Abstract
In mammalian species, acquisition of sperm fertilization competence is dependent on the phenomenon of sperm capacitation. One of the key elements of capacitation is protein tyrosine phosphorylation (TP) in various sperm membrane regions. In previous studies performed, the pattern of TP was examined in human sperm bound to zona pellucida of oocytes. In the present comparative study, TP patterns upon sperm binding to the zona pellucida or hyaluronic acid (HA) were investigated in spermatozoa arising from the same semen samples. Tyrosine phosphorylation, visualized by immunofluorescence, was localized within the acrosomal cap, equatorial head region, neck, and the principal piece. Tyrosine phosphorylation has increased in a time-related manner as capacitation progressed, and the phosphorylation pattern was identical within the principal piece and neck, regardless of the sperm bound to the zona pellucida or HA. Thus, the data demonstrated that the patterns of sperm activation-related TP were similar regardless of the spermatozoa bound to zona pellucida or HA. Further, sperm with incomplete development, as detected by excess cytoplasmic retention, failed to exhibit TP.
Keywords: human sperm, phosphotyrosine, capacitation, zona pellucida, hyaluronic acid
Introduction
Mammalian spermatozoa will undergo capacitation in order to acquire the ability for oocyte fertilization.1 Capacitation is a complex event that in part occurs in situ within the female reproductive tract and is associated with several sperm cellular changes, including increases in intracellular calcium, chloride ions, and cyclic adenosine monophosphate levels, efflux of cholesterol, and changes in protein kinase and phosphatase activities.2–10 Recently, it has also been shown that 2 sialidases on mammalian sperm are shed from sperm during capacitation and might play a role in the sperm binding to the zona pellucida of the ovum.11 Therefore, several in vitro protocols for assessing capacitation and acrosome reaction of human spermatozoa have been developed.12 Indeed, an increase in tyrosine phosphorylation (TP) of sperm membrane proteins was shown to be an essential element of sperm capacitation.13,14 Capacitation-associated changes in sperm has been demonstrated in several species.10,14–18
Immunofluorescence studies of capacitated sperm showed the localization of tyrosine phosphorylated proteins in the flagellum of sperm, particularly in those cells participating in zona pellucida binding, oolemma binding, and gamete fusion.19 Characteristic patterns of TP in the flagellum and neck were observed upon binding to zonae pellucidae.20 Tyrosine phosphorylated sperm proteins in the flagellum are linked to hyperactivated motility.21 Hyperactivated motility and the associated TP in the flagellum are initiated during in vitro capacitation, and their maintenance in zona pellucida-bound spermatozoa is of importance.22 Therefore, although the role of TP in capacitation is unclear, the level of TP in human sperm is strongly related to the sperm–zona-binding capacity,23 and changes in TP has been detected in subfertile patients,24 indicating its physiological role in fertilization.
In human sperm, creatine kinase (creatine phosphokinase, CK) is a marker of cytoplasmic retention and, thus, arrested sperm cellular maturation\development.25,26 Our earlier studies showed that in line with cytoplasmic extrusion, there is also a spermiogenetic sperm plasma membrane remodeling that facilitates the formation of zona pellucida- and hyaluronic acid (HA)-binding sites during spermiogenesis.27,28 Sperm with arrested spermiogenesis fails to bind to the zona pellucida and HA. Apparently, the lack of sperm membrane remodeling affects the formation of the binding sites for both the zona pellucida and the HA. The HA-bound sperm also exhibits other attributes of normal sperm development, such as lack of cytoplasmic retention, lack of persistent histones, and high levels of DNA chain integrity.29 Further, HA-bound sperm is enriched in the sperm of Tygerberg normal morphology; in addition, the frequencies of chromosomal aneuploidies in HA-bound spermatozoa are within the normal range, regardless of their frequencies in the original semen sperm fractions.30,31 It was demonstrated that HA-bound sperm showed almost exclusive presence of green acridine orange fluorescence, reflecting high DNA integrity compared to their respective semen fractions.32 In the past few years, the sperm HA-binding assessment in the Andrology Laboratory, and the intracytoplasmic sperm injection sperm selection device, the so-called PICSI dish (an in vitro fertilization Petri dish that carries an HA spot), has been increasingly accepted and used worldwide with excellent results in pregnancy rates and decline in early miscarriages.33,34
In the present study, we examined the changes occurring in human sperm TP in relation to the interaction between sperm and HA. Earlier, Sakkas et al investigated the TP patterns in capacitated and zona pellucida-bound sperm fractions.20 We have now examined various sperm regions for the pattern of phosphorylations in order to compare TP associated with the binding to HA or zona pellucida. Further, we examined the pattern of TP in mature developed sperm and in sperm with arrested maturation, as evidenced by surplus cytoplasmic retention. In overview, we have observed the sperm response to binding to zona pellucida or HA in three paradigms: (1) compared the pattern of TP in response to binding to the zona pellucida or HA in spermatozoa arising from the same semen sample; (2) compared to the phosphorylation patterns in response to zona pellucida in our hands and in the previous report by Sakkas et al20; and (3) we also followed the rate of phosphorylation in normally developed sperm versus in sperm with arrested development/maturation, as detected by the presence of surplus cytoplasmic retention.
Materials and Methods
Sperm Preparation
The leftover de-identified semen samples after the routine semen analyses were studied. Samples were collected by masturbation after 2 days of abstinence and were allowed to liquefy for 60 minutes. Sperm concentration and motility were assessed using computer-assisted semen analysis (Hamilton Thorne Research, Beverly, Massachusetts).
