Abstract
Objective:
To determine the effect of intrauterine inflammation on fetal responses to umbilical cord occlusion (UCO).
Study Design:
In pregnant sheep, lipopolysaccharide (LPS) or saline (SAL) was infused intra-amniotically for 4 weeks from 80 days of gestation (d). At 110 d, fetuses were instrumented for UCOs (5 × 2-minutes, 30-minute intervals: LPS + UCO, n = 6; SAL + UCO, n = 8) or no UCO (sham, n = 6) on 117 and 118 d. Tissues were collected at 126 d.
Results:
Fetal physiological responses to UCO were similar between LPS + UCO and SAL + UCO. Histologic chorioamnionitis and increased amniotic fluid interleukin 8 (IL-8) were observed in LPS + UCO pregnancies (versus SAL + UCO, P < .05). CNPase-positive oligodendrocyte number in the cerebral white matter was lower in LPS + UCO and SAL + UCO than sham (P < .05); there was no effect on astrocytes or activated microglia/macrophages. Two of the SAL + UCO fetuses had white matter lesions; none were observed in LPS + UCO or sham.
Conclusion:
Chronic pre-existing intrauterine inflammation did not exacerbate fetal brain injury induced by intermittent UCO.
Keywords: chorioamnionitis, fetal inflammatory response, birth asphyxia, cerebral palsy, sheep
Introduction
Preterm birth and its postnatal comorbidities (including cerebral palsy and bronchopulmonary dysplasia) are associated with the presence of inflammation and/or infection within the pregnant uterus.1,2 This intrauterine infection/inflammation can manifest either as overt clinical chorioamnionitis or fetal inflammatory response syndrome (FIRS),3 both of which can be life-threatening to the fetus or as histologic chorioamnionitis that may be clinically silent.
We have described previously the effects on the fetal brain of infusion of lipopolysaccharide (LPS) into the amniotic fluid of pregnant sheep from 80 to 108 days of gestation (d; term is ~150 days).4 In those experiments, the fetus appeared to be uncompromised by inflammation resulting from amniotic fluid LPS administration.5–7 Mild brain inflammation occurred,4 but there were no white matter lesions or other profound neuropathologies that have been reported for other animal models of intrauterine inflammation.8–10 Fetal physiologic compromise, manifest as hypoxemia, acidemia, hypotension, or even death is commonly observed in animal experiments in which overt brain injury is associated with intrauterine inflammation.8–10 These features of fetal compromise may, by themselves, be capable of causing fetal brain injury.11,12 However, we have also shown that repeated fetal intravenous LPS infusions, in doses that do not cause hypoxemia, cause brain damage; but the injury is milder than when there is coincident inflammation-induced fetal hypoxemia.13
Intrauterine inflammation and fetal hypoxemia/ischemia may interact to increase the risk of brain injury. In human pregnancies, the risk of cerebral palsy is greatly increased by a combination of intrauterine infection/inflammation and perinatal asphyxia, relative to the presence of either in isolation.14 A similar synergistic interaction between inflammation and hypoxia/asphyxia has been observed in neonatal rats15–17 and mice.18 In light of these experimental observations and the human clinical experience, we hypothesized that preexisting chronic intrauterine inflammation, which we have previously shown capable of causing mild brain injury in fetal sheep,4 would exacerbate the potentially damaging effect on the fetal brain of hypoxemia/ischemia induced by intermittent umbilical cord occlusion (UCO).19 We reasoned that inflammation could exacerbate brain injury induced by hypoxia/ischemia by either a direct effect on neurodevelopment or indirectly by impairing the fetal physiological response to the hypoxic/ischemic insult.20 Thus, our aim was to determine the effects of pre-existing intrauterine inflammation on fetal physiologic (blood gas, arterial pressure, hypothalamic-pituitary-adrenal axis activation) and neuroanatomical (inflammation, myelination, apoptosis) responses to intermittent UCO.
Materials and Methods
The Animal Experimentation Ethics Committee at The University of Western Australia approved all experimental procedures.
At 80 days of pregnancy, ewes bearing single fetuses underwent aseptic surgery for the implantation of osmotic pumps (2ML4; Alzet Osmotic Pumps, Cupertino CA, USA) containing either Escherichia coli LPS (055:B5, Sigma Chemical, St. Louis MO, USA; 18 mg/mL; n = 6) or saline (SAL, n = 14).4,6 After induction of anesthesia with intramuscular ketamine (20 mg/kg: Parnell Laboratories, Australia) and xylazine (0.2 mg/kg; Troy Laboratories, Australia), ewes were intubated and ventilated; anesthesia was maintained with 1.5% to 2% halothane in O2. After maternal laparotomy, 1 fetal forelimb was delivered through a uterine incision and an osmotic pump was attached using a silk ligature. The 2 mL osmotic pumps had an infusion rate of 2.5 μL/h, resulting in the intra-amniotic delivery of 1.1 mg LPS/day (or SAL) for 28 days. Antibiotics (Benacillin—150 mg procaine penicillin, 150 mg benzathine penicillin, and 20 mg procaine HCl; Troy Laboratories, Australia) were administered intramuscularly to the fetus and the forelimb was returned to the uterus. After closure of the uterine incision the ewe’s abdomen was sutured closed in 2 layers. Ewes recovered from surgery in individual pens, with free access to food and water.
On day 110 of pregnancy, ewes and fetuses underwent a second surgical procedure using the same anesthesia as described above. After maternal laparotomy, 1 fetal hindlimb was delivered through a uterine incision and polyvinyl catheters containing heparinized SAL were inserted into the descending aorta and inferior vena cava via femoral vessels. Another catheter was attached to the fetus for subsequent recording of intra-amniotic pressure. In all fetuses, an inflatable umbilical cord occluder (16 mm; In Vivo Metric, Healdsburg CA, USA) was placed around the proximal end of the umbilical cord and secured to the skin. The volume of SAL required to completely inflate the occluder was determined at the time of surgery. The osmotic pumps that were implanted at 80 days of pregnancy were not removed. The fetus received antibiotics (as above) and was returned to the uterus. After closure of the uterine incision, catheters were exteriorized through a small incision in the ewe’s flank and the ewe’s abdomen was closed in 2 layers. Polyvinyl catheters were inserted into the ewe’s descending aorta and inferior vena cava via femoral vessels. The maternal catheters were tracked subcutaneously and were exteriorized through the same incision as the fetal catheters; this incision was then closed. Fetal arterial blood samples were collected daily after surgery to monitor well-being.
On day 116 of gestation, a fetal arterial blood sample was collected for the measurement of baseline blood gases and lactate (Rapidlab 865, Bayer Diagnostics, East Walpole MA, USA).
Umbilical Cord Occlusions
On day 117 of gestation, a second baseline blood sample was collected (for physiologic measurements, as at 116 days) and the fetal arterial catheter was attached to a pressure transducer to allow continuous recording of arterial pressure (Powerlab, ADInstruments, Australia). Fetuses were then subjected to either a series of 5 UCOs, each lasting for 2 minutes at 30 minute intervals, during which the umbilical cord occluder was completely inflated with SAL (LPS + UCO, n = 6; or SAL + UCO, n = 8) or ‘sham occlusions,’ during which the umbilical cord occluder was not inflated in a group of SAL-infused sheep (sham, n = 6). During these periods, fetal arterial blood was sampled 3 times: immediately prior; during (1.5 minutes after commencement of occlusion); and immediately after each 2-minute occlusion, for measurement of blood gases, pH, and lactate (Rapidlab 865, Bayer Diagnostics). Fetal arterial plasma was collected from samples taken immediately before and after the first and last occlusions, for measurement of cortisol and adrenocorticotrophic hormone (ACTH).7,21 The occlusion protocol was chosen (based on our experience)19 to induce submaximal brain injury, so that the hypothesized exacerbation of damage in the LPS group would be detectable.
