Abstract
Uterine serous carcinoma (USC) is a highly aggressive variant of endometrial cancer. Although it only represents less than 10% of all cases, it accounts for a disproportionate number of deaths from endometrial cancer. Comprehensive surgical staging followed by carboplatin and paclitaxel chemotherapy represents the mainstay of USC therapy. Vaginal cuff brachytherapy is also of potential benefit in USC. Recent whole-exome sequencing studies have demonstrated gain of function of the HER2/NEU gene, as well as driver mutations in the PIK3CA/AKT/mTOR and cyclin E/FBXW7 oncogenic pathways in a large number of USCs. These results emphasize the relevance of these novel therapeutic targets for biologic therapy of chemotherapy-resistant recurrent USC.
Keywords: chemotherapy, endometrial cancer, immunotherapy, novel therapies, uterine serous carcinoma
Endometrial cancer is the most common gynecologic malignancy in the developed world. In the year 2013, approximately 49,560 new cases of endometrial cancer and 8190 related deaths are predicted to take place in the USA [1]. Endometrial cancers are typically designed as type I and type II tumors. Type I endometrial cancer accounts for 80% of cases and is associated with endometrioid histology [2], younger age of onset [3], retention of estrogen receptor and progesterone receptor status, and a history of unopposed estrogen, and deletions in KRAS, PTEN, or mismatch repair mechanisms [4]. Type II endometrial cancers are associated with serous, clear-cell or grade 3 endometrioid histology [5], loss of estrogen/progesterone receptor [6], black race [7], absence of unopposed estrogen, presentation at later stage, reduced E-cadherin expression, aneuploidy, mutations in P53, and HER2/NEU overexpression [8,9]. Type II endometrial cancers are typically more aggressive than type I cancers and have a poorer prognosis. Of note, 52–70% of type II cancers exhibit extrauterine spread at the time of surgery, compared with 4.6% of low-grade tumors [10-12]. Overall, they account for approximately 20% of endometrial cancers but as many as 74% of associated deaths [13]. Uterine serous carcinoma (USC) accounts for 10% of all endometrial cancer; however, it carries the poorest prognosis, with 5-year survival rates as low as 55% [13].
Current standard of care
Optimal treatment for USC begins with comprehensive surgical staging by laparotomy or laparoscopy, and maximal cytoreduction to no residual disease in advanced stages [14]. Complete surgical staging consists of total hysterectomy, bilateral salpingo-oophorectomy, bilateral pelvic lymphadenectomy, para-aortic lymph adenectomy, complete omentectomy, and peritoneal washings with biopsies as indicated [15].
Currently, there are no data to suggest that surgical staging via a minimally invasive approach is inferior to laparotomy for management of early-stage USC. In the GOG LAP2 study, 2181 endometrial cancer patients (13% of whom had USC) with clinical stage I–IIA disease were randomized to laparoscopy versus laparotomy [16]. No significant increased risk of recurrence based on laparoscopy versus laparotomy was found in this study. Port site metastases were observed in only 0.24% of laparoscopic cases. As most patients in the LAP2 study had low-risk disease, it is difficult to draw any firm conclusions that laparoscopic staging for USC is an equally effective method of surgical management compared with laparotomy. However, a recently reported, retrospective, multi-institutional cohort study of patients with high-grade endometrial cancer (with approximately 30% serous histology) who were comprehensively staged by either minimally invasive surgery or laparotomy showed similar survival outcomes between the groups [17]. Undoubtedly, a large, prospective randomized controlled trial is needed to definitively answer this question, but no absolute contraindication for laparoscopic staging of USC patients exists at this time.
Following comprehensive surgical staging, adjuvant chemotherapy is generally recommended in all USC patients with Stage IA–IV disease, with the exception of stage IA patients showing no evidence of residual disease on the final surgical pathology specimen. Analyses of Gynecologic Oncology Group (GOG) protocol 209 (a noninferiority trial in advanced/recurrent endometrial cancer patients comparing carboplatin and paclitaxel vs paclitaxel, adriamycin and cisplatin) support the favorable side effect profile of six cycles of carboplatin (AUC 6) and paclitaxel (175 mg/m2) [18]. Five additional Phase III studies conducted by GOG also confirm these findings [19-23]. While GOG 94 concluded that whole-abdomen radiation is of little benefit in early-stage USC [24], adjuvant carboplatin and paclitaxel seems beneficial in terms of decreasing risk of recurrence and improving survival [25,26].
Vaginal cuff brachytherapy is often recommended as adjuvant treatment in USC patients. One retrospective review of 74 patients showed a significant decrease in the risk of vaginal cuff recurrence (0 vs 19%) among those who received vaginal cuff brachytherapy versus those who did not [27]. In addition, another retrospective review of stage I and II USC showed that six cycles of carboplatin and paclitaxel followed by vaginal cuff brachytherapy is well tolerated and is associated with a 5-year overall survival of 90% [28]. GOG 249 is a randomized Phase III clinical trial that is studying pelvic radiation therapy versus vaginal brachytherapy when used in conjunction with paclitaxel and carboplatin in treating patients with high-risk stage I or II endometrial cancers, including USC. The results of this study are eagerly awaited and will likely help to guide management of early-stage USC.
CA-125 is the biomarker most commonly used to monitor disease status [15]. Significant elevations are associated with advanced disease and lower levels with earlier-stage disease, as well as any type of recurrence. Preoperative CA-125 levels are not a good indicator for postoperative disease recurrence [29]. Serum Amyloid A, an acute-phase reactant, and human KLK6 and KLK10, which are trypsin-like serum proteases, have been proposed as biomarkers for USC as well but at this time are not considered standard of care [30-32].
Molecular pathogenesis
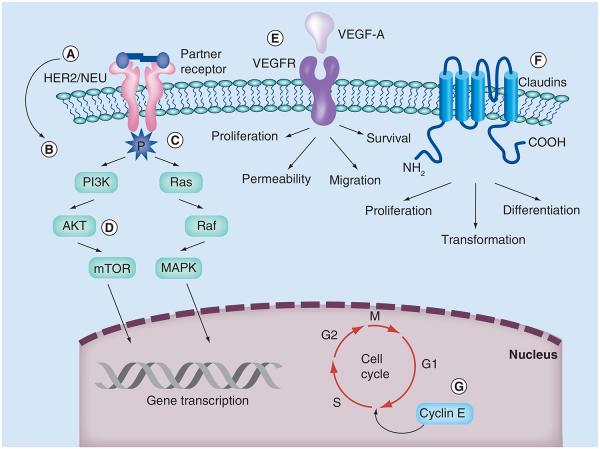
Type I and type II endometrial cancer differ in their molecular pathogenesis. Type I disease often expresses mutations in KRAS, PTEN and other mismatch repair mechanisms [8,9]. Type II disease, and USC specifically, exhibits aneuploidy [33,34] and the overexpression of HER2/NEU [35-38], as well as cyclin E [39] and claudin-3 and -4 [40,41]. They also have been shown to express mutations in TP53 and other proteins [42]. These mechanisms alter the cell cycle via defects in DNA damage repair, chromatin remodeling, cell cycle and cell proliferation. They also provide potential targets for therapy (Figure 1).
Figure 1. Targeted therapy in uterine serous carcinoma.
(A) HER2 antibodies and anti-HER2 vaccines, (B) antibody–drug conjugate, (C) tyrosine kinase inhibitors, (D) PI3K/AKT/mTOR pathway inhibitors, (E) monoclonal antibodies and small-molecule inhibitors, (F) Clostridium perfringens toxin-based therapeutic approaches and (G) CDK inhibitors and curcumin. VEGFR: VEGF receptor.
In 2012, Kuhn and colleagues examined 76 samples of USC [39]. Through whole-exome and Sanger sequencing, they identified that 81% of samples had somatic mutations in the tumor suppressor TP53. They also described mutations in PIK3CA (23%), FBXW7 (19%) and PPP2R1A (18%) in both carcinomas and matched precursor endometrial intraepithelial carcinoma. Furthermore, McConechy et al. showed mutations in PIK3CA, PPP2R1A and TP53 in 75.7% of USC samples, accounting for the majority of aberrations in this subtype and corroborating these findings [43].
TP53 is a transcriptional regulator that triggers apoptosis or cell cycle arrest in the setting of DNA damage. When defective, it is thought to contribute to half of all cancer cases [44]. In the case of USC, it regulates IGFR-1 [45]. PIK3CA plays a central role in cellular responses, such as proliferation, survival, mobility, metabolism and control of malignant cellular growth [46] via activation of the PTEN/AKT pathway. FBXW7 is an F-box protein that is critical in the ubiquitination and targeting of tumor-promoting proteins cyclin E (CCNE1) and PPP2R1A [47,48]. CCNE1 controls the G1 to S transition of the cell cycle [49], and PPP2R1A is a regulatory unit of serine/threonine protein phosphatase 2, which helps regulate growth. Mutations in PPP2R1A have been reported in up to 32% and CCNE1 in 57% of USC [50,51]. The identification of these alterations in both carcinoma and precursor tissue suggest that malignant transformation may happen earlier than was previously speculated.
HER2 & USC
The C-ERB2 gene encodes erbB2 (HER2), a member of the erbB receptor tyrosine kinase family. This family consists of four transmembrane glycoproteins: erbB1, erbB2, erbB3 and erbB4. The HER2 protein has a cysteine-rich extracellular ligand-binding domain, a hydrophobic membrane-spanning region and an intracellular tyrosine kinase domain. When HER2 is amplified, there is increased expression, and there may be up to 100 C-ERB2 genes per tumor cell [52-54] compared with the two copies that there are in normal cells. This amplification results in overexpression of HER2 at both the mRNA and protein levels. The overexpression of HER2 results in the phosphorylation of intracellular tyrosine kinase residues and ultimately modulates cell proliferation, differentiation, migration, and survival. In addition, the following pathways become activated: Ras/Raf/MAPK and PI3K/AKT/mTOR [55].