We studied the semen of 32 men (sperm concentrations: 64.2 ± 8.3 × 106 sperm/mL [min-max: 20-164], sperm motility 48.2 ± 3.4% [min-max: 30%-85%]). Of 32 men, 16 were used for the comparison of TP pattern in zona pellucida-bound and HA-bound sperm. Besides, the sample number expanded for the maturity studies (n = 13; sperm concentrations: 37.8 ± 6.2 × 106 sperm/mL, motility: 50.9% ± 4.1%; n = 13, not included with the other 32 study participants). Sperm was layered on a 45%/85% Isolate gradient (Irvine Scientific, Santa Ana, California) and centrifuged at 500g for 20 minutes. After resuspending the sperm pellet in human tubal fluid (HTF) medium (Irvine Sci, Irvine, California) containing 0.5% bovine serum albumin (BSA), an aliquot of 7 µL of the sperm suspension was smeared on glass slides and were fixed with 3.7% formaldehyde in phosphate buffer/sucrose (PB-suc) for 20 minutes at room temperature (noncapacitated sperm). This formaldehyde fixation methodology was employed in all experiments. Of the remaining sperm suspension, one aliquot was kept for the preparation of zona- and HA-bound sperm fractions, and the other part was diluted in HTF medium for study of capacitation and detection of phosphotyrosine. Sperm suspensions in HTF were incubated for 4 hours after the initial preparation at 37°C in 5% CO2 and fixed with formaldehyde (capacitated sperm). All studies were approved by the Human Investigation Committee, Yale School of Medicine.
Preparation and Study of HA-Bound Sperm Fractions
For the study of the HA-bound sperm, we applied 7-µL aliquots (approximately 0.5 × 106 motile sperm/mL) of the purified sperm suspension to the HA-coated surfaces of the sperm-HA-binding slides (HBA, Midatlantic Scientific, now Origio Co, Mount Laurel, New Jersey). The HA-bound sperm were recognized by the lack of progressive motility and by the characteristic sharp increase in tail cross-beat frequency. Some sperm did not exhibit HA-binding ability, most likely due to impaired/arrested spermatogenesis and spermiogenesis and thus lack of HA receptors, have failed to “perceive” the HA, and did not bind.28,29 Nonmotile sperm and sperm that do not exhibit the characteristic higher tail cross-beat frequency were not considered as HA-bound spermatozoa.
The sperm and the HA-coated slides (HBA slides) were observed for 10 minutes in a humidity chamber, and the unbound sperm were removed by slightly tilting the slides and applying HTF with a gentle drop-by-drop rinsing.29 The HA-selected bound sperm fraction was then air-dried and fixed with formaldehyde.
Preparation and Study of Spermatozoa Bound to Human Zonae Pellucidae
Zonae pellucidae of prophase 1 human oocytes that originated in postmortem ovarian tissue were obtained from Dr Denny Franken (Stellenbosch University, South Africa). These oocytes had no embryonic developmental potential following storage at 4°C in a Hepes buffer containing magnesium chloride and polyvinylpyrrolidone.35
Oocytes were washed in phosphate-buffered saline (PBS) and 5% BSA before use. Sperm were prepared as described earlier and capacitated for 4 hours in HTF medium at 37°C. From this suspension, different aliquots were used for preparation of HA-bound fraction, smeared to regular glass slide, and after fixation the phosphotyrosine patterns of capacitated sperm were determined prior to zona pellucida binding. After 4 hours of incubation, an aliquot of sperm suspension (0.5 × 106 motile sperm/mL) was added to droplets containing zona pellucida under oil. The zonae were then incubated for 3 hours with the sperm. The sperm loosely attached to the zonae pellucidae were removed by minimal pipetting, and the sperm–hemizona complexes were fixed with formaldehyde.36 After fixation, the sperm–hemizona complexes were washed in PBS and 5% BSA and incubated in 20 µL of propidium iodide (1 mg/mL; Sigma-Aldrich, St Louis, Missouri) for 10 minutes at room temperature. The sperm–hemizona complexes were gently washed and mounted on a slide prior to observation. The hemizonae protocol was performed by making minor modifications to the published protocol of Burkman et al.36 Therefore, the control incubation with matching hemizone was not used, since the aim was not to compare the fertilization capacity of sperm samples.36
Detection of TP by Immunofluoresence
Sperm from noncapacitated, capacitated, HA-bound, and zona-bound fractions were fixed with formaldehyde. After removal of the formalin, the slide was allowed to air-dry. Following 3 washing steps with PB-suc, the spermatozoa were exposed to a blocking solution of 3% BSA for 1 hour. In order to highlight the phoshotyrosine residues, the slides were incubated with a 1:100 dilution of monoclonal antiphosphotyrosine mouse antibody (clone 4G10, Millipore, Billerica, Massachusetts) overnight at 4°C. After more PB-suc washes, the slides were processed with a biotinylated secondary antibody at a 1:1000 dilution. Further, the slides were exposed to fluorescein isothiocyanate (FITC)-labeled avidin for 30 minutes. The specificity of the staining was established by using preimmune serum in place of the first antibody or by applying the second antibody only. Sperm were then examined under a fluorescence microscope using a ×100 objective. On each study slide, 200 sperm cells were evaluated, and the percentages of different expression patterns were compared between the slides from 0, 1, and 4 hours of incubation.
Double Labeling for Phosphotyrosine and CK
In order to visualize the simultaneous presence of phosphotyrosine and cytoplasmic retention characterizing sperm with arrested development, double immunolabeling for phosphotyrosine residues and CK (representing the presence of excess cytoplasm) was performed in the sperm suspension and in the HA-bound sperm fraction at the 0 and 4 hours. For CK immunostaining, fixed sperm cells were incubated with a 3% BSA (blocking solution) in PB-suc at room temperature. After washing with PB-suc, the sperm were exposed to a 1:1000 dilution of polyclonal anti-CK-B antiserum (Chemicon Co, Temecula, California) overnight at 4°C. After further PB-suc washes, the slides were processed with FITC-labeled anti-goat secondary antibody at a 1:500 dilution. After this step, the slides were incubated overnight at 4°C with a monoclonal antiphosphotyrosine mouse antibody. The phosphotyrosine immunoreaction was detected by using a biotinylated antimouse secondary antibody (Vector Laboratories, Burlingame, California) at a 1:1000 dilution and a 1:200 dilution of rhodamine-labeled avidin (Vector Laboratories).