At 118 days of gestation, 24 hours after the first series of UCOs, blood sampling, arterial pressure recording, and UCO (or sham) were repeated, identically to the day before. Thereafter, fetal arterial blood samples were collected daily for 7 days.
Deliveries, Tissue Collection, and Processing
At 126 days of gestation ewes and fetuses were humanely killed (sodium pentobarbitone, 130 mg/kg; Jurox, Australia), and the fetus was delivered. Fetal sex, body weight, crown-rump length, and thoracic girth were recorded. A sample of chorioamnion, from adjacent to the umbilical cord, was placed into 4% paraformaldehyde (PFA) in 0.1 mol/L phosphate buffer (PB, pH 7.4) overnight at 4°C and was subsequently paraffin-embedded for light microscopic assessment. The fetal brain was perfused (40-50 mm Hg pressure), via the ascending aorta, with heparinized physiological SAL (50 IU/mL) followed by 4% PFA in 0.1 mol/L PB (pH 7.4). The fetal brain was weighed and placed in fresh 4% PFA overnight at 4°C. Weights of the placenta, fetal liver, spleen, and thymus were recorded.
Four tissue blocks (5 mm thick) were collected from both cerebral hemispheres and embedded in paraffin; 2 from the frontal lobe, between the level of the coronal and ansate sulci (cerebral hemisphere regions 1-2) and 2 from the parietal and temporal lobes, between the level of the Sylvian sulcus and optic chiasm (cerebral hemisphere regions 3-4).4
Serial sections of paraffin-embedded cerebral hemispheres (regions 1-4, 6 μm, transverse) were cut, mounted (SuperFrost Plus slides, Menzel-Glaser, Germany), and dried at 37°C overnight. Two slides, 60 μm apart, from each tissue block were used for each of the staining procedures, which were performed on serial sections, enabling us to compare consecutive regions of white matter.
Immunohistochemistry
Rabbit anti-cow glial fibrillary acidic protein (GFAP) antibody (1:1000, DAKO Corporation, Carpinteria CA, USA) was used to stain astrocytes; mouse anti-CNPase (2′,3′-cyclic nucleotide 3′-phosphodiesterase) monoclonal antibody (1:500, Chemicon International) was used to stain myelinating oligodendrocytes and myelinated fibers; rabbit anti-Active Caspase 3 (AC-3) polyclonal antibody (1:100, Chemicon International, Temecula CA, USA) was used to stain cells undergoing apoptosis. Antigen retrieval was performed using: (1) Proteinase K (DAKO Corporation) for 5 minutes at room temperature, for sections to be stained with GFAP or (2) 0.1 mol/L citrate buffer (pH 8.5) at 80°C for 30 minutes for sections to be stained with CNPase or AC-3. Sections were then placed in 10% normal goat serum (NGS) in 0.1 mol/L PB for 30 minutes, washed (3 × 5-min) in 0.1 mol/L PB (pH 7.4) and the primary antisera prepared using 0.1 mol/L PB/2% NGS/0.3% Triton X-100 as diluent. Appropriate primary antisera were placed on the sections, which were incubated at 4°C overnight. Sections were then incubated for 60 minutes with biotinylated secondary antibodies diluted 1:100 in 0.1 mol/L PB/2% NGS: anti-rabbit IgG for GFAP, anti-mouse IgG for CNPase and AC-3. The reaction product was detected with the avidin-biotin peroxidase complex (Vector Laboratories, Burlingame CA, USA). For each primary antibody, tissues were stained simultaneously to reduce variability. Omission of the primary antibody resulted in no immunoreactive staining.
Lectin Histochemistry
Sections were incubated with biotinylated Lycopersicon Esculentum (tomato) lectin (1:500, Vector Laboratories) to stain-activated microglia and/or infiltrating peripheral macrophages as described previously.4 All sections were stained simultaneously to reduce variability. Omission of the biotinylated tomato lectin resulted in no staining.
All histological analyses were performed on coded slides so that the observer was blind to treatment group. Digital images for analysis were acquired using an Olympus BX51 microscope and Spot Advanced software (Diagnostic Instruments, Sterling Heights MI, USA). Images were analysed using ImagePro Plus (Media Cybernetics, Bethesda MD, USA).
Qualitative Analysis
A total of 8 sections from each fetus (paired sections from each tissue block, 60 μm apart) were stained with hematoxylin and eosin (H&E) for assessment of structure and gross examination of the cytoarchitecture. Sections were assessed for the presence of white matter lesions/cysts, perivascular cuffing, coagulative necrosis, subarachnoid and germinal layer hemorrhage, and ventriculomegaly. Sections were also examined to assess: myelination (CNPase-positive cell bodies and fibers) within the white matter at the level of the cingulate sulcus; and the extent of apoptosis (AC-3 positive cells) in the cerebral hemispheres (regions 1-4).
Quantitative Morphometric Analysis
Chorioamnion
Influx of inflammatory cells (mostly neutrophils) into the fetal membranes (ie, histologic chorioamnionitis) was graded by scoring a total of 30 random non-overlapping fields of view in three 5-μm H&E-stained sections (100 μm apart) as: 0 (no inflammatory cells); 1 (fewer than 50 inflammatory cells); 2 (50-100 inflammatory cells); or 3 (more than 100 inflammatory cells).4
Brain
The total cross-sectional area of white matter was measured using one H&E-stained section from each of the 4 tissue blocks (cerebral hemisphere regions 1-4) and the mean for each animal was calculated. Absolute values and white matter area expressed relative to total section area were compared. The proportion of white matter occupied by blood vessels was estimated, using point counting,12 in 4 fields of view (×20 magnification) from each of the 4 tissue blocks; a mean value was calculated for each animal: an increase is indicative of a cerebral response to an hypoxemic insult.12,19
Gyral formation was assessed by calculating the surface folding index (SFI), as described previously4: SFI = L2/A, where A is the area of the cerebral hemisphere dorsal to a line drawn perpendicular to the midline at the level of the upper limit of the lateral ventricles and L is the length of the surface. This index has no dimensions, is not affected by shrinkage, and gives an estimate of expansion of the surface relative to volume growth,22 possibly reflecting cortical and subcortical connectivity. Four H&E-stained sections from each fetus (cerebral hemisphere regions 1-4) were used to calculate a mean value for each animal.
The areal density of CNPase-positive oligodendrocytes and GFAP-positive astrocytes in the cerebral white matter was determined; in 1 section from each tissue block, 5 random nonoverlapping areas were assessed (2 areas in the subcortical white matter and 3 areas in the deep white matter). An alteration in the density of CNPase-positive cells would indicate a change in the normal developmental profile of the oligodendrocyte lineage possibly as a result of oxidative or nitrosative stress.23
The total cross-sectional area of all regions of the cerebral white matter (cerebral hemisphere regions 1-4) that were occupied by activated microglia/macrophages (lectin-positive cells) was measured and expressed as a percentage of the total white matter area in the same region. An activated microglia infiltration score (range 1-4) was assigned based on the number of lectin-positive cells within these regions: few (<20) lectin-positive cells (score 1); 20-50 lectin-positive cells (mild, score 2); 50-100 lectin-positive cells (moderate, score 3); >100 lectin-positive cells (extensive, score 4).4 Invasion of the neuropile by activated microglia/macrophages indicates underlying neural damage.