HER2 expression status is routinely determined by immunohistochemistry (IHC), followed with additional FISH assays to verify equivocal IHC results. Overexpression has been shown to correlate with prognosis in multiple tumor types [56,57]. In endometrial adenocarcinoma, the rates of HER2 overexpression and amplification range from 4 to 69% [58] and are more common in higher-grade and -stage tumors. USC has the highest rates of expression among the endometrial cancers [59]. Multiple research groups have shown that the HER2 receptor is overexpressed in USC (scores 2+ and 3+ on IHC), with expression rates from 18 to 80%, depending on the IHC technique used [36,60,61]. A higher frequency of HER2 amplification by FISH is found in African–Americans compared with Caucasians [62], and African–Americans have been found to have a considerably higher C-ERB2 gene mean copy number and worse overall survival compared with Caucasian patients [62]. Thus, HER2 overexpression may be an important molecular target in the treatment of USC.
Trastuzumab & pertuzumab
The HER2 receptor represents an additional target against USC by the use of antibodies targeting the extracellular domain of this receptor. Trastuzumab and pertuzumab are US FDA-approved humanized monoclonal antibodies targeting HER2 that work through recruitment of natural killer cells and initiation of antibody-dependent cell-mediated cytotoxicity or complement-dependent cytotoxicity, resulting in tumor lysis, as well as abrogation of downstream effectors [63-65].
Trastuzumab (Genentech, CA, USA) is an FDA-approved adjunct and adjuvant for the treatment of early-stage, HER2-positive, node-positive breast cancer [66]. The location of HER2 on the cell surface has greatly contributed to its appeal as an immunotherapy target for USC overexpressing HER2. However, despite encouraging case reports [67-69], when evaluated as a single agent in stage III/IV or recurrent endometrial cancers, trastuzumab did not initially demonstrate significant activity [70]. Combination therapy, including trastuzumab, however, proved more effective than single-agent trastuzumab. To evaluate the effect of progression-free survival in USC, a multi-institutional Phase II trial evaluating trastuzumabin combination with paclitaxel and carboplatin compared with paclitaxel and carboplatin alone is underway in patients with stage III/IV or recurrent USC that overexpress HER2 at 3+ level by IHC or positive by FISH [201].
Pertuzumab (Omnitarg®; Genentech) is a humanized IgG1 monoclonal antibody HER heterodimerization inhibitor that binds domain II of the erbB2 receptor. Compared with trastuzumab, pertuzumab inhibits a broader array of downstream signal transduction pathways through abrogation of lateral signal transduction [71-75].
Antibody-directed immunotherapy may also be further augmented and in particular natural killer cell function may be improved with the administration of IL-2 or an agonist monoclonal antibody for costimulatory receptors on natural killer cells. These are promising strategies, which have shown favorable results in preclinical studies [76].
Tyrosine kinase inhibitors
Small-molecule tyrosine kinase inhibitors target the HER2 intracellular pathway and have shown efficacy in HER2-positive trastuzumab-resistant cancers [77,78]. Lapatinib is a reversible dual inhibitor of both HER2 and EGFR, and in preclinical models has shown effectiveness in restoring trastuzumab sensitivity [79]. Accumulation of the truncated form of HER2, P95-HER2, which lacks the trastuzumab binding site but is able to maintain tyrosine kinase activity, is one mechanism of trastuzumab resistance. Lapatinib is also able to inhibit P95-HER2 phosphorylation and as a result reduce the growth of HER2-driven malignancies [80]. Pan-HER tyrosine kinase inhibitors, which inhibit epidermal growth factor family receptors and their downstream pathways, have also proved beneficial in solid tumor clinical trials and may have clinical benefit in HER2-positive USC [81].
Trastuzumab emtansine
Trastuzumab emtansine (T-DM1; Genentech) is a novel antibody–drug conjugate that combines trastuzumab with targeted delivery of the antimicrotubule agent DM1. DM1 belongs to the maytansine class of chemotherapeutic agents. On average, three to four molecules of DM1 bind to each trastuzumab molecule. T-DM1 is internalized by HER2 receptor-mediated endocytosis and, as such, its action is specific to HER2-expressing cells. After internalization, T-DM1 is then degraded by lysosomes, resulting in the release of free intracellular DM1. DM1 is a strong inhibitor of microtubule assembly and its activity leads to cell death as a result of G2/M phase cell cycle arrest [82-84]. T-DM1 also has the advantage of retaining the mechanism of action of trastuzumab with regard to reducing signaling in the HER2 pathway and initiation of antibody-dependent cell-mediated cytotoxicity [85,86]. T-DM1 could be a promising chemotherapeutic agent in HER2-positive USC, as it has been effective in HER2-positive breast cancer, as demonstrated in the EMILIA trial. In this trial, median progression-free survival was 9.6 months in the T-DM1 arm compared with 6.4 months in the capecitabine/lapatinib arm (HR: 0.650; 95% CI: 0.55–0.77; p < 0.001) [87]. Similarly, promising antitumor activity has been noted in HER2-positive gastric tumors with resistance to trastuzumab [88]; however, no clinical trials have yet been conducted in gynecologic cancer. Clinical trials exploring T-DM1 therapy in HER2-positive advanced/recurrent and/or refractory USC are warranted.
Anti-HER2 vaccine
While trastuzumab is an effective immunotherapeutic agent against a variety of tumors overexpressing HER2, it potentially has limitations of eventual drug resistance and risk of cardiotoxicity, especially in patients on previous anthracycline-based regimens. As experience with trastuzumab grew, so did interest in anti-HER2 vaccines. A potential advantage of a vaccine that induces or stimulates a pre-existing anti-HER2 immune response is fewer injections for patients. More importantly, however, is the possibility of establishing a memory immune response capable of preventing disease recurrence. Consistent with this view, several clinical trials are underway in patients with solid tumors with HER2 expression.
PI3K/mTOR inhibitors & anti-HER2 therapy
The PIK3/AKT/mTOR signaling cascade is critical to diverse cellular responses, including cell proliferation, survival, mobility and metabolism, and control of malignant cellular growth [46]. HER2/NEU is located upstream to the PIK3CA/AKT/mTOR pathway [89]. Also HER2/NEU and the PIK3CA/AKT/mTOR pathway are often constitutively activated in various human cancers secondary to gene amplifications (i.e., HER2/NEU) or activating mutations in the PIK3CA/AKT genes [90,91]. Importantly, multiple research groups have recently reported PIK3CA gene mutations and HER2/NEU gene amplifications in a relevant number of USCs by whole-exome sequencing [39,92,93]. As such, the use of PIK3/AKT/mTOR inhibitors may provide effective anticancer therapy in the naturally aggressive USC.
Stathmin 1 is an oncogene whose activity is influenced by PI3K/AKT pathway activation. High concentrations of stathmin are seen in PTEN-deficient tumors and have been associated with PI3K pathway alterations, such as amplification of the 3q26 region and increased PI3KCA copy number, and correlate with a poor prognosis [94]. The segregation of endometrial cancers into high and low phosphorylated stathmin at the serine 38 site has revealed transcriptional differences between these two subgroups of tumor. Based on these findings, the PI3K/mTOR pathway and heat shock protein 90 have all been suggested as potential effective targets in high pStathmin (S38) tumors [95]. The stratification of patients in clinical trials by phosphorylated stathmin status may help to further define the group of endometrial cancer patients most likely to benefit from PI3K/AKT/mTOR-targeted therapy.
There are very few clinical trials involving inhibitors of the PI3K/AKT/mTOR pathway and gynecologic malignancies. In 2012, Janku and colleagues evaluated breast and gynecology cancer patients with PIK3CA mutations who were in clinical trials using PI3K/AKT/mTOR inhibitors [96]. Of these patients, 9% had stable disease for greater than 6 months and 30% had a partial response. By comparison, 10% of patients with a wild-type PIK3CA showed a response (p = 0.04). In addition, 30% of patients with PIK3CA mutations expressed coexisting MAPK pathway (KRAS, NRAS and BRAF) mutations. Of these, 29% achieved a response.
In 2011, the National Cancer Institute of Canada clinical trials group reported favorable results with the use of temsirolimus, an mTOR inhibitor, in patients with all types of advanced endometrial cancer [97]. There were encouraging clinical results, especially in chemotherapy-naive patients: 14% had a partial response and 69% had stable disease, despite not being able to show a correlation of molecular markers of the PIK3/AKT/mTOR pathway with clinical outcomes. A Phase II randomized trial of carboplatin, paclitaxel and bevacizumab compared with carboplatin, paclitaxel and temsirolimus, or ixabepilone, carboplatin and bevacizumab in advanced and recurrent endometrial cancer inclusive of USC is now underway [202]. Several other mTOR and/or PIK3CA inhibitors are currently under evaluation in clinical trials against a variety of human cancers, inclusive of endometrial.
An additional area of research to be explored is the determination of whether HER2 expression/amplification or the presence of PI3KCA/AKT/mTOR pathway mutations affect prognosis or recurrence of USC.
Another type of anti-HER2 therapy on the horizon is radioimmunotherapy. Preclinical research has been performed on radioimmunotherapy in gynecologic cancer and other tumor types [98,99]. Currently underway is a Phase I trial evaluating lead-212 (212Pb)-trastuzumab in patients with HER2-positive cancers (including endometrial) with documented peritoneal studding or positive washings (intraperitoneal disease). This represents a potential treatment approach for patients with metastatic HER2-positive USC disease utilizing this lead isotope with a short path length specifically targeted to malignant cells by the trastuzumab antibody [203].