Statistics
Data analysis was carried out with Sigma-Stat 3.5 program (Jandel Corporation, San Rafael, California). The differences between initial, HA-bound, and zona-bound sperm fractions and also between the various TP patterns following various incubation times were compared by the student t test. The data are presented as mean ± standard error of the mean.
Results
Initiation of Sperm Capacitation and Localization of Phosphorylated Tyrosine Residues in Human Sperm
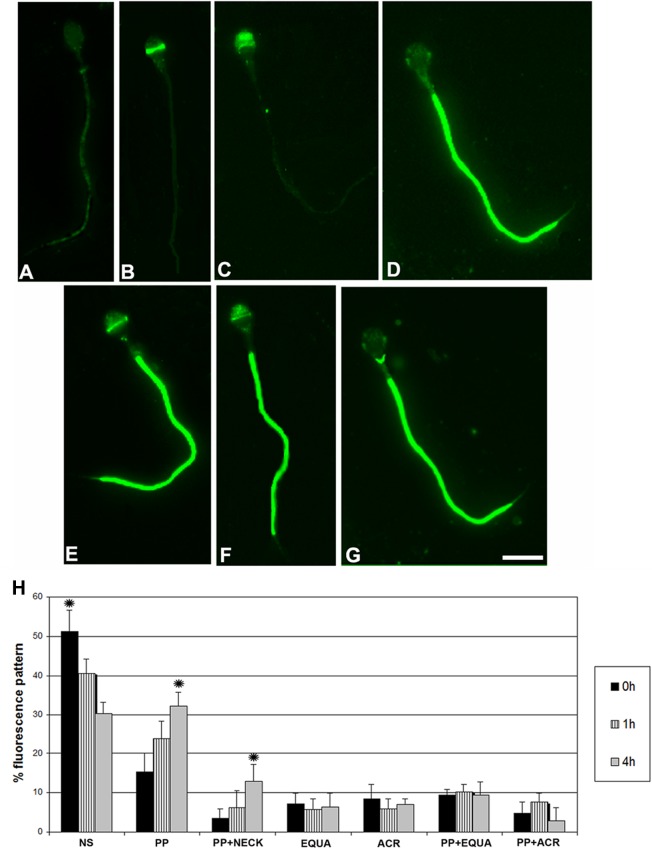
In the 32 men, we studied the variation in regional localization of phosphotyrosine in noncapacitated and capacitated human spermatozoa. The phosphorylated tyrosine residues were localized in the regions of (1) the acrosomal cap; (2) the equatorial segment; (3) the combined principal piece plus acrosomal region; (4) the principal piece plus equatorial segment; and (5) the principal piece plus neck region (Figure 1). The phosphotyrosine immunoreaction in the neck region was seen together with principal piece staining, but the equatorial region and acrosomal region were not always associated with principal piece staining. We compared the phosphotyrosine expression patterns during the 0, 1, and 4 hours of incubation and found that there were time-dependent changes in expression patterns shown by their varied percentages. It is of interest that a minor population of human spermatozoa showed tyrosine phosphorylation in the acrosomal cap and the equatorial region only (Figure 1H).
Figure 1.
Immunofluorescent localization of phosphotyrosine residues in human sperm. Fluorescence sperm regions: (A) nonstained spermatozoa; (B) equatorial region; (C) a cap region; (D) principal piece; (E) principal piece and equatorial region; (F) principal piece and acrosomal cap region; (G) principal piece and neck; (H) proportion of each tyrosine immunofluorescence pattern in noncapacitated and capacitated sperm within the 32 men (in each man 200 sperm were evaluated, 6400 sperm in all). Asterisks indicate significant differences (P < .05); NS, nonstaining, PP, principal piece; PP and neck, principal piece and neck region; EQUA, equatorial region; ACR, acrosomal region; PP + ACR, principal piece and acrosomal region.
The extent of phosphotyrosine significantly increased with time during the 0, 1, and 4 hours of incubation. Major phosphotyrosine changes were observed in the principal piece and the neck region of the mature sperm (Figure 1H and Figure 2). If the incubation time of the sperm was extended longer than 4 hours, the pattern and proportion of the phosphotyrosine did not change substantially in any of the 3 sperm groups observed (nonstaining, principal piece, principal piece and neck region). The proportion of sperm with principal piece and principal piece plus neck phosphorylation was significantly higher in the capacitated sperm fractions (P < .05; Figure 1H).
Figure 2.
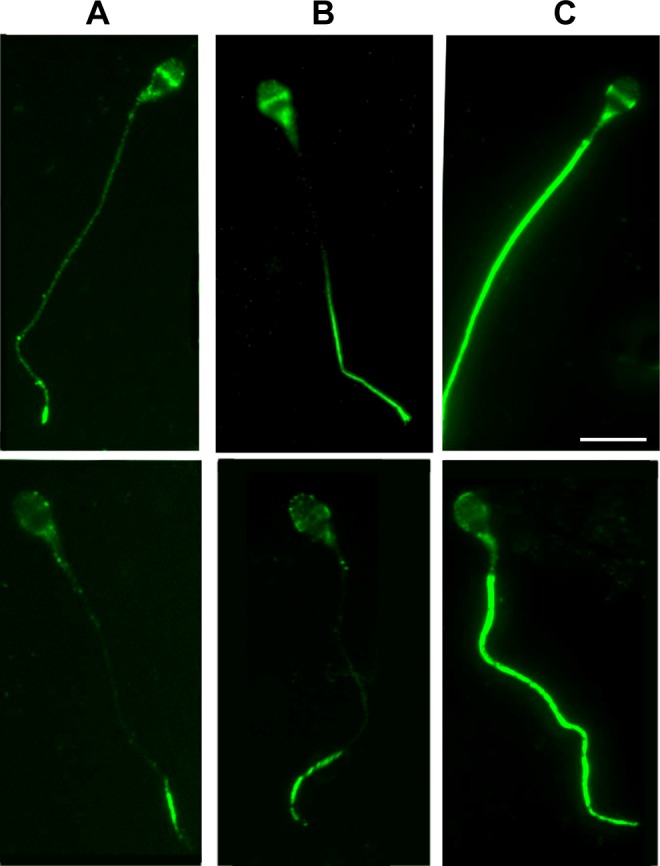
A representative figure for tyrosine phosphorylation (TP) at different time points. The extent of tyrosine phosphorylation is significantly increased at 1 hour (B) and 4 hours (C) versus at 0 time (A). Please observe major TP changes in the principal piece and the neck regions of mature sperm.