IL-6 and IL-8 ELISA
Concentrations of the proinflammatory cytokines IL-6 and IL-8 in samples of amniotic fluid collected on the day of tissue collection were measured by enzyme-linked immunosorbent assay (ELISA) using ovine-specific antibodies (Chemicon, USA), as described previously.24 Recombinant proteins kindly donated by the CSIRO Centre of Animal Biotechnology (Parkville, Victoria, Australia) were used as standards. Standard curves were sensitive at 0.05 to 50 ng/mL for IL-6 and 0.125 to 50 ng/mL for IL-8, with correlation coefficients of 0.98 for all assays.
White Blood Cell Counts
Blood cell counts were performed on maternal and fetal arterial blood samples collected on the day of tissue collection (126 days of gestation). Staff in the Department of Haematology, King Edward Memorial Hospital, Subiaco, Western Australia, performed the measurements using ovine-specific automated analysis. Manual differential counts were performed by a technician with experience in ovine hematology.
Statistical Analysis
Data are presented as mean ± SEM unless otherwise stated. Statistical comparisons were performed using Sigmastat (Systat Software, San Jose, California). Single time-point measurements were compared by analysis of variance (ANOVA; for normally distributed data) or Kruskal-Wallis ANOVA on ranks (if data could not be transformed to achieve equal variance and/or normality). Serial measures were compared using repeated measures ANOVA with treatment group and time (gestational age for baseline measurements; or time of sampling during cord occlusions or sham) as factors. Post hoc comparisons were made using Tukey test (for normally distributed data) or Dunn method.
Results
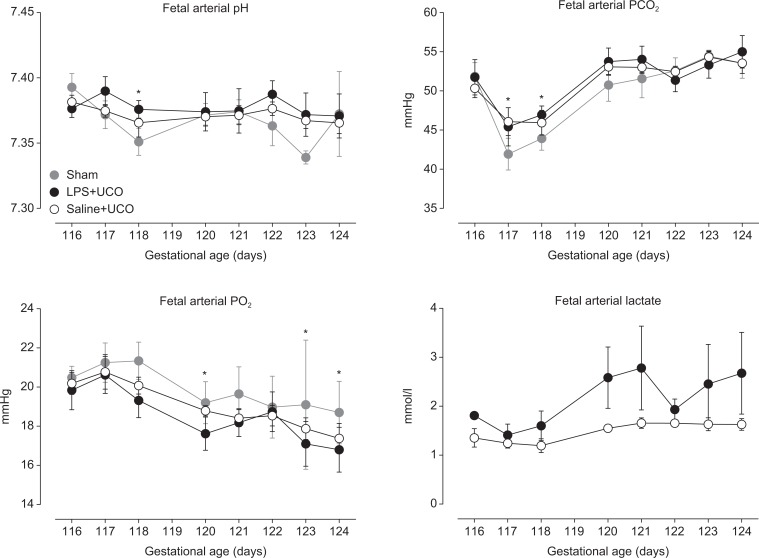
Baseline values of fetal arterial pH (pHa), PCO2 (PaCO2), PO2 (PaCO2), and lactate concentrations, from 116 to 124 days of gestation, are shown in Figure 1. Fetal pHa was not different between groups (P = .889); values at 118 days of gestation were lower than that at 116 days (P = .013). Fetal PaCO2 was not different between groups (P = .715) but was lower in all groups on the days of UCO (or sham; 117 and 118 days) than at other ages (P < .05). Fetal PaO2 was not different between groups (P = .343), and decreased with age in all groups (P < .001); arterial SO2 was similarly affected (data not shown). Fetal arterial lactate concentrations were not different between LPS + UCO and SAL + UCO groups (P = .167; insufficient data were obtained from the sham group for analysis of lactate).
Figure 1.
Baseline fetal arterial pH, PCO2, PO2, and lactate in sham (grey), SAL + UCO (white symbols), and LPS + UCO (black) groups. Values were not different between groups. *P < 0.05 versus 116 days of gestation.
Fetal body and organ weights at 126 days of gestation are shown in Table 1; there were no differences between groups. Thoracic girth was smaller in the LPS + UCO group (28.2 ± 0.6 cm) than in the SAL + UCO group (30.8 ± 0.7 cm; P = .027). Crown-rump length was not different between groups.
Table 1.
Fetal Body and Organ Weights and Indices of Inflammation at 126 Days of Gestation (Data are Mean ± SEM).
| LPS + UCO | SAL + UCO | Sham | |
|---|---|---|---|
| Number of fetuses (male:female) | 3:3 | 7:1 | 2:4 |
| Fetal body weight (kg) | 2.42 ± 0.21 | 2.97 ± 0.14 | 2.66 ± 0.24 |
| Fetal brain weight (g/kg) | 16.2 ± 1.4 | 13.6 ± 0.4 | 14.7 ± 0.6 |
| Thymus (g/kg) | 1.0 ± 0.2 | 1.1 ± 0.1 | 1.1 ± 0.1 |
| Spleen (g/kg) | 2.6 ± 0.4 | 2.4 ± 0.2 | 3.5 ± 0.5 |
| Liver (g/kg) | 42.6 ± 4.7 | 38.8 ± 3.3 | 42.9 ± 4.1 |
| Placenta (g) | 423 ± 73 | 531 ± 35 | 516 ± 50 |
| Maternal WBC (109 cells/L) | 12.2 ± 2.1 | 10.8 ± 1.1 | 7.8 ± 1.0 |
| Fetal WBC (109 cells/L) | 6.7 ± 2.8 | 5.4 ± 1.9 | 10.6 ± 4.5 |
| Amniotic fluid IL-6 (ng/mL) | 1.2 ± 0.5 | Undetected | 0.7 ± 0.4 |
| Amniotic fluid IL-8 (ng/mL) | 97.3 ± 8.0b | 2.5 ± 0.4 | 26.8 ± 23.1 |
| Chorioamnion inflammation score | 2.6 ± 0.2a,b | 1.2 ± 0.4 | 0.6 ± 0.1 |
Abbreviations: WBC, total circulating white blood cell count; IL, interleukin.
a P < .05 versus sham.
b P < .05 versus saline (ANOVA).
Both maternal and fetal circulating white blood cell counts at 126 days of gestation were not different between LPS + UCO, SAL + UCO, and sham groups (Table 1). Amniotic fluid IL-8 concentrations were higher in the LPS + UCO group than the SAL + UCO group (P < .05) but were not different between LPS + UCO and sham groups (owing to high variability in the sham group from a high value in one animal: Table 1). Amniotic fluid IL-6 concentrations were undetectable in the SAL + UCO group but there was no statistically significant difference between groups (P = .146). Chorioamnion inflammatory scores were higher in the LPS + UCO group than in the SAL + UCO (P = .028) and sham (P = .003) groups (Table 1).