Cyclin E & USC
Genome-wide analyses have recently provided additional insight into key aberrations in the molecular pathogenesis of USC [39,93] and potential new drug targets. In one study of 57 USCs analyzed by whole-exome sequencing, somatic focal amplification of chromosome 19 containing CCNE1 was identified in 44% [93]. CCNE1 encodes cyclin E1, the upregulation of which accelerates the cell cycle through the G1 phase via interactions with CDK-2 [49]. Accumulation of cyclin E has been described in a wide range of human cancers [100] besides endometrial [101]. In an additional 17% of USC, mutations in FBXW7, a member of the F-box family of proteins, were described. FBXW7 is responsible for ubiquitinization and targeting of cyclin E1 for proteosomal degradation. A related study corroborated the activation of cyclin E by either increased expression or impaired degradation in 57% of USC [39].
Over 50 CDK inhibitors have been described [102,103]; however, most exploit the ATP-binding domain and remain relatively nonselective, although newer agents have recently shown enhanced specificity for cyclin E or A [104]. Synthetic sulfonamides, such as E7070, had shown promising preclinical activity, but development did not progress beyond Phase II [105]. Curcumin has been proposed as a regulator of the proteasome and cyclin family cell cycle proteins [106]. Additional rational drug design is warranted.
VEGF & monoclonal antibodies
Angiogenesis is a rate-limiting step in tumor growth, progression and metastasis. It is often initiated when the core of a tumor mass attains a critical level of hypoxia. A number of substances promote neovascularization, including PDGF, FGF and angiopoietins, among others. Of these proteins, VEGF dominates. It enhances vascular permeability, capillary fenestration and vasodilatation. Production of VEGF is stimulated by inflammatory cytokines, such as IL-1α and IL-6, or hypoxia via HIF-1α. The family consists of six different members: VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E and P1GF [107]. These ligands bind several receptors: VEGFR-2 (Flk-1) appears to be the major mediator of the mitogenic properties of VEGF, whereas VEGFR-1 (Flt-1) signaling depends on developmental stage and cell type; VEGFR-3 binds only VEGF-C and VEGF-D. In endometrial cancer, VEGF-A expression is an important prognostic indicator. It is believed that increased expression indicated a poorer prognosis, as it is associated with advanced-grade, lymphovascular space invasion, lymphovascular spread [108,109] and the upregulation of P53 [110].
Bevacizumab (Avastin®; Genentech) is a recombinant human monoclonal IgG1 antibody that neutralizes VEGF [111]. In GOG 229G, a Phase II study of recurrent endometrial cancer, bevacizumab 15 mg/kg every 3 weeks produced a clinical response rate of 13.5%, including one complete and six partial responses among 52 patients with one (63.5%) or two (36.5%) prior lines of therapy [112]. Median progression-free and overall survival rates were 4.2 and 10.5 months, respectively. Despite representing only 27% of the study population, serous histology showed a significant response; serous histology was observed in 100% of complete responses and 50% of partial responses.
The promising results of single-agent therapy prompted interest in pursuit of combinatorial approaches. In GOG 229E, bevacizumab 10 mg/kg biweekly and temsirolimus 25 mg weekly was used in 49 patients with recurrent or persistent endometrial cancer treated with one or two prior lines of therapy [113]. There were four serous cancers represented (8.2%). In total, 23 (46.9%) patients achieved progression-free survival of 6 months or more. There were 12 (24.5%) clinical responses (one complete, 11 partial). Notably, there were two gastrointestinal perforations and three treatment-related deaths. Presently, bevacizumab in combination with paclitaxel and carboplatin is under study for advanced endometrial cancer [204]. There is also a three-arm Phase II trial investigating the relative efficacy of carboplatin/paclitaxel/bevacizumab, carboplatin/paclitaxel/temsirolimus and carboplatin/ixabepilone/bevacizumab for advanced or recurrent disease [202].
Other novel immunotherapies under investigation include VEGF Trap (Afibercept®; Sanofi-Aventis, Paris, France), a fusion protein containing VEGFR-1 and -2 components and a fully human IgG1 constant region [205], and ramucirumab, a monoclonal antibody targeting VEGFR-2, which is under study for a variety of solid tumors but not yet endometrial cancers [206-209].
Small-molecule inhibitors
Small-molecule inhibitors are tyrosine kinase modulators that offer an alternative oral strategy for targeting neoangiogenesis. Sunitinib is a multipathway agent that inhibits VEGFR-1, -2 and -3, as well as PDGF. This agent is currently under study at the Phase II level for recurrent or metastatic endometrial cancers, including USC, once-daily on days 1–28 of a 6-week cycle until progression or toxicity [210]. Results from this study are expected to be published in June 2014.
The triple-pathway inhibitor nintedanib (BIBF 1120; Boehringer Ingelheim Pharmaceuticals, Ingelheim am Rhein, Germany) against VEGF, PDGF and FGF has produced encouraging results at the Phase III level with pemetrexed in non-small-cell lung cancer [114,115] and Phase II level in renal cell carcinoma [116]. Unfortunately, it has been ineffective in gliomas [117], and a Phase II study in endometrial cancers has been suspended [211]. Pazopanib (against VEGF, PDGF and c-kit), sorafenib (against VEGF, PDGF, c-kit and Raf) and cediranib (against VEGFR) remain under study in ovarian but not yet endometrial cancers [118].
Inhibition of the prostaglandin cascade
Several authors have also posited that endometrial cancer may be promoted by a proinflammatory milieu of prostaglandins that promote angiogenesis, cell proliferation and DNA damage through modulation of cyclo-oxygenase-1 and -2 [119,120]. A large meta-analysis examining the chemoprevention afforded by aspirin and other nonsteroidal anti-inflammatory agents, including 1398 cases and 740 controls, showed that those patients who had used aspirin in the last 5 years had a significantly lower risk of endometrial cancer (OR: 0.78; 95% CI: 0.63–0.97) compared with those who did not [121]. There was a significant inverse dose response (p-trend < 0.001), such that women who reported using at least two aspirin/week had almost half the risk (OR: 0.54; 95% CI: 0.38–0.78). This was particularly notable among obese women and, interestingly, protective against both type I and II endometrial cancers, such as USC. These findings are overall consistent with the literature in supporting a role for aspirin in colorectal [122] and ovarian [123] cancer prevention.
Novel cytotoxic therapy: epothilones
Class III β-tubulin is capable of heterodimerizing with α-subunits to form microtubules critical to cell division. Resistance to paclitaxel has been tied to the upregulation of class III β-tubulin [124]. Paclitaxel not only binds preferentially to class I β-tubulin [125], but greater class III β-tubulin expression reduces the rate of microtubule assembly, further rendering cells less susceptible to paclitaxel [126]. Accordingly, aggressive biologic variants are associated with high levels of class III β-tubulin [127]. In a comparison of fresh frozen tissues using 28 USC and 20 ovarian serous carcinomas, USC overexpressed class III β-tubulin RNA (copy number: 552.9 ± 106.7 vs 202.0 ± 43.99; p = 0.01) [128]; upregulation was also observed in cell lines (copy number: 1701.0 ± 376.4 vs 645.1 ± 157.9; p = 0.02) and verified at the protein level using IHC (USC median IHC score: 3+; ovarian serous carcinomas median IHC score: 0–1+; p = 0.004). Overexpression of class III β-tubulin predicts poor overall survival in USC [128], ovarian serous [129] and clear-cell carcinomas [130], among a variety of other human cancers [131,132].
Epothilones are microtubule-stabilizing macrolides isolated from Sorangiumcellulosum [133]. They have activity in paclitaxel-resistant malignancies [125]. Patupilone (Novartis, Basel, Switzerland) and ixabepilone (Ixempra®/BMS-247550; Bristol-Meyers-Squibb, NJ, USA) are members of this group.
In vitro, patupilone has been shown to be highly effective relative to paclitaxel against USC cell lines expressing class III β-tubulin [128] and HER2/NEU [134], a marker of aggressive biologic behavior [35,37]. Patupilone has been studied in various clinical trials involving the treatment of ovarian carcinoma, but has not been validated in endometrial carcinomas [135-138].
Ixabepilone is FDA-approved for treating advanced and metastatic breast cancer, but is not yet approved for treating endometrial cancer. However, in GOG-129P, 50 patients with recurrent or persistent endometrial cancer (40% had USC and 2% had clear-cell carcinoma) who had already received at least one prior line of taxane-based chemotherapy were treated with ixabepilone and showed a favorable response [139]. An overall response rate of 12% was appreciated by using an ixabepilone dose of 40 mg/m2 every 21 days. In 60% of patients, there was disease stabilization for at least 8 weeks. Median progression-free and overall survival was 2.9 and 8.7 months, respectively.
In preclinical xenografts, synergism between bevacizumab with ixabepilone exceeded that of bevacizumab with paclitaxel [140]. In advanced non-small-cell lung cancer, the addition of bevacizumab to carboplatin and ixabepilone as first-line therapy produced a higher overall response rate (50 vs 29%) [141]. Median progression-free and overall survival in these cohorts was 6.7 versus 5.3 and 13.2 versus 9.3 months, respectively. Drawing from such experience, ixabepilone is currently under evaluation as first-line therapy with carboplatin and bevacizumab in stage III/IV primary or recurrent endometrial cancers [202].
