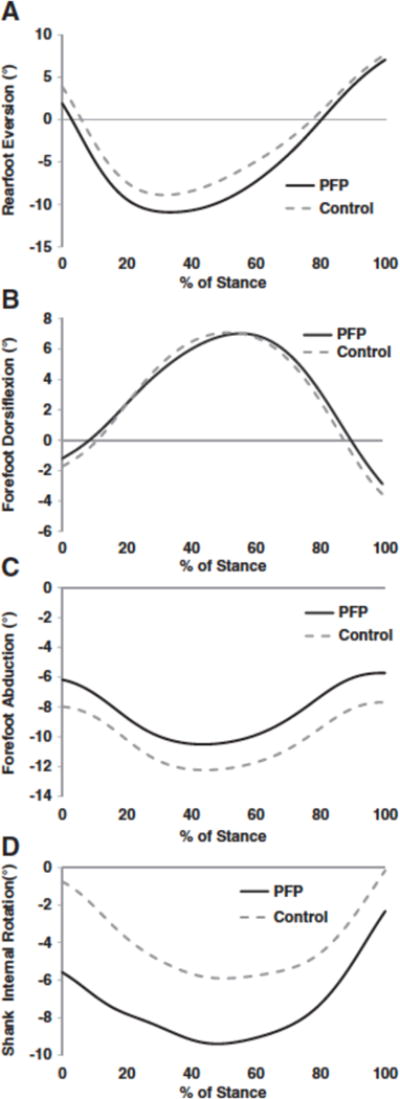
A) Rear foot eversion ensemble curves for the PFP group and healthy control group. Rear foot eversion is negative. B) Forefoot dorsiflexion curves for the PFP group and healthy control group. Forefoot dorsiflexion is positive. C) Forefoot abduction curves for the PFP and healthy control group. Forefoot abduction is negative. D) Shank internal rotation for the PFP and healthy control group. Shank internal rotation is negative.
4 Discussion
The purpose of this study was to determine if trunk, hip, and foot kinematics were significantly different between female runners with PFP and healthy female runners. We found that female runners with PFP had significantly greater peak hip adduction and hip internal rotation. Contrary to our expectations we found no differences in either peak contra-lateral trunk lean or pelvic drop. Additionally, with the exception of greater peak shank internal rotation we found no differences in foot mechanics.
We found significantly greater peak hip adduction in female runners with PFP (Fig. 1A). This finding is consistent with the work of Willson and Davis (2008). Conceptually, excessive hip adduction in runners with PFP increases the dynamic Q angle and may result in greater compressive stresses on the patellofemoral joint (Powers, 2010). Cadaveric simulations support this premise where increased hip adduction (Q angle) resulted in greater compression on the lateral aspect of the patella. Greater joint stress as the result of excessive hip adduction could be an especially important consideration in female runners where there is repetitive exposure to high loads. Caution should be emphasized in the interpretation of these results as the absolute magnitude of the difference in the groups was modest (2.2°). We found a much larger difference in hip internal rotation (4.6°), which was associated with a larger effect size.
Consistent with our hypothesis we found that female runners with PFP had greater peak hip internal rotation (Fig. 1B). This is in contrast to two previous studies, which reported no differences in hip internal rotation (Dierks et al., 2008; Willson and Davis, 2008). The discrepancy between these studies and ours may be attributable to a variety of confounding methodological factors such as normalizing joint angles to the standing angle, the time point at which the discrete value was selected in the stance phase, the kinematic models used and differences in the inclusion criteria of the participants. Our results though are consistent with the work of Souza et al., who used similar methodology to the current study (Souza and Powers, 2009a). The excessive hip internal rotation found in runners with PFP may have contributed to greater stress on the patella and subsequent pain. Recently, imaging studies have confirmed that greater internal rotation of the femur on the patella during a squatting task resulted in greater lateral patellar tilt and lateral displacement at the patellofemoral joint (Draper et al., 2009; Souza et al., 2010). Such kinematic alterations have been shown in cadaveric studies to shift the distribution of pressure laterally on the patella (Lee et al., 1994; Li et al., 2004).