Fetal Responses to UCO
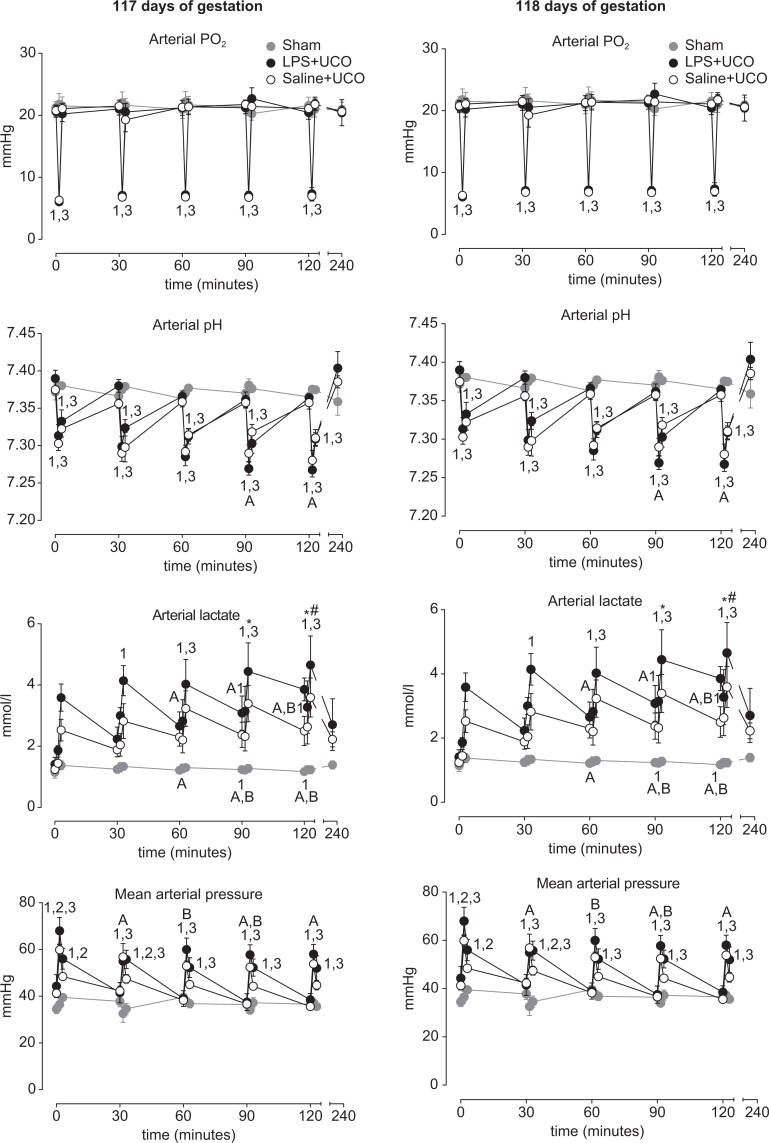
Umbilical cord occlusions caused transient reductions in fetal PaO2 and pHa, and increases in lactate and mean arterial pressure (Figure 2). Twenty-four hours after commencing UCOs, fetal PaO2, pHa, and lactate concentrations were not different between groups, or from pre-occlusion levels.
Figure 2.
Fetal arterial PO2, lactate concentration, pH, and mean arterial pressure in sham (grey), saline (SAL) + umbilical cord occlusion (UCO; white symbols), and LPS + UCO (black) groups, throughout the UCO (or sham) periods on days 117 and 118 of gestation. Responses to UCO were similar between SAL + UCO and LPS + UCO groups. 1 P < .05 LPS + UCO versus sham; 2 P < .05 LPS + UCO versus SAL + UCO; 3 P < .05 SAL + UCO versus sham; A P < .05 timepoint versus 0 minutes (LPS + UCO group); B P < .05 timepoint versus 0 minutes (SAL + UCO group).
Fetal PaO2 (Figure 2) fell consistently with each UCO in the LPS + UCO and SAL + UCO groups (P < .05 versus sham) but returned to pre-occlusion levels immediately after the occlusions. Fetal PaO2 responses to UCO were not different between SAL + UCO and LPS + UCO groups and were similar for UCOs performed on 117 and 118 days of gestation.
Fetal pHa (Figure 2) fell during UCOs (P < .05 versus sham) and returned toward pre-occlusion levels immediately after each UCO, but remained lower than in the sham group (P < .05). Fetal pHa was not different between the SAL + UCO or LPS + UCO groups immediately prior to any of the UCOs. Fetal pHa responses to UCO were not different between SAL + UCO and LPS + UCO groups. At 117 days of gestation fetal pHa was lower during the 4th and 5th UCO than during the 1st, in the LPS + UCO group (P < .05); this was not observed in the SAL + UCO group. There was no difference in pHa immediately after UCO between SAL + UCO and LPS + UCO groups. At 118 days of gestation, fetal pHa in the LPS + UCO group was lower during the 2nd, 3rd, 4th, and 5th occlusions than during the 1st. In the SAL + UCO group, fetal pHa was lower during the 2nd, 3rd, and 4th occlusions (but not the 5th) than during the 1st. Fetal pHa responses to UCOs on day 118 of gestation were not different between SAL + UCO and LPS + UCO groups.
Fetal arterial lactate concentrations (Figure 2) increased in response to each UCO, and over the course of the UCO periods on days 117 and 118 of gestation, in SAL + UCO and LPS + UCO groups (P < .05 versus sham). There was no difference in lactate responses to UCO between the SAL + UCO and LPS + UCO groups, but concentrations tended to be higher in the LPS + UCO group. At 117 days of gestation, but not at 118 days, fetal arterial lactate concentrations before, during, and/or after UCO were higher for the 3rd, 4th, and 5th occlusions than for the first in SAL + UCO and LPS + UCO groups (P < .05). At 117 and 118 days of gestation, fetal arterial lactate in the LPS + UCO group was higher than in the sham group (P < .05) at times when concentrations were not different between SAL + UCO and sham groups.
Fetal mean arterial pressure (MAP; Figure 2) increased during UCO in SAL + UCO and LPS + UCO groups (P < .05 versus sham). Mean arterial pressure returned toward pre-occlusion levels immediately after UCO but remained elevated, relative to the sham group. At 117 and 118 days of gestation, fetal MAP in the LPS + UCO group was greater than in the sham group (P < .05) at times when there was no difference between the SAL + UCO and sham groups. Mean arterial pressure in the LPS + UCO group was higher than in the SAL + UCO group (P < .05) during and immediately after the 1st UCO, and after the 2nd, on day 117 of gestation.
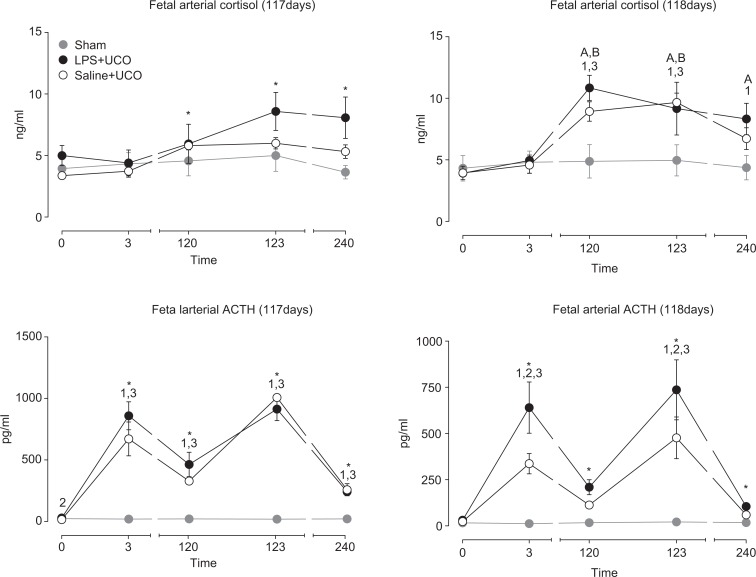
Fetal arterial cortisol concentrations over the course of the UCO period on day 117 of gestation (Figure 3) were higher at 120, 123, and 240 minutes compared to that immediately before the first occlusion (P < .003) but were not different between LPS + UCO and SAL + UCO groups (P = .11). Fetal arterial ACTH concentrations were higher in the LPS + UCO group (28.8 ±5.0 pg/mL) than in the SAL + UCO group (15.1 ±3.1 pg/mL; P = .017), but not the sham group (23.1 ±3.7 pg/mL), immediately prior to the first occlusion on day 117 of gestation. Fetal arterial ACTH concentrations (Figure 3) on day 117 were higher than that immediately before the first occlusion at all subsequent times in all groups (P < .05). ACTH concentrations were not different between the LPS + UCO and SAL + UCO groups at these times, but concentrations were higher in both groups than in the sham group (P < .05). On day 118 of gestation, fetal cortisol increased during the UCO period in the LPS + UCO and SAL + UCO groups (P < .05) but not in the sham group (Figure 3). Fetal cortisol levels were higher in the LPS + UCO group than in the sham group at 120, 123, and 240 minutes (P < .05). Fetal cortisol levels in the SAL + UCO group were greater than the sham group at 120 and 123 minutes (P < .05) but not at 240 minutes. On 118 days of gestation, fetal arterial ACTH concentrations were not different between groups prior to UCOs (or sham; Figure 3). Adrenocorticotrophic hormone concentrations were higher than immediately before the first UCO at all subsequent times in all groups (P < .05). Immediately after the first and last UCO, fetal ACTH concentrations were higher in the LPS + UCO group than in both the SAL + UCO (P < .01) and sham (P < .001) groups; values in the SAL + UCO group were higher than in the sham group (P < .003) at these same times. Fetal ACTH concentrations were not different between groups at 120 and 240 minutes after initiating cord occlusions (or sham) on 118 days of gestation (Figure 3).