We also established that the phosphotyrosine reaction initiates at the tip of the sperm tail and propagates through the neck during capacitation (0, 1, and 4 hours; Figure 2). In some sperm, the reaction did not change substantially in the head region, but the tail changes were always distinguishable (Figure 2).
There was also variability among patients with respect to the TP in the initial semen-originated and HA-selected/-bound sperm fractions. However, the immunoreaction at the tip of the tail, acrosome, and neck regions was observed in at least 50% to 70% of the each sample studied.
Characteristics of TP Pattern in Sperm Bound to the Zona Pellucida
In order to determine the TP patterns of sperm bound to zonae pellucidae, 5 zona-binding experiments were performed (Figure 3). The phosphotyrosine was localized to principal piece, principal piece and neck regions, acrosomal cap region, and equatorial head region of the spermatozoa. When comparing capacitated human sperm incubated for 4 hours in suspension and in sperm bound to the zonae pellucidae, there was a significant increase (P < .05) in phosphorylation of zona pellucida-bound sperm, with phosphotyrosine residues localized to the neck and the principal piece (Figure 4C). Moreover, a lower proportion of sperm showed acrosomal and equatorial staining whether they were or were not associated with a phosphorylated principal piece.
Figure 3.
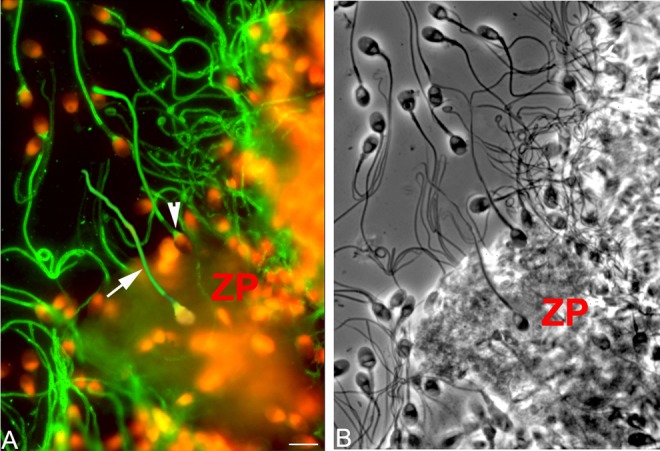
Immunofluorescent localization of phosphotyrosine in sperm bound to zonae pellucidae (ZP). (A) Tyrosine phosphorylation (TP) fluorescence (green) was mainly localized to the principal piece (arrow) and neck (arrowhead). Head of the sperm was labeled with propidium iodide (red). (B) Corresponding phase-contrast photomicrograph.
Figure 4.
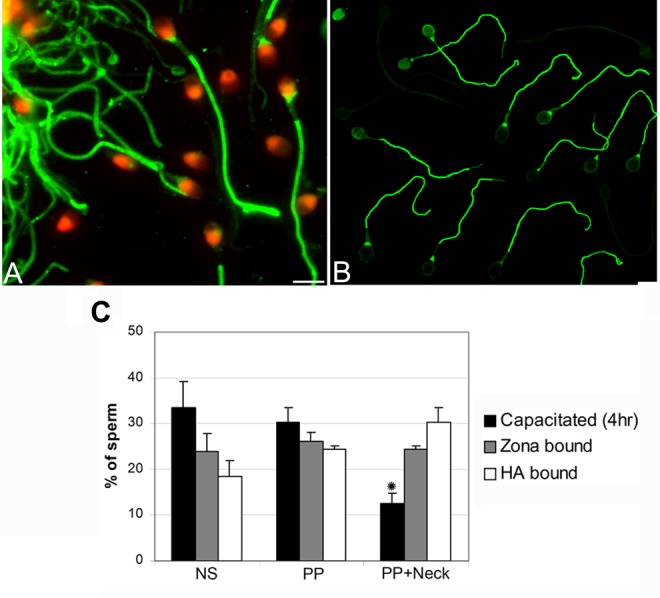
Comparison of tyrosine phosphorylation (TP) pattern in zona pellucida-bound (A) and HA-bound (B) sperm. Fluorescence was localized to the principal piece and neck region of spermatozoa in an identical pattern within both the zona pellucida- and the HA-bound sperm fractions. (C) Comparison of the extent of TP within the whole sperm population (motile and nonmotile) of capacitated sperm (4 hours), zona pellucida-bound and HA-bound sperm fractions. NS indicates nonstaining; PP, principal piece; PP + neck, principal piece and neck region. Data represent the mean proportion of each pattern within the 16 men. Two hundred sperm were examined for each patient (3200 sperm in all). Asterisk indicates significant differences (P < .05). No significant differences were found between the proportions of sperm with TP within the HA- and zona pellucid-bound sperm fractions.
Overall, there was a significant difference in the proportion of zona pellucida-bound sperm with positive phosphotyrosine response localized to the neck and the principal piece of the capacitated sperm compared to phosphotyrosine levels prior to the zona pellucida binding step (Figure 3 and Figure 4C). Although some variations were observed in different experiments, the number of the sperm showing a positive principal piece as well as principal piece and neck staining was always higher.