Claudins as a target
Normal epithelial cells are held together by tight junctions (TJs), adherens junctions and gap junctions, and their disruption is involved in the transformation from a benign to malignant state [142]. TJs block the diffusion of protein and lipids through the plasma membrane [143,144]. They are also associated with epithelial breakdown and promotion of the neoplastic process [145].
Claudins are membrane proteins that are involved in the formation of TJs. They also assist in recruiting cell-signaling proteins. In addition, they regulate cell proliferation, cell differentiation and neoplastic transformation [146,147]. They are composed of one intracellular amino terminal, one intracellular carboxy terminal, four transmembrane domains, and two extracellular loops [143,148,149]. One of the extracellular loops acts a binding site for Clostridium perfringens toxin (CPE) [150]. Claudin-3 is a low-affinity receptor for CPE and claudin-4 is a high-affinity receptor for CPE. Claudin-3 and -4 have been found to be among the highest differentially expressed genes in USC [41], in addition to a variety of other cancers [147,151].
Claudin-3 and -4 have been thought to represent a marker for biological aggressiveness. In one study of 20 USC samples, CPE receptors were identified in 100% of samples and significantly higher levels (p < 0.05) in metastatic USC when compared with primary tumor sites [41]. Thus, USC that is recurrent or refractory to standard treatments may be susceptible to CPE-based therapeutic approaches [41].
Conclusion & future perspective
USC is the most aggressive endometrial cancer, representing less than 10% of all cases, a disproportionate number of deaths and a poor 5-year overall survival of 55%. With such a dismal prognosis, these patients should be treated aggressively. Patients should receive complete surgical staging. Those who are identified to have residual USC in the uterus at the time of surgery should receive adjuvant carboplatin and paclitaxel chemotherapy, and a strong consideration should be given for vaginal cuff brachytherapy. In patients who present with advanced and/or recurrent chemotherapy-resistant disease, we expect whole-genome sequencing to soon represent a critical tool for the identification and rational design of targeted therapies in women diagnosed with USC. Prospective trials incorporating targeted therapies are warranted to define the optimal management approach for women with this biologically aggressive variant of endometrial cancer.
Executive summary.
Current
Uterine serous carcinoma (USC) is a highly aggressive type of endometrial cancer that is associated with a high rate of mortality.
Current standard of care
Surgical staging followed by treatment with platinum- and taxane-based chemotherapy may decrease the risk of recurrence, and may improve survival outcomes; vaginal cuff brachytherapy may also be of benefit in USC.
HER2 & USC
Approximately a third of USCs express high levels of HER2/NEU, a promising and rational target for biologic therapies based on trastuzumab and T-DM1.
PI3K/mTOR inhibitors & anti-HER2 therapy
Recent studies using whole-genome sequencing identified alterations in the PIK3CA/AKT/mTOR and cyclin E/FBXW7 pathways in a large number of USCs. These pathways may therefore represent novel therapeutic targets in USC.
Novel cytotoxic therapy: epothilones
Epothilones, a novel class of microtubule-stabilizing agents, are currently being evaluated for use against chemotherapy-resistant/recurrent USC in combination with bevacizumab.
Claudins as a target
Targeting of the tight junction protein claudin-4, the high-affinity receptor for Clostridium perfringens enterotoxin, may represent a novel approach for drug delivery against chemotherapy-resistant USC.
Acknowledgments
This work was supported in part by grants from the NIH RO1 CA122728-01A4 and RO1 CA154460-01A1 to AD Santin and the NIH research grant CA-16359 from the National Cancer Institute.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J. Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Felix AS, Weissfeld JL, Stone RA, et al. Factors associated with type I and type II endometrial cancer. Cancer Causes Control. 2010;21(11):1851–1856. doi: 10.1007/s10552-010-9612-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Creasman WT, Odicino F, Maisonneuve P, et al. Carcinoma of the corpus uteri. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int. J. Gynaecol. Obstet. 2006;95(Suppl. 1):S105–S143. doi: 10.1016/S0020-7292(06)60031-3. [DOI] [PubMed] [Google Scholar]
- 4.Lax SF, Pizer ES, Ronnett BM, Kurman RJ. Comparison of estrogen and progesterone receptor, Ki-67, and P53 immunoreactivity in uterine endometrioid carcinoma and endometrioid carcinoma with squamous, mucinous, secretory, and ciliated cell differentiation. Hum. Pathol. 1998;29(9):924–931. doi: 10.1016/s0046-8177(98)90197-6. [DOI] [PubMed] [Google Scholar]
- 5.Goff BA, Kato D, Schmidt RA, et al. Uterine papillary serous carcinoma: patterns of metastatic spread. Gynecol. Oncol. 1994;54(3):264–268. doi: 10.1006/gyno.1994.1208. [DOI] [PubMed] [Google Scholar]
- 6.Emons G, Fleckenstein G, Hinney B, Huschmand A, Heyl W. Hormonal interactions in endometrial cancer. Endocr. Relat. Cancer. 2000;7(4):227–242. doi: 10.1677/erc.0.0070227. [DOI] [PubMed] [Google Scholar]
- 7.Wilson TO, Podratz KC, Gaffey TA, Malkasian GD, Jr, O’Brien PC, Naessens JM. Evaluation of unfavorable histologic subtypes in endometrial adenocarcinoma. Am. J. Obstet. Gynecol. 1990;162(2):418–423. doi: 10.1016/0002-9378(90)90399-r. discussion 423-416. [DOI] [PubMed] [Google Scholar]
- 8.Sherman ME. Theories of endometrial carcinogenesis: a multidisciplinary approach. Modern Pathol. 2000;13(3):295–308. doi: 10.1038/modpathol.3880051. [DOI] [PubMed] [Google Scholar]
- 9.Hecht JL, Mutter GL. Molecular and pathologic aspects of endometrial carcinogenesis. J. Clin. Oncol. 2006;24(29):4783–4791. doi: 10.1200/JCO.2006.06.7173. [DOI] [PubMed] [Google Scholar]
- 10.Goff BA. Uterine papillary serous carcinoma: what have we learned over the past quarter century? Gynecol. Oncol. 2005;98(3):341–343. doi: 10.1016/j.ygyno.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Thomas M, Mariani A, Wright JD, et al. Surgical management and adjuvant therapy for patients with uterine clear cell carcinoma: a multi-institutional review. Gynecol. Oncol. 2008;108(2):293–297. doi: 10.1016/j.ygyno.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Yoon JH, Yoo SC, Kim WY, Chang SJ, Chang KH, Ryu HS. Para-aortic lymphadenectomy in the management of preoperative grade 1 endometrial cancer confined to the uterine corpus. Ann. Surg. Oncol. 2010;17(12):3234–3240. doi: 10.1245/s10434-010-1199-5. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton CA, Cheung MK, Osann K, et al. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br. J. Cancer. 2006;94(5):642–646. doi: 10.1038/sj.bjc.6603012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dizon DS. Treatment options for advanced endometrial carcinoma. Gynecol. Oncol. 2010;117(2):373–381. doi: 10.1016/j.ygyno.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz PE. The management of serous papillary uterine cancer. Curr. Opin. Oncol. 2006;18(5):494–499. doi: 10.1097/01.cco.0000239890.36408.75. [DOI] [PubMed] [Google Scholar]
- 16.Walker JL, Piedmonte MR, Spirtos NM, et al. Recurrence and survival after random assignment to laparoscopy versus laparotomy for comprehensive surgical staging of uterine cancer: Gynecologic Oncology Group LAP2 Study. J. Clin. Oncol. 2012;30(7):695–700. doi: 10.1200/JCO.2011.38.8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •.Important article that evaluates laparoscopy versus laparotomy for uterine cancer staging.
- 17.Fader AN, Seamon LG, Escobar PF, et al. Minimally invasive surgery versus laparotomy in women with high grade endometrial cancer: a multi-site study performed at high volume cancer centers. Gynecol. Oncol. 2012;126(2):180–185. doi: 10.1016/j.ygyno.2012.04.028. [DOI] [PubMed] [Google Scholar]
- 18.Miller D, Filiaci V, Fleming G, et al. Randomized Phase III noninferiority trial of first-line chemotherapy for metastatic or recurrent endometrial carcinoma: a Gynecologic Oncology Group study (late-breaking abstract); Presented at: Annual Meeting of the Society of Gynecologic Oncologists. Austin, TX, USA; Mar 24–27, 2012. [Google Scholar]
- •.Important study of the Gynecologic Oncology Group regarding treatment of metastatic and recurrent endometrial cancer.