Figure 3.
Fetal arterial cortisol and adrenocorticotrophic hormone (ACTH) concentrations in sham (grey), SAL + UCO (white symbols) and LPS + UCO (black) groups, throughout the cord occlusion (or sham) periods on days 117 and 118 of gestation. *P < .05 timepoint versus 0 minutes (all groups); 1 P < .05 LPS + UCO versus sham; 2 P < .05 LPS + UCO versus SAL + UCO; 3 P < .05 SAL + UCO versus sham; A P < .05 timepoint versus 0 minutes (LPS + UCO group); B P < .05 timepoint versus 0 minutes (SAL + UCO group).
Qualitative Assessment of the Fetal Brain
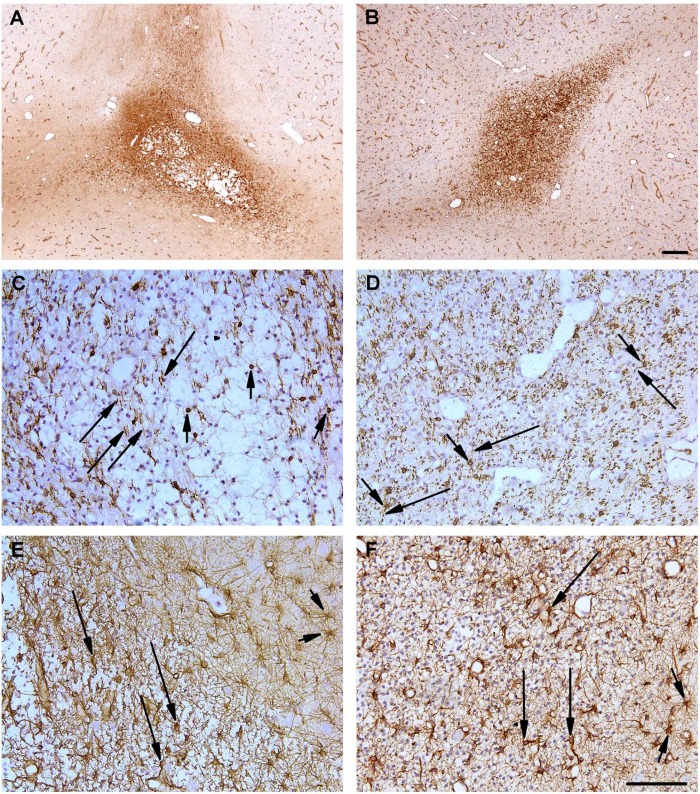
Regions of microglial/macrophage infiltration were present in all groups of fetuses (sham, SAL + UCO, LPS + UCO), typically in the subcortical white matter. In 2 of the 8 SAL + UCO fetuses cystic lesions were present within these regions of infiltration: in 1 fetus, the lesion was present in the temporal lobe, at the level of the optic chiasm; in the other fetus, the lesion was located in the frontal lobe at the level of the coronal sulcus (Figure 4A). Such lesions were not observed within the areas of microglial infiltration in the LPS + UCO (Figure 4B) or sham groups. In all fetuses, within areas of microglial infiltration, there appeared to be a decrease in the density of CNPase-positive oligodendrocytes (Figure 4C, SAL + UCO; Figure 4D, LPS + UCO); myelinated axons in these regions frequently appeared fragmented. The cell bodies of astrocytes within regions of infiltration were hypertrophic and exhibited attenuated and thickened processes (Figure 4E, SAL + UCO; 4F, LPS + UCO) although there appeared to be no increase in cell density. Apoptotic cells (AC-3 positive cells) were present in all groups of fetuses (sham, SAL + UCO, LPS + UCO) and were typically distributed sparsely throughout the grey and white matter and in the periventricular zone. Apoptotic cells were not located within the areas of micoglial infiltration (indicated by lectin-positive cells: data not shown). There was no perivascular cuffing, subarachnoid or germinal layer hemorrhage, or ventriculomegaly, in the regions of the brain we examined, in any group.
Figure 4.
Micrographs of the cerebral subcortical white matter from 1 SAL + UCO (A, C, E) and 1 LPS + UCO fetus (B, D, F) aged 126 days of gestation. (A) Lectin-positive cell infiltration (activated microglia/macrophages; brown staining) surrounding a subcortical, cystic lesion in a SAL + UCO-treated fetus; such pathology was observed in 2/8 fetuses from this group but not in other groups. (B) Mild lectin-positive cell infiltration (brown staining) of the subcortical white matter in an LPS + UCO fetus. (C, D) Oligodendrocytes (short arrows) were reduced in number and myelinated fibers were attenuated and fragmented (long arrows) in regions of infiltration in both UCO groups. (E, F) Reactive astrocytes (long arrows), within areas of microglial infiltration, were hypertrophic, intensely stained for GFAP and exhibited thickened and attenuated processes compared to outside the areas of infiltration (short arrows) in both groups. Scale bars in panels A-B are 200 µm; scale bars in panels C-F are 100 µm.