Thus, our TP findings in sperm bound to zona pellucida agree with those of Sakkas et al,20 and sperm bound to zona pellucida exhibited TP staining within the neck and principal piece, the acrosomal cap, and the equatorial region of the sperm head.
Comparative Study of TP Patterns in Spermatozoa Bound to Zonae Pellucidae or HA
In order to verify whether the TP immunostaining pattern is similar in zona pellucida- and HA-bound sperm fractions, 16 zona pellucida- and HA-binding experiments were performed. We used the sperm-HA-binding slides (HBA test), as described previously.29,31,32 Also, we compared the TP patterns in zona pellucida-bound and HA-bound spermatozoa originating within the same semen samples. The TP pattern in zona-bound and HA-bound sperm was similar (Figure 4 and 4C). The majority of sperm that are bound to HA have TP reaction on the principal piece and neck region, as described in the present study and in an earlier report for hemizona-bound spermatozoa.20 Moreover, the proportion of principal piece and neck region staining was similar in both the zona pellucida-bound and the HA-bound sperm fractions (24.5% ± 5.4% and 30.3% ± 5.3%, nonstaining; Figure 4C).
Although some variations was evident in some of the individual patterns obtained in different experiments (3 of the 16 patients), there were consistent increases with respect to principal piece plus neck region staining in both HA-bound and zona-bound sperm fractions compared to capacitated sperm incubated for 4 hours in suspension (P < .05, N = 16; Figure 4C).
Sperm Maturity and TP
In line with the previous studies, directed to the functional attribute differences in semen spermatozoa, we examined the sperm fraction originating in the HA-bound sperm fractions that underwent plasma membrane remodeling in 13 men. The question addressed whether or not there is a relationship between sperm with diminished cellular maturity/development as indicated by the presence of surplus cytoplasm and the response with TP during the capacitation process. In order to accomplish this, we double labeled the initial semen sperm and their respective HA-bound fractions with both CK and phosphotyrosine markers (Figure 5). Four sperm-staining patterns were categorized: only CK positive, only phosphotyrosine positive, both CK and phosphotyrosine positive, or lack of staining with either (Figure 5).
Figure 5.
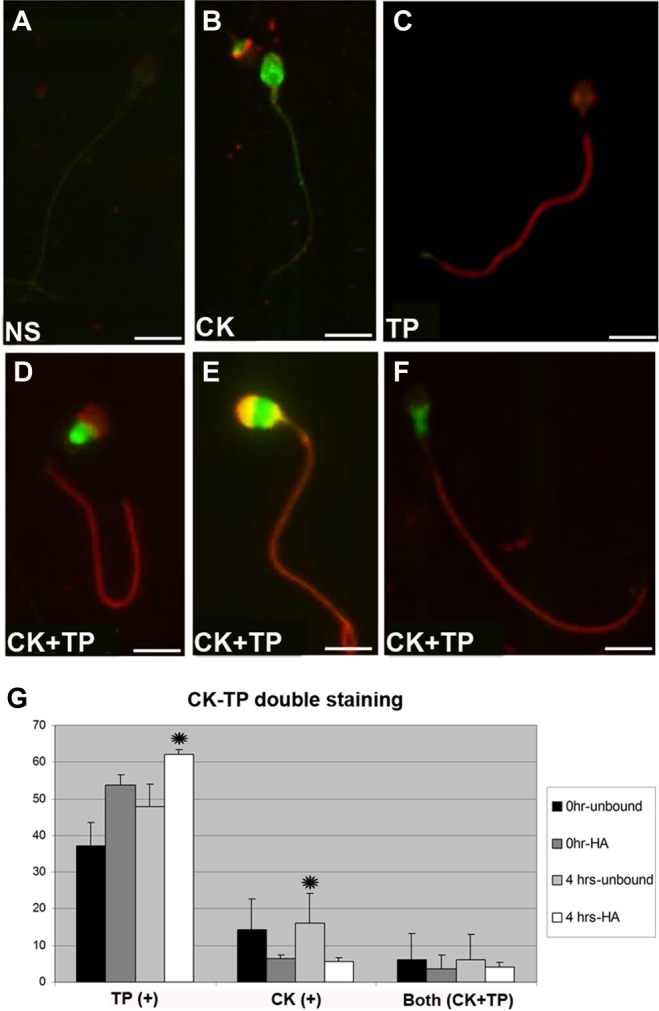
Immunofluorescent localization of creatine kinase (CK) and phosphotyrosine in human sperm. (A) Nonstained; (B) CK stained; (C) phosphotyrosine stained; (D-F) CK-phosphotyrosine (green-red) double-stained sperm; (G) proportion of double immunostaining of phosphotyrosine and CK in HA-bound and initial sperm fractions. Data represent the mean proportion of each pattern for the 13 patients. Two hundred sperm were examined for each patient (2600 sperm in all). Asterisks indicate significant differences (P < .05).
The spermatozoa stained light, intermediate, or dark with CK (reflecting the degree of cytoplasmic retention, thus arrested development) represent spermatozoa that show full development, intermediate development, or arrested development, respectively.37 Among the double-stained sperm, CK- and phosphotyrosine-labeled sperm was present only in diminished proportions, showing that immature sperm with CK staining surplus cytoplasmic retention exhibit limited TP (Figure G). However, the HA-bound sperm fractions that completed membrane remodeling showed very small number of CK-positive sperm along with a high proportion of sperm with extensive TP. After 4 hours, the rate of double-labeled sperm remained unchanged, although there was an increasing proportion of sperm with TP staining only.
Discussion
In the present study, we confirmed the previous observations that the levels of capacitation-related TP have increased in a time-related manner in the various regions of human sperm. For the first time, we showed that the extent of TP in the sperm neck and principal piece, a pattern that is a marker of sperm activation, increases in a time-related manner following sperm-HA-binding and sperm-HA interaction, similar to sperm that are bound to human zona pellucida. Furthermore, due to the common origin of spermiogenetic plasma membrane remodeling and formation of the respective receptors for both the zona pellucida- and the HA-binding sites,29,31,38 the sperm TP pattern changes were similar in the zona pellucida-bound and HA-bound sperm fractions (Figure 4). The proportion of TP in arrested maturity sperm with cytoplasmic retention was diminished. Conversely, the zona pellucida- and HA-bound mature sperm showed the highest degree of TP (Figure G).