- 19.Gallion HH, Brunetto VL, Cibull M, et al. Randomized Phase III trial of standard timed doxorubicin plus cisplatin versus circadian timed doxorubicin plus cisplatin in stage III and IV or recurrent endometrial carcinoma: a Gynecologic Oncology Group Study. J. Clin. Oncol. 2003;21(20):3808–3813. doi: 10.1200/JCO.2003.10.083. [DOI] [PubMed] [Google Scholar]
- 20.Fleming GF, Brunetto VL, Cella D, et al. Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study. J. Clin. Oncol. 2004;22(11):2159–2166. doi: 10.1200/JCO.2004.07.184. [DOI] [PubMed] [Google Scholar]
- 21.Fleming GF, Filiaci VL, Bentley RC, et al. Phase III randomized trial of doxorubicin + cisplatin versus doxorubicin + 24-h paclitaxel + filgrastim in endometrial carcinoma: a Gynecologic Oncology Group study. Ann. Oncol. 2004;15(8):1173–1178. doi: 10.1093/annonc/mdh316. [DOI] [PubMed] [Google Scholar]
- 22.Thigpen JT, Brady MF, Homesley HD, et al. Phase III trial of doxorubicin with or without cisplatin in advanced endometrial carcinoma: a gynecologic oncology group study. J. Clin. Oncol. 2004;22(19):3902–3908. doi: 10.1200/JCO.2004.02.088. [DOI] [PubMed] [Google Scholar]
- 23.Homesley HD, Filiaci V, Gibbons SK, et al. A randomized Phase III trial in advanced endometrial carcinoma of surgery and volume directed radiation followed by cisplatin and doxorubicin with or without paclitaxel: a Gynecologic Oncology Group study. Gynecol. Oncol. 2009;112(3):543–552. doi: 10.1016/j.ygyno.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sutton G, Axelrod JH, Bundy BN, et al. Adjuvant whole abdominal irradiation in clinical stages I and II papillary serous or clear cell carcinoma of the endometrium: a Phase II study of the Gynecologic Oncology Group. Gynecol. Oncol. 2006;100(2):349–354. doi: 10.1016/j.ygyno.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 25.Mahdavi A, Tajalli TR, Dalmar A, Vasilev SA, Lentz SE, Berman ML. Role of adjuvant chemotherapy in patients with early stage uterine papillary serous cancer. Int. J. Gynecol. Cancer. 2011;21(8):1436–1440. doi: 10.1097/IGC.0b013e31822e7588. [DOI] [PubMed] [Google Scholar]
- 26.Fader AN, Nagel C, Axtell AE, et al. Stage II uterine papillary serous carcinoma: carboplatin/paclitaxel chemotherapy improves recurrence and survival outcomes. Gynecol. Oncol. 2009;112(3):558–562. doi: 10.1016/j.ygyno.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 27.Kelly MG, O’Malley DM, Hui P, et al. Improved survival in surgical stage I patients with uterine papillary serous carcinoma (UPSC) treated with adjuvant platinum-based chemotherapy. Gynecol. Oncol. 2005;98(3):353–359. doi: 10.1016/j.ygyno.2005.06.012. [DOI] [PubMed] [Google Scholar]
- •.Important article for treatment of uterine serous carcinoma (USC)
- 28.Kiess AP, Damast S, Makker V, et al. Five-year outcomes of adjuvant carboplatin/paclitaxel chemotherapy and intravaginal radiation for stage I–II papillary serous endometrial cancer. Gynecol. Oncol. 2012;127(2):321–325. doi: 10.1016/j.ygyno.2012.07.112. [DOI] [PubMed] [Google Scholar]
- 29.Gupta D, Gunter MJ, Yang K, et al. Performance of serum CA125 as a prognostic biomarker in patients with uterine papillary serous carcinoma. Int. J. Gynecol. Cancer. 2011;21(3):529–534. doi: 10.1097/IGC.0b013e31821091b5. [DOI] [PubMed] [Google Scholar]
- 30.Cocco E, Bellone S, El-Sahwi K, et al. Serum amyloid A: a novel biomarker for endometrial cancer. Cancer. 2010;116(4):843–851. doi: 10.1002/cncr.24838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santin AD, Diamandis EP, Bellone S, et al. Human kallikrein 6: a new potential serum biomarker for uterine serous papillary cancer. Clin. Cancer Res. 2005;11(9):3320–3325. doi: 10.1158/1078-0432.CCR-04-2528. [DOI] [PubMed] [Google Scholar]
- 32.Santin AD, Diamandis EP, Bellone S, et al. Overexpression of kallikrein 10 (hK10) in uterine serous papillary carcinomas. Am. J. Obstet. Gynecol. 2006;194(5):1296–1302. doi: 10.1016/j.ajog.2005.10.794. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg P, Wingren S, Simonsen E, Stal O, Risberg B, Nordenskjold B. Flow cytometric measurements of DNA index and S-phase on paraffin-embedded early stage endometrial cancer: an important prognostic indicator. Gynecol. Oncol. 1989;35(1):50–54. doi: 10.1016/0090-8258(89)90010-3. [DOI] [PubMed] [Google Scholar]
- 34.Pradhan M, Abeler VM, Danielsen HE, Trope CG, Risberg BA. Image cytometry DNA ploidy correlates with histological subtypes in endometrial carcinomas. Modern Pathol. 2006;19(9):1227–1235. doi: 10.1038/modpathol.3800641. [DOI] [PubMed] [Google Scholar]
- 35.Santin AD, Bellone S, Van Stedum S, et al. Amplification of C-ERB2 oncogene: a major prognostic indicator in uterine serous papillary carcinoma. Cancer. 2005;104(7):1391–1397. doi: 10.1002/cncr.21308. [DOI] [PubMed] [Google Scholar]
- •.One of the articles that shows the importance of HER2 in USC.
- 36.Slomovitz BM, Broaddus RR, Burke TW, et al. Her-2/neu overexpression and amplification in uterine papillary serous carcinoma. J. Clin. Oncol. 2004;22(15):3126–3132. doi: 10.1200/JCO.2004.11.154. [DOI] [PubMed] [Google Scholar]
- 37.Grushko TA, Filiaci VL, Mundt AJ, Ridderstrale K, Olopade OI, Fleming GF. An exploratory analysis of HER-2 amplification and overexpression in advanced endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol. Oncol. 2008;108(1):3–9. doi: 10.1016/j.ygyno.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diaz-Montes TP, Ji H, Smith Sehdev AE, et al. Clinical significance of Her-2/neu overexpression in uterine serous carcinoma. Gynecol. Oncol. 2006;100(1):139–144. doi: 10.1016/j.ygyno.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 39.Kuhn E, Wu RC, Guan B, et al. Identification of molecular pathway aberrations in uterine serous carcinoma by genome-wide analyses. J. Natl Cancer Inst. 2012;104(19):1503–1513. doi: 10.1093/jnci/djs345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Konecny GE, Agarwal R, Keeney GA, et al. Claudin-3 and claudin-4 expression in serous papillary, clear-cell, and endometrioid endometrial cancer. Gynecol. Oncol. 2008;109(2):263–269. doi: 10.1016/j.ygyno.2008.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santin AD, Bellone S, Marizzoni M, et al. Overexpression of claudin-3 and claudin-4 receptors in uterine serous papillary carcinoma: novel targets for a type-specific therapy using Clostridium perfringens enterotoxin (CPE) Cancer. 2007;109(7):1312–1322. doi: 10.1002/cncr.22536. [DOI] [PubMed] [Google Scholar]
- •.Important article that explores the role of claudins in USC.
- 42.Llaurado M, Ruiz A, Majem B, et al. Molecular bases of endometrial cancer: new roles for new actors in the diagnosis and the therapy of the disease. Mol. Cell. Endocrinol. 2012;358(2):244–255. doi: 10.1016/j.mce.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 43.McConechy MK, Ding J, Cheang MC, et al. Use of mutation profiles to refine the classification of endometrial carcinomas. J. Pathol. 2012;228(1):20–30. doi: 10.1002/path.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goodsell DS. The molecular perspective: P53 tumor suppressor. Oncologist. 1999;4(2):138–139. [PubMed] [Google Scholar]
- 45.Attias-Geva Z, Bentov I, Kidron D, et al. P53 regulates insulin-like growth factor-I receptor gene expression in uterine serous carcinoma and predicts responsiveness to an insulin-like growth factor-I receptor-directed targeted therapy. Eur. J. Cancer. 2012;48(10):1570–1580. doi: 10.1016/j.ejca.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 46.Dancey JE. Therapeutic targets: MTOR and related pathways. Cancer Biol. Ther. 2006;5(9):1065–1073. doi: 10.4161/cbt.5.9.3175. [DOI] [PubMed] [Google Scholar]
- 47.Welcker M, Clurman BE. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat. Rev. Cancer. 2008;8(2):83–93. doi: 10.1038/nrc2290. [DOI] [PubMed] [Google Scholar]
- 48.Mao JH, Kim IJ, Wu D, et al. FBXW7 targets mTOR for degradation and cooperates with PTEN in tumor suppression. Science. 2008;321(5895):1499–1502. doi: 10.1126/science.1162981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cassia R, Moreno-Bueno G, Rodriguez-Perales S, Hardisson D, Cigudosa JC, Palacios J. Cyclin E gene (CCNE) amplification and hCDC4 mutations in endometrial carcinoma. J. Pathol. 2003;201(4):589–595. doi: 10.1002/path.1474. [DOI] [PubMed] [Google Scholar]
- 50.Shih Ie M, Panuganti PK, Kuo KT, et al. Somatic mutations of PPP2R1A in ovarian and uterine carcinomas. Am. J. Pathol. 2011;178(4):1442–1447. doi: 10.1016/j.ajpath.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nagendra DC, Burke J, 3rd, Maxwell GL, Risinger JI. PPP2R1A mutations are common in the serous type of endometrial cancer. Mol. Carcinog. 2012;51(10):826–831. doi: 10.1002/mc.20850. [DOI] [PubMed] [Google Scholar]
- •.Important in indentification of PPP2R1A mutations in USC.