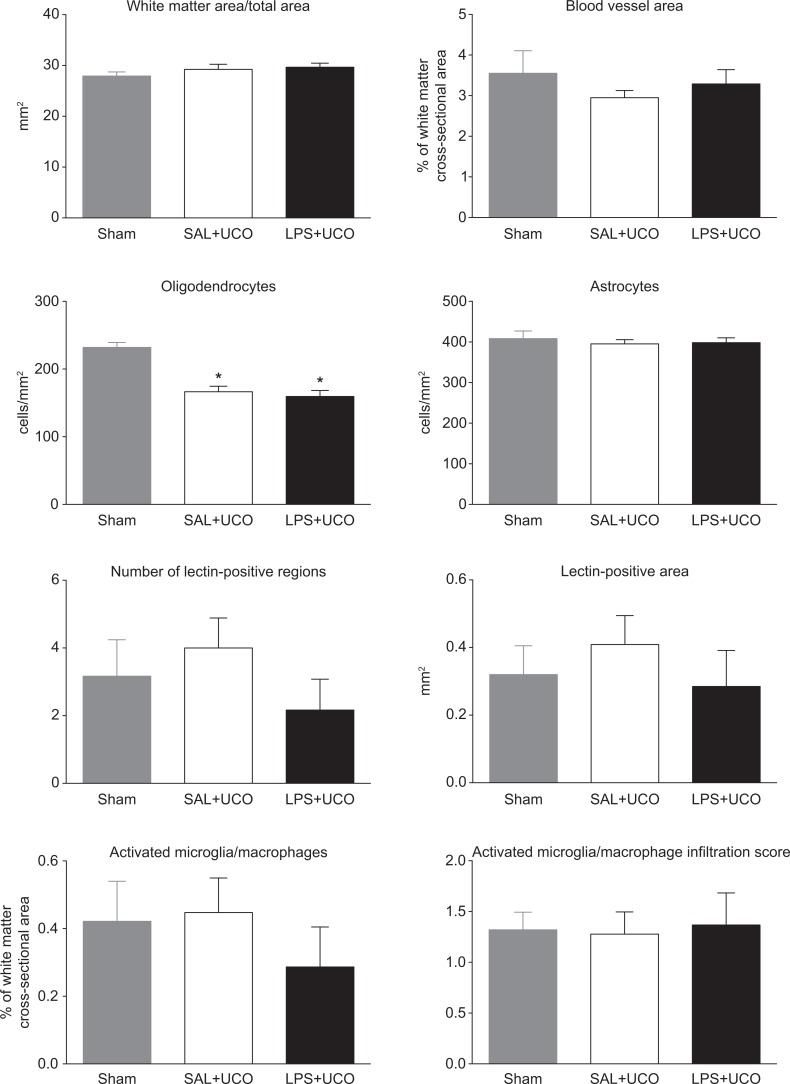
Quantitative Brain Morphometry
The cross-sectional area of white matter (expressed in absolute terms or relative to total section area) and the proportion of this area occupied by blood vessels were not different between groups (Figure 5). The areal density of CNPase-positive cells (ie, oligodendrocytes) in the brains of LPS + UCO and SAL + UCO fetuses was lower than in the sham group (P < .05; Figure 5). The areal density of GFAP-positive cells (ie, astrocytes) was not different between groups (Figure 5). The percentage of white matter area occupied by lectin-positive cells (ie, activated microglia/macrophages) and the activated microglia infiltration scores in these regions were not different between groups (Figure 5). The surface-folding index was not different between groups (LPS + UCO, 75.7 ± 2.0; SAL + UCO, 77.1 ± 2.8; sham, 73.3 ± 3.2: P = .6).
Figure 5.
Quantitative measures of brain inury/development in sham (grey), SAL + UCO (white) and LPS + UCO (black) groups. *P < .05 versus sham.
Comment
Our study has demonstrated that persistent intrauterine inflammation resulting from infusion of LPS into the amniotic cavity for a period of 4 weeks, commencing in mid-gestation, does not worsen subsequent physiologic or neurodevelopmental consequences of repeated intermittent UCOs in preterm fetal sheep.
Although our data do not support the hypothesis that preexisting intrauterine inflammation exacerbates fetal brain injury in response to UCO, they do not necessarily contradict the observations in humans14 that formed the rationale for our work. Systematic analysis of data from humans shows that clinical, but not histological, chorioamnionitis increases the risk of cerebral palsy.25 Clinical chorioamnionitis represents a severe, acute form of intrauterine inflammation; whereas histological chorioamniotitis is likely the result of longer term subclinical inflammation in many cases.2 Thus, the relative timing of inflammatory exposure of the fetus may be substantially different between these 2 manifestations of intrauterine inflammation. Distinction between such varied presentations of inflammation during pregnancy is critical for understanding effects on the fetus and potential neonatal outcomes. For example, histological chorioamnionitis does not increase the risk of cerebral palsy but it does increase the risk of bronchopulmonary dysplasia.26 Furthermore, the severity of the adverse neurological and pulmonary effects of intrauterine inflammation is related to the severity of the inflammation and the fetal involvement in the inflammatory process.27,28
The changes in fetal blood gas, arterial pressure, and circulating hormones we observed in response to UCO were similar to those of previous studies using brief UCOs in fetal sheep.19,29 Blood gas measurements during UCOs demonstrate that the fetal insult was similar in LPS + UCO and SAL + UCO groups. We had considered the possibility that UCO might result in different responses between LPS + UCO and SAL + UCO groups but this does not appear to have occurred. Although the magnitude of the effect of UCO appeared similar between groups, we observed some small but potentially significant differences in responses between groups. LPS + UCO fetuses tended to have greater increases in mean arterial pressure and circulating lactate in response to UCOs than SAL + UCO fetuses. None of our experimental groups showed any sign of deterioration in fetal condition in the days between the UCOs and delivery for tissue collection, indicating that longer term fetal well-being after hypoxemic/ischemic episodes, like the acute responses, is not adversely affected by pre-existing intrauterine inflammation. The low fetal PaCO2 levels observed on days 117 and 118 of gestation (from samples collected before commencement of UCOs or sham) probably reflect maternal hyperventilation as a result of the mild stress associated with investigators' presence during preparations for physiological recordings.
At 126 days of gestation, 1 week after UCOs (or sham), the degree of white matter infiltration by activated microglia/macrophages was similar in LPS + UCO, SAL + UCO, and sham groups. We showed previously that intra-amniotic infusion of LPS, as used in our current study, was accompanied by increased microglia/macrophage infiltration into the fetal white matter at 110 days of gestation, relative to SAL-infused controls; these regions showed damage to the tissue architecture.4 In comparison to that study, indices of microglial infiltration in our present experiment were considerably lower. This difference in the magnitude of the microglial response likely relates to the different time intervals between cessation of LPS infusion and tissue collection in the 2 studies (ie, 2 versus 18 days). The half-life of LPS in the amniotic cavity after intra-amniotic injection in sheep is ~1.7 days.30 Amniotic fluid LPS levels at 100 days of gestation during infusion, as in our present study, reach ~2 × 104 endotoxin units/mL.31 Thus, if the 100 d level represents steady state, we estimate: at the time of surgery there would be ~9 × 103 EU/mL; on the first day of UCOs there would be ~750 EU/mL. Such a low level probably provides little, if any, proinflammatory stimulus to a fetus that has presumably become somewhat tolerant32 by this time. Thus, it appears that when mild fetal brain inflammation occurs in response to intra-amniotic LPS administration, it can resolve spontaneously with time, as we have observed in the fetal lungs.6 Both UCO groups in our present study showed a reduction in CNP-ase immunostaining in the brain white matter, relative to the sham group, indicating a similar effect of UCO in reducing the number of CNP-ase positive oligodendrocytes and, hence, delaying or arresting myelination.
This paper shows that the effects of intermittent UCO on the fetal brain are not exacerbated by preexisting chronic intrauterine inflammation. We are cautious in our interpretation of the different frequencies of white matter lesions observed in the SAL + UCO (2/8 fetuses) and LPS + UCO (0/6 fetuses) groups. Not only is the number of participants in our experiment too low to provide sufficient power to detect statistical differences, but the distribution of sexes between the treatment groups is also different. The 2 fetuses in the SAL + UCO group with cerebral white matter lesions were both male, but only 3 of the 6 LPS + UCO fetuses we studied were male. It is known that males have higher rates of cerebral palsy and other neurodevelopmental disorders.33 Using a mouse model of preterm hypoxic brain injury, experiments specifically designed to examine sex differences showed greater loss of hippocampal volume and reductions in myelination in males than in females.34 Thus, the apparent difference in the incidence of white matter lesions in the brains of SAL + UCO and LPS + UCO fetuses in our study may be due to the higher number of males in the SAL + UCO group.