Various membrane regions of human spermatozoa, in line with the changes in the physiological state and sperm activation, undergo TP during both capacitation and binding to the zona pellucida or HA. We report, as observed previously by others, within the zona pellucida-bound spermatozoa, that the tyrosine-phosphorylated proteins were localized mainly in the principal piece of human sperm.20,39,40 It has been postulated that TP in the principal piece is related to hyperactivated motility, a motility pattern exhibited in association with the penetration of the cumulus oophorus and the zona pellucida of oocyte leading to fertilization.21 The proteins subjected to TP that affect sperm motility include structural components of the flagellum, the A-kinase-anchoring proteins (AKAPs); however, the precise complement of proteins phosphorylated during capacitation is not yet identified in men and in other species, such as rodents, but likely include signaling molecules, chaperone-like proteins, glycolytic enzymes, and also a protein that participates in transcriptional regulation.41 The α and β tubulins in the sperm tail were also found subjected to TP.42 Recently, Grizard et al have shown that adhesion of prostasomes, vesicles present in human semen, also known to be important in male fertility, to spermatozoa could negatively affect TP.43
The primary increase in the level of TP, following binding to zonae pellucidae or HA, occurred in the sperm neck region. Binding to the zona pellucida or HA, in addition to the increase in principal piece phosphorylation, further promoted TP in the sperm neck. This finding further supports that zonae pellucidae and hyaluronic acid elicit similar changes in the pattern of TP in the respective bound spermatozoa.
Various studies indicated that human sperm bound to HA exhibit attributes similar to that of zona pellucida-bound sperm, including lack of cytoplasmic retention and persistent histones, high DNA integrity shown with DNA in situ nick translation or with acridine orange fluorescence,31,32 and improvement in the proportion of sperm with Tygerberg normal morphology.31,38,44 Indeed, in the present study, we report similarities in TP in sperm bound to the zona pellucida or HA.20 Additionally, HA-bound sperm displayed the highest proportion of TP in contrast to sperm with arrested development and cytoplasmic retention, as detected by the presence of surplus CK.
In a previous study, a tyrosine kinase receptor was localized to the surface of the acrosomal cap in human sperm that exhibited TP following binding to the zonae pellucidae.45 In addition, inhibition of TP prevented the occurrence of acrosome reaction. This suggests the necessity of TP step for the zona pellucida-induced acrosome reaction.46–48 It has also been suggested that head TP is a subsurface event that occurs early during capacitation.49 In contrast to TP in the flagellum, we did not find the proportion of spermatozoa displaying TP in the acrosomal region to have increased with the process of capacitation. Recent findings on the roles of protein TP in many sperm-related processes (from spermatogenesis to epididymal maturation, capacitation, acrosomal exocytosis, and fertilization) are also summarized by Ijiri et al.50
It is of note that approximately 50% of spermatozoa failed to undergo phosphorylation after capacitation, suggesting that various subpopulations of spermatozoa may exhibit different degrees of responsiveness regarding TP upon interaction with the zona pellucida or HA. The possibility that the spermatozoa that exhibit a higher phosphorylation ability have a competitive advantage in the fertilization process remains to be elucidated. The findings of the present report may have implications for certain types of male subfertility/infertility, as unresponsive spermatozoa in an ejaculate, which fail to progress with the physiological sequence of phosphorylation and capacitation, are likely to show diminished oocyte binding, thus fertilizing potential.24
In conclusion, the current data, in agreement with previous findings, showed that various regions of human spermatozoa undergo a specific TP during both capacitation and the binding process regardless of zona pellucida or HA. Based on the similarity in phophorylation patterns of sperm bound either to zona pellucida or to HA, we suggest that there is a common regulatory pathway of TP related to sperm ability. We believe that such regulatory pathway originated in the synchronous formation of the zona pellucida and HA receptors in the sperm plasma membrane following the remodeling process related to the progress of spermiogenesis.
Acknowledgments
The authors wish to acknowledge the generous contributions by Dr. Denny Franken (Stellenbosch University, South Africa) and Jill Stronk, Sperm Physiology Laboratory, Yale Fertility Center.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received the following financial support for the research, authorship, and/or publication of this article : This study was supported by the NIH (OH-04061).