We were surprised to observe the greater fluctuation in hip transverse plane motion in the PFP group when compared to the healthy control group (Fig. 1B). Upon closer evaluation of the PFP group we noticed two distinct kinematic patterns that may help explain this finding (Fig. 2). We observed that all PFP subjects started out in more hip internal rotation. Then, half of the PFP group gradually external rotated, whereas the other half oscillated between externally and then internally rotating the hip (Fig. 2). Neither of these two patterns was evident in the control group and both are potentially deleterious to the patellofemoral joint. The subgroup of PFP subjects who went through a period of rapid external rotation may have done so due to poor proximal motor control or strength. In contrast the subgroup of PFP participants that started in greater hip internal rotation and gradually externally rotated may have done such because of mechanical reasons such as greater femoral anteversion. A recent study, however, found that structural parameters were poor predictors of hip internal rotation (Souza and Powers, 2009b). Future studies should consider whether these predictors are strengthened when analyzing subsets of those with excessive hip internal rotation.
Interestingly, we found no significant differences in peak contra-lateral pelvic drop (Fig. 1C). Greater peak contra-lateral pelvic drop is hypothesized to shift the center of mass away from the stance leg increasing the demand on the muscles that control hip adduction (Powers, 2010). Our findings are in contrast to Willson and Davis who reported greater contra-lateral pelvic drop in those with PFP (Willson and Davis, 2008). Within our group of habitual runners with PFP, some may have attempted to decrease the demand on weak hip abductors and lateral core weakness by leaning towards the involved side and reducing the magnitude of contra-lateral pelvic drop. In support of this we did find a trend towards reduced contra-lateral trunk lean. In addition, numerous studies have reported that females with PFP have weak hip abductor muscles. (Ireland et al., 2003; Robinson and Nee, 2007; Souza and Powers, 2009b). However, this theory is speculative at this point, as strength was not measured in this study. Additional studies are needed comparing hip and trunk strength to kinematics in this population.
In contrast to our expected hypothesis, we found a trend (p= 0.07) towards greater peak ipsilateral trunk lean over the stance leg (Fig. 1D). However, the differences in trunk lean between the groups were modest (1.5°). Several possibilities exist for why this was observed. First, the body may attempt to minimize lateral displacement of the trunk in order to decrease energy demands (Saunders et al., 1953). Second, reduced core stability may have resulted in individuals compensating by leaning towards the involved side, further decreasing the demands on the lateral trunk stabilizers such as the quadratus lumborum, lateral obliques abdominous muscles. Indeed, weaker trunk muscles have been previously reported in those with PFP (Cowan et al., 2009). Further studies are needed to substantiate this theory and assess the relationship of trunk kinematics and strength in those with PFP. Third, the subjects in the PFP group may have leaned to the involved side to reduce the demands on weak hip abductors that were previously discussed. Lastly, these results suggest that the body may attempt to minimize disruption of trunk mechanics at the expense of hip mechanics. Minimizing the sway of the trunk would be important for gaze stabilization and balance while running.
We found no differences between the PFP and control groups in terms of rearfoot or forefoot mechanics. Our results in rearfoot mechanics are in agreement with Dierks et al. (2008) but differ from several other studies (Levinger and Gilleard, 2005; Levinger and Gilleard, 2007; Powers et al., 2002). Direct comparisons to these latter studies are difficult as they were done during barefoot walking using different foot models. Although our results indicated no association between foot mechanics and PFP, the importance of the foot in the etiology of the condition should not be discounted. For example, several recent studies have shown good success in treating PFP with the use of foot orthotics (Barton et al., 2010; Collins et al., 2008). Moreover, a recent case control study demonstrated that PFP patients who responded positively to an orthotic intervention had significantly greater rearfoot eversion and a trend (although not statistically significant) towards greater forefoot dorsiflexion and abduction during walking compared to those who did not respond (Barton et al., 2010). This is suggestive of the presence of subgroups within the PFP population which is worthy of further investigation.