- 52.Karunagaran D, Tzahar E, Beerli RR, et al. ErbB-2 is a common auxiliary subunit of NDF and EGF receptors: implications for breast cancer. EMBO J. 1996;15(2):254–264. [PMC free article] [PubMed] [Google Scholar]
- 53.Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997;16(7):1647–1655. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Busse D, Doughty RS, Arteaga CL. HER-2/neu (erbB-2) and the cell cycle. Semin. Oncol. 2000;27(6 Suppl. 11):3–8. discussion 92–100. [PubMed] [Google Scholar]
- 55.Okines A, Cunningham D, Chau I. Targeting the human EGFR family in esophagogastric cancer. Nat. Rev. Clin. Oncol. 2011;8(8):492–503. doi: 10.1038/nrclinonc.2011.45. [DOI] [PubMed] [Google Scholar]
- 56.Hynes NE, Stern DF. The biology of erbB-2/neu/HER-2 and its role in cancer. Biochim. Biophys. Acta. 1994;1198(2–3):165–184. doi: 10.1016/0304-419x(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 57.Ravdin PM, Chamness GC. The c-erbB-2 proto-oncogene as a prognostic and predictive marker in breast cancer: a paradigm for the development of other macromolecular markers – a review. Gene. 1995;159(1):19–27. doi: 10.1016/0378-1119(94)00866-q. [DOI] [PubMed] [Google Scholar]
- 58.Peiro G, Mayr D, Hillemanns P, Lohrs U, Diebold J. Analysis of HER-2/neu amplification in endometrial carcinoma by chromogenic in situ hybridization. Correlation with fluorescence in situ hybridization, HER-2/neu, P53 and Ki-67 protein expression, and outcome. Mod. Pathol. 2004;17(3):227–287. doi: 10.1038/modpathol.3800006. [DOI] [PubMed] [Google Scholar]
- 59.Morrison C, Zanagnolo V, Ramirez N, et al. HER-2 is an independent prognostic factor in endometrial cancer: association with outcome in a large cohort of surgically staged patients. J. Clin. Oncol. 2006;24(15):2376–2385. doi: 10.1200/JCO.2005.03.4827. [DOI] [PubMed] [Google Scholar]
- 60.Santin AD, Bellone S, Gokden M, et al. Overexpression of HER-2/neu in uterine serous papillary cancer. Clin. Cancer Res. 2002;8(5):1271–1279. [PubMed] [Google Scholar]
- 61.Rolitsky CD, Theil KS, McGaughy VR, Copeland LJ, Niemann TH. HER-2/neu amplification and overexpression in endometrial carcinoma. Int. J. Gynecol. Pathol. 1999;18(2):138–143. doi: 10.1097/00004347-199904000-00007. [DOI] [PubMed] [Google Scholar]
- 62.Santin AD, Bellone S, Siegel ER, et al. Racial differences in the overexpression of epidermal growth factor type II receptor (HER2/NEU): a major prognostic indicator in uterine serous papillary cancer. Am. J. Obstet. Gynecol. 2005;192(3):813–818. doi: 10.1016/j.ajog.2004.10.605. [DOI] [PubMed] [Google Scholar]
- 63.Arnould L, Gelly M, Penault-Llorca F, et al. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br. J. Cancer. 2006;94(2):259–267. doi: 10.1038/sj.bjc.6602930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat. Med. 2000;6(4):443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 65.Gennari R, Menard S, Fagnoni F, et al. Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin. Cancer Res. 2004;10(17):5650–5655. doi: 10.1158/1078-0432.CCR-04-0225. [DOI] [PubMed] [Google Scholar]
- 66.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N. Engl. J. Med. 2005;353(16):1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 67.Jewell E, Secord AA, Brotherton T, Berchuck A. Use of trastuzumab in the treatment of metastatic endometrial cancer. Int. J. Gynecol. Cancer. 2006;16(3):1370–1373. doi: 10.1111/j.1525-1438.2006.00543.x. [DOI] [PubMed] [Google Scholar]
- 68.Villella JA, Cohen S, Smith DH, Hibshoosh H, Hershman D. HER-2/neu overexpression in uterine papillary serous cancers and its possible therapeutic implications. Int. J. Gynecol. Cancer. 2006;16(5):1897–1902. doi: 10.1111/j.1525-1438.2006.00664.x. [DOI] [PubMed] [Google Scholar]
- 69.Santin AD, Bellone S, Roman JJ, Mckenney JK, Pecorelli S. Trastuzumab treatment in patients with advanced or recurrent endometrial carcinoma overexpressing HER2/NEU. Int. J. Gynaecol. Obstet. 2008;102(2):128–131. doi: 10.1016/j.ijgo.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 70.Fleming GF, Sill MW, Darcy KM, et al. Phase II trial of trastuzumab in women with advanced or recurrent, HER2-positive endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol. Oncol. 2010;116(1):15–20. doi: 10.1016/j.ygyno.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mullen P, Cameron DA, Hasmann M, Smyth JF, Langdon SP. Sensitivity to pertuzumab (2C4) in ovarian cancer models: cross-talk with estrogen receptor signaling. Mol. Cancer Ther. 2007;6(1):93–100. doi: 10.1158/1535-7163.MCT-06-0401. [DOI] [PubMed] [Google Scholar]
- 72.Agus DB, Akita RW, Fox WD, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2(2):127–137. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 73.Jackson JG, St Clair P, Sliwkowski MX, Brattain MG. Blockade of epidermal growth factor- or heregulin-dependent ErbB2 activation with the anti-ErbB2 monoclonal antibody 2C4 has divergent downstream signaling and growth effects. Cancer Res. 2004;64(7):2601–2609. doi: 10.1158/0008-5472.can-03-3106. [DOI] [PubMed] [Google Scholar]
- 74.Nahta R, Hung MC, Esteva FJ. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 2004;64(7):2343–2346. doi: 10.1158/0008-5472.can-03-3856. [DOI] [PubMed] [Google Scholar]
- 75.Takai N, Jain A, Kawamata N, et al. 2C4, a monoclonal antibody against HER2, disrupts the HER kinase signaling pathway and inhibits ovarian carcinoma cell growth. Cancer. 2005;104(12):2701–2708. doi: 10.1002/cncr.21533. [DOI] [PubMed] [Google Scholar]
- 76.Kohrt HE, Houot R, Weiskopf K, et al. Stimulation of natural killer cells with a CD137-specific antibody enhances trastuzumab efficacy in xenotransplant models of breast cancer. J. Clin. Invest. 2012;122(3):1066–1075. doi: 10.1172/JCI61226. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 77.Lang SA, Hackl C, Moser C, et al. Implication of RICTOR in the mTOR inhibitor-mediated induction of insulin-like growth factor-I receptor (IGF-IR) and human epidermal growth factor receptor-2 (HER2) expression in gastrointestinal cancer cells. Biochim. Biophys. Acta. 2010;1803(4):435–442. doi: 10.1016/j.bbamcr.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 78.Kim JW, Kim HP, Im SA, et al. The growth inhibitory effect of lapatinib, a dual inhibitor of EGFR and HER2 tyrosine kinase, in gastric cancer cell lines. Cancer Lett. 2008;272(2):296–306. doi: 10.1016/j.canlet.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 79.Ritter CA, Perez-Torres M, Rinehart C, et al. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin. Cancer Res. 2007;13(16):4909–4919. doi: 10.1158/1078-0432.CCR-07-0701. [DOI] [PubMed] [Google Scholar]
- 80.Scaltriti M, Rojo F, Ocana A, et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J. Natl Cancer Inst. 2007;99(8):628–638. doi: 10.1093/jnci/djk134. [DOI] [PubMed] [Google Scholar]
- 81.Wilken JA, Badri T, Cross S, et al. EGFR/HER-targeted therapeutics in ovarian cancer. Future Med. Chem. 2012;4(4):447–469. doi: 10.4155/fmc.12.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lewis Phillips GD, Li G, Dugger DL, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68(22):9280–9290. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- 83.Remillard S, Rebhun LI, Howie GA, Kupchan SM. Antimitotic activity of the potent tumor inhibitor maytansine. Science. 1975;189(4207):1002–1005. doi: 10.1126/science.1241159. [DOI] [PubMed] [Google Scholar]
- 84.Barok M, Tanner M, Koninki K, Isola J. Trastuzumab-DM1 causes tumour growth inhibition by mitotic catastrophe in trastuzumab-resistant breast cancer cells in vivo. Breast Cancer Res. 2011;13(2):R46. doi: 10.1186/bcr2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Junttila TT, Li G, Parsons K, Phillips GL, Sliwkowski MX. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res. Treat. 2011;128(2):347–356. doi: 10.1007/s10549-010-1090-x. [DOI] [PubMed] [Google Scholar]
- 86.Wilken JA, Maihle NJ. Primary trastuzumab resistance: new tricks for an old drug. Ann. NY Acad. Sci. 2010;1210:53–65. doi: 10.1111/j.1749-6632.2010.05782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boyraz B, Sendur MA, Aksoy S, et al. Trastuzumab emtansine (T-DM1) for HER2-positive breast cancer. Curr. Med. Res. Opin. 2013;29(4):405–414. doi: 10.1185/03007995.2013.775113. [DOI] [PubMed] [Google Scholar]
- 88.Barok M, Tanner M, Koninki K, Isola J. Trastuzumab-DM1 is highly effective in preclinical models of HER2-positive gastric cancer. Cancer Lett. 2011;306(2):171–179. doi: 10.1016/j.canlet.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 89.English DP, Roque DM, Santin AD. HER2 expression beyond breast cancer: therapeutic implications for gynecologic malignancies. Mol. Diag. Ther. 2013;17(2):85–99. doi: 10.1007/s40291-013-0024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.She QB, Chandarlapaty S, Ye Q, et al. Breast tumor cells with PI3K mutation or HER2 amplification are selectively addicted to Akt signaling. PLoS ONE. 2008;3(8):e3065. doi: 10.1371/journal.pone.0003065. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 91.Brachmann SM, Hofmann I, Schnell C, et al. Specific apoptosis induction by the dual PI3K/mTor inhibitor NVP-BEZ235 in HER2 amplified and PIK3CA mutant breast cancer cells. Proc. Natl Acad. Sci. USA. 2009;106(52):22299–22304. doi: 10.1073/pnas.0905152106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Le Gallo M, O’Hara AJ, Rudd ML, et al. Exome sequencing of serous endometrial tumors identifies recurrent somatic mutations in chromatin-remodeling and ubiquitin ligase complex genes. Nat. Genet. 2012;44(12):1310–1315. doi: 10.1038/ng.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao S, Choi M, Overton JD, et al. Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma. Proc. Natl Acad. Sci. USA. 2013;110(8):2916–2921. doi: 10.1073/pnas.1222577110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••.Novel article on the landscape of mutations in USC.