References
- 1. Yanagimachi R. Fertility of mammalian spermatozoa: its development and relativity. Zygote. 1994;2(4):371–372. [DOI] [PubMed] [Google Scholar]
- 2. Travis AJ, Kopf GS. The role of cholesterol efflux in regulating the fertilization potential of mammalian spermatozoa. J Clin Invest. 2002;110(6):731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Demarco IA, Espinosa F, Edwards J, et al. Involvement of a Na+/HCO-3 cotransporter in mouse sperm capacitation. J Biol Chem. 2003;278(9):7001–7009. [DOI] [PubMed] [Google Scholar]
- 4. Harayama H, Muroga M, Miyake M. A cyclic adenosine 3',5'-monophosphate-induced tyrosine phosphorylation of Syk protein tyrosine kinase in the flagella of boar spermatozoa. Mol Reprod Dev. 2004;69(4):436–447. [DOI] [PubMed] [Google Scholar]
- 5. Harayama H, Nakamura K. Changes of PKA and PDK1 in the principal piece of boar spermatozoa treated with a cell-permeable cAMP analog to induce flagellar hyperactivation. Mol Reprod Dev. 2008;75(9):1396–1407. [DOI] [PubMed] [Google Scholar]
- 6. Barbonetti A, Vassallo MR, Cinque B, et al. Dynamics of the global tyrosine phosphorylation during capacitation and acquisition of the ability to fuse with oocytes in human spermatozoa. Biol Reprod. 2008;79(4):649–656. [DOI] [PubMed] [Google Scholar]
- 7. Wertheimer EV, Salicioni AM, Liu W, et al. Chloride is essential for capacitation and for the capacitation-associated increase in tyrosine phosphorylation. J Biol Chem. 2008;283(51):35539–35550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sagare-Patil V, Modi D. Progesterone activates Janus Kinase 1/2 and activators of transcription 1 (JAK1-2/STAT1) pathway in human spermatozoa. Andrologia. 2013;45(3):178–186. [DOI] [PubMed] [Google Scholar]
- 9. Orta G, Ferreira G, Jose O, Trevino CL, Beltran C, Darszon A. Human spermatozoa possess a calcium-dependent chloride channel that may participate in the acrosomal reaction. J Physiol. 2012;590(pt 11):2659–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Signorelli J, Diaz ES, Morales P. Kinases, phosphatases and proteases during sperm capacitation. Cell Tissue Res. 2012;349(3):765–782. [DOI] [PubMed] [Google Scholar]
- 11. Ma F, Wu D, Deng L, et al. Sialidases on mammalian sperm mediate deciduous sialylation during capacitation. J Biol Chem. 2012;287(45):38073–38079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Jonge CJ, Barratt CL. Methods for the assessment of sperm capacitation and acrosome reaction excluding the sperm penetration assay. Methods Mol Biol. 2013;927:113–118. [DOI] [PubMed] [Google Scholar]
- 13. Arcelay E, Salicioni AM, Wertheimer E, Visconti PE. Identification of proteins undergoing tyrosine phosphorylation during mouse sperm capacitation. Int J Dev Biol. 2008;52(5-6):463–472. [DOI] [PubMed] [Google Scholar]
- 14. Visconti PE, Moore GD, Bailey JL, et al. Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development. 1995;121(4):1139–1150. [DOI] [PubMed] [Google Scholar]
- 15. Lewis B, Aitken RJ. Impact of epididymal maturation on the tyrosine phosphorylation patterns exhibited by rat spermatozoa. Biol Reprod. 2001;64(5):1545–1556. [DOI] [PubMed] [Google Scholar]
- 16. Si Y, Okuno M. Role of tyrosine phosphorylation of flagellar proteins in hamster sperm hyperactivation. Biol Reprod. 1999;61(1):240–246. [DOI] [PubMed] [Google Scholar]
- 17. Kulanand J, Shivaji S. Capacitation-associated changes in protein tyrosine phosphorylation, hyperactivation and acrosome reaction in hamster spermatozoa. Andrologia. 2001;33(2):95–104. [DOI] [PubMed] [Google Scholar]
- 18. Bailey JL. Factors regulating sperm capacitation. Syst Biol Reprod Med. 2010;56(5):334–348. [DOI] [PubMed] [Google Scholar]
- 19. Urner F, Leppens-Luisier G, Sakkas D. Protein tyrosine phosphorylation in sperm during gamete interaction in the mouse: the influence of glucose. Biol Reprod. 2001;64(5):1350–1357. [DOI] [PubMed] [Google Scholar]
- 20. Sakkas D, Leppens-Luisier G, Lucas H, et al. Localization of tyrosine phosphorylated proteins in human sperm and relation to capacitation and zona pellucida binding. Biol Reprod. 2003;68(4):1463–1469. [DOI] [PubMed] [Google Scholar]
- 21. Nassar A, Mahony M, Morshedi M, Lin MH, Srisombut C, Oehninger S. Modulation of sperm tail protein tyrosine phosphorylation by pentoxifylline and its correlation with hyperactivated motility. Fertil Steril. 1999;71(5):919–923. [DOI] [PubMed] [Google Scholar]
- 22. Stauss CR, Votta TJ, Suarez SS. Sperm motility hyperactivation facilitates penetration of the hamster zona pellucida. Biol Reprod. 1995;53(6):1280–1285. [DOI] [PubMed] [Google Scholar]
- 23. Liu DY, Clarke GN, Baker HW. Tyrosine phosphorylation on capacitated human sperm tail detected by immunofluorescence correlates strongly with sperm-zona pellucida (ZP) binding but not with the ZP-induced acrosome reaction. Hum Reprod. 2006;21(4):1002–1008. [DOI] [PubMed] [Google Scholar]
- 24. Buffone MG, Verstraeten SV, Calamera JC, Doncel GF. High cholesterol content and decreased membrane fluidity in human spermatozoa are associated with protein tyrosine phosphorylation and functional deficiencies. J Androl. 2009;30(5):552–558. [DOI] [PubMed] [Google Scholar]
- 25. Huszar G, Vigue L, Corrales M. Sperm creatine kinase activity in fertile and infertile oligospermic men. J Androl. 1990;11(1):40–46. [PubMed] [Google Scholar]
- 26. Huszar G, Vigue L. Incomplete development of human spermatozoa is associated with increased creatine phosphokinase concentration and abnormal head morphology. Mol Reprod Dev. 1993;34(3):292–298. [DOI] [PubMed] [Google Scholar]
- 27. Cayli S, Jakab A, Ovari L, et al. Biochemical markers of sperm function: male fertility and sperm selection for ICSI. Reprod Biomed Online. 2003;7(4):462–468. [DOI] [PubMed] [Google Scholar]