We did find greater shank internal rotation in the PFP group. Excessive shank internal rotation has often been affiliated with excessive rearfoot eversion given the documented kinematic coupling relationship between the two segments (Pohl et al., 2007). However, given that we did not find any group differences in rearfoot eversion our results suggest that foot mechanics were not responsible for the greater shank internal rotation in PFP subjects. Another possibility is that abnormal hip mechanics might be driving the shank into greater internal rotation. Indeed, the PFP group displayed greater hip internal rotation, implying that proximal factors may be more important than distal factors in terms of controlling transverse shank rotation.
The results of this study support the continued development of interventions to improve hip control in female runners with PFP. Several recent studies have shown promising results using a combination of hip strengthening and functional exercises to reduce pain in those with PFP (Boling et al., 2006; Nakagawa et al., 2008; Tyler et al., 2006). To date only one study has directly attempted to improve hip mechanics in female runners (Noehren et al., 2011). These authors found that a real-time gait retraining program focused on restoring normal hip adduction motion resulted in a complete resolution of symptoms at a one month follow-up (Noehren et al., 2011). Clinical treatment approaches, therefore, may benefit from considering a global program focusing on restoring normal hip kinematics in both the frontal and transverse plane. Such a program may include hip muscle strength training along with specific exercises to improve the patients’ awareness of how they are controlling the hip (Earl and Hoch, 2011).
There are several limitations to our study that should be noted. First the trunk model consisted of a single segment to represent the entire trunk. Alterations in the lumbar spine kinematics may have been compensated for by the thoracic spine resulting in no net change in overall trunk position. Additional studies using more sophisticated trunk models are needed to explore this possibility. Secondly, the runners in the control group had a greater weekly training volume than the PFP subjects. Although not assessed quantitatively, many of the runners with PFP reported decreasing their training volume due to pain. However, all subjects were running a minimum of 16 km/week, ensuring they were habitual runners. Thirdly, because of the cross-sectional nature of the study we are unable to establish cause and effect. Prospective studies are needed to confirm the observations in this study. Fourthly, the sample size used in the present study was relatively small. Studies containing a greater sample size are needed before strong conclusions can be made regarding whether proximal or distal factors have a greater association with PFP. Lastly, PFP is a multifactorial disorder. We addressed only one possible component pertaining to biomechanics, and it was beyond the scope of this study to consider other possible factors such as training volume, shoe wear, experience and other factors. We also did not assess trunk or hip strength thus limiting our ability to directly test possible mechanisms for the observed altered mechanics. Future studies should consider which variables in addition to the biomechanical variables are most likely to contribute to PFP.
In conclusion, we found greater hip adduction and internal rotation in habitual female runners with PFP when compared to healthy control runners. Such deficits suggest a multi-planar loss of hip control in this cohort. No significant differences were observed at either the trunk or pelvis although there was a trend towards greater ipsilateral trunk lean in the PFP group. This may be a compensatory mechanism for weak hip and trunk muscles. Lastly, we did not find any significant difference in either rearfoot or forefoot variables. However, we did find that shank internal rotation was significantly greater in the PFP group. These results provide some of the first comprehensive quantification of the role of proximal and distal kinematics in PFP and help fill in literature gaps previously highlighted by the patellofemoral pain syndrome retreat.
Acknowledgments
No funding was received for this study. Salary support for Michael Pohl was received from Alberta Innovates—Health Solutions and salary support for Christian Lattermann was received from NIH/NIAMS grant K23 AR060275.
Appendix A. Definitions of Anatomical Terms Used and Description of How They Were Assessed
Rearfoot
Sagittal plane was defined using the superior and inferior calcaneus together with the midpoint of the medial and lateral malleoli. The antero-posterior axis (y) was in the sagittal plane parallel to the floor with its positive direction anterior. The medio-lateral axis (x) was orthogonal to the sagittal plane with its positive direction lateral (medial for a left foot). The cross-product of these two axes gave the vertical axis (z) with its positive direction proximal.