- 94.Salvesen HB, Carter SL, Mannelqvist M, et al. Integrated genomic profiling of endometrial carcinoma associates aggressive tumors with indicators of PI3 kinase activation. Proc. Natl Acad. Sci. USA. 2009;106(12):4834–4839. doi: 10.1073/pnas.0806514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wik E, Birkeland E, Trovik J, et al. High phospho-Stathmin(Serine38) expression identifies aggressive endometrial cancer and suggests an association with PI3K inhibition. Clin. Cancer Res. 2013;19(9):2331–2341. doi: 10.1158/1078-0432.CCR-12-3413. [DOI] [PubMed] [Google Scholar]
- 96.Janku F, Wheler JJ, Westin SN, et al. PI3K/AKT/mTOR inhibitors in patients with breast and gynecologic malignancies harboring PIK3CA mutations. J. Clin. Oncol. 2012;30(8):777–782. doi: 10.1200/JCO.2011.36.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Oza AM, Elit L, Tsao MS, et al. Phase II study of temsirolimus in women with recurrent or metastatic endometrial cancer: a trial of the NCIC Clinical Trials Group. J. Clin. Oncol. 2011;29(24):3278–3285. doi: 10.1200/JCO.2010.34.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Heyerdahl H, Abbas N, Brevik EM, Mollatt C, Dahle J. Fractionated therapy of HER2-expressing breast and ovarian cancer xenografts in mice with targeted alpha emitting 227Th-DOTAp-benzyl-trastuzumab. PLoS ONE. 2012;7(8):e42345. doi: 10.1371/journal.pone.0042345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ray GL, Baidoo KE, Keller LM, Albert PS, Brechbiel MW, Milenic DE. Pre-clinical assessment of lu-labeled trastuzumab targeting HER2 for treatment and management of cancer patients with disseminated intraperitoneal disease. Pharmaceuticals (Basel) 2011;5(1):1–15. doi: 10.3390/ph5010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Donnellan R, Chetty R. Cyclin E in human cancers. FASEB J. 1999;13(8):773–780. doi: 10.1096/fasebj.13.8.773. [DOI] [PubMed] [Google Scholar]
- 101.Gezginc ST, Celik C, Dogan NU, Toy H, Tazegul A, Colakoglu MC. Expression of cyclin A, cyclin E and p27 in normal, hyperplastic and frankly malignant endometrial samples. J. Obstet. Gynaecol. 2013;33(5):508–511. doi: 10.3109/01443615.2013.776024. [DOI] [PubMed] [Google Scholar]
- 102.Knockaert M, Greengard P, Meijer L. Pharmacological inhibitors of cyclin-dependent kinases. Trends Pharmacol. Sci. 2002;23(9):417–425. doi: 10.1016/s0165-6147(02)02071-0. [DOI] [PubMed] [Google Scholar]
- 103.Pasha FA, Neaz MM. Molecular dynamics and QM/MM-based 3D interaction analyses of cyclin-E inhibitors. J. Mol. Model. 2013;19(2):879–891. doi: 10.1007/s00894-012-1620-z. [DOI] [PubMed] [Google Scholar]
- 104.Dai L, Liu Y, Liu J, et al. A novel cyclinE/cyclinA–CDK inhibitor targets p27(Kip1) degradation, cell cycle progression and cell survival: implications in cancer therapy. Cancer Lett. 2013;333(1):103–112. doi: 10.1016/j.canlet.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 105.Van Kesteren C, Beijnen JH, Schellens JH. E7070: a novel synthetic sulfonamide targeting the cell cycle progression for the treatment of cancer. Anti Cancer Drugs. 2002;13(10):989–997. doi: 10.1097/00001813-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 106.Hasima N, Aggarwal BB. Targeting proteasomal pathways by dietary curcumin for cancer prevention and treatment. Curr. Med. Chem. 2013 doi: 10.2174/09298673113206660135. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 107.Ferrara N, Gerber HP, Lecouter J. The biology of VEGF and its receptors. Nat. Med. 2003;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 108.Kamat AA, Merritt WM, Coffey D, et al. Clinical and biological significance of vascular endothelial growth factor in endometrial cancer. Clin. Cancer Res. 2007;13(24):7487–7495. doi: 10.1158/1078-0432.CCR-07-1017. [DOI] [PubMed] [Google Scholar]
- 109.Hirai M, Nakagawara A, Oosaki T, Hayashi Y, Hirono M, Yoshihara T. Expression of vascular endothelial growth factors (VEGF-A/VEGF-1 and VEGF-C/VEGF-2) in postmenopausal uterine endometrial carcinoma. Gynecol. Oncol. 2001;80(2):181–188. doi: 10.1006/gyno.2000.6056. [DOI] [PubMed] [Google Scholar]
- 110.Mazurek A, Pierzynski P, Kuc P, et al. Evaluation of angiogenesis, p-53 tissue protein expression and serum VEGF in patients with endometrial cancer. Neoplasma. 2004;51(3):193–197. [PubMed] [Google Scholar]
- 111.Gerber HP, Ferrara N. Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res. 2005;65(3):671–680. [PubMed] [Google Scholar]
- 112.Aghajanian C, Sill MW, Darcy KM, et al. Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. J. Clin. Oncol. 2011;29(16):2259–2265. doi: 10.1200/JCO.2010.32.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •.Important study for the role of alternative treatments for USC.
- 113.Alvarez EA, Brady WE, Walker JL, et al. Phase II trial of combination bevacizumab and temsirolimus in the treatment of recurrent or persistent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol. Oncol. 2013;129(1):22–27. doi: 10.1016/j.ygyno.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 114.Reck M, Kaiser R, Eschbach C, et al. A Phase II double-blind study to investigate efficacy and safety of two doses of the triple angiokinase inhibitor BIBF 1120 in patients with relapsed advanced non-small-cell lung cancer. Ann. Oncol. 2011;22(6):1374–1381. doi: 10.1093/annonc/mdq618. [DOI] [PubMed] [Google Scholar]
- 115.Hanna NH, Rolf K, Sullivan RN, et al. Lume-lung 2: a multicenter, randomized, double-blind, Phase III study of nintedanib plus pemetrexed versus placebo plus pemetrexed in patients with advanced nonsquamous non-small cell lung cancer (NSCLC) after failure of first-line chemotherapy; Presented at: American Society of Clinical Oncology Annual Meeting. Chicago, IL, USA; 2013. 31 May–4 June. (Abstract 8034) [Google Scholar]
- 116.Eisen T, Shparyk Y, Macleod NJ, et al. Phase II efficacy study of nintedanib versus sunitinib in previously untreated renal cell carcinoma patients; Presented at: American Society of Clinical Oncology Annual Meeting. Chicago, IL, USA; 2013. 31 May–4 June. (Abstract 4506) [Google Scholar]
- 117.Norden AD, Schiff D, Ahluwalia MS, et al. Phase II trial of triple-receptor tyrosine kinase receptor inhibitor nintedanib (BIBF 1120) in recurrent high-grade gliomas; Presented at: American Society of Clinical Oncology Annual Meeting. Chicago, IL, USA; 2013. 31 May–4 June. (Abstract TPS2104) [Google Scholar]
- 118.Wei XW, Zhang ZR, Wei YQ. Anti-angiogenic drugs currently in Phase II clinical trials for gynecological cancer treatment. Expert Opin. Investig. Drugs. 2013;22(9):1181–1192. doi: 10.1517/13543784.2013.812071. [DOI] [PubMed] [Google Scholar]
- 119.Modugno F, Ness RB, Chen C, Weiss NS. Inflammation and endometrial cancer: a hypothesis. Cancer Epidemiol. Biomarkers Prev. 2005;14(12):2840–2847. doi: 10.1158/1055-9965.EPI-05-0493. [DOI] [PubMed] [Google Scholar]
- 120.O’Byrne KJ, Dalgleish AG. Chronic immune activation and inflammation as the cause of malignancy. Br. J. Cancer. 2001;85(4):473–483. doi: 10.1054/bjoc.2001.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Neill AS, Nagle CM, Protani MM, Obermair A, Spurdle AB, Webb PM. Aspirin, nonsteroidal anti-inflammatory drugs, paracetamol and risk of endometrial cancer: a case–control study, systematic review and meta-analysis. Int. J. Cancer. 2013;132(5):1146–1155. doi: 10.1002/ijc.27717. [DOI] [PubMed] [Google Scholar]
- 122.Rothwell PM, Wilson M, Elwin CE, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376(9754):1741–1750. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 123.Baandrup L, Faber MT, Christensen J, et al. Nonsteroidal anti-inflammatory drugs and risk of ovarian cancer: systematic review and meta-analysis of observational studies. Acta Obstet. Gynecol. Scand. 2013;92(3):245–255. doi: 10.1111/aogs.12069. [DOI] [PubMed] [Google Scholar]
- 124.Kavallaris M, Kuo DY, Burkhart CA, et al. Taxol-resistant epithelial ovarian tumors are associated with altered expression of specific beta-tubulin isotypes. J. Clin. Invest. 1997;100(5):1282–1293. doi: 10.1172/JCI119642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Magnani M, Ortuso F, Soro S, Alcaro S, Tramontano A, Botta M. The betaI/betaIII-tubulin isoforms and their complexes with antimitotic agents. Docking and molecular dynamics studies. FEBS J. 2006;273(14):3301–3310. doi: 10.1111/j.1742-4658.2006.05340.x. [DOI] [PubMed] [Google Scholar]
- 126.Hari M, Yang H, Zeng C, Canizales M, Cabral F. Expression of class III beta-tubulin reduces microtubule assembly and confers resistance to paclitaxel. Cell Motil. Cytoskeleton. 2003;56(1):45–56. doi: 10.1002/cm.10132. [DOI] [PubMed] [Google Scholar]
- 127.Carrara L, Guzzo F, Roque DM, et al. Differential in vitro sensitivity to patupilone versus paclitaxel in uterine and ovarian carcinosarcoma cell lines is linked to tubulin-beta-III expression. Gynecol. Oncol. 2012;125(1):231–236. doi: 10.1016/j.ygyno.2011.12.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Roque DM, Bellone S, English DP, et al. Tubulin-beta-III overexpression by uterine serous carcinomas is a marker for poor overall survival after platinum/taxane chemotherapy and sensitivity to epothilones. Cancer. 2013;119(14):2582–2592. doi: 10.1002/cncr.28017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •.Identifies tubulin-β III expression and novel treatment with epothilones.