Evidence suggests that proinflammatory stimuli can protect against, or exacerbate, hypoxemic/ischemic brain injury depending on the relative timing of the insults. In rodents, brain injury was reduced in 7-day-old pups if the hypoxemic/ischemic insult occurred 24 hours after LPS administration; if hypoxia/ischemia occurred either 6 or 72 hours after LPS administration, the brain injury was exacerbated.15 The temporal effect of LPS exposure on vulnerability to hypoxemic/ischemic brain injury appears to be related to corresponding inflammation-induced changes in gene expression patterns in the brain.35 This ability of inflammation to protect against subsequent hypoxic damage is well established for many tissues; not just the brain.36 Such protective “preconditioning” is not restricted to the effects of proinflammatory stimuli on hypoxic insults (eg, hyper- and hypothermic stimuli also have preconditioning effects), but inflammatory signaling mechanisms may be common to all preconditioning stimuli.36
Neuroprotection conferred by LPS exposure 24 hours prior to hypoxia/ischemia in 7-day-old rats coincides with elevated circulating levels of corticosterone (the principal endogenous glucocorticoid in this species); this has been suggested as a potential factor underlying the protective effect of inflammation.37 Our data show that fetal sheep exposed to chronic inflammation do not have altered circulating cortisol levels at rest, despite slightly higher ACTH levels prior to commencing UCOs. Activation of the HPA axis, which has an integral role in the fetal response to UCO,20 was altered by exposure to chronic inflammation. We observed a slightly increased cortisol response to UCO in fetuses exposed to inflammation because concentrations in the LPS + UCO group tended to be higher than in the SAL group after UCOs on day 117 of gestation and were elevated for longer than the SAL + UCO group on day 118. Further, the ACTH response to UCO at 118 days of gestation was elevated in fetuses exposed to chronic inflammation. Previous studies in rats have demonstrated the capacity for prenatal and early postnatal proinflammatory challenges to result in subsequent increases in postnatal hypothalamic-pituitary-adrenal (HPA) axis responsiveness38–41; this is consistent with our present observations. To our knowledge, the timing of onset of the inflammation-induced hyperresponsiveness of the HPA axis is not known, but our data suggest the effect might be present in utero. Further examination of the effect of intrauterine inflammation on fetal HPA axis development and function is warranted, given the suggestion from our present data, the role of the fetal HPA axis in parturition,42 and the association between preterm birth and intrauterine inflammation in humans.43
The UCO regimen we used was chosen because we considered it likely to induce mild-to-moderate brain injury in SAL + UCO fetuses given our prior experience19 and the work of others using late gestation fetal sheep,44 allowing us to detect our hypothesized exacerbation of damage in LPS + UCO fetuses. Given our results, investigation of the effects of intrauterine inflammation on the neurodevelopmental consequences of a more severe hypoxic/ischemic insult seems warranted to further explore the potential protective effect suggested by the previous studies in neonatal rats.15,35
Our study aimed to mimic the common clinical scenario of intermittent UCO in labor manifest clinically in delivery wards as variable decelerations on electronic fetal heart rate monitoring. Much obstetric effort is directed at the management of such fetal heart rate abnormalities for the purpose of preventing fetal death and fetal brain injury. Epidemiological studies, however, have provided evidence that short-term insults such as intermittent UCO are unlikely to be associated with subsequent cerebral palsy, although those studies have also shown increased risks of brain injury in the presence of infection.14,45 The pregnancies in the present study had features of histologic chorioamnionitis (ie, elevated white cell counts in fetal membranes and higher amniotic fluid IL-8 levels), but amniotic fluid IL-6 levels were not elevated, indicating inflammation at the level of the membranes but not of a severity that, in humans, would be described as FIRS.28 The absence of overt fetal brain injury in the present study therefore provides evidence that intermittent UCO in the presence of histologic chorioamnionitis, but without systemic fetal inflammation meeting the criteria of FIRS, is unlikely to produce brain injury that in humans may be manifested later as cerebral palsy. Further studies are required to investigate if similar intermittent UCO in the presence of overt FIRS would be more injurious to the fetal brain.
Acknowledgment
The authors thank Mr Adrian Jonker for expert technical assistance.
Footnotes
Declaration of Conflicting Interests: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research and/or authorship of this article: National Health and Medical Research Council of Australia (grants 254502, 458577, 303261, 384100).
References
- 1. Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342(20):1500–1507. [DOI] [PubMed] [Google Scholar]
- 2. Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med. 2006;11(5):317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gotsch F, Romero R, Kusanovic JP, et al. The fetal inflammatory response syndrome. Clin Obstetr Gynecol. 2007;50(3):652–683. [DOI] [PubMed] [Google Scholar]
- 4. Nitsos I, Rees SM, Duncan J, et al. Chronic exposure to intra-amniotic lipopolysaccharide affects the ovine fetal brain. J Soc Gynecol Investig. 2006;13(4):239–247. [DOI] [PubMed] [Google Scholar]
- 5. Moss TJM, Nitsos I, Newnham JP, Ikegami M, Jobe AH. Chorioamnionitis induced by subchorionic endotoxin infusion in sheep. Am J Obstet Gynecol. 2003;189(6):1771–1776. [DOI] [PubMed] [Google Scholar]
- 6. Moss TJM, Newnham JP, Willett KE, Kramer BW, Jobe AH, Ikegami M. Early gestational intra-amniotic endotoxin: lung function, surfactant, and morphometry. Am J Respir Crit Care Med. 2002;165(6):805–811. [DOI] [PubMed] [Google Scholar]
- 7. Nitsos I, Moss TJM, Cock ML, Harding R, Newnham JP. Fetal responses to intra-amniotic endotoxin in sheep. J Soc Gynecol Investig. 2002;9(2):80–85. [DOI] [PubMed] [Google Scholar]
- 8. Debillon T, Gras-Leguen C, Vérielle V, et al. Intrauterine infection induces programmed cell death in rabbit periventricular white matter. Pediatr Res. 2000;47(6):736–742. [DOI] [PubMed] [Google Scholar]
- 9. Duncan JR, Cock ML, Scheerlinck JP, et al. White matter injury after repeated endotoxin exposure in the preterm ovine fetus. Pediatr Res. 2002;52(6):941–949. [DOI] [PubMed] [Google Scholar]
- 10. Yoon BH, Kim CJ, Romero R, et al. Experimentally induced intrauterine infection causes fetal brain white matter lesions in rabbits. Am J Obstet Gynecol. 1997;177(4):797–802. [DOI] [PubMed] [Google Scholar]
- 11. Rees S, Mallard C, Breen S, Stringer M, Cock M, Harding R. Fetal brain injury following prolonged hypoxemia and placental insufficiency: a review. Comp Biochem Physiol. 1998;119(3):653–660. [DOI] [PubMed] [Google Scholar]