Forefoot
The antero-posterior axis (y) ran parallel with the floor with its direction passing through the midpoints of first and fifth metatarsal bases and heads. The vertical axis (z) was perpendicular to the floor with its positive direction upwards. The medio-lateral axis (x) was the cross-product of the proceeding axes with the lateral direction positive (medial for a left foot).
Shank
The vertical axis (z) ran in the direction between the midpoint of the malleoli and the midpoint of the femoral condyles with positive defined as proximal. The antero-posterior axis (y) was perpendicular to the plane formed by the femoral condyle and malleoli markers with the anterior direction positive. The cross-product of the two former axes gave the medio-lateral axis (x) with its positive direction lateral (medial in a left limb).
Thigh
The vertical axis (z) was defined as the vector starting between the midpoint of the femoral condyles to the functional hip joint center (defined in the methods section) with its positive direction defined as proximal. The antero-posterior axis (y) was perpendicular to the plane formed by the femoral condyles and the hip joint center with its positive direction anterior. Lastly, the cross product of the first two axes defined the medio-lateral axis (x) with its positive direction lateral (medial in the left limb).
Pelvis
The vertical axis (z) was defined as the vector originating from the midpoint of the greater trochanters and passing through the midpoint of the iliac crests with its positive direction defined as proximal. The frontal plane was then defined by the bilateral greater trochanter and iliac crest markers using the least squares plane fit employed by visual 3D with its positive direction anterior. The anterior posterior axis (y) was projected forward from this plane. The cross product of the first two axes gave the medio-lateral axis (x) with its positive direction towards the right limb.
Trunk
The vertical axis (z) was defined as the vector originating from the midpoints of the iliac crest markers and passing through the midpoint of the acromion process markers with its positive direction defined as proximal. The frontal plane was then defined by the bilateral iliac crest and acromion process markers using the least squares plane fit employed by visual 3D with its positive direction anterior. The anterior posterior axis (y) was projected forward from this plane. The cross product of the first two axes gave the medio-lateral axis (x) with its positive direction towards the right limb.
Footnotes
Conflict of interest
The authors have no conflicts of interest to report.
References
- Barton CJ. Kinematic gait characteristics associated with patellofemoral pain syndrome: a systematic review. Gait & Posture. 2009;30:405–416. doi: 10.1016/j.gaitpost.2009.07.109. [DOI] [PubMed] [Google Scholar]
- Barton CJ. Greater peak rearfoot eversion predicts foot orthoses efficacy in individuals with patellofemoral pain syndrome. Br J Sports Med. 2010 doi: 10.1136/bjsm.2010.077644. [DOI] [PubMed] [Google Scholar]
- Blond L, Hansen L. Patellofemoral pain syndrome in athletes: a 5.7-year retrospective follow-up study of 250 athletes. Acta Orthop Belg. 1998;64:393–400. [PubMed] [Google Scholar]
- Boling MC. Outcomes of a weight-bearing rehabilitation program for patients diagnosed with patellofemoral pain syndrome. Arch Phys Med Rehabil. 2006;87:1428–1435. doi: 10.1016/j.apmr.2006.07.264. [DOI] [PubMed] [Google Scholar]
- Boling M. Gender differences in the incidence and prevalence of patellofemoral pain syndrome. Scand J Med Sci Sports. 2009:725–730. doi: 10.1111/j.1600-0838.2009.00996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan MJ, Baltzopoulos V. Gait analysis in patients with anterior knee pain. Clin Biomech. 1994;9:79–84. doi: 10.1016/0268-0033(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Carson MC. Kinematic analysis of a multi-segment foot model for research and clinical applications: a repeatability analysis. J Biomech. 2001;34:1299–1307. doi: 10.1016/s0021-9290(01)00101-4. [DOI] [PubMed] [Google Scholar]