- 28. Huszar G, Sbracia M, Vigue L, Miller DJ, Shur BD. Sperm plasma membrane remodeling during spermiogenetic maturation in men: relationship among plasma membrane beta 1,4-galactosyltransferase, cytoplasmic creatine phosphokinase, and creatine phosphokinase isoform ratios. Biol Reprod. 1997;56(4):1020–1024. [DOI] [PubMed] [Google Scholar]
- 29. Huszar G, Ozenci CC, Cayli S, Zavaczki Z, Hansch E, Vigue L. Hyaluronic acid binding by human sperm indicates cellular maturity, viability, and unreacted acrosomal status. Fertil Steril. 2003;79(suppl 3):1616–1624. [DOI] [PubMed] [Google Scholar]
- 30. Jakab A, Sakkas D, Delpiano E, et al. Intracytoplasmic sperm injection: a novel selection method for sperm with normal frequency of chromosomal aneuploidies. Fertil Steril. 2005;84(6):1665–1673. [DOI] [PubMed] [Google Scholar]
- 31. Huszar G, Jakab A, Sakkas D, et al. Fertility testing and ICSI sperm selection by hyaluronic acid binding: clinical and genetic aspects. Reprod Biomed online. 2007;14(5):650–663. [DOI] [PubMed] [Google Scholar]
- 32. Yagci A, Murk W, Stronk J, Huszar G. Spermatozoa bound to solid state hyaluronic acid show chromatin structure with high DNA chain integrity: an acridine orange fluorescence study. J Androl. 2010;31(6):566–572. [DOI] [PubMed] [Google Scholar]
- 33. Worrilow K, Eld S, Woodhouse J. Prospective, multi-center, double-blind, randomized clinical trial evaluating the use of hyaluronan-bound sperm in ICSI: statistically significant improvement in clinical outcomes. ASRM annual meeting. Orlando, Florida: 2011. [Google Scholar]
- 34. Worrilow KC, Eid S, Woodhouse D, et al. Use of hyaluronan in the selection of sperm for intracytoplasmic sperm injection (ICSI): significant improvement in clinical outcomes—multicenter, double-blinded and randomized controlled trial. Hum Reprod. 2013;28(2):306–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Franken DR, Acosta AA, Kruger TF, Lombard CJ, Oehninger S, Hodgen GD. The hemizona assay: its role in identifying male factor infertility in assisted reproduction. Fertil Steril. 1993;59(5):1075–1080. [DOI] [PubMed] [Google Scholar]
- 36. Burkman LJ, Coddington CC, Franken DR, Krugen TF, Rosenwaks Z, Hogen GD. The hemizona assay (HZA): development of a diagnostic test for the binding of human spermatozoa to the human hemizona pellucida to predict fertilization potential. Fertil Steril. 1988;49(4):688–697. [PubMed] [Google Scholar]
- 37. Sati L, Ovari L, Bennett D, Simon SD, Demir R, Huszar G. Double probing of human spermatozoa for persistent histones, surplus cytoplasm, apoptosis and DNA fragmentation. Reprod Biomed online. 2008;16(4):570–579. [DOI] [PubMed] [Google Scholar]
- 38. Huszar G, Patrizio P, Vigue L, et al. Cytoplasmic extrusion and the switch from creatine kinase B to M isoform are completed by the commencement of epididymal transport in human and stallion spermatozoa. J Androl. 1998;19(1):11–20. [PubMed] [Google Scholar]
- 39. Carrera A, Moos J, Ning XP, et al. Regulation of protein tyrosine phosphorylation in human sperm by a calcium/calmodulin-dependent mechanism: identification of A kinase anchor proteins as major substrates for tyrosine phosphorylation. Dev Biol. 1996;180(1):284–296. [DOI] [PubMed] [Google Scholar]
- 40. Leclerc P, de Lamirande E, Gagnon C. Regulation of protein-tyrosine phosphorylation and human sperm capacitation by reactive oxygen derivatives. Free Radic Biol Med. 1997;22(4):643–656. [DOI] [PubMed] [Google Scholar]
- 41. Tang JB, Chen YH. Identification of a tyrosine-phosphorylated CCCTC-binding nuclear factor in capacitated mouse spermatozoa. Proteomics. 2006;6(17):4800–4807. [DOI] [PubMed] [Google Scholar]
- 42. Mitchell LA, Nixon B, Baker MA, Aitken RJ. Investigation of the role of SRC in capacitation-associated tyrosine phosphorylation of human spermatozoa. Mol Hum Reprod. 2008;14(4):235–243. [DOI] [PubMed] [Google Scholar]
- 43. Bechoua S, Rieu I, Sion B, Grizard G. Prostasomes as potential modulators of tyrosine phosphorylation in human spermatozoa. Syst Biol Reprod Med. 2011;57(3):139–148. [DOI] [PubMed] [Google Scholar]
- 44. Huszar G, Vigue L, Oehninger S. Creatine kinase immunocytochemistry of human sperm-hemizona complexes: selective binding of sperm with mature creatine kinase-staining pattern. Fertil Steril. 1994;61(1):136–142. [DOI] [PubMed] [Google Scholar]
- 45. Burks DJ, Carballada R, Moore HD, Saling PM. Interaction of a tyrosine kinase from human sperm with the zona pellucida at fertilization. Science. 1995;269(5220):83–86. [DOI] [PubMed] [Google Scholar]
- 46. Leyton L, LeGuen P, Bunch D, Saling PM. Regulation of mouse gamete interaction by a sperm tyrosine kinase. Proc Natl Acad Sci U S A. 1992;89(24):11692–11695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pukazhenthi BS, Wildt DE, Ottinger MA, Howard J. Inhibition of domestic cat spermatozoa acrosome reaction and zona pellucida penetration by tyrosine kinase inhibitors. Mol Reprod Dev. 1998;49(1):48–57. [DOI] [PubMed] [Google Scholar]
- 48. Ickowicz D, Finkelstein M, Breitbart H. Mechanism of sperm capacitation and the acrosome reaction: role of protein kinases. Asian J Androl. 2012;14(6):816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Barbonetti A, Vassallo MR, Cordeschi G, et al. Protein tyrosine phosphorylation of the human sperm head during capacitation: immunolocalization and relationship with acquisition of sperm-fertilizing ability. Asian J Androl. 2010;12(6):853–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ijiri TW, Mahbub Hasan AK, Sato K. Protein-tyrosine kinase signaling in the biological functions associated with sperm. J Signal Transduct. 2012;2012:181560. [DOI] [PMC free article] [PubMed] [Google Scholar]