- 12. Rees S, Stringer M, Just Y, Hooper SB, Harding R. The vulnerability of the fetal sheep brain to hypoxemia at mid-gestation. Brain Res Dev Brain Res. 1997;103(2):103–118. [DOI] [PubMed] [Google Scholar]
- 13. Duncan JR, Cock ML, Suzuki K, Scheerlinck JP, Harding R, Rees SM. Chronic endotoxin exposure causes brain injury in the ovine fetus in the absence of hypoxemia. J Soc Gynecol Investig. 2006;13(2):87–96. [DOI] [PubMed] [Google Scholar]
- 14. Nelson KB, Grether JK. Potentially asphyxiating conditions and spastic cerebral palsy in infants of normal birth weight. Am J Obstet Gynecol. 1998;179(2):507–513. [DOI] [PubMed] [Google Scholar]
- 15. Eklind S, Mallard C, Arvidsson P, Hagberg H. Lipopolysaccharide induces both a primary and a secondary phase of sensitization in the developing rat brain. Pediatr Res. 2005;58(1):112–116. [DOI] [PubMed] [Google Scholar]
- 16. Eklind S, Mallard C, Leverin AL, et al. Bacterial endotoxin sensitizes the immature brain to hypoxic–ischaemic injury. Eur J Neurosci. 2001;13(6):1101–1106. [DOI] [PubMed] [Google Scholar]
- 17. Wang X, Hagberg H, Nie C, Zhu C, Ikeda T, Mallard C. Dual role of intrauterine immune challenge on neonatal and adult brain vulnerability to hypoxia-ischemia. J Neuropathol Exp Neurol. 2007;66(6):552–561. [DOI] [PubMed] [Google Scholar]
- 18. Lehnardt S, Massillon L, Follett P, et al. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci USA. 2003;100(14):8514–8519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Loeliger M, Watson CS, Reynolds JD, et al. Extracellular glutamate levels and neuropathology in cerebral white matter following repeated umbilical cord occlusion in the near term fetal sheep. Neuroscience. 2003;116(3):705–714. [DOI] [PubMed] [Google Scholar]
- 20. Roelfsema V, Gunn AJ, Fraser M, Quaedackers JS, Bennet L. Cortisol and ACTH responses to severe asphyxia in preterm fetal sheep. Exp Physiol. 2005;90(4):545–555. [DOI] [PubMed] [Google Scholar]
- 21. Sloboda DM, Moss TJM, Li S, et al. Prenatal betamethasone exposure results in pituitary-adrenal hyporesponsiveness in adult sheep. Am J Physiol Endocrinol Metab. 2007;292(1):E61–E70. [DOI] [PubMed] [Google Scholar]
- 22. Conradi NG, Muntzing K. Cerebellar foliation in rats. 2. Effects of maternal malnutrition on the formation of fissures in foetal rats. Acta Pathol Microbiol Immunol Scand [A]. 1985;93(6):391–395. [PubMed] [Google Scholar]
- 23. Haynes RL, Folkerth RD, Keefe RJ, et al. Nitrosative and oxidative injury to premyelinating oligodendrocytes in periventricular leukomalacia. J Neuropathol Exp Neurol. 2003;62(5):441–450. [DOI] [PubMed] [Google Scholar]
- 24. Kramer BW, Moss TJM, Willet KE, et al. Dose and time response after intraamniotic endotoxin in preterm lambs. Am J Respir Crit Care Med. 2001;164(6):982–988. [DOI] [PubMed] [Google Scholar]
- 25. Wu YW. Systematic review of chorioamnionitis and cerebral palsy. MRDD Res Rev. 2002;8(1):25–29. [DOI] [PubMed] [Google Scholar]
- 26. Viscardi RM, Muhumuza CK, Rodriguez A, et al. Inflammatory markers in intrauterine and fetal blood and cerebrospinal fluid compartments are associated with adverse pulmonary and neurologic outcomes in preterm infants. Pediatr Res. 2004;55(6):1009–1017. [DOI] [PubMed] [Google Scholar]
- 27. Park CW, Moon KC, Park JS, Jun JK, Romero R, Yoon BH. The involvement of human amnion in histologic chorioamnionitis is an indicator that a fetal and an intra-amniotic inflammatory response is more likely and severe: clinical implications. Placenta. 2009;30(1):56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yoon BH, Romero R, Park JS, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000;182(3):675–681. [DOI] [PubMed] [Google Scholar]
- 29. Green LR, Homan J, White SE, Richardson BS. Cardiovascular and metabolic responses to intermittent umbilical cord occlusion in the preterm ovine fetus. J Soc Gynecol Investig. 1999;6(2):56–63. [DOI] [PubMed] [Google Scholar]
- 30. Newnham JP, Kallapur SG, Kramer BW, et al. Betamethasone effects on chorioamnionitis induced by intra-amniotic endotoxin in sheep. Am J Obstet Gynecol. 2003;189(5):1458–1466. [DOI] [PubMed] [Google Scholar]
- 31. Kallapur SG, Nitsos I, Moss TJM, et al. Chronic endotoxin exposure does not cause sustained structural abnormalities in the fetal sheep lungs. Am J Physiol Lung Cell Mol Physiol. 2005;288(5):L966–L974. [DOI] [PubMed] [Google Scholar]
- 32. Kallapur SG, Jobe AH, Ball MK, et al. Pulmonary and systemic endotoxin tolerance in preterm fetal sheep exposed to chorioamnionitis. J Immunol. 2007;179(12):8491–8499. [DOI] [PubMed] [Google Scholar]
- 33. Johnston MV, Hagberg H. Sex and the pathogenesis of cerebral palsy. Dev Med Child Neurol. 2007;49(1):74–78. [DOI] [PubMed] [Google Scholar]
- 34. Mayoral SR, Omar G, Penn AA. Sex differences in a hypoxia model of preterm brain damage. Pediatr Res. 2009;66(3):248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mallard C, Hagberg H. Inflammation-induced preconditioning in the immature brain. Semin Fetal Neonatal Med. 2007;12(4):280–286. [DOI] [PubMed] [Google Scholar]
- 36. Karikó K, Weissman D, Welsh FA. Inhibition of toll-like receptor and cytokine signaling--a unifying theme in ischemic tolerance. J Cereb Blood Flow Metab. 2004;24(11):1288–1304. [DOI] [PubMed] [Google Scholar]
- 37. Ikeda T, Yang L, Ikenoue T, Mallard C, Hagberg H. Endotoxin-induced hypoxic-ischemic tolerance is mediated by up-regulation of corticosterone in neonatal rat. Pediatr Res. 2006;59(1):56–60. [DOI] [PubMed] [Google Scholar]
- 38. del Rey A, Furukawa H, Monge-Arditi G, Kabiersch A, Voigt KH, Besedovsky HO. Alterations in the pituitary-adrenal axis of adult mice following neonatal exposure to interleukin-1. Brain Behav Immun. 1996;10(3):235–248. [DOI] [PubMed] [Google Scholar]
- 39. Hodgson DM, Knott B, Walker FR. Neonatal endotoxin exposure influences HPA responsivity and impairs tumor immunity in Fischer 344 rats in adulthood. Pediatr Res. 2001;50(6):750–755. [DOI] [PubMed] [Google Scholar]
- 40. Reul JM, Stec I, Wiegers GJ, et al. Prenatal immune challenge alters the hypothalamic-pituitary-adrenocortical axis in adult rats. J Clin Invest. 1994;93(6):2600–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Samuelsson AM, Ohrn I, Dahlgren J, et al. Prenatal exposure to interleukin-6 results in hypertension and increased hypothalamic-pituitary-adrenal axis activity in adult rats. Endocrinology. 2004;145(11):4897–4911. [DOI] [PubMed] [Google Scholar]
- 42. Challis JR, Sloboda D, Matthews SG, et al. The fetal placental hypothalamic-pituitary-adrenal (HPA) axis, parturition and post natal health. Mol Cell Endocrinol. 2001;185(1-2):135–144. [DOI] [PubMed] [Google Scholar]
- 43. Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2007;65(12 Pt 2):S194–S202. [DOI] [PubMed] [Google Scholar]
- 44. Mallard EC, Williams CE, Johnston BM, Gunning MI, Davis S, Gluckman PD. Repeated episodes of umbilical cord occlusion in fetal sheep lead to preferential damage to the striatum and sensitize the heart to further insults. Pediatr Res. 1995;37(6):707–713. [DOI] [PubMed] [Google Scholar]
- 45. Nelson KB. Causative factors in cerebral palsy. Clin Obstet Gynecol. 2008;51(4):749–762. [DOI] [PubMed] [Google Scholar]