- Collins N. Foot orthoses and physiotherapy in the treatment of patellofemoral pain syndrome: randomised clinical trial. Br Med J. 2008;337:a1735. doi: 10.1136/bmj.a1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan SM. Altered hip and trunk muscle function in individuals with patellofemoral pain. Br J Sports Med. 2009;43:584–588. doi: 10.1136/bjsm.2008.053553. [DOI] [PubMed] [Google Scholar]
- Davis IS, Powers CM. Patellofemoral pain syndrome: proximal, distal, and local factors, an international retreat, April 30–May 2, 2009, Fells Point, Baltimore, MD. J Orthop Sports Phys Ther. 2010;40:A1–A16. doi: 10.2519/jospt.2010.0302. [DOI] [PubMed] [Google Scholar]
- Dierks TA. Proximal and distal influences on hip and knee kinematics in runners with patellofemoral pain during a prolonged run. J Orthop Sports Phys Ther. 2008;38:448–456. doi: 10.2519/jospt.2008.2490. [DOI] [PubMed] [Google Scholar]
- Dierks TA. Lower extremity kinematics in runners with patellofemoral pain during a prolonged run. Med Sci Sports Exerc. 2011;43:693–700. doi: 10.1249/MSS.0b013e3181f744f5. [DOI] [PubMed] [Google Scholar]
- Draper CE. Using real-time MRI to quantify altered joint kinematics in subjects with patellofemoral pain and to evaluate the effects of a patellar brace or sleeve on joint motion. J Orthop Res. 2009;27:571–577. doi: 10.1002/jor.20790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffey MJ. Etiologic factors associated with anterior knee pain in distance runners. Med Sci Sports Exercise. 2000;32:1825–1832. doi: 10.1097/00005768-200011000-00003. [DOI] [PubMed] [Google Scholar]
- Earl JE, Hoch AZ. A proximal strengthening program improves pain, function, and biomechanics in women with patellofemoral pain syndrome. Am J Sports Med. 2011;39:154–163. doi: 10.1177/0363546510379967. [DOI] [PubMed] [Google Scholar]
- Eng JJ, Pierrynowski MR. Evaluation of soft foot orthotics in the treatment of patellofemoral pain syndrome. Phys Ther. 1993;73:62–70. doi: 10.1093/ptj/73.2.62. [DOI] [PubMed] [Google Scholar]
- Ferber R. Gender differences in lower extremity mechanics during running. Clin Biomech. 2003;18:350–357. doi: 10.1016/s0268-0033(03)00025-1. [DOI] [PubMed] [Google Scholar]
- Ireland ML. Hip strength in females with and without patellofemoral pain. J Orthop Sports Phys Ther. 2003;33:671–676. doi: 10.2519/jospt.2003.33.11.671. [DOI] [PubMed] [Google Scholar]
- Johnston LB, Gross MT. Effects of foot orthoses on quality of life for individuals with patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2004;34:440–448. doi: 10.2519/jospt.2004.34.8.440. [DOI] [PubMed] [Google Scholar]
- Lee TQ. The influence of fixed rotational deformities of the femur on the patellofemoral contact pressures in human cadaver knees. Clin Orthop Relat Res. 1994:69–74. [PubMed] [Google Scholar]
- Levinger P, Gilleard W. The heel strike transient during walking in subjects with patellofemoral pain syndrome. Phys Ther Sport. 2005;6:83–88. [Google Scholar]
- Levinger P, Gilleard W. Tibia and rearfoot motion and ground reaction forces in subjects with patellofemoral pain syndrome during walking. Gait Posture. 2007;25:2–8. doi: 10.1016/j.gaitpost.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Li G. The effect of tibiofemoral joint kinematics on patellofemoral contact pressures under simulated muscle loads. J Orthop Res. 2004;22:801–806. doi: 10.1016/j.orthres.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Nakagawa TH. The effect of additional strengthening of hip abductor and lateral rotator muscles in patellofemoral pain syndrome: a randomized controlled pilot study. Clin Rehabil. 2008;22:1051–1060. doi: 10.1177/0269215508095357. [DOI] [PubMed] [Google Scholar]
- Ness ME. Foot and ankle kinematics in patients with posterior tibial tendon dysfunction. Gait & Posture. 2008;27:331–339. doi: 10.1016/j.gaitpost.2007.04.014. [DOI] [PubMed] [Google Scholar]