- 129.Ferrandina G, Zannoni GF, Martinelli E, et al. Class III beta-tubulin overexpression is a marker of poor clinical outcome in advanced ovarian cancer patients. Clin. Cancer Res. 2006;12(9):2774–2779. doi: 10.1158/1078-0432.CCR-05-2715. [DOI] [PubMed] [Google Scholar]
- 130.Roque DM, Bellone S, Buza N, et al. Class III beta-tubulin overexpression in ovarian clear cell and serous carcinoma as a maker for poor overall survival after platinum/taxane chemotherapy and sensitivity to patupilone. Am. J. Obstet. Gynecol. 2013;209(1):62.e1–62.e9. doi: 10.1016/j.ajog.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 131.Seve P, Isaac S, Tredan O, et al. Expression of class III {beta}-tubulin is predictive of patient outcome in patients with non-small cell lung cancer receiving vinorelbine-based chemotherapy. Clin. Cancer Res. 2005;11(15):5481–5486. doi: 10.1158/1078-0432.CCR-05-0285. [DOI] [PubMed] [Google Scholar]
- 132.Paradiso A, Mangia A, Chiriatti A, et al. Biomarkers predictive for clinical efficacy of taxol-based chemotherapy in advanced breast cancer. Ann. Oncol. 2005;16(Suppl. 4):iv14–iv19. doi: 10.1093/annonc/mdi902. [DOI] [PubMed] [Google Scholar]
- 133.Bollag DM, Mcqueney PA, Zhu J, et al. Epothilones, a new class of microtubule-stabilizing agents with a taxol-like mechanism of action. Cancer Res. 1995;55(11):2325–2333. [PubMed] [Google Scholar]
- 134.Paik D, Cocco E, Bellone S, et al. Higher sensitivity to patupilone versus paclitaxel chemotherapy in primary uterine serous papillary carcinoma cell lines with high versus low HER-2/neu expression in vitro. Gynecol. Oncol. 2010;119(1):140–145. doi: 10.1016/j.ygyno.2010.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rubin EH, Rothermel J, Tesfaye F, et al. Phase I dose-finding study of weekly single-agent patupilone in patients with advanced solid tumors. J. Clin. Oncol. 2005;23(36):9120–9129. doi: 10.1200/JCO.2005.03.0981. [DOI] [PubMed] [Google Scholar]
- 136.Ten Bokkel Huinink WW, Sufliarsky J, Smit WM, et al. Safety and efficacy of patupilone in patients with advanced ovarian, primary fallopian, or primary peritoneal cancer: a Phase I, open-label, dose-escalation study. J. Clin. Oncol. 2009;27(19):3097–3103. doi: 10.1200/JCO.2008.20.4826. [DOI] [PubMed] [Google Scholar]
- 137.Bystricky B, Chau I. Patupilone in cancer treatment. Expert Opin. Invest. Drugs. 2011;20(1):107–117. doi: 10.1517/13543784.2011.542148. [DOI] [PubMed] [Google Scholar]
- 138.Colombo N, Kutarska E, Dimopoulos M, et al. Randomized, open-label, Phase III study comparing patupilone (EPO906) with pegylated liposomal doxorubicin in platinum-refractory or -resistant patients with recurrent epithelial ovarian, primary fallopian tube, or primary peritoneal cancer. J. Clin. Oncol. 2012;30(31):3841–3847. doi: 10.1200/JCO.2011.38.8082. [DOI] [PubMed] [Google Scholar]
- •.Important study about targeted therapy in USC.
- 139.Dizon DS, Blessing JA, Mcmeekin DS, Sharma SK, Disilvestro P, Alvarez RD. Phase II trial of ixabepilone as second-line treatment in advanced endometrial cancer: gynecologic oncology group trial 129-P. J. Clin. Oncol. 2009;27(19):3104–3108. doi: 10.1200/JCO.2008.20.6995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lee FY, Covello KL, Castaneda S, et al. Synergistic antitumor activity of ixabepilone (BMS-247550) plus bevacizumab in multiple in vivo tumor models. Clin. Cancer Res. 2008;14(24):8123–8131. doi: 10.1158/1078-0432.CCR-08-0025. [DOI] [PubMed] [Google Scholar]
- 141.Spigel DR, Greco FA, Rubin MS, et al. Phase II study of maintenance sunitinib following irinotecan and carboplatin as first-line treatment for patients with extensive-stage small-cell lung cancer. Lung Cancer. 2012;77(2):359–364. doi: 10.1016/j.lungcan.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 142.Martin TA, Watkins G, Mansel RE, Jiang WG. Loss of tight junction plaque molecules in breast cancer tissues is associated with a poor prognosis in patients with breast cancer. Eur. J. Cancer. 2004;40(18):2717–2725. doi: 10.1016/j.ejca.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 143.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2001;2(4):285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 144.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell Biol. 1998;141(7):1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Swift JG, Mukherjee TM, Rowland R. Intercellular junctions in hepatocellular carcinoma. J. Submicrosc. Cytol. 1983;15(3):799–810. [PubMed] [Google Scholar]
- 146.Mitic LL, Anderson JM. Molecular architecture of tight junctions. Annu. Rev. Physiol. 1998;60:121–142. doi: 10.1146/annurev.physiol.60.1.121. [DOI] [PubMed] [Google Scholar]
- 147.Morin PJ. Claudin proteins in human cancer: promising new targets for diagnosis and therapy. Cancer Res. 2005;65(21):9603–9606. doi: 10.1158/0008-5472.CAN-05-2782. [DOI] [PubMed] [Google Scholar]
- 148.Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog. Biophys. Mol. Biol. 2003;81(1):1–44. doi: 10.1016/s0079-6107(02)00037-8. [DOI] [PubMed] [Google Scholar]
- 149.Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu. Rev. Physiol. 2006;68:403–429. doi: 10.1146/annurev.physiol.68.040104.131404. [DOI] [PubMed] [Google Scholar]
- 150.Morita K, Furuse M, Fujimoto K, Tsukita S. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc. Natl Acad. Sci. USA. 1999;96(2):511–516. doi: 10.1073/pnas.96.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hewitt KJ, Agarwal R, Morin PJ. The claudin gene family: expression in normal and neoplastic tissues. BMC Cancer. 2006;6:186. doi: 10.1186/1471-2407-6-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 201.Evaluation of Carboplatin/Paclitaxel With and Without Trastuzumab (Herceptin) in Uterine Serous Cancer. http://clinicaltrials.gov/show/NCT01367002.
- 202.Paclitaxel, Carboplatin, and Bevacizumab or Paclitaxel, Carboplatin, and Temsirolimus or Ixabepilone, Carboplatin, and Bevacizumab in Treating Patients With Stage III, Stage IV, or Recurrent Endometrial Cancer. http://clinicaltrials.gov/show/NCT00977574.
- 203.Safety Study of 212Pb-TCMC-Trastuzumab Radio Immunotherapy. http://clinicaltrials.gov/show/NCT01384253.
- 204.Evaluation of Carboplatin/Paclitaxel/Bevacizumab in the Treatment of Advanced Stage Endometrial Carcinoma. http://clinicaltrials.gov/show/NCT00513786.
- 205.VEGF Trap in Treating Patients With Recurrent or Persistent Endometrial Cancer. http://clinicaltrials.gov/show/NCT00462826.
- 206.Study of Ramucirumab in Ovarian Cancer. http://clinicaltrials.gov/show/NCT00721162.
- 207.A Study of Ramucirumab (IMC-1121B) in Patients With Breast Cancer. http://clinicaltrials.gov/show/NCT01256567.
- 208.A Study of Paclitaxel With or Without Ramucirumab in Metastatic Gastric Adenocarcinoma (RAINBOW) http://clinicaltrials.gov/show/NCT01170663.
- 209.A Study of Chemotherapy and Ramucirumab vs. Chemotherapy Alone in Second Line Non-Small Cell Lung Cancer Participants Who Received Prior First Line Platinum Based Chemotherapy. http://clinicaltrials.gov/show/NCT01168973.
- 210.Sunitinib in Treating Patients With Recurrent or Metastatic Endometrial Cancer. http://clinicaltrials.gov/show/NCT00478426.
- 211.BIBF 1120 in Treating Patients With Recurrent or Persistent Endometrial Cancer. http://clinicaltrials.gov/show/NCT01225887.