- Noehren B, Manal K, Davis I, et al. improving between-day kinematic reliability using a marker placement device. J Orthop Res. 2010;28:1405–1410. doi: 10.1002/jor.21172. [DOI] [PubMed] [Google Scholar]
- Noehren B. The effect of real-time gait retraining on hip kinematics, pain and function in subjects with patellofemoral pain syndrome. Br J Sports Med. 2011;45:691–696. doi: 10.1136/bjsm.2009.069112. [DOI] [PubMed] [Google Scholar]
- Pohl MB. Forefoot, rearfoot and shank coupling: effect of variation in speed and mode of gait. Gait Posture. 2007;25:295–302. doi: 10.1016/j.gaitpost.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practice. Norwalk, Conn: Appleton & Lange; 1993. [Google Scholar]
- Powers CM. The influence of abnormal hip mechanics on knee injury: a biomechanical perspective. J Orthop Sports Phys Ther. 2010;40:42–51. doi: 10.2519/jospt.2010.3337. [DOI] [PubMed] [Google Scholar]
- Powers CM. Comparison of foot pronation and lower extremity rotation in persons with and without patellofemoral pain. Foot & Ankle International. 2002;23:634–640. doi: 10.1177/107110070202300709. [DOI] [PubMed] [Google Scholar]
- Robinson RL, Nee RJ. Analysis of hip strength in females seeking physical therapy treatment for unilateral patellofemoral pain syndrome. J Orthop Sports Phys Ther. 2007;37:232–238. doi: 10.2519/jospt.2007.2439. [DOI] [PubMed] [Google Scholar]
- Saunders JB. The major determinants in normal and pathological gait. J Bone Joint Surg Am. 1953;35-A:543–558. [PubMed] [Google Scholar]
- Schwartz MH, Rozumalski A. A new method for estimating joint parameters from motion data. J Biomech. 2005;38:107–116. doi: 10.1016/j.jbiomech.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Souza RB, Powers CM. Differences in hip kinematics, muscle strength, and muscle activation between subjects with and without patellofemoral pain. J Orthop Sports Phys Ther. 2009;39:12–19. doi: 10.2519/jospt.2009.2885. [DOI] [PubMed] [Google Scholar]
- Souza RB, Powers CM. Predictors of hip internal rotation during running: an evaluation of hip strength and femoral structure in women with and without patellofemoral pain. Am J Sports Med. 2009;37:579–587. doi: 10.1177/0363546508326711. [DOI] [PubMed] [Google Scholar]
- Sutlive TG. Identification of individuals with patellofemoral pain whose symptoms improved after a combined program of foot orthosis use and modified activity: a preliminary investigation. Phys Ther. 2004;84:49–61. [PubMed] [Google Scholar]
- Taunton JE. A retrospective case-control analysis of 2002 running injuries. Br J Sports Med. 2002;36:95–101. doi: 10.1136/bjsm.36.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler TF. The role of hip muscle function in the treatment of patellofemoral pain syndrome. Am J Sports Med. 2006;34:630–636. doi: 10.1177/0363546505281808. [DOI] [PubMed] [Google Scholar]
- Van Gent RN. Incidence and determinants of lower extremity running injuries in long distance runners: a systematic review. Br J Sports Med. 2007;41:469–480. doi: 10.1136/bjsm.2006.033548. discussion 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodburn J. Multisegment foot motion during gait: proof of concept in rheumatoid arthritis. J Rheumatol. 2004;31:1918–1927. [PubMed] [Google Scholar]
- Van Mechelen W. Running injuries: a review of the epidemiological literature. Sports Med. 1992;14:320–335. doi: 10.2165/00007256-199214050-00004. [DOI] [PubMed] [Google Scholar]
- Willson JD, Davis IS. Lower extremity mechanics of females with and without patellofemoral pain across activities with progressively greater task demands. Clin Biomech. 2008;23:203–211. doi: 10.1016/j.clinbiomech.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Zazulak BT. The effects of core proprioception on knee injury: a prospective biomechanical-epidemiological study. Am J Sports Med. 2007;35:368–373. doi: 10.1177/0363546506297909. [DOI] [PubMed] [Google Scholar]


